Abstract
Perivascular adipose tissue (PVAT) mediates buffering of vasoconstriction through activation of endothelium-derived factors. We hypothesized that the PVAT of Dahl salt-sensitive (Dahl SS) rats has reduced ability to buffer vasoconstriction. Vascular reactivity experiments were performed on aortic rings with PVAT intact (+PVAT) or removed (−PVAT), and endothelium intact (+ENDO) or removed (−ENDO) from Dahl SS rats and control SS.13BN rats (Dahl SS rats that have had chromosome 13 completely replaced with that of the Brown Norway rat, rendering this strain insensitive to high-salt or high-fat diet-induced hypertension). Endothelial dysfunction, assessed by ACh-mediated vasorelaxation, was confirmed in aortic rings of Dahl SS rats. The +PVAT+ENDO aortic rings had indistinguishable phenylephrine-induced vasoconstriction between genotypes. In both strains, removal of PVAT significantly enhanced vasoconstriction. Dahl SS rat −PVAT+ENDO aortic rings displayed exaggerated vasoconstriction to phenylephrine vs. SS.13BN rats, indicating that PVAT-mediated buffering of vasoconstriction was greater in Dahl SS rats. Removal of both the ENDO and PVAT restored vasoconstriction in both strains. The nitric oxide synthase (NOS) inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME), produced a similar effect as that seen with −ENDO. These data indicate that the function of the PVAT to activate endothelium-derived NOS is enhanced in Dahl SS compared with SS.13BN rats and, most likely, occurs through a pathway that is distinct from ACh-mediated activation of NOS. PVAT weight and total PVAT leptin levels were greater in Dahl SS rats. Leptin induced a significantly decreased vasoconstriction in −PVAT+ENDO aortic rings from Dahl SS rats, but not SS.13BN rats. In contrast to our initial hypothesis, PVAT in Dahl SS rats buffers vasoconstriction by activating endothelial NOS via mechanisms that may include the involvement of leptin. Thus, the PVAT serves a vasoprotective role in Dahl SS rats on normal-salt diet.
Keywords: aorta, endothelium, nitric oxide synthase, perivascular adipose tissue, phenylephrine, salt-senstive
the blood vessel wall has the capability to regulate vascular smooth muscle tone. Notably, the endothelium, which is the single-cell lining that covers the lumen of all blood vessels, is known for its role in maintaining vascular tone homeostasis (17). This is evidenced by findings that endothelial dysfunction or removal of the endothelium exaggerates responsiveness of the vessel to numerous vasoconstrictor agents (18, 36). Furthermore, particularly in large arteries like the aorta, the endothelium buffers vascular smooth muscle reactivity mainly via the action of nitric oxide (NO) produced by endothelial NO synthase (NOS3, eNOS) (2, 38).
More recently, it has become apparent that the endothelium is not the only component in the vessel wall that is capable of regulating smooth muscle tone. The perivascular adipose tissue (PVAT), once thought to be functionally benign, has been shown to modulate vascular tone in an endothelium-derived NOS-dependent manner (6, 22). Studies from humans and animal models have demonstrated that isolated vascular rings with both the PVAT and the endothelium intact (+PVAT+ENDO) display blunted vasoconstriction in response to adrenergic agonists compared with vascular rings without the PVAT but with the endothelium still intact (−PVAT+ENDO) (15, 29). Additionally, studies using aortic rings from adult male Wistar rats demonstrated that the PVAT promotes vasorelaxation in an endothelium-dependent manner (22). Furthermore, treatment with the nonselective NOS inhibitor, NG-monomethyl-l-arginine (l-NMMA), completely abrogated the PVAT-mediated buffering of norepinephrine-induced vasoconstriction in aortas from Wistar rats (39). These findings indicate that the PVAT assists the endothelium in regulating aortic vascular tone by enhancing NOS function.
The knowledge that the PVAT functionally tempers vascular reactivity raises the question of whether it becomes dysfunctional alongside the endothelium in cardiovascular disease. One of the most well-established rodent models that has a genetic predisposition to vascular disease is the Dahl salt-sensitive (SS) rat strain (3, 44). Indeed, on a normal-salt diet regimen, these animals develop endothelial dysfunction as adults. We have shown that at ∼16 wk of age, Dahl SS rats maintained on a standard, normal-salt chow presented with significantly reduced endothelium-dependent vasorelaxation in isolated aortic rings compared with the genetic control strain, the SS.13BN rat (38). The SS.13BN is in essence the Dahl SS rat with the exception that chromosome 13 has been completely replaced with that of the Brown Norway rat, rendering this strain insensitive to high-salt or high-fat diet-induced hypertension (5, 8, 37). Therefore, we hypothesized that under normal-salt conditions that the PVAT from Dahl SS rats is dysfunctional in its ability to buffer vasoconstriction compared with SS.13BN rats. Experiments were designed to examine the endothelial and NOS dependence of PVAT-mediated buffering of vasoconstriction in aortic tissue of Dahl SS and SS.13BN rats. Furthermore, it is known that leptin derived from the PVAT activates NOS in the endothelium and aids in mediating PVAT-buffering capacity in aortic tissue (12, 17); therefore, we examined the PVAT weight and adipocyte area, levels of leptin in the PVAT, leptin receptor expression, and leptin sensitivity of vasoconstriction in aortic tissue from both rat strains.
MATERIALS AND METHODS
Animals.
All animal experiments were conducted in accordance with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals” with all animal use protocols approved by the Georgia Regents University and the University of Alabama at Birmingham Institutional Animal Care and Use Committee. SS.13BN rats were purchased from Charles River Laboratories (Kingston, NY). Dahl SS rats were obtained from Harlan Laboratories (Indianapolis, IN) or bred in-house from purchased Dahl SS breeders (Charles River Laboratories, Wilmington, MA). The genetic background of the Dahl SS breeders was confirmed by a genome-wide scan described by Moreno et al. (31). For this purpose, rat tail DNA was extracted, and the presence of microsatellite markers specific against the Dahl SS genome was assessed with PCR using simple sequence-length polymorphism primers. Rats were maintained on Harlan Teklad 8,604 or 8,640 grain-based diet having 0.3–0.4% Na (Teklad, Madison, WI), and water was provided ad libitum. Male rats were used for all experiments.
Animals were age-matched, and at 16–21 wk old, body weight and epididymal fat mass was measured, and rats were euthanized under pentobarbital sodium (Nembutal; 0.5 mg/kg; Abbott Laboratories, Abbott Park, IL) anesthesia. Adiposity index was calculated by dividing epididymal fat mass by body weight, then multiplying by 100, and presented as a percentage. The thoracic aorta and accompanying PVAT were isolated and weighed separately. PVAT weight was normalized to aortic weight and expressed as PVAT:aorta weight. Tissues were snap-frozen in liquid N2.
Mean arterial blood pressure.
The day before vascular reactivity experiments, rats were placed under isoflurane anesthesia (Butler Schein Animal Health, Dublin, OH) for an average of 20 min to implant indwelling catheters in the left carotid artery, which were then exposed at the nape of the neck. Catheters consisted of V/1 tubing attached to V/3 tubing (Scientific Commodities, Lake Havasu City, AZ). Approximately 2.5 cm of the V/3 end of the catheter was inserted into the carotid. Catheters were filled with sterile heparin-saline solution (300 mg/ml; Pfizer, New York City, NY) and stoppered with a metal plug. Rats were placed in restraint cages, and catheters were connected to pressure transducers (MLT0699; ADInstruments, Colorado Springs, CO) coupled to a computerized data acquisition system (PowerLab, ADInstruments). Animals were allowed to acclimate to restraint for at least 1 h. Once hemodynamic readings stabilized, mean arterial blood pressure data were collected; n = 3 rats/group.
Vascular reactivity studies.
