Abstract
AIM: To investigate the relationship between lactate dehydrogenase (LDH) activity or LDH isoenzyme patterns and the pathogenesis of colorectal cancer.
METHODS: Activities of tissue LDH and LDH isoenzyme patterns in 16 patients with colorectal cancer were assayed using spectrophotometric procedures and agarose gel electrophoresis, respectively.
RESULTS: The total and specific activities of LDH were significantly higher in colorectal cancer tissues than those in adjacent noncancerous tissues (P < 0.001). The LDH isoenzyme pattern was also different from that in the control. The percentage of LDH5 doubled and the ratio of LDH4 + LDH5/LDH1 + LDH2 was 3.6 ± 1.4 in cancer tissue, significantly greater than in the control.
CONCLUSIONS: The increased LDH activity in colorectal cancer tissues resulted mainly from the increased LDH5, suggesting that the alteration of LDH activity and isoenzyme patterns were related to the pathogenesis of colorectal cancer.
Keywords: Colonic neoplasms, Rectal neoplasms, Lactate dehydrogenase, Lactate dehydrogenase isoenzymes
INTRODUCTION
Studies on lactate dehydrogenase (LDH) isoenzyme patterns in colorectal cancer tissues have rarely been reported although its total and specific activities have been measured by many authors[1,2]. To study the pathogenesis of colorectal cancer and provide a certain theoretical basis for diagnosis, in the present study we determined the total and specific activities and isoenzyme patterns of LDH in colorectal cancer tissues and in adjacent noncancerous tissues.
MATERIALS AND METHODS
Materials
All samples were obtained surgically and histological examinations were made routinely. The samples were washed with ice cold normal saline to remove contaminated blood and stored at 30 °C.
In our experiment specimens were obtained from rectal cancer (13 cases), colonic cancer (3 cases) and noncancerous tissues taken at 5-8 cm proximal or distal to the edges of the tumor of the same patient. Nine men and seven women were included in the group. All reagents used were “Anala R” grade.
Methods
Preparation of tissue homogenate supernatants 0.3 g tissues were homogenised in 3 mL of 0.01 mol/L Tris HCl buffer (containing 0.001 mol/L DTT, 0.001 mol/L EDTA, pH7.5) and centrifuged at 20000 × g for 20 min at 4 °C (DUPONT RC5C). The supernatants were collected for assay.
Determination of LDH activity Enzymatic activities in tissue extracts were measured by spectrophometric procedures with 2,4-dinitrophenylhydrazine[3]. 1 μmol pyruvate produced at 37 °C for 15 min represented one unit.
Isoenzyme patterns Isoenzyme patterns were assayed by agarose electrophoresis modified according to Lou et al[4]. Gels were scanned at 500 nm using a Dual Wavelength Chromato Scanner (Shimadzu CS-930).
Determination of protein content Protein content was measured by the method of Bradford[5], with bovine serum albumin as standard.
Statistical analysis
All values were expressed as ¯x ± s and Student’s t test was used for intergroup comparison.
RESULTS
LDH activities
Table 1 shows that both the total and specific LDH activities in tumors were significantly higher than those in the adjacent noncancerous tissues (P < 0.001).
Table 1.
Lactate dehydrogenase activities in colorectal cancer tissues and adjacent noncancerous tissues (¯x ± s)
| Tissues | n | Total activities (u/g tissue) | Specific activities (u/mg protein) |
| Cancer tissue | 16 | 62.70 ± 13.50 | 63.41 ± 12.41 |
| Adjacent Control | |||
| Proximal tissue | 16 | 43.15 ± 22.95d | 38.22 ± 19.77b |
| Distal tissue | 16 | 44.81 ± 17.24b | 39.92 ± 15.15b |
P < 0.01,
P < 0.01 vs cancer tissue
LDH isoenzyme patterns
The electrophoretograms of LDH isoenzymes in the diseased foci showed a shift towards the M type (Figure 1). The percentages of LDH1 and LDH2 in tumors decreased significantly in comparison with proximal and distal noncancerous tissues; the percentage of LDH3 decreased while that of LDH4 increased in comparison with distal tissues; the percentage of LDH5 was 2.2 and 2.4-fold higher than that in proximal and distal tissues, respectively. The ratio of LDH4 + LDH5/LDH1 + LDH2 was 3.6 ± 1.4, above the control (Table 2).
Figure 1.
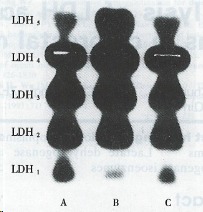
Lactate dehydrogenase isoenzyme patterns. A: proximal tissue; B: cancer tissue; C: distal tissue. LDH: lactate dehydrogenase.
Table 2.
