Abstract
The United States Pharmacopeia (USP) General Chapters Dissolution 〈711〉 and Disintegration and Dissolution of Dietary Supplements 〈2040〉 allows the use of enzymes in dissolution media when gelatin capsules do not conform to dissolution specifications due to cross linking. Possible interactions between enzymes and surfactants when used together in dissolution media could result in loss of the enzymatic activity. Pepsin is an enzyme commonly used in dissolution media, and in this work, the activity of pepsin was determined in the presence of different surfactants as usually found in case of dissolution tests of certain gelatin capsule formulations.
Pepsin enzymatic activity was determined according to the Ninth Edition of the Food Chemicals Codex (FCC) 9 method, in dissolution conditions: simulated gastric fluid, 37 °C and 50 rpm. Sodium dodecyl sulfate (SDS), cetyltrimethyl ammonium bromide (CTAB), polysorbate 80 (Tween 80) and octoxynol 9 (Triton X100) in concentrations above and below their critical micellar concentrations were selected. Results showed a significant reduction in the activity of pepsin at all the concentrations of SDS assayed. On the contrary, CTAB, Tween 80, and Triton X100 did not alter the enzymatic activity at of pepsin any of the concentration assayed.
This data demonstrates a rational selection of the surfactant to be used when pepsin is required in dissolution test.
Keywords: Enzymes, Surfactants, Dissolution test, Pepsin
Graphical abstract
1. Introduction
The United States Pharmacopeia (USP) General Chapters Dissolution 〈711〉 [29] and Disintegration and Dissolution of Dietary Supplements 〈2040〉 allow the addition of enzymes to the dissolution medium when gelatin capsules and gelatin-coated tablets do not conform to the dissolution specification due to cross-linking of gelatin. Cross-linking entails the formation of strong chemical linkages between gelatin chains due to interactions with the filling material or between the gelatin and the environment during storage [32]. The covalent bonding produced with this type of cross-linking is, for all practical purposes, irreversible, and will eventually render the gelatin insoluble.
Cross-linking typically results in the formation of a pellicle on the internal or external surface of the gelatin capsule shell that prevents the capsule fill from being released. In vitro dissolution testing of cross-linked capsules can result in slower or incomplete release of the active ingredient or no release at all [14], [7]. The degree of cross-linking is not usually uniform within one capsule or among different capsules. As consequence, dissolution results will have higher variability when gelatin capsules are cross-linked [10], [16], [5], [7].
When the gelatin is no longer soluble in water, dissolution of the shell must involve the breaking of other bonds, e.g., by enzyme-mediated breaking of peptide bonds in protein chains. The pH of the dissolution medium determines the appropriate enzyme to be used according to 〈711〉 and 〈2040〉 [31].
Pepsin is the enzyme commonly used in acidic dissolution media (pH 1 to pH 4) to break peptide bonds in gelatin capsules that are affected by cross linking. Pepsin is a monomeric, two domain, mainly l-protein, with a high percentage of acid residues (43 out of 327) leading to its very low isoelectric point (IEP)≈1. It catalyzes the hydrolysis of peptide bonds between two hydrophobic amino acids [1]. The catalytic site of pepsin is formed by two aspartate residues, Asp32 and Asp215 (pKa values about 1.4 and 4.5, respectively), one of which has to be protonated, and the other deprotonated, for the protein to be active. This occurs in the pH range between 1 and 5 [6].
The proteolytic activity of pepsin is affected by the conditions of the dissolution medium. In acidic dissolution media like simulated gastric fluid (SGF, pH 1.2, 37 °C), pepsin shows its maximum activity. In fact, porcine pepsin has optimal activity at pH approximately 2.2. At pH 4.5 it decays to about 35% [3]. Within a pH range of 2–4, the enzymatic activity of pepsin is not affected by temperature changes between 4 °C and 37 °C [13]. The denaturation temperature of pepsin in solution at pH 2 is 67.96±0.015 °C [13]. The effect of ionic force (µ) on pepsin denaturation may be explained by changes in the ionizable groups and alterations of the electrostatic interactions. It has also been previously demonstrated that the alkaline denaturation rate constant is accelerated by the ionic strength [11].