Depending on the experimental question, PVAT was dissected away from the vessel and/or vascular endothelium was removed by gently rubbing the vessel lumen with curved forceps. Aortic rings (∼3 mm) were mounted on pins for isometric wire myography (Danish Myo Technology A/S, Aarhus, Denmark) in physiological saline solution (PSS; in mM): 130 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 14.9 NaHCO3, 5.6 dextrose, 0.024 EDTA tetrasodium salt dehydrate, and 1.6 CaCl2 (Sigma, St. Louis, MO), as previously described (20). The baseline force was set to 28 mN, and all aortic rings were within ∼5% of each other prior to confirming viability of vascular segments by preconstricting with 10−6 M phenylephrine (PE) followed by relaxation using 10−4 M of ACh. Only those vessels that relaxed >80% to ACh were considered to have a sufficiently functional endothelium to proceed with generating concentration-response curves. A lack of ACh-dependent vasorelaxation was confirmed in all of the endothelium-denuded vessels. Aortic rings were incubated for 15 min in the presence or absence of the nonselective nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 10−4 M; Sigma), or in the presence or absence of rat leptin (20 ng/ml; Sigma) or indomethacin (10−5 M; Sigma) for 20 min. Following the incubation period, a cumulative-concentration response curve was generated to PE (1 × 10−9 to 3 × 10−4.5 M; Sigma). Vessel baths were then washed with PSS followed by assessment of constriction curves to KCl-induced depolarization. In a separate group of constriction studies, +PVAT+ENDO or −PVAT+ENDO aortic rings were submaximally preconstricted with 10−7 M PE followed by the addition of cumulative concentrations of l-NAME (1 × 10−12 to 1 × 10−3 M) to assess the effect of PVAT on endogenous NO bioavailability. Constriction data are presented as % increase in force as calculated using the equation [(response to vasoconstrictor − baseline prior to constriction)/baseline prior to constriction] × 100. N values are given in the results section and figure legends.
Additional experiments were performed to assess endothelial-dependent vasorelaxation to ACh (ACh; 1 × 10−9 to 3 × 10−4.5 M; Sigma) in aortic rings constricted with 10−6 M PE. These same aortic rings were washed with PSS, incubated with 10−4 M l-NAME for 15 min, and then constricted with 10−6 M PE for the purpose of conducting endothelium-independent vasorelaxation curves generated with the NO-donor sodium nitroprusside (SNP; 1 × 10−10 to 3 × 10−4.5 M; Sigma). These data are presented as maximum relaxation (Emax) as a percentage of PE and sensitivity as logEC50. N values are given in the results section and figure legends.
Aortic histology.
Paraffin-embedded aortas with adherent PVAT were cross-sectioned into 4-μm-thick sections and mounted on Superfrost slides. Adipocytes were stained with Gomori's blue trichrome and visualized using brightfield microscopy (Olympus BX40; Olympus America, Melville, NY). Photographs were obtained with a digital camera (Olympus DP12; Olympus America). In an experimenter-blinded fashion, the area (μm2) of individual adipocytes (≥36 adipocytes per animal) was determined for each animal. The average adipocyte area was determined for each rat by calculating the mean of the areas of all individual adipocytes counted. Adipocyte area was determined using Metamorph software (Molecular Devices, Sunnyvale, CA).
Tissue homogenization.
Thoracic aortas were isolated from anesthetized animals, and PVAT was dissected away from the vessels. Tissues were snap-frozen in liquid nitrogen and stored at −80°C until assays were performed. Using a hand-held motorized pestle, tissues were homogenized in lysis buffer [50 mM Tris (pH 7.4), 250 mM sucrose, 0.1 mM EDTA, 0.1 mM EGTA, 10% glycerol, 0.1% SDS, 0.5% Triton X-100, 0.5% sodium deoxycholate, 0.1% BME, 0.001 mg/ml phenylmethanesulfonyl fluoride (PMSF), and 0.01 mg/ml each of leupeptin, pepstatin, and aprotinin] at a ratio of 100 mg tissue/ml buffer. The samples were then snap-frozen, thawed, and sonicated for 10 × 1 s bursts on ice. Additional PMSF was added to the homogenate prior to incubation on a rocker at 4°C for 30 min. After centrifugation at 17,000 g at 4°C for 20 min, supernatant was collected and stored at −80°C for enzyme immunoassay (EIA) or Western blot analysis.
Leptin peptide and receptor level determinations.
Quantification of leptin peptide levels in PVAT was determined by EIA (kit no. 1007609; Cayman Chemicals, Ann Arbor, MI). PVAT samples (n = 6 per group) were prepared as described above and diluted 1:10 prior to performing the assay. Absorbance was measured using an Epoch colorimetric plate reader (Bio-Tek Instruments, Winooski, VT), and protein concentrations were calculated using Gen 5 Data Analysis Software (version 2.04, Bio-Tek Instruments,). Total leptin levels in the PVAT were calculated by normalizing leptin levels to milligrams of total PVAT protein and then multiplied by relative PVAT:aorta weight.
Leptin receptor density of thoracic aortas was assayed by Western blot analysis. Samples were run on 8% SDS-polyacrylamide gels, transferred to polyvinylidene fluoride membranes (Immobilon-FL) and blotted using antibodies against the long and short leptin receptor (Ob-R) (no. 3583 rabbit polyclonal; Abcam, Cambridge, MA), and GAPDH (no. 47724 mouse monoclonal; Santa Cruz Biotechnology, Dallas, TX). The following secondary antibodies were used: goat anti-mouse (1 μg/ml) for monoclonal antibodies, and goat anti-rabbit (1 μg/ml) for polyclonal antibodies (Rockland Immunochemicals, Gilbertsville, PA). Membranes were imaged using the Odyssey CLx Infared Imaging System (Li-Cor Imaging Systems, Lincoln, NE). Band intensities were quantified using Image Studio Software (Li-Cor Imaging Systems); n = 6 rats/group.
Statistical analysis.
All data are expressed as means ± SE. Data were graphed and statistically analyzed using a t-test or two-way ANOVA with Bonferroni post hoc tests using GraphPad Prism software (La Jolla, CA), where appropriate. To examine total response to vasoconstrictive agonists in aortic ring preparations, area under the curve (AUC) was calculated using GraphPad Prism. Statistical significance was defined as P < 0.05. The critical value of P was two-tailed.
RESULTS
Body weight, adiposity index, and blood pressure.
Body weight was significantly less in the Dahl SS (n = 8) vs. SS.13BN rats (n = 8) (380 ± 8 g vs. 465 ± 5 g, P = 0.04), whereas epididymal fat mass was similar between rat strains (4.1 ± 0.3 g vs. 4.0 ± 0.2 g, respectively). There was no significant difference in the adiposity index in Dahl SS vs. SS.13BN rats (1.05 ± 0.06% vs. 0.88 ± 0.07%, P = 0.09). Mean arterial blood pressure was similar between SS.13BN and Dahl SS rats (142 ± 5 mmHg vs. 140 ± 8 mmHg, respectively).
PVAT-mediated buffering of vasoconstriction in SS.13BN and Dahl SS rat aortas.
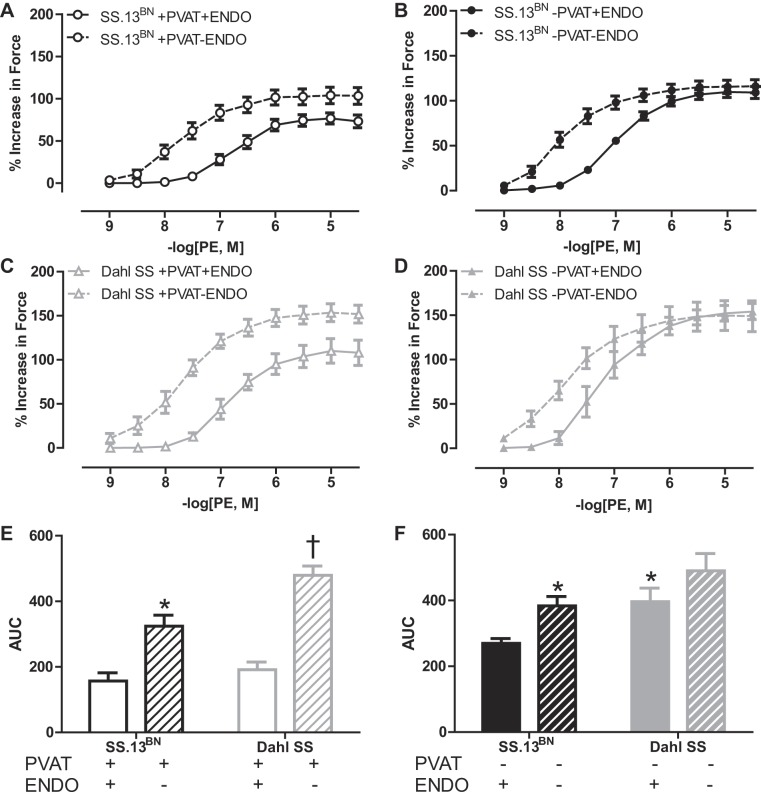
Aortic rings with an intact PVAT and endothelium (+PVAT+ENDO) had significantly reduced maximal (Emax) and sensitivity (logEC50) to PE-induced vasoconstriction in both SS.13BN (Fig. 1A, Tables 1 and 3) and Dahl SS rats (Fig. 1B, Tables 1 and 3) compared with aortic rings with the PVAT removed (−PVAT+ENDO). Interestingly, this PVAT-mediated buffering was more pronounced in the Dahl SS rats when assessed using area under the curve (AUC) analysis (Fig. 1C, Table 2). As a result, although aorta rings of −PVAT+ENDO constricted more in the Dahl SS rat group (Fig. 1C, Tables 1–3), the presence of the PVAT resulted in there being similar vasoconstriction between SS.13BN and Dahl SS rat strains (Fig. 1C, Tables 1–3).
