Abstract
Clinical data suggest that renal denervation (RDNX) may be an effective treatment for human hypertension; however, it is unclear whether this therapeutic effect is due to ablation of afferent or efferent renal nerves. We have previously shown that RDNX lowers arterial pressure in hypertensive Dahl salt-sensitive (S) rats to a similar degree observed in clinical trials. In addition, we have recently developed a method for selective ablation of afferent renal nerves (renal-CAP). In the present study, we tested the hypothesis that the antihypertensive effect of RDNX in the Dahl S rat is due to ablation of afferent renal nerves by comparing the effect of complete RDNX to renal-CAP during two phases of hypertension in the Dahl S rat. In the early phase, rats underwent treatment after 3 wk of high-NaCl feeding when mean arterial pressure (MAP) was ∼140 mmHg. In the late phase, rats underwent treatment after 9 wk of high NaCl feeding, when MAP was ∼170 mmHg. RDNX reduced MAP ∼10 mmHg compared with sham surgery in both the early and late phase, whereas renal-CAP had no antihypertensive effect. These results suggest that, in the Dahl S rat, the antihypertensive effect of RDNX is not dependent on pretreatment arterial pressure, nor is it due to ablation of afferent renal nerves.
Keywords: renal denervation, afferent renal nerves, efferent renal nerves, Dahl salt-sensitive rat
despite the staggering worldwide prevalence of hypertension and its significant association with death and disability, little progress has been made in developing more effective treatments for the disease (16, 20). However, recent technological advances have led to the development of catheter-based ablation of renal nerves (renal denervation, RDNX) as a possible treatment for drug-resistant hypertension in humans. The majority of clinical data that have been published using this technique have demonstrated a significant antihypertensive effect of RDNX, although a number of studies have failed to show any effect (4). This discrepancy has highlighted the need for further investigations into the mechanisms underlying the effects of RDNX.
One mechanistic question regarding this treatment is whether RDNX lowers arterial pressure (AP) secondary to ablation of afferent or efferent renal nerves. Because efferent renal nerves influence renin release, tubular sodium reabsorption, and renal vascular resistance, it has been proposed that the antihypertensive effect of RDNX may result from ablation of efferent renal nerves (5). Indeed, data from one clinical study suggest that RDNX reduces renal vascular resistance, as indicated by a reduction in renal resistive index (17). However, one study has found no correlation between the blood pressure lower effect of RDNX and sodium excretion (21), and data regarding the effect of RDNX on plasma renin concentration are limited and mixed (6, 27).
An alternative possibility is that the antihypertensive response to RDNX is due to ablation of sympathoexcitatory afferent renal nerves, resulting in decreased nonrenal sympathetic nerve activity (SNA). This hypothesis is supported by clinical studies demonstrating reductions in muscle SNA, plasma glucose, cardiac arrhythmias, and events of sleep apnea following RDNX (10, 23, 28). Although these studies suggest that there are physiological consequences of afferent renal nerve ablation, it is still unclear whether ablation of afferent renal nerves is responsible for the decrease in AP following RDNX. It should also be noted, that at least one study has failed to show reductions in SNA following RDNX (2).
We have recently established the Dahl salt-sensitive (DS) rat as an animal model of hypertension in which RDNX lowers AP to a degree similar to that reported in humans (7). This commonly used model is widely accepted as a good model of human hypertension, as it displays many comorbidities commonly observed in hypertensive patients, such as renal pathology, impaired glucose metabolism, and overactivity of the sympathetic nervous system (30). Although we have shown that RDNX decreases AP in DS rats, it is unknown whether this response is due to ablation of afferent or efferent renal nerves.
To address this question, we recently developed and validated a novel method for selective ablation of afferent renal nerves in the rat, termed renal-CAP (8). In the present study, we tested the hypothesis that the antihypertensive response to RDNX in the DS rat is due to ablation of afferent renal nerves specifically by comparing the antihypertensive effect of complete RDNX to that of renal-CAP. Moreover, because pretreatment AP has been suggested to be a predictor of the antihypertensive effect of RDNX (24), we tested this hypothesis in both the early phase (after 3 wk of high NaCl feeding when hypertension is moderate) and the late phase (after 9 wk of high NaCl feeding when hypertension is more severe) of hypertension.
MATERIALS AND METHODS
Animals and General Procedures
A total of 56 male DS rats were purchased from Charles River Laboratories (Wilmington, MA) and housed in pairs in a temperature- and light-controlled room until the beginning of the study. Rats were allowed access to standard rat chow and distilled water ad libitum during this preexperimental period. All procedures were approved by the University of Minnesota Animal Care and Use Committee and were conducted in accordance with the institutional and National Institutes of Health guidelines. For all surgeries, rats were anesthetized with 2.0% isoflurane (Phoenix Pharmaceutical, St. Joseph, MO). Atropine sulfate (0.4 mg/kg ip; West-Ward Pharmaceuticals, Eatontown, NJ), gentamicin sulfate (10 mg/kg im; Hospira, Lake Forest, IL) and ketoprofen (5 mg/kg sc; Fort Dodge Animal Health, Overland Park, KS) were administered prior to surgery. For three days following surgery, ketoprofen (2.5 mg/kg sc) was given once per day, and the drinking water was supplemented with amoxicillin (1 mg/ml; Sandoz International, Holzkirchen, Germany).
