Abstract
The purpose of this study was to determine whether trauma-induced coagulopathy is due to changes in 1) thrombin activity, 2) plasmin activity, and/or 3) factors that stimulate or inhibit thrombin or plasmin. Sprague-Dawley rats were anesthetized with 1–2% isoflurane/100% oxygen, and their left femoral artery and vein were cannulated. Polytrauma included right femur fracture, and damage to the small intestines, the left and medial liver lobes, and right leg skeletal muscle. Rats were then bled 40% of blood volume. Plasma samples were taken before trauma, and at 30, 60, 120, and 240 min. Polytrauma and hemorrhage led to a significant fall in prothrombin levels. However, circulating thrombin activity did not change significantly over time. Antithrombin III and α2 macroglobulin fell significantly by 2 h, then rose by 4 h. Soluble thrombomodulin was significantly elevated over the 4 h. Circulating plasmin activity, plasminogen, and D-dimers were elevated for the entire 4 h. Tissue plasminogen activator (tPA) was elevated at 30 min, then decreased below baseline levels after 1 h. Plasminogen activator inhibitor-1 was significantly elevated at 2–4 h. Neither tissue factor pathway inhibitor nor thrombin activatable fibrinolysis inhibitor changed significantly over time. The levels of prothrombin and plasminogen were 30–100 times higher than their respective active enzymes. Polytrauma and hemorrhage in rats lead to a fibrinolytic coagulopathy, as demonstrated by an elevation in plasmin activity, D-dimers, and tPA. These results are consistent with the observed clinical benefit of tranexamic acid in trauma patients.
Keywords: thrombin, plasmin, fibrinolysis, coagulation, hemorrhage
polytrauma and hemorrhage are leading causes of morbidity and mortality in patients younger than 45 yr in both the United States and world-wide (21, 31). Up to one-third of severely injured patients develop a coagulopathy, and these patients have a higher mortality rate than those without coagulopathic symptoms (4, 22, 23, 27). These patients receive more transfusions, spend more time in critical care, and are more likely to develop multiple organ failure (2, 35). The mechanism underlining acute traumatic coagulopathy (ATC) is not well understood. The clinical findings that define ATC include a prolongation of prothrombin time (PT) or activated partial thromboplastin time (aPTT). There is experimental and clinical evidence showing activation of both the anticoagulant, protein C, and fibrinolytic systems, as well as a decrease in platelet function (2, 3, 7, 19, 29, 34, 38). Overall, these observations suggest an imbalance between clot formation and breakdown in ATC.
Thrombin and plasmin are serine proteases that are converted from their inactive zymogen forms (prothrombin and plasminogen) by the prothrombinase complex, and by tissue plasminogen activator (tPA), respectively (17, 30, 37). Suppression of thrombin and plasmin is accomplished by inhibiting the generation of the active enzymes, and/or by removing the active enzymes, from the circulation. Thrombin generation can be suppressed by inactivation of FVa and FVIIIa by activated protein C (aPC). aPC is generated after PC binds endothelial PC receptor and is acted on by the thrombin-thrombomodulin complex. Thrombin is removed from the circulation after binding inhibitory proteins such as α2 macroglobulin (α2-MG), antithrombin III (ATIII), fibrin, and thrombomodulin (TM). Thrombin generation can be attenuated by thrombin binding ATIII, or by tissue factor pathway inhibitor (TFPI) binding FXa, or tissue factor-FVIIa complex. Plasmin generation can be suppressed by factors that inhibit tissue plasminogen activator (PA), including plasminogen activator inhibitor-1 (PAI-1) or thrombin activatable fibrinolysis inhibitor (TAFI). PAI-1 is produced by platelets, adipose tissue, and activated endothelium, and it is a strong inhibitor of tPA and urokinase. TAFI is synthesized in the liver and activated by the thrombin-TM complex to downregulate fibrinolysis. α2-MG can also inhibit fibrinolysis by binding plasmin. The proper orchestration of these factors defines how the procoagulant and fibrinolytic elements respond to polytrauma and hemorrhage in a manner that restores homeostasis.
We have previously developed a rat model of polytrauma and hemorrhage that is coagulopathic and demonstrates an elevation in PT and aPTT (9). This model is not resuscitated so as to simulate the prehospital conditions of the traumatized patient. In the present study, this model was used to characterize the effect of trauma and hemorrhage on the balance of thrombin and/or plasmin activity during the postinjury period.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee of the U.S. Army Institute of Surgical Research. This study has been conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the “Guide for the Care and Use of Laboratory Animals.”