Fig. 1.
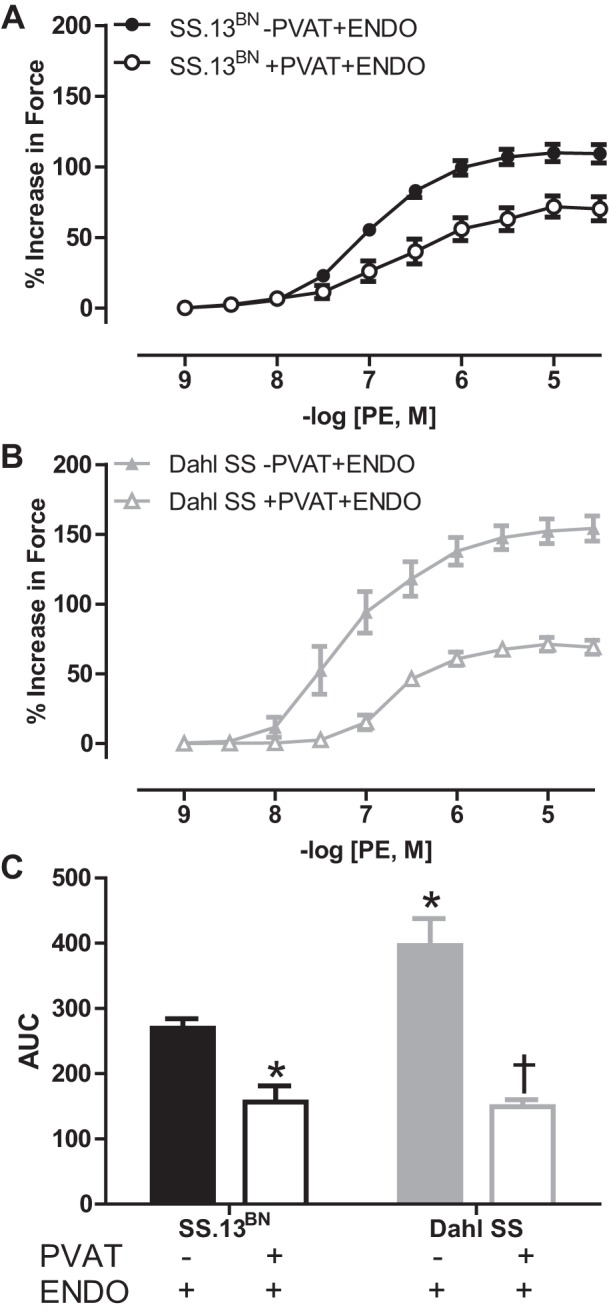
Phenylephrine (PE)-induced vasoconstriction curves in aortic rings with the perivascular adipose tissue (PVAT) removed (−PVAT) or intact (+PVAT) and the endothelium intact (+ENDO) from SS.13BN rats (n = 8–10) (A) and Dahl SS rats (n = 5–9) (B). C: area under the curve (AUC) analysis to examine total vascular response to vasoconstriction in aortic ring preparations. Number of multiple comparisons = 2. *P < 0.05 vs. SS.13BN aortic rings–PVAT+ENDO; †P < 0.05 vs. Dahl SS aortic rings–PVAT+ENDO.
Table 1.
Maximum vasoconstriction response (Emax) to phenylephrine in aortic preparations from SS.13BN and Dahl SS rats with or without the perivascular adipose tissue or the endothelium in the presence or absence of NOS inhibition with l-NAME
| Aortic Preparation | SS.13BN | Dahl SS |
|---|---|---|
| Emax; % Increase in Force Response to Phenylephrine | ||
| −PVAT+ENDO | 109.2 ± 6.6 | 154.2 ± 9.0* |
| +PVAT+ENDO | 70.6 ± 8.5† | 69.0 ± 5.0† |
| +PVAT-ENDO | 103.3 ± 10.0§ | 151.8 ± 10.1*§ |
| −PVAT-ENDO | 116.1 ± 7.4 | 148.9 ± 17.3 |
| +PVAT+ENDO+l-NAME | 97.9 ± 10.8§ | 174.8 ± 14.9*§ |
| −PVAT+ENDO+l-NAME | 121.2 ± 12.3 | 139.6 ± 10.4 |
Values are expressed as means ± SE. PVAT, perivascular adipose tissue; ENDO, endothelium; NOS, nitric oxide synthase; l-NAME, Nω-Nitro-[SCAP]L[R]-arginine methyl ester.
P < 0.05 vs. respective SS.13BN aortic preparation;
P < 0.05 vs. −PVAT+ENDO within same rat strain;
P < 0.05 vs. +PVAT+ENDO within same rat strain.
For SS.13BN rats, n = 8–10. For Dahl SS rats, n = 5–9.
Table 3.
Vascular sensitivity reported as logEC50 to phenylephrine in aortic preparations from SS.13BN rats and Dahl SS rats with or without PVAT or ENDO in the presence or absence of NOS inhibition with l-NAME
| Aortic Preparation | SS-13BN | Dahl SS |
|---|---|---|
| LogEC50 (M) Determined From the % Increase in Force to Phenylephrine | ||
| −PVAT+ENDO | −7.06 ± 0.08 | −7.55 ± 0.19* |
| +PVAT+ENDO | −5.97 ± 0.30† | −6.09 ± 0.14† |
| +PVAT-ENDO | −7.62 ± 0.21§ | −8.21 ± 0.17*§ |
| −PVAT-ENDO | −8.01 ± 0.16† | −8.31 ± 0.14*† |
| +PVAT+ENDO+l-NAME | −7.13 ± 0.19§ | −7.79±.025§ |
| −PVAT+ENDO+l-NAME | −7.83 ± 0.13† | −7.79 ± 0.09 |
Values are expressed as means ± SE.
P < 0.05 vs. respective SS.13BN aortic preparation;
P < 0.05 vs. −PVAT+ENDO within same rat strain;
P < 0.05 vs. +PVAT+ENDO within same rat strain.
For SS.13BN rats, n = 8–10. For Dahl SS rats, n = 5–9.
Table 2.
Area under the curve analysis in response to phenylephrine-induced vasoconstriction in aortic preparations from SS.13BN rats and Dahl SS rats with or without PVAT or the ENDO and in the presence or absence of NOS inhibition with l-NAME
| Aortic Preparation | SS.13BN | Dahl SS |
|---|---|---|
| AUC; % Increase in Force, Response to Phenylephrine | ||
| −PVAT+ENDO | 269.9 ± 14.4 | 396.3 ± 41.2* |
| +PVAT+ENDO | 156.6 ± 25.1† | 149.2 ± 11.2† |
| +PVAT-ENDO | 324.3 ± 33.3§ | 479.5 ± 28.2*§ |
| −PVAT-ENDO | 383.7 ± 28.7† | 490.1 ± 52.3* |
| +PVAT+ENDO+l-NAME | 273.9 ± 30.3§ | 481.4 ± 69.3*§ |
| −PVAT+ENDO+l-NAME | 376.1 ± 34.8† | 469.1 ± 49.1 |
Values are expressed as means ± SE.
AUC, area under the curve.
P < 0.05 vs. SS.13BN;
P < 0.05 vs. −PVAT+ENDO within same rat strain;
P < 0.05 vs. +PVAT+ENDO within same rat strain. For SS.13BN rats, n = 8–10. For Dahl SS rats, n = 5–9.