Lactate dehydrogenase isoenzyme patterns in colorectal cancer tissues and adjacent noncancerous tissues (¯x ± s)
| Tissues | n |
LDH isoenzyme (%) |
LDH4 + LDH5/LDH1 + LDH2 | ||||
| 1 | 2 | 3 | 4 | 5 | |||
| Cancer tissue | 16 | 1.65 ± 1.42 | 15.54 ± 3.80 | 26.59 ± 6.25 | 36.63 ± 6.80 | 19.13 ± 8.05 | 3.6 ± 1.4 |
| Adjacent control | |||||||
| Proximal tissue | 16 | 4.93 ± 6.19 | 19.18 ± 5.29a | 30.05 ± 4.04 | 37.03 ± 8.24 | 8.76 ± 6.04e | 2.3 ± 1.2a |
| Distal tissue | 16 | 4.28 ± 2.55b | 22.17 ± 4.57b | 34.38 ± 5.75e | 30.76 ± 5.83a | 8.11 ± 6.32e | 1.7 ± 0.9e |
P < 0.05,
P < 0.01,
P < 0.001 vs cancer tissue. LDH: lactate dehydrogenase.
DISCUSSION
It is well known that glycolysis in cancer tissue increases significantly as a consequence of an important enzyme of the glycolytic pathway LDH that may manifest with a higher activity in a cancer patient’s serum and tissues. Our data showed a significant increase of total and specific LDH activities in cancer tissues, about 140% of the control. These results were consistent with the reports by Carda-Abella et al[6] and Hong et al[7].
Because of the tissue distribution specificity, LDH isoenzymes may be expressed in different levels. It was necessary to assay LDH isoenzyme patterns while total and specific activities were determined. Our results indicated that the increased LDH5 contributes to the increase of total LDH activity in tumors; the ratio of LDH4 + LDH5/LDH1 + LDH2 also increased greatly, i.e. 3.6 ± 1.4, suggesting that LDH isoenzyme pattern shifts towards the M type. It is the M type LDH that promotes the conversion of pyruvate to lactate, while the H type LDH mainly catabolizes the utilization of lactate. Therefore M type LDH can be found predominantly in colorectal cancer tissues in which anaerobic glycolysis is increased abnormally. Market et al thought that the patterns of isoenzymes were biochemical phenotypes of genes. H and M subunits were controlled by A and B genes, respectively. The findings that LDH isoenzyme patterns shift towards the M type may be related to its abnormal expression of genes, suggesting that studying the expression of LDH genes in colorectal tumors will help to elucidate its pathogenesis. In the comparison of malignant tissues with the control at the distance of 1, 2, 4, 6 and 8 cm from the edge of cancer, Onos[8] found that LDH activity in cancer tissues was very high and it gradually decreased in control tissues surrounding the tumor with a distance from cancer. By studying LDH isoenzyme patterns in precancerous polyps, Onos also found that it shifts towards the M type, indicating that the deviation of LDH isoenzyme patterns in normal tissue could be regarded as early signs of malignancy before the morphological changes.
Our results suggest that the alteration of LDH activity and its isoenzyme patterns are related to the pathogenesis of colorectal cancer and more details will be studied in our laboratory.
Footnotes
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-)
Presented at Acta Academiae Medicinae Shandong, 1996; 34 (1):12-14 (in Chinese)
S- Editor: Yang ZD L- Editor: Ma JY E- Editor: Liu WX
References
- 1.Munjal DD. Concurrent measurements of carcinoembryonic antigen, glucosephosphate isomerase, gamma-glutamyltransferase, and lactate dehydrogenase in malignant, normal adult, and fetal colon tissues. Clin Chem. 1980;26:1809–1812. [PubMed] [Google Scholar]
- 2.Han B, Yu JP, Shen ZX, Luo HS, Yang YM, Wang ZW. Enzymatic analysis of colorectal biopsy specimens in polyps and carcinomas. Shijie Huaren Xiaohua Zazhi. 1989;9:342–345. [Google Scholar]
- 3.Li QY, Xu MZ, Kong XY. Practices of Medical Laboratory Sciences. Wuhan: Hubei People’s Publishing House; 1980. pp. 341–343. [Google Scholar]
- 4.Luo L, Yang ZH. A high sensitive method for determination of LDH isoenzymes. Zhonghua Jianyan Yixue Zazhi. 1992;15:6–7. [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 6.Carda-Abella P, Perez-Cuadrado S, Lara-Baruque S, Gil-Grande L, Nuñez-Puertas A. LDH isoenzyme patterns in tumors, polyps, and uninvolved mucosa of human cancerous colon. Cancer. 1982;49:80–83. doi: 10.1002/1097-0142(19820101)49:1<80::aid-cncr2820490118>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Hong GY, Li JW, Xiao NQ. Systematic studies of human LDH isoenzymes. Acta Sci Nat Univ Pekin. 1988;24:195–201. [Google Scholar]
- 8.Ono S. [Studies on carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), and LDH isozymes in the tissue of colorectal carcinoma] Nihon Geka Gakkai Zasshi. 1983;84:336–348. [PubMed] [Google Scholar]


