Abstract
Sympathoexcitation is associated with ventricular arrhythmogenesis. The aim of this study was to determine the role of thoracic dorsal root afferent neural inputs to the spinal cord in modulating ventricular sympathetic control of normal heart electrophysiology. We hypothesize that dorsal root afferent input tonically modulates basal and evoked efferent sympathetic control of the heart. A 56-electrode sock placed on the epicardial ventricle in anesthetized Yorkshire pigs (n = 17) recorded electrophysiological function, as well as activation recovery interval (ARI) and dispersion in ARI, at baseline conditions and during stellate ganglion electrical stimulation. Measures were compared between intact states and sequential unilateral T1–T4 dorsal root transection (DRTx), ipsilateral ventral root transection (VRTx), and contralateral dorsal and ventral root transections (DVRTx). Left or right DRTx decreased global basal ARI [Lt.DRTx: 369 ± 12 to 319 ± 13 ms (P < 0.01) and Rt.DRTx: 388 ± 19 to 356 ± 15 ms (P < 0.01)]. Subsequent unilateral VRTx followed by contralateral DRx+VRTx induced no further change. In intact states, left and right stellate ganglion stimulation shortened ARIs (6 ± 2% vs. 17 ± 3%), while increasing dispersion (+139% vs. +88%). There was no difference in magnitude of ARI or dispersion change with stellate stimulation following spinal root transections. Interruption of thoracic spinal afferent signaling results in enhanced basal cardiac sympathoexcitability without diminishing the sympathetic response to stellate ganglion stimulation. This suggests spinal dorsal root transection releases spinal cord-mediated tonic inhibitory control of efferent sympathetic tone, while maintaining intrathoracic cardiocentric neural networks.
Keywords: cardiac neuronal hierarchy, spinal cord, stellate ganglion, sympathetic nervous system, ventricular excitability
dynamic interactions between peripheral and central aspects of the cardiac nervous system are essential for the maintenance of adequate cardiac function (4, 6). Imbalances in autonomic neural processing are fundamental in the progression of cardiac pathology, including ventricular arrhythmias (34, 35). Increased sympathoexcitation in ischemic and nonischemic heart disease is associated with arrhythmia development (18). Correspondingly, modulation of sympathetic output via surgical sympathectomy, thoracic epidural anesthesia, or spinal cord stimulation has been demonstrated to be important therapeutic avenues for control of cardiac arrhythmias (13, 16, 17).
It is known that cardiac afferent neurons, projecting to high thoracic dorsal root ganglia, transmit tonic afferent neural input into the thoracic spinal cord to reflexly modulate efferent sympathetic outflow to the heart (11, 25, 29, 31, 38). Thoracic cardiac afferents likewise project to intrathoracic autonomic ganglia subserving intrathoracic short-loop feedback loops for cardiac control (7, 8). Intrathoracic reflexes work in conjunction with higher central control from the brain stem and spinal cord to provide dynamic autonomic neural regulation of cardiac electrophysiology (4, 6). However, what is not known is the effect that this tonic cardiac afferent signaling has on efferent sympathetic outflow and cardiac electrophysiological control in normal hearts. This is essential as a benchmark for subsequent studies in evaluating states of cardiac pathology. Further, with the emerging applications of neuromodulation and bioelectric medicine to treat cardiovascular diseases, the potential for central-peripheral neural interactions associated with electrical stimuli applied to specific nodes of the hierarchy for cardiac control needs be defined (14).
The aim of this study was to determine the role of cardiac afferent input to the spinal cord in modulating sympathetic control of ventricular electrophysiology in normal porcine hearts by examining the effects of sequential left and right dorsal root (afferent) and ventral (efferent) root transection on ventricular electrophysiological response at rest and in response to bioelectric stellate ganglion stimulation. We hypothesize that dorsal root afferent input tonically modulates basal and evoked efferent sympathetic control of the heart.
METHODS
All animal experimental protocols were devised in accordance with guidelines set by the University of California Institutional Animal Care and Use Committee and the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.”
Animal preparation.
