Abstract
Adipose triglyceride lipase (ATGL) catalyzes the rate-limiting removal of the first fatty acid from a triglyceride. ATGL is activated by comparative gene identification-58 and inhibited by G(0)/G(1) switch gene-2 protein (G0S2). Research in other tissues and cell culture indicates that inhibition is dependent on relative G0S2-to-ATGL protein content. G0S2 may also have several roles within mitochondria; however, this has yet to be observed in skeletal muscle. The purpose of this study was to determine if muscle G0S2 relative to ATGL content would decrease to facilitate intramuscular lipolysis following endurance training. Male Sprague-Dawley rats (n = 10; age 51–53 days old) were progressively treadmill trained at a 10% incline for 8 wk ending with 25 m/min for 1 h compared with control. Sciatic nerve stimulation for hind-limb muscle contraction (and lipolysis) was administered for 30 min to one leg, leaving the opposing leg as a resting control. Soleus (SOL), red gastrocnemius (RG), and white gastrocnemius were excised from both legs following stimulation or control. ATGL protein increased in all trained muscles. Unexpectedly, G0S2 protein was greater in the trained SOL and RG. In RG-isolated mitochondria, G0S2 also increased with training, yet mitochondrial G0S2 content was unaltered with acute contraction; therefore, any role of G0S2 in the mitochondria does not appear to be acutely mediated by content alone. In summary, G0S2 increased with training in oxidative muscles and mitochondria but not following acute contraction, suggesting that inhibition is not through relative G0S2-to-ATGL content but through more complicated intracellular mechanisms.
Keywords: adipose triglyceride lipase, CGI-58, G0S2, endurance training, muscle lipolysis, stimulated contraction
adipose triglyceride lipase (ATGL) is thought to be the first and rate-limiting step in lipolysis. It is responsible for catalyzing the reaction of removing the first fatty acid from a triglyceride molecule, releasing the fatty acid for various metabolic fates, and subsequently, producing a diglyceride (46). ATGL activity is modulated by two regulatory proteins (24). It is activated by association with comparative gene identification-58 (CGI-58), increasing ATGL catalytic activity by >20-fold (20), and putatively inhibited by the G(0)/G(1) switch gene-2 (G0S2) protein. It appears that this inhibition can override the activating effects of CGI-58 (24). Whereas the mechanisms of G0S2 inhibition of lipolysis are currently unknown, it appears that ATGL catalytic rates are dose dependently decreased as relative increases of G0S2 are introduced in HeLa cells (44). It was originally speculated that ATGL and G0S2 are always bound together (24, 44); yet in fasted human adipose tissue, there was an increase in ATGL protein content with a decrease in G0S2 protein content, suggesting, at least, that there is not a one-to-one stoichiometry between ATGL and G0S2 and that they do not follow coordinate expression (26).
Intramuscular triglycerides (IMTGs) have been viewed as an important source of energy during prolonged, rigorous exercise. Froberg and Mossfeldt (9) measured IMTG content before and after exhaustive cross-country skiing for >4 h in human vastus lateralis (9). IMTG content was decreased significantly following a single bout of exercise, identifying IMTGs as an important fuel source during prolonged rigorous endurance exercise. Similarly, with chronic endurance training, it is generally believed that there is an increase in reliance on lipolysis of IMTG stores for energy provision within the muscle. Following a 12-wk endurance training protocol, Hurley et al. (14) reported a lower respiratory exchange ratio post-training, coinciding with a decrease in plasma glycerol and free fatty acids following an acute bout of exercise, suggesting a greater reliance toward intramuscular fat stores for energy provision, since exogenous influences were decreased. They also noticed sparing of muscle glycogen and a greater drop in IMTG concentration during acute exercise following training, along with greater stored IMTG post-training. This suggests an increased reliance on muscle IMTG use with chronic endurance training.
The use of IMTGs during exercise appears to be muscle specific. In Sprague-Dawley rats, following exhaustive swimming, there was a reduction of IMTG content by 48% in the soleus (SOL), a predominantly type I fiber tissue, and a 29% reduction in the red gastrocnemius (RG), a predominantly mixed type I and type IIa fiber (36). There was no reduction in IMTG content in the white gastrocnemius (WG), almost entirely type IIb and therefore, relying predominantly on glycolysis (36). Therefore, oxidative SOL and RG have a higher reliance on IMTG during endurance exercise compared with the WG. In human mixed-fiber vastus lateralis, it has been observed that in skeletal muscle from both healthy and obese individuals, ATGL protein content increases with endurance training, whereas there is no change in CGI-58 protein content, suggesting that muscle lipolytic capacity goes up with endurance training (1, 23, 45). G0S2 has only been measured in skeletal muscles from obese men, and there are no apparent effects of endurance training on G0S2 protein content (1, 23). However, there have been no studies examining the effects of endurance training on G0S2 protein content in healthy skeletal muscle nor whether there are specific changes in this protein to facilitate the differences in lipolytic capacity observed among muscles with distinctly different metabolic characteristics. Similarly, no study has examined the effects of endurance training toward a specific change in ATGL or CGI-58 in a spectrum of muscles with varying oxidative capacity and metabolic profiles.
Whereas a definitive role for G0S2 within skeletal muscle has yet to be elucidated, it appears that G0S2 is also present in mitochondria, with the speculation of several possible functions. It appears that G0S2 functions as a proapoptotic protein, binding to Bcl-2 in cells treated with TNF-α, which in turn, increases mitochondrial membrane potential and cytochrome c release (43). Alternatively, G0S2 is thought to be a positive regulator of complex V (F0F1 ATPase) under hypoxic-induced environments, increasing oxidative phosphorylation in cell culture (19). However, this has not been investigated in skeletal muscle. Whereas G0S2 appears to increase ATP production through complex V, it has not been determined whether mitochondrial G0S2 content can be altered with increasing energy demand, such as acute muscle contraction or endurance training.
