Abstract
The aim of this study was to evaluate the efficacy of unfractionated bone marrow cells (BMCs) in attenuating acute kidney injury (AKI) induced by paraquat (PQ) in a mouse model. PQ (55 mg/kg BW) was intraperitoneally injected into C57BL/6 female mice to induce AKI, including renal function failure, glomerular damage and renal tubule injury. Glomerular podocytes were the first target damaged by PQ, which led to glomerular injury. Upon immunofluorescence staining, podocytes depletion was validated and accompanied by increased urinary podocin levels, measured on days 1 and 6. A total of 5.4 × 106 BMCs obtained from the same strain of male mice were injected into AKI mice through the tail vein at 3, 24, and 48 hours after PQ administration. As a result, renal function increased, tubular and glomerular injury were ameliorated, podocytes loss improved, and recipient mortality decreased. In addition, BMCs co-treatment decreased the extent of neutrophil infiltration and modulated the inflammatory response by shifting from pro-inflammatory Th1 to an anti-inflammatory Th2 profile, where IL-1β, TNF-α, IL-6 and IFN-γ levels declined and IL-10 and IL-4 levels increased. The present study provides a platform to investigate PQ-induced AKI and repeated BMCs injection represents an efficient therapeutic strategy.
Paraquat (1,1′-dimethyl-4,4′-bipyridylium dichloride; PQ) is a non-selective herbicide, defoliant, and desiccant that has been widely and commonly used worldwide since the 1960 s1,2. Most cases of PQ intoxication result from suicide attempts or accidental exposure3. Following ingestion, PQ is rapidly distributed to various tissues, especially the kidney, lung and liver4. At 3 hours after PQ exposure, the concentration is highest in the kidney and leads to acute kidney injury (AKI), which magnifies the toxicity in the lung and other organs5,6. In PQ-induced AKI, patients usually develop tubular degeneration and formation of granular eosinophilic cytoplasm in the proximal and distal convoluted tubules5. In clinical practice, immunosuppressive therapy including methylprednisolone (MP), cyclophosphamide (CP), and dexamethasone (DEX) has been prescribed for the treatment of PQ poisoning, although the mortality rate remains high7,8. PQ is not removed by dialysis, and hemodialysis is used only as a supportive treatment for patients who develop kidney failure9. Thus, maintaining renal function in patients suffering from PQ poisoning remains a therapeutically important treatment strategy10,11. However, there is no specific antidote or effective therapy for PQ poisoning; therefore, effective therapies are urgently needed.
The mechanism of PQ toxicity involves a series of cyclic reduction-oxidation reactions, with sequential depletion of reduced nicotinamide adenine dinucleotide phosphate (NADPH) and generation of PQ radicals and reactive oxygen species (ROS)12,13. The following pathological changes can be observed after PQ exposure: (1) the renal tubules lose their characteristic appearance, and their lining epithelial cells develop cytoplasmic vacuolation; (2) the glomerulus becomes degenerated, and the renal blood vessels become congested; and (3) the inter-tubular spaces are infiltrated by inflammatory leukocytes14. However, the specific mechanisms of PQ-induced AKI have not been fully elucidated.
The concept of bone marrow-derived cells engraftment involves the differentiation of these cells into functional somatic cells capable of repairing injured tissue. Additionally, bone marrow-derived cells transplantation may support the healing of damaged tissues by exerting potent immunosuppressive effects and secreting soluble factors that regulate the pro-inflammatory cascade to promote tissue remodeling and cellular regeneration15. Many studies have shown that bone marrow-derived cells may be involved in tissue turnover and regeneration, including in the kidney. In particular, unfractionated bone marrow cells (BMCs) have been shown to regenerate tissue after cisplatin-induced acute tubular injury, improve renal function and ameliorate inflammation16. The selective recruitment and localization of bone marrow-derived cells to the kidney vasculature result in structural and functional recovery, as well as increased survival17. Bone marrow cells are incorporated into the glomerulus during recovery from experimentally induced glomerulonephritis18, and bone marrow-derived cells transplantation led to the formation of bone marrow-derived podocytes and mesangial cells in a mouse model of Alport’s syndrome, a hereditary nephropathy19. Given the poor prognosis of AKI, the promise of bone marrow-derived cells transplantation therapy to aid regeneration and organ recovery is an attractive therapeutic strategy.
Oxidative stress and renal tubule injury are important factors in AKI caused by PQ poisoning. Currently, anti-oxidative stress therapy and glucocorticoid treatments are clinically applied. However, the survival rates of patients with PQ poisoning still remain as low. Novel insights into the toxicity mechanisms and potential therapeutic strategy developments are promising to reduce the high mortality of PQ poisoning. In the present study, we first attempted to characterize the mechanisms of PQ-induced toxicity in C57BL/6 mice, including the survival rate, degree of renal function, and pathological alterations. Furthermore, we sought to evaluate whether BMCs were capable of minimizing or preventing kidney injuries induced by PQ. Inflammatory cytokine and chemokine analysis was performed to assess the potential of BMCs transplantation in reducing PQ-induced nephrotoxicity.
Results
BMCs improved survival in PQ-treated mice
PQ exposure is a risk factor for the development of nephrotoxicity. Our experiments first evaluated and determined the dose of PQ that would cause AKI following intraperitoneal (ip) administration in C57BL/6 female mice. Based on previous reports and to simulate the clinical phenomenon of PQ poisoning, PQ was administered ip at doses of 55, 65, and 70 mg/kg BW to evaluate acute toxicity in C57BL/6 female mice. The survival rate was recorded on days 1–6 following administration of PQ. The survival rates of C57BL/6 female mice treated with 55, 60 and 70 mg/kg BW of PQ were 20, 10, and 0%, respectively, on day 6 (data not shown). These results were similar to the mortality rate of clinical patients with PQ-induced nephrotoxicity20. After that PQ treatment at a dose of 55 mg/kg BW, the survival rates of mice that also received BMCs were evaluated from day 1–6. BMCs were identified using flow cytometry analysis showed that most of the BMCs were positive for mesenchymal markers CD105, CD29, and CD90 and negative for markers of hematopoietic lineages CD31, CD34, and CD45 (Supplementary Fig. 1A). After 24 h of adherent growth, the BMCs morphologically resembled fibroblasts (Supplementary Fig. S1B). The treatment process included single or triple BMCs co-treatment, and the detailed protocol is described in the Material and Methods section. Transplanted BMCs being detected on day 3 in PQ + BMCs (3) mice by using PKH26 pre-labeled BMCs in kidney section (Supplementary Fig. S2). After 6 days, the survival rate of PQ-treated animals was 20%, whereas normal saline treatment in the control group had no adverse effect on survival. In the single BMCs co-treatment group (PQ + BMCs (1)), the survival rate was also 20%. However, the triple BMCs co-treatment group (PQ + BMCs (3)) showed a significantly improved survival rate of 60%. Additionally, DEX, an anti-inflammatory steroid drug, did not improve the survival rate of PQ-treated animals (22%) (PQ + DEX) (Fig. 1). This result showed that anti-inflammatory drug therapy with DEX did not efficiently improve survival following PQ poisoning, whereas repeated BMCs co-treatment exhibited a positive effect on survival.
Figure 1. The survival rate of C57BL/6 mice in the various treatment groups.