Male and female Yorkshire pigs (n = 17) weighing 42 ± 3 kg were sedated with telazol (8–10 mg/kg im). Following intubation and initiation of positive-pressure ventilation, general anesthesia was induced and maintained with isoflurane (1–1.5%, inhalation) concomitant with intermittent boluses of fentanyl (1–3 μg/kg iv). Following completion of the surgery, anesthesia was changed to α-chloralose (50 mg/kg intravenous bolus administration followed by continuous infusion at 10 mg·kg−1·h−1 iv). Heart rate (HR) was monitored throughout the experiments via a standard limb lead electrocardiogram. The right femoral artery was catheterized for monitoring aortic blood pressure. To measure left ventricular (LV) pressure, a 5-Fr pig-tail 12-pole conductance pressure catheter was inserted into the LV chamber via the left carotid artery. This catheter was connected to a MPVS Ultra Pressure Volume Loop System (Millar Instruments, Houston, TX). The right internal jugular vein was cannulated to allow fluid replacement and drug administration. Arterial blood gases were evaluated hourly, and adjustments of tidal volume and/or infusions of sodium bicarbonate were applied, as required, to maintain arterial blood gas homeostasis.
Surgical procedure.
Dorsal spinal laminectomy was performed in the prone position to expose the T1–T4 ventral and dorsal roots bilaterally. Animals were then placed in the supine position and a midline sternotomy was performed to expose the heart and both stellate ganglia. After completion of surgery, animals were stabilized for 1 h.
Stellate ganglion sympathetic stimulation.
Each left (LSS) and right (RSS) stellate ganglion was stimulated individually via bipolar needle electrodes implanted in the ganglia that were connected to a Grass S88 Stimulator (Grass, Warwick, RI) via PSIU6 constant current isolation units. Square wave stimulation pulses (4-ms duration, 4-Hz frequency) were delivered individually to each ganglion. Stimulus threshold was defined as the stimulation current strength that was sufficient to elicit a 10% increase of left ventricular end-systolic pressure (LVESP) or heart rate. Stimulus intensity was increased to 1.5 times threshold for all subsequent stellate ganglion stimulations, while maintaining the 4-Hz frequency and 4-ms pulse width.
Cardiac electrophysiology: activation recovery interval and dispersion of its analyses.
A custom 56-electrode sock, placed over both ventricles, was attached to a Prucka CardioLab (GE Healthcare, Fairfield, CT) to identify regional activation recovery intervals. Global ventricular activation recovery intervals (ARIs) were calculated via customized software ScalDyn M (University of Utah, Salt Lake City, UT), as described previously (37). Briefly, localized ventricular epicardial activation times (ATs) were measured from the beginning of the QRS complex to the first minimal dV/dt in the QRS complex. Localized epicardial recovery times (RT) were computed from the beginning of the QRS complex to the first maximal dV/dt of the T wave. Activation recovery intervals were derived from subtracting ATs from these RTs. This parameter has been shown to correlate with local ventricular action potential durations (36, 37). Global dispersion in ARI was calculated using the variance of all 56-electrode ARIs to identify spatial dispersion of regional ventricular epicardial repolarization.
Experimental protocol.
The effects of sequential spinal transection, at rest and with left (LSS) or right (RSS) stellate ganglion stimulation, on hemodynamic parameters and ventricular electrical indices were recorded. Stellate ganglia were stimulated for 30-s periods, with 10-min separating each stimulation sequence to allow the return of hemodynamic and regional ventricular electrical indices to baseline values. Animals were randomized with either left or right unilateral root transection first. For the left-sided protocol group (n = 9), the transection sequence was 1) all spinal roots intact, 2) left T1–T4 dorsal roots cut (Lt. DRTx), 3) left T1–T4 ventral roots cut (Lt. VRTx), and 4) right T1–T4 dorsal and ventral roots cut (Rt. DVRTx). For the right-side protocol group (n = 8), the transection sequence was 1) all spinal roots intact, 2) right T1–T4 dorsal roots cut (Rt. DRTx), 3) right T1–T4 ventral roots cut (Rt. VRTx), and 4) left T1–T4 dorsal and ventral roots cut (Lt. DVRTx). To allow for stabilization for neural networks, a 60-min observation period elapsed between each successive dorsal or ventral spinal cord root transection before stellate ganglion stimulation sequences were initiated.
Statistical analysis.
Data are reported as means ± SE. Repeated-measures one-way ANOVA with post hoc correction for multiple hypothesis testing was performed for comparing electrophysiological and hemodynamic data obtained during baseline and stellate ganglion stimulations in each of the experimental conditions before and after spinal root transection. Change in ARI and dispersion, by RSS and LSS, between four experimental conditions were also analyzed by repeated-measures one-way ANOVA. Stats were analyzed by using SigmaStat (version 3.1) and JMP (version 11). A P value less than 0.05 was considered to be statistically significant.
RESULTS
Effects of Thoracic Spinal Root Transections on Regional Ventricular Electrical Indices
Effects of sequential transection in the baseline (unstimulated) state.