Therefore, the purpose of our study was to characterize whole skeletal muscle and isolated mitochondrial G0S2 content in response to muscle contraction and endurance training in three skeletal muscles with varying oxidative capacities and metabolic profiles (SOL, RG, and WG). Our specific aims were the following: 1) to determine if endurance training would increase the capacity for lipolysis through alterations in muscle ATGL, CGI-58, and G0S2 protein content; 2) to determine if an acute, electrically stimulated contraction, which induced significant muscle lipolysis in whole muscle (30, 32), would decrease G0S2 relative to ATGL and CGI-58 to regulate lipolysis acutely; and 3) to examine whether mitochondrial G0S2 content would increase with acute muscle contraction and endurance training to increase energy production.
METHODS
Animals.
Male Sprague-Dawley rats (51–53 days old; Charles River Laboratories, Quebec, Canada) were randomly assigned to either the trained condition or the control condition (n = 10/group); however, one animal was unable to complete the training protocol, leaving the final experiments to an n = 9. All animals were housed in a reverse 12:12 light-dark cycle in the Brock University Comparative Bioscience Facility. All animals had access to ad libitum food and water at all times. All experimental protocols and procedures were approved by Brock University Animal Care and Utilization Committee and conformed to all Canadian Council on Animal Care Guidelines (27).
Training protocol.
Rats randomized into the training group started at 18 m/min for 30 min and by wk 8, were at 25 m/min for 1 h; all training was performed at a 10% incline (5, 7). Rats were caged in pairs with all cagemates in the same condition (either trained or sedentary). Food intake and body weight were measured weekly. Food intake was calculated using the following equation: food intake = (food provided − food remaining/2). Rats were housed two/cage.
Anesthesia.
Rats were anesthetized by isoflurane inhalant [induced at 5%; monitored between 3 and 5% during in vivo dual-energy X-ray absorptiometry (DXA) scans and surgical procedures].
Determination of lean and fat mass.
Three days before euthanasia, fat mass and lean mass were measured using pDEXA SABRE DXA (Orthometrix, White Plains, NY). Under isoflurane anesthesia, rats were placed supine with all limbs stretched onto the DXA and a region of interest (ROI) 10 cm wide × 4 cm long, starting from the proximal heads of the femur, extending cranially toward the thoracic vertebra. All scans were analyzed using specialized software (Host Software version 3.9.4; Orthometrix, White Plains, NY; Scanner Software version 1.2.0). Scans were performed at a speed of 20 mm/s with a resolution of 0.5 × 0.5 mm (16). Lean mass and fat mass are reported as a percentage of total ROIs.
Sciatic nerve stimulation.
Under isoflurane-induced anesthesia, sciatic nerve stimulation was applied to stimulate the left leg, whereas the right leg served as a resting internal control (12, 38). This stimulus has been established previously to induce a decrease in IMTG content and has been published previously by our lab (30). Briefly, a small incision was made on the left side, exposing the sciatic nerve, where platinum electrodes were attached, transmitting a pulse stimuli (20 V, 100 ms train duration) once every 3 s for 13 min, followed by a 4-min rest with no stimulation applied, with another 13 min of a pulse stimuli every 3 s.
Muscle excision.
Immediately after 30 min sciatic stimulation, the SOL, RG, and WG were removed and immediately snap frozen and subsequently stored in liquid nitrogen from the stimulated leg first, followed by the resting leg. The rats were not fasted before the surgery; therefore, they were in a fed state.
Mitochondrial isolation.
Primarily subsarcolemmal mitochondrial isolation has been described previously and characterized for purity in our lab by Western blotting for proteins found in other subcellular compartments (30) and adapted from previous studies (15, 28, 39). Briefly, fresh RG muscle tissue was minced manually on ice. Following mincing, samples were placed in 20× (vol/wt) of solution 1 (100 mM KCl, 40 mM Tris·HCl, 10 mM Tris base, 5 mM MgSO4, 5 mM EDTA, and 1 mM ATP) and further homogenized in glass potters. Then homogenized, samples were centrifuged at 700 g for 10 min, collecting the supernatant, and spun again at 14,000 g to pellet-suspended mitochondria. The mitochondrial pellet was resuspended in solution 2 (100 mM KCl, 40 mM Tris·HCl, 5 mM Tris base, 1 mM MgSO4, 0.01 mM EDTA, 1% BSA, and 0.25 mM ATP) and solution 3 (100 mM KCl, 40 mM Tris·HCl, 5 mM Tris base, 1 mM MgSO4, 0.01 mM EDTA, and 0.25 mM ATP) and spun at 7,000 g for 10 min, respectively. Mitochondria were purified with a 60% Percoll (P1644; Sigma-Aldrich, St. Louis, MO) gradient with final resuspension in sucrose and mannitol solution (220 mM sucrose, 70 mM mannitol, 10 mM Tris·HCl, and 0.1 mM EDTA). Samples were then stored in a −80°C freezer until analysis.
Citrate synthase.
The SOL, RG, and WG from the rested (nonstimulated) leg were assayed for citrate synthase (CS) activity from both sedentary and endurance-trained rats to confirm the effectiveness of the training protocol and the oxidative content of the surgical removal of the three muscles, as previously conducted in our lab (22). Briefly, tissue was homogenized in 1 M K2HPO4 buffer (pH = 8.1) and underwent two freeze/thaw cycles. In a cuvette, Triton, acetyl CoA, oxaloacetate, and the tissue homogenate were added and free CoA produced, reacted with 5,5′-dithiobis-2-nitrobenzoic acid. All analyses were conducted at 412 nm in an Ultrospec 2100 pro spectrophotometer (GE Healthcare, Baie-D'Urfé, Quebec, Canada) (37).