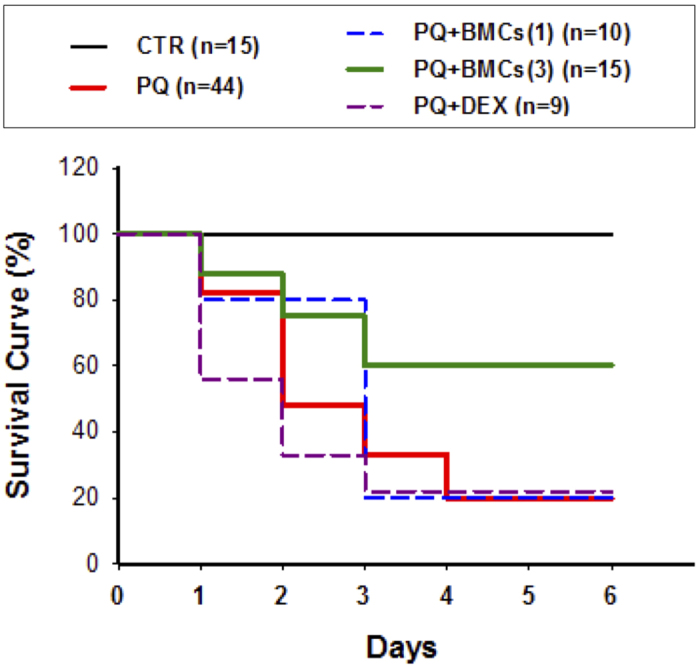
The treatment groups included the control group (CTR, normal saline), PQ group (55 mg/kg BW), PQ + BMCs (1) (single BMCs co-treatment with PQ), PQ + BMCs (3) group (triple BMCs co-treatment with PQ) and PQ + DEX group (10 mg/kg BW DEX co-treatment with PQ). Survival rate was recorded from day 1 to day 6 after PQ treatment.
Triple BMCs co-treatment improved the biochemical profiles of PQ-induced mice
To study the therapeutic effects of BMCs in PQ-induced AKI, renal function was analyzed in PQ-only and triple BMCs co-treated mice. Serum blood urea nitrogen (BUN) and Creatinine (CRE) levels were significantly elevated compared to the control group after PQ administration, with a peak at day 1; the serum BUN level was 145.81 ± 8.66 mg/dl (p < 0.001), and the CRE level was 0.84 ± 0.47 mg/dl (p < 0.001). Cell therapy with triple BMCs injection significantly decreased the serum BUN and CRE levels compared with the PQ-only group (Table 1).
Table 1. The BUN, CRE and total protein levels in serum and urine of the control group, PQ-only group and triple BMCs co-treatment with PQ group.
| Serum BUN (mg/dl) |
Serum CRE (mg/dl) |
uPCR |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | PQ | PQ + BMCs (3) | CTR | PQ | PQ + BMCs (3) | CTR | PQ | PQ + BMCs (3) | |
| Day 1 | 27.26 ± 1.39 | 145.81 ± 8.66** | 54.21 ± 22.71## | 0.29 ± 0.09 | 0.84 ± 0.47** | 0.37 ± 0.03**,# | 3.94 ± 1.83 | 15.18 ± 4.15** | 6.19 ± 1.31*,## |
| Day 2 | 25.80 ± 1.49 | 114.38 ± 23.07** | 52.11 ± 25.11## | 0.20 ± 0.05 | 0.71 ± 0.57** | 0.43 ± 0.03## | 3.27 ± 2.07 | 10.78 ± 5.45** | 7.54 ± 1.36*,## |
| Day 3 | 27.73 ± 1.52 | 107.78 ± 25.10** | 40.47 ± 38.40**,## | 0.24 ± 0.05 | 0.73 ± 0.61** | 0.47 ± 0.09** | 2.78 ± 2.09 | 6.63 ± 3.78** | 4.56 ± 1.21*,## |
| Day 6 | 19.93 ± 1.24 | 55.02 ± 5.00** | 34.74 ± 2.51**,## | 0.20 ± 0.01 | 0.63 ± 0.40** | 0.28 ± 0.02**,## | 3.52 ± 2.38 | 6.53 ± 3.36 | 4.35 ± 0.68*,## |
Data are expressed as the mean ± SE n = 6. uPCR, urine protein and CRE ratio.
*p < 0.05 relative to control group; **p < 0.01 relative to control group.
#p < 0.05 relative to PQ group; ##p < 0.01 relative to PQ group.
CTR: control group; PQ: PQ-only group; PQ + BMCs (3): triple BMCs co-treatment with PQ groups.
The urine protein and CRE ratio (uPCR) was significantly higher in PQ-only mice; this ratio reached its highest level of 15.18 ± 4.15 (p < 0.001) following PQ treatment on day 1. Additionally, in triple BMCs co-treated mice, the uPCR was significantly lower than in the PQ-only group and was similar to that in control mice (Table 1). These results showed that triple BMCs co-treatment markedly improved renal function by lowering serum BUN and CRE levels and reducing the uPCR.
Effect of triple BMCs co-treatment on PQ-induced glomerular and tubular injury
Histopathological examination was performed on days 1 and 6 following PQ-only administration, triple BMCs co-treatment and control groups. Kidney sections of control group displayed standard kidney histopathology, such as complete Bowman’s capsule, well-organized glomerulus, renal tubule with distributed brush border and regular nuclear arrangement (Fig. 2A). Kidney sections of PQ-only administration displayed severe histopathological alterations. In the glomerulus, PQ administration induced Bowman’s urinary space decrease, vacuolization in the glomerulus and glomerulus atrophy on days 1 and 6. In the renal tubule, PQ-induced mice displayed severe loss of the brush border, depletion of tubular polarity, and loss of cell nuclei in proximal convoluted tubules on day 6 (Fig. 2B). However, triple BMCs co-treatment markedly attenuated glomerular and tubular injuries by maintaining glomerulus architecture and retaining brush border polarity in renal tubule (Fig. 2C). Quantification of histopathological alterations of PQ administered mice were significantly increased and triple BMCs co-treatment markedly ameliorated histopathological score (Fig. 2D). These results indicated that PQ-induced kidney damage targeted both the glomerulus and renal tubule, although triple BMCs co-treatment successfully attenuated PQ-induced kidney injury.
Figure 2. Histopathological examination of kidney tissue by HE staining.
