Abstract
Neuronostatin (NST) is a recently described peptide that is produced from the somatostatin preprohormone in pancreatic δ-cells. NST has been shown to increase glucagon secretion from primary rat pancreatic islets in low-glucose conditions. Here, we demonstrate that NST increases proglucagon message in α-cells and identify a potential mechanism for NST's cellular activities, including the phosphorylation of PKA following activation of the G protein-coupled receptor, GPR107. GPR107 is abundantly expressed in the pancreas, particularly, in rodent and human α-cells. Compromise of GPR107 in pancreatic α-cells results in failure of NST to increase PKA phosphorylation and proglucagon mRNA levels. We also demonstrate colocalization of GPR107 and NST on both mouse and human pancreatic α-cells. Taken together with our group's observation that NST infusion in conscious rats impairs glucose clearance in response to a glucose challenge and that plasma levels of the peptide are elevated in the fasted compared with the fed or fasted-refed state, these studies support the hypothesis that endogenous NST regulates islet cell function by interacting with GPR107 and initiating signaling in glucagon-producing α-cells.
Keywords: neuronostatin, glucose homeostasis, islet function, glucagon, GPR107, cAMP-independent PKA activation
the failure to maintain normal glucose homeostasis during the development of Type 2 diabetes mellitus (T2DM) is associated with an inability of the pancreatic β-cells to sufficiently compensate for an increased insulin demand. It has been shown repeatedly in recent years that the increase in circulating levels of glucagon in the immediate postprandial state is an early sign of diabetes (7, 34, 42, 43), suggesting that cell-cell communication within the islets of Langerhans may be impaired. In healthy subjects, insulin inhibits glucagon secretion from α-cells. When this signal is defective, insulin released postprandially fails to sufficiently inhibit glucagon secretion, resulting in abnormally elevated blood glucose levels. The loss of regulation in the cell-cell communication between α- and β-cells highlights the importance of endocrine hormones, specifically the regulation of glucagon production, as a novel target to explore in prediabetic (individuals with impaired glucose tolerance and/or impaired fasting glucose) and diabetic patients early in the disease (i.e., patients with functioning β-cells). The cause of the imbalance of glucagon-to-insulin ratios in prediabetes has not been fully established, but has been associated with a decrease in the number and function of β-cells and defective insulin signaling (12, 32). Alternatively, a decrease in β-cell mass may lead to α-cell hyperplasia and, consequently, excessive glucagon release (29). The problem may also originate in the α-cell, either by development of insulin resistance (31) or some other alteration of normal α-cell function.
Neuronostatin (NST), a recently discovered peptide derived from the somatostatin (SST) preprohormone (corresponding to ProS6-19), has been shown to increase glucagon expression and secretion in pancreatic α-cells (35, 36). Although NST and SST are encoded by the same preprohormone, NST does not activate any of the five known SST receptors, suggesting that NST and SST exert activities that are independent from each other (36). In cardiomyocytes, it was demonstrated that NST signaled via PKA and JNK-dependent mechanisms (15), suggesting that NST interacted with a G protein-coupled receptor (GPCR). Although NST was shown to activate PKA in pancreatic α-cells as well (35), this effect was not due to a cAMP-mediated signaling, since exposure of α-cells to NST did not result in elevated intracellular levels of cAMP (35). These data suggested that NST signaled via a GPCR that was coupled to a G protein other than Gαs. Yosten et al. (47) demonstrated that in hypothalamic tissue, as well as in a gastric tumor cell line, NST signaled through the previously orphaned GPCR, GPR107.
In isolated rat islets, NST stimulated glucagon release and attenuated glucose-stimulated insulin secretion. The effect of NST on insulin secretion appeared to be indirect, requiring the presence of other islet cell types, in addition to β-cells, suggesting that NST stimulated the release of a locally acting mediator, which could interfere with glucose-stimulated insulin secretion from the β-cell. Furthermore, intravenous infusion of NST in adult male rats delayed glucose clearance and decreased insulin release compared with saline-infused controls following a glucose challenge, suggesting that NST may play a role in glucose homeostasis in vivo (35). An improved understanding of NST's contribution to the physiological regulation of glucose homeostasis may lead to better prevention strategies and therapies for diabetic patients. Here, we explore NST signaling in pancreatic α-cells across multiple species.
MATERIALS AND METHODS
Cell culture.
Mouse pancreatic αTC1-9 cells were purchased from American Type Culture Collection (ATTC) and cultured according to the supplier's instructions and growth medium recommendations (ATCC, Manassas, VA), with 1.0% penicillin-streptomycin (Gibco, Grand Island, NY). Cells were maintained at 37°C under atmosphere of 95% air-5% CO2, as described previously (24). Mouse pancreatic β-Min6 cells were cultured in DMEM (Cellgro, Manassas, VA), 10% FBS (20% with initial thawing of cells), additional 20 mM l-glutamine, additional 10 mM sodium pyruvate, 25 μM β-mercaptoethanol, and 1.0% penicillin-streptomycin, as previously described (25). KATOIII cells (ATCC) were maintained in Iscove's Modified Dulbecco's Medium with 20% FBS and 1.0% penicillin-streptomycin.
Islet isolation.
Pancreatic islets were isolated from 250–300 g male Sprague-Dawley rats by collagenase digestion, as previously described (20). Islets were cultured overnight in complete CMRL-1066 media: CMRL 1066 (Life Technologies, Grand Island, NY; cat. no. 11530), 2 mM l-glutamine, 10% heat-inactivated FCS, and 1.0% penicillin/streptomycin. Islets were maintained at 37°C under atmosphere of 95% air-5% CO2 before experimentation.
Neuronostatin treatment.
αTC1-9 cells were exposed to a low-glucose DMEM media 16 h prior to neuronostatin treatment. DMEM media without glucose was purchased from Sigma (St. Louis, MO), and glucose was added to a final concentration of 2 mM. Rat islets were exposed to a low-glucose CMRL1066 media for 30 min prior to neuronostatin treatment. CMRL1066 media without glucose for islet pretreatment was purchased from U.S. Biological Life Sciences, and d-glucose was added to a final concentration of 2 mM. Lyophilized neuronostatin (HPLC purified; purity ≥ 95%; Phoenix Pharmaceuticals, Burlingame, CA) was solubilized in saline to a concentration of 20 μM immediately prior to the addition to cells in low-glucose media. αTC1-9 cells were treated with 100 nM neuronostatin for 4 h for gene expression experiments and 40 min for PKA phosphorylation experiments. Rat islets were treated with neuronostatin for 3 h, as previously reported (35).
PCR and quantitative PCR.
Total RNA was isolated using the RNeasy RNA isolation kit (Qiagen, Valencia, CA). First-strand cDNA synthesis was performed using Oligo(dT) (Life Technologies) and MML-V reverse transcriptase (Promega, Madison, WI), according to the manufacturer's instructions. Standard PCR was performed and analyzed on a 1% agarose gel containing ethidium bromide, as described previously (46). Brightness and contrast of whole gel images were adjusted using Bio-Rad Image Lab software. Real-time PCR was performed using iQ SYBR Green Master Mix (Bio-Rad, Hercules, CA) and an annealing temperature of 60°C, as previously described (47). Primer sets are listed in Table 1.
Table 1.
Primer sequences
| Target Name | Ref. Sequence | Species | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|---|---|---|---|
| GAPDH | NM_001289726.1 | Mouse | GGATGAAGGGATGATGTTC | TGCACCACCAACTGCTTAG |
| GPR107 | NM_178760.4 | Mouse | GAACAAGGCCATGGAAACAT | AGGCTATCACAGAGCGATCC |
| GCG | NM_008100.3 | Mouse | GATCATTCCCAGCTTCCCAG | CTGGTAAAGGTCCCTTCAGC |
| GPR56 | NM_018882/NM_152242 | Mouse/Rat | ACTTCCTTCCAAGGCTTCCTCATC | AGCTAGGTATCTACCTACCAGAGCA |
| HPRT1 | NM_012583.2 | Rat | AGTCCCAGCGTCGTGATTAGTGAT | CTCGAGCAAGTCTTTCAGTCCTGT |
| GPR107 | NM_001107828.1 | Rat | TCCAGAAGTGTTCTCCAGTTGGCT | TTAGAGACTACCCTTTCTGTCCTCGG |
| GCG | NM_012707.2 | Rat | TGAATTTGAGAGGCATGCTG | TGGTGCTCATCTCGTCAGAG |
Data are presented as a percentage of control, normalized to vehicle-transfected, and vehicle-treated cells. Relative Ct values were calculated as relative Ctsample = Ctproglucagon/GPR107/GPR56 − CtGAPDH/HPRT. Changes in expression were calculated as fold change = 2^(Rel CtControl − Rel CtExp), where Rel CtControl is the relative Ct of the vehicle-transfected, vehicle-treated cells, and Rel CtExp is the relative Ct of the treatment group, as previously described (47). For each replicate, fold change was converted to percent change, and means were calculated as the average percent change for each treatment group across experiments.
Transfection.
