Abstract
AIM: To explore whether the presence of a sliding hiatus hernia influences gastroesophageal reflux.
METHODS: Endoscopy and 24 h pH monitoring were performed for 197 outpatients with gastroesophageal reflux symptoms.
RESULTS: Of the 197 patients with symptoms of gastroesophageal reflux, patients with hiatus hernia accounted for 36%. The incidence of esophagitis in patients with hiatus hernia was significantly higher than that in patients without hiatus hernia. The results of 24 h pH monitoring showed that 84 patients had physiological reflux, 37 had pathological reflux without esophagitis, 64 had reflux esophagitis and 12 had physiological reflux concomitant with esophagitis. All the patients with hiatus hernia had a longer percentage time with supine reflux and a higher frequency of episodes lasting over 5 min at night compared to those without hiatus hernia. The incidence of combined daytime and nocturnal reflux in patients with hiatus hernia was significantly higher than that in patients without hiatus hernia.
CONCLUSION: Pathological reflux and reflux esophagitis in some patients with symptoms of gastroesophageal reflux represent two different stages of gastroesophageal reflux disease. Pathological reflux is the first stage, in which the lower esophageal sphincter is incompetent but the esophageal mucosal resistance effectively prevents regurgitated acid from damaging the esophageal mucosa. Reflux esophagitis represents the second stage, in which the aggression of the regurgitated acid is so strong that the esophageal mucosa fails to resist it and the epithelium of the esophagus is damaged. Patients with hiatus hernia have a high incidence of combined daytime and nocturnal reflux, with the latter being responsible for esophagitis.
Keywords: Hernia, hiatal; Gastroesophageal reflux; Endoscopy, gastrointestinal; Hydrogen ion concentration; Esophagitis, peptic
INTRODUCTION
The relationship between hiatus hernia (HH) and gastroesophageal reflux (GER) disease remains controversial[1-4]. One opinion was that the demonstration of HH would frequently imply the presence of reflux esophagitis. Some investigators, for example, have claimed that HH can be found in almost all cases of esophagitis[5-6]. As a result, some authors believed that it was necessary to excise HH to cure GER disease. Another opinion was that the existence of HH did not affect esophageal sphincter competence[7-10]. The third opinion was that HH might play only a partial role in the development of esophagitis, that is, the absence of HH could exclude more severe forms of reflux esophagitis[11-14]. This study was, therefore, designed to evaluate the influence of the existence of HH on GER.
MATERIALS AND METHODS
Patients
One hundred and ninety-seven consecutive outpatients (female, 75; male, 122) who had experienced the symptoms of heartburn, regurgitation and chest pain for at least 6 mon were included in this study. None had a past history of surgery or had taken H2 receptor blockers or proton pump inhibitors during the 4 wk prior to endoscopy and 24 h esophageal pH monitoring.
Endoscopy and 24 h pH monitoring
Endoscopy was performed a week before 24 h esophageal pH monitoring. Esophagitis was graded from I to IV according to the Savary-Miller classification[15]. Only a few patients presented with grades II and III and therefore grades I and II, III and IV were grouped together, respectively. Twenty-four hour intraesophageal pH monitoring was carried out in accordance with a method described elsewhere[16,17]. Patients were advised to take a standard meal with approximately 2200 kilocalories during 24 h intraesophageal pH monitoring. A glass pH electrode with an incorporated potassium chloride reference electrode (Ingold Electrode, No.440) was passed by the nasoesophageal route and positioned with the tip 5 cm above the gastroesophageal junction identified by a pH meter. The results from the pH probe were recorded on a solid state recorder (Autronicord CM18), which was carried by the patients on a belt. A computer based analysis was used for the interpretation of the 24 h pH monitoring data. The parameters recorded included the number and percentage time of GER episodes and the number of GER episodes lasting over 5 min. A pathological reflux (PR) was diagnosed if 1) the pH value in the regurgitated contents was less than 4.0 and 2) the complete reflux duration was more than 7% in 24 h[16,17]; physiological reflux (PhR) was defined if a reflux event did not fulfill the above criteria; reflux esophagitis (RE) means a pathological reflux event associated with esophageal inflammatory lesions; and physiological reflux esophagitis concomitant with esophagitis (PhRE) means an esophageal inflammatory lesion without pathological GER.