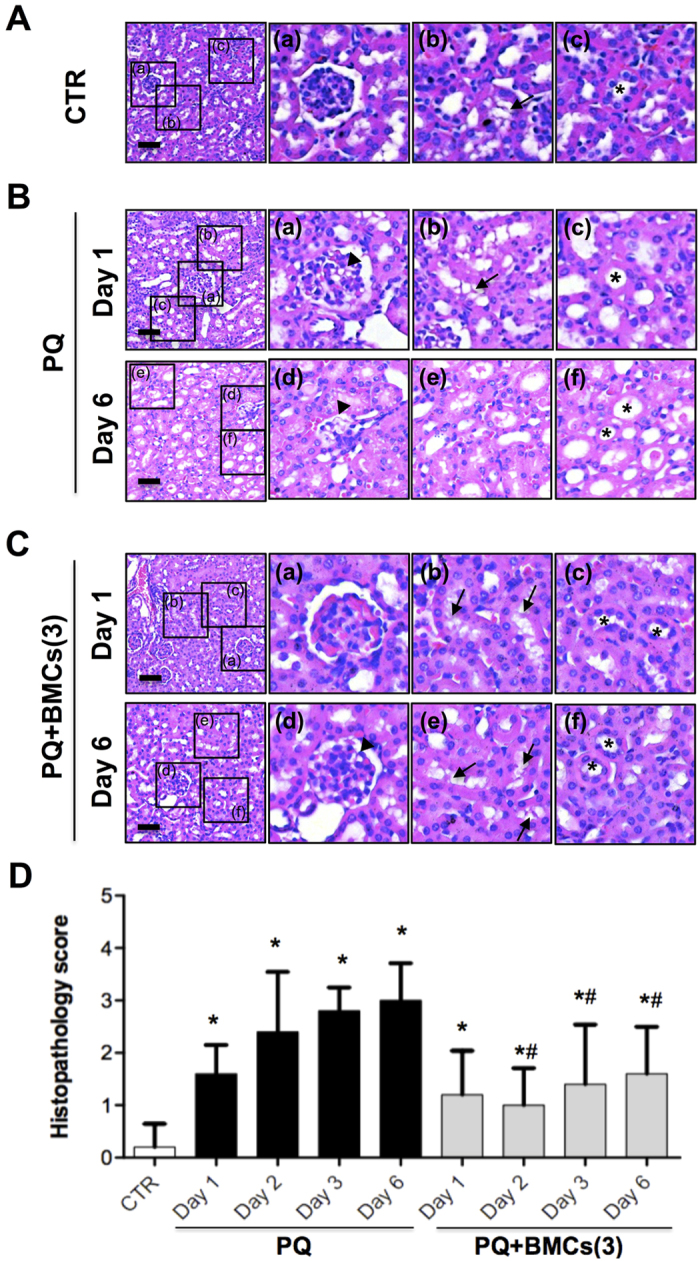
(A) The control group displayed standard kidney histopathology. a, Complete Bowman’s capsule and glomerulus architecture. b, Renal tubule with distributed brush border (arrow). c, Regular nuclear arrangement in renal tubule (asterisk). (B) The PQ-only group displayed histopathological changes. On day 1, kidneys showed mild changes: a, incomplete Bowman’s capsule with vacuolization in the glomerulus (arrowhead); b, mild loss of brush border in the renal tubule; and c, loss of polarity of tubular cells. On day 6, kidneys showed severe histopathological change: d, glomerular shrinkage and decreased space between the glomerulus and Bowman’s capsule (arrowhead); e, loss of brush border in the renal tubule, along with cytoplasmic flattening and loss of the epithelial cells; and f, loss of polarity of tubular cells and degeneration in the renal tubule (asterisk). (C) Triple BMCs administration improved kidney histopathology following PQ treatment. a and d, Complete Bowman’s capsule and glomerulus architecture on day 1 and mild vacuolization in the glomerulus on day 6 (arrowhead). b and e, Renal tubule with distributed brush border (arrows). c and f, Regular nuclear arrangement, and well-development renal tubule (asterisk). Scale bar is 50 μm. (D) Histopathological examination of kidney tissue of PQ administrated mice. Histopathology scoring of renal glomerular degeneration and renal tubule necrosis by a veterinary pathologist: grade 0: no injury; grade 1: minimal injury with less than 10% of cells exhibiting degeneration or necrosis; grade 2: mild injury involving 10–25% of cells; grade 3: moderate injury involving 25–40% of cells; grade 4: marked injury involving 40–50% of cells = grade 4; grade 5: severe injury involving greater than 50% of cells. N = 5, *p < 0.05 vs. control group and #p < 0.05 vs. PQ-only group.
Triple BMCs co-treatment resumed podocytes damage induced by PQ administration
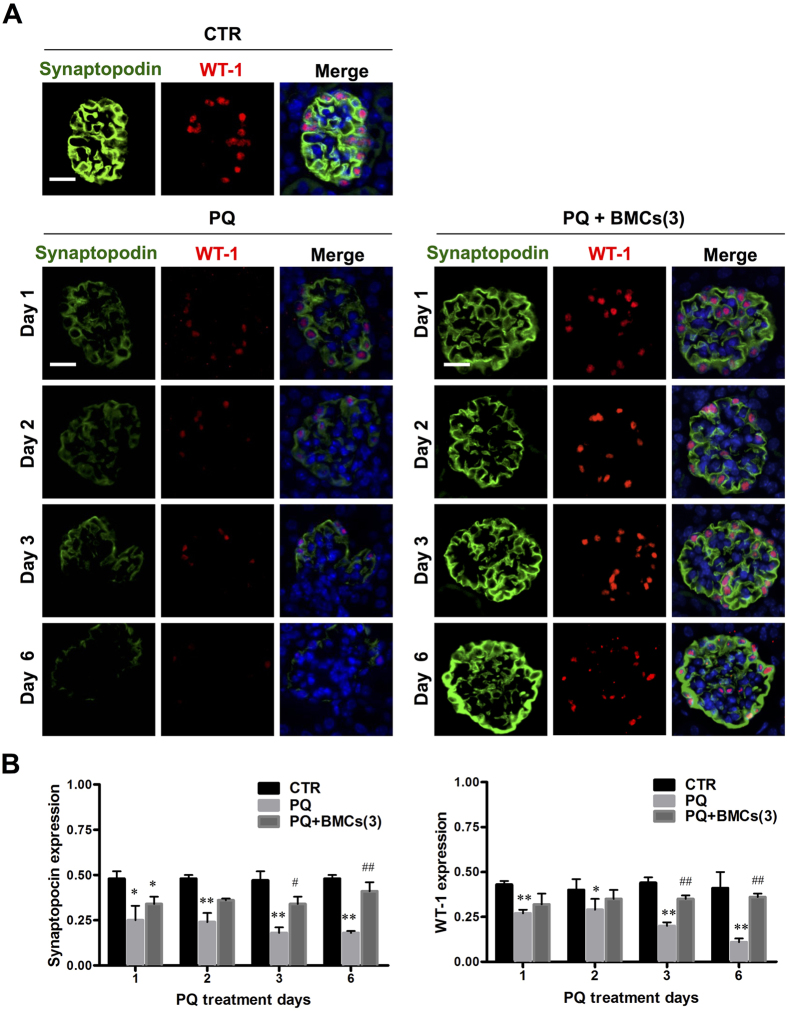
Glomerular injury is often characterized by the effacement of podocytes and loss of slit diaphragms21. Renal biopsies of patients with proteinuria and kidney disease most often are associated with immunofluorescence staining was performed for WT-1 and synaptopodin expression, and the results showed that PQ-only mice displayed severe loss of WT-1 and synaptopodin expression, whereas triple BMCs co-treatment resumed this expression level (Fig. 3A). According to fluorescence quantification, synaptopodin expression in the glomerulus was 0.48 ± 0.02 in the control group, whereas following PQ treatment, synaptopodin expression dramatically decreased to 0.10 ± 0.01 on day 6. WT-1 expression in the glomerulus was 0.41 ± 0.09 in the control group and remarkably decreased to 0.11 ± 0.02 after 6 days of PQ treatment. Triple BMCs co-treatment significantly improved WT-1 and synaptopodin expression compared to the PQ-only treatment group, whereas the levels were similar to those of the control group (Fig. 3B). In vitro, PQ cytotoxicity was evaluated in renal cell lines, including glomerulus podocytes (PDCs), glomerulus mesangial cells (GMCs), renal proximal tubule epithelial cells (PTECs), and renal distal convoluted tubule epithelial cells (DCTCs). Cellular viability analysis showed glomerulus podocytes was the most susceptible to PQ cytotoxicity. The EC50 value of PQ was 70.5 ± 6.3 μM for PDCs, which are approximately 2–12 fold lower than that of the GMCs, PTECs, and DCTCs (Fig. 4). These results showed that glomerular podocytes were the first target for PQ damage and led to glomerular injury, whereas triple BMCs co-treatment markedly attenuated this pathological damage.
