Abstract
Epidermal growth factor receptor (EGFR) Tyrosine kinase inhibitor (TKI) is an effective targeted therapy for advanced non-small cell lung cancer (NSCLC) but also causes adverse drug reactions (ADRs) e.g., skin rash and diarrhea. SNPs in the EGFR signal pathway, drug metabolism/ transport pathways and miRNA might contribute to the interpersonal difference in ADRs but biomarkers for therapeutic responses and ADRs to TKIs in Chinese population are yet to be fully investigated. We recruited 226 Chinese advanced NSCLC patients who received TKIs erlotinib, gefitinib and icotinib hydrochloride and systematically studied the genetic factors associated with therapeutic responses and ADRs. Rs884225 (T > C) in EGFR 3′ UTR was significantly associated with lower risk of ADRs to erlotinib (p value = 0.0010, adjusted p value = 0.042). A multivariant interaction four-SNP model (rs884225 in EGFR 3′UTR, rs7787082 in ABCB1 intron, rs38845 in MET intron and rs3803300 in AKT1 5′UTR) was associated with ADRs in general and the more specific drug induced skin injury. The SNPs associated with both therapeutic responses and ADRs indicates they might share a common genetic basis. Our study provided potential biomarkers and clues for further research of biomarkers for therapeutic responses and ADRs in Chinese NSCLC patients.
Non-Small Cell Lung Cancers (NSCLC) make up the major part of lung cancers and are more resistant to chemotherapy and radiation therapy than small cell lung cancers1. Previous research has proved that the hyperactivation of epidermal growth factor receptor (EGFR) pathway is the keystone in NSCLC oncogenesis2,3. EGFR, located on the cell surface, activates proliferative and cell-survival signals by triggering the downstream kinase (such as AKT1)4. Based on the above molecular mechanism, targeted drug EGFR tyrosine kinase inhibitors (TKIs) (e.g. erlotinib, gefitinib and icotinib hydrochloride) were developed to treat patients with activating mutations in EGFR5 . Clinical trials show that patients with activating mutations in EGFR responded better when treated with TKI than with chemotherapy6.
TKIs have a distinguishing adverse drug reaction (ADR) profile from chemotherapy and radiation therapy. They significantly lower the risk of typical severe ADRs to chemotherapy (e.g., neutropenia, thrombocytopenia, anaemia, nausea, constipation, increased ALT, fatigue). However, TKIs increase the risk of skin injury (mainly skin rash) and digestive tract injury (mainly diarrhea)7,8, both of which still cause considerable discomfort.
Identifying genetic biomarkers for drug response can facilitate personalized medication, which aims to maximize the therapeutic effect and minimize ADRs according to each individual’s profile, e.g., genetic information. So far, studies have mainly focused on the activating mutations in the tyrosine kinase domain of EGFR and have proved that they are predictive biomarkers of therapeutic response to TKIs9,10,11. However the proper biomarkers for TKIs induced ADRs have not yet been fully investigated.
Previous studies have revealed the mechanism of skin rash and diarrhea and their possible correlations with therapeutic responses. The potential for skin rash to be used as a predictor of therapeutic response to TKIs6,12,13 lies in the fact that skin injuries are “on-target” effects caused by the down-stream inhibition of EGFR signaling that interferes the proper function of epidermal cells14,15,16. Unlike skin rash which is the specific response to the inhibition of EGFR signaling, TKI-induced diarrhea is the general result from interference caused by TKI drug molecules7.
Evidence has shown that SNPs in the EGFR signal pathway, drug metabolism/ transport pathways and miRNA SNPs might contribute to the interpersonal difference of therapeutic responses and ADRs to TKIs. A gene polymorphism that could influence the EGFR tyrosine kinase signaling might also affect the response to TKIs. Besides the coding SNPs in EGFR, the mutations in the regulation sequences of EGFR (promoter17, intron18, 5′ UTR19) also play a role in carcinogenesis by influencing the expression of EGFR. Moreover, the variations in EGFR 5′UTR have been shown to be associated with skin rash (−216G/T)19 and diarrhea (−216 G/T and −191 C/A)20 in NSCLC patients.