There is widespread acceptance of the view that vagal stimulation evokes the secretion of acid gastric juice rich in pepsin. According to that, variability in human gastric secretion is high. The SGF is an artificial dissolution medium that is intended to represent a standardized way the stomach acid secretion in fasted state. The composition for SGF is given in the section Reagents: Test Solutions in USP-NF.
The dissolution medium may additionally contain a percentage of surfactants, dispersing agents, or solubility enhancers when either the capsule fill or the active ingredient (or both), are hydrophobic or water-insoluble. They may also be used if the media as described in 〈1092〉 [30] is ineffective in dispersing the capsule fill or in achieving proper sink conditions for the active ingredient.
The need for a surfactant and its particular concentration can be justified from drug substance solubility investigations, that include all common surfactant types, anionic (i.e., sodium dodecyl sulfate (SDS)), cationic (i.e., cetyltrimethyl ammonium bromide (CTAB)) and nonionic (i.e., polysorbate 80 (Tween 80) or (octoxynol 9 (Triton X100))) [17], [25], [28], [8], [9], [26]. When a suitable surfactant has been identified, different concentrations should be investigated to identify the lowest concentration needed to achieve sink conditions for the dissolution test. Typically, such concentration is above the surfactant׳s critical micellar concentration (CMC).
When the concomitant use of surfactants and enzymes are required for dissolution tests, a rational selection of the surfactant to be used should be performed in order to reach reliable results. Among the reasons that should be considered with this selection are the interactions between the surfactants and enzymes. The interaction between enzymes and surfactants has been widely described in the literature [24]; however, this topic has not been discussed for its implication in dissolution tests and no systematic studies have been reported about the effect of surfactants on the activity of the pepsin under the conditions used in USP dissolution tests, i. e. temperature, pH, stirring and time. Besides, this interaction depends on both the type and the concentration of the surfactant used. The monomeric or micellar form of the surfactants, which is related with their CMC, is one of the main factors that can affect the type of interaction with the enzymes. For water-soluble proteins, interactions with surfactants can be broadly split up into two regions: below and above the CMC [24]. Depending on the concentration of the surfactant and its ionic character, an unfolding or denaturation process of the enzyme could occur as a consequence of their interaction [24].
The goal of this study was to evaluate the impact in pepsin activity produced by the concomitant presence of surfactants currently used in dissolution studies. USP-SGF was selected as dissolution medium for this study.
2. Materials and methodology
2.1. Reagents
USP Pepsin for assay RS, Lot FOM 228, having an activity of 7.7 U/mg (USP 2015 Pepsin Activity) was used. Pepsin from porcine gastric mucosa lyophilized powder, 3200–4500 U/mg protein from SIGMA-ALDRICH® (Lot. SLBL 1721V, 26.0 U/mg) was used as reagent. Milli-Q water was used for all solutions preparations. The SGF was prepared according to specifications described in USP 38 (USP, 2015 Reagent, test solutions). The 4.0% w/v trichloroacetic acid (TCA) solution was prepared by diluting 5% TCA solution (RICCA®, reagent grade) with water, and the diluted hydrochloric acid solution (HCl Sol) was prepared by diluting 2.5 mL of 37% hydrochloric acid (Fisher®) to 1 l with water. The pH of the final solution was adjusted to 1.6±0.1 with 37% hydrochloric acid.
The substrate solution consisted of 2% w/v hemoglobin protease substrate USP RS, Lot FOM 231 in HCl Sol. The pH of the final solution was adjusted to a pH of 1.6±0.1 with 1 M HCl.
SDS (Spectrum®, Lot 2DH221), CTAB (MP Biomedicals, Lot MR31911), Tween 80 (Fisher®, Lot 132307) and Triton X100 (Fisher®, Lot 136597) were selected since they are the surfactants most commonly used un dissolution media [17], [25], [26], [28], [8], [9].