Figure 3. Podocytes depletion of C57BL/6 mice in the treatment groups.
(A) Kidney tissue from mice in the control, PQ-only and PQ + BMCs (3) groups were collected on days 1, 2, 3 and 6. Kidney sections underwent double immunofluorescence staining to validate synaptopodin and WT-1 expression in the glomerulus. Synaptopodin is labeled in green (Alex488nm fluorescence) and distributed within the cytoplasm of podocytes. WT-1, a podocytes nuclear marker, is shown in red (Cy3 fluorescence). DAPI was used to stain the nucleus. These images are representative of each group. The control group displayed standard synaptopodin and WT-1 distribution. Following PQ-only treatment, synaptopodin and WT-1 expression dramatically diminished, whereas triple BMCs co-treatment resulted in markedly recovered synaptopodin and WT-1 expression. Scale bar is 50 μm, n = 6. (B) Quantification of glomerular synaptopodin and WT-1 expression in kidneys of the control, PQ-only and PQ + BMCs (3) groups. *p < 0.05, **p < 0.01 vs. control group. #p < 0.05, ##p < 0.01 vs. PQ-only group.
Figure 4. In vitro cytotoxicity of PQ on viability of the glomerulus and renal tubular epithelial cells.
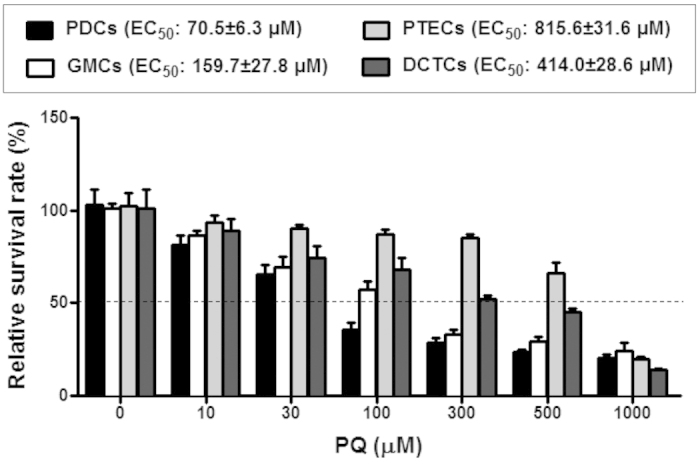
Effects of PQ on cell viability of glomerulus podocytes (PDCs), glomerulus mesangial cells (GMCs), proximal tubule epithelial cells (PTECs), and distal convoluted tubule epithelial cells (DCTCs) determined using the WST-1 assay. Cells were treated with PQ (10, 30, 100, 300, 500, and 1000 μM) for 24 h. Results are expressed as the percent of cell viability compared to the control cells. *p < 0.05, **p < 0.01 vs. control cells.
After glomerulus injury, podocytes can undergo depletion with the appearance in urine of structural and functional podocyte components. Monitoring podocyte loss by measuring urinary specific podocyte antigens may be promising clinically application for AKI diagnosis. The molecular component in podocyte foot processes and slit diaphragms are in the process of being understood, such as podocin, which involved in maintaining slit diaphragms22. In our study, urinary podocin levels were significantly increased following PQ-only treatment (the highest peak was observed at 27.19 ± 5.03 on day 1) and were markedly increased compared with the control group (7.22 ± 2.35, p < 0.001). In contrast, the urinary podocin level was reduced in the triple BMCs co-treatment group (12.72 ± 1.11, p < 0.001) at day 1 and was similar to the level observed in the control group at day 6 (7.26 ± 2.08, p < 0.58 relative to control group) (Fig. 5). These data show that triple BMCs co-treatment resumed podocytes loss induced by PQ administration.
Figure 5. Quantification of urinary podocin in kidneys of the control, PQ-only and PQ + BMCs (3) groups.
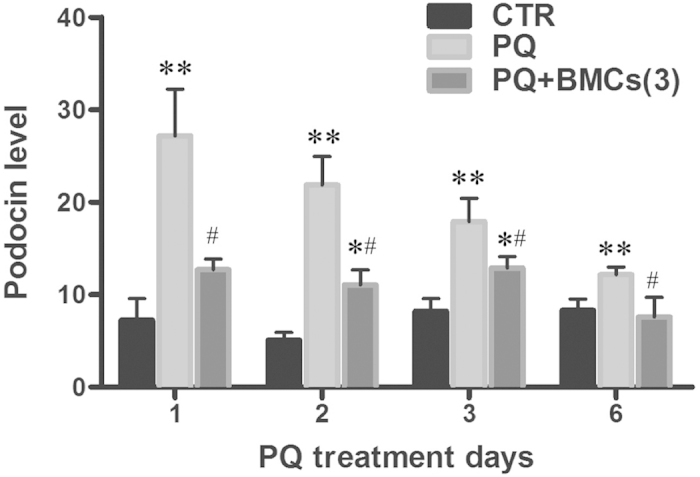
Urine samples from the control, PQ-only, and PQ + BMCs (3) groups were collected on days 1, 2, 3, and 6. Urinary podocin level examined using ELISA. The urinary podocin content was normalized to the urinary CRE concentration. All samples were assayed in triplicate. *p < 0.05, **p < 0.01 vs. control group. #p < 0.05 vs. PQ-only group.
Triple BMCs co-treatment reduced neutrophil infiltration and inflammation in the kidney
Based on immunofluorescence staining, neutrophil infiltration was increased following PQ treatment but was significantly reduced by triple BMCs co-treatment. The number of Ly6G-positive neutrophils in the PQ-only group was elevated, and the highest peak was observed as 4.82 ± 0.28 cells/mm2 on day 3, which was significantly higher than that observed in the control group (0.84 ± 0.03/mm2, p < 0.001). In the triple BMCs co-treatment group, there were significantly fewer Ly6G-positive cells (1.17 ± 0.02/mm2) compared to the PQ-only group (Table 2). Thus, PQ-induced AKI was accompanied with neutrophil infiltration, and triple BMCs co-treatment showed a significant therapeutic effect against this type of inflammation.
