Abstract
AIM: To study the uptake of bacterial lipopolysaccharides (LPS) and expression of tumor necrosis factor α-mRNA (TNF-α-mRNA) with cultured rat intrahepatic bile duct epithelial cells.
METHODS: By using fluorescent, immunohistochemical and in situ hybridization techniques, the uptake of Escherichia coli LPS and expression of TNF-α-mRNA with isolated rat intrahepatic bile duct epithelial cells were observed with confocal laser scanning microscopy.
RESULTS: Positive reactions to LPS were found in the cytoplasm of isolated intrahepatic bile duct epithelial cells after incubation with LPS for 15 min and the FITC fluorescent intensity against LPS was significantly higher than that of the controls (121.45 μFI/μm2 ± 15.62 μFI/μm2 vs 32.12 μFI/μm2 ± 9.64 μFI/μm2, P < 0.01). After incubation with LPS for 3 h, fluorescein isocyanate (FITC) fluorescent intensities of the expression of TNF-α-mRNA with fluorescent in situ hybridization in the cytoplasm and nuclei of the cultured bile duct epithelial cells were significantly higher than those of the controls (189.15 μFI/μm2 ± 21.33 μFI/μm2 vs 10.00 μFI/μm2 ± 8.99 μFI/μm2, 64.85 μFI/μm2 ± 14.99 μFI/μm2 vs 21.20 μFI/μm2 ± 2.04 μFI/μm2, respectively (P < 0.01)). The increase of FITC fluorescent intensity of TNF-α-mRNA expression in the cytoplasm peaked at 6 h after incubation (221.38 μFI/μm2 ± 22.99 μFI/μm2). At various time points after incubation with LPS, the increase of fluorescent intensities of TNF-α-mRNA in the cytoplasm were much higher than those in the nuclei (P < 0.01).
CONCLUSION: LPS can act on and enter into isolated intrahepatic bile duct epithelial cells and stimulate the expression of TNF-α-mRNA.
Keywords: Lipopolysaccharides, Epithelial cell bile ducts, Tumor necrosis factor, In situ hybridization
INTRODUCTION
Endotoxins, mainly consisting of lipopolysaccharides (LPS) and proteins, can induce a number of reactions, both beneficial and harmful to the body[1]. It is generally known that the cells of the reticuloendothelial system, mainly the Kupffer cells of the liver, are responsible for the clearance of LPS[2]. LPS that have undergone degradation in the liver are subsequently excreted mainly into the gut through the bile ducts. Although the LPS present in the liver after the injection exhibited a lower fatty acid to carbohydrate ratio than the original LPS, lipid A was still present, covalently bound to the polysaccharide, thus showing that the overall macromolecular structure of LPS had remained unaltered[3]. Besides, the toxic activity of LPS in the bile was the same as the original LPS, indicating that LPS in the bile are biologically active[4]. The bile duct epithelial cells have been postulated to be involved in absorptive and secretory activities, including the transport of water, electrolytes, sugars, amino acids, bile acids and proteins[5-6], and might play a role in the liver immune system, but only a little information exists about their interactions with LPS. In the present study, by using a monoclonal antibody specific to the core lipid A region of LPS and a fluorescein isocyanate (FITC) labelled oligonucleotide as a probe for TNF-α-mRNA, the uptake of LPS and expression of TNF-α-mRNA with isolated rat intrahepatic bile duct epithelial cells were observed with immunofluorescence and fluorescent in situ hybridization techniques followed by confocal laser scanning microscopy.
MATERIALS AND METHODS
Materials
Collagenase (Type I), trypsin, insulin, heparin and wild form LPS for Escherichia coli stereotype 0111:B4 were purchased from Sigma Chemical Company. Monoclonal antibody against LPS specific to the core lipid A region was a gift from Dr. Noguchi. Fluorescent (FITC) labelled affinity purified goat anti mouse IgM was from O.E.M. Concepts Incorporation. Eagles’ MeM was obtained from Nissui Pharmaceutical Co. William’s Medium E. was from ICN Biomedicals Incorporation. Low LPS fetal bovine serum (FBS) was from Life Technologies. The endotoxin concentration in the prepared media with 10% FBS used in the experiments was as low as 0.6 ng/mL by measurement using the Endotoxin Species Test Kit (Seikagakce Kogyo LtD.). Percoll (sterile) was purchased from Pharmacia Bioprocess Technology AE. All other reagents were of analytical grade from the usual commercial sources. Male Wistar rats under specific pathogen free conditions were used (each 150 to 200 mg). Twenty-four hours before the experiments, the rats were given kanamycin (1 mg), lactulose (100 mg), sodium picosulfate (0.125 mg) and magnesium citrate (50 mg) diluted in 2 mL sterile water orally every other hour. All the rats were deprived of food but allowed free access to sterile water.