The maximal KCl-induced constriction (Fig. 2) was similarly reduced in +PVAT+ENDO compared with −PVAT+ENDO aortic rings in both SS.13BN rats (Emax, % increase in force: 94.8 ± 9.0 vs. 130.4 ± 11.5, P = 0.03) and Dahl SS rats (Emax, % increase in force: 96.9 ± 4.6 vs. 130.2 ± 11.1, P = 0.02). The AUC was significantly reduced in +PVAT+ENDO compared with −PVAT+ENDO aortic rings in Dahl SS rats (6,426.0 ± 353.9 vs. 10,019.4 ± 1,210.2, P < 0.05) but not SS.13BN rats (5,109.6 ± 471.2 vs. 6,785.4 ± 484.0). Statistical analysis of AUC by two-way ANOVA indicated that there was an effect of PVAT to blunt vasoconstriction in both rat strains with the post hoc test showing only a significant difference within the Dahl SS strain. KCl-induced constriction (Emax and AUC) from −PVAT+ENDO aortic rings was similar in both strains.
Fig. 2.
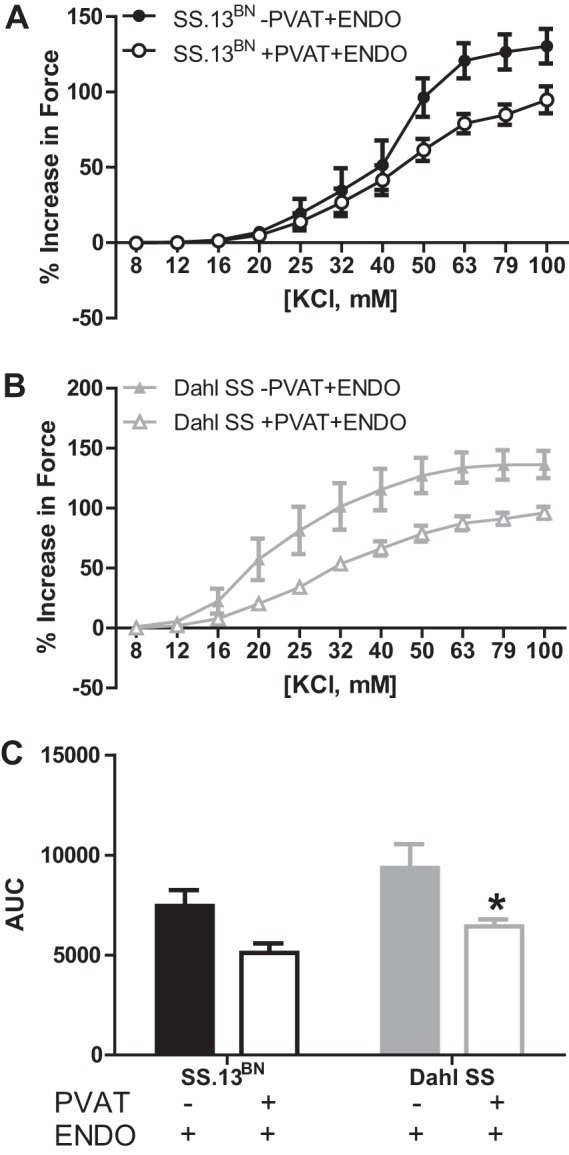
KCl-induced vasoconstriction curves in aortic rings with the PVAT removed (−PVAT) or intact (+PVAT) and the endothelium intact (+ENDO) from SS.13BN rats (n = 8) (A) and Dahl SS rats (n = 6) (B). C: area under the curve (AUC) analysis to examine total vascular constriction in aortic ring preparations. Number of multiple comparisons = 2. KCl-induced constriction (Emax and AUC) from −PVAT+ENDO aortic rings was similar in both strains. The two-way ANOVA indicated there was an effect of PVAT to blunt vasoconstriction in both rat strains with the post hoc test showing a significant difference only within the Dahl SS strain.*P < 0.05 vs. Dahl SS aortic rings −PVAT+ENDO.
Endothelium dependency of PVAT-mediated buffering of vasoconstriction in SS.13BN and Dahl SS rat aortas.
Removing the endothelium (−ENDO) enhanced the Emax, AUC, and logEC50 of vasoconstriction to PE in aortic rings +PVAT from SS.13BN (Fig. 3A, Tables 1–3) and Dahl SS rats (Fig. 3C, Tables 1–3), although this increase was greater in the Dahl SS rat strain (Fig. 3E, Tables 1–3). In aortic rings without PVAT (−PVAT), −ENDO did not alter Emax in either rat strain (Fig. 3, B and D, Table 1), whereas denudation significantly increased the AUC only in the SS.13BN rats (Fig. 3F, Table 2). These data show that the PVAT-mediated buffering of PE-induced vasoconstriction in aortic rings from both SS.13BN and Dahl SS rats is dependent on the endothelium and that this endothelium dependency was greater in the Dahl SS rats. It is noted that experiments in Figs. 1 and 3 were conducted at different times, resulting in a quantitatively different value of maximum constriction in the +PVAT+ENDO aortic rings of Dahl SS rats; however, all experiments were conducted in a paired manner of both rat strains with or without PVAT and/or ENDO. Qualitative findings were consistent among all experiments.
Fig. 3.
Phenylephrine (PE)-induced vasoconstriction curves in aortic rings with the PVAT removed (−PVAT) or intact (+PVAT) and the endothelium removed (−ENDO) or intact (+ENDO) in SS.13BN rats (n = 8–10) (A, B) and Dahl SS rats (n = 5–9) (C, D). AUC examining the effect of endothelial denudation on the vasoconstriction in aortic rings +PVAT (E) and −PVAT (F) in SS.13BN and Dahl SS rats. Number of multiple comparisons = 2. *P < 0.05 vs. SS.13BN aortic ring controls; †P < 0.05 vs. Dahl SS aortic ring controls.
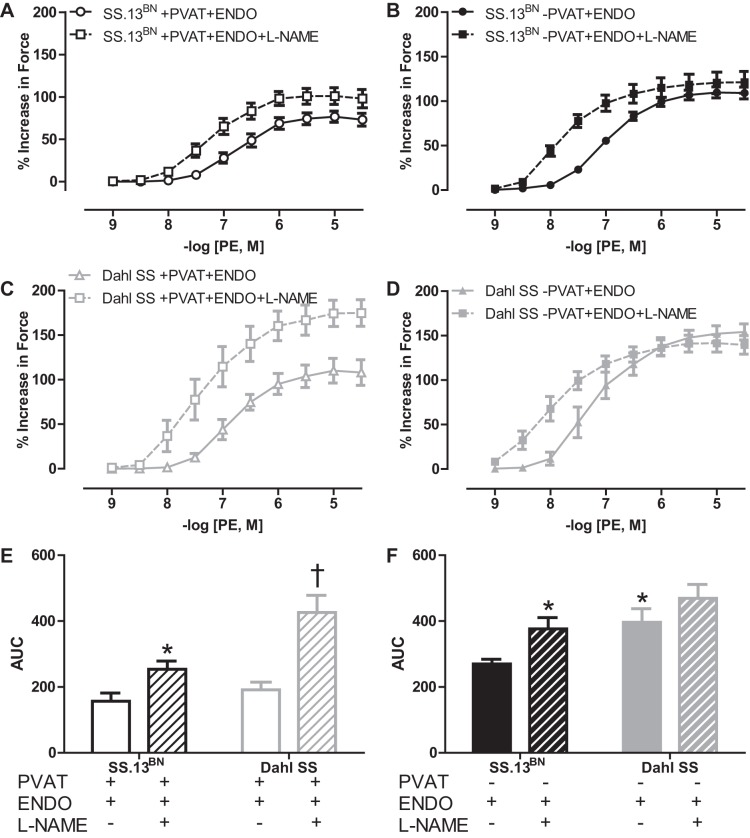
NOS dependency of PVAT-mediated buffering of vasoconstriction in SS.13BN and Dahl SS rat aortas.