Baseline global ventricular ARIs decreased following unilateral dorsal root transection (DRTx) from 369 ± 12 to 319 ± 13 ms (P < 0.01) by left DRTx (Lt. DRTx) and from 388 ± 19 to 356 ± 15 ms (P < 0.01) by right DRTx (Rt. DRTx). Figure 1 shows representative examples in baseline ARIs for both transection sequences. Subsequent ipsilateral ventral root transections (Lt. VRTx or Rt. VRTx) or contralateral transections (Rt. DVRTx and LT. DVRTx) evoked minimal additional effects on ventricular ARI (Figs. 1–3). In contrast, ventricular dispersion in ARI at baseline conditions remained relatively unaffected by any of these sequential spinal root transections (Figs. 2 and 3).
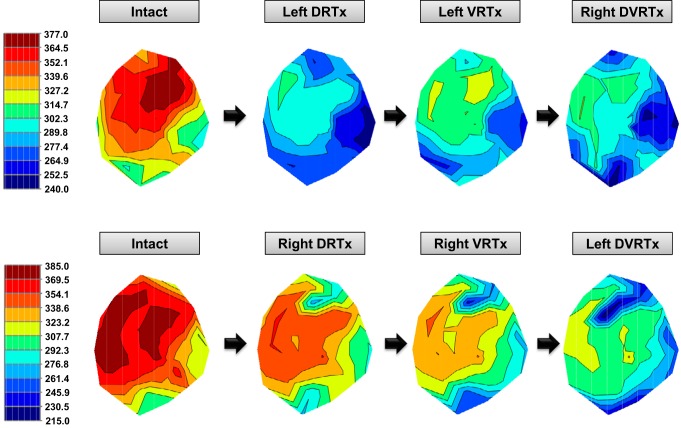
Fig. 1.
Effects of the sequential T1–T4 root transection on the baseline Activation Recovery Intervals (ARIs) in a representative animal. Top panels show left-right transection protocol, while bottom panels show right-left protocol. Dorsal root transection shortened basal ARI homogenously in both left- and right-sided protocols. Reduction in global ventricular ARI is seen after initial ipsilateral dorsal root transection.
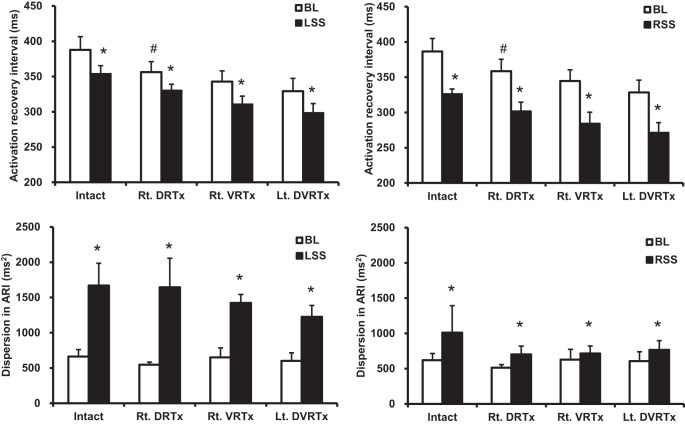
Fig. 3.
Summary effects of sequential spinal root transections in right-sided experimental protocol on stellate ganglion stimulation-induced changes in global ventricular activation recovery intervals, along with dispersion in ARI. After right (T1–T4) dorsal root transections (Rt. DRTx), global ventricular ARI shortened. Subsequent transection of ipsilateral ventral roots (Rt. VRTx) and transection of contralateral dorsal and ventral roots (Lt. DVRTx) evoked no further effects on whole heart ARI. Spinal cord dorsal and ventral root transections exerted no significant effects on overall ventricular dispersion. #P < 0.05, significant difference in baseline from preceding condition. *P < 0.05, significant difference from baseline with stellate ganglion stimulation.
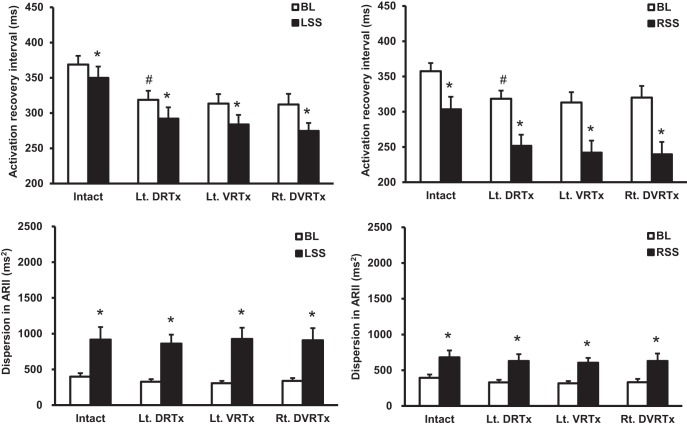
Fig. 2.