Sprague-Dawley rats (300–400 g) were anesthetized with 1–2% isoflurane/100% oxygen through a nose cone and allowed to breathe spontaneously. Cannulas were placed in the left femoral artery and vein for monitoring arterial blood pressure and for withdrawing blood. Polytrauma was performed, as previously described (9). Briefly, a midline incision was made through the abdominal skin and underlying muscle layers. The right and medial lobes of the liver received three crush injuries each using a clamp covered with Silastic tubing. A 10-cm section of small intestines anterior to the cecum was isolated and run gently through the same clamp. The intestines and liver were replaced, and the abdominal incision closed in two layers with sutures. Femur fracture was accomplished by dropping six stainless-steel balls (65 g each) stacked together, from 36′′ through a guide tube (1′′ internal diameter) to impact on a rounded aluminum blade resting on the mid-right femur of the right leg that was suspended on two aluminum stands, one under the hip and one under the knee. A large hemostat (5-inch tongs) was used to clamp the muscle of the right leg 10 times. The rats were then bled in a controlled manner through the arterial cannula to a mean arterial pressure of 40 mmHg within 5 min and maintained at 40 mmHg until 40% of estimated blood volume was removed. Hemorrhage was usually completed between 30 and 45 min after trauma. Hemorrhage was then discontinued, and blood pressure and heart rate were allowed to freely compensate. No resuscitation was given. Blood volume was estimated as 7% of body wt (10, 11). All rats survived the 240-min experimental period, which was immediately followed by euthanasia. Blood samples (in 20 mM sodium citrate with normal saline) were taken before trauma (after cannulation) and at 30, 60, 120, and 240 min after trauma from the femoral vein. The volume of sample was calculated into the hemorrhage volume. Blood samples were immediately spun at 10,000 g for 10 min, and the plasma was separated and stored at −80°C.
Plasma levels of rat active tPA and active PAI-1 were determined by ELISA (Molecular Innovations, Novi, MI). Plasma levels of rat ATIII, α2-MG, and soluble TM were measured by ELISA (Kamiya Biomedical, Tukwila, WA). Plasma levels of rat TFPI and TAFI were measured by ELISA (CUSABIO, Wuhan, PR China). Plasma levels of rat D-dimer was measured by ELISA (USCN Life Science, Wuhan, PR China). All ELISAs were run according to the manufacturer's procedures.
Plasma aPC was measured by OLIGOBIND aPC activity assay (American Diagnostica, Pfungstardt, Germany). Blood sample was immediately treated with aprotinin (10 μmol/l, Sigma-Aldrich, St. Louis, MO) and recombinant-Hirudin (15 μg/ml; Hyphen BioMed, Neuville-Sur-Oise, France) during collection. Total PC was determined by converting all PC to aPC after incubation with 0.2 unit/ml of PROTAC (ANIARA) for 10 min at 37°C. Collection of blood over aprotinin and hirudin, as well as performance of ELISA, was in accordance with the manufacturer's instructions. aPC provided from the manufacturer was used to generate standard curves. PC was calculated by subtracting aPC from the total PC.
Thrombin activity.
Thrombin activity was measured by monitoring the conversion of artificial substrate (SN-20; Hematologic Technologies) to a fluorogenic product over time using a fluorometer. Thrombin (Sigma-Aldrich) was used to generate standard curves. Total thrombin was determined by converting all prothrombin to thrombin after incubating plasma with 20 μg/ml of rat FXa (Molecular Innovations) at 37°C for 60 min. Prothrombin was calculated as the total minus the thrombin activity.
Plasmin activity.
Plasmin activity was measured by monitoring the conversion of chromogenic substrate (S-2403; Chromogenix, DiaPharma Group, West Chester, OH) to a colored product over time using a spectrophotometer at 405 nm. Plasmin (Sigma-Aldrich) was used to generate standard curves. Total plasmin was determined by converting all plasminogen to plasmin after incubating plasma with 1 μg/ml of rat tPA (Abcam, Cambridge, MA) at 37°C for 60 min. Plasminogen was calculated as the total minus the plasmin activity.
As the amount of blood taken during sampling was limited in this model, the total measurements were assigned to two identical groups of rats undergoing the same procedures (n = 8 for each group). One group of rats was used for measurement of ATIII, α2-MG, TM, TFPI, TAFI, tPA, PAI-1, and D-dimer. A separate group of rats was used for measurement of aPC, PC, thrombin, prothrombin, plasminogen, and plasmin.
Data analysis.
Comparisons between groups were analyzed by one-way ANOVA corrected for repeated measures followed by Holms-Sidac post hoc test. If the data population failed the normality test (Shapiro-Wilk), then group comparisons were performed by Friedman repeated-measures ANOVA on ranks, followed by post hoc comparisons by the method of Dunn. All statistical calculations were performed using SigmaPlot (Systat Software).
RESULTS
Description of the injury.
After euthanasia, we visually examined all of the injured organs and tissues to determine the extent of injury (visual) and if any free bleeding occurred after injury. Generally, the crush injury did not completely break apart any of the organs or tissues affected. The amount of free bleeding was assessed by measuring the size of clots formed near the site of injury and was found to be minimal to none. At 4 h after injury, gentle crush to the intestine showed petechial hemorrhaging along the length of the intestine that was injured. A line of hematoma was observed at each liver lobe crush injury. Again no free bleeding into the abdominal cavity was seen. No apparent bleeding occurred after crush in the leg skeletal muscle, although the injured skeletal muscle was paler than the noninjured leg. A small hematoma (approximately 0.25 ml) was seen at the site of the femur break. For all of the injured sites, clotting appeared to occur quickly, was stable, and showed little to no uncontrolled bleeding. Therefore, we believe that hemostasis was normal at the time of injury, allowing for stable clots to form prior to the development of coagulopathy over the next 4 h.
