Abstract
Recent studies have implicated a role of norepinephrine (NE) in the activation of the sodium chloride cotransporter (NCC) to drive the development of salt-sensitive hypertension. However, the interaction between NE and increased salt intake on blood pressure remains to be fully elucidated. This study examined the impact of a continuous NE infusion on sodium homeostasis and blood pressure in conscious Sprague-Dawley rats challenged with a normal (NS; 0.6% NaCl) or high-salt (HS; 8% NaCl) diet for 14 days. Naïve and saline-infused Sprague-Dawley rats remained normotensive when placed on HS and exhibited dietary sodium-evoked suppression of peak natriuresis to hydrochlorothiazide. NE infusion resulted in the development of hypertension, which was exacerbated by HS, demonstrating the development of the salt sensitivity of blood pressure [MAP (mmHg) NE+NS: 151 ± 3 vs. NE+HS: 172 ± 4; P < 0.05]. In these salt-sensitive animals, increased NE prevented dietary sodium-evoked suppression of peak natriuresis to hydrochlorothiazide, suggesting impaired NCC activity contributes to the development of salt sensitivity [peak natriuresis to hydrochlorothiazide (μeq/min) Naïve+NS: 9.4 ± 0.2 vs. Naïve+HS: 7 ± 0.1; P < 0.05; NE+NS: 11.1 ± 1.1; NE+HS: 10.8 ± 0.4). NE infusion did not alter NCC expression in animals maintained on NS; however, dietary sodium-evoked suppression of NCC expression was prevented in animals challenged with NE. Chronic NCC antagonism abolished the salt-sensitive component of NE-mediated hypertension, while chronic ANG II type 1 receptor antagonism significantly attenuated NE-evoked hypertension without restoring NCC function. These data demonstrate that increased levels of NE prevent dietary sodium-evoked suppression of the NCC, via an ANG II-independent mechanism, to stimulate the development of salt-sensitive hypertension.
Keywords: NCC, salt-sensitive hypertension, norepinephrine, sodium homeostasis, sympathetic nervous system
hypertension, a significant public health burden, contributes to deaths from stroke, myocardial infarction, and kidney failure, making hypertension the single greatest risk factor for premature mortality. Salt-sensitive hypertension occurs in ∼50% of hypertensive patients and results in a three-fold increase in the risk of adverse cardiovascular events (9, 19, 39). It is well established that multiple factors contribute, in an integrated fashion, to the pathophysiology of salt-sensitive hypertension, and current evidence points to the kidney playing a pivotal role in the long-term control of blood pressure through its essential role in regulating sodium homeostasis (2, 7, 13). Recently, there has been increased interest in delineating the interactions between the sympathetic nervous system and the kidney, which function to regulate sodium reabsorption. Increased dietary salt intake in salt-resistant phenotypes results in the suppression of neural, humoral, and renal sodium-retaining mechanisms (5, 16, 24). In contrast, increased activity of the sympathetic nervous system is thought to play a key role in the pathogenesis of salt-sensitive hypertension by triggering an increase in renal sodium and water retention (8, 18, 25).
The epithelial sodium channel (ENaC) is an amiloride-sensitive sodium channel located at the apical membrane of polarized epithelial cells in the collecting tubules in the kidney (29). Mutations in ENaC result in Liddle's syndrome and have been linked to salt sensitivity in human patient populations (27, 31). The sodium chloride cotransporter (NCC) is a thiazide-sensitive transporter that is predominantly found on the apical side of the distal convoluted tubule (DCT; Ref. 12). The importance of the NCC in blood pressure regulation and sodium homeostasis is showcased in the genetic disorder Gitelman's syndrome, in which loss-of-function mutations in the NCC result in salt-wasting, hypokalemia, and hypotension (28). In the salt-resistant Sprague-Dawley rat, elevations in dietary salt intake suppress sympathetic outflow and circulating norepinephrine (NE) levels (14, 15), in addition to rapidly and persistently reducing the expression of the NCC (40). The physiological process of downregulating the NCC in response to increased dietary salt intake is hypothesized to facilitate sodium homeostasis and normotension (40). However, investigations into the direct impact of increased NE levels on the expression of the NCC during normal dietary salt intake have produced conflicting evidence. In the Sprague-Dawley rat, increased plasma NE content, achieved via a subcutaneous osmotic minipump infusion of NE, in animals maintained on a normal salt intake evoked hypertension without impacting the renal expression of the NCC (30). In contrast, recent studies conducted in C57BL/6J mice have reported that a chronic subcutaneous NE infusion results in the development of hypertension and the upregulation of both total and phosphorylated NCC expression during normal salt intake (22, 32). However, in these studies, the impact of a high-salt intake in combination with a subcutaneous NE infusion on NCC expression was not investigated. In addition to the recent focus of the actions of NE on the NCC, ANG II, a potent vasoconstrictor and sodium-retaining hormone (20), has been reported to alter both the activity and phosphorylation of the NCC via actions on the angiotensin type 1 receptor (AT1; Refs. 26, 34, 36).
In this study, we hypothesize that salt-sensitive hypertension is driven, in part, by a failure to downregulate renal NCC activity in the presence of excess circulating NE during high dietary salt intake. The following studies are designed to examine the impact of excess circulating NE and increased dietary salt intake, both individually and in combination, on sodium homeostasis, blood pressure regulation, and NCC function and expression in conscious male Sprague-Dawley rats. Furthermore, chronic pharmacological antagonism of the AT1 receptor examined the potential role of the renin-angiotensin system (RAS) in the development of NE-evoked salt-sensitive hypertension.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN; 275–299 g body wt) were individually housed in a temperature (range 68–79°F)- and humidity (range 30–70%)-controlled environment under a 12:12-h light-dark cycle. Following the completion of surgical procedures, rats were randomly assigned to a standard normal salt (NS) rodent diet [Teklad Global Diet, Harlan Laboratories, Indianapolis, IN; Teklad Global 18% protein rodent diet no. 2918, 18% protein, 5% crude fat, 5% fiber, total NaCl content 0.6% (102 meq Na+/kg)] or a high-salt (HS) rodent diet [Test Diet, St. Louis, MO; basal diet no. 5G01, 22% protein, 5.5% crude fat, 5% fiber, modified to contain total NaCl content 8% (1,378 meq Na+/kg)] and tap water ad libitum for a 14-day experimental period. All protocols were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee, and all procedures were conducted in accordance with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals.”
Surgical Procedures
Subcutaneous osmotic minipump implantation.
Animals were anesthetized (sodium methohexital, 20 mg/kg ip) and surgically instrumented with an osmotic minipump (Alzet, osmotic pump model 2ML2, Palo Alto, CA) that was placed subcutaneously in the subscapular region. Following subcutaneous osmotic minipump surgical placement, all animals were returned to their home cage following administration of penicillin (300,000 units/ml, 0.3 ml im).
Acute femoral vein, artery, and bladder cannulation.
Following 14 days of NS or HS intake, all animals were anesthetized (sodium methohexital, 20 mg/kg ip, supplemented with 10 mg/kg iv, as required). Once anesthetized, rats were instrumented with catheters in the left femoral artery, left femoral vein, and bladder for the measurement of arterial blood pressure, intravenous administration of saline and/or drugs, and renal function, respectively (15, 37, 38). Rats were then placed in a Plexiglas holder, and an intravenous infusion of isotonic saline (20 μl/min) was maintained for a 2-h surgical recovery period prior to experimentation to enable the animal to regain full consciousness and for cardiovascular/renal excretory functions to stabilize (15, 37, 38). Mean arterial pressure (MAP) and heart rate (HR) were continuously recorded via the surgically implanted femoral artery cannula using computer-driven BIOPAC data acquisition software (MP150 and AcqKnowledge 3.8.2; BIOPAC Systems, Goleta, CA) connected to an external pressure transducer (P23XL; Viggo Spectramed, Oxnard, CA; Refs. 15, 37, 38). Note that the zero level of the pressure transducer corresponded to heart level.
Experimental Treatment Groups
Naïve animals.
Naïve animals were randomly assigned to receive a NS or HS diet for a 14-day experimental period (n = 6/group).
Isotonic saline vehicle infusion.