In addition to the polymorphism of the EGFR gene, mutations in other genes have also been found to influence the EGFR pathway. The activation of hepatocyte growth factor receptor MET mediates resistance to EGFR TKIs21. As important regulators of gene expression, miRNAs greatly influence the process of carcinogenesis22. Therefore we decided to include miRNA SNPs in our study.
In terms of pharmacokinetics, metabolism (mainly by CYP and UGT family) and transport (mainly by ABC family) of TKIs influenced both therapeutic responses and ADRs. After absorption and distribution, erlotinib and gefitinib are both transported by ATP-binding cassette family protein ABCB1 and ABCG2 and then metabolized in liver by CYP450 family. Erlotinib is metabolized primarily by CYP3A4 and CYP1A1 and marginally by CYP3A5, gefitinib primarily by CYP3A4 and marginally by CYP3A5 and CYP2D6. UGT1A1 is inhibited by erlotinib, CYP2C19 by gefitinib23. CYP2C19 has also been reported to be associated with the pharmacokinetics of icotinib hydrochloride24.
Studies have found the association between drug metabolism/transport genes and ADRs to TKIs. The polymorphisms of ABCG2 were found to be associated with gefitinib induced diarrhea25,26. CYP2D6 genotype of reduced activity were associated with gefitinib-induced skin rash27. However, a study conducted with 31 Japanese samples found that diarrhea were associated with exposure to gefitinib in plasma but not with common variations in metabolism and transport genes28.
So far the pharmacogenetics association studies of TKIs have mainly focused on the single aspect of either therapeutic response or ADRs, and have been conducted mainly among Caucasian populations. In order to facilitate personalized medication among the Chinese population, we conducted biomarker study of therapeutic response and ADRs in 226 Chinese advance NSCLC patients. Based on the previous findings, we selected SNPs from EGFR signal pathway, drug metabolism/ transport pathway and miRNA SNPs for analysis.
Results
Patient Characteristics
The general characteristics of the patients are shown in Table 1. The patients who took different TKIs had similar age, progression free survival (PFS), occurrence rate of adverse reaction, objective responses. However, the gender ratio varied in the 3 groups. The patients who had objective response to icotinib hydrochloride showed lower occurrence rate of skin injury but the association between skin rash and therapeutic response still existed among these patients (Table 2).
Table 1. The characteristics of the patients.
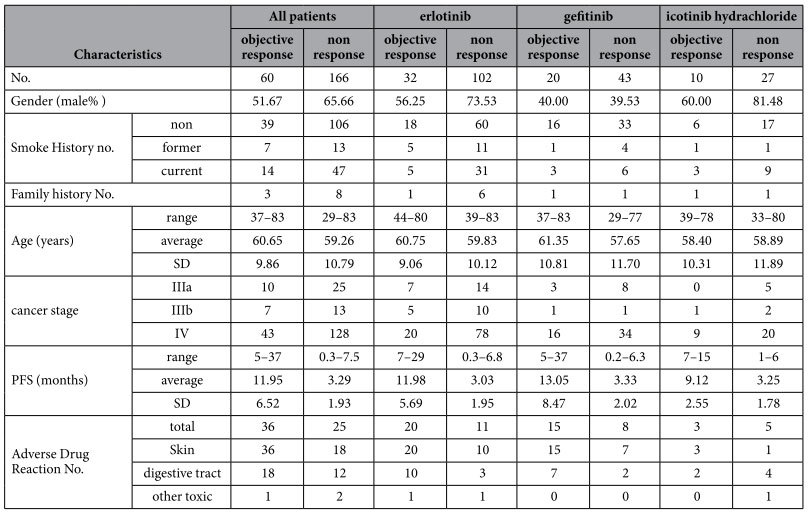
SD: standard deviation; No. : the number of.