Summary effects of sequential spinal root transections in left-sided experimental protocol on stellate ganglion stimulation-induced changes in global ventricular activation recovery intervals along with dispersion in ARI. After left (T1–T4) dorsal root transections (Lt. DRTx), global ventricular ARI shortened. Subsequent transection of ipsilateral ventral roots (Lt. VRTx) and transection of contralateral dorsal and ventral roots (Rt. DVRTx) evoked no further effects on global ventricular ARI. Spinal cord dorsal and ventral root transections exerted no significant effects on overall ventricular dispersion. #P < 0.05, significant difference in baseline from preceding condition. *P < 0.05, significant difference from baseline with stellate ganglion stimulation (LSS, left stellate ganglion stimulation; RSS, right stellate ganglion stimulation).
Activation recovery intervals and spatial dispersion in ARI elicited by stellate ganglion stimulation.
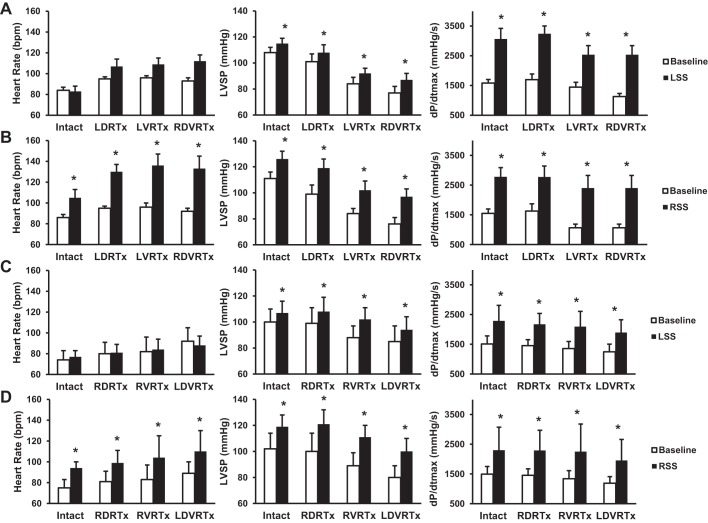
LSS stimulation preferentially shortened ARIs particularly in the left lateral and posterior ventricular walls (Fig. 4, middle). In contrast, RSS stimulation preferentially shortened ARIs in the right and anterior left ventricular walls (Fig. 4, right). This regional alteration in ARI, induced by unilateral stellate ganglion stimulation, is consistent with our previous study (40). Unilateral T1–T4 dorsal root transections (Lt. DRTx or Rt. DRTx) shortened baseline ARI; however, there was no change in the magnitude of ARI shortening with stellate stimulation and dorsal root transection (Figs. 2–4). These ARI responses were not modified by subsequent unilateral ventral root transection, or by contralateral dorsal and ventral transection. Moreover, while enhancement of whole ventricular dispersion in ARI occurred during right or left stellate ganglion stimulation, alterations in this index elicited by ganglionic stimulation were not changed by any of the staged spinal transections (Figs. 2 and 3).
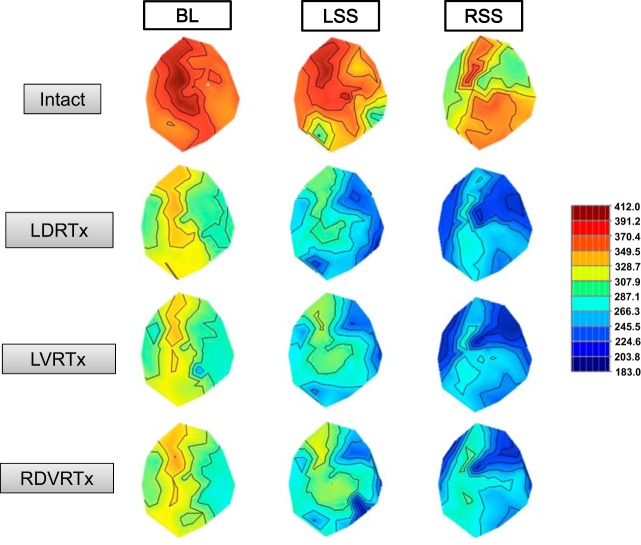
Fig. 4.