αTC1-9 cells were plated onto 12-well plates in a volume of 1 ml/well in antibiotic-free media and incubated for 48 h at 37°C. Primary rat islets were dispersed into 24-well nontissue culture-treated plates with ∼60 islets per well and incubated in antibiotic-free media for 24 h. Cells were then exposed to vehicle alone (Lipofectamine 2000 in OPTI-MEM media; Life Technologies), GPR107 siRNA (1, 10, 100, or 400 nM), GPR56 siRNA (1, 10, or 100 nM) or enhanced green fluorescent protein (eGFP) siRNA (1 nM) using Lipofectamine 2000 (Life Technologies) for 48 h according to the manufacturer's instructions and as previously described (47). If cells or islets were to be treated with neuronostatin, media were changed to low-glucose media, as stated in Neuronostatin treatment. Experiments were performed in triplicate and repeated three times. Sequences of siRNA constructs (Integrated DNA Technologies, Coralville, IA) are reported in Table 2.
Table 2.
siRNA sequences
| Target Name | Ref. Sequence | Species | siRNA Sequences, 5′-3′ |
|---|---|---|---|
| GPR107 | NM_178760.4 | Mouse | GAUCGGACUAAGAACGAUGGCUTT |
| GGCUGUUGUAUACUACAUAACUCAC | |||
| AGUCCGUAGUGACGACAUCGGGUGT | |||
| GPR56 | NM_018882 | Mouse | CUAUGAUCAAUCUUCAGAGCCUCAC |
| CCAGGACAAGAAUUCUAGCCAAGTC | |||
| GCACCUUCCAGCUUGUCAUCCUCTA | |||
| GPR107 | NM_001107828.1 | Rat | AGACUAUAGCAGAGAAAUUCUUCTC |
| GGAGUGUCGAGGAGACAAGGUGUTA | |||
| GUCUCUAAACAAACACUUAGGUUAA | |||
| GPR56 | NM_152242 | Rat | GGCUGAAGACCCUGUAAAGCUUCGC |
| GGAACCUAGAGGACUCUAUCAUUTC | |||
| CCUAGGCUUGGAGGUAUAAUGGCTG | |||
| eGFP | AY157666.1 | Heteractis magnifica | ACCCUGAAGUUCAUCUGCACCACCG |
Confocal microscopy.
Immunohistochemistry was performed on αTC1-9 cells and 5-μm sections of human autopsy samples. Because human samples were not associated with identifiers, these experiments were deemed not to constitute human subjects research by the Saint Louis University Institutional Review Board. Plated αTC1-9 cells or human pancreas sections were incubated for 5 min with exogenous neuronostatin (1 μM) in PBS. Slides then were fixed in cold acetone for 10 min and subsequently washed three times in PBS. Samples were blocked with 10% human serum in PBS containing 0.5% BSA. Primary antibodies to GPR107, neuronostatin, and glucagon were incubated with Fab fragments labeled with separate fluorophores (Zenon tricolor rabbit IgG labeling kit no. 1, Molecular Probes, Life Technologies), according to the manufacturer's recommendations. Final concentrations of labeled antibodies were 1:100 for anti-GPR107 (Abcam, Cambridge, MA), 1:100 for anti-neuronostatin (Phoenix Pharmaceuticals), and 1:200 for anti-glucagon (Abcam). Specificity of the neuronostatin antibody was demonstrated previously using preabsorption assays (11) and as detailed below. After incubation with primary antibodies, slides were washed 3 times in PBS, and SlowFade Gold (Life Technologies, Burlingame, CA) was used to mount cover slips to the slides. Slides were visualized on an Olympus FV1000 confocal microscope (Olympus America, Center Valley, PA) with differential interference contrast and fluorescence or an Olympus 41BX upright microscope with fluorescence (Olympus America) and photographed with a DP72 camera (Olympus America). Images were further processed with the FIJI image-processing application (37).
Preabsorption analysis.
Following perfusion with 4% paraformaldehyde, rat hypothalamus was removed and sectioned at 10 μm using a cryostat. Sections were mounted onto slides, postfixed with 4% paraformaldehyde, and incubated with antineuronostatin antibody (1:100) alone or with antibody preincubated with 20 μg neuronostatin (Phoenix Pharmaceuticals) for 1 h. Sections then were incubated with anti-rabbit Cy3 (Jackson ImmunoResearch, West Grove, PA) and visualized using an Olympus epifluorescence microscope. Brightness and contrast were adjusted globally using ImageJ to equalize background staining based on tanycyte autofluorescence.
Protein isolation and quantification.
Cells were lysed on ice with PhosphoSafe Extraction Reagent (Novagen) with the addition of 1 nM protease and phosphatase inhibitor (Sigma) and processed according to the manufacturer's instructions. Protein quantification was determined using Bio-Rad's protein assay reagent (Hercules, CA), according to the manufacturer's instructions.
Electrophoresis and Western blot analysis.
Cellular proteins (100 picograms total protein/sample) were separated by SDS-PAGE, transferred to PVDF membranes, blocked overnight with 5% BSA (Sigma), and incubated with primary antibody overnight and then secondary antibody for 2 h. PKAc (protein kinase A catalytic subunit, T197) antibody, PKAc alpha antibody and secondary HRP-linked antibody were diluted 1:1,000 (Cell Signaling, Beverly MA). Experiments were performed in duplicate and repeated three times. Brightness and contrast of whole gel images were adjusted using Bio-Rad Image Lab software. GPR107 antibody (Abcam) was diluted 1:500 for use in the SNAP ID Western blot analysis system (Fisher Scientific, Waltham, MA).
Inhibitor experiments.
αTC1-9 cells were exposed to a low-glucose DMEM media 16 h prior to neuronostatin treatment. DMEM media without glucose were purchased from Sigma (St. Louis, MO), and glucose (d-glucose standard reference material, National Institute of Standards and Technology, Gaithersburg, MD) was added to a final concentration of 2 mM. Cells were pretreated with inhibitors solubilized in DMSO for 30 min. H89 (PKA inhibitor; Ki = 0.048 μM) (26); KT5720 (PKA inhibitor; Ki = 60 nM) (19); wedelolactone (IκB inhibitor; IC50 = 10–20 μM) (22) were purchased from Sigma. (E)-2-Fluoro-4′-methoxystilbene (a resveratrol analog and NF-κB inhibitor; IC50 = 0.85–1.42 μM) (4) was purchased from EMD Millipore. αTC1-9 cells were treated with neuronostatin for 4 h, and then lysed for RNA isolation. Experiments were performed in triplicate and repeated at least three times.
Animals and tissue collection.
All protocols and procedures have been approved by the Saint Louis University Animal Care and Use Committee. Adult, male Sprague-Dawley rats (250–300 g; Harlan Laboratories, Indianapolis, IN) were maintained under controlled conditions (lights on 0600–1800; 23–25°C) with free access to standard lab chow and municipal water. To examine whether fed or fasted-state effects NST protein levels, rats were categorized as either fed, fasted, or refed. Fed rats were allowed ad libitum feeding throughout the night, and tissue and plasma were collected in the morning. Fasted rats were fasted for 24 h (with access to municipal water) followed by tissue and plasma collection. Refed rats were fasted for 24 h (with access to municipal water), and then food was reintroduced for 4 h followed by tissue and plasma collection. Each treatment group consisted of 4–6 animals. Animals were killed by rapid decapitation, and tissues were dissected. Dissected tissues were frozen on dry ice and stored at −80. Tissues were sonicated in cold lysis buffer, and total RNA was isolated using the RNeasy RNA isolation kit (Qiagen, Valencia, CA).
Neuronostatin immunoassay.
As previously reported (36), rat neuronostatin-13 peptide levels were determined using a commercially available radioimmunoassay (Phoenix Pharmaceuticals). The RIA for rat neuronostatin-13 showed intra-assay variability of 10%, detection limit of 14 pg/ml, and EC50 of 68 pg/ml. For the determination of neuronostatin tissue content, tissues from male rats were collected into 1.0 ml of 0.2 N acetic acid on ice before homogenization. After centrifugation for 30 min at 3,000 g (4°C), an aliquot of the supernatant was collected for protein content determination, and the supernatants were dried in a rotary evaporator and subsequently solubilized in assay buffer (Phoenix Pharmaceuticals). Plasma levels of immunoreactive neuronostatin were determined following purification of the sample by C-18 column chromatography, as recommended by the supplier of the RIA kit (Phoenix Pharmaceuticals).
Statistical analyses.
RIA results for tissue peptide levels are reported as picograms of peptide per milligram of extracted protein, and levels of plasma were reported as picograms of neuronostatin immunoreactivity per milliliter plasma. Results were analyzed by one-way ANOVA (between groups) with Scheffé's multiple comparisons (post hoc testing). All other data sets were transformed data sets, and therefore, they were analyzed using the Mann-Whitney U nonparametric test. Data are expressed as means ± SE.