Table 2. The correlation of therapeutic responses and ADRs among patients.
| Drug | PFS | objective reaction | skin injury | |
|---|---|---|---|---|
| erlotinib | PFS | — | 0.766** | — |
| ADR | 0.559** | 0.540** | — | |
| skin injury | 0.559** | 0.540** | — | |
| digestive tract injury | 0.533** | 0.438** | 0.584** | |
| geftinat | PFS | — | 0.676** | — |
| ADR | 0.390** | 0.545** | — | |
| skin injury | 0.415** | 0.573** | — | |
| digestive tract injury | 0.223 | 0.404** | 0.462** | |
| icotinib hydrachloride | PFS | — | 0.801** | — |
| ADR | 0.120 | 0.172 | — | |
| skin injury | 0.325* | 0.376* | — | |
| digestive tract injury | 0.110 | 0.062 | 0.555** |
*p < 0.05,
**p < 0.01.
We found that the therapeutic responses and ADRs were correlated among the patients as shown in Table 2. As expected, PFS and objective response, which are both indicators of therapeutic response, were highly correlated: among the patients who responded, their PFSs were similar no matter which drug they took. The same went with patients who did not respond. Patients who objectively responded to TKIs had approximately 1 year FPS, while PFS of those who did not was approximately 3 months. ADRs, especially skin injury were correlated with therapeutic reactions. However, digestive tract injury was less correlated. This tendency was more obvious among patients who took icotinib hydrochloride.
SNPs Associated with drug response and adverse drug reactions
As shown in Fig. 1, we found 9 SNPs from EGFR pathway and drug metabolism genes associated with objective response, 13 SNPs mainly from drug metabolism and transport genes associated with ADRs. 4 SNPs located in EGFR, CYP2C9, CYP2C19 and miRNA MIR141 were shared by the objective response group and ADR group. However, most associations found in this study did not survive multiple testing correction.
Figure 1. The SNPs associated with therapeutic responses and ADRs.
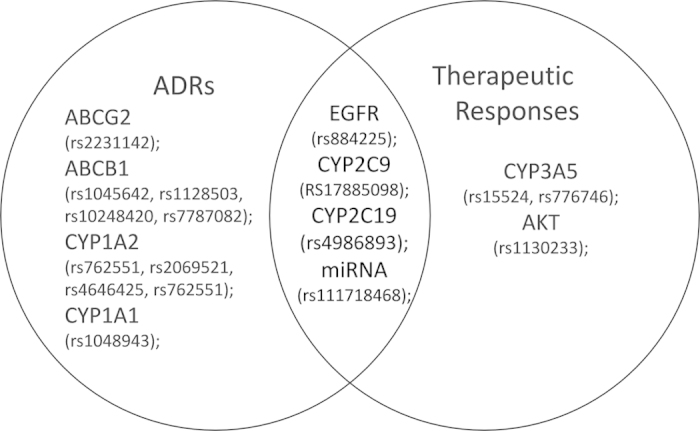
EGFR 3′UTR rs884225 was most significantly associated with both objective response to drug and ADR of all the SNPs analyzed in this study (Table 3). The association of its T > C allele with lower risk of ADR induced by erlotinib survived Bonferroni correction and FDR correction (unadjusted p value = 0.0010; adjusted p value = 0.042).
Table 3. SNP sites associated with therapeutic responses and ADRs.