To examine the mechanisms whereby the endothelium mediates the PVAT-mediated buffering of PE-induced vasoconstriction in aortas and the greater dependency of this effect in Dahl SS rats, vascular reactivity experiments were conducted in aortic rings +PVAT+ENDO in the presence or absence of the nonselective NOS inhibitor l-NAME. l-NAME enhanced the PE vasoconstriction (Emax, AUC, and logEC50) in aortic rings +PVAT+ENDO from SS.13BN (Fig. 4A, Tables 1–3) with this increase being even greater in the Dahl SS rats (Fig. 4, C and E, Tables 1–3). Thus, there was not a difference in the effect of l-NAME to augment PE-induced vasoconstriction in aortic rings having either +PVAT+ENDO or −PVAT+ENDO in SS.13BN rats, but this effect was more pronounced in Dahl SS rats. In aortic rings −PVAT+ENDO, l-NAME induced an increase in AUC and logEC50 but not Emax in the SS.13BN rats only (Fig. 4, B and D, Tables 1–3).
Fig. 4.
Phenylephrine (PE)-induced vasoconstriction curves in aortic rings with the PVAT removed (−PVAT) or intact (+PVAT) and in the presence or absence of the nonselective NOS inhibitor l-NAME in SS.13BN rats (n = 8–10) (A, B) and Dahl SS rats (n = 5–9) (C, D). AUC examining the effect of NOS inhibition on the vasoconstriction in aortic rings +PVAT (E) and −PVAT (F) without (−) or with (+) corresponding treatments. Number of multiple comparisons = 2. *P < 0.05 vs. SS.13BN aortic ring controls; †P < 0.05 vs. Dahl SS aortic ring controls.
To examine whether the PVAT affected the endogenous ability of NOS to control vascular tone, aortic rings were preconstricted with a submaximal dose of PE followed by generation of constriction curves to l-NAME. It was found that the Emax response to NOS inhibition was similar between −PVAT+ENDO and +PVAT+ENDO aortic preparations, respectively, from both SS.13BN rats (34.9 ± 3.3%, n = 8 vs. 31.8 ± 2.6%, n = 7) and Dahl SS rats (29.5 ± 4.2%, n = 6 vs. 23.6 ± 4.1%, n = 6). Also, the sensitivity to l-NAME (logEC50) was similar in −PVAT+ENDO and +PVAT+ENDO aortic rings, respectively, within SS.13BN (−2.9 ± 0.1 M vs. −2.8 ± 0.1 M) and Dahl SS (−2.6 ± 0.2 M vs. −2.6 ± 0.1 M) rat strains. However, the endogenous ability of NOS to regulate vascular tone was significantly less in Dahl SS compared with the SS.13BN rat genotype (P < 0.05).
To determine whether PVAT may also lead to the release of NOS-independent vasodilator prostanoids, PE constriction curves were generated in aortic rings +PVAT+ENDO in the absence or presence of indomethacin, respectively. Emax was similar in aortic rings from SS.13BN rats (127.9 ± 11.0%, n = 3 vs. 112.8 ± 14.7%, n = 5) and Dahl SS rats (120.2 ± 3.7%, n = 3 vs. 114.7 ± 11.3%, n = 5), as well as sensitivity to indomethacin treatment among aortic rings from SS.13BN rats (logEC50, −7.3 ± 0.1 M vs. −7.1 ± 0.1 M) or Dahl SS rats (logEC50, −7.2 ± 0.3 M vs. −7.6 ± 0.2 M).
Effect of PVAT on endothelial-dependent vasorelaxation in SS.13BN and Dahl SS rat aortas.
We assessed whether the action of PVAT in buffering vasoconstriction was also accompanied by augmentation of endothelium-dependent relaxation. ACh-induced endothelial function was similar in the presence and absence of PVAT in aortic rings +ENDO from SS.13BN rats (Emax, 84.6 ± 3.9% vs. 81.3 ± 3.1%; logEC50, −6.5 ± 0.2 M vs. −6.6 ± 0.1 M, respectively) and Dahl SS rats (Emax, 75.6 ± 2.2% vs. 75.4 ± 3.0%; logEC50, −6.1 ± 0.1 M vs. −6.3 ± 0.1 M, respectively). Statistical analysis by two-way ANOVA indicated that Dahl SS rat strain as a whole showed reduced maximum relaxation and sensitivity to ACh (P < 0.05). Endothelial-independent vasorelaxation induced by SNP was unaltered in the presence and absence of PVAT in SS.13BN rats (Emax, 98.5 ± 2.5% vs. 97.4 ± 0.6%; logEC50, M: −7.6 ± 0.1 M vs. −7.7 ± 0.1 M), as well as Dahl SS rats (Emax, 106.2 ± 3.3% vs. 98.9 ± 0.7%; logEC50, −7.7 ± 0.2 M vs. −7.7 ± 0.2 M, respectively), and these responses were not different between the rat strains.
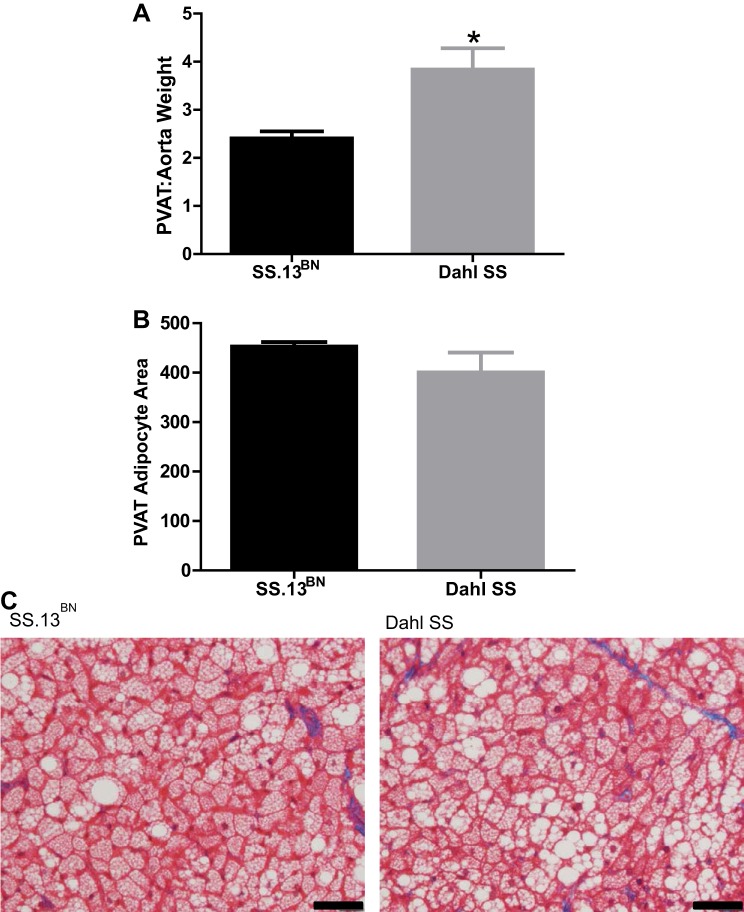
Aortic PVAT weight and adipocyte size in SS.13BN and Dahl SS rats.
PVAT weight was significantly greater in the Dahl SS rats compared with the SS.13BN control rat strain (Fig. 5A); however, there was no significant difference in adipocyte size between SS.13BN or Dahl SS rats as quantified in Fig. 5B and depicted in the representative histology images in Fig. 5C.
Fig. 5.
A: perivascular adipose tissue (PVAT) weight normalized to aorta weight (PVAT:aorta weight) in SS.13BN rats (n = 9) and Dahl SS rats (n = 5). B: quantification of PVAT adipocyte area. C: representative histological images of PVAT adipocytes in SS.13BN rats and Dahl SS rats. *P < 0.05 vs. SS.13BN rats.
Leptin levels in PVAT and vascular leptin receptor expression in SS.13BN and Dahl SS rat aortas.
Leptin levels when normalized to total PVAT protein were not different in the PVAT between rat strains (SS.13BN: 1,714 ± 224 pg/mg total protein vs. Dahl SS: 1,343 ± 163 pg/mg total protein, P > 0.05). However, when the greater PVAT weight in the Dahl SS rat group was taken into consideration, total tissue leptin levels were found to be elevated in the Dahl SS compared with SS.13BN rats (Fig. 6A). Whole thoracic aorta (−PVAT+ENDO) levels of the long and short forms of the leptin receptor (Ob-R) were similar between rat strains (Fig. 6B), as pictured in the representative Western blot in Fig. 6C.
Fig. 6.