Representative ARI effects evoked by stellate ganglion stimulation prior to (intact) and following successive T1–T4 root transections. Unilateral T1–T4 dorsal root transections shortened baseline ARI; however, there was no change in the magnitude of ARI shortening with stellate stimulation and unilateral dorsal root (LDRTx), ventral root (LVRTx), or contralateral dorsal/ventral root transections (RDVRTx). Left stellate ganglion stimulation (LSS) preferentially shortened ARIs, particularly in the left lateral and posterior ventricular walls. In contrast, right stellate ganglia stimulation (RSS) preferentially shortened ARIs in the right and anterior left ventricular walls.
Effects of successive thoracic spinal root transections on hemodynamic measurements.
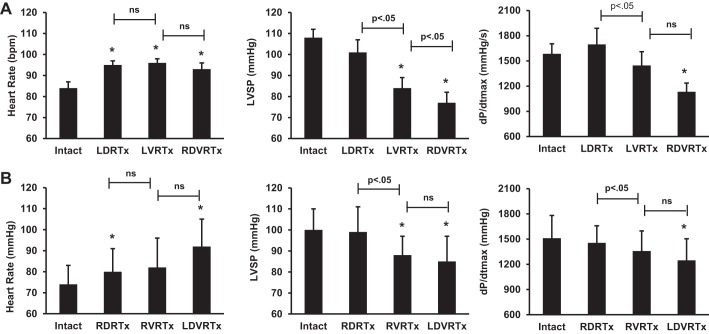
Basal heart rate was increased by T1–T4 dorsal root transection, with right or left unilateral transection (Fig. 5). Subsequent root transection (ipsilateral VRx and contralateral DVRx) evoked no further significant changes in basal heart rate. Positive chronotropic responses were evoked in response to right, but not left, stellate stimulation (Fig. 6). Right stellate evoked effects on heart rate were maintained following each of the successive root transections. Left stellate stimulation did not change heart rate during any stage of the successive root transections.
Fig. 5.
Hemodynamic data are presented as means ± SE. A: hemodynamic results from left dorsal root transection protocol. B: hemodynamic results from right dorsal root transection protocol. *P < 0.05, significant difference from baseline. Significant differences between conditions are displayed above bar. ns, nonsignificant.
Fig. 6.
Hemodynamic data are presented as means ± SE. A: hemodynamic changes observed with left dorsal root transection-baseline compared with LSS. B: left dorsal root transection-baseline compared with RSS. C: right dorsal root transection-baseline compared with LSS. D: right dorsal root transection-baseline compared with RSS. *P < 0.05, significant difference from baseline with LSS and RSS.
Indices of basal LV inotropic function, as assessed by the first derivative of LV pressure (+dP/dt), were maintained following dorsal root transection, decreasing from baseline only with subsequent bilateral dorsal and ventral root transection. LVSP had no change after unilateral dorsal root transection but decreased from baseline with subsequent ventral root transections (Fig. 5). LV pressure and inotropy increased with stellate stimulation and were unchanged with spinal root transections (Fig. 6). Time control showed stable conditions throughout the duration of the experimental protocol with only minimal changes in HR, LVSP, and dP/dt max from baseline to end of protocol (HR: 75–83 bpm, LVSP: 90-81 mmHg, and dP/dt max: 1247-1159 mmHg/s).
DISCUSSION
The primary findings of this study are 1) interruption of unilateral cardiac afferent input to the spinal cord increases basal sympathetic tone to the heart, as demonstrated by a reduction in ARI duration and tachycardia; and 2) intrathoracic (cardiocentric) sympathetic control was functionally maintained following bilateral transection of dorsal and ventral spinal roots, as there was no difference in the magnitude of ARI shortening or increase in dispersion associated with subsequent stellate ganglion stimulation.
The results of this study suggest that dorsal afferent neural input into the spinal cord exerts tonic inhibitory control of basal efferent sympathetic output. Dorsal root transection interrupts the nerve fibers transmitting tonic afferent neural impulses from the heart (11). The cessation of this afferent input to the thoracic spinal cord resulted in a reduction in ARI duration and increased HR, indicating that afferent cardiac neural input tonically modulates efferent sympathetic signaling, and release of this negative feedback signal results in increased cardiac sympathetic tone. ARI shortening occurred only after the initial dorsal root transection, with no additional changes seen after subsequent spinal root transections. Initial dorsal root transection was also associated with an increase in HR with no additional changes seen with subsequent transections.