Treatment Surgeries
Renal denervation.
Complete denervation of the kidneys was performed as previously described (8). Briefly, the left renal nerves were exposed through a midline abdominal incision. All visible renal nerve fibers were sectioned, and the renal artery and vein were brushed with a small piece of gauze soaked in 10% phenol in ethanol. The area was then dried, and the procedure was repeated on the contralateral side.
Renal-CAP treatment.
Selective ablation of afferent renal nerves was performed as previously described (7). Briefly, a midline abdominal incision was made, and the left renal artery and vein were exposed through a small hole in the peritoneal membrane. The fat surrounding the renal artery and vein was then dissected away, and parafilm was placed under the vessels. A small piece of gauze soaked in a capsaicin solution (33 mM in 5% ethanol, 5% Tween 80 and 90% normal saline) was wrapped around the renal artery and vein for 15 min. The gauze and parafilm were removed, the area was dried, and the procedure was repeated on the contralateral side.
Sham surgery.
The left and right renal nerves were visualized through a midline abdominal incision without physical disruption of the area.
Experimental Protocol
Early-phase protocol.
Sixty-seven to seventy-four-day-old rats were placed on a low-salt diet (0.1% NaCl; Research Diets, New Brunswick, NJ) and instrumented with radio telemeters [model TA11PA-C40; Data Sciences International (DSI), St. Paul, MN] for monitoring of mean arterial pressure (MAP) and heart rate (HR), as previously described (25). After a 7-day recovery period, rats were individually housed in metabolic cages (Techniplast 3701M001; Buguggiate, Italy) and were allowed to acclimate for 4 days. Three days of baseline data were then collected (see below for details), and rats were placed on a high-salt diet (4.0% NaCl; Research Diets) for the remainder of the protocol. After 21 days of high-salt intake, rats were anesthetized with isoflurane and, via a midline approach, subjected to sham surgery (Sham; n = 10), renal denervation (RDNX; n = 10), or selective ablation of afferent renal nerves (renal-CAP; n = 10), as described above. Rats were returned to their cages and monitored for an additional 4 wk.
Late-phase protocol.
Sixty-two to sixty-four-day-old rats were placed on a high-salt diet (4.0% NaCl; Research Diets), and after 49–50 days, they were instrumented with radio telemeters (DSI) for monitoring of MAP and HR. After a 6–7-day recovery period, rats were individually housed in metabolic cages (Techniplast) and allowed to acclimate for 4 days. Three days of baseline data were then collected. After 9 wk total of a high-salt diet, rats were anesthetized with isoflurane and, via a midline approach, subjected to a sham surgery (Sham; n = 8), renal denervation (RDNX; n = 9), or selective ablation of afferent renal nerves (renal-CAP; n = 9), as described above. Rats were returned to their cages and monitored for an additional 2 wk.
At the end of the study, rats were anesthetized and exsanguinated, and the kidneys were removed immediately after death. The renal artery, renal vein, ureter, and capsule were removed, and the kidneys were placed in a cold normal saline bath for further dissection. Renal parenchymal samples were taken from the poles and lateral portion of the kidney, and they were flash frozen in liquid nitrogen. The renal pelvis was then carefully dissected from the remaining portion of kidney and flash frozen in liquid nitrogen. All frozen samples were stored at −80°C until being assayed.
The parenchymal samples were assayed for norepinephrine (NE) using HPLC, as previously described (15). The detection limit of this assay is 100 pg/g with a intra-assay coefficient of variation (CV) of 4.4% and interassay CV of 6.7%. The isolated renal pelvic samples were assayed for calcitonin gene-related peptide (CGRP) using a commercially available ELISA kit (Cayman Chemicals, Ann Arbor, MI; item no. 589001). Tissues were homogenized in 1 M acetic acid, and CGRP was extracted using C18 Sep-columns (Peninsula Laboratories, San Carlos, CA; item no. Y-1000). To eliminate any interassay variance, all pelvic samples were run on a single 96-well ELISA plate.
Daily Measurements
The transmitter signal was monitored by a receiver (Data Sciences, model no. RPC-1) mounted on the side of the metabolic cage and connected to a Data Exchange Matrix (DSI). The AP signal was sampled at 500 samples/s for 10 s every 4 min using commercially available software (DSI). HR was determined from the AP profile using the same software. Twenty-four-hour averages of MAP and HR were determined and plotted for each day of the study.