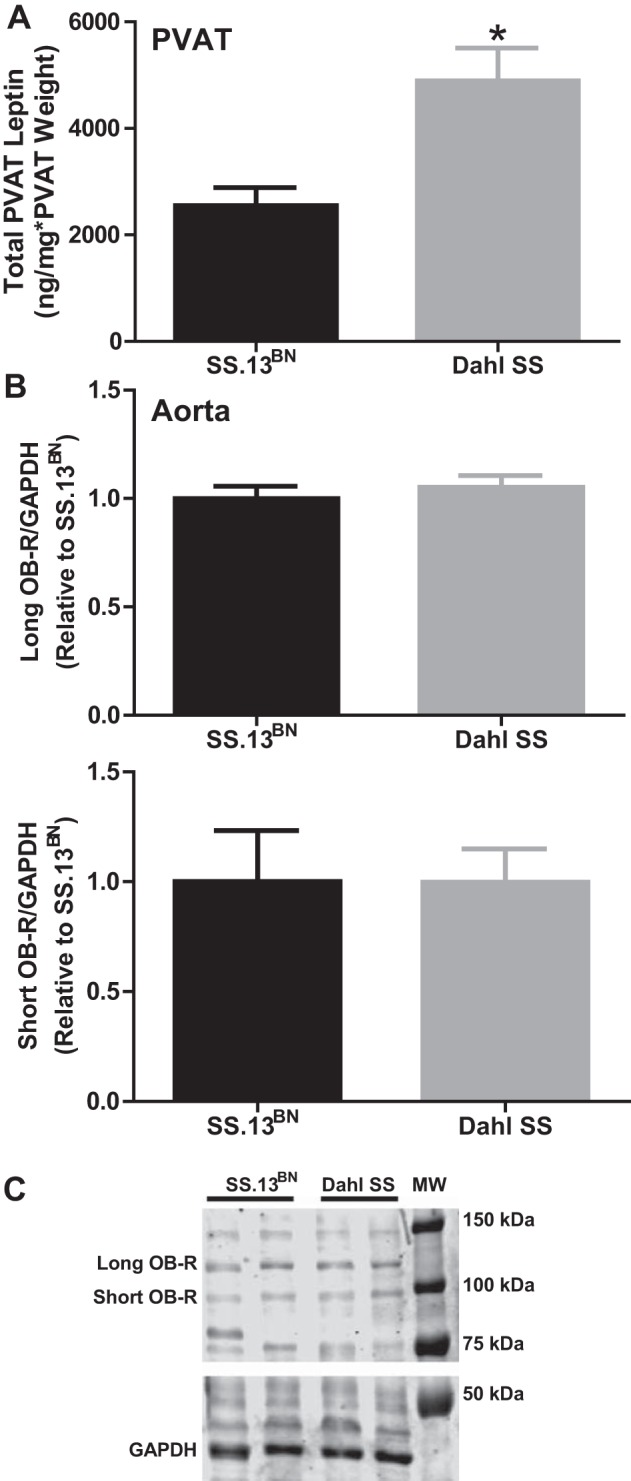
A: total leptin peptide levels in the PVAT from SS.13BN rats and Dahl SS rats. These levels were calculated by normalizing leptin levels to milligrams of total PVAT protein and then multiplied by relative PVAT:aorta weight. B: semiquantification of results from Western blot analyses examining expression of the long Ob-R (top) and short Ob-R (bottom) forms of the leptin receptor in aortic vascular tissue. C: representative Western blot. n = 6 rats/group. *P < 0.05 vs. SS.13BN rats.
Effects of leptin on PE-induced constriction in SS.13BN and Dahl SS −PVAT+ENDO rat aortic rings.
On the basis of the above findings, we further determined whether −PVAT+ENDO aortic rings from Dahl SS rats compared with those of SS.13BN rats would have greater leptin-mediated buffering of PE-induced vasoconstriction. Indeed, Fig. 7 shows that leptin preincubation significantly blunted PE-induced vasoconstriction only in aortic rings from Dahl SS rats (Emax, Dahl SS rats: 138.8 ± 7.9% vs. 122.6 ± 5.1%, P < 0.05; SS.13BN: 143.5 ± 3.5% vs. 135.1 ± 12.6%). The logEC50 responses were similar between untreated and treated aortic rings, respectively, in Dahl SS rats (−7.6 ± 0.1 M vs. −7.6 ± 0.04 M) and SS.13BN rats (−7.6 ± 0.06 M vs. −7.6 ± 0.1 M). The AUC was similar between untreated and treated aortic rings, respectively, in Dahl SS rats (369.6 ± 28.6 vs. 338.9 ± 12.2) and SS.13BN rats (373.5 ± 12.8 vs. 364.3 ± 36.2).
Fig. 7.
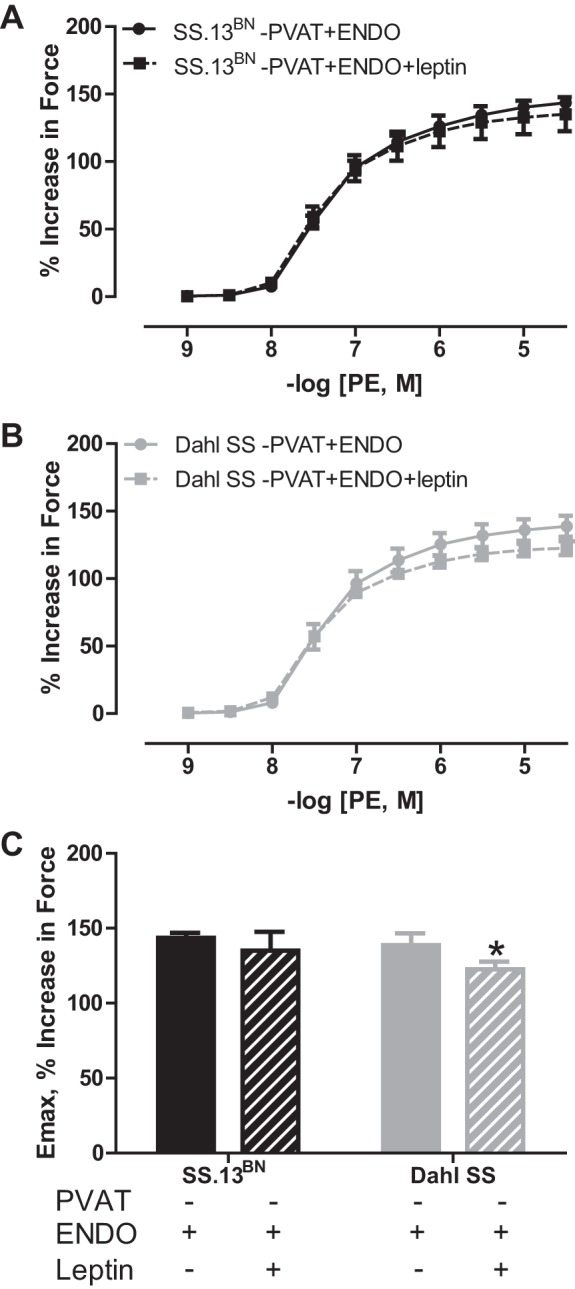
PE-induced vasoconstriction curves in aortic rings with the PVAT removed (−PVAT) and the endothelium intact (+ENDO) from SS.13BN rats (A) and Dahl SS rats (B) incubated in the presence or absence of leptin at 20 ng/ml for 20 min. C: maximum constriction (Emax) to −log [4.5, M] of PE. n = 5 rats/group. Number of multiple comparisons = 2. *P < 0.05 vs. Dahl SS aortic ring without leptin.
DISCUSSION
The main finding of our study is that, in contrast to our hypothesis, the PVAT from Dahl SS rats is functionally capable of blunting the response to adrenergic receptor-induced vasoconstriction in aortic tissue. Moreover, the PVAT-buffering effect exceeded that observed in the SS.13BN rat strain. These data provide evidence that PVAT function is present and enhanced in Dahl SS rats on standard chow diet. As a result, although aortic rings without the PVAT but with an intact endothelium constricted more in response to phenylephrine in the Dahl SS rats than in the SS.13BN rats, when the PVAT remained intact, there was an equalization of the vasoconstriction between the two rat strains. Mechanistically, in both rat strains, the PVAT function was entirely dependent on the endothelium. In both rat strains having both an intact PVAT and endothelium, treatment of the vascular ring segments with the nonselective NOS inhibitor produced a similar response as with endothelial denudation alone. In aortic rings without PVAT, endothelial denudation or l-NAME treatment, significantly increased vasoconstriction only in the SS.13BN rat strain. These data provide compelling evidence that PVAT-mediated buffering of vasoconstriction is enhanced in Dahl SS rats, where it works to control vascular tone in a manner that is dependent on signaling to NOS in the endothelium.