Thrombin and thrombin inhibitors.
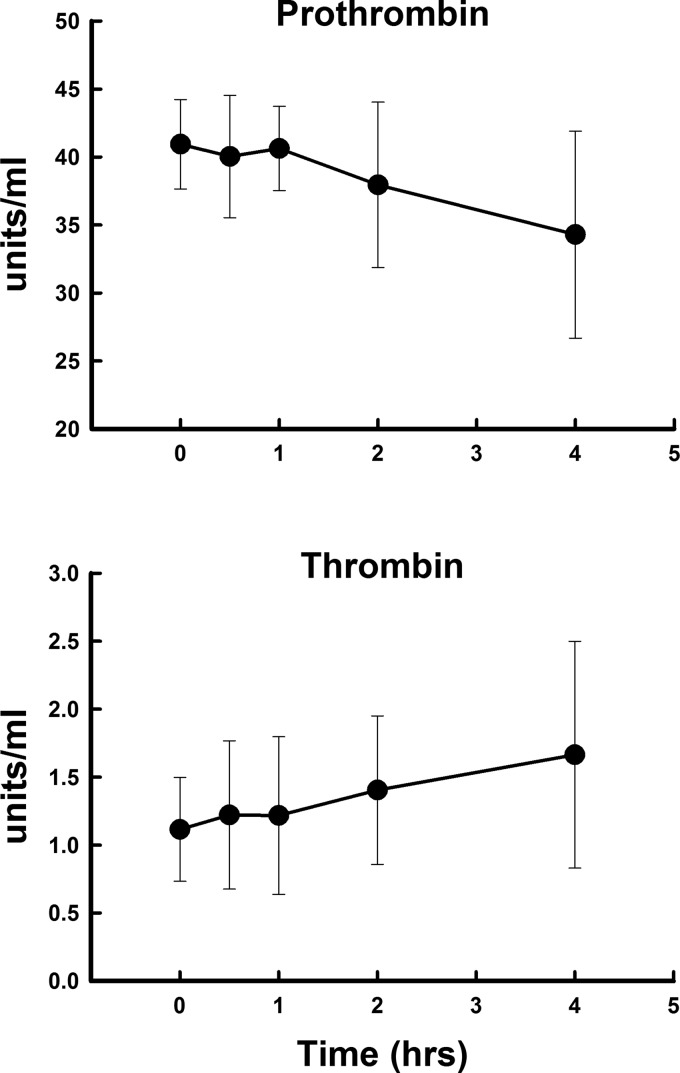
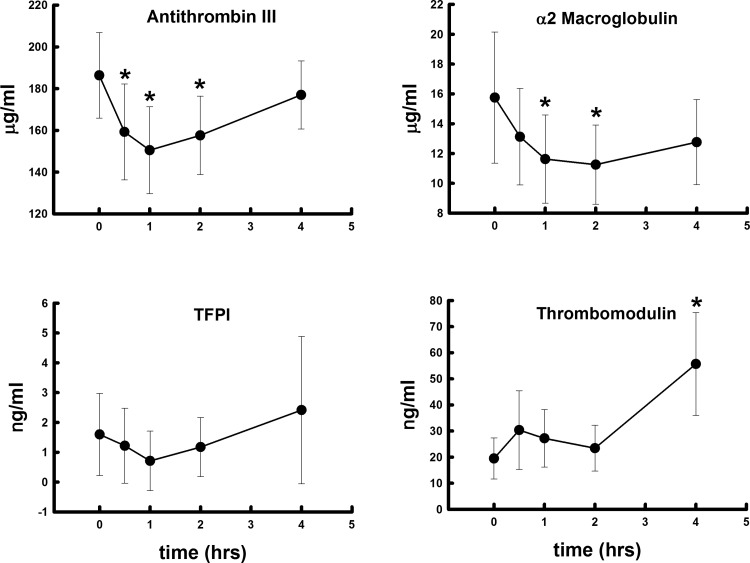
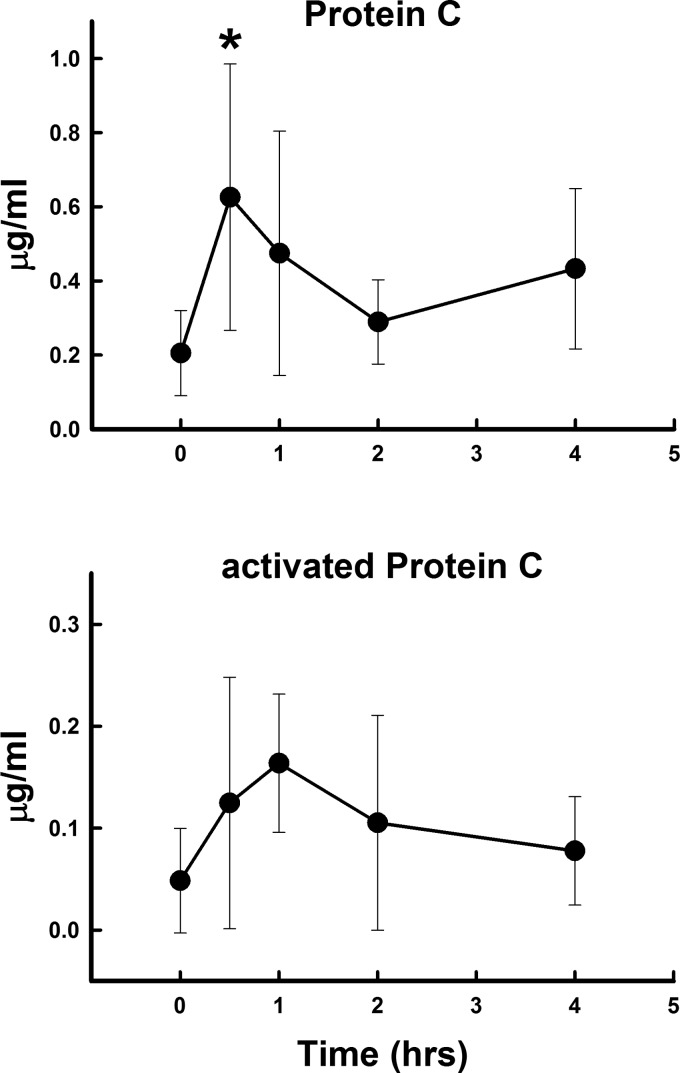
Polytrauma and hemorrhage did not lead to a significant rise in thrombin activity over the 4 h studied (Fig. 1). However, prothrombin levels fell by 4 h, suggesting that thrombin was being produced and prothrombin was being consumed. However, this fall was not significant. Prothrombin levels were more than 30–40 times higher than thrombin levels at each time point. α2-MG and ATIII both fell significantly (Fig. 2) at 2 h and then rose toward baseline. TFPI did not change significantly over the 4 h, but tended to mirror α2-MG and ATIII. Soluble TM rose significantly and progressively, more than doubling in concentration over the 4 h (Fig. 2). Protein C increased at 30 min and was 2–6-fold higher than aPC (Fig. 3). The rise in aPC was not significant by ANOVA, and the measured levels were at the lower detection limits of the assay.
Fig. 1.
Changes in plasma prothrombin and thrombin concentrations after polytrauma and hemorrhage over 4 h. Values represent means ± SD.
Fig. 2.