In this study, even partial loss of the cardiac afferent inputs, as with unilateral T1–T4 dorsal root transection, resulted in the release of thoracic afferent-modulated basal sympathetic tone inhibition. An increase in sympathetic tone was identified following unilateral dorsal transection, regardless of whether it was left or right dorsal roots that were transected first. Interruption of dorsal afferent signaling from either side resulted in ARI shortening likely due to increased efferent signaling, similar to how patients with unilateral stroke or traumatic brain injury may experience paroxysmal sympathetic hyperreactivity (32). It is also consistent with recent findings that showed unilateral cardiac infarctions evoked bilateral changes in stellate ganglion structure/function (2), an effect that is mediated by sympathetic afferent neurons. While unilateral afferent transection increased basal sympathetic tone, there was no significant change in LV mechanical function, likely due to compensatory neural inputs from the contralateral spinal nerve roots and intact baroreflexes (3, 4, 31).
The results of this study show that sympathetic cardiocentric control was functionally maintained following surgical spinal root transection, as there was no difference in the magnitude of ARI shortening or increase in dispersion associated with subsequent stellate ganglion stimulation, despite the difference observed in basal tone with spinal root transection. Stellate ganglion stimulation leads to an increase in cardiac sympathetic outflow leading to ARI shortening, increased dispersion in ARI, and increased LV inotropy independent of spinal cord modulation (29, 37). As previously reported, RSS, compared with LSS, is associated with a greater chronotropic response (40). In this study, RSS was associated with significant increases in heart rate, whereas LSS was not. The components of cardiac neural reflexes exist at multiple levels, with reflexes mediated by neurons of the intrinsic cardiac nervous system (5, 12), extra-cardiac intrathoracic sympathetic ganglia (6–8), spinal cord (17), brain stem (19), and higher centers (20). The results of this study support the concept that intrathoracic neural networks are capable on their own of integrated cardiac control, even when disconnected from the rest of nervous system (7, 28). This finding has important implications with respect to residual autonomic control of cardiac function in patients following therapeutic bilateral stellate decentralization (14, 34).
While ventricular electrophysiological changes were identified after unilateral dorsal root transection, mechanical changes were not observed until both dorsal and ventral roots were transected. With removal of bilateral afferent and efferent neural inputs to and from the spinal cord, there was a significant reduction in LV dP/dt max and systolic blood pressure. This reduction in function may have been due to several reasons. First, high thoracic dorsal root transection uncouples central-peripheral control elements in the cardiac neuroaxis. Following bilateral transection of the dorsal and ventral roots, central aspects of the cardiovascular control system have lost important afferent signals from the heart. Thus, the normal spinal/higher center modulation of sympathetic outflows transitions toward intrathoracic cardiocentric reflex control. Without central control, sympathetic efferent activity to the heart and peripheral vasculature is lost, including that which is necessary to support inotropic function and blood pressure. Second, the degree of changes seen in cardiac mechanical function might not follow a linear relationship with changes in ventricular electrical indices following spinal root transections, similarly to the differential relationship in sympathetic control of chronotropism and inotropism in subjects with spinal cord injury (24). Third, vagal projections, both afferent and efferent, remained intact in our study. These residual neural circuits could have subserved important reflex adjustments to the stress imposed by spinal cord root transections. Future studies should consider the potential ramifications of differential effects of targeted neuromodulaton interventions on ventricular electrical vs. mechanical function.
Study Limitations
This study was designed to determine the effect of cardiac afferent signaling on ventricular electrical indices. As such, dorsal root transections were performed as the primary procedure, with sequential ventral root transections being performed later. The effects of ventral root transection alone remain to be evaluated. Furthermore, root transections were done in healthy preparations. It is known that chronic cardiac disease induces remodeling of peripheral and central aspects of the cardiac nervous system (2, 22, 23), and, as such, chronic disease models will need to be evaluated. Cardiac control via the cardiac nervous system involves both sympathetic and parasympathetic outflows. In this study, the parasympathetic networks remained intact, although they too can be impacted by alterations in afferent inputs secondary to dorsal root transection (11, 15, 27). We have recently shown that the vagus can have profound influence on cardiac electrophysiology reflective of interactions between afferent and efferent components of the cervical vagus (39). Future studies should consider the dynamic interplay between different levels of the hierarchy for cardiac control in response to the disease process, particularly as influenced by targeted neuromodulation-based therapeutic approaches. Finally, general anesthesia can suppress sympathetic nerve activity and could have impacted our results, although we kept the concentrations of anesthetic agents constant and similar across all animals. This study was powered for the primary outcome of changes in ARI with spinal transection and, therefore, may not be adequately powered to detect all hemodynamic responses associated with nerve root transection or, for that matter, stellate ganglion stimulation.