RESULTS
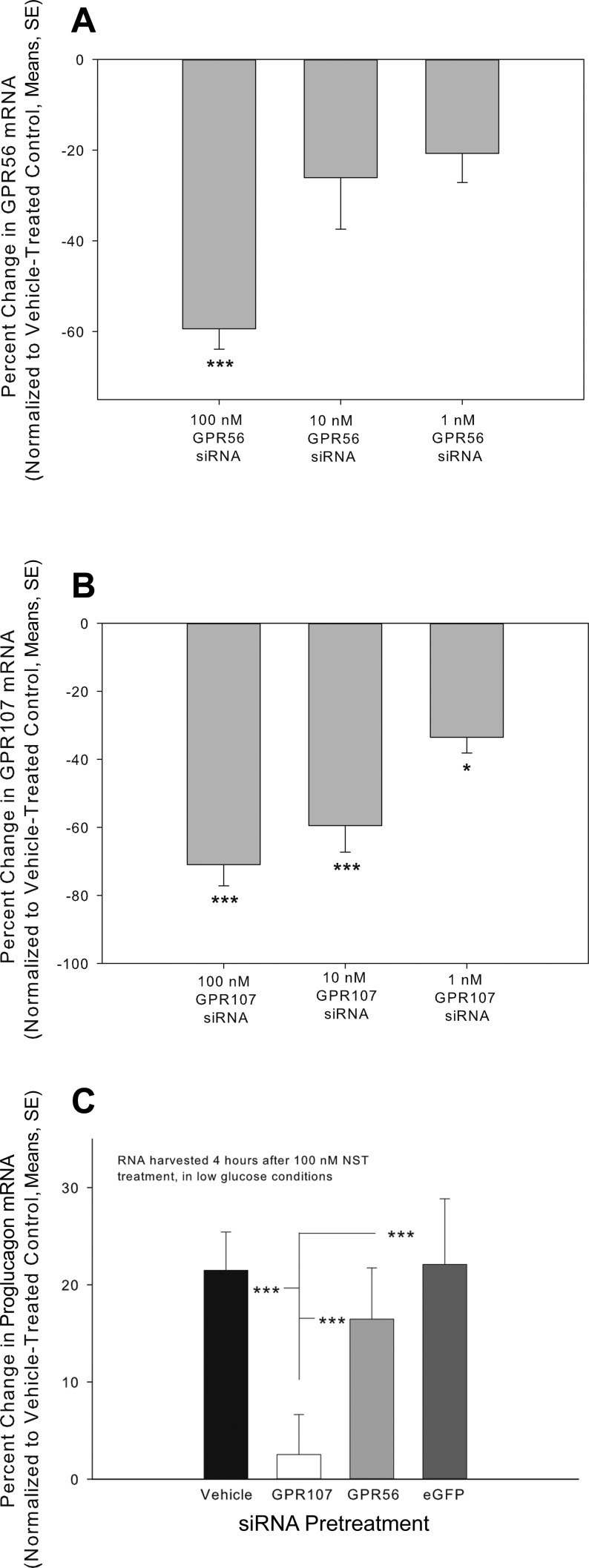
GPR56 and GPR107 previously were identified as possible neuronostatin receptor candidates (47), and thus, involvement of these receptors in NST signaling was explored in pancreatic cells. Pretreatment with siRNAs (Table 2) targeting GPR56 resulted in a concentration-related compromise of GPR56 mRNA levels (Fig. 1A). Transfection with 1 nM, 10 nM, and 100 nM siRNA resulted in decreases in GPR56 gene expression of 20.7 ± 6.4%, 26.1 ± 11.4%, and 59.4 ± 4.5%, respectively, compared with Lipofectamine-treated controls. Treatment of αTC1-9 cells with siRNA targeting GPR107 resulted in a concentration-related compromise of GPR107 mRNA (Fig. 1B). Exposure to 1 nM, 10 nM, and 100 nM GPR107 siRNA resulted in decreases in GPR107 mRNA levels of 33.6% ± 4.6, 59.5% ± 7.8, and 71.0% ± 6.2, respectively, compared with Lipofectamine-treated controls. Pretreatment with siRNA targeting GPR107 mRNA abrogated the cellular response to neuronostatin, as measured by changes in proglucagon mRNA levels compared with those observed in Lipofectamine-treated cells (Fig. 1C). siRNA pretreatment of these cells decreased GPR107 mRNA levels by 76.0 ± 6.9% compared with Lipofectamine-treated controls. Pretreatment with siRNA targeting GPR56 did not alter the cellular response to neuronostatin but did decrease significantly GPR56 mRNA levels 56.0 ± 4.3% compared with Lipofectamine-treated controls. Cells pretreated with siRNA targeting eGFP (an siRNA control) remained responsive to neuronostatin.
Fig. 1.
Knockdown of GPR107, but not GPR56, abrogates neuronostatin-induced proglucagon mRNA expression in αTC1-9 cells. Mouse pancreatic αTC1-9 clone cells were incubated for 48 h and then changed to fresh media. Mouse-specific siRNA complexes targeting either GPR56 (A) or GPR107 (B) mRNA were mixed with Lipofectamine 2000 and added to the cells at the above concentrations. After 48 h, cells were lysed, RNA was isolated, cDNA was generated, and gene expression was measured by qPCR. C: Mouse pancreatic αTC1-9 clone cells were incubated for 48 h, pretreated with siRNA (targeting the mRNA encoding GPR107, GPR56, or eGFP) and then changed to low glucose media overnight. Neuronostatin (100 nM final concentration) or saline was then added to the media for 4 h, and proglucagon mRNA expression was determined by qPCR. GPR56, GPR107, and proglucagon mRNA levels were normalized to those of the housekeeping gene GAPDH. Significant reductions in GPR mRNA levels A and B: *P < 0.05, ***P < 0.001. Significance in percent change in proglucagon mRNA levels compared with vehicle pretreated controls. C: ***P < 0.001.
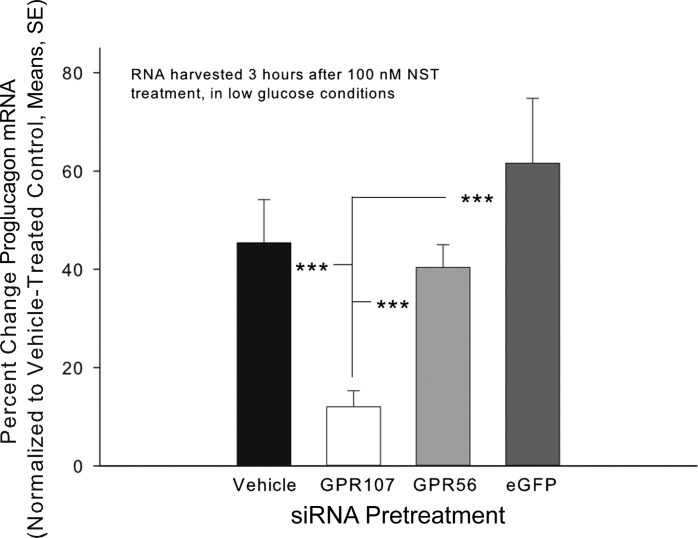
To further establish GPR107's role in neuronostatin-driven proglucagon expression, similar experiments were performed using freshly isolated rat islets. Islets were pretreated for 48 h with rat-specific siRNA complexes targeting GPR107 mRNA (100 nM), as previously described (3). Transfection with 100 nM GPR107 siRNA resulted in a significant compromise (43.7 ± 14.7%) of GPR107 mRNA levels compared with controls. Additional islets then were pretreated with 100 nM (GPR107 or GPR56) or 1 nM (eGFP) siRNA, switched to low glucose, and subsequently treated with 100 nM neuronostatin in low-glucose media (Fig. 2). While pretreatment with siRNA targeting GPR56 mRNA did not alter the cellular response to neuronostatin, pretreatment with siRNA targeting GPR107 mRNA prevented the response to neuronostatin. Knockdown for GPR107 and GPR56 in these samples were confirmed with mRNA reductions of 56.2 ± 12.5% and 56.1 ± 22.2%, respectively, compared with Lipofectamine-treated controls. Housekeeping genes GAPDH and HPRT1 were used to calculate percent change in the pancreatic mouse cell line and rat islets, respectively, as their mRNA levels remained unchanged by siRNA pretreatment or neuronostatin exposure.
Fig. 2.
Knockdown of GPR107, but not GPR56, inhibits neuronostatin-induced proglucagon mRNA expression in primary rat islets. Freshly isolated primary rat islets were incubated for 24 h in media with antibiotic and then for 24 h in antibiotic free media. Rat-specific siRNA complexes targeting the GPR107, GPR56, or eGFP mRNAs were premixed with Lipofectamine and added to the cells for 48 h. Media were changed to low glucose for 30 min, and then 100 nM neuronostatin or saline vehicle was added to the samples for 3 h. Cells were lysed, RNA was isolated, cDNA was generated, and gene expression was measured by quantitative PCR. Proglucagon mRNA levels were normalized to those of the housekeeping gene HPRT1. Significance to vehicle/NST-treated controls is indicated by ***P < 0.001) as determined by a Mann-Whitney U-nonparametric test for transformed data.
Many ligands that act via G protein-coupled receptors influence expression of their cognate receptors (5, 28). Therefore, αTC1-9 cells were pretreated with low glucose overnight followed by 100 nM neuronostatin for 2, 4, or 6 h. Neuronostatin treatment for 6 h, but not 2 or 4 h, resulted in significantly increased GPR107 mRNA levels (60.1 ± 6.6%, P < 0.001) compared with vehicle-treated control.