Changes in plasma antithrombin III, α2 macroglobulin, tissue factor pathway inhibitor (TFPI), and soluble thrombomodulin concentrations after polytrauma and hemorrhage over 4 h. Values represent means ± SD. *P < 0.05 significant difference compared with time 0.
Fig. 3.
Changes in plasma protein C and activated protein C concentrations after polytrauma and hemorrhage over 4 h. Values represent means ± SD. *P < 0.05 significant difference compared with time 0 after ANOVA.
Plasmin and plasmin inhibitors.
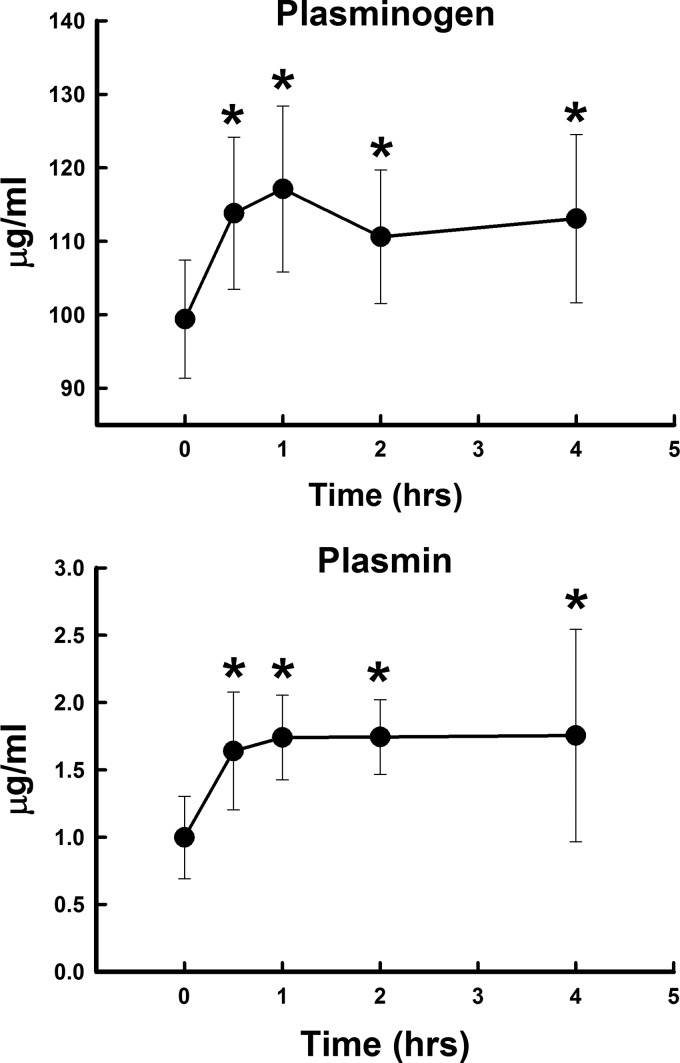
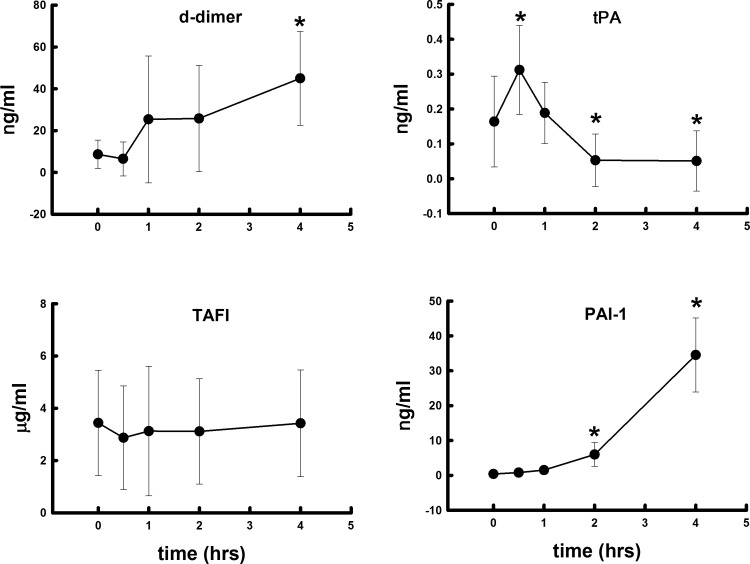
Polytrauma and hemorrhage led to a significant rise in plasmin activity starting at 30 min and continued throughout the 4 h studied (Fig. 4). Also, plasminogen levels were significantly elevated at all times measured, with the greatest change at 1 h. Plasminogen levels were more than 100 times higher than plasmin activity at each time point. Plasma D-dimer levels were significantly elevated after polytrauma and hemorrhage and paralleled the rise in plasmin activity (Fig. 5). Polytrauma and hemorrhage led to a significant rise in tPA at 30 min, then fell below baseline by 2–4 h. This fall was likely due to the significant rise in PAI-1 at 2–4 h. TAFI did not change significantly over time (Fig. 5).