Statistical analysis
The Wilcoxon test was used for the analysis of GER parameters in the patients with and without HH. Student’s t test was applied to analyze the age and the duration of GER symptoms. The remaining data were analyzed using the Chi square test.
RESULTS
Of 197 patients with symptoms of GER, patients with HH accounted for 36%. Demographic data and endoscopic findings in patients with and without HH are listed in Table 1. The incidence of esophagitis in patients with HH was significantly higher than that in patients without HH (grade III esophagitis, P < 0.05; and grade III-IV esophagitis, P < 0.001). There was no significant difference between patients with HH and without HH concerning sex, GER symptoms, smoking and alcohol consumption (P > 0.05).
Table 1.
Demographic data and endoscopic findings in patients with and without hiatus hernia
| Patients with HH (n = 71) | Patients without HH (n = 126) | P value | |
| Age (yr) | |||
| Patients with esophagitis | 51.1 ± 1.5 | 40.7 ± 2.9 | < 0.001 |
| Patients without esophagitis | 51.8 ± 4.2 | 41.0 ± 2.2 | < 0.001 |
| Sex (n, f/m) | 25/46 | 50/76 | > 0.05 |
| Heartburn (%) | 80.2 | 89.6 | > 0.05 |
| Regurgitation (%) | 54.3 | 54.8 | > 0.05 |
| Chest pain (%) | 60.6 | 61.1 | > 0.05 |
| Smoker (%) | 21.1 | 25.4 | > 0.05 |
| Alcohol consumer (%) | 36.6 | 27.0 | > 0.05 |
| Endoscopic findings (%) | |||
| Normal | 42.3 | 72.2 | < 0.001 |
| Grade I-II esophagitis | 42.3 | 26.2 | < 0.05 |
| Grade III-IV esophagitis | 15.4 | 1.6 | < 0.001 |
HH = Hiatus hernia
On the basis of the results of 24 h pH monitoring and endoscopy, the patients with HH and without HH were divided into 8 subgroups: HH patients with RE, non HH patients with RE, HH patients with PR, non HH patients with PR, HH patients with PhR, non HH patients with PhR, HH patients with PhRE and non HH patients with PhRE. GER parameters in patients with and without HH are shown in Table 2. The statistical analysis for the GER parameters in patients with and without HH is given in Table 3. A mean percentage time with GER in HH patients with PR was longer than that in non HH patients with PR and a frequency of nocturnal reflux lasting over 5 min was higher in HH patients with PR than that in non HH patients with PR.
Table 2.
Gastroesophageal reflux parameters in patients with and without hiatus hernia (¯x ± s)
| RE (n = 64) | PR (n = 37) | PhR (n = 84) | PhRE (n = 12) | |
| Patients with HH | ||||
| % time of reflux | ||||
| 24-h | 18.8 ± 15.4 | 14.9 ± 9.8 | 2.5 ± 0.2 | 3.9 ± 2.6 |
| Upright | 17.9 ± 5.0 | 14.4 ± 9.3 | 2.7 ± 2.2 | 4.4 ± 3.7 |
| Supine | 19.1 ± 2.2 | 15.1 ± 0.0 | 2.1 ± 3.5 | 3.6 ± 4.2 |
| No. of episodes > 5 min | ||||
| 24-h | 9.1 ± 4.3 | 7.5 ± 3.2 | 0.9 ± 1.4 | 2.6 ± 4.1 |
| Upright | 5.8 ± 4.4 | 4.2 ± 3.2 | 0.5 ± 1.0 | 1.5 ± 1.3 |
| Supine | 3.3 ± 2.1 | 3.2 ± 2.3 | 0.3 ± 0.6 | 1.5 ± 1.8 |
| Patients without HH | ||||
| % time of reflux | ||||
| 24-h | 17.1 ± 4.8 | 13.3 ± 6.7 | 2.7 ± 2.2 | 1.5 ± 1.7 |
| Upright | 18.9 ± 3.5 | 7.0 ± 8.0 | 3.4 ± 2.8 | 0.3 ± 0.5 |
| Supine | 13.5 ± 1.7 | 2.7 ± 2.2 | 1.5 ± 3.0 | 1.1 ± 1.6 |
| No. of episodes > 5 min | ||||
| 24-h | 10.5 ± 8.0 | 6.1 ± 3.5 | 0.9 ± 1.4 | 2.0 ± 2.7 |
| Upright | 8.1 ± 6.9 | 4.5 ± 3.0 | 0.5 ± 1.0 | 1.6 ± 2.0 |
| Supine | 2.3 ± 2.1 | 1.6 ± 1.4 | 0.3 ± 0.6 | 0.3 ± 0.8 |
HH = Hiatus hernia; RE = Reflux esophagitis; PR = Pathological reflux; PhR = Physiological reflux; PhRE = Esophagitis with physiological reflux.
