Abstract
Renal artery stenosis is increasing in prevalence. Angioplasty plus stenting has not proven to be better than medical management. There has been a reluctance to use available denervation methodologies in this condition. We studied conscious, chronically instrumented, two-kidney, one-clip (2K-1C) Goldblatt rats, a model of renovascular hypertension, to test the hypothesis that renal denervation by cryoablation (cryo-DNX) of the renal nerve to the clipped kidney decreases mean arterial pressure (MAP), plasma and tissue ANG II, and contralateral renal sympathetic nerve activity (RSNA). Five-week-old male Sprague-Dawley rats underwent sham (ShC) or right renal artery clipping (2K-1C), placement of telemetry transmitters, and pair-feeding with a 0.4% NaCl diet. After 6 wk, rats were randomly assigned to cryo-DNX or sham cryotreatment (sham DNX) of the renal nerve to the clipped kidney. MAP was elevated in 2K-1C and decreased significantly in both ShC cryo-DNX and 2K-1C cryo-DNX. Tissue norepinephrine was ∼85% lower in cryo-DNX kidneys. Plasma ANG II was higher in 2K-1C sham DNX but not in 2K-1C cryo-DNX vs ShC. Renal tissue ANG II in the clipped kidney decreased after cryo-DNX. Baseline integrated RSNA of the unclipped kidney was threefold higher in 2K-1C versus ShC and decreased in 2K-1C cryo-DNX to values similar to ShC. Maximum reflex response of RSNA to baroreceptor unloading in 2K-1C was lower after cryo-DNX. Thus, denervation by cryoablation of the renal nerve to the clipped kidney decreases not only MAP but also plasma and renal tissue ANG II levels and RSNA to the contralateral kidney in conscious, freely moving 2K-1C rats.
Keywords: angiotensin, baroreflex, Goldblatt kidney, renovascular hypertension, sympathetic nerve activity
activation of the sympathetic nervous system plays an important role in the development and maintenance of several forms of human hypertension (16, 23, 53) and animal hypertension (31, 42, 45, 46). Specifically, neuroexcitation contributes to the high arterial pressure and high plasma renin and angiotensin II (ANG II) levels in the two-kidney, one-clip (2K-1C) Goldblatt rat, a model of renovascular hypertension (17, 26, 39, 51). Early in the course of 2K-1C hypertension, renin secretion by the clipped kidney increases, and that of the contralateral kidney is suppressed by the elevated blood pressure. Approximately 6 wk after the renal artery was clipped, the elevated arterial pressure transitions from a primarily renin-dependent to a more neurogenically mediated mechanism (40).
Stimulation of the renal sympathetic nerves results in renin secretion as well as renal tubular sodium reabsorption (4, 34). Inhibitory renorenal reflexes are impaired in 2K-1C rats, thereby leading to enhanced efferent renal sympathetic nerve activity (RSNA) and urinary sodium retention (30). Denervation of the clipped kidney by surgical transection of the nerve with application of phenol to the nerve and artery of the clipped kidney interrupts nerve activity to the ischemic kidney. In addition, physiological indices consistent with diminished RSNA to the contralateral kidney such as renin secretion and renal sodium reabsorption are reduced (7, 19, 26, 28, 30, 50). Potential decreases in other sympathetic outputs resulting from interruption of afferent signals from the kidney may result in vasodilation of other vascular beds as well (32). Except for one study showing decreased RSNA in the contralateral kidney only 1.5 h after recovery from anesthesia (30), direct measurements of contralateral RSNA, certainly in fully awake and freely moving rats several days after recovery, have not been reported. Together, inhibition of the renin-angiotensin system, higher urinary sodium excretion, and vasodilation of selective vascular beds contribute to the reduction in arterial pressure observed with renal denervation of the stenotic kidney.
Over the last few years, renal sympathetic denervation for uncontrolled essential hypertension in humans has become an area of intense study (6, 33, 53), but significant renal artery stenosis has typically been an exclusion criterion (24). Importantly, renovascular hypertension due to atherosclerotic disease is increasing, with 20–54% prevalence in high-risk groups with diabetes mellitus, heart failure, or peripheral vascular disease (1, 15). Many such patients suffer from hypertension resistant to multidrug therapy (8). Nearly one-third of these individuals die within 5 yr, even with current interventional modalities (2). Moreover, angioplasty with or without stenting of the renal arteries has proven to be of little benefit in controlling arterial pressure, decreasing cardiac or renal events, or reducing mortality (2, 13, 61). A recent study in a limited number of patients who had already undergone renal artery stenting reported some success in further decreasing systolic pressure after radio frequency denervation (3). Given technical considerations such as avoiding the area near the stenosis and delivery of lower-power radio frequency energies, there is still concern as to whether renovascular patients are suitable for current approaches for renal sympathetic denervation (60).
Cryotherapies are used to ablate aberrant cardiac conduction pathways (52), treat malignancies (14), and ameliorate peripheral vascular disease (35). Therefore, it is possible that emerging endovascular cryotechnologies using very low temperatures may successfully ablate renal sympathetic nerves (36). The present studies were designed as proof-of-principle experiments to test the hypothesis that cryotreatment of the renal nerve to the clipped kidney will decrease systemic arterial pressure, reduce plasma ANG II levels, and decrease contralateral RSNA in conscious, chronically instrumented 2K-1C hypertensive rats. Since changes in baseline arterial pressure as well as alteration in afferent inputs from the kidney could very well modulate the arterial baroreflex, whenever possible, the baroreflex response of RSNA before and after cryotreatment was assessed in the 2K-1C rats.
METHODS
Male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) were used in all protocols. Rats were permitted to acclimate after being placed in the vivarium for a minimum of 3 days. They were housed under controlled conditions (ambient temperature 21–23°C, lights on from 0700 to 1900). They were permitted free access to water and standard rat chow containing 0.4% NaCl, except where noted by protocol. All rats were cared for in compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (8th edition, 2011). All procedures and protocols were reviewed and approved by the Wayne State University Institutional Animal Care and Use Committee.
Renal artery clipping.
Five-week-old rats were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (8 mg/kg). Additional doses (25–50% of the initial dose) were administered if needed to maintain a plane of anesthesia. The right renal artery was exposed via a right flank incision and visualized under a stereomicroscope so that the renal nerves as well as blood vessels could be carefully identified. Then, a 0.2-mm silver clip was placed around the artery (2K-1C), carefully avoiding the renal nerves. This resulted in the clip typically being placed more proximal to the aorta and avoiding the hilus of the kidney. Sham clipped rats (ShC) underwent identical surgery, but no clip was placed. The flank incision was closed with surgical staples.
Hemodynamic radiotelemetry transmitter placement.
Immediately after renal artery clipping was completed, the femoral artery was exposed via a groin incision and the proximal end occluded briefly so that the gel-filled catheter attached to the radiotelemetry transducer (TA11PA-C40; Data Sciences International, St. Paul, MN) could be inserted into the artery and then advanced into the distal aorta. The catheter was secured with medical adhesive and the transmitter device placed subcutaneously and secured to the underlying muscle. The skin was closed with surgical staples. The rat then received a dose of buprenorphine SR (0.3 mg/kg ip) for analgesia. Each rat was returned to its home cage with its individual receiver and permitted to recover for 3 days prior to hemodynamic recordings being initiated.
Dual hemodynamic and renal nerve radiotelemetry transmitter placement.
Rats were anesthetized with pentobarbital sodium (50 mg/kg ip). If required, supplemental doses were given as needed. The telemetry unit (Telemetry Research TR46S, Auckland, New Zealand), which has both blood pressure and nerve electrode components, was placed using a modification of the technique described by Stocker and Muntzel (57). Briefly, two incisions were made: one over the left flank and the other in the left groin. A separate venous catheter was first inserted into the left femoral vein, secured, and then tunneled subcutaneously and exteriorized posteriorly at the base of the neck. To maintain patency, the catheter was filled with heparinized saline (100 U/ml). Then, a tunnel was made subcutaneously from the femoral area to the flank. The electrode wires from the transmitter were passed through this tunnel for subsequent placement. First, the catheter with the arterial pressure transducer was inserted into the left femoral artery and secured with sutures. Then, the left renal nerve was identified under a stereomicroscope. The nerve was carefully placed onto the exposed ends of the electrode wires from the transmitter. The silicone casing of the proximal ends of the wires was then stabilized by anchoring it with 6-0 sutures to the adventitia of the aortic wall. Placement of the electrodes and quality of the nerve signal were established by evaluating the nerve sound using an audio monitor and verified using an oscilloscope (Hameg, New Meadow, NY). Then, the nerve and electrodes were encased with silicone gel (Kwik-Sil; World Precision Instruments, Sarasota, FL). The muscles were then sutured closed in layers, and the body of the transmitter was inserted subcutaneously over the lower abdomen. The skin was closed with surgical staples. The rat was then returned to its home cage and permitted to recover.