Table 2. Quantification of Ly6G expression in kidneys of the control group, PQ-only group and triple BMCs co-treatment with PQ group.
| Neutrophil/mm2 |
|||
|---|---|---|---|
| CTR | PQ | PQ + BMCs (3) | |
| Day 1 | 0.83 ± 0.03 | 1.97 ± 0.18** | 1.63 ± 0.25* |
| Day 2 | 0.84 ± 0.02 | 3.00 ± 0.15** | 1.17 ± 0.03** |
| Day 3 | 0.84 ± 0.03 | 4.82 ± 0.28** | 1.17 ± 0.02**,## |
| Day 6 | 0.83 ± 0.02 | 3.83 ± 0.11** | 1.10 ± 0.03**,## |
Data are expressed as the mean ± SE n = 6.
*p < 0.05 relative to control group; **p < 0.01 relative to control group.
#p < 0.05 relative to PQ group; ##p < 0.01 relative to PQ group.
CTR: control group; PQ: PQ-only group; PQ + BMCs (3): triple BMCs co-treatment with PQ group.
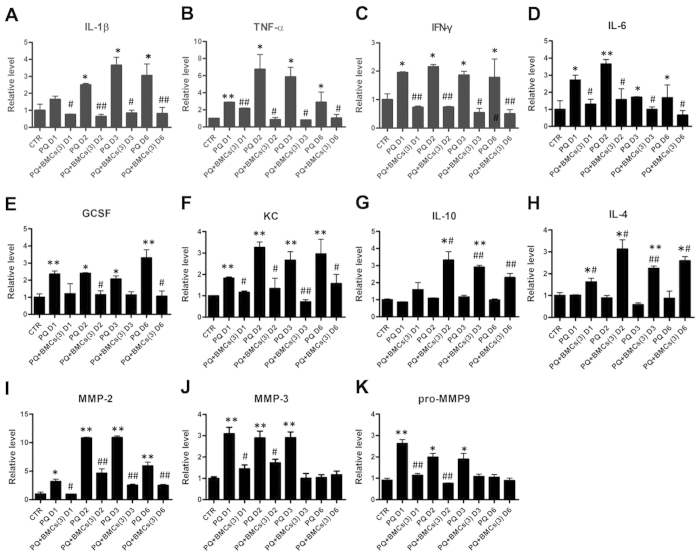
We further evaluated pro-inflammatory cytokines, including interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and interferon-γ (IFN-γ) in kidney supernatants of treated animals. These results showed increased level of various cytokines in the PQ-only group compared with the control group but significantly decreased level in the triple BMCs co-treatment group (Fig. 6A–C). Moreover, as neutrophil recruitment and activation is associated with interleukin 6 (IL-6), granulocyte colony-stimulating factor (GCSF) and keratinocyte-derived chemokine (KC), we observed an increased level of these factors in the PQ-only group (Fig. 6D–F). However, triple BMCs co-treatment efficiently inhibited these factors and induced the production of IL-10 and IL-4 (Fig. 6G,H). In addition to inflammatory factors, matrix metalloproteinases (MMPs) mediate micro-vascular disruption and progression of AKI. In our results, PQ treatment resulted in significantly increased MMP2, MMP3 and pro-MMP9 levels (Fig. 6I–K), whereas levels of the endogenous inhibitors TIMP1 and TIMP2 were decreased (data not shown). Moreover, these MMP level were diminished following triple BMCs co-treatment. Together, these results showed that PQ treatment induced elevated level of numerous inflammatory factors and neutrophil infiltration, whereas triple BMCs co-treatment diminished this level of inflammation cytokines and up-regulated the anti-inflammatory factors, IL-10 and IL-4.
Figure 6. Kidney inflammatory cytokine and chemokine levels in C57BL/6 mice from the different treatment groups.
Kidney supernatants from the control, PQ-only and PQ + BMCs (3) groups were collected on days 1, 2, 3 and 6. The expression levels of (A), IL-1β; (B), TNF-α; (C), IFN-γ; (D), IL-6; (E), GCSF; (F), KC; (G), IL-10; (H), IL-4; (I), MMP2; (J), MMP3; (K), pro-MMP9 were shown relative to the control group. *p < 0.05, **p < 0.01 vs. control group. #p < 0.05, ##p < 0.01 vs. PQ-only group.
Discussion
PQ poisoning is a major public health issue in many developing countries, and acute PQ poisoning leads to a high mortality rate (60–80%) within one week due to AKI23. In the present study, the survival rate of C57BL/6 mice that received triple BMCs co-treatment significantly increased the survival rate to 60%. In our study, PQ administration resulted in acute renal function failure, podocytes depletion, and glomerular injury, which were reversed following triple BMCs injections. Thus, the present study provides a platform to investigate PQ-induced AKI and develop efficient therapeutic strategies.
Many inflammatory mediators produced in response to PQ poisoning are secreted by neutrophils, including elastase, proteases and oxidants. Thus, immunosuppressive therapy has been used in the treatment of PQ poisoning, particularly glucocorticoids, which are widely used therapeutically to achieve immune suppression by inhibiting the NF-κB-mediated transcription of pro-inflammatory cytokines24. However, the utility of these immunosuppressive agents in protecting against PQ poisoning remains controversial8. Many clinical studies have also shown that glucocorticoid treatment did not improve the survival rate of patients with fulminant poisoning20. In recent years, several studies have shown that bone marrow derived cells transplantation has the ability to reduce mortality resulting from AKI15,25. In the present study, C57BL/6 mice with PQ-induced AKI, the conventional immunosuppressive drug DEX had no effect on survival (22%), whereas triple BMCs co-treatment was efficiently improved the survival rate up to 60%, although single administration had no such effect. The ability of BMCs to ameliorate AKI was closely related with the frequency and dosage of BMCs treatment, which indicates that an optimal engraftment protocol warrants further investigation.
In pathological examination, we observed the loss of polarity and brush borders in the proximal convoluted tubules, as well as glomerular injury. Many previous studies of PQ-induced nephrotoxicity have focused on the renal tubule, as kidney damage is primarily localized in the proximal tubule. However, PQ exposure has been associated with various glomerular diseases, such as glomerulonephritis26. Upon histology examination, glomerular injuries were observed in the PQ-only group, with incomplete Bowman’s capsules and vacuolization in the glomerulus on day 1, which further progressed to glomerular shrinkage and decreased the space between the glomerulus and Bowman’s capsule on day 6. As glomerular podocytes play a critical role in numerous glomerular diseases21, we were interested in whether podocytes participated in PQ-induced glomerular injury. Upon immunofluorescence staining, podocytes depletion was immediately identified after PQ treatment from day 1 until day 6, indicated by the loss of expressed synaptopodin and WT-1 protein, two specific podocyte antigens. This scenario is also supported by our in vitro cytotoxicity studies showing that podocytes displayed more sensitivity to PQ treatment than renal tubular epithelial cells or glomerular mesangial cells. Podocytes are highly specialized cells with important roles in maintaining the glomerular filtration barrier and producing growth factors for both mesangial and endothelial cells22,27. Synaptopodin is essential for the integrity of the podocyte actin cytoskeleton and for regulating podocyte migration. Moreover, synaptopodin serves as a marker of podocyte differentiation and maturation28. The WT-1 protein is highly expressed in podocytes and plays an important role in the maintenance of podocytes function22. Previous studies have involved immunofluorescence staining of synaptopodin and WT-1 to monitor damage to podocytes29,30. According to the above discussion results, PQ toxicity targeted and damaged podocytes, leading to glomerular injury. Podocyte loss has been proposed as a more specific marker for ongoing glomerular damage than proteinuria and albuminuria31. In the present study, podocyte loss paralleled the increase in urinary podocin levels, suggesting that PQ-induced AKI is closely associated with the pathological alteration of podocytes. Urinary podocin would thus be a suitable biomarker for the diagnosis and risk stratification of AKI in PQ poisoning. This present study was the first to investigate the involvement of podocytes in PQ-induced glomerular injury. The significance of these findings in relation to PQ induced podocytes damage and AKI deserves further exploration.