Table 3.
Statistical significance of gastroesophageal reflux parameters between patients with and without hiatus hernia in Table 2
| RE | PR | PhR | PhRE | |
| % time of reflux | ||||
| 24-h | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| Upright | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| Supine | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| No. of episodes | ||||
| 24-h | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| Upright | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| Supine | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| No. of episodes > 5 min | ||||
| 24-h | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| Upright | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| Supine | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
HH = Hiatus hernia; RE = Reflux esophagitis; PR = Pathological reflux; PhR = Physiological reflux; PhRE = Esophagitis with physiological reflux.
The incidences of reflux esophagitis, pathological reflux, physiological reflux and physiological reflux with esophagitis in patients with and without HH are shown in Figure 1. The incidence of reflux esophagitis in patients with HH was significantly higher than that in patients without HH (P < 0.01); on the other hand, the incidence of physiological reflux in patients without HH was significantly higher than that in patients with HH (P < 0.01). The incidence of pathological reflux and physiological reflux with esophagitis in the two groups showed no statistically significant difference (P > 0.05). Figure 2 shows the percentages of upright, supine and combined upright and supine reflux in patients with and without HH. There was a significant difference between patients with and without HH regarding upright and combined GER (P < 0.01). There was no statistically significant difference in supine GER in patients with and without HH (P > 0.05).
Figure 1.
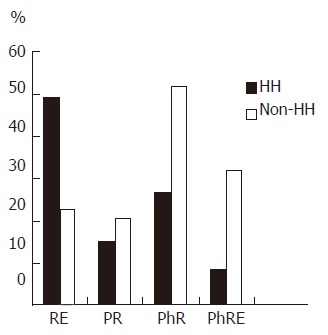
The incidences of Reflux esophagitis, Pathological reflux, Physiological reflux and Esophagitis with physiological reflux in patients with and without hiatus hernia. RE: reflux esophagitis, PR: Pathological reflux; PhR: Physiological reflux; PhRE: esophagitis with physiological reflux; HH: Hiatus hernia, Non HH: Patients without HH.
Figure 2.
Percentages of upright, supine and combined reflux in patients with and without hiatus hernia. U: Upright reflux, S: Supine reflux; C: Combined daytime and nocturnal reflux, HH: Hiatus hernia, Non HH: Patients without HH.
DISCUSSION
Because the relationship between HH and GER disease is still controversial, it is necessary to calculate the incidence of sliding HH and to compare GER parameters in HH patients with those without HH. Clagett et al[18] reported that the incidence of HH in the general population is far more than the number of patients who present clinically with symptoms of GER. In 95 asymptomatic subjects examined by Dyer et al[19], the incidence of HH was 33% and only 16% of the subjects complained of symptoms of GER. Of 102 patients with symptoms of GER studied by DeMeester, 52% had endoscopic evidence of HH[20]. Kaul et al[11] reported that HH was found in 50 of 101 patients with symptoms of GER. In our 197 patients with symptoms of GER, 36% had HH. The incidence of reflux esophagitis in patients with HH was significantly higher than in patients without HH, while the incidence of physiological GER was significantly higher in patients without HH than that in patients with HH.