Cryotreatment of the renal nerve.
Cryotreatment was performed using the argon-based CryoCare system with a PERC-15 Percryo cryoprobe (Endocare, Austin, TX). Rats were anesthetized with ketamine and xylazine as before. A right flank incision was made along the scar from the previous surgery. The right renal nerve was identified and isolated under a stereomicroscope. The tip of the cryoprobe was carefully held under the nerve such that the tip was isolated from other tissues as the nerve lay on the tip. Freezing was initiated at 100% power. Once the probe temperature reached −155 ± 5°C, it was held for 30 s, followed by a thawing cycle (maximum temperature not exceeding 7°C) of 1 min. The freeze-thaw cycle was repeated a total of three times (cryo-DNX). Then, the muscle and skin layers were closed. Sham-treated rats underwent the same procedures, but without freezing or thawing (sham DNX). Rats were returned to their home cages and received acetaminophen (6 mg/ml) in their drinking water for 3 days for analgesia to avoid potential effects of long-acting opioids on blood pressure.
Protocol 1.
The experimental design in protocol 1 is depicted in Fig. 1. All protocol measurements were performed on conscious rats. After renal artery clipping or sham clipping completed and hemodynamic telemetry transmitters were placed, arterial pressure and heart rate were monitored for 6 wk by telemetry. At the end of the 6th wk, each rat was randomly assigned to undergo cryotreatment of the right renal nerve or sham cryotreatment. Hemodynamic parameters were then monitored for an additional 2 wk in the resulting four groups of rats (ShC cryo-DNX, ShC sham DNX, 2K-1C cryo-DNX, and 2K-1C sham DNX). Each rat was then placed into a metabolic cage and permitted to acclimate for 3 days. During the entire time, they were provided with free access to water. Food intake was measured daily. The 2K-1C rats typically ingest ∼2–5 g less food than ShC rats, so on the third day pair-feeding was begun so that each ShC rat was pair-fed to receive the same amount of 0.4% NaCl diet as its 2K-1C counterpart. Urine was collected daily from days 4 to 7 for measurement of volume, Na, K, creatinine, and protein. Upon completion of the balance studies, the rats were returned to their home cages, and hemodynamic recording was resumed for 1 wk. At the end of the observation period, each rat was anesthetized with ketamine and xylazine as described above, the aorta was exposed via a midline abdominal incision, and aortic blood was obtained for measurement of Na, K, creatinine, and ANG II. Both kidneys were harvested and weighed, and cortical tissue was snap-frozen for determination of tissue ANG II and norepinephrine.
Fig. 1.
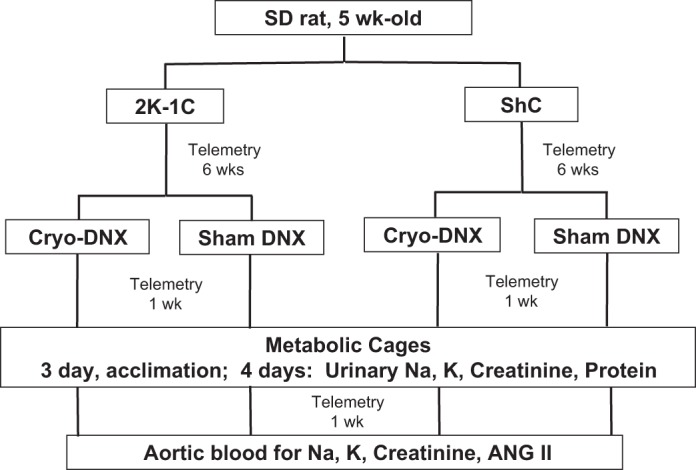
Timeline and procedures in the experimental design for rats in protocol 1 (see methods). 2K-1C, two-kidney, one-clip; ShC, sham clipped; Cryo-DNX, cryo-treated nerve on clipped side; sham DNX, sham-treated nerve on clipped side; ANG II, angiotensin II.
For comparison with previously published data regarding renal tissue ANG II in 2K-1C rats (44), separate groups of sham clipped and 2K-1C rats were anesthetized as above, and renal cortical tissue was taken for assessment of tissue ANG II 1 wk after sham or renal artery clipping.
Protocol 2.
After renal artery clipping or sham clipping was completed, rats were returned to their home cages. Five and a half weeks later, each rat was equipped with a dual hemodynamic and renal nerve telemetry transmitter and a venous catheter for infusions. Arterial pressure, heart rate, and RSNA were recorded daily from 0800 to 1200 via a TR161 receiver. Data was digitized, recorded, and analyzed using an analog-to-digital converter and software platform (Power Lab 8/30 and LabChart Pro 7; ADInstruments, Colorado Springs, CO). Four days after placement of the transmitter, ShC and 2K-1C rats then underwent either cryotreatment or sham cryotreatment of the right renal nerve. This resulted in four groups: ShC cryo-DNX, ShC sham DNX, 2K-1C cryo-DNX, and 2K-1C sham DNX. Recording of hemodynamic and RSNA parameters continued for ≤10 days or until the nerve recording lost fidelity. Values for RSNA were used only for analysis from day 3 after surgery to permit full recovery from anesthesia. In addition, RSNA was monitored daily, and if the recording failed on a given day (e.g., day 6), values only from ≥24 h prior to the failure (day 4) were used to ensure validity. On average, our nerve activity lasted 5 days.
Baroreflex testing of the heart rate and RSNA responses was performed on unrestrained, conscious 2K-1C rats in their home cages 2 days prior to and 3 days after cryo or sham treatment of the renal nerve. Baroreflex curves were generated by inducing ramp decreases and increases in arterial pressure. Nitroprusside (200 μg/ml; Ohmeda) was infused intravenously at an increasing rate of 7.5–100 μg·kg−1·min−1 and phenylephrine at 5–50 μg·kg−1·min−1 (200 μg/ml; Sigma Aldrich, St. Louis, MO) so as to result in a ramp decrease or increase, respectively, over a 2-min period. Fifteen to 30 min were permitted between infusions for all parameters to return to baseline values. At the end of each experiment, trimethaphan camsylate (20 mg/kg iv; Hoffman-La Roche) was administered as a bolus dose to assess background noise.
Plasma and urinary measurements.
Urine volume was measured gravimetrically. Plasma and urinary sodium concentrations were measured by flame photometry (Model 2655-10; Cole-Parmer, Vernon Hills, IL). Creatinine in plasma and urine was measured using a modified colorimetric Jaffe reaction (Pointe Scientific, Canton, MI) (47). Urinary protein was assessed by the method of Lowry et al. (38).
Plasma and tissue ANG II radioimmunoassay.
Plasma was assayed for ANG II by the method reported by Navar et al. (44) and adapted by our laboratory (39). In brief, 1 ml of plasma was extracted with 90% methanol in water. The extracts were taken to dryness under nitrogen and stored at −70°C overnight. Renal cortical tissue was processed by homogenizing 50–100 mg tissue in ice-cold methanol, followed by centrifugation at 4,000 g for 30 min at 0°C. The supernatant was aspirated into the assay tubes and the pellet resuspended in ice-cold methanol, homogenized, and centrifuged as before. The supernatants were then combined, dried down under nitrogen, and stored at −70°C until assay.
Plasma and tissue extracts were reconstituted in assay buffer containing 50 mM sodium phosphate, 1 mM EDTA, 0.25 mM thimerosal, and 0.25% peptidase-free human serum albumin. Each sample was assayed in duplicate. [125I]ANG II (Perkin-Elmer, Billerica, MA) was used as the tracer. The anti-ANG II antibody (Peninsula Laboratories, San Carlos, CA) was at a final dilution of 1:660,000. Nonspecific binding was 2.3%; the lower limit of detection was 0.6 fmol/tube; 50% binding was 16.0 fmol/tube.
Tissue norepinephrine assay.
Renal tissue was homogenized in 0.1 N perchloric acid with 1 μM EGTA and centrifuged at 4,000 g for 10 min. An aliquot of the supernatant was diluted 1:10 in perchloric-EGTA for assay, and the remainder was frozen at −70°C. The diluted sample was shipped on dry ice for analysis at the Vanderbilt Hormone Assay Core (http://hormone.mc.vanderbilt.edu). Analysis was accomplished by high-pressure liquid chromatography via electrochemical detection. Dehydroxylbenzylamine was used as the internal standard with each extraction to monitor recovery and determine quantitation.