In AKI, pro-inflammatory cytokines and chemokines are important factors that initiate inflammation; they also play a leading role in the progression of AKI. PQ poisoning induces glomerulus injury, renal tubular epithelium damage, and leukocyte infiltration generates mediators that potentiate inflammation, including IL-1β, TNF-α, IL-6 and IFN-γ, which favor a Th1 type inflammatory response. Triple BMCs administration following PQ injury resulted in a significant decrease in pro-inflammatory cytokine levels but increased IL-10 and IL-4 level, which are associated with the Th2 anti-inflammatory response. Although they remain poorly understood, BMCs have been shown to possess immunomodulatory properties that result in the inhibition or modulation of the T cell response, and these cells also secrete various growth factors and cytokines. Previous studies have shown that mesenchymal stem cell triggering of the Th2 immune response is critical for renal regeneration following ischemic injury32,33. Our findings indicate that BMCs can modulate the inflammatory response by shifting it from a pro-inflammatory Th1 profile to an anti-inflammatory Th2 profile. Moreover, the beneficial effects of BMCs are primarily mediated via complex paracrine actions that promote a protective response important for tissue regeneration in PQ-induced AKI.
MMPs are a family of zinc-dependent proteases responsible for extracellular matrix turnover, and they are also known to modulate the tissue microenvironment34. Recently, MMPs, particularly MMP2 and MMP9, have been shown to play major roles in pathological lesions of various organs35,36. MMP3 is induced by pro-inflammatory cytokines such as TNF-α and IL-1β and cleaves pro-MMP9 to produce active MMP937. In addition, activated MMP2 and MMP9 are involved in the pathogenesis of kidney injury mediated by PQ administration38. In our studies, we found that PQ treatment induced MMP2, MMP3 and pro-MMP9 activation, which may be regulated by IL-1β, TNF-α, IFN-γ.
In conclusion, PQ treatment targeted and damaged podocytes, leading to glomerular injury. Podocyte damage is considered not only a cause of proteinuria, but also a critical step in the progression of various glomerular diseases. Recent studies indicated that podocytes may aggravate glomerular injury by their capacity to modulate inflammation and produce ROS. Podocytes express the chemokine receptor Toll-like receptor 4, which is up-regulated in glomerulonephritis, where it may mediate glomerular injury by modulating chemokine expression and recruitment of inflammatory cells39,40. Increased ROS generation by podocytes is implicated in the progression of various glomerular diseases such as type I diabetes and puromycin nephrosis. Severe inflammation and oxidative stress are the prime factors in the pathogenesis of kidney injury and high mortality in PQ poisoning. Therefore, to further elucidate the critical role of podocytes would provide new insights into PQ poisoning therapy. Triple BMCs co-treatments attenuated kidney injury, improved renal function, and reduced mortality in PQ-induced AKI. In particular, the anti-inflammatory effects of BMCs on cytokine and chemokine production were likely responsible for the amelioration of kidney injury following PQ administration. Additionally, we found that a single dose of PQ (55 mg/kg BW) administration induced central airways resistance and pulmonary parenchyma resistance elevation and compliance reduction with most significant changes in day 3, in our unpublished data. Triple BMCs co-treatment restored the respiratory function, which also related to the survival benefits. Our study, however, showed that the anti-inflammatory effect of exogenous BMCs was closely related to the frequency of administration. This present study provides substantial evidence for the development of BMCs transplantation therapy for AKI patients, although an optimal BMCs transplantation protocol, long-term safety data, and further analysis of changes to the kidney microenvironment deserve additional investigation.
Materials and Methods
Animal experimental protocol and survival analysis
All animal procedures were reviewed and approved by the Animal Care Committee of the National Taiwan University, College of Medicine (IACUC’s number: 20120432), and the Committee recognizes that the proposed animal experiment follows the Animal Protection Law by the Council of Agriculture, Executive Yuan, R.O.C. and the guideline as shown in the Guide for the Care and Use of Laboratory Animals as promulgated by the Institute of Laboratory Animal Resources, National Research Council, USA. Female C57BL/6 mice aged eight weeks were obtained from the National Taiwan University, College of Medicine, Laboratory Animal Center (Taipei, Taiwan). The animals were randomly divided into experimental groups, and the five groups were treated as follows: (1) the control group (n = 15) was administered 10 ml/kg saline; (2) the PQ-only group (n = 44) was administered PQ (55 mg/kg BW) by ip injection; (3) PQ + BMCs (1) group (n = 10) was treated with single injection of BMCs (1.8 × 106 cells) intravenously at 3 hours after PQ administration; (4) the PQ + BMCs (3) group (n = 15) was treated with triple injection of BMCs (5.4 × 106 cells) intravenously at 3, 24 and 48 hours after PQ administration; and (5) the PQ + DEX group (n = 9) was ip injected with 10 mg/kg of DEX at 3 hours after PQ administration. The survival rate was recorded at days 1–6. The mice were sacrificed on days 1, 2, 3 and 6 after PQ exposure to evaluate the therapeutic efficiency of BMCs and DEX.
BMCs isolation
According recently studies and our unpublished data, VEGF pre-stimulated mesenchymal stem cells (MSCs) existed a better migration capacity, which promoted us to choose the VEGF pre-stimulated BMCs in PQ induced acute kidney injury41,42,43. Ten-week-old C57BL/6 male mice were treated with recombinant mouse vascular endothelial growth factor (VEGF) (80 ng in 100 μl PBS; ip) once per day for 5 days. BMCs were harvested on day 6, the tibias and femurs were flushed three times with ice-cold PBS, and the samples were pooled. The pooled samples were centrifuged at 1,300 g for 5 min, re-suspended in PBS, sieved through a 70-μm mesh, re-suspended in PBS at 1.8 × 108 cells/ml, and kept on ice until use.
The surface marker expression of BMCs was assessed by flow cytometry (Beckman Coulter). BMSCs were suspended (1 × 106 cells/ml) and stained with Allophycocyanin (APC)-conjugated CD105 (Biolegend), APC-conjugated CD90 (Biolegend), R-phycoerythrin (PE)-conjugated CD29 (Biolegend), APC-conjugated CD31 (Biolegend), PE-conjugated CD34 (Biolegend), or PE-conjugated CD45 (Biolegend). After 24 hours adherent culture, the morphology of BMCs were recorded by microscope.
To trace the BMCs in vivo, the BMCs were labeled with the lipophilic fluorescent PKH dye (PKH26, Sigma-Aldrich) according to manufacturer’s instructions, and 96.53 ± 0.91% of BMCs labeled with PKH26 demonstrated evidence of labeling by flow cytometry (Supplementary Fig. S2A). PKH 26 fluorescence detection was performed on frozen sections of kidney on day 3 in PQ + BMCs (3) mice.