2.2. Standard curve of pepsin RS activity
Enzymatic activity of pepsin was performed using the method “Activity of pepsin” described in the FCC 9 [33]. In this assay, acidified hemoglobin is hydrolyzed by pepsin at 25 °C. This gives TCA soluble peptides, which are detected by UV absorbance at 280 nm. Enzymatic activity is expressed as U/mg protein.
In order to construct a calibration reference curve, fresh standard solutions (SS) of 0.7, 0.9, 1.0 and 1.30 U/mL (0.09–0.17 mg/mL) were prepared from a 0.2 mg/mL stock solution of pepsin RS in HCl Sol.
2.2.1. Enzymatic reaction
SS tubes: Tubes (in duplicate) containing 1.0 mL of each of the SS were placed into a water bath maintained at 25±0.1 °C, added with 5.0 mL of the hemoglobin substrate solution and mixed by vortexing. Exactly 10 min after that, the reaction was stopped by the addition of 10.0 mL of the TCA Solution.
SS blank tubes (SSB): Tubes (in duplicate) containing 1.0 mL of each of the SS were placed into a water bath maintained at 25±0.1 °C, added with 10.0 mL of TCA and mixed by vortexing. Then, an aliquot of 5.0 mL of the hemoglobin substrate solution was also added.
After 25 min, the content of SS and SSB tubes were filtered twice through a Whatman No. 41 ashless filter circle with a diameter of 150 mm. The absorbance of each of the filtrates was measured at 280 nm using an AGILENT single beam UV–visible spectrophotometer, in a 1-cm quartz cell. A blank solution was prepared by transferring 1.0 mL HCl Sol into a single tube and processed as described for SSB.
The net absorbance for the SS was calculated by subtracting the average absorbance of the SSB from the average absorbance of the corresponding SS. A standard curve of the net absorbance for each SS versus its concentration (mg/ml) was established. The calibration curve was performed six times, and the results of these curves were averaged in order to obtain an equation to be used to determine the activity of the pepsin.
The Pepsin RS enzymatic activity showed linearity in the concentration range between 0.7 and 1.3 U/mL (0.09–0.17 mg/mL), with a correlation coefficient of 0.9997 (R2). The equation of the average adjusted curve was calculated as y=4.6647x−0.00104. This equation was used to calculate the activity of pepsin.
Pepsin activity can be determined using different techniques. Thus, in different studies either edestin, gelatin, egg albumin, hemoglobin or human plasma has been used as substrates. Also, the time of digestion, pH, temperature, and the parameters of proteolytic activity varied in different studies.
USP and FCC methods specify hemoglobin as substrate and therefore, the results using this method generally cannot be extrapolated to other substrates or different testing conditions.
2.3. Activity of pepsin in SGF
In order to determine the activity of pepsin in similar conditions to a dissolution procedure, 500 mL of a pepsin solution in SGF (1.9 mg/mL) were stirred to 50 rpm and 37 °C using common dissolution equipment (Vankel, VK 7010, Apparatus 2). After 30 min, an aliquot was withdrawn and diluted 1/50 mL with HCl Sol in order to perform the enzymatic reaction (Section 2.2). This conservative sampling time was selected based on the knowledge that the opening time for crosslinked gelatin capsules was 15 min [12].
The activity of pepsin was calculated according to Eq. (1):
| (1) |
AS=average absorbance for the sample corrected for the average absorbance of the sample blanks
b=y-intercept of the standard curve
P=activity of the Pepsin RS
m=slope of the standard curve
C=concentration of the pepsin solution (mg/mL).
This assay was performed by fourteen fold. For comparison, the activity of pepsin in HCl Sol (0.03 mg/mL) was also determined (in duplicate).
2.4. Activity of pepsin in SGF in the presence of surfactants
The activity of pepsin was determined under the conditions specified in Section 2.3 (the dissolution medium, stirring, and temperature), with the addition of different amounts of surfactants, according to Table 1. For this purpose, 500 mL of pepsin solution in SGF (1.9 mg/mL) were transferred into a dissolution vessel and heated at 37 °C and then, the desired amount of surfactant was added. After 30 min, 1 mL of the solution containing the pepsin and surfactant was diluted to 50 mL with HCl Sol and subjected to the enzymatic reaction described in Section 2.2. The assay was conducted for each surfactant concentration in triplicate. A control solution containing pepsin in SGF without surfactant was also run with each experiment. The activity of pepsin was calculated according to Eq. (1).