Isolation in culture of intrahepatic bile duct epithelial cells
The intrahepatic bile duct epithelial cells were isolated from the rat livers by means of a modified technique described by Ishii et al[7]. The rats were anesthetized and their livers were perfused in situ at a flow rate of 10 mL/min with the perfused liquid (heparin 2.5 μ/mL, Ca2+ and Mg2+ free) followed with perfusion of the 0.05% collagenase solution for 15 min. After perfusion, the livers were then carefully removed and placed in ice coated Eagles’ MEM. The intact hyperplastic bile ductular tissue was gently separated from the enzyme dissociated hepatic parenchymal and sinusoidal components. An essentially pure bile ductular tissue fraction was obtained by shaking the tissue fragments for 50 min in Hank’s medium containing 0.1% collagenase and 0.25% trypsin in order to remove any residual hepatic parenchymal cells that might have remained attached. Following centrifugation at 467 × g for 5 min at 4 °C, the cells were then resuspended in Eagles’ MEM and laid as 5 mL aliquot on top of 15 mL of 30% and 50% Percoll in Hank’s medium. Following centrifugation at 1870 g for 30 min at 4 °C, viable intrahepatic bile duct epithelial cells were obtained and viability was determined by the methods of trypan blue dye exclusion. The cell count was made (1-3 × 105 cell/mL). After incubation at 37 °C for 24 h in 35 mm plastic dishes in a 5% CO2 and 40% O2 atmosphere ( in William’s Medium E with 10% FBS), the media were changed to William’s medium E (FBS free) with LPS (10 mg/L). After incubation for 15 min, the cells were fixed with 99% methanol for 10 min, washed with sterile saline and then underwent the immunohistochemistry procedure.
In a separate experiment, the isolated bile duct epithelial cells were set in glass dishes. After incubation at 37 °C for 24 h in a 5% CO2 and 40% O2 atmosphere (in William’s Medium E with 10% FBS), the media were changed to William’s medium E (FBS free) with LPS (10 mg/L). After incubation for 3, 6 and 9 h, the cells were fixed with PHA, washed with sterile saline and then the fluorescent in situ hybridization procedure was done to determine the expression of TNF-α-mRNA.
Immunofluorescence staining and confocal laser scanning microscopic observation
To localize LPS in the cells, the indirect staining procedure was followed by using the monoclonal antibody against lipid A of LPS (1:800) and FITC labelled immunoglobulin as the secondary antibody (1:30). After immunofluorescence staining, the dishes were mounted with VECTASHIELD mounting medium for fluorescence under the coverslip. With a confocal laser scanning microscope (LSM-GB 200, Olympus, Japan) and an analysis system of LSM-SB 200 Ver. 2.01 provided by Olympus Company, the fluorescent intensities of FITC in the cytoplasm of the intrahepatic bile duct epithelial cells were measured under the same conditions as the analysis system. For each dish, 20 to 30 cells were measured.
Fluorescent in situ hybridization procedure and confocal laser scanning microscopic observation
A fluorescein-dUTP labelled oligonucleotide as a fluorescent in situ hybridization probe for TNF-α-mRNA (5’-GCCCACGTCGTAGCCAAACCACCAAGTGG-3’) was used in this study. After fixation with PHA, the cells were hybridized with the probe in the hybridization mixture for 20 h at 45 °C. After hybridization, the dishes were rinsed three times in phosphate buffered saline and dehydrated. FITC activities against the expression of TNF-α-mRNA in the cytoplasm and nuclei were observed and measured with a confocal laser scanning microscope by the same method as described above.
Statistical analysis
All the data were expressed as -x ± s from three to six separate experiments and were analyzed with the StatView program. Student’s t test and ANOVA F test were applied where appropriate.
RESULTS
Uptake of LPS by cultured intrahepatic bile duct epithelial cells
Cultured intrahepatic bile duct epithelial cells showed a strong positive reaction to LPS after 15 min of incubation. Positive reactions were found in the cytoplasm but not in the nuclei (Figure 1). The fluorescent intensities in the cytoplasm of the bile duct epithelial cells were significantly higher than that of the controls (121.45 μFI/μm2 ± 15.62 μFI/μm2 vs 32.12 μFI/μm2 ± 9.64 μFI/μm2, P < 0.01). No fluorescence was found in the negative controls using only the monoclonal antibody or FITC labelled secondary antibody.
Figure 1.