The number of GER episodes, percentage time with GER and the number of episodes lasting over 5 min in patients with HH were compared with those in patients without HH. The results showed that the percentage time with nocturnal reflux in HH patients with PR was longer than that in non HH patients with PR and the frequency of episodes lasting over 5 min at night in HH patients with PR was higher than that in non HH patients with PR, whereas there was no significant difference in the frequency of GER, percentage time with GER and number of episodes lasting over 5 min between HH patients and non HH patients with RE, PhR and PhRE. In a previous study, Sloan et al[21] found that impaired emptying in patients with HH was attributable to an early retrograde flow, occurring immediately after lower esophageal sphincter relaxation. The results reported by Mittal et al[22] showed that acid clearance at 5 cm above the lower esophageal sphincter was faster in non HH patients than in HH patients with GER. Our data revealed that the presence of HH in patients with PR impaired the acid clearance function of the esophagus, while in patients with RE, no noteworthy influence of HH on the parameters of GER was observed. One question arising from our results is why the presence of HH had a different influence on the parameters of GER in patients with PR and RE. It seems that PR and RE in some patients with symptoms of GER represented two different stages of GER disease, with PR being the first stage, in which the lower esophageal sphincter was incompetent but the esophageal mucosal resistance effectively prevented regurgitated acid from damaging the esophageal mucosa. RE in some patients with symptoms of GER represented the second stage, in which the aggression of the regurgitated acid was so strong that the esophageal mucosa failed to resist it and the epithelium of the esophagus was damaged. There is evidence that in the first stage HH had considerable influence on GER and esophageal clearance of refluxed acid. In the second stage, the interaction between lower esophageal sphincter incompetence and esophagitis might be involved in the pathogenesis, i.e. GER might develop into esophagitis, which, in turn, impairs lower esophageal sphincter competence and aggravates the GER. In this stage, a vicious cycle of the conditions might have a crucial influence on GER[23], whereas the effect of etiological factors such as HH on GER in this stage appeared to be less important.
It is currently accepted that there are a number of factors involved in the pathogenesis of GER disease but the most important one is the contact time between regurgitated acid and the esophageal mucosa[24-27]. In the present study, the exposure time of esophageal mucosa to regurgitated acid and patterns of GER in patients with HH were compared with those without HH. However, we were unable to demonstrate any significant difference in the percentage time with GER between HH patients with RE and those without HH. Our results revealed that the incidence of combined daytime and nocturnal reflux was significantly higher in patients with HH than that in patients without HH and that the incidence of upright reflux, on the other hand, was significantly higher in patients without HH than that in patients with HH. Attention has long been paid to the relationship between the development of esophagitis and patterns of GER since the advent of 24 h pH monitoring. Some investigators, for example, have revealed that the development of esophagitis is related to increased supine reflux[5,24,28-30]. Others have found evidence to support the opinion that daytime gastroesophageal reflux plays a more important role in the development of esophagitis[31-34]. Although there are different opinions as to the relationship between patterns of GER and esophagitis, it is generally recognised that a difference in patterns of GER is related to the development of esophagitis. Our results, however, suggested that combined daytime and nocturnal reflux may be responsible for the increased incidence of esophagitis in HH patients.