Table 1.
Surfactants and the concentration used.
| Type | Surfactant | Concentration |
|---|---|---|
| Non ionic | Polysorbate 80 (Tween 80) | 0.0005% |
| 0.005%[21]a | ||
| 0.1% | ||
| 0.5% | ||
| Polyethylene glycoltert-octylphenyl ether (Triton X-100) | 0.0005% | |
| 0.015% [27]a | ||
| 0.1% | ||
| 0.5% | ||
| Anionic | Sodium dodecyl sulfate (SDS) | 0.05% |
| 0.20% [19]a | ||
| 0.5% | ||
| 0.8% | ||
| Cationic | Cetyltrimethyl ammonium bromide (CTAB) | 0.005 |
| 0.03%[21]a | ||
| 0.05% | ||
| 0.1% |
CMC (critical micellar concentration).
3. Results and discussion
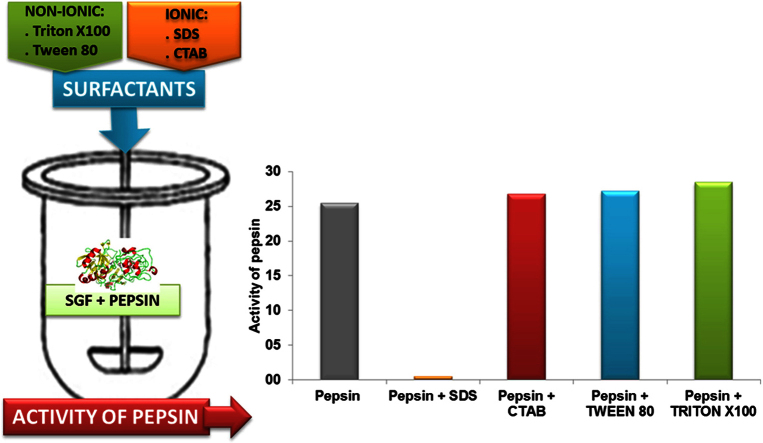
The activity of pepsin in SGF determined under conditions similar to a dissolution procedure was 25.4±1.3 U/mg. The value obtained for pepsin in HCl Sol was 26.9±0.5 U/mg. Notice that for the enzymatic activity a relative standard deviation of ±10% from the average is considered acceptable and therefore, in case of a comparison, the values obtained in SGF and HCl Sol are not considered to be different.
3.1. Activity of pepsin in SGF in the presence of surfactants
3.1.1. Anionic surfactant
SDS is one of the most common and frequently used surfactant in dissolution tests. SDS concentrations commonly used as solubility enhancer of poorly water soluble drug substances are in the range of 0.2–1% w/v (Oxcarbazepine Tablets, Tadalafil Tablets, Fenofibrate Capsules, Efavirenz Capsules (USP monographs, 2015).