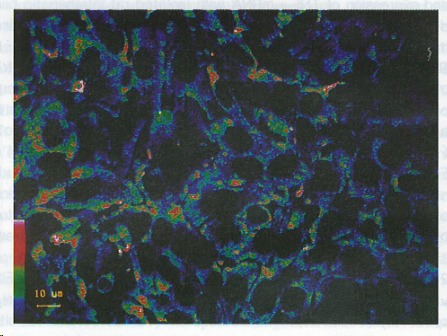
Fluorescent reactions to lipopolysaccharides in cultured intrahepatic bile duct epithelial cells. Positive reactions were obvious in the cytoplasm. The color bar in the bottom on the left side shows the fluorescent intensities (the highest is red and the lowest green).
Expression of TNF-α-mRNA
Positive FITC reactions to TNF-α-mRNA were found in the cytoplasm of the cultured intrahepatic bile duct epithelial cells after incubation with LPS for 3 h. Positive reactions were also seen in the nuclei. The positive FITC reactions became stronger in the cytoplasm after 6 h of incubation (Figure 2) and decreased after 9 h of incubation. Changes of FITC intensities in the cytoplasm and nuclei of the cultured cells are shown in Table 1.
Figure 2.
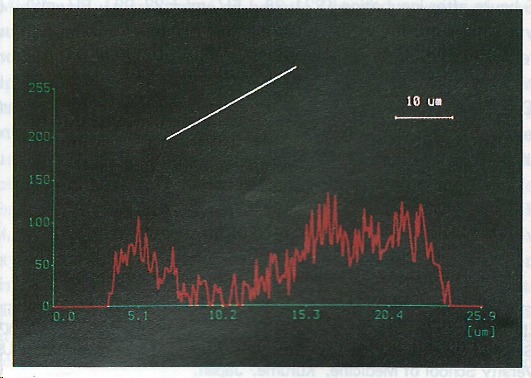
Fluorescent reactions to TNF-α-mRNA in cultured intrahepatic bile duct epithelial cells. Positive reactions were found in the cytoplasm and nuclei of the cells. The chart shows the fluorescent intensities along the line through the cells as indicated.
Table 1.
Fluorescein isocyanate fluorescent intensities of TNF-α-mRNA expression in isolated intrahepatic bile duct epithelial cells after incubation with lipopolysaccharides (μFI/μm2, -x ± s)
|
FITC fluorescent intensities |
||
| Cytoplasm | Nuclei | |
| Controls | 10.00 ± 8.99 | 2.20 ± 2.04 |
| LPS incubations | ||
| 3 h | 189.15 ± 21.33b | 64.85 ± 14.99b |
| 6 h | 221.38 ± 22.99bd | 138.15 ± 36.54bd |
| 9 h | 170.00 ± 15.25b | 70.42 ± 9.08b |
Compared with the controls (P < 0.01);
Compared with those of LPS incubations for 3 and 9 h (P < 0.01). FITC: fluorescein isocyanate.
The FITC fluorescent intensities in the cytoplasm at various time points were significantly higher than those in the nuclei.
DISCUSSION
Endotoxins mainly consist of LPS and some proteins. It is LPS which inflicts all the ill, toxic effects from the endotoxins[1]. It is generally known that most endotoxins in the bile flow excreted from the liver remain to exhibit the overall macromolecular structure of LPS and are biologically active[3-4]. The LPS injected through the portal vein could be detected in the intrahepatic bile duct epithelial cells in rats[8]. In the present study, strong positive FITC reactions against LPS could be seen in the cytoplasm of the cultured intrahepatic bile duct epithelial cells after incubation with LPS for 15 min, indicating that biologically active LPS could act on and get into the bile duct epithelial cells. Two different kinds of mechanisms, i.e. specific and nonspecific, are suggested to be involved in the initial interaction of LPS with the cells[9]. The specific interactions result from the binding of LPS to a specific receptor on the plasma membrane. On the other hand, nonspecific interactions result from the binding of LPS macromolecules to any membrane constituents other than the receptors. Both mechanisms are involved in the uptake process of LPS by cells[10,11]. A number of receptors in the membranes of various cells specific to different parts of LPS such as a 73 kDa membrane localized protein, CD14 and high lipoprotein receptor were recently reported[12,13]. Whether there are such receptors in the membranes of the bile duct epithelial cells and with which mechanism the biologically active LPS enter those cells are still to be clarified.