Animals underwent implantation of an osmotic minipump delivering a subcutaneous infusion of isotonic saline (flow rate 5 μl/h) prior to random assignment to either a NS or HS diet for a 14-day experimental period (n = 6/group).
Norepinephrine infusion.
Animals underwent implantation of an osmotic minipump delivering a subcutaneous infusion of NE (Sigma, St. Louis, MO; cat. no. A7256) dissolved in isotonic saline (NE; 600 ng/min, flow rate 5 μl/h; Ref. 30) prior to random assignment to either a NS or HS diet for a 14-day experimental period (n = 6/group).
DMSO/saline vehicle infusion.
Animals underwent implantation of an osmotic minipump delivering a subcutaneous infusion of DMSO/isotonic saline (50:50 solution, flow rate 5 μl/h) prior to random assignment to either a NS or HS diet for a 14-day experimental period (n = 6/group).
Hydrochlorothiazide infusion.
Animals underwent implantation of an osmotic minipump delivering a subcutaneous infusion of NE (NE: 600 ng/min, flow rate 5 μl/h; Ref. 30), in combination with hydrochlorothiazide (HCTZ; Sigma, St. Louis, MO; cat. no. H4759) dissolved in DMSO/isotonic saline (50:50 solution; HCTZ: 4 mg·kg−1·day−1; flow rate 5 μl/h; Ref. 4) prior to random assignment to either a NS or HS for a 14-day experimental period (n = 6/group).
Losartan infusion.
Animals underwent implantation of an osmotic mini-pump delivering a subcutaneous infusion of NE (NE: 600 ng/min, flow rate 5 μl/h; Ref. 30) in combination with losartan (Tokyo Chemical Industry, Tokyo, Japan; cat. no. L2032) dissolved in DMSO/isotonic saline (50:50 solution; losartan: 3 mg/kg/day, flow rate 5 μl/h; Ref. 17) prior to random assignment to either a NS or HS for a 14-day experimental period (n = 6/group).
Acute Experimental Protocols
The following cardiovascular, renal sodium transporter activity, and autonomic function protocols were performed consecutively in a single experiment in each animal following 14 days of NS or HS intake.
Cardiovascular function.
Following a 2-h surgical recovery period, baseline MAP was recorded continuously over a 30-min period in conscious rats via the surgically implanted femoral artery cannula (n = 6/group; Refs. 15, 38). In Figs. 1A, 4A, and 6A, the presented value for MAP represents the average obtained over the entire 30-min period in which baseline MAP was recorded.
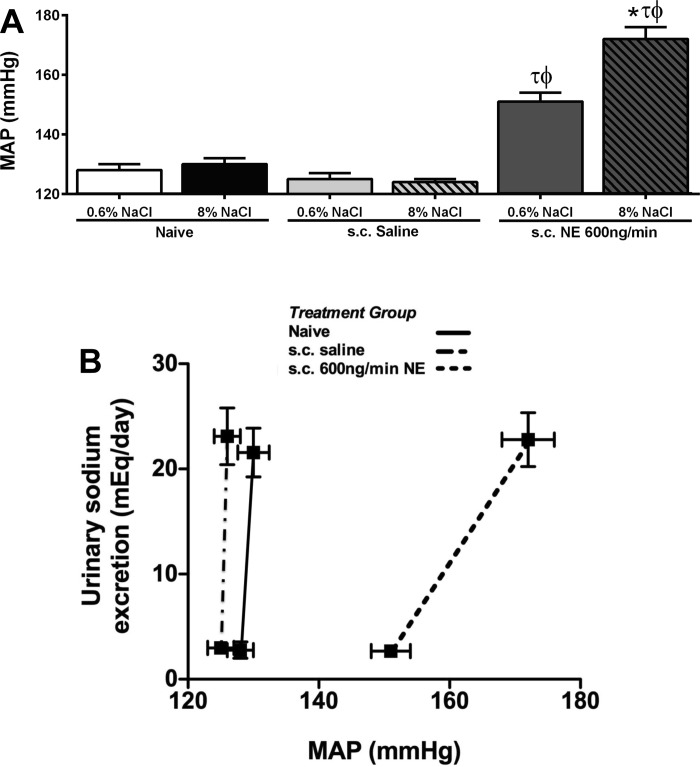
Fig. 1.
Basal mean arterial pressure (MAP, mmHg) (A) and index of salt-sensitivity (B) were determined from a 24-h sodium excretion and baseline MAP in naïve conscious male Sprague-Dawley rats and in Sprague-Dawley rats receiving either a subcutaneous isotonic saline infusion or a subcutaneous norepinephrine (NE; 600 ng/min) infusion maintained on either a normal (0.6% NaCl) or a high-salt (8% NaCl) diet for 14 days. Data are expressed as means ± SE; n = 6/group. *P < 0.05 vs. respective 0.6% NaCl group value. τP < 0.05 vs. respective naive group value. ϕP < 0.05 vs. respective saline group value.
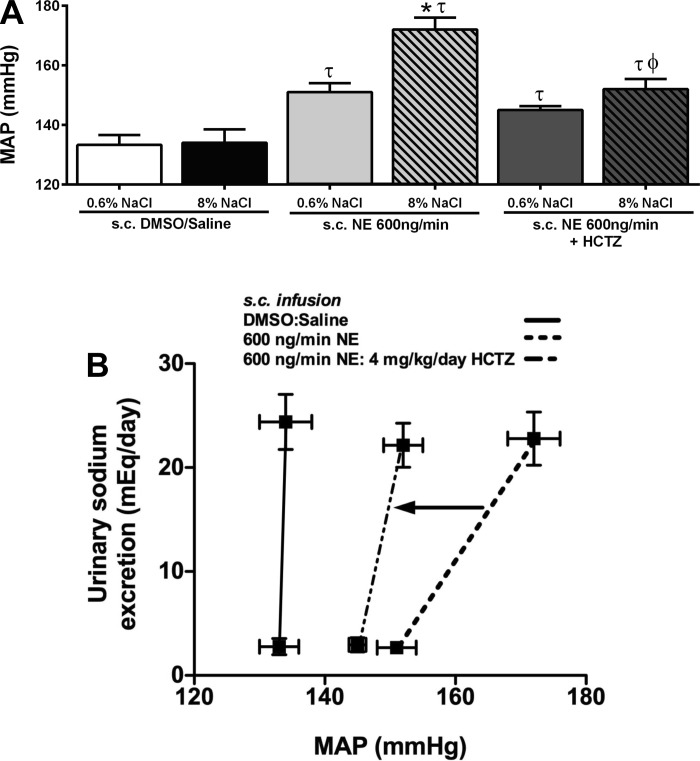
Fig. 4.
MAP (mmHg) (A) and an index of salt sensitivity (B) determined from 24-h sodium excretion and baseline MAP in conscious male Sprague-Dawley rats receiving either a subcutaneous 50:50 DMSO/isotonic vehicle saline infusion, a subcutaneous NE (600 ng/min) infusion or a subcutaneous NE (600 ng/min) and HCTZ (4 mg·kg−1·day−1) infusion maintained on either a normal (0.6% NaCl) or high-salt (8% NaCl) diet for 14 days. Data are expressed as means ± SE (n = 6/group) *P < 0.05 vs. respective 0.6% NaCl group value; τP < 0.05 vs. respective DMSO/saline group value; ϕP < 0.05 vs. respective NE group value. Data for subcutaneous NE infusion reproduced from Fig. 1 for clarity.