Western blotting.
SDS-PAGE was performed using 8% (for CGI-58), 10% (for ATGL), or 15% (for G0S2) running gels. Electrophoresis was performed for 85 min at 120 V and transferred onto a 0.45-μm polyvinylidene difluoride (PVDF) membrane for ATGL and CGI-58 (Amersham Biosciences, Piscataway, NJ) or a 0.20-μm PVDF membrane (Bio-Rad Laboratories, Hercules, CA) for G0S2. Anti-ATGL primary antibody (Cat. no. 2439s; Cell Signaling Technology, Beverly, MA) was diluted at 1:700 μl in 5% BSA in Tris-buffered saline-Tween 20 (TBST). Anti-CGI-58 primary antibody (Cat. no. NB110-41576; Novus Biologicals, Oakville, ON, Canada) was diluted at 1:1,000 μl in 2% fat-free powdered milk in TBST. As previously described and validated by our lab (41), two antibodies were used for G0S2 incubation: anti-G0S2 at a dilution of 1:2,000 μl NH2 terminus (Cat. no. sc-133424; Santa Cruz Biotechnology, Santa Cruz, CA) and a dilution of 1:2,000 μl Internal (Cat. no. sc-133423; Santa Cruz Biotechnology) in 5% fat-free powdered milk in TBST. Band density was determined using ImageJ software (National Institutes of Health, Bethesda, MD). To ensure equal loading, all blots were made relative to Ponceau staining (Sigma-Aldrich) as a loading control, which has been validated as a suitable loading control (8, 34). Variability in the Ponceau staining was approximately <15%.
Statistics.
Body weights, composition, and food weights were analyzed as a two-way ANOVA for training status × time (week). SOL and RG G0S2 protein content [training status (trained vs. sedentary) × contraction status (rested leg vs. stimulated leg)] were analyzed using a two-way ANOVA with a Student-Newman-Keuls post hoc test on SigmaStat (Systat Software, San Jose, CA). All other statistics were conducted within a given muscle (e.g., SOL, RG, WG) using a Student's t-test (trained vs. sedentary), and graphs were prepared on GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, CA). All graphical representation of Western blot data is made relative to the average of the rested sedentary control so that significance does not change compared with arbitrary units. Significance is reported as P < 0.05.
RESULTS
Body weights and food intake.
Although both sedentary and trained groups increased in size, the sedentary group was consistently heavier than the trained group, from week 1 to 7, with the same initial starting weights (week 0; P < 0.001; Fig. 1A). The trained group ate ∼87.5 g less/cage (i.e., 2 rats)/wk than the sedentary group throughout the duration of the study, starting at the first week (P < 0.001; Fig. 2).
Fig. 1.
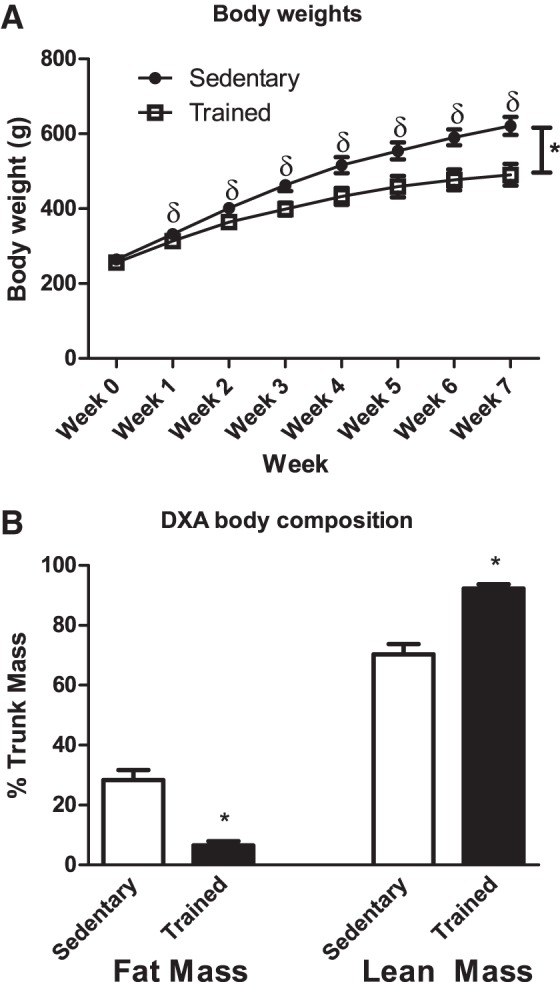
A: body weights of sedentary compared with trained rats from wk 0 to final body weights at the end of the training protocol (n = 9). Weights were recorded weekly. Data are reported as means ± SE; δsignificant differences between trained and sedentary for each individual week; *main effect for training status. Closed circles, sedentary; open squares, trained. B: lean mass and fat mass (n = 9). Analysis was conducted on the region of interest, which comprised lean mass + fat mass + bone mineral content. Data are reported as means ± SE; *significant differences between trained and sedentary (P < 0.05). Open bars, sedentary group; closed bars, trained group. DXA, dual-energy X-ray absorptiometry.
Fig. 2.
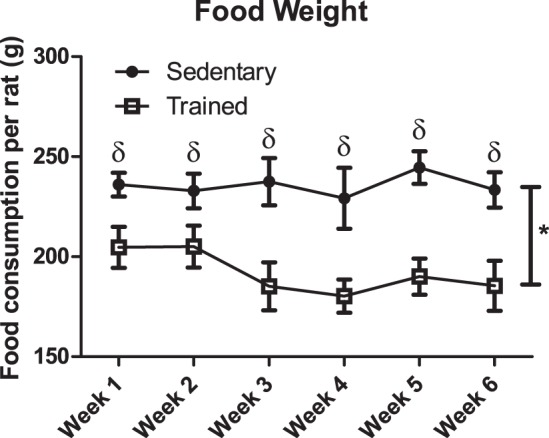
Food intake of sedentary compared with trained rats from the end of week 1 until week 7. Food intake = (food provided − food consumed/2 animals/cage); n = 9/group. Data are reported as means ± SE; δsignificant differences between trained and sedentary for each individual week; *main effect for training status (P < 0.05). Closed circles, sedentary; open squares, trained.