Twenty-four hour food intake, water intake, and urine output were measured gravimetrically. Twenty-four-hour sodium intake was calculated by multiplying food intake (grams) and sodium content of the diet (0.1% NaCl = 0.01711 mmol Na+/g food; 4.0% NaCl = 0.6844 mmol Na+/g food). Twenty-four-hour sodium excretion was calculated by multiplying 24-h urine output (ml) and urinary sodium concentration (mmol Na+/ml), which was measured using an ion-specific electrode (NOVA-5+ electrolyte analyzer, Nova Biomedical, Waltham, MA). Twenty-four-hour sodium and water balances were calculated as 24-h intake minus 24-h excretion. Cumulative sodium and water balance were then calculated by sequential summation of daily balances beginning on the day of treatment surgery.
Statistical Analysis
MAP, HR, and cumulative Na+/H2O balance data were analyzed by 2-way ANOVA for repeated measures followed by the Bonferroni method for post hoc comparisons using GraphPad Prism v6 (GraphPad Software, San Diego, CA). Baseline MAP and HR, as well as CGRP and NE data, were analyzed by one-way ANOVA. A P value less than 0.05 was considered to be statistically significant.
RESULTS
Early-Phase Protocol
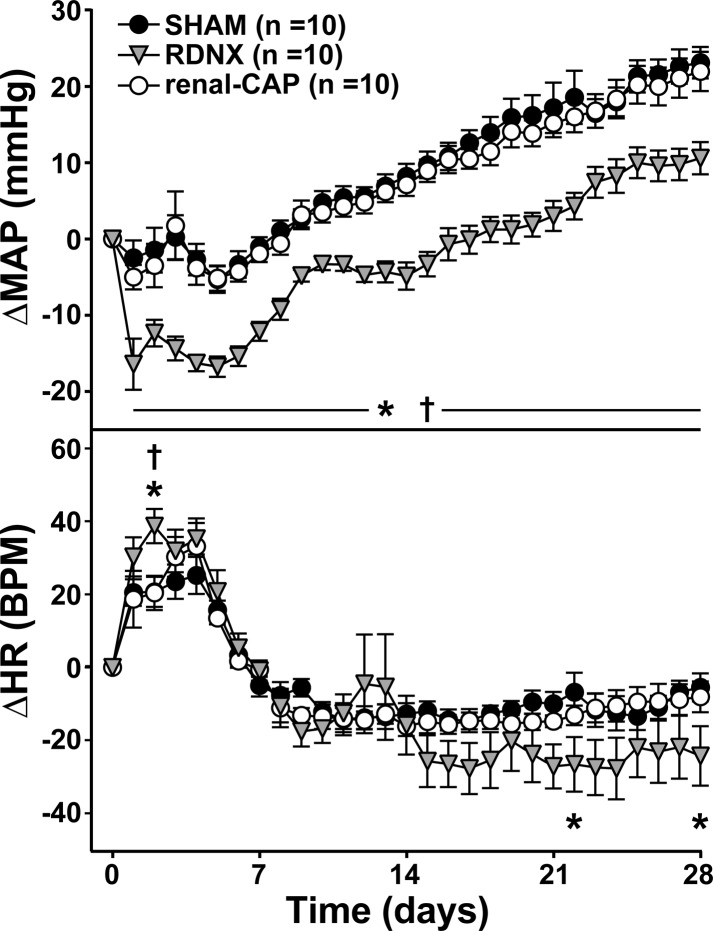
As shown in Table 1, early-phase pretreatment MAP (after 3 wk of high-NaCl feeding) was similar in all groups (∼140 mmHg). Figure 1 shows the change in MAP from the day before treatment (ΔMAP). Following treatment, MAP dropped transiently in all three groups and then continued to rise throughout the protocol. This drop was significantly greater on the day of surgery in RDNX (−16 ± 3 mmHg, P < 0.05) compared with Sham (−3 ± 2 mmHg) and renal-CAP (−5 ± 2 mmHg), and on the final day of the protocol, MAP had risen significantly less in RDNX (11 ± 2 mmHg, P < 0.05) than Sham (23 ± 2 mmHg) and renal-CAP (22 ± 3 mmHg).
Table 1.
Baseline cardiovascular measurements
| Early Phase |
Late Phase |
|||||
|---|---|---|---|---|---|---|
| Sham (n = 10) | RDNX (n = 10) | Renal-CAP (n = 10) | Sham (n = 8) | RDNX (n = 9) | Renal-CAP (n = 9) | |
| MAP, mmHg | 139 ± 3 | 140 ± 3 | 138 ± 2 | 166 ± 4 | 169 ± 6 | 169 ± 5 |
| HR, bpm | 396 ± 3 | 401 ± 4 | 396 ± 4 | 386 ± 3 | 392 ± 5 | 396 ± 6 |
Mean arterial pressure (MAP) and heart rate (HR) on the day before treatment surgery in Sham, renal denervation (RDNX), and renal-CAP (selective ablation of afferent renal nerves) rats in both the early-phase and late-phase protocols. There were no statistical differences in MAP or HR between groups in either protocol.
Fig. 1.