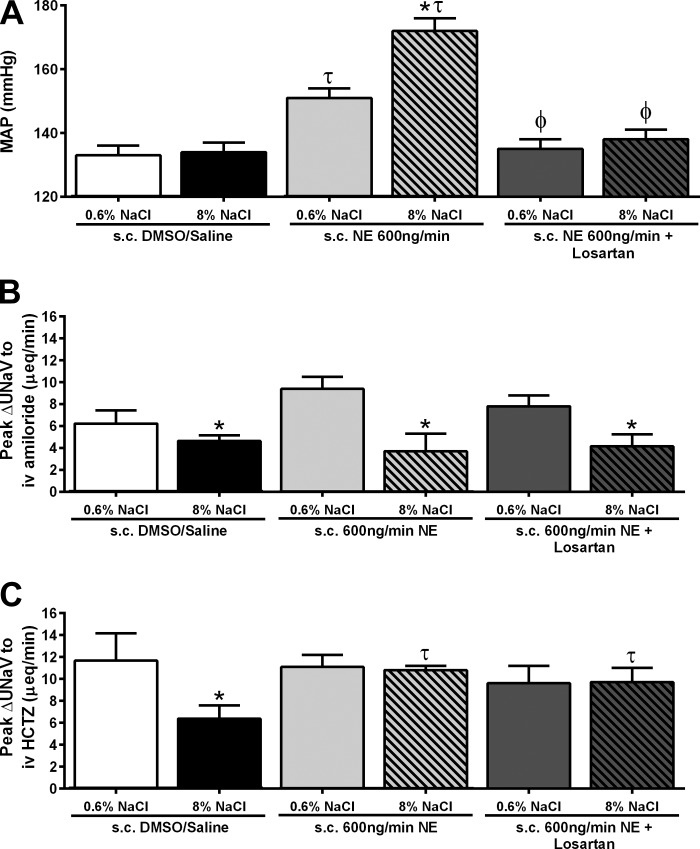
Fig. 6.
Basal MAP (mmHg) (A), peak natriuresis (UNaV; μeq/min) in response to an intravenous amiloride infusion (2 mg/kg) (B), and peak natriuresis (UNaV; μeq/min) in response to an intravenous infusion of amiloride (2 mg/kg) in combination with HCTZ (2 mg/kg) (C), in conscious male Sprague-Dawley rats receiving either a subcutaneous 50:50 DMSO/isotonic saline vehicle infusion, a subcutaneous NE (600 ng/min) infusion, or a subcutaneous NE (600 ng/min) and losartan (3 mg·kg−1·day−1) infusion maintained on either a normal (0.6% NaCl) or high-salt (8% NaCl) diet for 14 days. Data are expressed as means ± SE (n = 6/group). *P < 0.05 vs. respective 0.6% NaCl group value; τP < 0.05 vs. respective DMSO/saline group value; ϕP < 0.05 vs. respective NE group value. Data for subcutaneous DMSO/saline and subcutaneous NE infusion reproduced from Figs. 4 and 5 for clarity.
Renal sodium transporter activity.
Following the completion of the cardiovascular function protocol, a renal sodium transporter activity protocol was initiated. All animals received an intravenous infusion of isotonic saline (flow rate 20 μl/min) for 1 h, followed by an intravenous bolus of amiloride (2 mg/kg) preceding a 1-h intravenous infusion of amiloride (2 mg/kg, flow rate 20 μl/min; Ref. 4), and an intravenous bolus of HCTZ (2 mg/kg) preceding a 1-h intravenous infusion of amiloride (2 mg/kg, flow rate 20 μl/min; Ref. 4) in combination with HCTZ (2 mg/kg, flow rate 20 μl/min; Ref. 4). Throughout the 3-h protocol, HR and MAP were continually monitored, and urine was collected in 10-min intervals to assess peak natriuresis to intravenous amiloride or HCTZ (n = 6/group). The peak natriuretic response (ΔUNaV; μeq/min) was determined by subtracting the baseline UNaV value from the maximum natriuretic value observed during each hour of drug infusion (i.e., intravenous amiloride or intravenous amiloride+HCTZ). Baseline UNaV values were determined by averaging the UNaV values from the last two 10-min time points during the previous hours of the study [hour 1: intravenous saline (40–50 min, 50–60 min) or hour 2: intravenous amiloride (100–110 min, 110–120 min)]. The maximum natriuretic response to amiloride and amiloride+HCTZ occurred during the 10–20 min and 20–30 min time points post-drug infusion, respectively.
Autonomic function protocol.
Following completion of the renal sodium transporter activity protocol, the peak change in MAP in response to an intravenous bolus of hexamethonium (30 mg/kg; Refs. 15 and 38) was assessed. Baseline MAP was determined as the average MAP recorded over a 10-min control period prior to hexamethonium injection. After baseline MAP measurement, animals received an intravenous bolus of hexamethonium, and blood pressure was monitored for an additional 30-min period. The peak depressor response, assessed over a 60-s period, occurred within 5 min postinjection. Following protocol completion, rats were decapitated while conscious, and both kidneys were collected and immediately frozen at −80°C for measurement of the NCC in the kidney cortex.
Metabolic Balance Studies
Metabolic balance studies were conducted in all treatment groups on day 13 of their respective dietary sodium intake period following a 48-h acclimatization to the metabolic cages. Rats were individually housed in metabolic cages (model 18cv, Fenco, Cataumet, MA), with external food containers and water bottles. Metabolic cages were equipped with a double-fine mesh screen that allowed separation of food and feces from urine that was collected into beakers that contained a layer of mineral oil to prevent evaporation. Rats were given ad libitum access to their respective assigned diet and tap water for 24 h. Measurements were made for food and water consumption and urine output during the 24-h period (15, 38). Daily water balance was determined by calculating the difference between water intake and urine output. Daily sodium balance was determined by calculating the difference between sodium intake (dietary sodium intake) and sodium output (urinary sodium excretion).
Analytical Techniques
Urine volume was determined gravimetrically, assuming 1 g = 1 ml. Urinary and plasma sodium concentration was measured by flame photometry (model 943; Instrumentation Laboratories, Bedford, MA; Refs. 15, 37, 38). Plasma hematocrit (Hct) was determined using a micro-hematocrit centrifuge (Adams Readacrit, Clay Adams, NJ). Hct was used to calculate estimated plasma volume (EPV) and estimated blood volume (EBV) using the following equations: EPV = [0.065 × body wt (kg)] × (100 − Hct), EBV = (EPV × 100)/(100 − Hct) (37).
Plasma Renin Activity and Plasma Norepinephrine Measurement
In separate groups of animals, naïve Sprague-Dawley and NE-infused Sprague-Dawley rats were placed on a NS or HS diet for the 14-day experimental protocol. At the end of the 14-day experimental period, animals were decapitated while conscious and whole blood was collected. Plasma renin activity was determined via ELISA (Immuno-Biological Labs America, Minneapolis, MN; cat. no. IB59131). Plasma NE content was determined via ELISA (Immuno-Biological Labs America, Minneapolis, MN; cat. no. IB89552).
Kidney Cortical Membrane Preparation
Kidneys were harvested from animals following completion of acute experimental protocols and stored at −80°C. Kidney cortex tissue (∼200 mg) was homogenized on ice using a hand-held pestle in a homogenizing buffer (10 mM triethanolamine, 250 mM sucrose, 100 mM NaN3, 10 mM PMSF, and 1 mM leupeptin). The resulting homogenate was centrifuged at 4,000 g for 10 min at 4°C. The supernatant was collected and centrifuged at 17,000 g for 60 min at 4°C. Following centrifugation, the membrane pellet was resuspended in 400 μl of homogenizing buffer, and protein content was quantified via the BCA assay. Membrane preparations were stored at −80°C prior to use in immunoblotting studies.
Determination of Renal Sodium Chloride Cotransporter Levels
Protein extracted from membrane preparations of kidney cortex tissue were loaded at a concentration of 20 μg of protein per lane. Membranes were blocked in 5% milk for 1-h and probed overnight at 4°C with anti-NCC (1:2,000; Millipore, Billerica, MA; cat. no. AB3553) or anti-β-actin (1:5,000; Sigma, St. Louis, MO; cat. no. A5316) in 0.1% PBS-Tween. Subsequently, membranes were exposed to either a secondary horseradish peroxidase donkey anti-rabbit IgG (H+L) (1:5,000; Promega, Madison, WI; cat. no. V7951) or anti-mouse IgG peroxidase antibody (1:10,000; Sigma; cat. no. A9044) in 0.1% PBS-Tween for 1 h at room temperature. Bound antibodies were visualized using chemiluminescence (GE signal enhancer; GE, Buckinghamshire, UK). Densitometric analysis was performed using Quantity One software (Bio-Rad, Hercules, CA), and band densities were normalized to β-actin.