Lean and fat mass.
There was a 1.3-fold increase in lean mass in the trained group compared with the sedentary group (P < 0.001; Fig. 1B). There was a greater than four-fold decrease in fat mass in the trained group compared with the sedentary group (P < 0.001; Fig. 1B).
Citrate synthase.
As expected, before training, SOL and RG had similar CS activity, which was two-fold higher than WG. As a result of endurance training, CS activity increased ∼1.5-fold in all three muscles, such that SOL > RG > WG (Table 1).
Table 1.
Maximal citrate synthase activity
| CS Activity | SOL | RG | WG |
|---|---|---|---|
| Sedentary | 32.5 ± 1.8* | 29.4 ± 4.5* | 15.3 ± 3.1 |
| Trained | 54.8 ± 4.1 | 42.1 ± 3.9 | 27.9 ± 3.1 |
Results are means ± SE in μmol · min−1 · g wet weight−1 (n = 10). CS, citrate synthase; SOL, soleus; RG, red gastrocnemius; WG, white gastrocnemius (n = 9).
Means are not significantly different (P < 0.05).
ATGL and CGI-58 protein content.
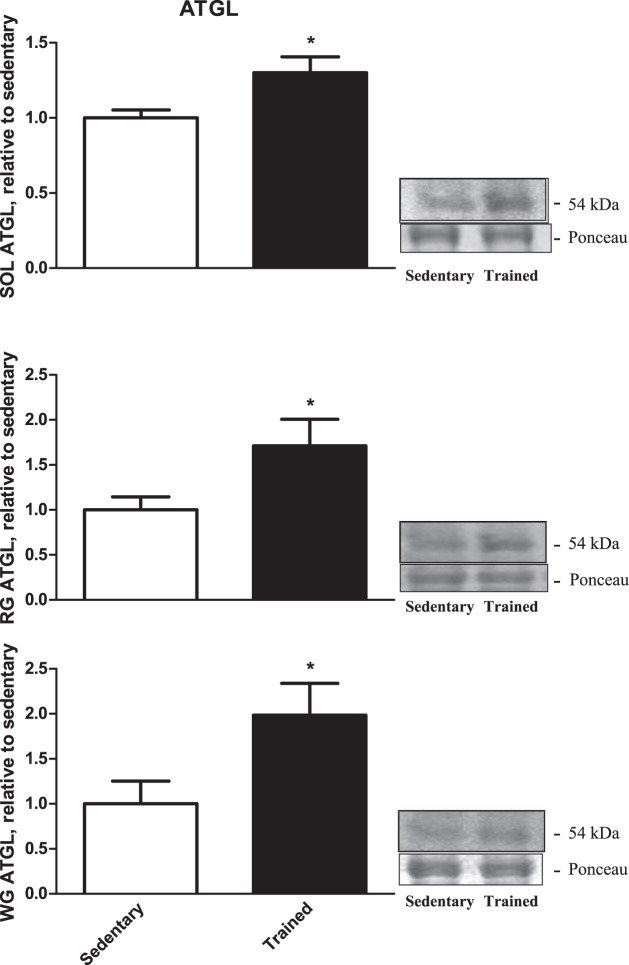
There was a 1.3-fold increase of ATGL protein content in the trained SOL muscle compared with the sedentary group (P = 0.02; Fig. 3, top), a 1.7-fold increase of ATGL protein content in trained RG compared with the sedentary group (P = 0.04; Fig. 3, middle), and almost a twofold increase in ATGL protein content in trained WG compared with the sedentary group (P = 0.04; Fig. 3, bottom).
Fig. 3.
Adipose triglyceride lipase (ATGL) protein content from our 3 rested skeletal muscles [soleus (SOL; top), red gastrocnemius (RG; middle), white gastrocnemius (WG; bottom); n = 9]. Data are reported as means ± SE; *significant differences between trained and sedentary (P < 0.05). Open bars, sedentary-rested group; closed bars, trained-rested group.
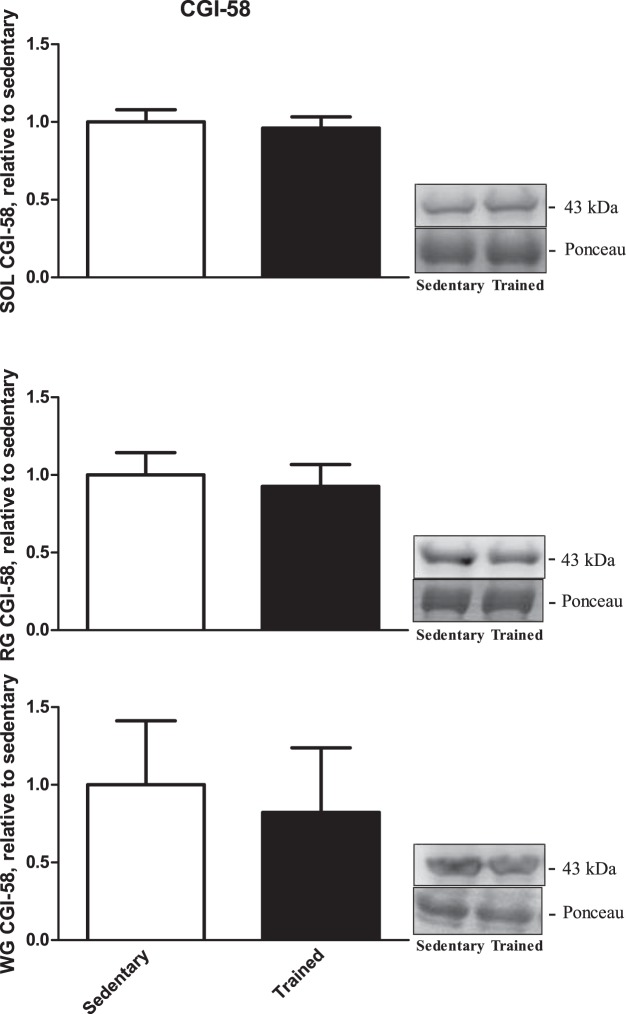
There were no significant differences in CGI-58 protein content due to endurance training compared with the sedentary group in SOL (P = 0.7; Fig. 4, top), RG (P = 0.7; Fig. 4, middle), or WG (P = 0.8; Fig. 4, bottom).