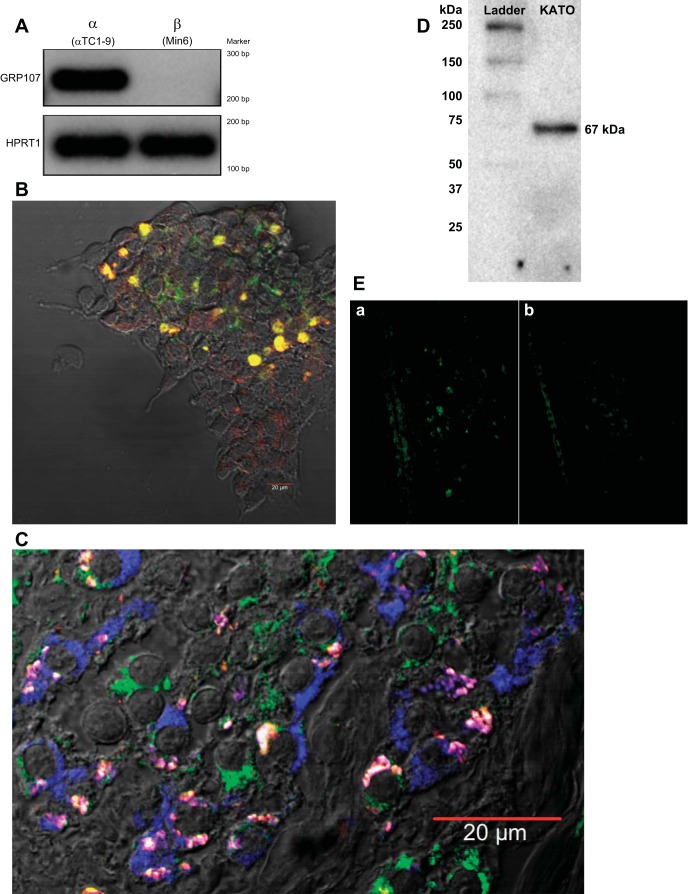
We previously reported that the effect of neuronostatin on glucose homeostasis was due to a direct action on α-, but not β-cells (35). Therefore, we evaluated the expression of GPR107 in α- and β-cell lines using PCR (Fig. 3A). While the mRNA levels of the housekeeping gene HPRT1 are similar between cell lines, GPR107 mRNA was detected in α-cell (αTC1-9, left lanes) but not β-cell (Min6, right lanes) extracts.
Fig. 3.
GPR107 colocalizes with exogenous neuronostatin on α-cell membranes. A: GPR107 mRNA was detected by PCR in the mouse pancreatic αTC1-9 cell line, but not in the mouse pancreatic β-cell line (Min6). B: αTC1-9 cells were incubated with synthetic neuronostatin (1 μM) for 30 min, then fixed and stained using antibodies against neuronostatin (red) and GPR107 (green). Areas of colocalization are indicated by yellow. C: human pancreatic tissue was sectioned and incubated with 1 μM synthetic, exogenous neuronostatin prior to fixation and labeling using antibodies against neuronostatin (red), GPR107 (green), and glucagon (blue). Areas of triple colocalization between all three fluorophores are indicated by pink/white. Colocalization between neuronostatin and GPR107 was not observed on any glucagon-negative cells, suggesting that exogenous neuronostatin interacts with GPR107 specifically on α-cell membranes. D: Western blot using GPR107 antibody for KATOIII whole cell lysate. A single band was detected at 67 kDa. E: rat hypothalamus incubated with antibody against neuronostatin (a) or neuronostatin preincubated with neuronostatin peptide (b).
To further explore the relationship between GPR107 and neuronostatin on the pancreatic α-cell, αTC1-9 cells were treated with neuronostatin and imaged by confocal microscopy. Colocalization between neuronostatin and GPR107 was observed on multiple αTC1-9 cells, as indicated by yellow (Fig. 3B). To confirm that colocalization between neuronostatin and GPR107 was an α-cell-specific event, human pancreatic tissue was exposed to exogenous neuronostatin, fixed, and stained using antibodies targeted against neuronostatin, GPR107, and glucagon (Fig. 3C). Triple colocalization of GPR107, neuronostatin, and glucagon was observed on multiple cells in these human samples. No instances of colocalization between GPR107 and neuronostatin were observed on cells that did not exhibit glucagon immunoreactivity, providing further evidence that GPR107 and neuronostatin interact on the pancreatic α- but not β-cell.
To evaluate the specificity of the GPR107 antibody, whole cell lysates of KATOIII cells, which were used in the identification of GPR107 as the putative receptor of neuronostatin (47), were subjected to gel electrophoresis, transferred to a PVDF membrane, and blotted using the GPR107 antibody. A single band was observed at 67 kDa, the predicted size of GPR107 (Fig. 3D). To evaluate the specificity of the neuronostatin antibody, sections of rat hypothalamus were incubated with either the neuronostatin antibody alone or with antibody preincubated with neuronostatin peptide. Sections stained with antibody that was preincubated with neuronostatin peptide exhibited reduced fluorescence intensity (Fig. 3E).
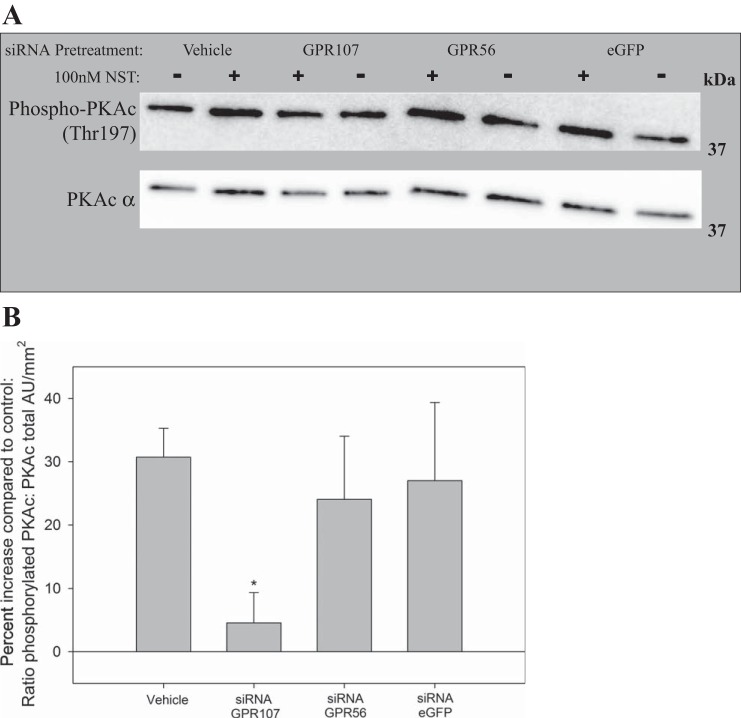
To determine the importance of GPR107 in PKAc phosphorylation, αTC1-9 cells were pretreated with siRNA and neuronostatin, and then PKAc phosphorylation was determined by Western blot analysis (Fig. 4A). Densitometry was conducted by ImageQuant LAS 4000 software, and a ratio is reported for phosphorylated PKAc (Thr-197): PKAc (α) (Fig. 4B). Treatment with neuronostatin increased PKAc phosphorylation compared with that observed with exposure to vehicle control (30.7 ± 4.6%). Cells pretreated with GPR107 siRNA showed an attenuated increase in PKAc phosphorylation compared with controls (4.6 ± 4.8%). Pretreatment with siRNA to GPR56 or eGFP did not alter the cells' ability to respond to neuronostatin, which exhibited an increase in PKAc phosphorylation of 24.0 ± 10.0% and 27.0 ± 12.4%, respectively.
Fig. 4.
Pretreatment with GPR107 siRNA prevents neuronostatin-induced PKA phosphorylation in mouse pancreatic cell line. Mouse pancreatic αTC1-9 clone cells were incubated for 48 h, pretreated with GPR107, GPR56, or eGFP siRNA for 28 h and then changed to low glucose media overnight (∼16 h). Neuronostatin or saline vehicle were added to the media for 40 min. Cells were lysed, protein was isolated and quantified, and 100 pg/sample was loaded on to a gradient gel and transferred to a PVDF membrane. The membrane was probed for phosphorylated PKAc (Thr-197), stripped, and reprobed for total PKAc (α). A: representative Western blot. Note that the order of treatment group (±) differs in vehicle-pretreated cells from other siRNA groups. B: average densitometry for separate experiments performed in duplicate. Significance to vehicle-treated controls is indicated by * (P < 0.05), as determined by a Mann-Whitney U-nonparametric test for transformed data.