Fig. 4.
Changes in plasma plasminogen and plasmin concentrations after polytrauma and hemorrhage over 4 h. Values represent means ± SD. *P < 0.05, significant difference compared with time 0.
Fig. 5.
Changes in plasma D-dimer, tissue plasminogen activator (tPA), thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor-1 (PAI-1) concentrations after polytrauma and hemorrhage over 4 h. Values represent mean ± SD. *P < 0.05 significant difference compared with time 0.
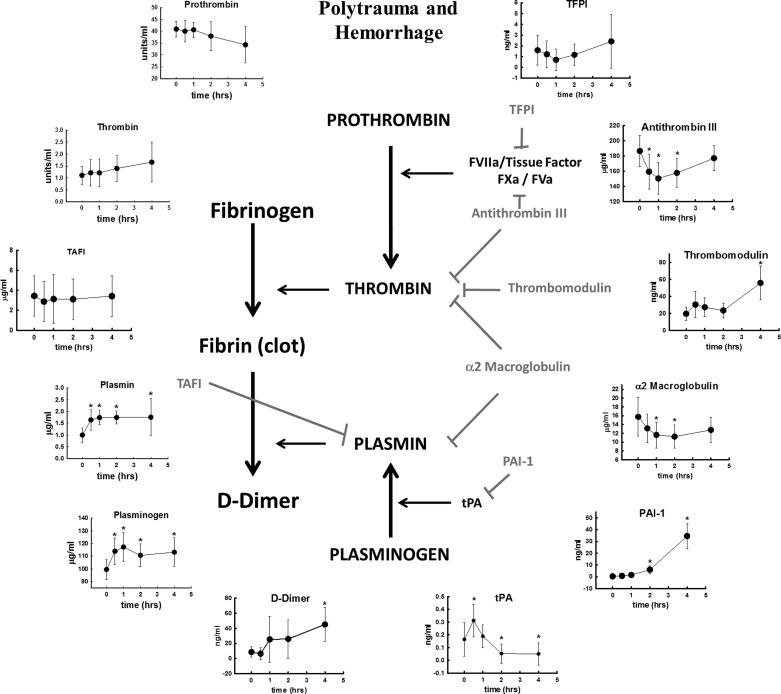
A complete overview of this entire study is presented in Fig. 6. For simplicity, we have left out the contributions of platelets and other cellular components in the formation of the clot. Fibrinogen is converted to fibrin by thrombin and enzymatically digested by plasmin. Thrombin and plasmin are derived from the inactive precursers prothrombin and plasminogen, respectively, by the action of tissue factor/factor VIIa and prothrombinase (FXa/FVa), and by tPA. All of the inhibitors measured in this study are shown in gray. All factors measured in this study are arranged in graphic form around the outside. Each graph represents the changes in that factor following polytrauma and hemorrhage.