Analyses and statistics.
Heart rate and arterial pressure were averaged over 7 consecutive days unless specified otherwise. Resting integrated RSNA consisted of 1-s sequential averages over the 4-h recording period. Values were expressed as means ± SE. Protocol 1 was designed to assess a difference of 10 mmHg with a standard deviation of 5 mmHg, with 95% power at an α-level of 0.05, thus requiring nine rats per group. The study in protocol 2 was powered to assess a 50% change in resting integrated RSNA of the 2K-1C group (∼4.5 μV/s with a standard deviation of 2 μV/s) 3 days after cryotreatment, with 95% power and an α-level of 0.05, thus requiring four rats per group. Comparison with baseline values in the same animal was accomplished by using the paired t-test. Comparisons among groups were made by one-way ANOVA followed by Tukey-Kramer analysis. Comparison with baseline over time among groups was done by two-way ANOVA followed by Dunnett's test.
Baroreflex curves were constructed by using maximum activity of the RSNA as the 100% value as well as the raw integrated nerve activity. RSNA versus mean arterial pressure (MAP) curves were constructed for each rat, as described previously (39). Resting values recorded before nitroprusside and phenylephrine were averaged for each curve. All data are reported as means ± SE. One-way ANOVA followed by Tukey-Kramer analysis was used for comparisons among groups.
Urinary sodium excretion was calculated as the product of urine sodium concentration and urine flow rate; sodium intake was the product of the weight of food ingested and its sodium content. Sodium intake and urine sodium excretion were averaged for each day (54). Creatinine clearance was calculated using the standard clearance formula (58). Body weights, kidney weights, plasma ANG II, tissue ANG II, and tissue NE concentrations were averaged for each group. All data were expressed as means ± SE. Two-way ANOVA for independent measurements was used for comparisons across time in the four groups of rats. For comparisons of individual means among groups, one-way ANOVA followed by Tukey-Kramer post hoc analysis was used. In all cases, a P value of <0.05 was accepted as significant.
RESULTS
Baseline values for the rats in protocol 1 are shown in Table 1. Body weights did not differ among the groups. The right (clipped) kidney weighed significantly less than the left kidney in both groups of 2K-1C rats; kidneys from ShC rats were of similar weight.
Table 1.
Body weights, kidney weights, and cortical NE content in the 4 groups of rats in protocol 1
| Group | Body Weight g | Left Kidney Weight, g | Right Kidney Weight, g | Left Kidney NE, ng/g | Right Kidney NE, ng/g |
|---|---|---|---|---|---|
| ShC sham cryo (n = 11) | 442 ± 10 | 1.291 ± 0.098 | 1.278 ± 0.087 | 100 ± 6 | 92 ± 6 |
| ShC cryo-DNX (n = 9) | 446 ± 6 | 1.267 ± 0.024 | 1.273 ± 0.083 | 106 ± 6 | 16 ± 4*† |
| 2K-1C sham cryo (n = 9) | 415 ± 8 | 1.264 ± 0.030 | 1.146 ± 0.026* | 88 ± 8 | 82 ± 8 |
| 2K-1C cryo-DNX (n = 11) | 432 ± 15 | 1.369 ± 0.201 | 1.181 ± 0.038* | 104 ± 11 | 24 ± 6*† |
Values are means ± SE. NE, norepinephrine; ShC, sham clipped; 2K-1C, two-kidney, one-clip; DNX, denervated; cryo-DNX, cryotreatment; sham cryo, sham cryotreatment.
P < 0.05 vs. left kidney;
P < 0.001 vs. corresponding sham cryo-DNX group.
Figure 2 shows daily MAP and heart rate 1 day prior to cryo- or sham treatment and daily averages over the next 7 days. Although MAP declined in the 2K-1C cryo-DNX group as soon as 1 day after treatment, the decrease in MAP did not achieve significance until day 5. Thereafter, MAP tended to stabilize at this new level until euthanasia on day 21; MAP on day 7 was 129.8 ± 4.1 vs. 130.5 ± 5.6 mmHg on days 18–21 (see Fig. 3). MAP after cryo-DNX in the ShC group did not decrease over the first 7 days. Heart rate did not differ among the groups or over time (P > 0.05). The average MAP during the 3 days prior to sham cryotreatment or cryotreatment was significantly higher in both groups of 2K-1C rats compared with ShC rats. MAP averaged over days 18–21 after cryotreatment of the nerve to the clipped kidney decreased MAP significantly in 2K-1C rats. Notably, ShC cryo-DNX rats also displayed a significant decrease in MAP by the end of the 3 wk. Sham cryotreatment of the renal nerve resulted in no change in MAP in either ShC or 2K-1C rats (Fig. 3). Heart rate was similar to that observed in the first 7 days of the protocol and did not differ among the four groups at the end of the 3 wk (data not shown).
Fig. 2.
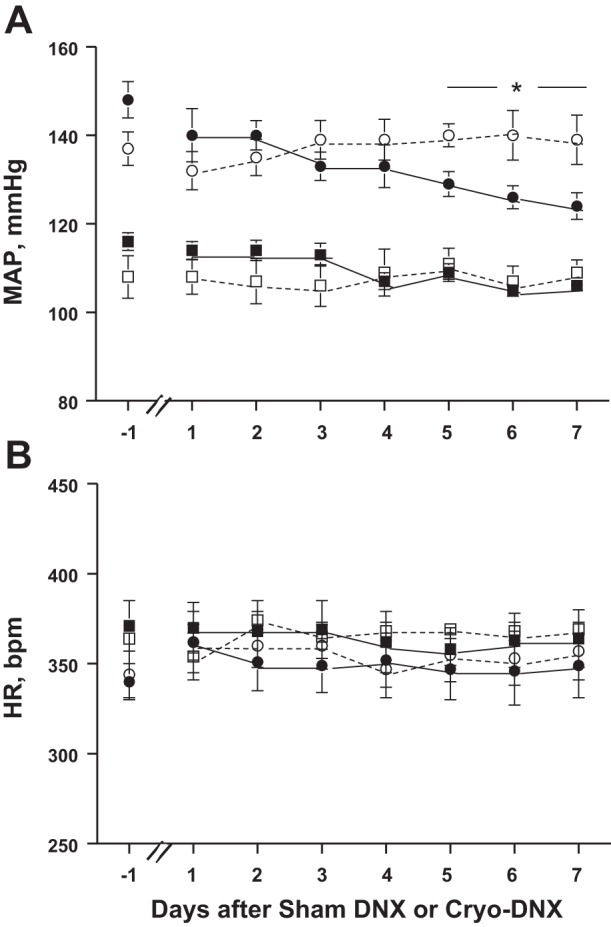
Changes in mean arterial pressure (MAP; A) and heart rate (HR; B) as measured by telemetry 1 day prior to and daily for 7 days after cryo- or sham treatment of the renal nerve to the clipped kidney in 2K-1C and ShC rats. ShC sham DNX (□; n = 11), ShC Cryo-DNX (■; n = 9), 2K-1C sham DNX (□; n = 9), and 2K-1C Cryo-DNX (■; n = 11). *P < 0.05 vs. baseline day −1.
Fig. 3.
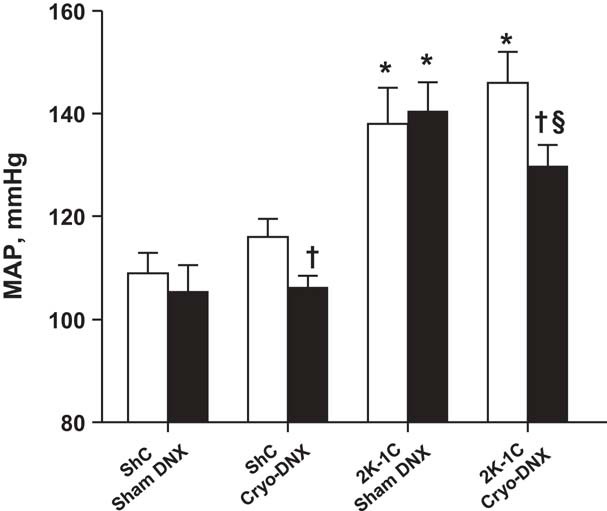
MAP by telemetry averaged over the 3 days prior to cryotreatment (open bars) and 3 wk later averaged over the 3 days prior to euthanasia (black bars); sham treatment (sham DNX) or cryotreatment (Cryo-DNX) of the renal nerve to the clipped kidney in 2K-1C rats or the sham clipped kidney in ShC rats, respectively. Values are means ± SE; n = 11, 9, 9, and 11. *P < 0.01 vs. the corresponding ShC group; †P < 0.05 vs. before cryo-DNX (same group); §P < 0.05 vs. 2K-1C sham DNX posttreatment.