Effect of sham surgery, renal denervation (RDNX), and selective ablation of afferent renal nerves (renal-CAP) on mean arterial pressure (MAP; top) and heart rate (HR; bottom) in the early phase protocol. *P < 0.05 for RDNX vs. Sham. †P < 0.05 for RDNX vs. renal-CAP.
As shown in Table 1, early-phase pretreatment HR was similar in all groups (∼400 bpm). Figure 1 shows the change in HR from the day before treatment (ΔHR). In all three groups, HR transiently increased following treatment and then fell slightly below baseline. Although there were no differences between Sham and renal-CAP throughout the protocol, ΔHR on day 2 after surgery was greater in RDNX (30 ± 5 BPM) compared with Sham (21 ± 4 BPM, P < 0.05) and renal-CAP (19 ± 8 BPM, P < 0.05). Additionally, HR fell more toward the end of the protocol in RDNX, and ΔHR was significantly lower in RDNX than Sham on days 22 (−27 ± 8 vs. −7 ± 5 BPM, P < 0.05) and 28 (−24 ± 8 vs. −6 ± 4 BPM, P < 0.05).
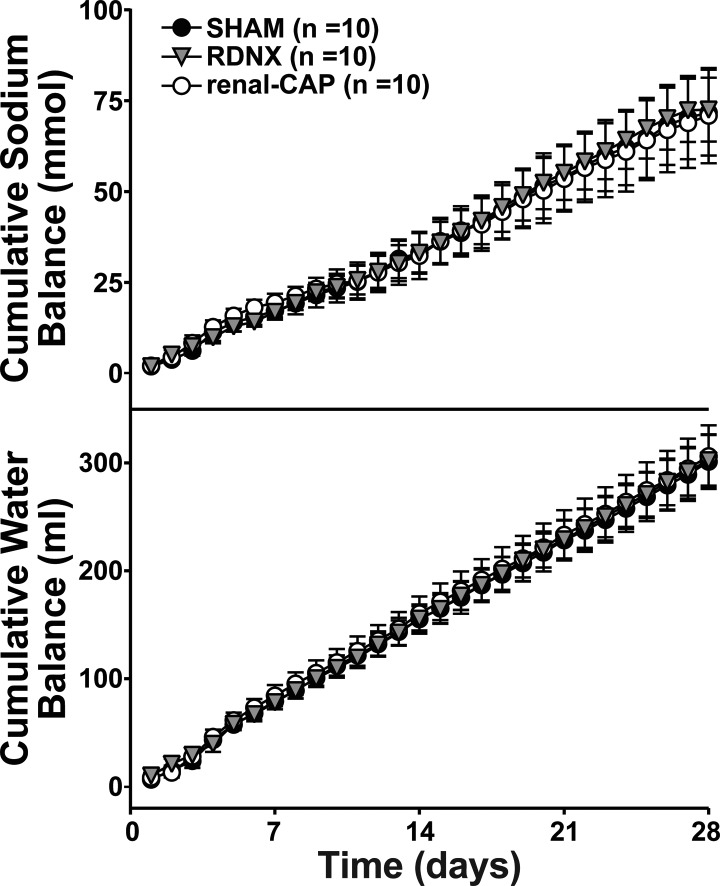
As shown in Fig. 2, there were no differences in cumulative sodium or water balance between groups at any time following treatment.
Fig. 2.
Effect of sham surgery, RDNX, and renal-CAP on cumulative sodium (top) and water (bottom) balance in the early phase protocol. Treatment surgery was performed on day 0. There were no statistical differences between groups at any time point.