Statistical Analysis
Data are expressed as means ± SE. Differences between groups were assessed by a one-way ANOVA, followed by a Tukey post hoc test, to compare variations among the groups. A two-tailed Student's t-test was used to assess the difference between diet groups for the NCC expression quantification. Statistical analysis was carried out using a software program (GraphPad Prism version 6; GraphPad software, La Jolla, CA). Statistical significance is defined as P < 0.05.
RESULTS
High Salt Intake Exacerbates Norepinephrine-Induced Hypertension
When naïve or subcutaneous infused saline-treated Sprague-Dawley rats were challenged with a HS diet for 14 days, we did not detect any difference in baseline MAP compared with animals maintained on a NS diet (Fig. 1A). In contrast to a subcutaneous saline infusion, a 14-day subcutaneous infusion of NE at a rate of 600 ng/min resulted in the development of hypertension in animals maintained on a NS diet [Fig. 1A; MAP (mmHg) Naïve+NS: 128 ± 2; subcutaneous saline+NS: 125 ± 2 vs. subcutaneous NE+NS: 151 ± 3; P < 0.05)]. The magnitude of hypertension evoked by a subcutaneous NE infusion was exacerbated when the animals were maintained on a HS diet [Fig. 1A; MAP (mmHg) subcutaneous NE+NS: 151 ± 3 vs. subcutaneous NE+HS: 172 ± 4; P < 0.05]. Illustrated as an index of the salt sensitivity of blood pressure, both naïve and subcutaneous saline-infused Sprague-Dawley rats exhibit a classical salt-resistant phenotype. The subcutaneous infusion of NE caused a significant reduction in the slope of the chronic pressure-natriuresis relationship in Sprague-Dawley rats (Fig. 1B), reflecting the increased salt sensitivity of blood pressure. Additionally, Sprague-Dawley rats placed on a HS diet or receiving a subcutaneous NE infusion showed no significant differences in 24-h sodium or water balance (Table 1). Furthermore, the combination of a subcutaneous NE infusion and increased salt intake did not significantly alter 24-h sodium or water balance (Table 1). When estimated plasma (EPV) and blood volume (EBV) was calculated in these groups of animals, we observed no significant effect of increased dietary sodium intake or NE infusion, alone or in combination, on EPV or EBV (Table 2).
Table 1.
Twenty-four hour water and sodium balance listed for naïve Sprague-Dawley rats and Sprague-Dawley rats receiving a subcutaneous isotonic saline, 50:50 solution of DMSO and isotonic saline, NE, or NE and HCTZ infusion maintained on either a normal (0.6% NaCl) or high-salt (8% NaCl) diet for 14 days
| 24-h H2O Balance, ml |
24-h Sodium Balance, meq |
|||
|---|---|---|---|---|
| Treatment | 0.6% NaCl | 8% NaCl | 0.6% NaCl | 8% NaCl |
| Naïve | 19.1 ± 3.2 | 24.3 ± 4.0 | 0.6 ± 0.3 | 0.7 ± 0.2 |
| Saline | 26.2 ± 2.6 | 33.2 ± 4.2 | 0.5 ± 0.2 | 0.8 ± 0.2 |
| DMSO Saline | 21.5 ± 4.2 | 34.1 ± 6.0 | 1.0 ± 0.3 | 0.9 ± 0.2 |
| NE, 600 ng/min | 13.7 ± 2.6 | 19.5 ± 2.5 | 0.5 ± 0.2 | 0.5 ± 0.3 |
| NE, 600 ng/min, and HCTZ, 4 mg·kg−1·day−1 | 16.3 ± 4.3 | 13.8 ± 2.3 | 0.2 ± 0.6 | 032 ± 0.6 |
Data are expressed as means ± SE (n = 6/group). NE, norepinephrine; HCTZ, hydrochlorothiazide.
Table 2.
EPV and EBV in milliliters listed for naive Sprague-Dawley rats and Sprague-Dawley rats receiving a subcutaneous infusion of isotonic saline or 600 ng/min NE, maintained on either a normal or a high-salt diet for 14 days
| Treatment | EPV, ml | EBV, ml |
|---|---|---|
| Naïve 0.6% NaCl | 12.2 ± 0.4 | 21.0 ± 0.2 |
| Naïve 8% NaCl | 12.5 ± 0.2 | 21.7 ± 0.2 |
| Saline 0.6% NaCl | 11.9 ± 0.2 | 22.0 ± 0.4 |
| Saline 8% NaCl | 11.8 ± 0.3 | 21.1 ± 0.2 |
| NE 0.6% NaCl | 12.2 ± 0.4 | 21.8 ± 0.7 |
| NE 8% NaCl | 11.9 ± 0.2 | 21.6 ± 0.2 |
Data are expressed as means ± SE (n = 5 or 6/group). EPV, estimated plasma volume; EBV, estimated blood volume.
Norepinephrine Infusion Prevents Dietary Sodium-Evoked Suppression of NCC Activity
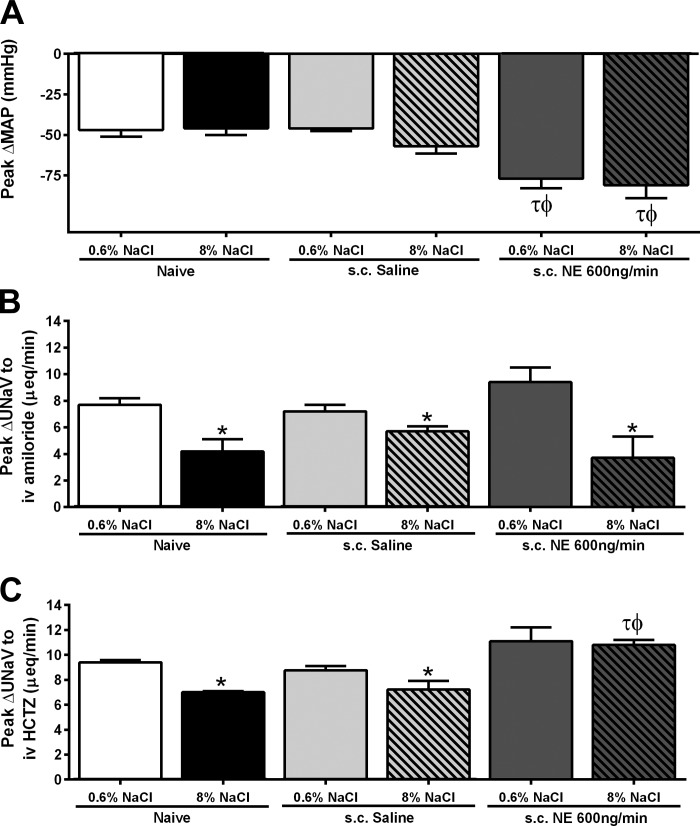
To assess the impact of NE infusion on vascular tone, we challenged animals with the ganglionic blocker hexamethonium via an acute intravenous bolus injection. As illustrated (Fig. 2A), increased dietary sodium intake did not alter the peak depressor response following ganglionic blockade in naïve and subcutaneous saline-infused Sprague-Dawley rats. However, NE infusion significantly increased the peak depressor response to hexamethonium, a response that was not altered following 14 days of HS intake (Fig. 2A). Next, we assessed the impact of NE infusion on the activity of two key renal sodium transporters, ENaC and NCC. The peak natriuretic response to amiloride in naïve and saline-treated groups was suppressed on a HS diet [Fig. 2B; peak ΔUNaV to intravenous amiloride (μeq/min) Naïve+NS: 7.7 ± 0.5 vs. Naïve+HS: 4.2 ± 1; P < 0.05]. NE infusion did not impact the dietary sodium-evoked suppression of the peak natriuretic response to amiloride (Fig. 2B; peak ΔUNaV to intravenous amiloride (μeq/min): subcutaneous NE+NS: 9.4 ± 1 vs. subcutaneous NE+HS: 3.7 ± 1.6; P < 0.05). Both naïve and subcutaneous saline-infused rats exhibited dietary sodium-evoked suppression of the peak natriuretic response to HCTZ [Fig. 2C; peak ΔUNaV to intravenous HCTZ (μeq/min) Naïve+NS: 9.4 ± 0.2 vs. Naïve+HS: 7 ± 0.1; P < 0.05; subcutaneous saline+NS: 8.8 ± 0.3 vs. subcutaneous saline+HS: 7.2 ± 0.7; P < 0.05]. Significantly, dietary sodium-evoked suppression of the peak natriuresis to HCTZ was not observed in NE-infused animals [Fig. 2C; peak ΔUNaV to intravenous HCTZ (μeq/min) subcutaneous NE+NS: 11.1 ± 1 vs. NE+HS: 10.8 ± 0.4]. Both saline and NE infusion in Sprague-Dawley rats had no impact on plasma sodium content irrespective of dietary sodium intake [plasma Na+ (mmol/l) subcutaneous saline+NS: 138 ± 2; subcutaneous saline+HS: 138 ± 1; subcutaneous NE+NS: 137 ± 1; subcutaneous NE+HS: 136 ± 2]. Additionally, plasma renin activity (PRA) and plasma NE content were determined in naïve and NE-infused Sprague-Dawley rats. Naïve rats showed a significant decrease in PRA and plasma NE content when challenged with a HS diet (Table 3). Rats receiving a NE infusion maintained on either a NS or HS diet showed a significant decrease in PRA compared with naïve Sprague-Dawley rats on NS (Table 3). In NE-infused rats, we observed increased plasma NE levels in animals maintained on both NS and HS compared with naïve Sprague-Dawley rats (Table 3).