Fig. 6.
Clotting pathways studied in this manuscript. At the top, is a graphic depiction of polytrauma and hemorrhage in our rat model. In the center is the clotting cascade. Fibrin (clot) is created from the conversion of fibrinogen by thrombin, and broken down by plasmin into D-dimers and other breakdown products. Prothrombin and plasminogen are the inactive precursors to thrombin and plasmin, respectively. Prothrombin is converted by prothrombin complex (FVIIa/tissue factor and FXa/FVa). Plasminogen is converted by tPA. All inhibitors of this process are represented in gray. Along the outside are the changes that occur in these factors over 4 h after polytrauma and hemorrhage.
DISCUSSION
After trauma to the intestines and liver, we visually observed rapid formation of clot, suggesting that thrombin generation was rapidly initiated by the trauma. The fall in plasma prothombin also suggests that thrombin was being generated from its precursor. Thrombin activity did not rise significantly in the systemic circulation. The failure to detect a rise in thrombin activity may be due to the presence of endogenous inhibitors that bind thrombin or inhibit its generation. Indeed, the levels of α2-MG and ATIII are orders of magnitude higher than plasmin or thrombin. The fall in α2-MG and ATIII after trauma suggests that these inhibitors are binding thrombin, which interferes with the ability of the assay to detect the inhibitors. It is likely that thrombin is rapidly generated in large amounts at the site of injury, and excess thrombin is neutralized by inhibitors to prevent inappropriate clot formation in the general circulation. Thrombin activity changes little in the peripheral circulation after trauma and indicates that the procoagulant side of hemostasis is tightly regulated.
Thrombomodulin is an integral membrane protein expressed on the surface of endothelial cells and acts as a thrombin receptor. TM binding to thrombin creates a complex that inhibits the coagulation system by promoting conversion of protein C to aPC, which proteolytically inactivates factors Va and VIIIa, limiting further thrombin generation. TM binding to thrombin also activates TAFI, and TAFI inhibits fibrinolysis (36). The appearance of soluble TM in plasma may be related to the degree of endothelial damage, as neutrophil elastase cleaves surface TM and releases the soluble form into the circulation (13). Although soluble TM has been shown to bind thrombin and convert PC into its active form (15), soluble TM is less effective in promoting thrombin inhibition compared with endothelial membrane-bound TM (13). Since soluble TM is less active than membrane-bound TM, the rise in soluble TM may reflect a decrease in the capacity to generate aPC, as well as a decrease in the capacity to activate TAFI. Indeed, we did not detect significant changes in plasma TAFI and aPC over time.
It has been shown that depletion of PC in patients with severe sepsis is caused by a combination of degradation of PC by neutrophil elastase (14) and an inadequate biosynthesis of PC in the liver (12). In this study, Protein C significantly rose immediately after trauma and hemorrhage, suggesting that trauma and hemorrhage lead to release of protein C into the circulation, although the source of the PC remains unclear.
Polytrauma and hemorrhage led to a rise in the circulating levels of plasminogen, plasmin activity, and D-dimer concentration. The early rise in tPA may be the main driver for the elevated plasmin activity. The late rise in PAI-1 is most likely responsible for the inhibition of tPA at 2–4 h. The fact that TAFI was not elevated suggests that TAFI does not significantly affect the elevation in plasmin activity. The early rise in plasminogen, like the rise in PC, suggests a release from a reservoir into the circulation.
The rise in plasmin activity and D-dimers suggests that a fibrinolytic coagulopathy develops over time in this model and mirrors the developing elevation in PT and aPTT that we have previously reported (9). Plasmin not only enzymatically breaks down clots, but also inhibits procoagulant acitivity by proteolysis of several coagulation proteins, including factor Va (20, 25), Factor IXa (33), Factor X (28), and Factor VIII (16, 24, 32). Therefore, an elevation of plasmin activity not only causes fibrinolysis, but also inhibits thrombin formation, and thus could contribute to the prolongation of PT and aPTT.