Late-Phase Protocol
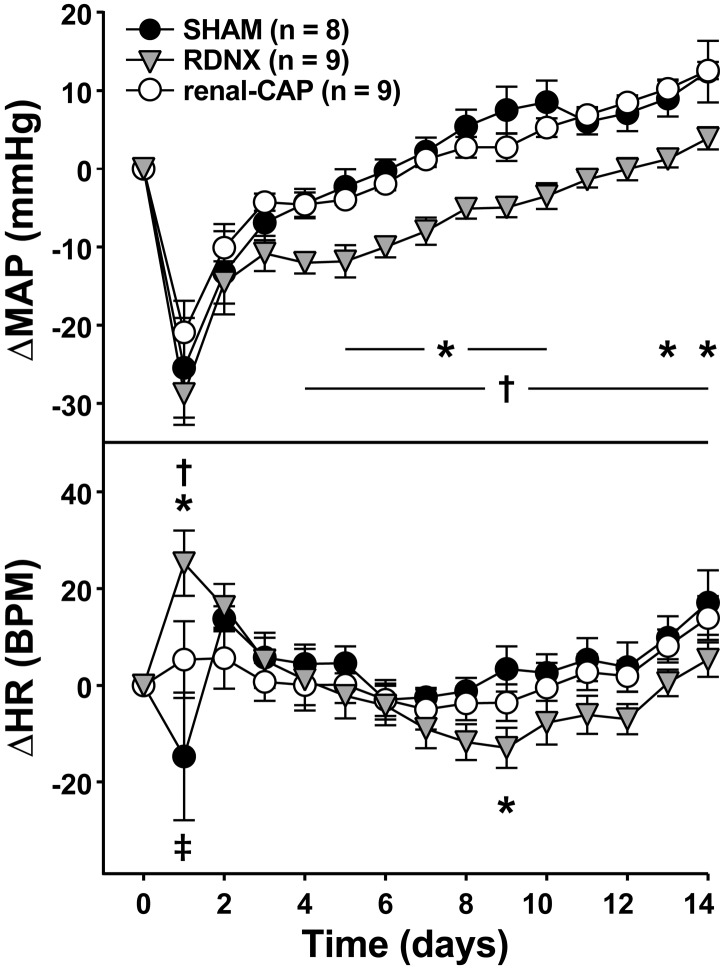
As shown in Table 1, late-phase pretreatment MAP (after 9 wk of high NaCl feeding) was similar in all groups (∼170 mmHg) and nearly 30 mmHg higher than rats fed a high-NaCl diet for 3 wk. MAP dropped transiently in all three groups following treatment and then continued to rise throughout the remainder of the protocol (Fig. 3). While the initial drop was similar in all groups (∼25 mmHg), MAP increased less in RDNX, such that ΔMAP was lower in RDNX than Sham and renal-CAP throughout much of the protocol. On the final day, ΔMAP was significantly lower in RDNX (4 ± 1 mmHg) than Sham (12 ± 4 mmHg, P < 0.05) and renal-CAP (13 ± 1 mmHg, P < 0.05).
Fig. 3.
Effect of sham surgery, RDNX, and renal-CAP on MAP (top) and HR (bottom) in the late-phase protocol. *P < 0.05 for RDNX vs. Sham. †P < 0.05 for RDNX vs. renal-CAP. ‡P < 0.05 for renal-CAP vs. Sham.
As shown in Table 1, late-phase pretreatment HR was similar in all groups and similar to the pretreatment HR in the early-phase protocol (∼400 bpm). As shown in Fig. 3, HR initially increased in RDNX (25 ± 8 bpm), decreased in Sham (−15 ± 13 bpm), and was largely unchanged in renal-CAP (5 ± 8 bpm). HR in all groups then returned roughly to baseline levels for the remainder of the protocol, and there were no differences between groups except on day 9 when RDNX (−13 ± 4 bpm) was significantly less that of Sham (3 ± 5 bpm, P < 0.05).
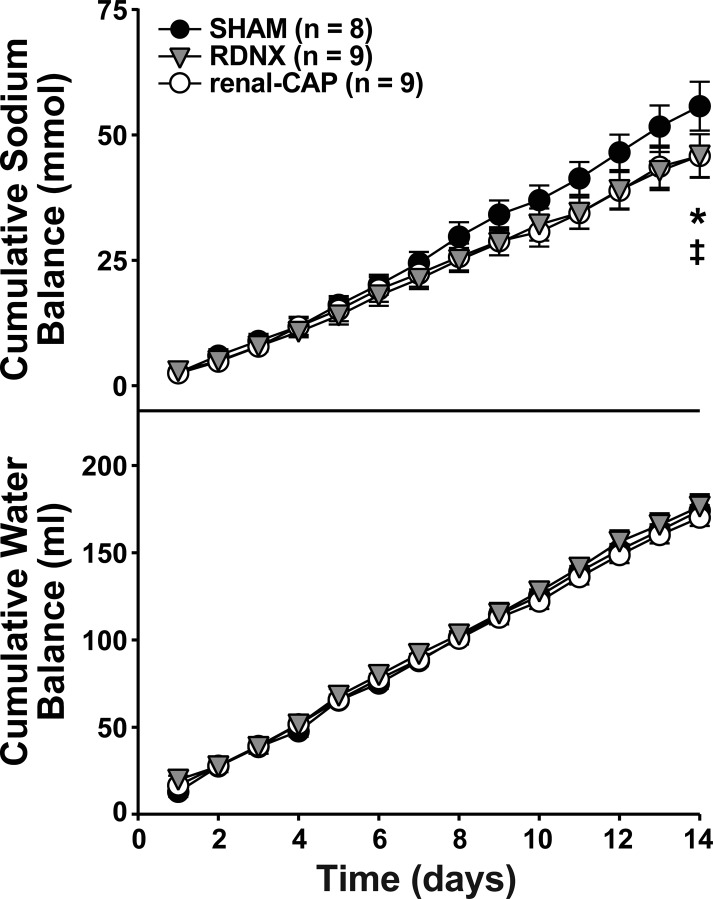
As with the early-phase protocol, there were no differences in cumulative water balance between groups (Fig. 4). However, cumulative sodium balance trended slightly lower in RDNX and renal-CAP rats compared with Sham, but these differences were only significant between RDNX (58.1 ± 4.7 mmol) and Sham (68.3 ± 6.0 mmol, P < 0.05) on the final day of the protocol.