Three weeks after sham or cryotreatment, tissue norepinephrine values from the right kidney were significantly lower in both ShC and 2K-1C cryo-treated groups (Table 1). Thus, renal tissue norepinephrine content was ∼85% lower in the cryo-treated kidney compared with either the corresponding sham cryo-treated kidney or the contralateral kidney in the same rat. In a separate set of rats, tissue norepinephrine content was 86 ± 3% lower at 1 wk (n = 5) and 81 + 3% (n = 7) at 3 wk after cryotreatment of the renal nerve.
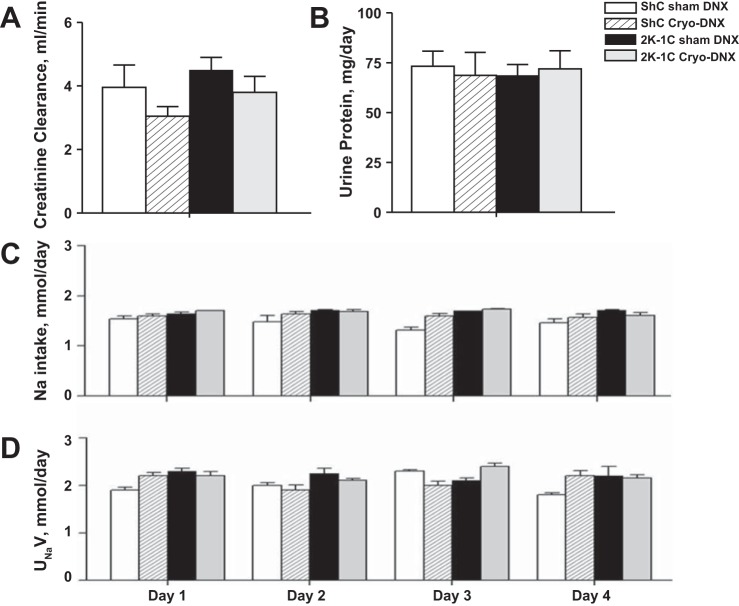
Creatinine clearance and protein excretion were similar among the groups (Fig. 4, A and B). Sodium intake and urinary sodium excretion did not change over the 4 days of measurement and were similar among the groups (Fig. 4, C and D). After completion of the metabolic studies, MAP remained significantly lower in the 2K-1C cryo-DNX rats compared with pretreatment MAP (−16 ± 5 mmHg, P < 0.05). No differences were evident in ShC sham DNX (−1.9 ± 3.9 mmHg) or 2K-1C sham cryo-DNX rats (+3.0 ± 3.0 mmHg). The ShC cryo-DNX group did display a decrease in MAP compared with pre-cryotreatment MAP (−7.0 ± 3.5 mmHg, P < 0.05).
Fig. 4.
Renal parameters in ShC sham DNX (n = 11), ShC Cryo-DNX (n = 9), 2K-1C sham DNX (n = 9), and 2K-1C Cryo-DNX (n = 11) in metabolic cages. Creatinine clearance (A), 24-h protein excretion on day 4 (B), and Na intake (C) and Na excretion (D) on days 1–4. Values are means ± SE.
Figure 5 shows that plasma ANG II values were approximately threefold greater in the 2K-1C sham cryo-DNX rats compared with either ShC group. Cryotreatment of the renal nerve to the clipped kidney decreased plasma ANG II values significantly in the 2K-1C rats to values no different from plasma ANG II concentrations in the ShC rats. Notably, renal tissue ANG II was similar in ShC rats at both 1 and 9 wk. Renal tissue ANG II content was increased significantly 2K-1C rats 1 wk after renal artery clipping; however, this elevation was not evident in the 2K-1C rats after 9 wk. Renal cortical tissue ANG II values were similar from both right and left kidneys of both groups of ShC rats. The clipped kidney of the 2K-1C sham cryo-treated rats displayed significantly higher tissue ANG II content compared with the nonclipped kidney; this increase was prevented by cryotreatment (Fig. 6).
Fig. 5.
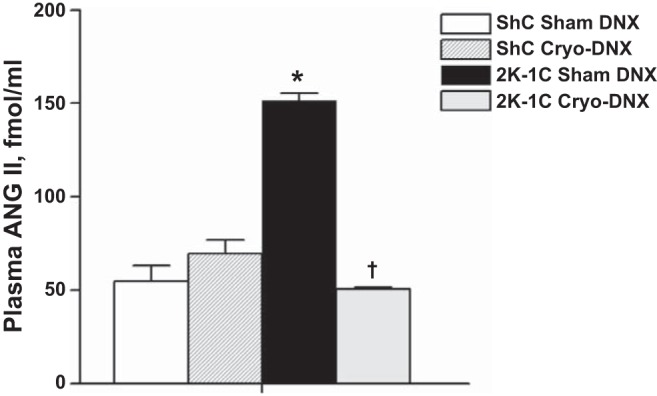
Plasma ANG II values in ShC sham DNX (n = 11), ShC Cryo-DNX (n = 9), 2K-1C sham DNX (n = 9), and 2K-1C Cryo-DNX (n = 11) at the end of the protocol (week 9). Values are means ± SE. *P < 0.001 vs. both ShC groups; †P < 0.001 vs 2K-1C sham DNX.
Fig. 6.
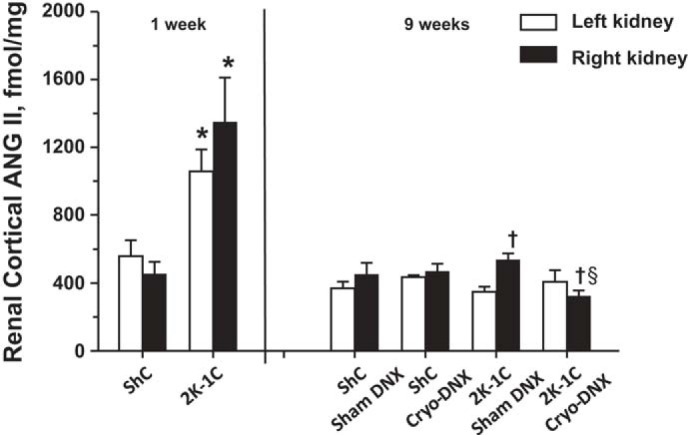
Renal cortical tissue ANG II values in kidneys from ShC and 2K-1C rats 1 wk after clipping (2K-1C) or sham clipping (ShC) and 9 wk after clipping or sham clipping the right renal artery. Tissues at week 9 were obtained from rats with data depicted in Figs. 2–4. Values are means ± SE; for week 1, ShC (n = 6) and 2K-1C (n = 6); for week 9, ShC sham DNX (n = 11), ShC Cryo-DNX (n = 9), 2K-1C sham DNX (n = 9), and 2K-1C Cryo-DNX (n = 11). *P < 0.05 vs. ShC, left kidney in 2K-1C compared with left kidney in ShC and right kidney in 2K-1C compared with right kidney in ShC; †P < 0.05 vs. left kidney, same treatment; §P < 0.05 vs. right kidney, 2K-1C sham DNX.
Table 2 shows the values for MAP at baseline and 4 days after sham cryotreatment or cryotreatment in protocol 2. Cryotreatment lowered MAP significantly in the 2K-1C group; sham cryotreatment had no effect. As in protocol 1, sham cryotreatment did not change tissue norepinephrine content of the right kidney, but cryotreatment decreased norepinephrine content of the right kidney in both ShC and 2K-1C rats.
Table 2.
Mean arterial pressure, renal tissue NE content, and renal sympathetic nerve activity before and 3 days after cryotreatment in protocol 2
| MAP, mmHg |
Integrated RSNA, μV/s |
|||||
|---|---|---|---|---|---|---|
| Group | Baseline | Post-DNX | Left Kidney NE, ng/g | Right Kidney NE, ng/g | Baseline | Post-DNX |
| ShC sham DNX | 105.0 ± 4.9 | 108.7 ± 6.7 | 100 ± 3 | 87 ± 2 | 2.8 ± 2.2 | 3.3 ± 2.0 |
| ShC cryo-DNX | 107.3 ± 2.6 | 105.0 ± 4.9 | 134 ± 19 | 19 ± 4#† | 3.1 ± 1.2 | 4.7 ± 1.1 |
| 2K-1C sham DNX | 161.0 ± 12.6* | 168.0 ± 8.7* | 96 ± 2 | 75 ± 5 | 9.1 ± 1.3* | 11.2 ± 3.5* |
| 2K-1C cryo-DNX | 162.0 ± 5.4* | 152.0 ± 5.4*†§ | 101 ± 3 | 17 ± 2#† | 12.2 ± 1.5* | 3.1 ± 1.5†§ |
Values are means ± SE; n = 4 for each group. MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity.