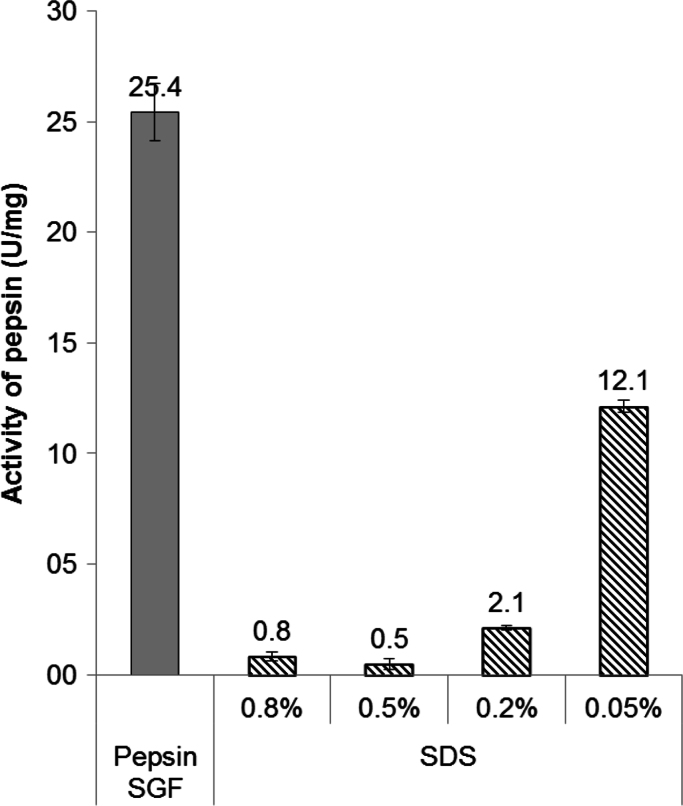
Fig. 1 shows the enzymatic activity of pepsin in presence of SDS. When SDS concentration is below its CMC (0.05%), the pepsin activity was reduced about 50% of its initial value. When SDS concentration is at its CMC or above, a 10% or less of its initial value was obtained. In both cases, the formation of a precipitate which conferred turbidity to the solution was observed.
Fig. 1.
Activity of pepsin in the presence of SDS.
The reduction of the activity in the presence of SDS has already been described for several enzymes and ascribed to the disruption of their structures due to interaction with SDS [22], [23]. The precipitate observed in our experiments is in agreement with the results obtained by Komarov et al. [15], which found the inhibition of pepsin by SDS to be due to precipitation of the enzyme which is maximal at the isoelectric point for pepsin, pH 2.5. Additionally, Nelson [20], has previously shown a reduction of 43% (2 h) and 67% (4 h) in the activity of the pepsin in presence of SDS 1.4% (at pH 1.6 and with albumin as the substrate), suggesting an important kinetic step in the unfolding enzyme process.
This behavior may negatively impact on dissolution studies of crosslinked capsules. Indeed, Marchais et al. [18] observed that no dissolution of carbamazepine occurred despite the presence of pepsin in SGF. A possible explanation may be the existence of an interaction between SDS and pepsin not allowing capsule disintegration.
Based on the above information, the concomitant use of SDS and pepsin should be avoided in dissolution studies. Alternativelya so called “pretreatment” should be applied, as it is stated in USP General Chapters 〈711〉, 〈1092〉, 〈1094〉 and 〈2040〉 [29], [30], [31], [32], when the use of both pepsin and SDS is required for the dissolution test.
3.1.2. Cationic surfactants
There is currently no information in the literature regarding the activity of pepsin in presence of CTAB. An interaction between pepsin and CTAB was reported by Chakraborty et al. [4], although its impact in the enzymatic activity was not quantified, and also, different conditions than those described in this study where used. They found that pepsin-induced interaction produced complexes, aggregates, and micelles of CTAB with distinct physicochemical features. At very low surfactant concentrations, much below CMC, the monomers preferentially adsorb on to the oppositely charged peripheral pepsin sites. Addition of further CTAB led to coacervation of the solution. The unfolding of pepsin induced further surfactant adsorption on to the pepsin sites, both in monomeric and aggregated forms.