Fig. 4.
Effect of sham surgery, RDNX, and renal-CAP on cumulative sodium (top) and water (bottom) balance in the late-phase protocol. Treatment surgery was performed on day 0. *P < 0.05 for RDNX vs. Sham. ‡P < 0.05 for renal-CAP vs. Sham.
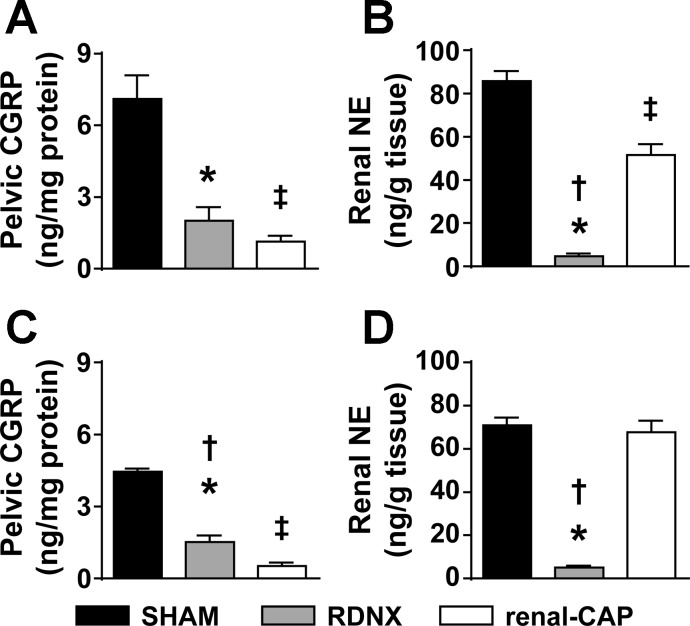
Renal NE and CGRP Content
To confirm the denervation procedures, we measured renal tissue content of the afferent nerve marker, CGRP, and the efferent nerve marker, NE (Fig. 5). In the early-phase protocol, both RDNX and renal-CAP resulted in significant reductions in CGRP compared with Sham. Renal NE was significantly reduced in the kidneys of both RDNX and renal-CAP compared with Sham; however, NE was significantly less in RDNX than renal-CAP.
Fig. 5.
Effect of sham surgery, RDNX, and renal-CAP on the content of nerve markers in the kidneys. A: renal pelvic content of the afferent nerve marker calcitonin gene-related peptide (CGRP) in the early-phase protocol (Sham = 14, RDNX = 18, renal-CAP = 16 kidneys). B: renal content of the efferent nerve marker norepinephrine (NE) in the early-phase protocol (Sham = 14, RDNX = 18, renal-CAP = 16 kidneys). C: renal pelvic content of CGRP in the late-phase protocol (Sham = 16, RDNX = 16, renal-CAP = 18 kidneys). D: renal content of NE in the late-phase protocol (Sham = 16, RDNX = 18, renal-CAP = 17 kidneys). Because some samples were lost in processing, the number of kidneys does not necessarily correspond with the number of rats in each group. *P < 0.05 for RDNX vs. SHAM. †P < 0.05 for RDNX vs. renal-CAP. ‡P < 0.05 for renal-CAP vs. Sham.
In the late-phase protocol, both RDNX and renal-CAP resulted in significant reductions in CGRP compared with Sham. Renal NE was significantly reduced in the kidneys of RDNX but not renal-CAP compared with Sham.
DISCUSSION
Although both preclinical and clinical studies suggest that RDNX has the potential for treating hypertension, it is unclear whether the antihypertensive effect of RDNX is due to ablation of afferent or efferent renal nerves. This distinction is an important one, as it may be feasible to target one subset of renal nerves selectively to optimize efficacy and reduce possible side effects of RDNX. For instance, if the antihypertensive effect of RDNX is due solely to ablation of afferent renal nerves, an ideal ablation procedure would target afferent renal nerves specifically preserving efferent function for situations in which neural control of the kidney may become beneficial for the preservation of water and electrolyte homeostasis, such as during severe dehydration or hemorrhage. Likewise, if RDNX lowers AP secondary to ablation of efferent renal nerves specifically, it would be preferable to preserve renal afferent signaling, which may be important under certain conditions, such as ureteral obstruction due to a kidney stone. Moreover, pinpointing which subset of renal nerves is involved in hypertension could lead to other avenues of treatment that could target pathways either upstream or downstream of activation of those nerves. The current study is an important step in elucidating the differential role of afferent and efferent renal nerves in the antihypertensive effect of RDNX.