P < 0.05 vs. corresponding ShC group;
P < 0.001 vs. left kidney;
P < 0.001 vs. corresponding sham DNX group;
P < 0.05 vs. baseline, same group.
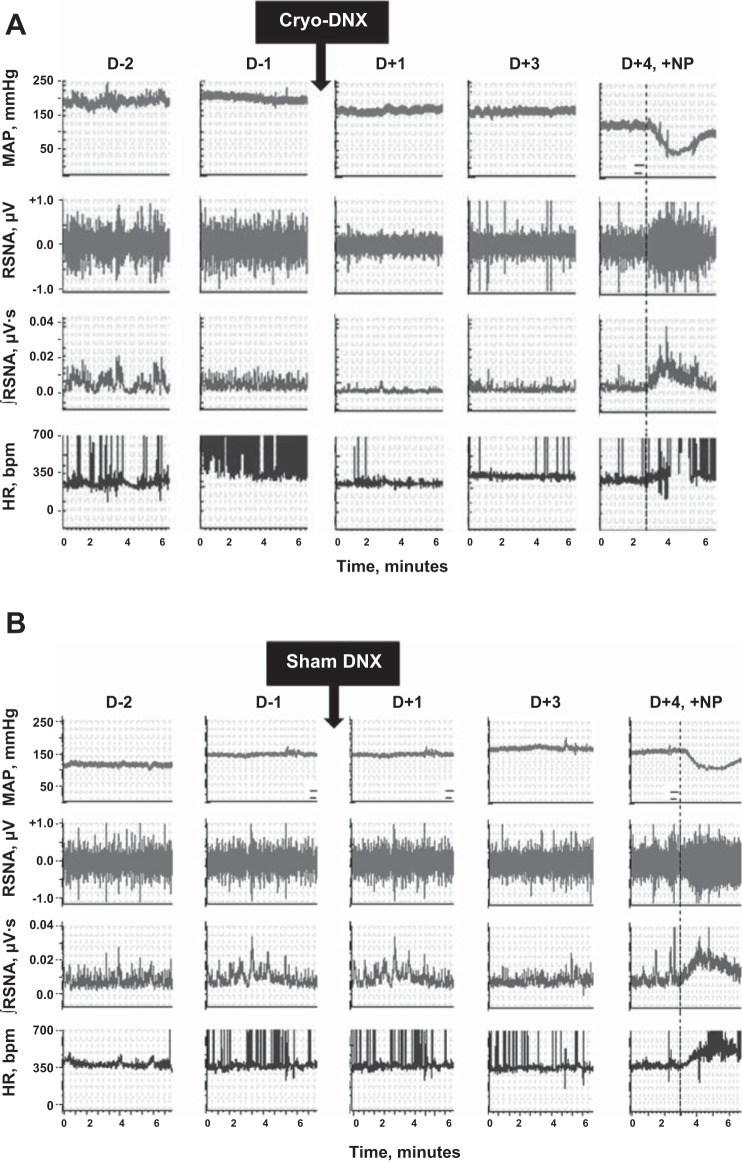
Raw telemetry recordings of MAP, raw RSNA, integrated RSNA, and heart rate before and after cryotreatment from a freely moving 2K-1C are shown in Fig. 7A. Both MAP and RSNA decreased 1 day after cryotreatment and continued to decline up to day 4. The decrease in RSNA was not due to electrode malfunction or decreased nerve viability since a decrease in MAP induced by injection of nitroprusside 4 days after cryotreatment evoked increases in both heart rate and RSNA. Sham cryotreatment of a 2K-1C rat in Fig. 7B shows that MAP continued to rise over time and that RSNA was unchanged. Overall, integrated RSNA prior to cryotreatment was three- to fourfold higher in both groups of 2K-1C rats compared with the ShC groups. Integrated RSNA decreased significantly in the 2K-1C cryo-treated group to a value no different from that in the ShC groups (Table 2).
Fig. 7.
Representative recordings of MAP, renal sympathetic nerve activity (RSNA), integrated RSNA (∫RSNA), and HR 2 days (D −2) and 1 day (D −1) prior to treatment and then 1 (D +1), 3 (D +3), and 4 days (D +4) in individual 2K-1C rats after either cryotreatment (Cryo-DNX; A) or sham cryotreatment (sham DNX; B). Dotted line indicates injection of nitroprusside (+NP) to verify nerve response.
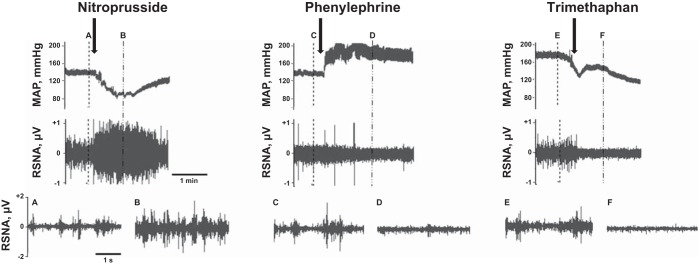
Examples of the integrity of the telemetric nerve recordings at baseline and after responses to nitroprusside or phenylephrine are depicted in Fig. 8. These recordings are from the same rat shown in Fig. 9, C and D, 9 days after cryotreatment. Background RSNA after ganglionic blockade with trimethaphan camsylate is also shown.
Fig. 8.
Representative MAP and RSNA recordings in a single 2K-1C rat immediately before and after injection with either nitroprusside, phenylephrine, or trimethaphan camsylate during baroreflex testing. The expanded recordings were taken at the points of the dashed lines with the corresponding letters. Arrows indicate the time at the beginning of the injections.
Fig. 9.
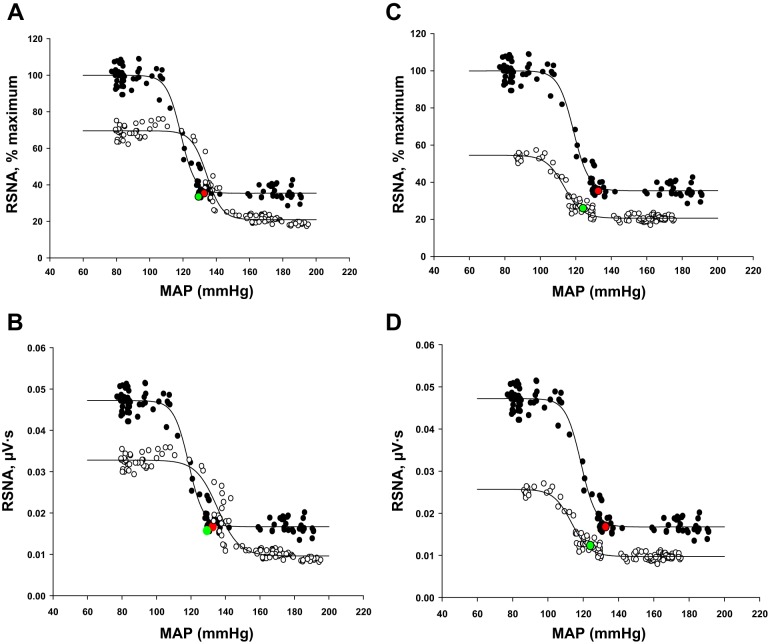
Baroreflex curves of RSNA in a single 2K-1C rat before (baseline; ●) and on days 3 (A and B) and 9 (C and D) after cryotreatment (cryo-DNX; ○) of the nerve to the clipped kidney. Values are plotted as %maximum RSNA (A and C) and as raw nerve activity (μV/s; B and D). Resting values are shown in red for pretreatment and green for posttreatment.
Typical baroreflex curves of RSNA expressed as percent maximum RSNA as well as in absolute units in a single 2K-1C rat before and after cryotreatment are presented in Fig. 9. In this rat, both upper and lower plateaus decreased. Baroreflex testing was not part of the original hypothesis and design but was performed as an addendum to the study. Thus, full baroreflex curves were completed on day 3 in three of four 2K-1C rats; these results are shown in Table 3. In one rat, were able to complete baroreflex curves after cryotreatment on day 3 (Fig. 9, A and B) and as late as day 9 (Fig. 9, C and D). The major difference was a further decrease in resting MAP and range and a shift in the curve to the left. The upper plateau decreased significantly after cryotreatment, resulting in a concurrent decrease in the range. In contrast, the upper plateau, if anything, displayed a tendency to rise in the sham cryo-treated 2K-1C rats (Table 3), but this did not achieve significance since the study was not powered to evaluate baroreflex parameters.