Fig. 2.
A: peak change in MAP (mmHg) in response to an intravenous bolus of hexamethonium (30 mg/kg). B: peak natriuresis (UNaV; μeq/min) in response to an intravenous amiloride infusion (2 mg/kg). C: peak natriuresis (UNaV; μeq/min) in response to an intravenous infusion of amiloride (2 mg/kg) in combination with hydrochlorothiazide (HCTZ; 2 mg/kg), in naïve conscious male Sprague-Dawley rats and in Sprague-Dawley rats receiving either a subcutaneous isotonic saline infusion or a subcutaneous NE (600 ng/min) infusion maintained on either a normal (0.6% NaCl) or high-salt (8% NaCl) diet for 14 days. Data are expressed as means ± SE (n = 6/group). *P < 0.05 vs. respective 0.6% NaCl group value; τP < 0.05 vs. respective naive group value; ϕP < 0.05 vs. respective saline group value.
Table 3.
Estimated plasma renin activity expressed as ANG I generation and plasma NE content listed for naïve Sprague-Dawley rats and Sprague-Dawley rats receiving a subcutaneous infusion of 600 ng/min NE maintained on a normal or high-salt diet for 14 days
| Treatment | PRA Expressed as ANG I Generation, ng·ml−1·h−1 | Plasma NE, nmol/l |
|---|---|---|
| Naïve 0.6% NaCl | 5.1 ± 0.3 | 61.6 ± 4.5 |
| Naïve 8% NaCl | 1.8 ± 0.3 | 36.7 ± 4.2* |
| NE 0.6% NaCl | 1.3 ± 0.2 | 104.3 ± 8.2τ |
| NE 8% NaCl | 0.8 ± 0.1 | 118.4 ± 7.3ϕ |
Data are expressed as means ± SE (n = 5/group). PRA, plasma renin activity.
P < 0.05 respective group 0.6% NaCl group value, τP < 0.05 vs. naïve 0.6% NaCl group value. ϕP < 0.05 vs. naïve 8% NaCl group value.
Norepinephrine Infusion Prevents Dietary Sodium-Evoked Suppression of NCC Expression
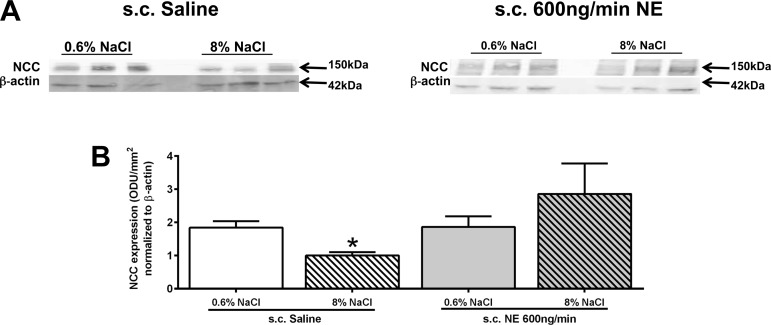
Following the completion of all acute experiments, kidney cortex tissue was harvested for analysis of NCC expression from the same saline- and NE-infused rats for which physiological data are presented in Figs. 1 and 2. When challenged with HS for 14 days, normotensive saline-treated animals showed dietary sodium-evoked suppression of renal NCC expression (Fig. 3, A and B), mirroring our physiology results. Chronic NE infusion did not significantly change NCC expression in animals maintained on a NS intake. In contrast to the result obtained in saline-infused rats, animals receiving a NE infusion and maintained on HS failed to show a dietary sodium-evoked decrease in NCC expression (Fig. 3, A and B).
Fig. 3.
A: representative immunoblots illustrating sodium chloride cotransporter (NCC) expression in kidney cortex tissue. B: quantification of renal NCC expression in male Sprague-Dawley rats receiving either a subcutaneous isotonic saline or NE (600 ng/min sc) infusion maintained for 14 days on a normal (0.6% NaCl) or high-salt (8% NaCl) diet. Data are expressed as means ± SE (n = 6/group). *P < 0.05 vs. respective 0.6% NaCl group value.
Chronic NCC Antagonism Abolishes the Salt-Sensitive Component of Norepinephrine-Evoked Hypertension
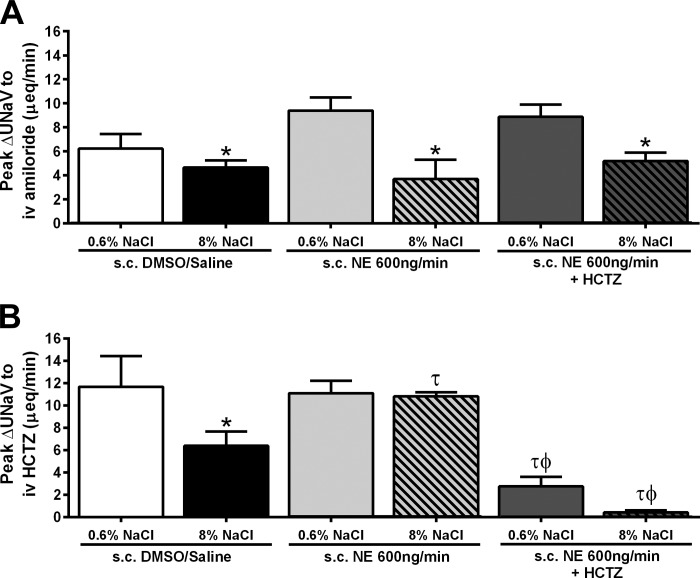
Following our observation that a NE infusion prevented the sodium-evoked suppression of NCC function and expression (Figs. 2C, 3, A and B), we elected to chronically antagonize the NCC with HCTZ during subcutaneous NE infusion. A subcutaneous DMSO/saline vehicle infusion did not alter the salt-resistant phenotype of the Sprague-Dawley rat (Fig. 4, A and B) or the sodium-evoked suppression of the peak natriuresis to HCTZ (Fig. 5B). The coinfusion of HCTZ with NE did not prevent the development of hypertension in animals maintained on a NS diet. In these animals, the magnitude of hypertension was comparable to that observed in NE-infused animals [Fig. 4A; MAP (mmHg) subcutaneous NE+NS: 151 ± 3; subcutaneous NE+HCTZ+NS: 145 ± 1]. Significantly, the coinfusion of NE with HCTZ during a HS intake abolished the salt-sensitive component of NE-evoked hypertension, reducing the MAP to a level similar to that observed in NE-treated animals maintained on NS (Fig. 4A). When plotted as an index of the salt sensitivity of blood pressure, coinfusion of NE and HCTZ significantly increased the slope of the chronic pressure-natriuresis relationship vs. animals receiving a subcutaneous infusion of NE alone (Fig. 4B), indicating a reduction in the salt sensitivity of blood pressure. The NE+HCTZ coinfusion did not prevent dietary sodium-evoked suppression of the peak natriuretic response to amiloride (Fig. 5A). Demonstrating the efficacy of our chronic antagonism of the NCC, an acute intravenous infusion of HCTZ did not evoke natriuresis in animals receiving a subcutaneous NE+HCTZ coinfusion (Fig. 5B). Additionally, DMSO/saline vehicle or coinfusion of NE+HCTZ did not significantly alter 24-h sodium or water balance regardless of salt intake (Table 1). When EPV and EBV were calculated in these groups of animals, we observed no significant effect of increased dietary sodium intake or drug infusion on EPV or EBV (Table 4).