Renal Denervation, but not Selective Ablation of Afferent Renal Nerves, Attenuates DS Hypertension
To test whether the antihypertensive effect of RDNX in the DS rat is due to ablation of afferent renal nerves, we compared the effect of RDNX and renal-CAP on MAP in both the early and late phase of DS hypertension. In both protocols, RDNX lowered MAP ∼10 mmHg compared with Sham. Importantly, selective ablation of afferent renal nerves by renal-CAP had no effect on MAP compared with sham surgery in either protocol. These data suggest that the antihypertensive effect of RDNX in the DS rat is not due to ablation of afferent renal nerves. This is in contrast to other animal models of hypertension to which afferent renal nerves appear to contribute. Specifically, we have shown that selective ablation of afferent renal nerves attenuates the development of DOCA-salt hypertension to a similar extent as RDNX (8). Moreover, others have implicated afferent renal nerves in the pathogenesis of hypertension in various other animal models (3, 29). Still others have suggested that, under conditions of dietary sodium loading, hypertension may result from impaired afferent renal nerve activity (14, 26).
The reason for this discrepancy is presently unknown; however, many of the preclinical models in which afferent renal nerves appear to play a role involve severe renal pathology, such as reduced renal mass, renal hypoxia, and even renal necrosis. In the case of the DOCA-salt model used in this study, renal mass is reduced by unilateral nephrectomy, which results in compensatory renal hypertrophy of the remaining kidney. This is followed by chronic administration of a salt-retaining hormone and excessive salt intake induced by replacing drinking water with a saline solution. This results in a profound inflammatory state in the kidney that includes marked leukocyte infiltration and increased tissue levels of inflammatory cytokines such as TNF-α, IFNγ, and IL-6 (1, 22). In contrast, although some renal inflammation has been reported in the Dahl S rat, it involves moderate infiltration of T lymphocytes into the kidneys with no increase in either IFNγ or IL-6 (9). Since numerous cytokines have been shown to chronically modulate the activity of visceral afferent nerve function (19), the difference in the renal inflammatory profile may explain why ablation of renal afferent nerves attenuates hypertension in DOCA-salt model, but not Dahl S model, of hypertension. Our laboratory is currently investigating in greater detail the mechanisms linking renal inflammation to modulation of afferent renal nerves in various preclinical models of hypertension.
Antihypertensive Effect of RDNX Is Similar in Both the Early and Late Phase of DS Hypertension
Because pretreatment AP has been suggested to be a predictor of the antihypertensive effect of RDNX (24), we subjected DS rats to RDNX and renal-CAP in both the early phase (after 3 wk of high NaCl feeding), when MAP was ∼140 mmHg, and in the late phase (after 9 wk of high NaCl feeding), when MAP was ∼170 mmHg. Interestingly, the antihypertensive effect of RDNX was nearly identical in both the early and late phase of DS hypertension, suggesting that, unlike in humans with resistant hypertension, pretreatment MAP may not always be a good predictor of response to RDNX (24).
Antihypertensive Effect of RDNX Is not Due to Sodium and Water Unloading
While total assessment of the efferent renal nerve-specific responses to RDNX (i.e., plasma renin concentration and renal vascular resistance) was outside of the scope of this study, we did measure whole body cumulative sodium and water balance. These measurements revealed that the antihypertensive effect of RDNX did not correspond with a reduction in sodium or water balance, thus demonstrating that a reduction in MAP can occur in the absence of an enhanced natriuresis and diuresis. This finding is consistent with our earlier report on the effect of RDNX in DS rats (7). Combined with our findings that afferent renal nerves do not play a role in this model, these data suggest that the antihypertensive effect of RDNX in the DS rat is likely due to reduced activity of the renin angiotensin system, a reduction in renal vascular resistance, or another effect of efferent renal nerve ablation. Further investigation will be needed to test these possibilities.
Renal Nerves May Play a Minor Role in the Regulation of Heart Rate
Our previous work has suggested that renal nerves may play a minor role in the long-term regulation of heart rate. Specifically, renal-CAP resulted in a blunting of the bradycardic response to dietary sodium loading in normotensive Sprague-Dawley rats (8). Alternatively, the bradycardic response to DOCA-salt hypertension was actually enhanced in RDNX rats compared with Sham rats, despite the antihypertensive effect of RDNX (12). Interestingly, there was a slightly enhanced bradycardia in the early phase of DS hypertension in this study. While the effect was minor and only significant on a few days, these results are consistent with some minor role of the renal nerves in heart rate regulation. The mechanisms underlying this effect are unclear but may involve changes in baroreceptor reflex sensitivity, possibly secondary to decreased circulating ANG II following RDNX. This recurring phenomenon merits further investigation to uncover the mechanisms involved, as it may have important implications for the possible effects—both therapeutic and detrimental—of RDNX on the heart.
Although RDNX appears to be a promising treatment option for drug-resistant hypertension, the mechanisms underlying this therapeutic effect remain unclear. To further improve this treatment, it is important to determine whether the antihypertensive effect is due to ablation of afferent or efferent renal nerves. To this end, we have shown that the antihypertensive effect of RDNX in the DS rat is not due to ablation of afferent renal nerves. This is in contrast to our previous study in the DOCA-salt model in which renal-CAP blunted the development of hypertension. These results are critically important because they suggest that the mechanisms underlying the antihypertensive effect of RDNX will likely vary from patient to patient. Therefore, it may be ideal to develop selective ablation techniques, which could be used to target either subset of renal nerves to improve efficacy and reduce side effects. Furthermore, it may be possible to develop clinical tests to identify patients that would benefit from such selective ablations. Further animal studies will be critical in developing such tests.