In the present study, we compared the PVAT-mediated buffering of vasoconstriction in Dahl SS rats vs. their genetic control counterpart, the SS.13BN rat. We initially hypothesized that Dahl SS rats may exhibit PVAT dysfunction, that is, a reduced effect of the PVAT to buffer vasoconstriction. The Dahl SS rat strain is well known for its predisposition to develop cardiovascular disease as adults with endothelial dysfunction (3). This occurs in adults, even though they are maintained on a normal-salt chow. Indeed, we previously showed that endothelial function, as assessed by ACh-induced vasorelaxation, was significantly attenuated in Dahl SS rats vs. SS.13BN rats that were maintained under standard chow diet conditions (38). In the present study and contrary to our hypothesis, we found that the PVAT blunted phenylephrine-induced vasoconstriction in aortic ring preparations from both rat strains compared with their respective aortic rings with the PVAT removed. Interestingly, this response was observed to be greater in the Dahl SS rat strain. As a result, in vascular preparations having both an intact PVAT and an endothelium, the adrenergic vasoconstriction was similar between both rat strains.
Intriguingly, work from Allyn Mark's group demonstrated in situ experiments that there is similar aortic distensibility, as assessed by constructing pressure-volume curves, between Dahl SS rats and their salt-resistant counterparts while on a lower-salt (0.13% NaCl) diet (16). Vascular distensibility allows large arteries like the aorta to accommodate pulsatile cardiac output. With the aorta having native PVAT in vivo, we speculate that the PVAT is an important regulator of aortic response to factors that increase tension of the blood vessel wall.
Studies in Sprague-Dawley (10, 11, 23, 24, 26) and Wistar-Kyoto rats (13–15, 24, 42) have demonstrated that the PVAT attenuates vasoconstriction by stimulating NO release from the endothelium (15). However, this has not been examined in Dahl SS rats. We found that the NOS inhibitor l-NAME significantly enhanced PE-induced vasoconstriction in aortic rings that have both the endothelium and PVAT intact. This occurred not only in the control SS.13BN rat strain, but also in Dahl SS rats on the normal-chow regimen. NOS inhibition produced a similar effect to that observed with endothelial denudation. Therefore, our data confirm that PVAT blunts vasoconstriction in aortic tissue in the SS.13BN rat similar to other control strains via activation of NOS in the endothelium. Our findings also show that the Dahl SS rat uses a similar mechanism in that the PVAT activates NOS signaling in the endothelium to mediate enhanced buffering capacity. In contrast, we found that the presence of the PVAT did not alter endothelium-dependent vasorelaxation to ACh, endothelium-independent vasorelaxation to sodium nitroprusside, nor did its presence impact l-NAME-induced vasoconstriction in aortic rings from either rat strain. Thus, we propose that the PVAT-mediated stimulation of NOS in the endothelium is, most likely, through a pathway that is distinct from the ACh-induced activation of endothelial NOS or any alteration of basal endogenous NO bioavailability.
Verlohren et al. (40) found that longitudinal removal of 50% of the PVAT attenuates the PVAT-mediated buffering of vasoconstriction by approximately half in mesenteric arteries. These data suggest that the extent to which the PVAT is able to buffer vasoconstriction is dependent on its mass. Their study has particular relevance to our study, since we found that Dahl SS rats had a greater amount of PVAT weight and PVAT buffering of vasoconstriction than control rats. Furthermore, we found that the total levels of leptin in the PVAT surrounding the thoracic aortas, which was calculated by multiplying leptin per gram of total protein by the PVAT weight, were greater in the Dahl SS vs. SS.13BN rats. However, when leptin levels were quantified on the basis of grams of total protein, there was no difference in leptin levels in the PVAT. As leptin production from adipocytes is based on size, it follows that we observed no difference between groups in PVAT adipocyte size but found increased PVAT weight. In functional experiments, we found that incubation with rat leptin blunted PE-induced vasoconstriction in aortic rings −PVAT+ENDO from Dahl SS but not SS.13BN rats. The leptin concentration of 20 ng/ml chosen for use in our study was based on existing literature reporting a 50–100% maximum relaxation in ex vivo vascular preparations of aorta and mesenteric arteries without PVAT from rats (19, 25, 34). This indicates that PVAT-derived leptin may be involved in the greater effect that PVAT has on buffering vasoconstriction in the Dahl SS rat strain. However, the effect of leptin alone, although significant for the higher concentrations of phenylephrine, was considerably smaller than the effect of PVAT in suppressing vasoconstriction. Thus, this leptin treatment did not completely recapitulate the buffering effect seen in +PVAT+ENDO aortic segments. Leptin may act in combination with other factors derived from the PVAT to produce the more pronounced PVAT buffering capacity in Dahl SS rats; thus, further work is needed to decipher these mechanisms.
A study by Galvez-Prieto et al. showed that the periaortic adipose tissue from WKY rats expresses leptin (12). This study went on to demonstrate that exogenous application of leptin elicited vasorelaxation in isolated aortic rings with an intact endothelium without the PVAT. Interestingly, Benkhoff et al. (4) showed that leptin treatment in vivo, which increased plasma leptin levels 5 × to ∼10 ng/ml, restored aortic endothelial function in a hypertensive mouse model that displays endothelial dysfunction. We showed that the total leptin levels in the PVAT from Dahl SS rats are greater than that found in SS.13BN rats. This was calculated on the basis of the fact that PVAT was greater in the Dahl SS rats. There was not a difference in the level of either the long or short form of the leptin receptor in the aortic vascular tissue. Collectively, these data suggest that the adipocyte hormone leptin is a player in mediating the greater PVAT function present in Dahl SS rats.
Although the Dahl SS rats had greater amounts of aortic PVAT, their body weight was less than that of the SS.13BN rat strain. The rats in this study were age-matched, resulting in this disparity in body weight. We speculate that this is a result of the genetic difference between the two strains. Previous publications find that the Dahl SS rat is hyperlipidemic (1, 37). This altered metabolic state may promote the greater adiposity around the blood vessels to preserve the vascular function in the face of these increased circulating lipids, without changing adiposity in the other fat pads. Future studies should compare the effects of changes in body weight, such as in response to high-fat diet feeding or caloric restriction, on PVAT function between these rat strains.
Although we have previously found that Dahl SS rats at this age and on standard chow have higher blood pressure than their SS.13BN controls (38), we did not detect a difference in mean arterial blood pressure between rat strains in the present study. This may be due to the acute methods used to collect blood pressure measurements compared with telemetry in the previous study (38). However, it was verified that endothelium-dependent relaxation was reduced in the Dahl SS rats vs. SS.13BN rats under the standard chow conditions of this study, supporting the observation that the Dahl SS rat strain is predisposed to cardiovascular disease under normal-salt chow conditions. With that being said, it is interesting to speculate what may happen to PVAT function in Dahl SS rats in the context of hypertension. The Dahl SS rat is known for its defined hypertensive response to a high-salt diet (44). Intriguingly, it was demonstrated in a form of essential hypertension, namely the spontaneously hypertensive rat (SHR), that the aortic PVAT levels of leptin are significantly reduced along with a loss of its anticontractile effect (12). Therefore, we speculate that the hypertension produced by high-salt diet in Dahl SS rats may produce PVAT dysfunction as found in the SHR. Although it is not yet understood how hypertension in the Dahl SS rat affects PVAT-mediated buffering of vasoconstriction, what is known is that high-salt diet-induced hypertension elicits dramatic endothelial dysfunction in their aortas but not in their salt-resistant counterparts (28). Under certain conditions such as a prooxidant environment, NOS becomes uncoupled and generates reactive oxygen species, specifically superoxide, instead of NO (7, 9, 21, 27, 30, 41, 43). Because PVAT function was observed to be dependent on the endothelium in the Dahl SS rat, this knowledge further leads us to propose that PVAT function would be attenuated in Dahl SS rats in the face of a high-salt diet. Future studies will be designed to test this hypothesis.