Our animal model of coagulopathy is consistent with clinical observations in trauma patients that show an increase in PT, aPTT, tPA, and D-dimer, and fibrinolysis. This suggests that ATC is due, at least in part, to an elevation in fibrinolysis (6, 18, 26). The early rise in tPA shown in this study may be the key to preventing this coagulopathy. Inhibitors of plasmin generation, such as tranexamic acid, could prevent the rise in plasmin activity. Indeed, clinical trials have shown that tranexamic acid reduces bleeding in surgery and reduces mortality after trauma (1, 5, 8). However, tPA was not elevated throughout the 4 h and, therefore, cannot explain the continued rise in plasmin. It is possible that urokinase is involved. Endothelial or other cell surfaces express urokinase receptors and/or annexion II, which binds uPA, tPA and plasminogen, and may contribute to the sustained elevation in plasmin.
The rise in both plasminogen and PC suggests that these zymogens are actively secreted or released into the general circulation from hepatocytes or endothelial cells in response to polytrauma and hemorrhage. It is interesting to note that the levels of zymogens measured in this study (prothrombin, plasminogen, and PC) are many times higher than their respective active enzymes. In the case of plasminogen and prothrombin, the levels are 30–100 times higher. This suggests that these pools of precursors will not be depleted in the acute setting (4 h), ensuring that active enzymes can be produced in response to severe trauma.
Conclusion.
This study shows that polytrauma with hemorrhage leads to a sustained elevation in plasmin activity and plasminogen concentration, but no significant change in circulating thrombin activity. The significant rise in plasmin activity, D-dimers, and tPA suggests that the coagulopathy in this model is fibrinolytic, which is consistent with numerous clinical studies (3, 7, 29, 34, 38). The fall in fibrinogen is likely due to consumption and/or dilution by fluid moving into the vascular space. The data in this article suggest that the coagulopathy is due to a number of factors that may include dilution, consumption, and an elevation in fibrinolysis. The fall in ATIII and α2-MG suggests that thrombin is elevated and being neutralized by these inhibitors. It is possible that ATIII or α2-MG may be useful as a therapy to dampen elevated thrombin generation in situations in which thrombin generation is uncontrolled.
Perspectives and Significance
The most significant finding in this study is that polytrauma and hemorrhage lead to an elevation of plasmin, D-dimers, and tPA, as measured in the peripheral circulation. However, what is surprising is that thrombin levels did not change in the peripheral circulation. But is this really surprising? Although the damage in the model was severe and extensive, it was localized. We believe that thrombin is elevated proximal to the damage, and any thrombin spillover from the injury is quickly sequestered by inhibitors. This would prevent the formation of inappropriate clots distal to the injury and preserves homeostasis.
However, plasmin levels were elevated systemically. We propose that the coagulopathy, as measured by PT in the same peripheral samples, is caused by this rise in plasmin, and, therefore, a rise in fibrinolysis. The rise in plasmin may simply be a physiological compensatory response to prevent the formation of “inappropriate” clots peripheral to the injury. Clot formation is dynamic and involves formation by thrombin and breakdown by plasmin. The formation of the clot depends on the concentration of each enzyme and the environmental conditions that exist in the tissue where the clot forms. The rise in plasmin and the ensuing fibrinolysis (and coagulopathy) may simply be the normal physiological compensation to severe trauma. However, the medical literature has demonstrated that the coagulopathy is pathological and needs to be treated. Unfortunately, for the patient with acute traumatic coagulopathy, any ensuing surgical intervention to repair the damage could cause further bleeding, shock, and potentially death. Treatment of the coagulopathy prior to surgical intervention is, therefore, imperative.
GRANTS
This study was funded by the U.S. Army Medical Research Material Command.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.W., D.N.D., and A.P.C. conception and design of research; X.W. performed experiments; X.W., D.N.D., and A.P.C. analyzed data; X.W., D.N.D., and A.P.C. interpreted results of experiments; X.W. and D.N.D. prepared figures; X.W. and D.N.D. drafted manuscript; X.W., D.N.D., and A.P.C. edited and revised manuscript; X.W., D.N.D., and A.P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
This material was presented in part at Military Health System Research Symposium 2013 and 2014. The opinions or assertions expressed herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Department of the Army or the U.S. Department of Defense.