Conclusion
We demonstrate that the dorsal root afferent inputs from the heart to the spinal cord exert tonic inhibitory control of efferent sympathetic tone to the heart. Interruption of thoracic spinal afferent signaling results in enhanced basal cardiac sympathoexcitability without mitigating the functional sympathetic response to direct stellate ganglion stimulation. This indicates that spinal dorsal root transection releases centrally mediated reflex modulation of efferent sympathetic tone to the heart while maintaining intrathoracic cardiocentric neural networks. The mechanistic insight into spinal cord control of cardiac sympathoexcitation may aid in developing future therapeutic modalities aimed at spinal modulation of ventricular arrhythmias.
Perspectives and Significance
The finding of functional intrathoracic, cardiocentric sympathetic control is of important clinical relevance, especially as related to residual neural control of cardiac function after stellate ganglion decentralization for intractable ventricular tachycardia (13, 30, 33). Stellate ganglion decentralization has been shown to protect against ventricular arrhythmias in humans (1, 30, 34). This surgical procedure removes most, if not all, cardiac afferent inputs to spinal cord neurons and preserves most intrathoracic cardio-cardiac reflexes (10, 21), including those confined to the intrinsic cardiac nervous system (7, 9, 21). The data from our study demonstrate the functional capacity of intrathoracic neural networks to coordinate cardiac electrical and mechanical function, even when disconnected from the central nervous system. It is through understanding the inherent network interactions that exist throughout the cardiac neuroaxis that one can mechanistically understand its acquired adaptations to cardiac disease and, thus, devise novel autonomic regulatory approaches to optimize outcomes. Stellate decentralization for intractable VT (13, 30) and spinal cord stimulation for angina (26) are just some of the ongoing areas of clinically impactful manifestations of this principle.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01 HL-084261 to K. Shivkumar and RO1 HL-71830 to J. Laurence-Ardell.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.Y., K.H.-Q., W.Z., P.S.R., D.Y., M.V., and O.A.A. performed experiments; K.Y., K.H.-Q., W.Z., P.S.R., D.Y., M.V., O.A.A., J.A.A., K.S., J.L.A., and A.M. analyzed data; K.Y., K.H.-Q., W.Z., P.S.R., D.Y., M.V., O.A.A., J.A.A., K.S., J.L.A., and A.M. interpreted results of experiments; K.Y., K.H.-Q., W.Z., D.Y., J.L.A., and A.M. prepared figures; K.Y., K.H.-Q., J.L.A., and A.M. drafted manuscript; K.Y., K.H.-Q., M.V., O.A.A., J.A.A., K.S., J.L.A., and A.M. edited and revised manuscript; K.Y., K.H.-Q., W.Z., P.S.R., D.Y., M.V., O.A.A., J.A.A., K.S., J.L.A., and A.M. approved final version of manuscript; K.H.-Q., P.S.R., M.V., O.A.A., J.A.A., K.S., J.L.A., and A.M. conception and design of research.
REFERENCES
- 1.Ajijola OA, Lellouche N, Bourke T, Tung R, Ahn S, Mahajan A, Shivkumar K. Bilateral cardiac sympathetic denervation for the management of electrical storm. J Am Coll Cardiol 59: 91–92, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: Neuropeptide and morphologic changes. Heart Rhythm 12: 1027–1035, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardell JL. The cardiac neuronal hierarchy and susceptibility to arrhythmias. Heart Rhythm 8: 590–591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardell JL. Intrathoracic neuronal regulation of cardiac function In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL, New York: Oxford University Press, 2004, p. 118–152. [Google Scholar]
- 5.Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol Heart Circ Physiol 260: H713–H721, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Ardell JL, Cardinal R, Vermeulen M, Armour JA. Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia. Am J Physiol Regul Integr Comp Physiol 297: R470–R477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armour JA. Activity of in situ stellate ganglion neurons of dogs recorded extracellularly. Can J Physiol Pharmacol 64: 101–111, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Armour JA. Synaptic transmission in the chronically decentralized middle cervical and stellate ganglia of the dog. Can J Physiol Pharmacol 61: 1149–1155, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Armour JA, Collier K, Kember G, Ardell JL. Differential selectivity of cardiac neurons in separate intrathoracic autonomic ganglia. Am J Physiol Regul Integr Comp Physiol 274: R939–R949, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Armour JA, Hopkins DA. Localization of sympathetic postganglionic neurons of physiologically identified cardiac nerves in the dog. J Comp Neurol 202: 169–184, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Armour JA, Kember G. Cardiac sensory neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 79–117. [Google Scholar]