Fig. 4.
Comparative gene identification-58 (CGI-58) protein content from our 3 rested skeletal muscles [SOL (top), RG (middle), WG (bottom); n = 9]. Data are reported as means ± SE; there was no significant difference in CGI-58 in any of the muscles. Open bars, sedentary-rested group; closed bars, trained-rested group.
Whole muscle G0S2 protein content.
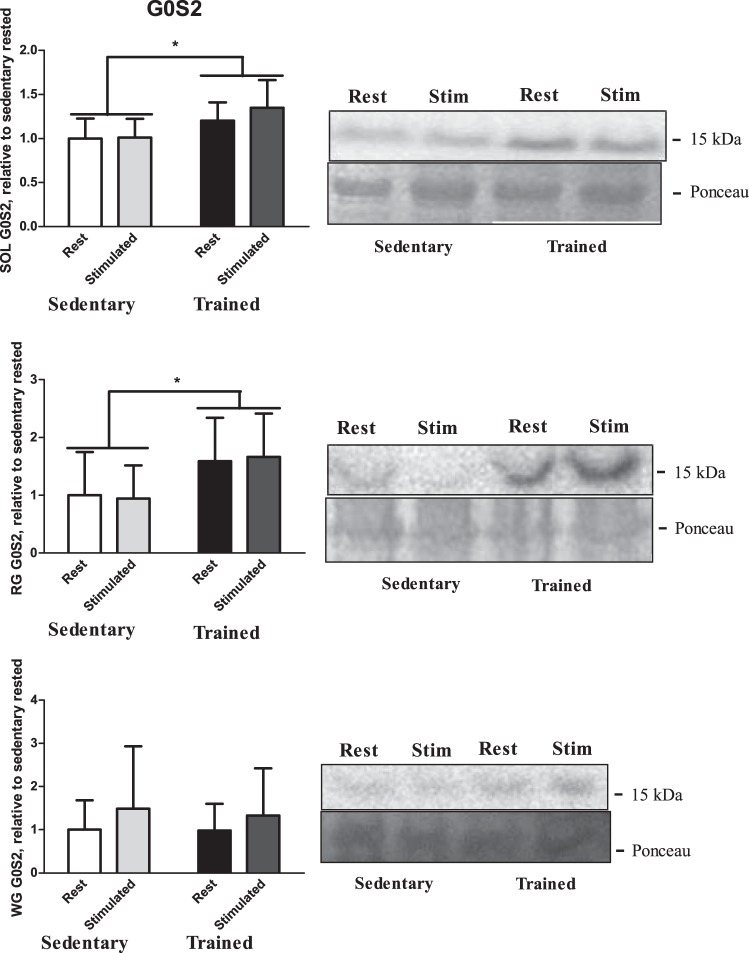
There were increases in G0S2 protein content in response to training in the SOL and RG. Protein content was assessed both for training status (trained vs. sedentary) and sciatic stimulation (rest vs. stimulated leg). A main effect was observed for training status, with the trained group having a greater G0S2 protein content compared with the sedentary group in both the SOL (P < 0.001; Fig. 5, top) and RG (P = 0.005; Fig. 5, middle), with no main effect observed for sciatic stimulation. There was no difference in G0S2 protein content in the WG in between the trained group and sedentary group (P = 0.9; Fig. 5, bottom).
Fig. 5.
G(0)/G(1) switch gene-2 (G0S2) protein content from both rested and stimulated legs of SOL (top), RG (middle), and WG (bottom) in both trained and sedentary groups (n = 9). Data are reported as means ± SE; *significant differences (P < 0.05). Open bars, sedentary-rested groups; light gray bars, sedentary-stimulated groups; black bars, trained-rested group; dark gray bars, trained-stimulated group.
Mitochondrial G0S2 protein content.
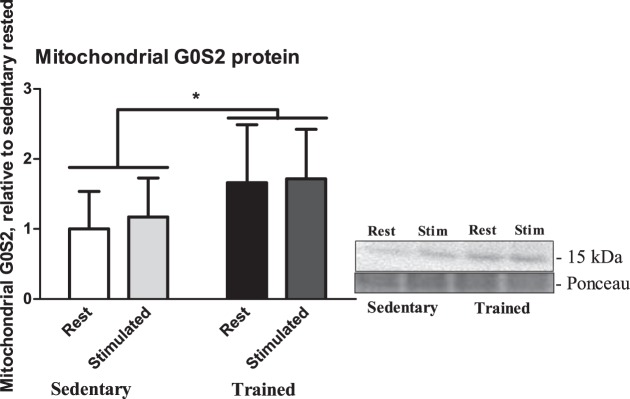
Because there were increases in whole muscle G0S2 in RG, and there was sufficient tissue to extract mitochondria, we examined whether there was any change in mitochondrial enrichment of G0S2 following training and whether this was altered by an acute bout of muscle contraction. There was a main effect for training status, such that there was a twofold increase in G0S2 protein content in mitochondria from trained RG muscle (P = 0.01; Fig. 6) compared with mitochondria from sedentary RG muscle, but there were no differences observed with an acute 30-min contraction in either group (P = 0.6).