REFERENCES
- 1.Ausset S, Glassberg E, Nadler R, Sunde G, Cap AP, Hoffmann C, Plang S, Sailliol A. Tranexamic acid as part of remote damage-control resuscitation in the prehospital setting: A critical appraisal of the medical literature and available alternatives. J Trauma Acute Care Surg 78: S70–S75, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 13: 680–685, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma 64: 1211–1217; discussion 1217, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma 54: 1127–1130, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma 71: S9–S14, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock 41: 514–521, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg 255: 379–385, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CRASH-2 Collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 377: 1096–1101, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Darlington DN, Craig T, Gonzales MD, Schwacha MG, Cap AP, Dubick MA. Acute coagulopathy of trauma in the rat. Shock 39: 440–446, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Darlington DN, Jones RO, Magnuson TA, Gann DS. Role of intestinal fluid in restitution of blood volume and plasma protein after hemorrhage in awake rats. Am J Physiol Regul Integr Comp Physiol 268: R715–R722, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Darlington DN, Jones RO, Marzella L, Gann DS. Changes in regional vascular resistance and blood volume after hemorrhage in fed and fasted awake rats. J Appl Physiol 78: 2025–2032, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med 29: S42–S47, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Dhainaut JF, Yan SB, Cariou A, Mira JP. Soluble thrombomodulin, plasma-derived unactivated protein C, and recombinant human activated protein C in sepsis. Crit Care Med 30: S318–S324, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Eckle I, Seitz R, Egbring R, Kolb G, Havemann K. Protein C degradation in vitro by neutrophil elastase. Biol Chem Hoppe Seyler 372: 1007–1013, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh J, O'Donnell M, Weitz JI. New anticoagulants. Blood 105: 453–463, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Holmberg L, Ljung R, Nilsson IM. The effects of plasmin and protein Ca on factor VIII:C and VIII:CAg. Thromb Res 31: 41–50, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Huntington JA. Thrombin inhibition by the serpins. J Thromb Haemost 11 Suppl 1: 254–264, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, Lam L, Shulman I, Nelson J, Demetriades D. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg 215: 496–502, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma 51: 639–647, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Kalafatis M, Mann KG. The role of the membrane in the inactivation of factor Va by plasmin. Amino acid region 307–348 of factor V plays a critical role in factor Va cofactor function. J Biol Chem 276: 18,614–18,623, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Krug EG, Sharma GK, Lozano R. The global burden of injuries. Am J Public Health 90: 523–526, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maegele M, Paffrath T, Bouillon B. Acute traumatic coagulopathy in severe injury: incidence, risk stratification, and treatment options. Dtsch Arztebl Intl 108: 827–835, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma 64: 1459–1463; discussion 1463– 1455, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Nogami K, Shima M, Matsumoto T, Nishiya K, Tanaka I, Yoshioka A. Mechanisms of plasmin-catalyzed inactivation of factor VIII: a crucial role for proteolytic cleavage at Arg336 responsible for plasmin-catalyzed factor VIII inactivation. J Biol Chem 282: 5287–5295, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Omar MN, Mann KG. Inactivation of factor Va by plasmin. J Biol Chem 262: 9750–9755, 1987. [PubMed] [Google Scholar]
- 26.Ostrowski SR, Sorensen AM, Larsen CF, Johansson PI. Thrombelastography and biomarker profiles in acute coagulopathy of trauma: a prospective study. Scand J Trauma Resus Emerg Med 19: 64, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pidcoke HF, Aden JK, Mora AG, Borgman MA, Spinella PC, Dubick MA, Blackbourne LH, Cap AP. Ten-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: increased plasma and platelet use correlates with improved survival. J Trauma Acute Care Surg 73: S445–S452, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Pryzdial EL, Lavigne N, Dupuis N, Kessler GE. Plasmin converts factor X from coagulation zymogen to fibrinolysis cofactor. J Biol Chem 274: 8500–8505, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De'Ath HD, Allard S, Hart DP, Pasi KJ, Hunt BJ, Stanworth S, MacCallum PK, Brohi K. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost 11: 307–314, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Rehman AA, Ahsan H, Khan FH. α-2-Macroglobulin: a physiological guardian. J Cell Physiol 228: 1665–1675, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg 260: 13–21, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Rick ME, Krizek DM. Platelets modulate the proteolysis of factor VIII: C protein by plasmin. Blood 67: 1649–1654, 1986. [PubMed] [Google Scholar]
- 33.Samis JA, Ramsey GD, Walker JB, Nesheim ME, Giles AR. Proteolytic processing of human coagulation factor IX by plasmin. Blood 95: 943–951, 2000. [PubMed] [Google Scholar]
- 34.Schochl H, Voelckel W, Maegele M, Solomon C. Trauma-associated hyperfibrinolysis. Hamostaseologie 32: 22–27, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Sixta SL, Hatch QM, Matijevic N, Wade CE, Holcomb JB, Cotton BA. Mechanistic determinates of the acute coagulopathy of trauma (ACoT) in patients requiring emergency surgery. Int J Burns Trauma 2: 158–166, 2012. [PMC free article] [PubMed] [Google Scholar]
- 36.Vercauteren E, Mutch NJ, Declerck PJ, Gils A. Plasmin and the thrombin-thrombomodulin complex both contribute to thrombin-activatable fibrinolysis inhibitor activation in whole blood model thrombi. J Thromb Haemost 11: 190–192, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev 93: 327–358, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Yanagida Y, Gando S, Sawamura A, Hayakawa M, Uegaki S, Kubota N, Homma T, Ono Y, Honma Y, Wada T, Jesmin S. Normal prothrombinase activity, increased systemic thrombin activity, and lower antithrombin levels in patients with disseminated intravascular coagulation at an early phase of trauma: comparison with acute coagulopathy of trauma-shock. Surgery 154: 48–57, 2013. [DOI] [PubMed] [Google Scholar]