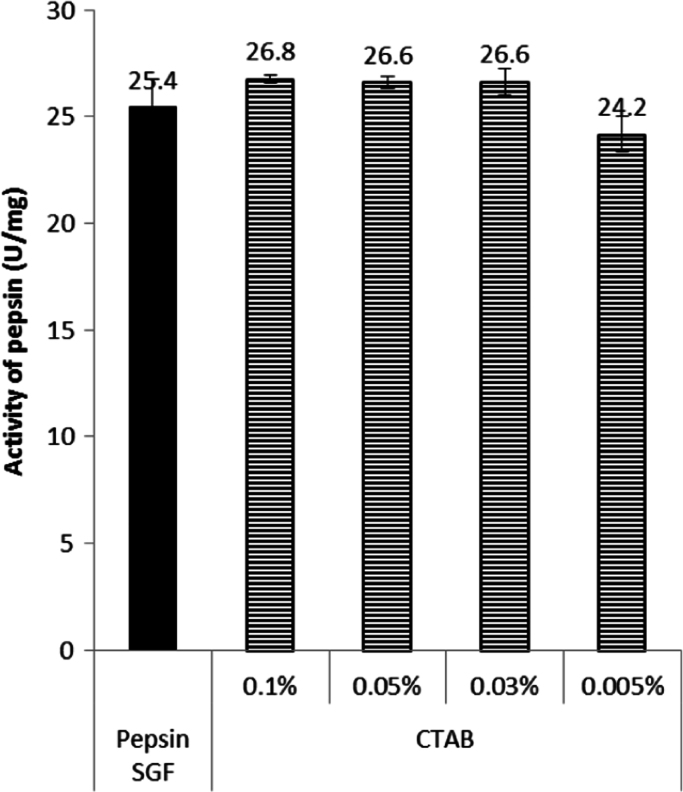
In our experiments, the pepsin activity was not affected by any of the CTAB concentration used in the dissolution medium, either those above or below its CMC (Fig. 2). However, based on Chakraborty et al. [4] report, an interaction between pepsin and CTAB cannot be ruled out. Taking this information into account, the concentration of CTAB to be used as a solubility enhancer, and the conditions of the assay should be considered as important factors to avoid changes in the activity of the pepsin. More research is required if different conditions than those described here are need to be used.
Fig. 2.
Activity of pepsin in the presence of CTAB.
3.1.3. Non-ionic surfactants
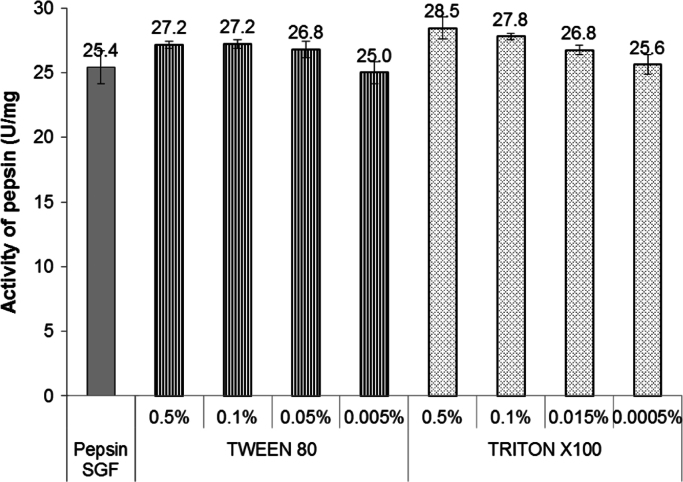
Non-ionic surfactants generally do not cause inactivation or denaturation of enzymes [2], [34]. In accordance to this, our study showed that Tween 80 and Triton X100, in all concentrations assayed, did not produce changes in the enzymatic activity of pepsin compared to the reference (Fig. 3).
Fig. 3.
Activity of pepsin in the presence of Tween 80 and Triton X100.
Indeed, according to Jahan [13], polysorbates act as stabilizers for pepsin. It is believed that such surfactants are preferentially adsorbed at the interface and prevent protein to be adsorbed to it and its subsequent unfolding.
4. Conclusion
When SGF was used as the dissolution media, a marked reduction in the activity of pepsin was observed in presence of SDS, in concentrations commonly used as a solubility enhancer. In contrast, no changes in pepsin activity were observed under similar conditions when the cationic and non-ionic surfactant models were used. The results reported here can contribute toward a more rational basis when selecting appropriate surfactants during required concomitant use of pepsin, and/or to describe a specific approach in setting up the dissolution test.