Fig. 6.
Mitochondrial G0S2 protein content from both rested and stimulated legs of isolated RG mitochondria in both trained and sedentary groups (n = 9). Data are reported as means ± SE; *significant differences between trained and sedentary (P < 0.05). Open bar, sedentary-rested groups; light gray bar, sedentary-stimulated groups; black bar, trained-rested group; dark gray bar, trained-stimulated group.
DISCUSSION
This study is the first to examine the effects of endurance training in several metabolically different skeletal muscles (SOL, RG, and WG) on the relative protein expression of ATGL and its activator CGI-58 and putative inhibitor G0S2. The use of rat muscles with varying metabolic profiles allowed us to examine any potential differences in fiber type, as the SOL is primarily a type I (slow oxidative) fiber tissue and depends heavily on fat use (84% type Ia, 7% type IIa, and 9% type IIb/x), the RG is representative of mostly a mix of type I and type IIa fibers that are also highly oxidative (51% type Ia, 35% type IIa, and 14% type IIb/x), and the glycolytic WG is almost entirely type IIb fibers (0% type Ia, 0% type IIa, and 100% type IIb/x) (3).
Several novel findings have been identified from this study: 1) ATGL protein increased in all three skeletal muscles in response to training, yet the largest relative increase is in glycolytic WG, which typically relies the least on fat as a fuel source; 2) CGI-58 protein content does not change regardless of skeletal muscle type and training status; 3) whereas there were no changes with acute muscle contraction, whole muscle G0S2 protein is higher following endurance training but only in the SOL and RG, with no change observed in the glycolytic WG; and 4) in subsarcolemmal mitochondria from RG, G0S2 protein is higher in the trained group compared with the sedentary, although there were no observable differences in mitochondrial content of G0S2 with a 30-min contraction.
Effectiveness of training protocol.
Our training protocol caused whole-body and muscle adaptations. As expected, the trained group had a higher lean mass and lower fat mass compared with the sedentary group. In confirmation of muscle oxidative capacity, CS activities increased in SOL, RG, and WG muscles, as previously observed with training (21).
The sedentary group weighed significantly more compared with the trained group consistently throughout the training after the first week. This appeared to be due to both a higher energy expenditure and lower energy consumption. One possibility is that the daily rigorous exercise suppressed the appetite of the endurance-trained group. It has been observed in human adult men that there was a brief appetite suppression after rigorous exercise, which delayed the onset of feeding, such that repeated daily exercise (such as endurance training) leads to a significant decrease in energy consumption compared with energy expenditure over time (18).
ATGL and CGI-58 protein content.
We previously demonstrated that ATGL has the greatest expression in SOL (predominantly type I fibers) (3), but to a lesser degree, ATGL is also expressed in the WG (no type I fibers) (3). In this study, ATGL protein content was increased in the trained group compared with the sedentary group, as has been reported before (1, 2, 23, 45), and this is evident in all three of the muscle types investigated here. However, we observed tissue differences, with strong increases in the RG and SOL and the largest relative increase (approximately two-fold) in the glycolytic WG. This is contrary to previous work that suggested that ATGL was absent in IIb fibers (17) and demonstrates that it can be increased in glycolytic muscle, in addition to more oxidative muscle, probably due to a fiber-type shift toward more oxidative fiber types with a decrease in type IIb fibers [as reviewed by Scott et al. (35)].
CGI-58 protein content was unchanged regardless of training status; this was consistent across all of the studied skeletal muscles. This is consistent with previous work demonstrating that CGI-58 protein content does not change, regardless of training status in mixed human vastus lateralis (1). Thus we have added to this information by demonstrating that regardless of the skeletal muscle metabolic profile (i.e., oxidative vs. glycolytic), CGI-58 protein content is unaltered, not only among muscles with distinctly different metabolic characteristics (41) but also following 8 wk of endurance training in a variety of muscle types.
G0S2 protein content.
We are the first to demonstrate that G0S2 protein content increases following training in skeletal muscle, but the effects of training appear to be muscle specific, since G0S2 protein content only increased in the SOL and RG and remains unchanged regardless of training status in the WG. Given the small molecular weight of G0S2 (∼15 kDa), we previously determined a protein expression pattern across the SOL, RG, and WG, as well as verified the detection of G0S2 using Western blotting (41). Through a global G0S2 knockout, we proved specificity of the antibodies, as well as demonstrated a higher amount of G0S2 in SOL compared with WG, which suggests greater G0S2 in the more oxidative tissues (41). With endurance training, there is increased demand for IMTG lipolysis for energy production (6, 10, 11, 13, 14, 29), and this is consistent with the observed increase in ATGL protein to facilitate this process. However, contrary to our hypothesis that G0S2 content would decrease to release inhibitory control over ATGL, it increased in the two muscles that rely most heavily on fat oxidation and lipolysis. With the use of the plantaris muscle, we were able to determine IMTG content and that there was an increase in stored muscle lipids with training and no inhibition of lipolysis (as witnessed by the delta of the rested vs. stimulated leg), despite the apparent increase in G0S2 content. Whereas there was not a significant increase in IMTG use, these data demonstrate that despite the increase in G0S2 content, there was no inhibition of lipolysis (32). This suggests that lipolysis is under more complex regulation by G0S2 and not simply by G0S2 content. It is possible that as the lipolytic capacity increases in RG and SOL, it is also important to increase the potential for inhibition to ensure tighter regulation of fatty acid release to match with the rates of oxidation.