Table 3.
Arterial baroreflex parameters of renal sympathetic nerve activity before and 3 days after sham or cryotreatment of the renal nerve in 2K-1C rats
| 2K-1C |
||||
|---|---|---|---|---|
| Sham Cryo |
Cryo-DNX |
|||
| Pretreatment | Posttreatment | Pretreatment | Posttreatment | |
| Upper plateau, % | 100 ± 0 | 142.8 ± 55.8* | 100 ± 0 | 55.6 ± 9.1*† |
| Lower plateau, % | 50.3 ± 13.0 | 41.0 ± 3.0 | 34.6 ± 1.0 | 28.4 ± 5.6 |
| Range, % | 49.5 ± 12.8 | 101.8 ± 53.7 | 65.4 ± 1.0 | 27.2 ± 6.1*† |
| BP50, mmHg | 140.9 ± 21.7 | 139.7 ± 14.9 | 130.6 ± 12.5 | 137.7 ± 18.9 |
| Slope coefficient, −1/mmHg | 0.17 ± 0.04 | 0.12 ± 0.02 | 0.14 ± 0.05 | 0.16 ± 0.02 |
| Gmax, %/mmHg | 2.45 ± 0.51 | 3.02 ± 1.17 | 2.25 ± 0.86 | 1.16 ± 0.35 |
Values are means ± SE; n = 3 for each group. BP50, midrange of the baroreflex curve; Gmax, maximum gain.
P < 0.05 vs. pretreatment;
P < 0.05 vs. sham cryo posttreatment.
DISCUSSION
The present findings support the hypothesis that cryotreatment of the renal nerve to the clipped kidney in 2K-1C rats decreases systemic arterial pressure comparable with decreases in pressure observed with surgical denervation. Arterial pressure in sham clipped rats was also significantly decreased by unilateral cryoablation of the renal nerve. In addition, these studies demonstrate for the first time that denervation of the renal nerves by cryoabation reduces plasma ANG II levels, lowers renal tissue ANG II content of the clipped kidney, and decreases contralateral RSNA in conscious, chronically instrumented 2K-1C hypertensive rats. The decline in renal tissue norepinephrine content of the clipped kidney by as much as 85% provides proof of principle that cryotherapy may be used to effect sympathetic renal denervation comparable with that reported 2 wk after severing of the renal nerve and application of phenol in spontaneously hypertensive rats and suggests that reinnervation may be limited or delayed with this approach (43). Heart rate did not differ among the groups either before or after sham or cryoablative therapy. Moreover, a limited number of baroreflex observations suggests that denervation of the renal nerves by cryoablation results in a decreased reflex renal sympathoexcitatory response to a decline in arterial pressure.
Cryoablation of the renal nerve to the clipped kidney reproducibly decreased mean arterial pressure by ∼15 mmHg in 2K-1C rats 6 wk after clipping in both protocols rats in both protocols. This decline in arterial pressure after cryotreatment was comparable with that observed 6 days after standard surgical denervation of the ischemic kidney in this model (26). The decrease in pressure was sustained through the third week after cryotreatment and is similar in magnitude to the attenuation seen with bilateral renal denervation in the development of hypertension in spontaneously hypertensive rats (27) and DOCA salt rats (22) or after the nerve to the unclipped kidney is severed in 2K-1C rats (50). Arterial pressure also declined significantly in the sham clipped rats in protocol 1, which was powered to assess mean arterial pressure, but not in protocol 2, which was powered to evaluate RSNA. This is consistent with the findings of Jacob et al. (21) who found an ∼10-mmHg difference in mean arterial pressure after standard denervation in normal rats. They were able to observe this difference as early as 7 days after bilateral renal denervation. In the present studies, mean arterial pressure in the sham clipped group was not different at 7 days (Fig. 2 and Table 2) but did achieve significance 3 wk after cryoablation of the renal nerve (Fig. 3). It may well be that unilateral denervation requires a longer period for its effect on normotensive rats. Moreover, the study in protocol 2 was powered to evaluate RSNA and not mean arterial pressure. Taken together, these observations in both 2K-1C and sham clipped rats show that cryoablation of the renal nerve is as effective as surgical denervation to decrease arterial pressure.
Just as with surgical denervation, several mechanisms contribute to the decrease in systemic pressure after cryoablation in the 2K-1C rats. Low renal perfusion pressure to the clipped kidney stimulates renin secretion via renal baroreceptor and macula densa mechanisms. Efferent renal sympathetic stimulation potentiates the renin secretory response to low perfusion pressure (56). Thus, whereas high-plasma ANG II would otherwise depress renin secretion by a feedback mechanism (9), the concurrent sympathetic activation and low perfusion pressure in the clipped kidney together result in high-circulating ANG II. The substantial decrease in plasma ANG II after cryotreatment of the 2K-1C rats to levels no different from in sham clipped rats is consistent with the absence of a renin secretory response to renal hypoperfusion in humans after preganglionic sympathetic blockade due to epidural anesthesia (20).
The potential mechanisms involving ANG II and renal nerves in 2K-1C hypertension are depicted in Fig. 10. Besides raising systemic pressure by producing direct vasoconstriction via activation of AT1 receptors on vascular smooth muscle cells (5), circulating ANG II also results in differential activation of regional sympathetic nerve activities (46). In contrast to chronic exogenous ANG II infusion, which typically results in baroreflex inhibition of efferent RSNA (62), endogenous activation of the renin-angiotensin system in the 2K-1C model enhances cervical sympathetic nerve activity (59) and RSNA (7). Afferent inputs from the stenosed kidney in 2K-1C hypertension (30), as in other ischemic renal conditions (29), paradoxically exert an excitatory influence on contralateral efferent RSNA despite elevated endogenous ANG II. Studies have shown that inhibitory renorenal reflexes that occur in kidneys from normal rats (12) do not occur from the clipped kidney in 2K-1C rats but actually enhance efferent RSNA to the contralateral kidney (30). Moreover, afferent renal nerves also project to central structures that regulate sympathetic outputs to key vascular beds (55). Some of these nuclei, such as the subfornical organ and area postrema, lie outside the blood-brain barrier and possess abundant AT1 angiotensin receptors (49) so that plasma ANG II can then potentiate excitatory afferent renal nerve inputs, further enhancing efferent sympathetic activity (10, 11, 39). Cryotreatment performed in the present studies involved freezing the whole renal nerve so that afferent as well as efferent nerves from the stenosed kidney were interrupted. Thus, the combined decline in plasma ANG II and excitatory afferent inputs in awake, freely moving rats after cryotreatment contributed to the decrease in both systemic pressure and contralateral RSNA and confirm the earlier studies regarding surgical denervation by Kopp and Buckley-Bleiler (30) observed only 1.5 h after recovery from pentobarbital anesthesia.
Fig. 10.
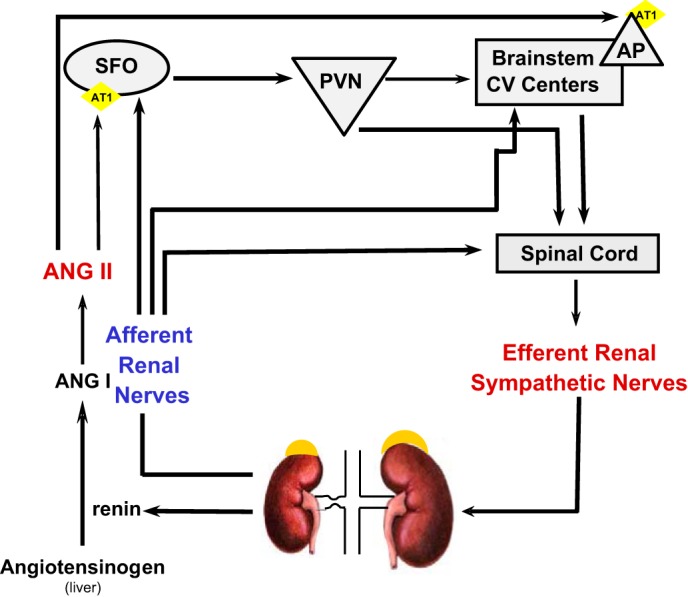
Diagram of potential mechanisms involved in increased ANG II and efferent RSNA to the contralateral kidney in 2K-1C hypertension. Hypoperfusion of the stenotic kidney results in increased secretion of renin, thereby initiating a cascade leading to elevated plasma ANG II. Plasma ANG II can act to increase vascular resistance directly (not shown) but can also act on brain nuclei that lie outside the blood-brain barrier and possess AT1 receptors such as the subfornical organ (SFO) or area postrema (AP). Projections from the AP or the SFO via paraventricular nucleus (PVN) can then send signals either via the brainstem cardiovascular regulatory centers or directly to the spinal cord to enhance efferent RSNA. Notably, efferent RSNA may increase renin secretion from the contralateral kidney as well. Afferent renal nerves from the clipped kidney also project to SFO and to brainstem centers. These inputs, together with spinal cord renorenal reflexes initiating from afferent nerves of the clipped kidney, lead to increased efferent RSNA to the contralateral kidney.