Dahl SS rats also have a hypertensive response to other dietary stressors. We and others have shown that these animals also become hypertensive with a high-fat, normal-salt diet compared with their genetic controls (5, 32, 37). This indicates that Dahl SS rats are also fat-sensitive. However, paradoxically, Dahl SS rats are protected from aortic endothelial dysfunction on both a short-term (4 wk) and long-term (20 wk) high-fat diet regimens, even though they are hypertensive (5, 38). These studies noted that endothelium-dependent vasorelaxation was increased following a high-fat diet along with elevated blood vessel expression of NOS3 and phosphorylation at the activation site Ser-1177 of NOS3 in Dahl SS rats. PVAT function was not examined in these studies. Because it has been shown that a high-fat diet enlarges adipocyte size and leptin levels in the PVAT of carotid arteries from mice (35), it is attractive to hypothesize that the PVAT plays an important role in the vasoprotective mechanisms observed with high-fat diet in Dahl SS rats.
Perspectives and Significance
The PVAT is a novel regulator of vascular function via blunting of vasoconstriction (33). This study demonstrated that PVAT function is present in Dahl SS and SS.13BN rats on a normal-salt diet. This function depends on NOS signaling in the endothelium. This function was greater in the Dahl SS rats than that observed in their genetic controls. These data suggest that the PVAT is a valid target of diet-induced alterations in vascular function in the Dahl SS rat strain. As understanding the regulation of vascular function in cardiovascular disease in humans is complicated due, in part, to complex dietary patterns, the Dahl SS rats may provide novel, clinically relevant information on mechanisms of PVAT function in pathological states.
GRANTS
This study was supported by National Institutes of Health (NIH) P01 HL-69999 (J. S. Pollock), NIH R01 HL-60653 (J. S. Pollock), NIH 2T32HL-076146 (F. T. Spradley), American Heart Association Grant 10PRE4010001 (F. T. Spradley), and NIH 1F32HL-116145 (D. H. Ho).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.T.S., D.H.H., and J.S.P. conception and design of research; F.T.S. and D.H.H. performed experiments; F.T.S., D.H.H., and J.S.P. analyzed data; F.T.S., D.H.H., and J.S.P. interpreted results of experiments; F.T.S. and D.H.H. prepared figures; F.T.S. drafted manuscript; F.T.S., D.H.H., and J.S.P. edited and revised manuscript; F.T.S., D.H.H., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Carol Moreno-Quinn and The Human and Molecular Genetics Center, Medical College of Wisconsin, for their generosity in genotyping our Dahl SS rat breeders. We thank Janet Hobbs for her histological expertise, Amy Dukes for her assistance with enzyme immunoassays, and Jacqueline Musall for her assistance with Western blot analyses.
Present addresses: F. T. Spradley, Cardiovascular-Renal Research Center, Women's Health Research Center, Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, MS; and D. H. Ho, Department of Clinical Investigation, Tripler Army Medical Center, Honolulu, HI 96859.
REFERENCES
- 1.Atarashi K, Ishiyama A, Takagi M, Minami M, Kimura K, Goto A, Omata M. Vitamin E ameliorates the renal injury of Dahl salt-sensitive rats. Am J Hypertens 10: 116S–119S, 1997. [PubMed] [Google Scholar]
- 2.Atochin DN, Huang PL. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflügers Arch 460: 965–974, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton M, Vos I, Shaw S, Boer P, D'Uscio LV, Grone HJ, Rabelink TJ, Lattmann T, Moreau P, Luscher TF. Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and glomerulosclerosis. J Am Soc Nephrol 11: 835–845, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Benkhoff S, Loot AE, Pierson I, Sturza A, Kohlstedt K, Fleming I, Shimokawa H, Grisk O, Brandes RP, Schroder K. Leptin potentiates endothelium-dependent relaxation by inducing endothelial expression of neuronal NO synthase. Arterioscler Thromb Vasc Biol 32: 1605–1612, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension 60: 404–410, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 34: 1621–1630, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 102: 9056–9061, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 286: H1107–H1113, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fang L, Zhao J, Chen Y, Ma T, Xu G, Tang C, Liu X, Geng B. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens 27: 2174–2185, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Galvez-Prieto B, Somoza B, Gil-Ortega M, Garcia-Prieto CF, de Las Heras AI, Gonzalez MC, Arribas S, Aranguez I, Bolbrinker J, Kreutz R, Ruiz-Gayo M, Fernandez-Alfonso MS. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol 3: 103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 26: 1297–1302, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee RM. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol 590: 264–268, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol 151: 323–331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon FJ, Mark AL. Mechanism of impaired baroreflex control in prehypertensive Dahl salt-sensitive rats. Circ Res 54: 378–387, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res 35: 1039–1047, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino J, Sakamaki T, Nakamura T, Kobayashi M, Kato M, Sakamoto H, Kurashina T, Yagi A, Sato K, Ono Z. Exaggerated vascular response due to endothelial dysfunction and role of the renin-angiotensin system at early stage of renal hypertension in rats. Circ Res 74: 130–138, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Jamroz-Wisniewska A, Gertler A, Solomon G, Wood ME, Whiteman M, Beltowski J. Leptin-induced endothelium-dependent vasorelaxation of peripheral arteries in lean and obese rats: role of nitric oxide and hydrogen sulfide. PLos One 9: e86744, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase-dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49: 893–901, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens 27: 782–790, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Lee RM, Lu C, Su LY, Werstuck G, Gao YJ. Effects of hyperglycemia on the modulation of vascular function by perivascular adipose tissue. J Hypertens 27: 118–131, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, Chen PY, Kuo JS, Lee TJ. Role of Perivascular Adipose Tissue-Derived Methyl Palmitate in Vascular Tone Regulation and Pathogenesis of Hypertension. Circulation 124: 1160–1171, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes 49: 293–297, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther 315: 1058–1064, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Luscher TF, Raij L, Vanhoutte PM. Endothelium-dependent vascular responses in normotensive and hypertensive Dahl rats. Hypertension 9: 157–163, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothorac Surg 33: 225–231, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Moreno C, Kennedy K, Andrae JW, Jacob HJ. Genome-wide scanning with SSLPs in the rat. Methods Mol Med 108: 131–138, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Effect of high fat loading in Dahl salt-sensitive rats. Clin Exp Hypertens 31: 451–461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol 10: 191–196, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahin AS, Bariskaner H, Gokbel H, Okudan N. The dual effects of leptin on aortic rings with and without endothelium isolated from streptozotocin-induced diabetic rats. Methods Find Exp Clin Pharmacol 31: 325–329, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Schroeter MR, Eschholz N, Herzberg S, Jerchel I, Leifheit-Nestler M, Czepluch FS, Chalikias G, Konstantinides S, Schafer K. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler Thromb Vasc Biol 33: 980–987, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Shastri S, Gopalakrishnan V, Poduri R, Di Wang H. Tempol selectively attenuates angiotensin II evoked vasoconstrictor responses in spontaneously hypertensive rats. J Hypertens 20: 1381–1391, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Spradley FT, De Miguel C, Hobbs J, Pollock DM, Pollock JS. Mycophenolate mofetil prevents high-fat diet-induced hypertension and renal glomerular injury in Dahl SS rats. Physiol Rep 1: e00137, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spradley FT, Kang KT, Pollock JS. Short-term hypercaloric diet induces blunted aortic vasoconstriction and enhanced vasorelaxation via increased nitric oxide synthase 3 activity and expression in Dahl salt-sensitive rats. Acta Physiol (Oxf) 207: 358–368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun X, Hou N, Han F, Guo Y, Hui Z, Du G, Zhang Y. Effect of high free fatty acids on the anti-contractile response of perivascular adipose tissue in rat aorta. J Mol Cell Cardiol 63: 169–174, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 44: 271–276, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng ZH, Zhang ZH, Luo BH, He WK, Liang LY, He CC, Su CJ. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin Exp Hypertens 31: 355–363, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Song P, Xu J, Zou MH. Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 31: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zicha J, Dobesova Z, Vokurkova M, Rauchova H, Hojna S, Kadlecova M, Behuliak M, Vaneckova I, Kunes J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res 61 Suppl 1: S35–S87, 2012. [DOI] [PubMed] [Google Scholar]