Footnotes
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-)
S- Editor: Ma JY L- Editor: Ma JY E- Editor: Liu WX
References
- 1.Pope CE. Pathophysiology and diagnosis of reflux esophagitis. Gastroenterology. 1976;70:445–454. [PubMed] [Google Scholar]
- 2.Dodds WJ, Hogan WJ, Miller WN. Reflux esophagitis. Am J Dig Dis. 1976;21:49–67. doi: 10.1007/BF01074140. [DOI] [PubMed] [Google Scholar]
- 3.Ott DJ, Dodds WJ, Wu WC, Gelfand DW, Hogan WJ, Stewart ET. Current status of radiology in evaluating for gastroesophageal reflux disease. J Clin Gastroenterol. 1982;4:365–375. doi: 10.1097/00004836-198208000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Dodds WJ. 1976 Walter B. Cannon Lecture: current concepts of esophageal motor function: clinical implications for radiology. AJR Am J Roentgenol. 1977;128:549–561. doi: 10.2214/ajr.128.4.549. [DOI] [PubMed] [Google Scholar]
- 5.Lichter I. Measurement of gastro-oesophageal acid reflux: its significance in hiatus hernia. Br J Surg. 1974;61:253–258. doi: 10.1002/bjs.1800610402. [DOI] [PubMed] [Google Scholar]
- 6.Brennan TG, Trindade LM, Rozycki ZJ, Giles GR. The influence of the lower oesophageal sphincter pressure on the outcome of hiatus hernia repair. Br J Surg. 1974;61:201–205. doi: 10.1002/bjs.1800610308. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Harris LD. Does hiatus hernia affect competence of the gastroesophageal sphincter. N Engl J Med. 1971;284:1053–1056. doi: 10.1056/NEJM197105132841902. [DOI] [PubMed] [Google Scholar]
- 8.HIEBERT CA, BELSEY R. Incompetency of the gastric cardia without radiologic evidence of hiatal hernia. The diagnosis and management of 71 cases. J Thorac Cardiovasc Surg. 1961;42:352–362. [PubMed] [Google Scholar]
- 9.Kramer P. Does a sliding hiatus hernia constitute a distinct clinical entity. Gastroenterology. 1969;57:442–448. [PubMed] [Google Scholar]
- 10.Field P, Stalker MJ. Incompetence of the cardiac sphincter without radiologic demonstration of hiatus hernia. Can J Surg. 1968;11:412–419. [PubMed] [Google Scholar]
- 11.Kaul B, Petersen H, Myrvold HE, Grette K, Røysland P, Halvorsen T. Hiatus hernia in gastroesophageal reflux disease. Scand J Gastroenterol. 1986;21:31–34. doi: 10.3109/00365528609034617. [DOI] [PubMed] [Google Scholar]
- 12.Berstad A, Weberg R, Frøyshov Larsen I, Hoel B, Hauer-Jensen M. Relationship of hiatus hernia to reflux oesophagitis. A prospective study of coincidence, using endoscopy. Scand J Gastroenterol. 1986;21:55–58. doi: 10.3109/00365528609034622. [DOI] [PubMed] [Google Scholar]
- 13.Ott DJ, Wu WC, Gelfand DW. Reflux esophagitis revisited: prospective analysis of radiologic accuracy. Gastrointest Radiol. 1981;6:1–7. doi: 10.1007/BF01890213. [DOI] [PubMed] [Google Scholar]
- 14.Wright RA, Hurwitz AL. Relationship of hiatal hernia to endoscopically proved reflux esophagitis. Dig Dis Sci. 1979;24:311–313. doi: 10.1007/BF01296546. [DOI] [PubMed] [Google Scholar]
- 15.Savary M, Miller G. The Esophagus. Handbook and Atlas of Endoscopy. Switzerland: Gassmann; 1978. pp. 125–132. [Google Scholar]
- 16.Bianchi Porro G, Pace F. Comparison of three methods of intraesophageal pH recordings in the diagnosis of gastroesophageal reflux. Scand J Gastroenterol. 1988;23:743–750. doi: 10.3109/00365528809093943. [DOI] [PubMed] [Google Scholar]
- 17.Pace F, Sangaletti O, Bianchi Porro G. Daytime reduction of gastro-oesophageal reflux after healing of oesophagitis and its value as an indicator of favourable response to maintenance treatment. Gut. 1990;31:1025–1029. doi: 10.1136/gut.31.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clagett OT. Present concepts regarding the surgical treatment of oesophageal hiatal hernia. Ann R Coll Surg Engl. 1966;38:195–209. [PMC free article] [PubMed] [Google Scholar]