The high levels of intrarenal ANG II content in both clipped and nonclipped kidneys 1 wk after clipping confirm the findings by Guan et al. (18), who also showed that by 25 days, tissue ANG II content, although significantly elevated, was not as pronounced. This present report is the first to show that there is no difference in renal tissue ANG II content between 2K-1C and sham clipped rats 9 wk after clipping. Nonetheless, similar to rats studied at 25 days (18), tissue ANG II content of the clipped kidney of 2K-1C rats was higher than that of the contralateral kidney and was significantly attenuated by cryotreatment of the nerve. Elevated intrarenal ANG II has been shown to decrease sodium excretion and may thereby further contribute to hypertension (48). The present data show that denervation decreases intrarenal ANG II in the stenosed kidney and confirm the earlier findings in anesthetized 2K-1C rats (30) that sympathetic inputs to the contralateral kidney are suppressed, leading to increased urinary sodium excretion. Thus, effects on sodium excretion by the reduced intrarenal ANG II and sympathetic drive may work in concert to lower systemic pressure and restore sodium balance chronically. We did not observe the increase in urinary sodium excretion that likely occurs early after renal denervation, as the metabolic studies were performed 10 days after denervation, at which time the rats were back into sodium balance. Since arterial pressure also declined in sham clipped rats, our findings are consistent with earlier studies that renal nerves influence basal arterial pressure even in the normotensive state (21). However, it is also apparent that in 2K-1C rats there is an additional decrease in pressure that is likely due to changes in systemic and intrarenal ANG II. Further studies are certainly warranted to ascertain the role of renal nerves in normotensive rats as well.
The limited number of baroreflex observations suggests that cryoablation of the renal nerve decreases the reflex renal sympathoexcitatory response to baroreceptor unloading. The present data cannot distinguish whether this modulation of the baroreflex is due to interruption of direct neural inputs from renal afferent nerves, lowering of circulating ANG II, or both. Since both the neural and ANG II inputs converge on the subfornical organ, it is of interest that the attenuation of the maximal response to baroreceptor unloading here is similar to that seen with electrolytic lesioning of the subfornical organ (39) or chronic administration of a centrally acting sympatholytic agent (7). In addition, although this study was not a priori powered to assess baroreflex function, there was no detectable difference in the lower plateau. The latter observation would be consistent with previous studies suggesting that the baroreflex is already maximally active to suppress arterial pressure in secondary hypertension (7, 37, 59) and suggests that further studies are warranted.
Limitations.
Cryotreatment resulted in a significant decrease in tissue norepinephrine content, but not to the extent reported with severing of the renal nerve and application of phenol, where tissue norepinephrine decreased by >95% (30). Without detailed longitudinal histological studies, it is not possible to distinguish definitively between lack of full denervation versus reinnervation. Previous studies with surgical denervation reported attenuation of the decrease in renal norepinephrine to 89% after 2 wk and 76% after 4 wk (27), whereas others have shown tissue norepinephrine to be only 50% of control values by 24 days (25). That the decrease in renal tissue norepinephrine was similar at 7 and 21 days post-cryotreatment is more consistent with cryoablation being initially less effective than surgical denervation. Nonetheless, if tissue norepinephrine is used as the index of denervation, reinnervation appears to be limited during the 3-wk course of the present experiments. This contrasts with studies after standard denervation showing ∼40% reinnervation after 3–4 wk (43). Thus, cryoablation may be less effective initially but could conceivably be more long lasting. Corroboration of this finding as well as identification of the mechanism of the sustained decrease in norepinephrine would provide valuable information. Importantly, sodium intake was carefully controlled across groups in the present study. No differences were seen in creatinine clearance or sodium excretion at the time of the balance studies, but this does not exclude the possibility that sodium excretion differed acutely after sham or cryotreatment such that cumulative sodium excretion may have differed prior to balance being restored. Split renal function would have provided additional insights but also would have entailed anesthesia. Baroreflex testing was a post hoc addition to the studies and hence, not adequately powered for definitive interpretation. Nonetheless, the findings indicate that the availability of nerve telemetry will prove pivotal in ascertaining the relationship between the arterial baroreflex in freely moving rats after chronic manipulations in disease models.
Perspectives and Significance
In summary, these studies support the proof of principle that cryoablation alone to the renal nerve is able to effect substantial sympathetic renal denervation to the clipped kidney in conscious, chronically instrumented 2K-1C rats, leading to reductions in systemic arterial pressure, plasma ANG II levels, renal tissue ANG II and norepinephrine content of the clipped kidney, and contralateral RSNA. Given that renovascular hypertension due to atherosclerotic disease is increasing in prevalence (15), it is noteworthy that standard approaches using angioplasty and stenting of the renal artery have proven to be ineffective in lowering systemic pressure (13). Because the time of onset of stenosis in humans is impossible to judge, it is likely that the sympathetic mechanisms are engaged by the time the stenosis is addressed. Despite the early reports of successful treatment with radiofrequency denervation in resistant essential hypertension (33, 53), renal artery stenosis has generally been considered an exclusionary factor (60). Moreover, issues have more recently have come to light regarding the limited effectiveness of radio frequency denervation, such as proximity to the vessel wall, medication compliance, and operator experience, among others. Other radiofrequency-based technology for renal denervation may improve on these technical aspects, but balloon cryoplasty has already been applied to peripheral vascular disease (41). Emerging cryotechnology has shown sufficient power to effect endovascular approaches to the highest heat sink scenario of aberrant cardiac conduction pathways. Thus, cryoablation of the renal nerves with or without concurrent angioplasty appears to be increasingly feasible for future endovascular approaches and may be of benefit in individuals with renovascular hypertension to relieve both the stenosis as well as sympathoexcitation.
GRANTS
This work was supported by a Department of Veterans Affairs Merit Award to N. F. Rossi.
DISCLOSURES
None of the authors have any conflicts of interest, financial or otherwise, to declare.
AUTHOR CONTRIBUTIONS
N.F.R. and P.J.L. conception and design of research; N.F.R., R.P., and H.C. performed experiments; N.F.R., R.P., and M.M.-S. analyzed data; N.F.R. and M.M.-S. interpreted results of experiments; N.F.R. prepared figures; N.F.R. drafted manuscript; N.F.R. and M.M.-S. edited and revised manuscript; N.F.R., R.P., H.C., P.J.L., and M.M.-S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the computer and technical assistance of Robert J. Pawlowicz.