| Classification | Gene | SNP | P value | phenotype | Genotype number(frequency) | HWE p value | ||
|---|---|---|---|---|---|---|---|---|
| objective response to drug | EGFR | rs884225 | 0.0226 | T T | T C | C C | ||
| positive | 20(0.333) | 31(0.517) | 9(0.150) | 0.5916 | ||||
| negative | 29(0.175) | 94(0.566) | 43(0.259) | 0.0700 | ||||
| ABCG2 | rs2231142 | 0.0828 | A A | A C | C C | |||
| positive | 8(0.138) | 32(0.552) | 18(0.310) | 0.2959 | ||||
| negative | 11(0.067) | 79(0.482) | 74(0.451) | 0.0955 | ||||
| C C | C T | T T | ||||||
| objective response to erlotinib | CYP2C9 | rs17885098 | 0.0191 | positive | 2(0.062) | 5(0.156) | 25(0.781) | 0.0456 |
| negative | 0(0.000) | 9(0.088) | 93(0.912) | 0.6411 | ||||
| A A | A G | G G | ||||||
| CYP2C19 | rs4986893 | 0.0332 | positive | 1(0.031) | 5(0.156) | 26(0.812) | 0.2628 | |
| negative | 0(0.000) | 5(0.052) | 91(0.948) | 0.7934 | ||||
| A A | A G | G G | ||||||
| AKT1 | rs1130233 | 0.0433 | positive | 2(0.100) | 15(0.750) | 3(0.150) | 0.0243 | |
| negative | 14(0.326) | 18(0.419) | 11(0.256) | 0.2981 | ||||
| C C | C T | C T | ||||||
| positive | — | 2(0.100) | 18(0.900) | 0.8139 | ||||
| miRNA | rs111718468 | 0.0373 | negative | — | 0(0.000) | 42(1.000) | 1.0000 | |
| T T | T C | C C | ||||||
| ADR to TKIs | EGFR | rs884225 | 0.0018 | positive | 23(0.377) | 27(0.443) | 11(0.180) | 0.5367 |
| negative | 26(0.158) | 98(0.594) | 41(0.248) | 0.0111 | ||||
| C C | C T | T T | ||||||
| ABCB1 | rs1045642 | 0.0462 | positive | 28(0.459) | 25(0.410) | 8(0.131) | 0.5239 | |
| negative | 47(0.285) | 92(0.558) | 26(0.158) | 0.0864 | ||||
| A A | A G | G G | ||||||
| ABCB1 | rs10248420 | 0.0434 | positive | 16(0.271) | 30(0.508) | 13(0.220) | 0.8804 | |
| negative | 62(0.403) | 76(0.494) | 16(0.104) | 0.2990 | ||||
| C C | C T | T T | ||||||
| ADR to erlotinib | ABCB1 | rs1128503 | 0.0344 | positive | 4(0.129) | 20(0.645) | 7(0.226) | 0.0922 |
| negative | 12(0.115) | 42(0.404) | 50(0.481) | 0.4889 | ||||
| T T | T C | C C | ||||||
| EGFR | rs884225 | 0.0010 | positive | 15(0.484) | 10(0.323) | 6(0.194) | 0.1000 | |
| negative | 17(0.163) | 60(0.577) | 27(0.260) | 0.0933 | ||||
| A A | A G | G G | ||||||
| ABCB1 | rs7787082 | 0.0356 | positive | 10(0.323) | 11(0.355) | 10(0.323) | 0.1061 | |
| negative | 13(0.125) | 51(0.490) | 40(0.385) | 0.5984 | ||||
| A A | A C | C C | ||||||
| CYP1A2 | rs762551 | 0.0126 | positive | 7(0.233) | 20(0.667) | 3(0.100) | 0.0503 | |
| negative | 50(0.510) | 36(0.367) | 12(0.122) | 0.1805 | ||||
| A A | A G | G G | ||||||
| ABCB1 | rs10248420 | 0.0474 | positive | 8(0.267) | 14(0.467) | 8(0.267) | 0.7150 | |
| negative | 37(0.385) | 50(0.521) | 9(0.094) | 0.1748 | ||||
| ADR to gefitinib | CYP1A1 | rs1048943 | 0.1076 | C C | C T | T T | ||
| positive | 3(0.130) | 10(0.435) | 10(0.435) | 0.8416 | ||||
| negative | 1(0.026) | 12(0.308) | 26(0.667) | 0.7804 | ||||