Fig. 5.
A: peak natriuresis (UNaV; μeq/min) in response to an intravenous amiloride infusion (2 mg/kg). B: peak natriuresis (UNaV; μeq/min) in response to an intravenous infusion of amiloride (2 mg/kg) in combination with HCTZ (2 mg/kg), in conscious male Sprague-Dawley rats receiving either a subcutaneous 50:50 DMSO/isotonic saline vehicle infusion, a subcutaneous NE (600 ng/min) infusion, or a subcutaneous NE (600 ng/min) and HCTZ (4 mg·kg−1·day−1) infusion maintained on either a normal (0.6% NaCl) or high-salt (8% NaCl) diet for 14 days. Data are expressed as means ± SE (n = 6/group). *P < 0.05 vs. respective 0.6% NaCl group value; τP < 0.05 vs. respective DMSO/saline group value; ϕP < 0.05 vs. respective NE group value. Data for subcutaneous NE infusion reproduced from Fig. 2 for clarity.
Table 4.
EPV and EBV listed for Sprague-Dawley rats receiving a subcutaneous infusion of a 50:50 solution of DMSO and isotonic saline or NE in combination with HCTZ, maintained on either a normal or high-salt diet for 14 days
| Treatment | EPV, ml | EBV, ml |
|---|---|---|
| DMSO/Saline 0.6% NaCl | 12.4 ± 0.3 | 22.1 ± 0.4 |
| DMSO/Saline 8% NaCl | 11.9 ± 0.4 | 21.2 ± 0.4 |
| NE, 600 mg/min, and HCTZ 0.6% NaCl, 4 mg·kg−1·day−1 | 11.8 ± 0.3 | 21.2 ± 0.3 |
| NE, 600 mg/min, and HCTZ 8% NaCl, 4 mg·kg−1·day−1 | 12.5 ± 0.3 | 22.4 ± 0.6 |
Data are expressed as means ± SE (n = 5 or 6/group).
Chronic AT1 Receptor Antagonism Prevents Norepinephrine-Evoked Hypertension, but Does not Restore Sodium-Evoked NCC Impairment
To determine the contribution of the RAS to impaired NCC function during NE infusion and HS intake, we coinfused Sprague-Dawley rats with NE and losartan, an AT1 receptor antagonist. A subcutaneous infusion of NE+losartan significantly reduced baseline MAP for animals on a NS diet compared with NE infusion alone [Fig. 6A; MAP (mmHg) subcutaneous NE+NS: 151 ± 3 vs. subcutaneous NE+losartan+NS: 135 ± 3; P < 0.05]. A NE+losartan coinfusion also prevented the development of salt sensitivity [Fig. 6A; MAP (mmHg) subcutaneous NE+HS: 172 ± 4 vs. subcutaneous NE+losartan+HS: 138 ± 3; P < 0.05]. Dietary sodium-evoked suppression of peak natriuresis to amiloride was observed in animals receiving a subcutaneous infusion of NE+losartan [Fig. 6B; peak ΔUNaV to intravenous amiloride (μeq/min) sucutaneous NE+losartan+NS: 7.8 ± 1 vs. subcutaneous NE+losartan+HS: 4.15 ± 1; P < 0.05). However, dietary sodium evoked suppression of natriuresis to HCTZ was not observed following NE+losartan coinfusion [Fig. 6C; peak ΔUNaV to intravenous HCTZ (μeq/min) subcutaneous NE+losartan+NS: 11.5 ± 2; subcutaneous NE+losartan+HS: 11.3 ± 2].
DISCUSSION
The key finding of this study is that excess plasma NE levels in combination with a high dietary salt intake results in the development of salt-sensitive hypertension in the Sprague-Dawley rat, which exhibits a salt-resistant phenotype (37, 38). In the present study, we demonstrate that in this animal model, a subcutaneous NE infusion alone evoked hypertension, the magnitude of which was exacerbated when NE-infused rats were placed on a HS diet, demonstrating the development of the salt sensitivity of blood pressure. In these animals, increased plasma NE content prevented dietary sodium-evoked suppression of NCC, but not ENaC activity, suggesting a role for the NCC in the observed development of salt-sensitive hypertension. In agreement with a prior report in rats (30), and in contrast to studies in mice (22, 32), NE infusion alone did not increase NCC protein expression in animals maintained on an NS diet. Significantly, dietary sodium-evoked suppression of NCC expression was abolished in animals challenged with NE and an elevated salt intake for 14 days, a response correlating with impaired NCC activity and salt sensitivity. Confirming a direct role of the NCC in the development of NE-evoked salt-sensitive hypertension, chronic NCC antagonism with HCTZ abolished the salt-sensitive component of NE-mediated hypertension. Chronic antagonism of AT1 receptors with losartan was unable to restore NCC function; however, AT1 receptor blockade was able to significantly reduce NE-evoked increases in MAP in animals maintained on either a NS or HS diet. Collectively, these data show a critical interaction between dietary salt intake and circulating levels of NE that prevent the downregulation of NCC activity and expression to evoke the development of salt-sensitive hypertension in the Sprague-Dawley rat.
Our results demonstrating NE-mediated hypertension are substantiated by the findings of Sonalker et al. (30), in which the same chronic infusion of NE (600 ng/min) resulted in the development of hypertension in Sprague-Dawley rats maintained on a NS diet. Extending the studies of Sonalker et al. (30), we provide evidence that increased plasma NE content—driven by the constant subcutaneous osmotic mini-pump infusion of NE—to levels comparable to those observed in the hypertensive Dahl salt-sensitive rat (38), results in the development of salt sensitivity in the classically salt-resistant Sprague-Dawley rat (14, 15). The development of salt sensitivity during chronic NE infusion is evidenced by a significant increase in MAP during HS intake and a rightward shift of the chronic pressure-natriuresis relationship, reflecting the long-term resetting of the chronic pressure-natriuresis relationship to a new higher set-point blood pressure. Notably, EPV and EBV were not impacted by 14 days of dietary sodium intake or NE infusion in these studies. These data suggest that the observed increases in MAP after the 14-day protocol are not dependent on a long-term increase in blood or plasma volume. However, these studies do not rule out the possibility that transient increases in EPV and/or EBV play a role in the induction and/or development of NE-evoked hypertension. To further investigate the mechanisms contributing to elevated MAP in these NE-infused animals, we assessed the impact of hexamethonium-mediated ganglionic blockade on the peak depressor response. In these studies, we observed no difference in the peak depressor response following ganglionic blockade between NE-infused animals maintained on either a NS or HS diet. In NE-infused animals, we observed a significantly greater drop in blood pressure vs. that observed in saline infused rats, indicating that increased vasoconstriction contributes to the development of NE-evoked hypertension. As observed in control (naïve and saline infused) animals, a HS intake did not significantly affect the depressor response to ganglionic blockade in NE-infused rats, suggesting that there is no impact of sodium intake to increase sympathetic outflow in NE-infused animals. As such, we believe the difference in basal blood pressure observed following ganglionic blockade between NE-infused animals on NS vs. HS is reflective of the baseline blood pressure differences observed prior to hexamethonium administration vs. intrinsic differences in sympathetic activity. The mechanism underlying our observation of an enhanced depressor response after ganglionic blockade in NE-infused animals remains to be determined and highlights the complexity of interpreting the results of ganglionic blockade in the setting of chronic hypertension. In the current experimental paradigm, it would be anticipated that NE infusion would not activate the sympathetic nervous system and may evoke reduced sympathetic outflow. Therefore, we speculate that vascular hypertrophy, as has been reported following ANG II-evoked persistent increases in blood pressure (21), may underlie the enhanced response to ganglionic blockade observed in these studies.