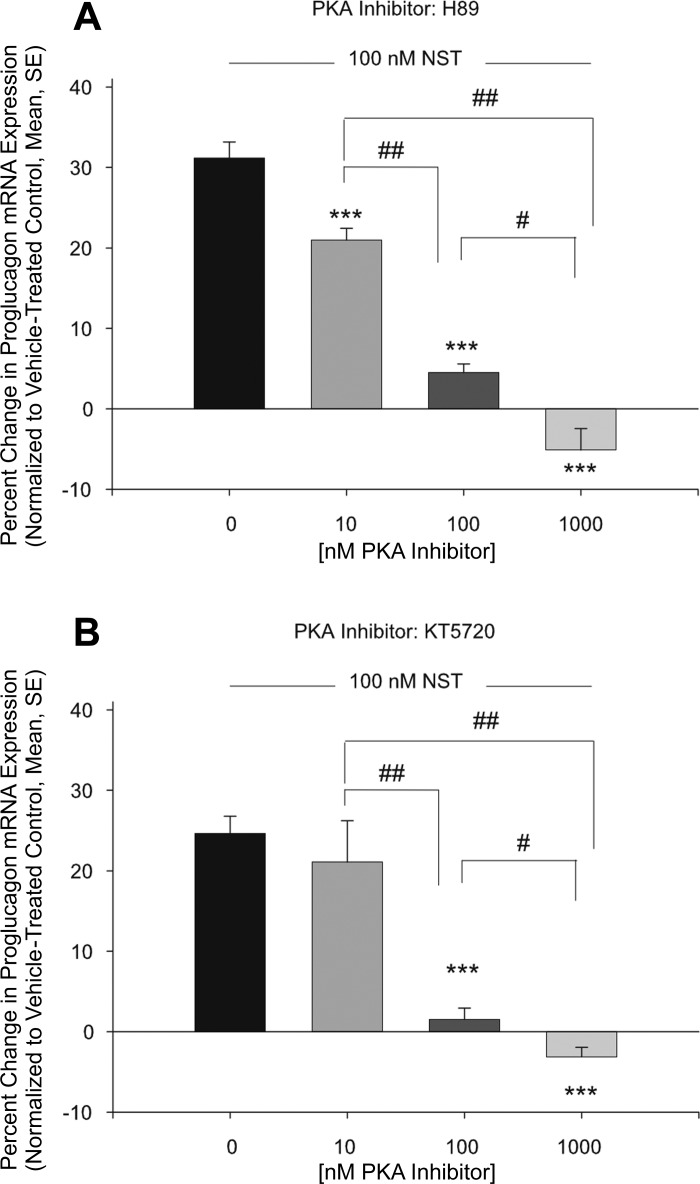
To further explore the role of PKA in NST signaling, αTC1-9 cells were pretreated with two PKA inhibitors that interfere with ATP-driven phosphorylation of PKA: H89 and KT5720 (26, 41). In these experiments, neuronostatin increased proglucagon mRNA levels by 31.2 ± 2.0% and 24.7 ± 2.1% (Fig. 5, A and B, respectively). Pretreatment with H89 led to a concentration-dependent reduction in the ability of neuronostatin to increase proglucagon message (Fig. 5A, 21.0 ± 1.5%, 4.5 ± 1.1%, and −5.1 ± 2.6% for 10,100 and 1,000 nM of H89, respectively). Similarly, pretreatment with KT5720 led to a reduction in the ability of neuronostatin to increase proglucagon mRNA levels of 21.1 ± 5.1%, 1.5 ± 1.4%, and −3.1 ± 1.2% for 10, 100, and 1,000 nM of KT5720, respectively (Fig. 5B).
Fig. 5.
Pretreatment with PKA inhibitors prevents neuronostatin-induced proglucagon mRNA expression. Mouse pancreatic αTC1-9 clone cells were incubated for 48 h and then changed to low-glucose media overnight. Cells were then pretreated with either H89 (A) or KT5720 (B) or DMSO for 30 min at the concentrations indicated. Neuronostatin (100 nM) or saline vehicle were added to the media for 4 h. Cells were lysed, RNA was isolated, cDNA was generated, and mRNA levels were determined by quantitative PCR. Proglucagon mRNA levels were normalized to those of the housekeeping gene GAPDH. Significance compared with vehicle treated controls was determined by the Mann Whitney U-test for nonparametric data. ***P < 0.001. Significance between groups #P < 0.05, ##P < 0.01.
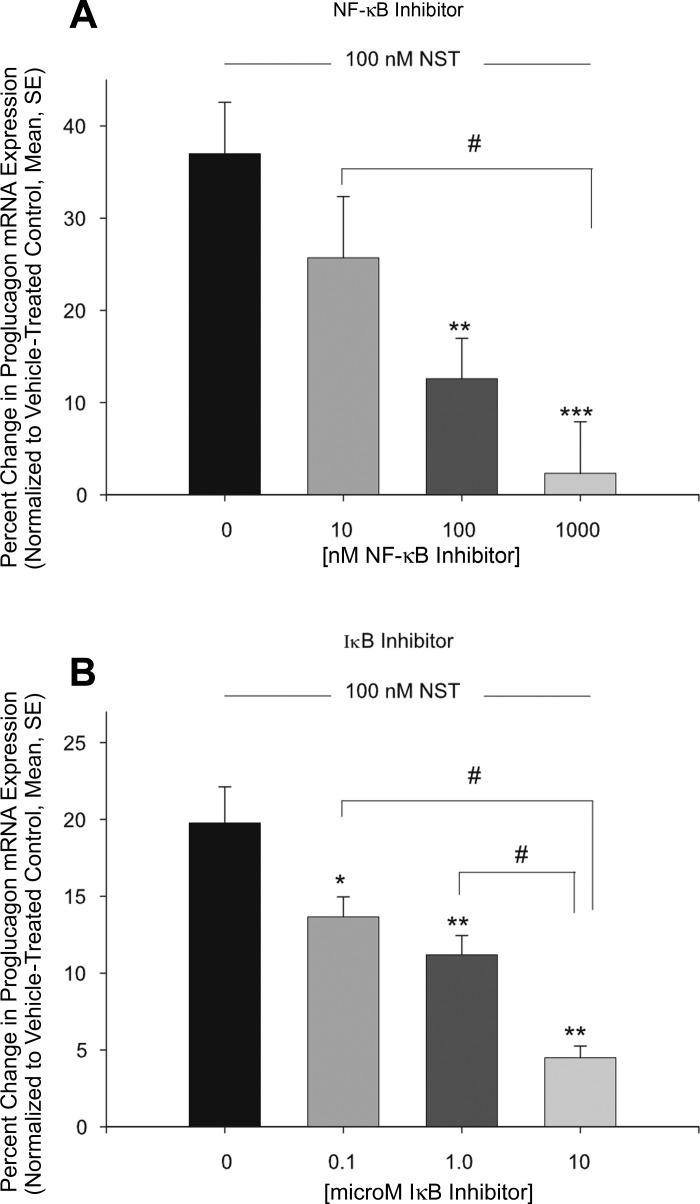
Previously, Salvatori et al. (35) reported that neuronostatin did not elevate cAMP levels in pancreatic islets or αTC1-9 cells, suggesting neuronostatin may activate PKA in a cAMP-independent manner, possibly through the NF-κB-IκB complex. To determine whether activation of the NF-κB-IκB complex played a role in neuronostatin's ability to induce proglucagon gene expression, αTC1-9 cells were pretreated with an NF-κB inhibitor [(E)-2-fluoro-4′-methoxystilbene (resveratrol analog)] and an IκB inhibitor wedelolactone (6). In these experiments, neuronostatin increased proglucagon mRNA levels by 37.0 ± 5.5% and 19.8 ± 2.3% (Fig. 6, A and B, respectively). Pretreatment with the NF-κB inhibitor led to a concentration-dependent reduction in the ability of neuronostatin to increase proglucagon message (Fig. 6A) (25.7 ± 6.6%, 12.6% ± 4.4%, and 2.3 ± 5.6% for 10, 100, and 1,000 nM of NF-κB inhibitor, respectively). Similarly, pretreatment with wedelolactone led to a reduction in the ability of neuronostatin to increase proglucagon mRNA levels (Fig. 6B) of 13.7 ± 1.3%, 11.2 ± 1.3%, and 4.5 ± 0.8% for 0.1, 1.0, and 10.0 μM of wedelolactone, respectively.
Fig. 6.
Pretreatment with NF-κB or IκB inhibitors prevents neuronostatin-induced proglucagon mRNA expression. Mouse pancreatic αTC1-9 clone cells were incubated for 48 h and then changed to low glucose media overnight. Cells were then pretreated with NF-κB inhibitor [(E)-2-fluoro-4′-methoxystilbene] (A), IκB inhibitor (wedelolactone) (B) or DMSO for 30 min at the concentrations indicated. Neuronostatin (100 nM) or saline vehicle were added to the media for 4 h. Cells were lysed, RNA was isolated, cDNA was generated, and mRNA levels were determined by quantitative PCR. Proglucagon mRNA levels were normalized to those of the housekeeping gene GAPDH. Significance compared with vehicle-treated controls was determined by the Mann Whitney U-test for nonparametric data. *P < 0.05, **P < 0.01, ***P < 0.001. Significance between groups #P < 0.05.