- 19.Dyer NH, Pridie RB. Incidence of hiatus hernia in asymptomatic subjects. Gut. 1968;9:696–699. doi: 10.1136/gut.9.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMeester TR, Lafontaine E, Joelsson BE, Skinner DB, Ryan JW, O'Sullivan GC, Brunsden BS, Johnson LF. Relationship of a hiatal hernia to the function of the body of the esophagus and the gastroesophageal junction. J Thorac Cardiovasc Surg. 1981;82:547–558. [PubMed] [Google Scholar]
- 21.Sloan S, Kahrilas PJ. Impairment of esophageal emptying with hiatal hernia. Gastroenterology. 1991;100:596–605. doi: 10.1016/0016-5085(91)80003-r. [DOI] [PubMed] [Google Scholar]
- 22.Mittal RK, Lange RC, McCallum RW. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology. 1987;92:130–135. doi: 10.1016/0016-5085(87)90849-3. [DOI] [PubMed] [Google Scholar]
- 23.Mueller-Lissner SA. When is oesophagitis healed In Tytgat GN (ed). The medical management of oesophageal reflux disease. Royal Society of MedicineaRound Table series N. 22-oxford: Alden Press; 1990. pp. 106–115. [Google Scholar]
- 24.Kruse-Andersen S, Wallin L, Madsen T. Reflux patterns and related oesophageal motor activity in gastro-oesophageal reflux disease. Gut. 1990;31:633–638. doi: 10.1136/gut.31.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demeester TR, Johnson LF, Joseph GJ, Toscano MS, Hall AW, Skinner DB. Patterns of gastroesophageal reflux in health and disease. Ann Surg. 1976;184:459–470. doi: 10.1097/00000658-197610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodds WJ, Hogan WJ, Helm JF, Dent J. Pathogenesis of reflux esophagitis. Gastroenterology. 1981;81:376–394. [PubMed] [Google Scholar]
- 27.Richter JE, Castell DO. Gastroesophageal reflux. Pathogenesis, diagnosis, and therapy. Ann Intern Med. 1982;97:93–103. doi: 10.7326/0003-4819-97-1-93. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D, Aldersley M, Shepherd H, Smith CL. Patterns of acid reflux in complicated oesophagitis. Gut. 1987;28:1484–1488. doi: 10.1136/gut.28.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pujol A, Grande L, Ros E, Pera C. Utility of inpatient 24-hour intraesophageal pH monitoring in diagnosis of gastroesophageal reflux. Dig Dis Sci. 1988;33:1134–1140. doi: 10.1007/BF01535790. [DOI] [PubMed] [Google Scholar]
- 30.Orr WC, Robinson MG, Johnson LF. Acid clearance during sleep in the pathogenesis of reflux esophagitis. Dig Dis Sci. 1981;26:423–427. doi: 10.1007/BF01313584. [DOI] [PubMed] [Google Scholar]
- 31.de Caestecker JS, Blackwell JN, Pryde A, Heading RC. Daytime gastro-oesophageal reflux is important in oesophagitis. Gut. 1987;28:519–526. doi: 10.1136/gut.28.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Branicki FJ, Evans DF, Jones JA, Ogilvie AL, Atkinson M, Hardcastle JD. A frequency-duration index (FDI) for the evaluation of ambulatory recordings of gastro-oesophageal reflux. Br J Surg. 1984;71:425–430. doi: 10.1002/bjs.1800710607. [DOI] [PubMed] [Google Scholar]
- 33.Blackwell JN, Heading RC. When does gastro-oesophageal reflux occur in patients with peptic oesophagitis. (Abstract) Gut. 1980;21:922. [Google Scholar]
- 34.Rokkas T, Anggiansah A, Uzoechina E, Owen WJ, Sladen GE. The role of shorter than 24-h pH monitoring periods in the diagnosis of gastro-oesophageal reflux. Scand J Gastroenterol. 1986;21:614–620. doi: 10.3109/00365528609003108. [DOI] [PubMed] [Google Scholar]