| skin injury induced by TKIs | EGFR | rs884225 | 0.0073 | T T | T C | C C | ||
| positive | 20(0.370) | 24(0.444) | 10(0.185) | 0.5589 | ||||
| negative | 29(0.169) | 101(0.587) | 42(0.244) | 0.0175 | ||||
| T T | T C | C C | ||||||
| skin injury induced by erlotinib | EGFR | rs884225 | 0.0033 | positive | 14(0.467) | 10(0.333) | 6(0.200) | 0.1221 |
| negative | 18(0.171) | 60(0.571) | 27(0.257) | 0.1211 | ||||
| C C | C T | T T | ||||||
| CYP2C9 | rs17885098 | 0.0222 | positive | 2(0.067) | 4(0.133) | 24(0.800) | 0.0205 | |
| negative | 0(0.000) | 10(0.095) | 95(0.905) | 0.6084 | ||||
| C C | C T | T T | ||||||
| ABCB1 | rs1128503 | 0.0305 | positive | 3(0.100) | 20(0.667) | 7(0.233) | 0.0503 | |
| negative | 13(0.124) | 42(0.400) | 50(0.476) | 0.3750 | ||||
| A A | A C | C C | ||||||
| CYP1A2 | rs762551 | 0.0058 | positive | 6(0.207) | 20(0.690) | 3(0.103) | 0.0338 | |
| negative | 51(0.515) | 36(0.364) | 12(0.121) | 0.1663 | ||||
| A A | A G | G G | ||||||
| CYP2C19 | rs4986893 | 0.0113 | positive | 1(0.036) | 5(0.179) | 22(0.786) | 0.3311 | |
| negative | 0(0.000) | 5(0.050) | 96(0.950) | 0.7987 | ||||
| A A | A G | G G | ||||||
| digestive tract injury induced by TKIs | CYP1A2 | rs2069521 | 0.0366 | positive | 1(0.033) | 3(0.100) | 26(0.867) | 0.0585 |
| negative | 0(0.000) | 24(0.123) | 171(0.877) | 0.3599 | ||||
| C C | C T | T T | ||||||
| CYP1A2 | rs4646425 | 0.0361 | positive | 26(0.867) | 3(0.100) | 1(0.033) | 0.0585 | |
| negative | 172(0.878) | 24(0.122) | 0(0.000) | 0.3613 | ||||
| C C | C T | C T | ||||||
| miRNA | rs111718468 | 0.0407 | positive | — | 3(0.100) | 27(0.900) | 0.7731 | |
| negative | — | 5(0.026) | 190(0.974) | 0.8561 | ||||
For the shared 4 SNPs, the alleles associated with more sensitive objective response were also associated with higher risk of ADR except CYP2C9 rs17885098 (T > C). Rs17885098 T allele was associated with objective response to gefitinib (unadjusted p value = 0.049193) while C allele was association with objective response to erlotinib (unadjusted p value = 0.0071) and skin injury induced by erlotinib (unadjusted p value = 0.0189).
For the 13 SNPs associated with ADRs, only 3 SNPs were associated with digestive tract injury (CYP1A2 SNPs rs2069521 G > A, rs4646425 C > T and miRNA SNP rs111718468).
Haplotype Associated with adverse drug reactions
After analyzing all the genotyped genes, 3 blocks were identified in ABCB1 (contain rs1045642, rs7787082, rs10248420, 26kb) CYP3A5-CYP3A4 (contain rs15524, rs776746, rs12333983, rs4646440, rs2242480, 115kb) and AKT1 (contain rs2494732, rs1130233, 18kb) respectively. Rs1045642 and rs7787082 in ABCB1 had a strong linkage with D’ = 96, r2 = 41; rs15524 and rs776746 in CYP3A5 have a linkage with D’ = 96, r2 = 88; rs2494732 and rs1130233 in ATK1 have a linkage with D’ = 94, r2 = 42.
As shown in Table 4, only weak association existed between the haplotypes and ADRs. None of the associations was significant after adjustment.