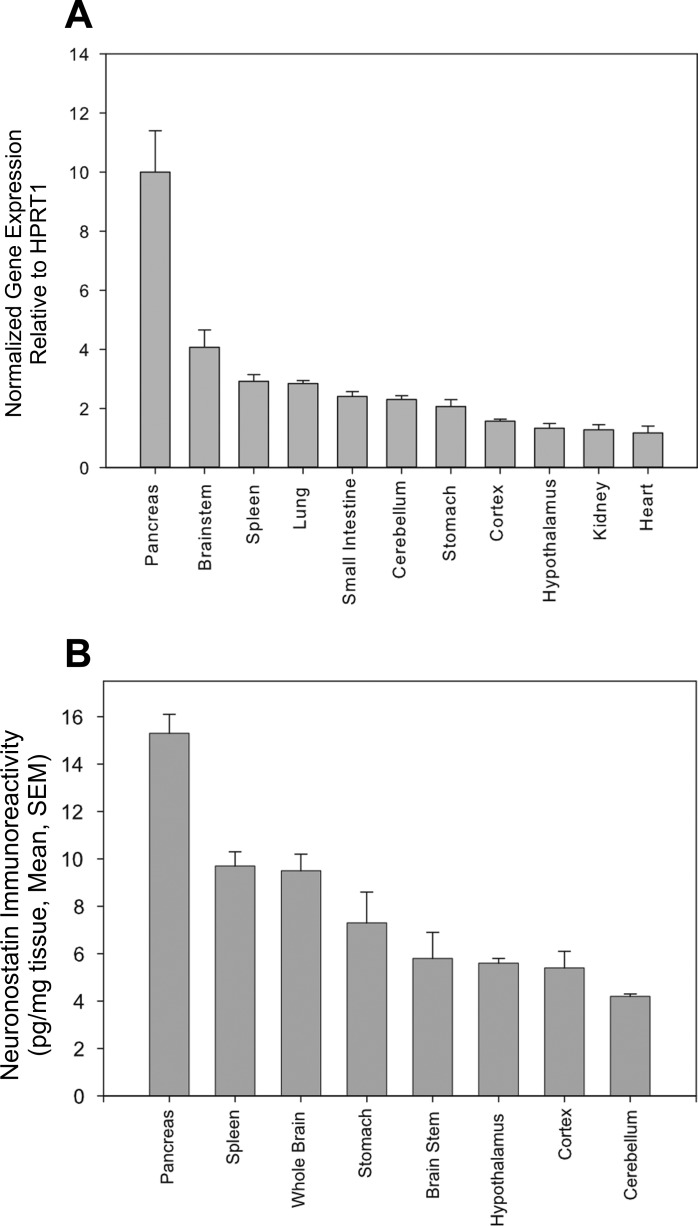
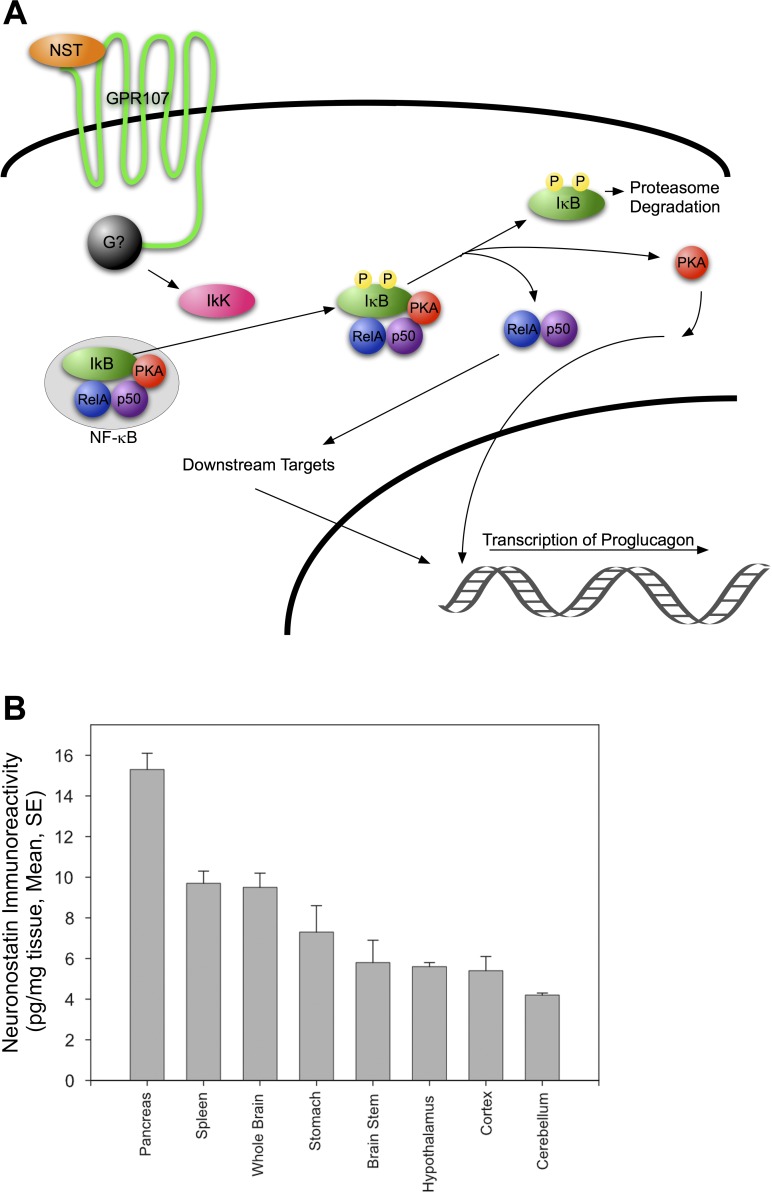
GPR107 mRNA and neuronostatin peptide levels in multiple tissues of male Sprague-Dawley rats are shown in Fig. 7, A and B. In these animals, the highest levels of GPR107 mRNA were detected in the pancreas, approximately three-fold higher than the next highest tissue, brain stem, and six-fold higher than the tissue with the lowest GPR107 expression (heart). GPR107 mRNA was detectable in all tissues collected. Measurements of preprosomatostatin mRNA levels would be a predictor of both somatostatin and neuronostatin protein levels, since they are encoded by the same gene (although processed differentially from their preprohormone). Therefore, neuronostatin protein levels were measured. Heart NST content was not assessed because previous immunohistochemistry findings by others (9) indicated that the somatostatin prohormone was localized to sparsely populated, cardiac afferents, suggesting content below detection limits of the radioimmunoassay. Neuronostatin content in picograms per milligram tissue is shown in Fig. 7B. Pancreas extracts contained the highest amount of neuronostatin immunoreactivity, 15.3 ± 0.8 pg/mg tissue, followed by spleen (9.7 ± 0.6) and whole brain (9.5 ± 0.7). Cerebellar extracts contained the lowest detectable levels of neuronostatin immunoreactivity (4.2 ± 0.1 pg/mg tissue). Remaining brain tissues (minus the hypothalamus, brain stem, and portions of the cerebellum and cerebral cortex) were pooled and are indicated as “whole brain.” Plasma neuronostatin levels were significantly higher (P < 0.001) in fasted animals (17.2 ± 1.2 pg/ml plasma) than in fed (12.2 ± 0.4 pg/ml plasma) and fasted-refed (11.4 ± 0.4 pg/ml plasma) rats.
Fig. 7.
GPR107 mRNA expression and neuronostatin protein content in rat tissues. A: GPR107 mRNA levels in the tissues of adult male Sprague-Dawley rats were determined by quantitative PCR (normalized to HPRT-1). B: neuronostatin protein content (picogram neuronostatin per milligram extracted protein) in rat tissues was determined by radioimmunoassay.
DISCUSSION
We have demonstrated previously that neuronostatin induced glucagon secretion in rat islets, as well as in a mouse pancreatic α-cell line (35). Glucagon release from pancreatic α-cells is controlled at the transcriptional level; therefore, changes in proglucagon gene expression are a measure of pancreatic α-cell activation (14). Here, we demonstrated that neuronostatin, in low-glucose conditions (i.e., when circulating insulin levels would be low), increased proglucagon mRNA levels in the pancreatic α-cell and that this action was prevented when GPR107 mRNA levels were reduced by pretreatment with selective siRNAs. We demonstrated this both in a pancreatic α-cell line (mouse-derived), as well as in primary rat islets; thus, this was not a species-specific or cell line-specific event. Many ligands are known to regulate the expression of their own receptor (5, 28). We demonstrated that neuronostatin treatment led to increased mRNA encoding GPR107. Taken together, these studies support a functional role for GPR107 in neuronostatin's ability to increase proglucagon mRNA levels in the pancreatic α-cell. These data are in agreement with previous reports that neuronostatin acts through GPR107 in a human gastric tumor cell line in vitro as well as in vivo in rat (47).
A 20% decrease in GPR107 mRNA levels in the brain was sufficient to significantly reduce neuronostatin's central cardiovascular actions (47). Therefore, it is not surprising that a 76% reduction in GPR107 mRNA levels in the α-cell line and a 56% reduction in primary rat islets would attenuate neuronostatin's actions on gene expression in the α-cell line and rat islets, respectively. If the GPR107 protein turns over rapidly (i.e., receptor recycling), the cumulative effect of gene inhibition over 48 h could lead to a functional decrease in receptors on the cell surface. Additionally, if there are only a few receptors on the surface of the cell, any decrease in the number of receptors may lead to an impact on receptor function. Preliminary data suggest a decrease in GPR107 protein due to pretreatment with siRNA, as measured by Western blot analysis (data not shown).
The maximum knockdown achieved by pretreatment with siRNA targeting GPR56 mRNA levels was 59% in the αTC1-9 cell line and 56% in rat primary islets. It could be argued that the knockdown was insufficient to inhibit the neuronostatin response and that neuronostatin may be acting through GPR56. During the course of these experiments, the orphan G protein-coupled receptor GPR56 was identified to be the receptor for collagen 3 and was demonstrated to be critical to regulation of neuronal migration during cortical development (27). Therefore, GPR56, while originally explored as a possible neuronostatin receptor candidate, in the end served as an additional experimental control.