Acknowledgments
Maria Laura Guzman is a fellow of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
References
- 1.Antonov V.K., Ginodman L.M., Kapitannikov Y.V., Barshevskaya T.N., Gurova A.G., Rumsh L.D. Mechanism of pepsin catalysis: general base catalysis by the active-site carboxylate ion. FEBS Lett. 1978;88(1):87–90. doi: 10.1016/0014-5793(78)80613-9. [DOI] [PubMed] [Google Scholar]
- 2.Banga A.K. Therapeutic Peptides and Proteins Formulation, Processing and Delivery Systems. 2nd ed. Taylor & Francis; New York: 2006. [Google Scholar]
- 3.Bohak Z. Purification and characterization of chicken pepsinogen and chicken pepsin. J. Biol. Chem. 1969;244(17):4638–4648. [PubMed] [Google Scholar]
- 4.Chakraborty T., Chakraborty I., Moulik S.P., Ghosh S. Physicochemical Studies on pepsin-CTAB interaction: energetics and structural changes. J. Phys. Chem. B. 2007;111(10):2736–2746. doi: 10.1021/jp066051l. [DOI] [PubMed] [Google Scholar]
- 5.Chang C.K., Alvarez-Nunez F.A., Rinella J.V., Jr., Magnusson L.-E., Sueda K. Roller compaction, granulation and capsule product dissolution of drug formulations containing a lactose or mannitol filler, starch, and talc. AAPS PharmSciTech. 2008;9:597–604. doi: 10.1208/s12249-008-9088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement G.E. Catalytic activity of pepsin. In: Kaiser E.T., Kezdy F.J., editors. vol. 2. John Wiley & Sons; New York: 1973. pp. 177–239. (Progress in Bioorganic Chemistry). [Google Scholar]
- 7.Cole E.T., Cadé D., Benameur H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv. Drug Deliv. Rev. 2008;60:747–756. doi: 10.1016/j.addr.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Davydova N., Stippler E., Jin P., Giancaspro G. Development and validation of a dissolution test method for vitamin A in dietary supplement tablets. J. Pharm. Biomed. Anal. 2010;53(3):295–301. doi: 10.1016/j.jpba.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 9.El-Massik M.A., Darwish I.A., Hassan E.E., El-Khordagui L.K. Development of a dissolution medium for glibenclamide. Int. J. Pharm. 1996;140(1):69–76. [Google Scholar]
- 10.Elmeshad A.N., Darwish M.K. Stability studies of the effect of crosslinking on hydrochlorothiazide release. Drug Discov. Ther. 2009;3:136–142. [PubMed] [Google Scholar]
- 11.Favilla R., Parisoli A., Mazzini A. Alkaline denaturation and partial refolding of pepsin investigated with DAPI as an extrinsic probe. Biophys. Chem. 1997;67:75–83. doi: 10.1016/s0301-4622(97)00016-1. [DOI] [PubMed] [Google Scholar]
- 12.V. Gray, et al., Use of enzymes in the dissolution testing of gelatin capsules and gelatin-coated tablets—revisions to dissolution 〈711〉 and disintegration and dissolution of dietary supplements 〈2040〉. Stimuli to the Revision Process. The United States Pharmacopeial Convention. Dissolution Technologies. 2014, doi: dx.doi.org/10.14227/DT210414P6.
- 13.Jahan S.N. Uppsala University; 2009. Characterization and stabilization of Pepsin- A Systematic Approach in Formulating Therapeutic Proteins (Master of Science) (Degree Project in Applied Biotechnology) [Google Scholar]
- 14.Kalantzi L., Page R., Nicolaides E., Digenis G., Reppas C. In vitro methods can forecast the effects of intragastric residence on dosage form performance. Eur. J. Pharm. Sci. 2008;33:445–451. doi: 10.1016/j.ejps.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Komarov S.A., Siplet H., Shay H., Gruenstein M. A study of the effects and mechanism of action of sodium dodecyl sulphate on gastric secretion in rats. Br. J. Pharmacol. 1950;5(1):1–8. doi: 10.1111/j.1476-5381.1950.tb00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marques M.R.C., Cole E., Kruep D., Gray V., Murachanian D., Brown W.E., Giancaspro G.I. Liquid filled gelatin capsules. Pharm. Forum. 2009;35(4) Available from: 〈 www.usppf.com〉. [Google Scholar]