At first glance, our work seems contrary to a previous study in human skeletal muscle that did not observe a change in G0S2 (even with a large increase in ATGL) in the vastus lateralis of obese men following endurance training (23). However, in obese subjects, there is significantly less type I fibers with a greater proportion of glycolytic fast-twitch fibers (40). Therefore, these results would be closer to what we observed in the WG and underscore the important, specific role for G0S2 in metabolically different muscles that may rely heavily on lipolysis during exercise.
It is possible that G0S2 content in skeletal muscle increases with muscle lipid content, which is normally associated with endurance training in both human and rat muscles (2, 14, 25, 30). Previous work in heart muscle demonstrated that following physiological heart hypertrophy caused by endurance training, there was no increase in heart G0S2 protein, as well as no increase in cardiac lipid storage (4). This was in sharp contrast to pathological heart hypertrophy caused by aortic banding, where there was an increase in intracardiac triglycerides and diglycerides, as well as an increase in G0S2 protein content (4).
Interestingly, it has been postulated that ATGL and G0S2 are always bound together (24, 44); therefore, an increase in one protein would likely result in an increase in the other. However, we have demonstrated that if this is true, then this does not appear to be a one-to-one stoichiometric relationship, since the WG demonstrated the largest relative increase in ATGL protein content yet no change in G0S2.
Mitochondrial G0S2 content in response to training and contraction.
A novel finding from this study is that in isolated RG subsarcolemmal mitochondria, there was higher G0S2 protein content in our trained group compared with our sedentary group, similar to what was observed in whole muscle. However, there was no change in mitochondrial G0S2 content, due to an acute 30-min contraction, in either the trained or sedentary group. Interestingly, we were unable to detect ATGL protein content in these isolated mitochondria, only G0S2, suggesting that it perhaps has a role independent of lipolysis in the purified mitochondrial fraction. Kioka et al. (19) determined that G0S2 interacts with complex V (F0F1 ATPase), positively upregulating ATP production. Whereas we cannot comment on the apoptotic effects of G0S2 (43), the fact that we were able to detect G0S2 in the mitochondrial fraction would support the argument that it has a role in this organelle, potentially in regulating ATP production during exercise. However, because the mitochondrial content of G0S2 did not change following 30 min of muscle contraction, which would be expected to increase oxidative energy demand immediately, G0S2 does not appear to be moving to the mitochondria to cause acute changes in complex V activity. This suggests that regulation of complex V by G0S2 must be through more complicated mechanisms and not necessarily through changes in mitochondrial G0S2 content.
Perspectives and Significance
In summary, ATGL protein content increases due to endurance training, and the relative increase in ATGL is greatest in the WG, which typically relies more on substrates other than fat for energy production. However, WG does not support coordination of expression of G0S2 protein content, since the WG is the only one of the three skeletal muscles studied that did not demonstrate an increase in G0S2 protein content, despite the increase in ATGL protein content. Within the SOL and RG and isolated mitochondria from the RG, there were increases in G0S2 protein content due to training; however, there were no acute changes due to electrically stimulated muscle contraction. This suggests, perhaps, that there is a chronic regulation of G0S2 protein content in response to the prolonged stimuli of repeated daily dynamic treadmill exercise. Our study indicates that whereas G0S2 may have inhibitory effects on ATGL (24, 44) and alternate effects within the mitochondria (19, 43), these specific functions appear to be under more complicated control other than protein content alone, perhaps through post-translational or intracellular mechanisms.
GRANTS
Support for this research was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) grants (to B. D. Roy and S. J. Peters). W. E. Ward holds a Canada Research Chair in Bone and Muscle Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.C.T., A.B.L., S.V.R., B.D.R., W.E.W., and S.J.P. conception and design of research; P.C.T., A.B.L., and S.V.R. performed experiments; P.C.T. analyzed data; P.C.T., B.D.R., W.E.W., and S.J.P. interpreted results of experiments; P.C.T. prepared figures; P.C.T. drafted manuscript; P.C.T., A.B.L., S.V.R., B.D.R., W.E.W., and S.J.P. edited and revised manuscript; P.C.T. and S.J.P. approved final version of manuscript.