Although the bile duct epithelial cells were not considered to play a significant role in liver function, the involvement in immunological functions is being described more frequently now. The bile duct epithelial cells in the primary culture have been shown to activate T cells[14] and in cholestatic liver diseases they displayed aberrant expression of MHC class and antigens and of TNF-α receptors[15]. The expression of TNF-α-mRNA was detected in the intrahepatic bile duct epithelial cells of LPS perfused livers[16]. The results of the present study showed that strong expression of TNF-α-mRNA could be detected in the cultured bile duct epithelial cells after 3 h incubation with LPS, denoting that the bile duct epithelial cells could synthesize TNF-α-mRNA for the stimulation of LPS. TNF is generally considered to be an important cytokine which is associated with the injury of hepatocytes, the production of collagen and proliferation and differentiation of the cells. It plays a critical role in provoking the development of certain liver diseases. The proliferation and differentiation of the bile duct epithelial cells is likely to be a common feature related to quite a number of liver diseases. The oval cells are postulated to originate from the differentiation of the bile duct epithelial cells[5]. In vitro, the bile duct epithelial cells reacted to cytokines such as TGF, EGF and TNF to proliferate[17]. With the observations in our study, the authors tended to conclude that the interaction of the biologically active LPS in the bile flow with the bile duct epithelial cells might take part in the processes of forming the pathogical changes in the liver in some liver diseases.
Footnotes
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-)
Supported by The Natural Science Foundation for Youth of Shanxi Province, No. 95013.
Presented at the Annual Meeting of American Association for the Study of Liver Diseases, Chicago, 11-15 November, 1994 and the 1st China Japan International Congress of Pathophysiology, Dalian, 10-11 October, 1995
S- Editor: Feng CY L- Editor: Ma JY E- Editor: Liu WX
References
- 1.Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992;267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 2.Nakao A, Taki S, Yasui M, Kimura Y, Nonami T, Harada A, Takagi H. The fate of intravenously injected endotoxin in normal rats and in rats with liver failure. Hepatology. 1994;19:1251–1256. [PubMed] [Google Scholar]
- 3.Freudenberg MA, Galanos C. Alterations in rats in vivo of the chemical structure of lipopolysaccharide from Salmonella abortus equi. Eur J Biochem. 1985;152:353–359. doi: 10.1111/j.1432-1033.1985.tb09205.x. [DOI] [PubMed] [Google Scholar]
- 4.Freudenberg M, Galanos C. Metabolic fate of endotoxin in rat. In: Friedman H, Klein TW, Nakano M, Nowotny A, editors. editors. Advances in experimental medicine and biology. Vol 256. New York: Plenum Press; 1990. pp. 499–509. [DOI] [PubMed] [Google Scholar]
- 5.Tavoloni N. The intrahepatic biliary epithelium: an area of growing interest in hepatology. Semin Liver Dis. 1987;7:280–292. doi: 10.1055/s-2008-1040583. [DOI] [PubMed] [Google Scholar]
- 6.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen XM, Han DW, Noguchi K, Mimura Y, Ohishi M, Harada M. Selective uptake of two types of lipopolysaccharide (LPS) by Kupffer cells and other liver cells. Jpn J Pathophysiol. 1995;4:27. [Google Scholar]
- 9.Morrison DC. Nonspecific interactions of bacterial lipopolysaccharides with membranes and membrabe components. In: Berry IJ, editor. Cellular biology of endotoxin. New York: Elsevier; 1985. pp. 25–30. [Google Scholar]
- 10.Kriegsmann J, Gay S, Bräuer R. Endocytosis of lipopolysaccharide in mouse macrophages. Cell Mol Biol (Noisy-le-grand) 1993;39:791–800. [PubMed] [Google Scholar]
- 11.Kriegsmann J, Bräuer R. Lipopolysaccharide (LPS) binding in subpopulations of mouse peritoneal macrophages. Cell Mol Biol (Noisy-le-grand) 1993;39:783–789. [PubMed] [Google Scholar]
- 12.Lei MG, Stimpson SA, Morrison DC. Specific endotoxic lipopolysaccharide-binding receptors on murine splenocytes. III. Binding specificity and characterization. J Immunol. 1991;147:1925–1932. [PubMed] [Google Scholar]
- 13.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick D, Baranski B, Gourley G, Kreamer B, Loew N. T cell stimulation by normal mouse biliary duct cells in culture: a new model for graft versus host disease. Gastroenterol. 1991;96:A630. [Google Scholar]
- 15.Giroir BP, Johnson JH, Brown T, Allen GL, Beutler B. The tissue distribution of tumor necrosis factor biosynthesis during endotoxemia. J Clin Invest. 1992;90:693–698. doi: 10.1172/JCI115939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann R, Grewe M, Estler HC, Schulze-Specking A, Decker K. Regulation of tumor necrosis factor-alpha-mRNA synthesis and distribution of tumor necrosis factor-alpha-mRNA synthesizing cells in rat liver during experimental endotoxemia. J Hepatol. 1994;20:122–128. doi: 10.1016/s0168-8278(05)80478-7. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology. 1994;20:376–382. [PubMed] [Google Scholar]