Assessment of renal function
Blood and urine samples were collected from the control group, PQ-only group and the triple BMCs co-treatment group on days 1, 2, 3 and 6. Mouse orbital venous blood was obtained using a glass capillary. The urine of each mouse was collected from a metabolic cage on the same day. Serum was used to analyze BUN and CRE. Urine was obtained to analyze urinary total protein and CRE using a Hitachi 7070 automatic biochemical analyzer (Hitachi, Tokyo, Japan).
Histopathological examination
Kidney tissue was excised and fixed in 10% formaldehyde for routine histology techniques. These tissues were dehydrated in an ascending grade of ethanol, cleared in xylene and embedded in paraffin. The paraffin–embedded sample was cut into 3-μm sections for histopathological examination. Tissue sections were de-paraffinized in xylene and stained with hematoxylin and eosin (HE) (Muto Pure Chemicals, 3204–2). Histopathological examinations of the kidneys were performed by an independent pathologist and one of the investigators.
Immunofluorescence staining
Tissue sections (3 μm) were de-paraffinized in xylene and re-hydrated utilizing standard procedures. Antigen retrieval was conducted with citrate buffer (0.01 M, pH 6.0) through heating incubation, and treatment with 3% hydrogen peroxide removed endogenous peroxidase activity. Tissue sections were subjected to blocking in 3% bovine serum albumin (BSA) followed by incubation with the primary antibody overnight. Double immunofluorescence staining was performed using anti-WT-1 antibody (Santa Cruz, sc-14026; 1:500) as a nuclear marker of podocytes and anti-synaptopodin antibody (Santa Cruz, sc-21537; 1:200) as a marker of podocytes. Neutrophil infiltration of the kidney was assessed by anti-Ly6G antibody (1:1,000) identification. After washing, tissue sections were treated with the corresponding secondary antibody and prepared with a relative secondary antibody control. Finally, tissue sections were subjected to DAPI nuclear staining. Photographs were taken with inverted fluorescence microscope (ZEISS, Axiovert 200 M Inverted Microscope), and images were acquired using TissueQuest software (TissueGnostics, Vienna, Austria). Quantification of immunofluorescence was performed using TissueGnostics microscopy scanning and HistoQuest software analysis (TissueGnostics, Vienna, Austria). Quantification of glomerulus damage was performed by analyzing synaptopodin and WT-1 distribution ratios in the glomerulus. One hundred glomeruli were evaluated in each experimental group. Neutrophil infiltration of the kidney was observed by staining with Ly6G, a neutrophil marker. Ly6G-positive cell ratios were measured in five mice from each group.
Urinary podocin level
The urinary podocin level was determined using commercial mouse podocin enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource, MBS722280). The assay procedure was performed according to the manufacturer’s instructions. Urine samples were collected from the control group, PQ-only group and the triple BMCs co-treatment group on days 1, 2, 3 and 6. The urinary podocin content was normalized to the urinary CRE concentration. All samples were assayed in triplicate.
Cytokine level
The cytokine content of the kidneys was measured with a mouse cytokine array that included 97 cytokines (RayBiotech, Norcross, GA, AAM-CYT-3 and AAM-CYT-4). Kidney samples were collected from the control group, PQ-treated group and triple BMCs co-treatment group on days 1, 2, 3 and 6. Kidney supernatant was obtained as follows. First, the kidneys were removed, minced, and homogenized in lysis buffer. The homogenate was then centrifuged at 9,000 × g for 30 min at 4 °C, the pellet was discarded, and the supernatant was stored at –70 °C until use. The cytokine content of the kidney supernatant was performed according to the manufacturer’s instructions. Briefly, the samples were incubated with the membranes for 2 h at room temperature. After washing, the membranes were incubated with biotin-conjugated anti-cytokine primary antibodies, followed by washes and development with HRP-conjugated streptavidin. The membrane was exposed to X-ray film for 30–60 s. The intensities of the cytokine signals were quantified using Image J software (NIH, Bethesda). The densities of the cytokine spots were normalized according to the blank control spot, and a positive spot was used as the internal control to measure the cytokine content of individual samples.
Cell culture and cellular viability assay
Renal cell lines used in this study included murine podocyte (PDCs), murine glomerular mesangial cells (GMCs, MES-13), rat proximal tubular epithelial cells (PTECs, NRK-52E), and murine distal convoluted tubular epithelial cells (DCTCs). PDCs from a conditionally immortalized cell line were cultured, as described previously44. Cells were cultured under growth-permissive conditions at 33 °C in RPMI-1640 medium (Sigma) supplemented with 10% FBS (GIBCO) and 20 U/ml mouse recombinant interferon-gamma (IFN-γ, Sigma). To induce differentiation, podocytes were maintained in non-permissive conditions at 37 °C in the absence of IFN-γ for at least 2 weeks and were used for experiments. GMCs (MES-13) were cultured in DMEM medium (Sigma) supplemented with 10% FBS. PTECs (NRK-52E cells) were cultured in DMEM medium containing 5% FBS and 2 mM glutamine (Invitrogen). DCTCs were maintained in DMEM low glucose medium (Sigma) with 5% FBS. After serum starvation for 16 h, cells were treated with PQ for a range, as indicated. After 24 h, WST-1 assay (Roche) was performed to determine cell viability according to the manufacturer’s instructions. Cell viability was expressed as a percentage of the non-treated group, and the EC50 values were determined.
Statistical analysis
Each experiment was repeated at least three times, and the results are presented as the mean ± SE. Statistically significant differences between groups were determined using Student’s t test. P values less than 0.05 were considered significant.
Additional Information
How to cite this article: Gu, S.-Y. et al. Unfractionated bone marrow cells attenuate paraquat-induced glomerular injury and acute renal failure by modulating the inflammatory response. Sci. Rep. 6, 23287; doi: 10.1038/srep23287 (2016).
Supplementary Material
Acknowledgments
We thank Dr. Kuo-Huang Ling and Dr. Chih-Kang Chiang for helpful discussion and suggestions. This work was supported by research grants from National Science Council, Executive Yuan, Taiwan (NSC-100-2320-B-002-107).
Footnotes
Author Contributions F.-C.P. and S.-Y.G. planned the experiments. S.-Y.G. and S.-Y.L. Performed experiments and gathered data. S.-Y.L and T.-Y.Y. analyzed data and prepared figure. F.-C.P. and S.-Y.G. wrote the manuscript. All authors reviewed the manuscript.