Limitations
We recently validated the renal-CAP method as a novel technique to target afferent renal nerves while leaving efferent renal nerves intact (8). This was demonstrated by the observation that renal-CAP markedly reduces CGRP but has no effect on renal NE content. However, in the present study, we observed a modest reduction in renal NE content in the early-phase renal-CAP group, which raises the possibility of an off-target effect of this treatment. We feel this was not likely due to the capsaicin itself since sympathetic fibers lack receptors for capsaicin. However, it is possible that some efferent fibers were disrupted when the fat was dissected away from the renal vessels prior to renal-CAP treatment. Although there was a small decrease in renal NE content in the kidneys of these rats, the decrease appeared to be insufficient to have a physiological effect, as there was no effect on MAP or HR compared with Sham.
GRANTS
This research was supported by National Institutes of Health Grant HL-116476-01.
DISCLOSURES
J. W. Osborn was a paid consultant for Medtronic Cardiovascular, Inc., at the time this study was conducted. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.F. and J.W.O. conception and design of research; J.D.F. performed experiments; J.D.F. analyzed data; J.D.F., G.D.F., and J.W.O. interpreted results of experiments; J.D.F. prepared figures; J.D.F. drafted manuscript; J.D.F., G.D.F., and J.W.O. edited and revised manuscript; J.D.F., G.D.F., and J.W.O. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Dusty Van Helden, Jeff Larson, Eric Homan, Madeline Gauthier, and Marcos Kuroki for their help with metabolic data collection and analysis. We would also like to thank Robert Burnett for his work on the NE assay, as well as Ruijun Han and Xinying Yang for their work on the CGRP ELISA.
REFERENCES
- 1.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 63: 1791–1800, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension 60: 1485–1490, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 25: 878–882, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL, Joyal D. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol 62: 231–241, 2013. [DOI] [PubMed] [Google Scholar]
- 5.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Ezzahti M, Moelker A, Friesema EC, van der Linde NA, Krestin GP, van den Meiracker AH. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens 32: 135–141, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 61: 806–811, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308: R112–R122, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD Jr. Renal NF-κB activation and TNF-α upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 291: R1817–R1824, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61: 457–464, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol Heart Circ Physiol 284: H2302–H2310, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1529, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Jacob F, LaBine BG, Ariza P, Katz SA, Osborn JW. Renal denervation causes chronic hypotension in rats: Role of β1-adrenoceptor activity. Clin Exp Pharmacol Physiol 32: 255–262, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension 42: 968–973, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci 154: 66–73, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367: 1747–1757, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Boehm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 60: 419–424, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Bohm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 128: 132–140, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 417–449, 2009. [DOI] [PMC free article] [PubMed]
- 20.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 49: 69–75, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Poss J, Ewen S, Schmieder RE, Muhler S, Vonend O, Ott C, Linz D, Geisel J, Rump LC, Schlaich M, Bohm M, Mahfoud F. Effects of renal sympathetic denervation on urinary sodium excretion in patients with resistant hypertension. Clin Res Cardiol 104: 672–678, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Seifi B, Kadkhodaee M, Xu J, Soleimani M. Pro-inflammatory cytokines of rat vasculature in DOCA-salt treatment. Mol Biol Rep 37: 2111–2115, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg JS, Pokushalov E, Mittal S. Renal denervation for arrhythmias: hope or hype? Curr Cardiol Rep 15: 392, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 57: 911–917, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Veitenheimer B, Osborn JW. Role of spinal V1a receptors in regulation of arterial pressure during acute and chronic osmotic stress. Am J Physiol Regul Integr Comp Physiol 300: R460–R469, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang DH, Li J, Qiu J. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension 32: 649–653, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Lu CZ, Zhang X, Luo D, Zhao B, Yu X, Xia DS, Chen X, Zhao XD. [The effect of catheter based renal synthetic denervation on renin-angiotensin-aldosterone system in patients with resistant hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi 41: 3–7, 2013. [PubMed] [Google Scholar]
- 28.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, Michalowska I, Kabat M, Warchol E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 58: 559–565, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Wyss JM, Aboukarsh N, Oparil S. Sensory denervation of the kidney attenuates renovascular hypertension in the rat. Am J Physiol Heart Circ Physiol 250: H82–H86, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Zicha J, Dobesova Z, Vokurkova M, Rauchova H, Hojna S, Kadlecova M, Behuliak M, Vaneckova I, Kunes J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res 61 Suppl 1: S35–S87, 2012. [DOI] [PubMed] [Google Scholar]