Owing to reports that the Dahl salt-sensitive rat, which exhibits salt-sensitive hypertension and increased plasma NE content (38), exhibits a failure to downregulate certain ENaC subunits during high salt intake (1, 39), we elected to examine the activity of the ENaC in hypertensive NE-infused Sprague-Dawley rats. Our physiological data, generated in conscious animals, indicate that the combination of a NE infusion and a HS intake does not prevent sodium-evoked downregulation of the activity of the ENaC. These data suggest that in the Sprague-Dawley rat, failure to suppress ENaC activity (assessed via peak natriuresis to amiloride) is not involved in the development of NE-evoked salt-sensitive hypertension, a finding in contrast to the ENaC dysregulation observed in the Dahl salt-sensitive rat phenotype (1, 3). The differences between the previously reported impact of salt intake on ENaC in the Dahl salt-sensitive rat and our current report of a lack of effect during chronic NE infusion in the Sprague-Dawley rat may be attributed to 1) the genetic differences between the Dahl salt-sensitive and Sprague-Dawley rat and 2) the multifactorial nature of Dahl salt-sensitive hypertension featuring aberrations in central, vascular, cardiac, hormonal, and renal mechanisms. We elected not to determine renal ENaC subunit expression or to examine the impact of chronic ENaC inhibition on the development of NE-evoked salt sensitivity of blood pressure in the Sprague-Dawley rat for the following reasons: 1) we observed no significant physiological differences in our NE infusion model compared with control animals in terms of the response of ENaC function during HS intake and 2) a prior report stating that chronic ENaC inhibition did not prevent the development of NE-evoked salt-sensitive hypertension in mice (22).
Therefore, our next series of studies focused on the role of the NCC in the development of the salt sensitivity of blood pressure in the Sprague-Dawley rat. Our initial data demonstrate that on a NS intake, the infusion of NE, which evokes hypertension, does not alter the activity (assessed as peak natriuresis to HCTZ) or expression (assessed via immunoblotting) of the NCC. These data support the prior findings that chronic NE exposure does not alter NCC expression in the Sprague-Dawley rat (30) or in mice (33). These data are in direct contrast to data generated in mice reporting that total expression and phosphorylation of NCC are increased by both chronic (22) and acute increases in NE (32). We believe the discrepancy between our data and the data of other laboratories concerning the impact of NE on the expression of the NCC may reflect, in part, a potential species difference in the mechanistic effects of NE on NCC expression. In support of previous studies demonstrating that a HS intake evokes downregulation of the NCC (40), we observed the downregulation of NCC activity (assessed as peak natriuresis to HCTZ) and protein expression (assessed via immunoblotting) in naïve Sprague-Dawley rats challenged with a HS diet. In contrast, animals that received a NE infusion and a HS diet failed to downregulate the activity of the NCC during HS intake, suggesting a role of impaired NCC function in the pathophysiology of sympathetically mediated salt-sensitive hypertension. These data suggest, that in the Sprague-Dawley rat, contrary to prior observations in mice, the NCC evokes the development of salt-sensitive hypertension through a failure to be downregulated in response to an elevated salt intake vs. a direct action of NE to increase activity and expression of the NCC. We acknowledge that these studies cannot exclude a potential direct effect of increased blood pressure, occurring independently of an interaction between salt and NE, that is influencing the activity of the NCC. To confirm a direct role of the failure to downregulate the activity of the NCC in response to HS in the development of salt sensitivity, we conducted additional studies in which the NCC was chronically antagonized by coinfusing Sprague-Dawley rats with NE+HCTZ for 14 days. The chronic antagonism of the NCC abolished the salt-sensitive component of NE-mediated hypertension and significantly attenuated the reduction in the slope of the chronic pressure-natriuresis relationship. These data provide strong evidence that the NCC plays a key role in the sympathetically mediated regulation of the kidney to drive the development of salt-sensitive hypertension.
To determine the potential contribution of the RAS in this animal model of NE-mediated salt-sensitive hypertension, we coinfused Sprague-Dawley rats with NE+losartan, an AT1 receptor antagonist. The coinfusion of NE+losartan significantly decreased MAP in animals maintained on both NS and HS diets vs. animals receiving a NE infusion. Our data, indicating the suppression of PRA activity during NE infusion, raise the paradoxical issue of how AT1 antagonism reduces blood pressure in the setting of low renin/ANG II levels. These data are in line with prior studies that have demonstrated the efficacy of AT1 antagonism in multiple models of “low renin” hypertension, and we believe that further studies, beyond the scope of the current manuscript, are required to determine the mechanism underlying the apparent counterintuitive abolishment of hypertension during NE infusion by AT1 antagonism. However, when Sprague-Dawley rats receiving a NE+losartan coinfusion were acutely challenged with HCTZ, dietary sodium-evoked suppression of NCC activity was not observed. These data indicate that the failure of the NCC to be downregulated in response to increased dietary salt intake during NE infusion occurs independently of the actions of the RAS. Previous studies have reported that ANG II has direct effects on the abundance, activity, and regulation of the NCC (10, 35, 36). However, a recent study using ANG II receptor type 1a (AT1a) knockout mice reported that NE-induced effects on NCC expression and phosphorylation are not AT1a dependent (32). These data support our results, which also indicate that the effects of NE on the NCC are independent of ANG II. Further, the ability of losartan to prevent NE-evoked hypertension, but not restore HS stimulated downregulation of NCC activity, suggests that NCC activity is regulated by NE via a mechanism that is independent of increased blood pressure. Although low-dose ANG II infusion has been shown (23) to result in hyponatremia in Sprague-Dawley rats, in the current studies, plasma Na+ levels in NE-infused rats remained within the normal range (135–145 mmol/l), suggesting that in our NE infusion model, we are not evoking systemic increases in ANG II, at least not to a level that evokes hyponatremia, a hypothesis supported by the NE-evoked suppression of PRA. Our data on the suppression of PRA activity during NE infusion and HS intake substantiate a lack of activation of the RAS in our experimental paradigm. The actions of NE to impact the NCC have been suggested to be dependent on the actions of the β-adrenergic receptor (βAR) (22, 32). Mu et al. (22) reported that stimulation of the β2AR increased NCC activity and sodium retention during the development of salt-sensitive hypertension in mice. However, a recent study has suggested that the β1AR is primarily responsible for mediating the effects of NE on the NCC due to the fact that β1ARs are highly enriched in the DCT (32). Currently, the β-adrenergic receptor subtype responsible for mediating the effects of NE on the NCC remains to be definitively established, as does the impact of dietary salt intake and NE on the renal expression of β-adrenergic receptors.
In conclusion, our results highlight the importance of the interaction between NE and salt, and not just NE alone, to influence the function and expression of the NCC during the pathophysiology of salt-sensitive hypertension. Although we believe our results are based on an action of NE directly on NCC regulation, we cannot exclude a direct effect of blood pressure preventing the downregulation of the NCC independently of an interaction between salt and NE. In the current studies, NE is being increased systemically, not just locally at the level of the kidney; as such, we are unable to rule out extrarenal effects and acknowledge that circulating NE may not penetrate the kidney to the same extent as local release of NE from sympathetic nerve terminals. However, given that in salt-sensitive human and rat (Dahl salt-sensitive) subjects, there is global sympathoexcitation (6, 11) and that our model of subcutaneous NE infusion is directly comparable with prior studies published in rats (30) and mice (22, 33), we believe we have developed a suitable model to investigate the actions of both salt and NE on sodium homeostasis and blood pressure regulation. Despite the clinical use and well-defined transporter-specific diuretic actions of both HCTZ and amiloride, we recognize that this series of studies relies on their selectivity and that off-target effects must be considered when interpreting the results. Owing to the short-term infusion of amiloride and HCTZ during the acute experimental protocol, we speculate the off-targets actions of these drugs are likely limited. In regard to the 14-day infusion of HCTZ, we observed no overt signs of potential hypokalemia that could have impacted our studies. In future studies, beyond the scope of the current investigations, we plan to address the effects of NE and salt on NCC function and the expression in additional rat models of salt-sensitive hypertension (e.g., Dahl salt-sensitive rat vs. Dahl salt-resistant rat). Future studies will also be conducted to investigate the impact of salt and NE on the phosphorylation of NCC via SPAK (SPS1-related proline/alanine-rich kinase) and OxSR1 (oxidative stress response-1) in our animal model to allow for further clarification of the mechanism(s) contributing to the observed dysregulation of NCC activity in Sprague-Dawley rats during NE infusion and high dietary sodium intake.