The initial publication describing the discovery of neuronostatin reported a transcriptional response of islet cells to an intraperitoneal injection of neuronostatin, and on the basis of the localization of responsive cells, it was hypothesized that neuronostatin may have direct effects on α-cells (36). Rat islets, when treated with neuronostatin, showed an inhibition of insulin release under high-glucose conditions (35). However, when a clonally derived β-cell line designed to overexpress the insulin gene was treated with neuronostatin, insulin release in response to high levels of glucose was not altered, suggesting neuronostatin's ability to inhibit glucose-stimulated insulin release in whole islets was not due to a direct action on the β-cell but instead on another cell type present in the islet. GPR107 was reported to be expressed in the mouse pancreatic α-cell line, αTC1-9 (35, 47). In a mouse insulinoma β-cell line (Min6 cells) (16), we were unable to detect the presence of GPR107 mRNA either by traditional PCR as imaged bands on a gel (Fig. 4A) or by amplification with real-time PCR (data not shown). Confocal microscopy demonstrated colocalization of GPR107 and exogenous neuronostatin on both mouse pancreatic α-cell line (αTC1-9) and in human pancreatic tissue. Further evidence for neuronostatin's actions on the α-cell and not the β-cell was shown by the colocalization of glucagon (produced in the α-cell and not the β-cell) with cells that also express GPR107, and triple colocalization of neuronostatin, GPR107, and glucagon, suggesting that exogenous neuronostatin, and endogenous GPR107 colocalize on α-cell membranes in human islets. Additional neuronostatin was observed in the human pancreas tissue that did not colocalize with either GPR107 or glucagon. This staining likely is due to endogenous neuronostatin produced by the pancreatic δ-cells. Future confocal microscopy studies using a fourth fluorophore for insulin may further demonstrate the importance of the relationship with GPR107 and neuronostatin uniquely with the α- and not the β-cell.
Pretreatment with two separate PKA inhibitors blocked neuronostatin's action to increase proglucagon mRNA levels. The fact that both inhibitors blocked the action of neuronostatin in a concentration-dependent manner suggests PKA is necessary for neuronostatin signaling. Although it is known that these inhibitors are not completely selective for PKA, these are the most selective PKA inhibitors currently available (30). There is one common kinase that both inhibitors block, mitogen- and stress-activated protein kinase-1 (MSK1); however, inhibition of this enzyme is not predicted at the concentrations examined (26). An MSK1 inhibitor could be used to rule out the involvement of MSK1 in the inhibition of neuronostatin's action to increase proglucagon mRNA levels; however, currently there are no selective MSK1 inhibitors available. Furthermore, we demonstrated that neuronostatin-induced PKA activation was dependent upon neuronostatin signaling via GPR107. Taken together, our data suggest neuronostatin requires association with GPR107 and subsequent PKA activation to increase proglucagon mRNA levels in pancreatic α-cells.
It has been demonstrated that neuronostatin-driven PKA activation was not associated with an elevation in cAMP, and therefore, cAMP-independent (35). Both PKA inhibitors used here block PKA at the ATP-driven phosphorylation site of the PKA catalytic subunit (PKAc), and, therefore, support the possibility of cAMP-independent PKA activation. In the last several years, cAMP-independent activation of PKAc has been reported by multiple laboratories. Zhong et al. (48) were the first to characterize cAMP-independent activation of PKAc in rabbit lung cytosol extracts, as well as mouse and human lymphocytes. Although they were able to measure increased PKAc phosphorylation as a result of LPS treatment, they were unable to detect an elevation in cAMP. An elegant set of pharmacological experiments demonstrated that a portion of intracellular PKAc (but not PKA regulatory subunit) was bound to the NF-κB-IκB complex and that degradation of IκB with NF-κB inducers led to the release and activation of PKAc. These same NF-κB-IκB-PKAc isolated complexes were insensitive to cAMP inducers, and increased cAMP levels did not lead to increased PKAc activation. Since then, there have been several reports in the literature linking GPCRs with cAMP-independent PKAc activation. (10, 13, 18, 38, 39, 45). Pretreatment with an NF-κB inhibitor or an IκB inhibitor prevented neuronostatin's action to increase proglucagon mRNA levels, suggesting neuronostatin-induced PKA phosphorylation may require activation of the NF-κB-IκB-PKAc complex. Future experiments should explore the interactions of NF-κB-IκB-PKAc complex in primary islets by functional assays and coimmunoprecipitation experiments in αTC1-9 cells.
In vivo, GPR107 gene expression and neuronostatin protein content were highest in the pancreas. Neuronostatin also was measured in rat plasma in fed, fasted, and refed states, and circulating plasma neuronostatin levels were elevated in low-glucose conditions compared with high-glucose conditions (fasted vs. fed or refed). We hypothesize that increased neuronostatin plasma levels during fasting might reflect the peptide's pancreatic release, resulting in the stimulation of glucagon release from pancreatic α-cells. If the increase in plasma neuronostatin content originates from the pancreatic δ-cells, local levels at the adjacent α-cells would be much higher than the levels observed in the plasma. Glucagon release in response to a paracrine action of δ cell-derived neuronostatin would then act on the liver to stimulate gluconeogenesis and glycogenolysis, restoring circulating glucose levels.
Glucagon is released during low-glucose conditions when insulin levels are low. It was reported previously that glucagon stimulates the pancreatic δ-cell, which would lead to the release of both somatostatin and neuronstatin (8). We have shown that in the pancreas, NST is found at an increased ratio compared with that of SST, likely due to differences in posttranslational processing (36). Neuronostatin, secreted from the δ-cells of the pancreas during low-glucose conditions, could act in a paracrine fashion to stimulate proglucagon transcription and release from the neighboring α-cells. At the same time, somatostatin (although at a lower concentration compared with neuronostatin) could act on the β-cell to inhibit insulin release. It is possible that another α-cell-secreted peptide, such as GRPP (glicentin-related polypeptide), IP-1 (intervening peptide-1), or IP-2 (intervening peptide-2), could also be responsible for the inhibition of insulin secretion (21). Alternatively, there are cells present in the islet other than α, β, and δ cells, such as F cells (secrete pancreatic polypeptide) and epsilon cells (secrete ghrelin) (2), and neuronostatin could stimulate those cells to release a yet to be identified, insulin-inhibitory factor. We propose that posttranslational processing and secretion of neuronostatin from the pancreatic δ-cell is regulated by blood glucose levels, glucagon, insulin, unknown paracrine factors, or some combination of these factors. Alterations in δ-cell production or release of neuronostatin, or neuronostatin's action on the α-cell may be associated with impaired blood glucose regulation. Experiments now in progress are attempting to determine human plasma neuronostatin levels in the fed vs. fasted states, in healthy as well as diabetic individuals.
From the data presented herein and in our previous study (35), we have generated the following model for neuronostatin-induced proglucagon mRNA expression in pancreatic α-cells (Fig. 8). In this model, binding of neuronostatin to GPR107 initiates G protein signaling through an unknown G protein that leads to the activation of IκB kinase (IKK). IKK phosphorylates IκB, which targets IκB for proteosomal degradation, thus releasing the NF-κB complex, including Rel A and p50, and the NF-κB-associated pool of PKA. Through a series of, as yet, unidentified signaling events, activation of PKA ultimately leads to transcription of proglucagon mRNA.
Fig. 8.
Model of neuronostatin signaling in pancreatic α-cells. Activation of GPR107 by neuronostatin results in the signaling of an unknown G protein, leading to the downstream activation of IKK. IKK phosphorylates IκBα, targeting it for degradation by the proteasome. Dissociation of IκBα releases the NF-κB complex (Rel A and p50), and the NF-κB-associated pool of PKA. The catalytic subunit of PKA is thus exposed, allowing activation and downstream phosphorylation events that ultimately lead to the transcription of proglucagon mRNA.
Perspectives and Significance
In very early stage Type 2 diabetes, identified as prediabetes by the American Diabetes Association (1), patients are diagnosed as a result of impaired (elevated) fasting blood glucose or impaired (elevated) blood glucose during a glucose tolerance test. Prediabetic patients exhibit postprandial elevations in glucagon compared with healthy controls, as well as insufficient insulin secretion to maintain healthy blood glucose levels, resulting in elevated blood glucose levels (23, 34, 40). Progression of Type 2 diabetes is driven by a cycle of high-blood glucose levels, leading to increased β-cell insulin secretion and further exacerbation of insulin resistance in multiple tissues. We have shown previously that neuronostatin can increase glucagon release and decrease insulin release. Therefore, it is possible that alterations in neuronostatin release or action may contribute to the development of prediabetes. Even if neuronostatin is not responsible for the imbalance developed in prediabetes, a neuronostatin antagonist given to a prediabetic individual could be useful in preventing or forestalling the progression of the disease.