- 12.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, Shivkumar K. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 121: 2255–2262, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley U, Yamakawa K, Takamiya T, Andrew Armour J, Shivkumar K, Ardell JL. Targeted stellate decentralization: Implications for sympathetic control of ventricular electrophysiology. Heart Rhythm 13: 282–288, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coote JH. Myths and realities of the cardiac vagus. J Physiol 591: 4073–4085, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 61: 143–167, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Foreman RD, D MJL; Linderoth B. Integrative control of cardiac function by cervical and thoracic spinal neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL, New York: Oxford University Press, 2004, p. 153–186. [Google Scholar]
- 18.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 116: 2005–2019, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol 305: R187–R204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper RM, Kumar R, Ogren JA, Macey PM. Sleep-disordered breathing: effects on brain structure and function. Respir Physiol Neurobiol 188: 383–391, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins DA, Armour JA. Localization of sympathetic postganglionic and parasympathetic preganglionic neurons which innervate different regions of the dog heart. J Comp Neurol 229: 186–198, 1984. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins DA, Macdonald SE, Murphy DA, Armour JA. Pathology of intrinsic cardiac neurons from ischemic human hearts. Anat Rec 259: 424–436, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Nguyen HD, Ogren JA, Macey PM, Thompson PM, Fonarow GC, Hamilton MA, Harper RM, Woo MA. Global and regional putamen volume loss in patients with heart failure. Eur J Heart Fail 13: 651–655, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J Appl Physiol (1985) 113: 1332–1341, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malliani A, Recordati G, Schwartz PJ. Nervous activity of afferent cardiac sympathetic fibres with atrial and ventricular endings. J Physiol 229: 457–469, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Luscher T, Pasic M, Thelle D. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J 23: 355–370, 2002. [DOI] [PubMed] [Google Scholar]
- 27.McAllen RM, Salo LM, Paton JF, Pickering AE. Processing of central and reflex vagal drives by rat cardiac ganglion neurones: an intracellular analysis. J Physiol 589: 5801–5818, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy DA, Thompson GW, Ardell JL, McCraty R, Stevenson RS, Sangalang VE, Cardinal R, Wilkinson M, Craig S, Smith FM, Kingma JG, Armour JA. The heart reinnervates after transplantation. Ann Thorac Surg 69: 1769–1781, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Randall DC, Brown DR, Brown LV, Kilgore JM. Sympathetic nervous activity and arterial blood pressure control in conscious rat during rest and behavioral stress. Am J Physiol Regul Integr Comp Physiol 267: R1241–R1249, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz PJ. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol 11: 346–353, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz PJ, Foreman RD, Stone HL, Brown AM. Effect of dorsal root section on the arrhythmias associated with coronary occlusion. Am J Physiol 231: 923–928, 1976. [DOI] [PubMed] [Google Scholar]
- 32.Siefferman JW, Lai G. Propranolol for paroxysmal sympathetic hyperactivity with lateralizing hyperhidrosis after stroke. Case Rep Neurol Med 2015: 421563, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaseghi M, Boyle NG, Kedia R, Patel JK, Cesario DA, Wiener I, Kobashigawa JA, Shivkumar K. Supraventricular tachycardia after orthotopic cardiac transplantation. J Am Coll Cardiol 51: 2241–2249, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, Shivkumar K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm 11: 360–366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Progr Cardiovasc Dis 50: 404–419, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaseghi M, Yamakawa K, Sinha A, So EL, Zhou W, Ajijola OA, Lux RL, Laks M, Shivkumar K, Mahajan A. Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. Am J Physiol Heart Circ Physiol 305: H1020–H1030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaseghi M, Zhou W, Shi J, Ajijola OA, Hadaya J, Shivkumar K, Mahajan A. Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart Rhythm 9: 1303–1309, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Xi X, Randall WC, Wurster RD. Electrophysiological properties of canine cardiac ganglion cell types. J Auton Nerv Syst 47: 69–74, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Yamakawa K, Rajendran PS, Takamiya T, Yagishita D, So EL, Mahajan A, Shivkumar K, Vaseghi M. Vagal nerve stimulation activates vagal afferent fibers that reduce cardiac efferent parasympathetic effects. Am J Physiol Heart Circ Physiol 309: H1579–H1590, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou W, Yamakawa K, Benharash P, Ajijola O, Ennis D, Hadaya J, Vaseghi M, Shivkumar K, Mahajan A. Effect of stellate ganglia stimulation on global and regional left ventricular function as assessed by speckle tracking echocardiography. Am J Physiol Heart Circ Physiol 304: H840–H847, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]