Perspectives and Significance
The current data highlight the critical interaction between dietary salt intake and increased levels of NE on the activity of the NCC. This work extends our understanding of interactions between the sympathetic nervous system and the kidney in the long-term regulation of sodium homeostasis and blood pressure. These findings challenge recent reports generated in mice of a direct effect of NE to increase expression of the NCC and substantiate a species difference in the response of NCC protein levels to NE infusion. We demonstrate that there is no effect of NE infusion alone on the physiological activity of the NCC in vivo in conscious Sprague-Dawley rats. Our data suggest a crucial impact of NE in preventing the sodium-evoked suppression of NCC activity and expression and support a key role for the NCC in the pathophysiology of salt-sensitive hypertension via actions of the sympathetic nervous system on the kidney. These findings suggest that there are significant species differences in the response to NE and highlight the importance of using multiple animal models to investigate the origins and mechanisms underlying salt sensitivity. We speculate that in hypertensive patients with increased activity of the sympathetic nervous system, there may be an increased risk of developing salt sensitivity vs. patients in which sympathetic activity remains unchanged, possibly using elevated plasma NE levels as a marker of future salt sensitivity.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.R.W., J.T.K., and R.D.W. conception and design of research; K.R.W., J.T.K., J.W.S., and R.D.W. performed experiments; K.R.W., J.W.S., and R.D.W. analyzed data; K.R.W., J.T.K., and R.D.W. interpreted results of experiments; K.R.W. and R.D.W. prepared figures; K.R.W. and R.D.W. drafted manuscript; K.R.W., J.W.S., and R.D.W. edited and revised manuscript; K.R.W., J.T.K., and R.D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health (NIH) R01 HL-107330 and NIH K02HL-112718 (to R. D. Wainford), NIH T32 GM-008541 (to K. R. Walsh), and NIH T32 HL-007224 (to J. W. Shim).
REFERENCES
- 1.Amin MS, Reza E, El-Shahar E, Wang HW, Tession F, Leenen FH. Enhanced expression of epithelial sodium channels in renal medulla of Dahl S rats. Can J Physiol Pharmacol 89: 159–168, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Ando K, Fujita T. Pathophysiology of salt-sensitive hypertension. Ann Med Surg 44 Suppl 1: S119–S126, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Aoi W, Niisato N, Sawabe Y, Miyazaki H, Tokuda S, Nishio K, Yoshikawa T, Marunaka Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertension rats. Cell Biol Int 31: 1288–1291, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Ashek A, Menzies RI, Mullins LJ, Bellamy COC, Harmar AJ, Kenyon CJ, Flatman PW, Mullins JJ, Bailey MA. Activation of thiazide-sensitive co-transport by angiotensin II in cyp1a1-Ren2 hypertensive rat. PLoS One 7: e36311, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol 32: 426–432, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int 21: 371–378, 1982. [DOI] [PubMed] [Google Scholar]
- 7.Crowley SD, Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest 124: 2341–2347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiBona GF. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol 289: R633–R641, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr 25 Suppl 3: 247S–255S, 2006. [DOI] [PubMed] [Google Scholar]
- 10.San-Cristobal Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II singaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384–4389, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill JR, Gullner GR, Lake CR, Lakatua DJ, Lan G. Plasma and urinary catecholamines in salt-sensitive idiopathic hypertension. Hypertension 11: 312–319, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Glover M, Zuber AM, O'Shaughnessy KM. Hypertension, dietary salt intake, and role of the thiazide-sensitive sodium chloride cotransporter NCCT. Cardiovasc Ther 29: 68–76, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Depire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest 123: 2011–2023, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapusta DR, Pascale CL, Wainford RD. Brain heteromeric Gαi2-subunit protein-gated pathways mediate central sympathoinhibition to maintain fluid and electrolyte homeostasis during stress. J Fed Am Soc Exp Biol 26: 2776–2787, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapusta DR, Pascale CL, Kuwabara JT, Wainford RD. Central Nervous system Gαi2-subunit proteins maintain salt resistance via a renal nerve-dependent sympathoinhibitory pathway. Hypertension 61: 368–375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohmeier TE, Hildebrandt DA, Hood WA. Renal nerves promote sodium excretion during long-term salt intake. Hypertension 33: 487–492, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Martinez LA, Villalobos-Molina R. Early and chronic captopril or losartan therapy reduces infarct size and avoids congestive heart failure after myocardial infarction in rats. Arch Med Res 34: 357–361, 2003. [DOI] [PubMed] [Google Scholar]
- 18.May CN, Frithiof R, Hood SG, McAllen RM, McKinley MJ, Ramchandra R. Specific control of sympathetic nerve activity to the mammalian heart and kidney. Exp Physiol 95: 34–40, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell KD, Braam B, Navar LG. Hypertensinogenic mechanisms mediated by renal actions of renin-angiotensin system. Hypertension 19: I18–I17, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Moretti JL, Burke SL, Evans RG, Lambert GW, Head GA. Enhanced responses to ganglion blockade do not reflect sympathetic nervous system contribution to angiotensin II-induced hypertension. J Hypertens 27: 1838–1848. [DOI] [PubMed] [Google Scholar]
- 22.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 17: 573–580, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen MTX, Lee DH, Depire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osborn JW, Collister JP, Guzman P. Effects of peripheral sympathetic nerve dysfunction on salt sensitivity of arterial pressure. Clin Exp Pharmacol Physiol 35: 273–279, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Iturbe B, Vaziri ND. Salt-sensitive hypertension - update on novel findings. Nephrol Dial Transplant 22: 992–995, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule NaCl cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr., Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP. Liddle's syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP. Gitleman's variant of Barter's syndrome inherited hypokalemic alkalosis is caused by mutations in NCC. Nat Genet 12: 24–30, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Sonalker PA, Tofovic SP, Bastacky SI, Jackson EK. Chronic noradrenaline increases renal expression of NHE-3, NBC-1, BSC-1 and aquaporin-2. Clin Exp Pharmacol Physiol 35: 594–600, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Zhang J, Zhao D, Wang Q, GU Y, Ma H, Zhang Z. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol Sin 32: 789–797, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terker AS, Yang CL, McCormick JA, Meermeier NP, Rogers SL, Grossman S, Trompf K, Delpire E, Loffing J, Ellison DH. Sympathetic stimulation of thiazide-sensitive sodium chloride cotransport in the generation of salt-sensitive hypertension. Hypertension 64: 178–184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida S, Chiga M, Sohara E, Rai T, Sasaki S. Does a β2-adrenergic receptor-WNK4-Na-Cl co-transporter signal cascade exist in the in vivo kidney? Nat Med 18: 1324–1325, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zieste R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Van der Lubbe N, Meima ME, Lim CH, van Veghel R, Rosenbaek LL, Mutig K, Danser AHJ, Fenton RA, Zieste R, Hoorn EJ. Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. Pflügers Arch 463: 853–863, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Lubbe N, Zieste R, Hoorn EJ. Effects of angiotensin II on kinase-mediated sodium and potassium transport in the distal nephron. Curr Opin Nephrol Hypertens 22: 120–126, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Wainford RD, Pascale CL, Kuwabara JT. Brain Gαi2-subunit protein-gated pathways are required to mediate the centrally evoked sympathoinhibitory mechanisms activated to maintain sodium homeostasis. J Hypertens 31: 747–757, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Wainford RD, Carmichael CY, Pascale CL, Kuwabara JT. Gαi2-protein-mediated signal transduction: central nervous system molecular mechanism countering the development of sodium-dependent hypertension. Hypertension 65: 178–186, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol 295: F1003–F1016, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]