- 17.Maggi L., Torre M.L., Giunchedi P., Conte U. Supramicellar solutions of sodium dodecyl sulphate as dissolution media to study the in vitro release characteristics of sustained-release formulations containing an insoluble drug: nifedipine. Int. J. Pharm. 1996;135(1–2):73–79. [Google Scholar]
- 18.Marchais H., Cayzeele G., Legendre J.Y., Skiba M., Arnaud P. Cross-linking of hard gelatin carbamazepine capsules: effect of dissolution conditions on in vitro drug release. Eur. J. Pharm. Sci. 2003;19:129–132. doi: 10.1016/s0928-0987(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 19.Mihali C., Oprea G., Cical E. Determination of critical micelar concentration of anionic surfactants using surfactants—sensible electrodes. Chem. Bull. “Politehnica” Univ. (Timişoara) 2008;53:1–2. [Google Scholar]
- 20.Nelson C. The binding of detergents to proteins. I. The maximum amount of dodecyl sulfate bound to proteins and the resistance to binding of several proteins. J. Biol. Chem. 1971;246(12):3895–3901. [PubMed] [Google Scholar]
- 21.Neugebauer J.M. Detergents: an overview. Methods Enzymol. 1990;182:239–253. doi: 10.1016/0076-6879(90)82020-3. [DOI] [PubMed] [Google Scholar]
- 22.Otzen D.E., Christiansen L., Schülein M.A. A comparative study of the unfolding of the endoglucanase Cel45 from Humicola insolens in denaturant and surfactant. Protein Sci. 1999;8(9):1878–1887. doi: 10.1110/ps.8.9.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otzen D.E., Oliveberg M. Burst-phase expansion of native protein prior to global unfolding in SDS. J. Mol. Biol. 2002;315(5):1231–1240. doi: 10.1006/jmbi.2001.5300. [DOI] [PubMed] [Google Scholar]
- 24.Otzen D.E. Protein–surfactant interactions: a tale of many states. Biochim. Biophys. Acta. 2011;1814(5):562–591. doi: 10.1016/j.bbapap.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Paavera U., Lusta A., Mirzab S., Rantanenc J., Veskia P., Heinämäkia J., Kogermanna K. Insight into the solubility and dissolution behavior of piroxicam anhydrate and monohydrate forms. Int. J. Pharm. 2012;431(1–2):111–119. doi: 10.1016/j.ijpharm.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Park S.H., Choi H.K. The effects of surfactants on the dissolution profiles of poorly water-soluble acidic drugs. Int. J. Pharm. 2006;321(1–2):35–41. doi: 10.1016/j.ijpharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal K.S., Koussale F. Critical micelle concentration determination of non-ionic detergents with coomassie brilliant blue G-250. Anal Chem. 1983;55:1115–1117. [Google Scholar]
- 28.Shihab F.A., Ebian A.R., Mustafa R.M. Effect of polyethylene glycol, sodium lauryl sulfate and polysorbate-80 on the solubility of furosemide. Int. J. Pharm. 1979;4(1):13–20. [Google Scholar]
- 29.USP, 〈711〉 Dissolution. USP 38. In: USP 38–NF 33, United States Pharmacopeial Convention, Rockville, MD, 2015a, pp. 486–496.
- 30.USP, 〈1092〉 The dissolution procedure: development and validation. USP 38. In: USP 38–NF 33. United States Pharmacopeial Convention, Rockville, MD, 2015b, pp. 1090–1097.
- 31.USP, 〈1094〉 Capsules—dissolution testing and related quality attributes. USP 38. In: USP 38–NF 33, United States Pharmacopeial Convention, Rockville, MD, 2015c, pp. 1097–1105.
- 32.USP, 〈2040〉 Disintegration and dissolution of dietary supplements. USP 38. In USP 38–NF 33, United States Pharmacopeial Convention, Rockville, MD, 2015d, pp. 1774–1781.
- 33.USP, Pepsin activity. In: Ninth Edition of the Food Chemicals Codex (FCC 9). United States Pharmacopeial Convention, Rockville, MD, 2015e, pp. 1410–1411.
- 34.Zappone M., Kaziska A., Bogush G. Handbook of Detergents, Part E: Applications. CRC Press; Boca Raton, FL: 2009. Application of detergents in laundering; pp. 69–82. [Google Scholar]