REFERENCES
- 1.Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P, Zimmermann R, Zechner R, Kiens B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab 296: E445–E453, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60: 2588–2597, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Dobrzyn P, Pyrkowska A, Duda MK, Bednarski T, Maczewski M, Langfort J, Dobrzyn A. Expression of lipogenic genes is upregulated in the heart with exercise training-induced but not pressure overload-induced left ventricular hypertrophy. Am J Physiol Endocrinol Metab 304: E1348–E1358, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Duan C, Winder WW. Effect of endurance training on activators of glycolysis in muscle during exercise. J Appl Physiol 76: 846–852, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyck DJ, Miskovic D, Code L, Luiken JJ, Bonen A. Endurance training increases FFA oxidation and reduces triacylglycerol utilization in contracting rat soleus. Am J Physiol Endocrinol Metab 278: E778–E785, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PloS One 8: e72457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froberg SO, Mossfeldt F. Effect of prolonged strenuous exercise on the concentration of triglycerides, phospholipids and glycogen in muscle of man. Acta Physiol Scand 82: 167–171, 1971. [DOI] [PubMed] [Google Scholar]
- 10.Gollnick PD, Saltin B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol 2: 1–12, 1982. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Han XX, Chabowski A, Tandon NN, Calles-Escandon J, Glatz JF, Luiken JJ, Bonen A. Metabolic challenges reveal impaired fatty acid metabolism and translocation of FAT/CD36 but not FABPpm in obese Zucker rat muscle. Am J Physiol Endocrinol Metab 293: E566–E575, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Hurley BF, Nemeth PM, Martin WH 3rd, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol Respir 60: 562–567, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Jackman MR, Willis WT. Characteristics of mitochondria isolated from type I and type IIb skeletal muscle. Am J Physiol Cell Physiol 270: C673–C678, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Jiang JM, Sacco SM, Ward WE. Ovariectomy-induced hyperphagia does not modulate bone mineral density or bone strength in rats. J Nutr 138: 2106–2110, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Jocken JW, Smit E, Goossens GH, Essers YP, van Baak MA, Mensink M, Saris WH, Blaak EE. Adipose triglyceride lipase (ATGL) expression in human skeletal muscle is type I (oxidative) fiber specific. Histochem Cell Biol 129: 535–538, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur J Clin Nutr 48: 715–724, 1994. [PubMed] [Google Scholar]
- 19.Kioka H, Kato H, Fujikawa M, Tsukamoto O, Suzuki T, Imamura H, Nakano A, Higo S, Yamazaki S, Matsuzaki T, Takafuji K, Asanuma H, Asakura M, Minamino T, Shintani Y, Yoshida M, Noji H, Kitakaze M, Komuro I, Asano Y, Takashima S. Evaluation of intramitochondrial ATP levels identifies G0/G1 switch gene 2 as a positive regulator of oxidative phosphorylation. Proc Natl Acad Sci USA 111: 273–278, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3: 309–319, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc PJ, Harris RA, Peters SJ. Skeletal muscle fiber type comparison of pyruvate dehydrogenase phosphatase activity and isoform expression in fed and food-deprived rats. Am J Physiol Endocrinol Metab 292: E571–E576, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Leblanc PJ, Mulligan M, Antolic A, Macpherson L, Inglis JG, Martin D, Roy BD, Peters SJ. Skeletal muscle type comparison of pyruvate dehydrogenase phosphatase activity and isoform expression: effects of obesity and endurance training. Am J Physiol Regul Integr Comp Physiol 295: R1224–R1230, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Louche K, Badin PM, Montastier E, Laurens C, Bourlier V, de Glisezinski I, Thalamas C, Viguerie N, Langin D, Moro C. Endurance exercise training up-regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. J Clin Endocrinol Metab 98: 4863–4871, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Lu X, Yang X, Liu J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle 9: 2719–2725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan TE, Short FA, Cobb LA. Effect of long-term exercise on skeletal muscle lipid composition. Am J Physiol 216: 82–86, 1969. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen TS, Vendelbo MH, Jessen N, Pedersen SB, Jorgensen JO, Lund S, Moller N. Fasting, but not exercise, increases adipose triglyceride lipase (ATGL) protein and reduces G(0)/G(1) switch gene 2 (G0S2) protein and mRNA content in human adipose tissue. J Clin Endocrinol Metab 96: E1293–E1297, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Olfert ED, Cross BM, McWilliam AA. Guide to the Care and Use of Experimental Animals. Ottawa, Ontario: Canadian Council on Animal Care, 1993, vol. 1. [Google Scholar]
- 28.Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJ, Spriet LL. Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. Am J Physiol Endocrinol Metab 281: E1151–E1158, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol 81: 2182–2191, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Ramos SV, MacPherson RE, Turnbull PC, Bott KN, LeBlanc P, Ward WE, Peters SJ. Higher PLIN5 but not PLIN3 content in isolated skeletal muscle mitochondria following acute in vivo contraction in rat hindlimb. Physiol Rep 2: pii: e12154, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos SV, Turnbull PC, MacPherson RE, LeBlanc PJ, Ward WE, Peters SJ. Changes in mitochondrial perilipin 3 and perilipin 5 protein content in rat skeletal muscle following endurance training and acute stimulated contraction. Exp Physiol 100: 450–462, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther 81: 1810–1816, 2001. [PubMed] [Google Scholar]
- 36.Spriet LL, Peters SJ, Heigenhauser GJ, Jones NL. Rat skeletal muscle triacylglycerol utilization during exhaustive swimming. Can J Physiol Pharmacol 63: 614–618, 1985. [DOI] [PubMed] [Google Scholar]
- 37.Srere P. Citrate synthase. Methods Enzymol 13: 3–11, 1969. [Google Scholar]
- 38.Stefanyk LE, Bonen A, Dyck DJ. Insulin and contraction-induced movement of fatty acid transport proteins to skeletal muscle transverse-tubules is distinctly different than to the sarcolemma. Metabolism 61: 1518–1522, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Stefanyk LE, Coverdale N, Roy BD, Peters SJ, LeBlanc PJ. Skeletal muscle type comparison of subsarcolemmal mitochondrial membrane phospholipid fatty acid composition in rat. J Membr Biol 234: 207–215, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Turnbull PC, Ramos SV, MacPherson RE, Roy BD, Peters SJ. Characterization of lipolytic inhibitor G(0)/G(1) switch gene-2 protein (G0S2) expression in male Sprague-Dawley rat skeletal muscle compared to relative content of adipose triglyceride lipase (ATGL) and comparitive gene identification-58 (CGI-58). PloS One 10: e0120136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch C, Santra MK, El-Assaad W, Zhu X, Huber WE, Keys RA, Teodoro JG, Green MR. Identification of a protein, G0S2, that lacks Bcl-2 homology domains and interacts with and antagonizes Bcl-2. Cancer Res 69: 6782–6789, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 11: 194–205, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao-Borengasser A, Varma V, Coker RH, Ranganathan G, Phanavanh B, Rasouli N, Kern PA. Adipose triglyceride lipase expression in human adipose tissue and muscle. Role in insulin resistance and response to training and pioglitazone. Metabolism 60: 1012–1020, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–1386, 2004. [DOI] [PubMed] [Google Scholar]