References
- Wesseling C. et al. Paraquat in developing countries. Int J Occup Environ Health 7, 275–286, 10.1179/oeh.2001.7.4.275 (2001). [DOI] [PubMed] [Google Scholar]
- Wilks M. F. et al. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med 5, e49, 10.1371/journal.pmed.0050049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S. J. et al. Paraquat intoxication in subjects who attempt suicide: why they chose paraquat. Korean J Intern Med 24, 247–251, 10.3904/kjim.2009.24.3.247 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisaki E. & Sato H. Tissue distribution of paraquat and diquat after oral administration in rats. Forensic Sci Int 14, 165–170 (1979). [DOI] [PubMed] [Google Scholar]
- Bescol-Liversac J., Paquelin A. & Guillam C. [Ultrastructural study of a renal biopsy in a patient poisoned by paraquat]. Eur J Toxicol Environ Hyg 8, 236–246 (1975). [PubMed] [Google Scholar]
- Hawksworth G. M., Bennett P. N. & Davies D. S. Kinetics of paraquat elimination in the dog. Toxicol Appl Pharmacol 57, 139–145 (1981). [DOI] [PubMed] [Google Scholar]
- Afzali S. & Gholyaf M. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med 11, 387–391, 08114/AIM.009 (2008). [PubMed] [Google Scholar]
- Lin J. L., Lin-Tan D. T., Chen K. H. & Huang W. H. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med 34, 368–373 (2006). [DOI] [PubMed] [Google Scholar]
- Wong O. F., Fung H. T. & Kam C. W. Case series of paraquat poisoning in Tuen Mun Hospital. Hong Kong J Emerg Med 13, 155–160 (2006). [Google Scholar]
- Gawarammana I. B. & Buckley N. A. Medical management of paraquat ingestion. Br J Clin Pharmacol 72, 745–757, 10.1111/j.1365-2125.2011.04026.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samai M., Hague T., Naughton D. P., Gard P. R. & Chatterjee P. K. Reduction of paraquat-induced renal cytotoxicity by manganese and copper complexes of EGTA and EHPG. Free Radic Biol Med 44, 711–721, 10.1016/j.freeradbiomed.2007.11.001 (2008). [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira R. J. et al. Sodium salicylate prevents paraquat-induced apoptosis in the rat lung. Free Radic Biol Med 43, 48–61, 10.1016/j.freeradbiomed.2007.03.014 (2007). [DOI] [PubMed] [Google Scholar]
- Moran J. M., Ortiz-Ortiz M. A., Ruiz-Mesa L. M. & Fuentes J. M. Nitric oxide in paraquat-mediated toxicity: A review. J Biochem Mol Toxicol 24, 402–409, 10.1002/jbt.20348 (2010). [DOI] [PubMed] [Google Scholar]
- Lamfon H. A. A.-R. M. M. Effect of antox and paraquat-induced histological and biochemical changes in kidney of albino rats. Journal of Applied Sciences Research 3, 988–993 (2007). [Google Scholar]
- Wise A. F. & Ricardo S. D. Mesenchymal stem cells in kidney inflammation and repair. Nephrology (Carlton) 17, 1–10, 10.1111/j.1440-1797.2011.01501.x (2012). [DOI] [PubMed] [Google Scholar]
- Morigi M. et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 26, 2075–2082, 10.1634/stemcells.2007-0795 (2008). [DOI] [PubMed] [Google Scholar]
- Li B. et al. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 121, 2211–2220, 10.1161/CIRCULATIONAHA.109.928796 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M. et al. Role of bone marrow cells in the healing process of mouse experimental glomerulonephritis. Pediatr Res 58, 323–328, 10.1203/01.PDR.0000169997.45684.05 (2005). [DOI] [PubMed] [Google Scholar]
- Prodromidi E. I. et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24, 2448–2455, 10.1634/stemcells.2006-0201 (2006). [DOI] [PubMed] [Google Scholar]
- Lin J. L., Leu M. L., Liu Y. C. & Chen G. H. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med 159, 357–360, 10.1164/ajrccm.159.2.9803089 (1999). [DOI] [PubMed] [Google Scholar]
- Jefferson J. A., Alpers C. E. & Shankland S. J. Podocyte biology for the bedside. Am J Kidney Dis 58, 835–845, 10.1053/j.ajkd.2011.03.033 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M. A. Biology of the human podocyte. Nephron Exp Nephrol 95, e87–92, 74324 (2003). [DOI] [PubMed] [Google Scholar]
- Lin J. L., Lin Tan D. T., Chen K. H. & Huang W. H. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Critical care medicine 34, 368–373 (2006). [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira R. J. et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 38, 13–71, 10.1080/10408440701669959 (2008). [DOI] [PubMed] [Google Scholar]
- Marques F. S. et al. Transplantation of bone marrow mononuclear cells reduces mortality and improves renal function on mercury-induced kidney injury in mice. Ren Fail 35, 776–781, 10.3109/0886022X.2013.780660 (2013). [DOI] [PubMed] [Google Scholar]
- Stratta P., Mazzucco G., Griva S., Tetta C. & Monga G. Immune-mediated glomerulonephritis after exposure to paraquat. Nephron 48, 138–141 (1988). [DOI] [PubMed] [Google Scholar]
- Kawachi H. et al. Role of podocyte slit diaphragm as a filtration barrier. Nephrology (Carlton) 11, 274–281, 10.1111/j.1440-1797.2006.00583.x (2006). [DOI] [PubMed] [Google Scholar]
- Asanuma K. et al. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8, 485–491, 10.1038/ncb1400 (2006). [DOI] [PubMed] [Google Scholar]
- Yu D. et al. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 16, 1733–1741, 10.1681/ASN.2005020159 (2005). [DOI] [PubMed] [Google Scholar]
- Matsui K. et al. Clinical significance of tubular and podocyte biomarkers in acute kidney injury. Clin Exp Nephrol 15, 220–225, 10.1007/s10157-010-0384-y (2011). [DOI] [PubMed] [Google Scholar]
- Wickman L. et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol 24, 2081–2095, 10.1681/ASN.2013020173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togel F. et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289, F31–42, 10.1152/ajprenal.00007.2005 (2005). [DOI] [PubMed] [Google Scholar]
- Semedo P. et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol 9, 677–682, 10.1016/j.intimp.2008.12.008 (2009). [DOI] [PubMed] [Google Scholar]
- Nagase H., Visse R. & Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69, 562–573, 10.1016/j.cardiores.2005.12.002 (2006). [DOI] [PubMed] [Google Scholar]
- Rosell A. & Lo E. H. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol 8, 82–89, 10.1016/j.coph.2007.12.001 (2008). [DOI] [PubMed] [Google Scholar]
- Chow A. K., Cena J. & Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol 152, 189–205, 10.1038/sj.bjp.0707344 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock D. M. et al. Activation of matrix metalloproteinases following anti-Abeta immunotherapy; implications for microhemorrhage occurrence. J Neuroinflammation 8, 115, 10.1186/1742-2094-8-115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee L., Tuite N., Ryan M. P. & McMorrow T. TNF-alpha and IL-1 beta-mediated regulation of MMP-9 and TIMP-1 in human glomerular mesangial cells. Nephron Exp Nephrol 107, e73–86, 10.1159/000108645 (2007). [DOI] [PubMed] [Google Scholar]
- Banas M. C. et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 19, 704–713, 10.1681/ASN.2007040395 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping P. G. Are podocytes passive or provocative in proteinuric glomerular pathology? J Am Soc Nephrol 19, 651–653, 10.1681/ASN.2008020156 (2008). [DOI] [PubMed] [Google Scholar]
- Togel F., Zhang P., Hu Z. & Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 13, 2109–2114, 10.1111/j.1582-4934.2008.00641.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X. et al. [VEGF promotes the proliferation of bone marrow derived mesenchymal stem cells through ERK1/2 signal pathway]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 18, 1292–1296 (2010). [PubMed] [Google Scholar]
- Yuan L. et al. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol 300, F207–218, 10.1152/ajprenal.00073.2010 (2011). [DOI] [PubMed] [Google Scholar]
- Saleem M. A. et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13, 630–638 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.