REFERENCES
- 1.Aqel RA, Zoghbi GJ, Baldwin SA, Auda WS, Calhoun DA, Coffey CS, Perry GJ, Iskandrian AE. Prevalence of renal artery stenosis in high-risk veterans referred to cardiac catheterization. J Hypertens 21: 1157–1162, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bates MC, Campbell JE, Stone PA, Jaff MR, Broce M, Lavigne PS. Factors affecting long-term survival following renal artery stenting. Catheter Cardiovasc Interv 69: 1037–1043, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bausback Y, Friedenberger J, Hertting K, Werner M, Branzan D, Freitas B, Piorkowski M, Schmidt A, Scheinert D. Renal denervation for hypertension refractory to renal artery stenting. J Endovasc Ther 21: 181–190, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Beierwaltes WH. Sympathetic stimulation of renin is independent of direct regulation by renal nitric oxide. Vascul Pharmacol 40: 43–49, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bennett MA, Thurston H. Effect of angiotensin-converting enzyme inhibitors on resistance artery structure and endothelium-dependent relaxation in two-kidney, one-clip Goldblatt hypertensive and sham-operated rats. Clin Sci (Lond) 90: 21–29, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, Investigators SH . A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370: 1393–1401, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Burke SL, Evans RG, Head GA. Effects of chronic sympatho-inhibition on reflex control of renal blood flow and plasma renin activity in renovascular hypertension. Br J Pharmacol 159: 438–448, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51: 1403–1419, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Kim SM, Eisner C, Oppermann M, Huang Y, Mizel D, Li L, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Schnermann J, Briggs JP. Stimulation of renin secretion by angiotensin II blockade is Gsalpha-dependent. J Am Soc Nephrol 21: 986–992, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciriello J. Afferent renal inputs onto subfornical organ neurons responsive to angiotensin II. Am J Physiol Regul Integr Comp Physiol 272: R1684–R1689, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Ciriello J, Rosas-Arellano MP, Solano-Flores LP. Induction of c-fos in forebrain circumventricular organs after renal artery stenosis. Brain Res 995: 109–117, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Colindres RE, Spielman WS, Moss NG, Harrington WW, Gottschalk CW. Functional evidence for renorenal reflexes in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 239: F265–F270, 1980. [DOI] [PubMed] [Google Scholar]
- 13.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D'Agostino RB Sr, Dworkin LD; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelis F, Havez M, Lippa N, Al-Ammari S, Verdier D, Carteret T, Amoretti N, Gangi A, Palussiere J, Hauger O, Grenier N. Radiologically guided percutaneous cryotherapy for soft tissue tumours: A promising treatment. Diagn Interv Imaging 94: 364–370, 2013. [DOI] [PubMed] [Google Scholar]
- 15.de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens 27: 1333–1340, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Esler M. Sympathetic nervous system: contribution to human hypertension and related cardiovascular diseases. J Cardiovasc Pharmacol 26, Suppl 2: S24–S28, 1995. [PubMed] [Google Scholar]
- 17.Fink GD, Brody MJ. Impaired neurogenic control of renal vasculature in renal hypertensive rats. Am J Physiol Heart Circ Physiol 238: H770–H775, 1980. [DOI] [PubMed] [Google Scholar]
- 18.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension 20: 763–767, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Hesse IF, Johns EJ. The effect of graded renal nerve stimulation on renal function in the anaesthetized rabbit. Comp Biochem Physiol A Comp Physiol 79: 409–414, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Hopf HB, Schlaghecke R, Peters J. Sympathetic neural blockade by thoracic epidural anesthesia suppresses renin release in response to arterial hypotension. Anesthesiology 80: 992–999; discussion 927A–928A, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol Heart Circ Physiol 284: H2302–H2310, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1529, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation 99: 2537–2542, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Kandzari DE, Bhatt DL, Sobotka PA, O'Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 35: 528–535, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katholi RE, Naftilan AJ, Oparil S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension 2: 266–273, 1980. [DOI] [PubMed] [Google Scholar]
- 26.Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension 4: 166–174, 1982. [PubMed] [Google Scholar]
- 27.Kline RL, Stuart PJ, Mercer PF. Effect of renal denervation on arterial pressure and renal norepinephrine concentration in Wistar-Kyoto and spontaneously hypertensive rats. Can J Physiol Pharmacol 58: 1384–1388, 1980. [DOI] [PubMed] [Google Scholar]
- 28.Kopp U, Aurell M, Nilsson IM, Ablad B. The role of beta-1-adrenoceptors in the renin release response to graded renal sympathetic nerve stimulation. Pflugers Arch 387: 107–113, 1980. [DOI] [PubMed] [Google Scholar]
- 29.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol 308: R79–R95, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp UC, Buckley-Bleiler RL. Impaired renorenal reflexes in two-kidney, one clip hypertensive rats. Hypertension 14: 445–452, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Kopp UC, Cicha MZ, Smith LA. Impaired interaction between efferent and afferent renal nerve activity in SHR involves increased activation of alpha2-adrenoceptors. Hypertension 57: 640–647, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Krueger AD, Lee JY, Yang PC, Papaioannou SE, Walsh GM. Selective vasodilation produced by renal denervation in adult spontaneously hypertensive rats. Hypertension 8: 372–378, 1986. [DOI] [PubMed] [Google Scholar]
- 33.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275–1281, 2009. [DOI] [PubMed] [Google Scholar]
- 34.La Grange RG, Sloop CH, Schmid HE. Selective stimulation of renal nerves in the anesthetized dog. Effect on renin release during controlled changes in renal hemodynamics. Circ Res 33: 704–712, 1973. [DOI] [PubMed] [Google Scholar]
- 35.Laird J, Jaff MR, Biamino G, McNamara T, Scheinert D, Zetterlund P, Moen E, Joye JD. Cryoplasty for the treatment of femoropopliteal arterial disease: results of a prospective, multicenter registry. J Vasc Interv Radiol 16: 1067–1073, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Littrup PJ, Bang HJ, Currier BP, Goodrich DJ, Aoun HD, Heilbrun LK, Adam BA. Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes. J Vasc Interv Radiol 24: 1817–1825, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohmeier TE, Hildebrandt DA, Warren S, May PJ, Cunningham JT. Recent insights into the interactions between the baroreflex and the kidneys in hypertension. Am J Physiol Regul Integr Comp Physiol 288: R828–R836, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 39.Maliszewska-Scislo M, Chen H, Augustyniak RA, Seth D, Rossi NF. Subfornical organ differentially modulates baroreflex function in normotensive and two-kidney, one-clip hypertensive rats. Am J Physiol Regul Integr Comp Physiol 295: R741–R750, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension 17: 707–719, 1991. [DOI] [PubMed] [Google Scholar]
- 41.McCaslin JE, Andras A, Stansby G. Cryoplasty for peripheral arterial disease. Cochrane Database Syst Rev 8: CD005507, 2013. [DOI] [PubMed] [Google Scholar]
- 42.McCubbin JW, Page IH. Neurogenic Component of Chronic Renal Hypertension. Science 139: 210–215, 1963. [DOI] [PubMed] [Google Scholar]
- 43.Mulder J, Hokfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 304: R675–R682, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. J Renin Angiotensin Aldosterone Syst 2: S176–S184, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Donaughy TL, Brooks VL. Deoxycorticosterone acetate-salt rats: hypertension and sympathoexcitation driven by increased NaCl levels. Hypertension 47: 680–685, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol 95: 61–68, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez-Rojas JM, Kassem KM, Beierwaltes WH, Garvin JL, Herrera M. Nitric oxide produced by endothelial nitric oxide synthase promotes diuresis. Am J Physiol Regul Integr Comp Physiol 298: R1050–R1055, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pontes RB, Crajoinas RO, Nishi EE, Oliveira-Sales EB, Girardi AC, Campos RR, Bergamaschi CT. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am J Physiol Renal Physiol 308: F848–F856, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Premer C, Lamondin C, Mitzey A, Speth RC, Brownfield MS. Immunohistochemical Localization of AT1a, AT1b, and AT2 Angiotensin II Receptor Subtypes in the Rat Adrenal, Pituitary, and Brain with a Perspective Commentary. Int J Hypertens 2013: 175428, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rademacher R, Berecek KH, Ploth DW. Effects of angiotensin inhibition and renal denervation in two-kidney, one clip hypertensive rats. Hypertension 8: 1127–1134, 1986. [DOI] [PubMed] [Google Scholar]
- 51.Rossi NF, Maliszewska-Scislo M, Chen H, Black SM, Sharma S, Ravikov R, Augustyniak RA. Neuronal nitric oxide synthase within paraventricular nucleus: blood pressure and baroreflex in two-kidney, one-clip hypertensive rats. Exp Physiol 95: 845–857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santangeli P, Proietti R, Di Biase L, Bai R, Natale A. Cryoablation versus radiofrequency ablation of atrioventricular nodal reentrant tachycardia. J Interv Card Electrophysiol 39: 111–119, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361: 932–934, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Sigmon DH, Beierwaltes WH. Influence of nitric oxide derived from neuronal nitric oxide synthase on glomerular filtration. Gen Pharmacol 34: 95–100, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res 753: 102–119, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Stella A, Calaresu F, Zanchetti A. Neural factors contributing to renin release during reduction in renal perfusion pressure and blood flow in cats. Clin Sci Mol Med 51: 453–461, 1976. [DOI] [PubMed] [Google Scholar]
- 57.Stocker SD, Muntzel MS. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am J Physiol Heart Circ Physiol 305: H1407–H1416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomiyama H, Scicli AG, Scicli GM, Carretero OA. Renal effects of Fab fragments of kinin antibodies on deoxycorticosterone acetate-salt-treated rats. Hypertension 15: 761–766, 1990. [DOI] [PubMed] [Google Scholar]
- 59.Tsyrlin VA, Galagudza MM, Kuzmenko NV, Pliss MG, Rubanova NS, Shcherbin YI. Arterial baroreceptor reflex counteracts long-term blood pressure increase in the rat model of renovascular hypertension. PLoS One 8: e64788, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y. Patients with renal artery stenosis may not be suitable for renal denervation. Clin Res Cardiol 103: 585–586, 2014. [DOI] [PubMed] [Google Scholar]
- 61.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55: 644–651, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]