In both Type 1 and advanced-stage Type 2 diabetic patients, β-cell function is severely diminished or lost and, therefore, insulin must be injected to control blood glucose levels. A serious complication of insulin-dependent diabetes is insulin-induced hypoglycemia. Excess insulin can cause blood glucose levels to drop dangerously low. In healthy subjects, a drop in blood glucose levels leads to decreased insulin secretion, disinhibition of glucagon production, and release of glucagon from the α-cell (44). In insulin-induced hypoglycemia, these events may not occur, glucagon may not be secreted, and blood glucose levels may continue to fall (7). Intervention is required to restore blood glucose levels or loss of consciousness, and possibly death can ensue. Once a hypoglycemic event has occurred, the patient is at risk for recurrent hypoglycemia due to impaired glucagon response for the next 30 days (17, 33). During this time of increased susceptibility to a hypoglycemic event, a therapeutic intervention with a NST agonist may prevent recurrent and potentially life-threatening, insulin-induced hypoglycemia. Further elucidation of the actions and physiological relevance of neuronostatin both under normal and pathophysiological states (including diabetes) could lead to the development of neuronostatin analogs as potential, multifaceted diabetic therapeutics.
GRANTS
This work was supported by National Institutes of Health Grant HL-06623 to W. K. Samson and DK DK-052194 to J. A. Corbett. Portions of these data were presented at the Experimental Biology meeting, San Diego, CA, April 2014. G. L. C. Yosten is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.M.E., W.K.S., J.A.C., A.S.S., G.R.K., and G.L.C.Y. conception and design of research; M.M.E., W.K.S., A.S.S., L.M.S., G.R.K., A.N., and G.L.C.Y. performed experiments; M.M.E., W.K.S., J.A.C., G.R.K., and G.L.C.Y. analyzed data; M.M.E., W.K.S., J.A.C., A.S.S., G.R.K., and G.L.C.Y. interpreted results of experiments; M.M.E., G.R.K., and G.L.C.Y. prepared figures; M.M.E. and G.L.C.Y. drafted manuscript; M.M.E., W.K.S., J.A.C., A.S.S., L.M.S., G.R.K., A.N., and G.L.C.Y. edited and revised manuscript; M.M.E., W.K.S., J.A.C., A.S.S., L.M.S., G.R.K., A.N., and G.L.C.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge and thank Dr. Jane Turner and Mr. Jim Edwards (Department of Pathology, Saint Louis University) for providing the human autopsy tissue.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 37 Suppl 1: S81–S90, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Arnes L, Hill JT, Gross S, Magnuson MA, Sussel L. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLos One 7: e52026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley SP, Rastellini C, da Costa MA, Kowalik TF, Bloomenthal AB, Brown M, Cicalese L, Basadonna GP, Uknis ME. Gene silencing in the endocrine pancreas mediated by short-interfering RNA. Pancreas 31: 373–379, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Braeuning A, Vetter S. The nuclear factor-κB inhibitor (E)-2-fluoro-4′-methoxystilbene inhibits firefly luciferase. Biosci Rep 32: 531–537, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai MJ, Dong DJ, Wang Y, Liu PC, Liu W, Wang JX, Zhao XF. G-protein-coupled receptor participates in 20-hydroxyecdysone signaling on the plasma membrane. Cell Commun Signal 12: 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Sun X, Shen S, Zhang H, Ma X, Liu J, Kuang S, Yu Q. Wedelolactone, a naturally occurring coumestan, enhances interferon-gamma signaling through inhibiting STAT1 protein dephosphorylation. J Biol Chem 288: 14,417–14,427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 153: 1039–1048, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daley MS, Riddle MC. Glucose and glucagon do stimulate somatostatin release from isolated pancreatic islets. Diabetologia 21: 238–239, 1981. [DOI] [PubMed] [Google Scholar]
- 9.Day SM, Gu J, Polak JM, Bloom SR. Somatostatin in the human heart and comparison with guinea pig and rat heart. Br Heart J 53: 153–157, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulin NO, Niu J, Browning DD, Ye RD, Voyno-Yasenetskaya T. Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J Biol Chem 276: 20,827–20,830, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dun SL, Brailoiu GC, Tica AA, Yang J, Chang JK, Brailoiu E, Dun NJ. Neuronostatin is co-expressed with somatostatin and mobilizes calcium in cultured rat hypothalamic neurons. Neuroscience 166: 455–463, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folli F, Okada T, Perego C, Gunton J, Liew CW, Akiyama M, D'Amico A, La Rosa S, Placidi C, Lupi R, Marchetti P, Sesti G, Hellerstein M, Perego L, Kulkarni RN. Altered insulin receptor signalling and beta-cell cycle dynamics in type 2 diabetes mellitus. PloS One 6: e28050, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambaryan S, Kobsar A, Rukoyatkina N, Herterich S, Geiger J, Smolenski A, Lohmann SM, Walter U. Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of protein kinase A from an NF-κB-IκB complex. J Biol Chem 285: 18,352–18,363, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hua Y, Ma H, Samson WK, Ren J. Neuronostatin inhibits cardiac contractile function via a protein kinase A- and JNK-dependent mechanism in murine hearts. Am J Physiol Regul Integr Comp Physiol 297: R682–R689, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36: 1139–1145, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson L, Ansari T, McGuinness OP. Counterregulatory deficits occur within 24 h of a single hypoglycemic episode in conscious, unrestrained, chronically cannulated mice. Am J Physiol Endocrinol Metab 290: E678–E684, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji R, Sanchez CM, Chou CL, Chen XB, Woodward DF, Regan JW. Prostanoid EP1 receptors mediate up-regulation of the orphan nuclear receptor Nurr1 by cAMP-independent activation of protein kinase A, CREB, and NF-κB. Br J Pharmacol 166: 1033–1046, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun 142: 436–440, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Kelly CB, Blair LA, Corbett JA, Scarim AL. Isolation of islets of Langerhans from rodent pancreas. Methods Mol Med 83: 3–14, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 20: 876–913, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, Yuan J. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Different 11: 123–130, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Larsson H, Ahren B. Islet dysfunction in insulin resistance involves impaired insulin secretion and increased glucagon secretion in postmenopausal women with impaired glucose tolerance. Diabetes Care 23: 650–657, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Vaca Sanchez P, Leibiger IB, Berggren PO. Glucagon regulates its own synthesis by autocrine signaling. Proc Natl Acad Sci USA 109: 20,925–20,930, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lilla V, Webb G, Rickenbach K, Maturana A, Steiner DF, Halban PA, Irminger JC. Differential gene expression in well-regulated and dysregulated pancreatic β-cell (MIN6) sublines. Endocrinology 144: 1368–1379, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev 24: 261–274, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci USA 108: 12,925–12,930, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May LT, Lin Y, Sexton PM, Christopoulos A. Regulation of M2 muscarinic acetylcholine receptor expression and signaling by prolonged exposure to allosteric modulators. J Pharmacol Exp Ther 312: 382–390, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Menge BA, Gruber L, Jorgensen SM, Deacon CF, Schmidt WE, Veldhuis JD, Holst JJ, Meier JJ. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes 60: 2160–2168, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Science Signal 1: re4, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Rafacho A, Goncalves-Neto LM, Santos-Silva JC, Alonso-Magdalena P, Merino B, Taboga SR, Carneiro EM, Boschero AC, Nadal A, Quesada I. Pancreatic alpha-cell dysfunction contributes to the disruption of glucose homeostasis and compensatory insulin hypersecretion in glucocorticoid-treated rats. PloS One 9: e93531, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes 54: 757–764, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Reno CM, Litvin M, Clark AL, Fisher SJ. Defective counterregulation and hypoglycemia unawareness in diabetes: mechanisms and emerging treatments. Endocrinol Metab Clin NA 42: 15–38, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohrer S, Menge BA, Gruber L, Deacon CF, Schmidt WE, Veldhuis JD, Holst JJ, Meier JJ. Impaired crosstalk between pulsatile insulin and glucagon secretion in prediabetic individuals. J Clin Endocrinol Metab 97: E791–E795, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Salvatori AS, Elrick MM, Samson WK, Corbett JA, Yosten GL. Neuronostatin inhibits glucose-stimulated insulin secretion via direct action on the pancreatic alpha-cell. Am J Physiol Endocrinol Metab 306: E1257–E1263, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GL, Klein C, Lyu RM, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan XB, Chang JK, Hsueh AJ. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem 283: 31,949–31,959, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sriwai W, Mahavadi S, Al-Shboul O, Grider JR, Murthy KS. Distinctive G protein-dependent signaling by protease-activated receptor 2 (PAR2) in smooth muscle: feedback inhibition of RhoA by cAMP-independent PKA. PloS One 8: e66743, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sriwai W, Zhou H, Murthy KS. Gq-dependent signalling by the lysophosphatidic acid receptor LPA3 in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem J 411: 543–551, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 373: 2215–2221, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta 1834: 1271–1278, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toft I, Jenssen T. Type 2 diabetic patients have increased gluconeogenic efficiency to substrate availability, but intact autoregulation of endogenous glucose production. Scan J Clin Lab Invest 65: 307–320, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 122: 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unger RH, Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med 304: 1518–1524, 1981. [DOI] [PubMed] [Google Scholar]
- 45.Vinciguerra M, Deschenes G, Hasler U, Mordasini D, Rousselot M, Doucet A, Vandewalle A, Martin PY, Feraille E. Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell 14: 2677–2688, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber SM, Scarim AL, Corbett JA. Inhibition of IFN-γ -induced STAT1 activation by 15-deoxy-Δ 12,14-prostaglandin J2. Am J Physiol Endocrinol Metab 284: E883–E891, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Yosten GL, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol 303: R941–R949, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89: 413–424, 1997. [DOI] [PubMed] [Google Scholar]