Table 4. Haplotypes associated with ADRs.
| Phenotype | Haplotype | Case freq. % | Control freq. % | Fisher’s p value | adjusted p value | Odds Ratio [95%CI] |
|---|---|---|---|---|---|---|
| ADRs to TKIs | ABCB1: C A G | 41.4 | 31.5 | 0.046992 | 0.23496 | 1.562 [1.004~2.429] |
| ABCB1: T G A | 31.4 | 42.4 | 0.041685 | 0.208425 | 0.626 [0.398~0.984] | |
| Skin injury induced by TKIs | CYP3A5, CYP3A4: C A A C T | 4.6 | 1.2 | 0.034768 | 0.17384 | 3.816 [1.009~14.436] |
| AKT: C G | 23.8 | 15.2 | 0.03813 | 0.11439 | 1.755 [1.027~2.999] | |
| ADRs to erlotinib | CYP3A5, CYP3A4: C G T C C | 4.8 | 0.8 | 0.033093 | 0.198558 | 6.511 [0.911~46.559] |
| Skin injury induced by erlotinib | CYP3A5, CYP3A4: C G T C C | 5.0 | 0.8 | 0.027835 | 0.16701 | 6.835 [0.955~48.917] |
Multivariant interaction analysis of objective response and adverse drug reaction
We investigated the probable multivariate interactions associated with PFS, objective response, ADRs with multifactor dimensionality reduction (MDR). Of all the possible multivariant models consisting of 2–4 genes, a four-gene model (rs884225 in EGFR 3′UTR, rs7787082 in ABCB1 intron, rs38845 in MET intron and rs3803300 in AKT1 5′UTR) was found to be significantly associated with ADRs as a whole as well as more specific skin injury alone in all the patients undergoing this study (Table 5). None of the 2- and 3-gene models were statistically significant.
Table 5. Multivariant interaction of ADRs and skin injury to TKIs.
| P value | CVC | Bal. Acc. CV Training | Bal. Acc. CV Testing | Bal. Acc. Model Training | Bal. Acc. Model Testing | Bal. Acc. Overall | |
|---|---|---|---|---|---|---|---|
| skin | 0.021 | 9/10 | 0.8522 | 0.6343 | 0.852 | 0.6586 | 0.8472 |
| ADR | 0.032 | 9/10 | 0.835 | 0.6257 | 0.8349 | 0.6676 | 0.8296 |
CVC: cross-validation consistency.
Discussion
TKIs are an effective targeted therapy for advanced NSCLC patients with activating mutations in EGFR but can also cause ADRs, such as skin rash and diarrhea. According to previous findings, the adverse drug reactions (ADRs) of TKIs might be correlated with therapeutic response because of their shared mechanisms. We conducted this study to 1) further identify genetic biomarkers for predicting therapeutic responses and ADRs and 2) analyze the correlation between the therapeutic and adverse responses in Chinese Han population.
In terms of single SNPs analysis, we first identified a strong association between an SNP rs884225 C > T in 3′UTR of EGFR and increased risk of ADR to erlotinib. This association survived Bonferroni correction. SNP rs884225 C > T is very promising potential biomarkers for predicting ADRs to TKIs.
Various studies have shown that activating mutations in the EGFR pathway are associated with improved PFS and improved object response rate. The SNPs in the EGFR promoter and intron were also associated with ADRs to TKIs19,20, but to our knowledge no association between polymorphism in EGFR 3′ UTR and ADRs to TKIs has previously been found.
A previous study may reveal the mechanism underlying the association between rs884225 and responses to TKIs. Chu et al. discovered that rs884225 was significantly associated with bladder cancer risk. According to their bioinformatics analysis, rs884225 polymorphism lay within a predicted binding site for hsa-miR-214, but further in vitro validation found that the rs884225(T > C) alone would increase the expression of EGFR, not necessarily by the modulation of hsa-miR-21429. We predict that 1) SNP rs884225 might affect the response to erlotinib by influencing the expression of EGFR and 2) this influence might exist in normal tissue cells as well as cancer cells, which would lead to a significant association with ADR and much weaker associations with therapeutic response.
In terms of multiple phenotypes and multigenic analysis, we found that therapeutic responses and ADRs to TKIs are correlated, which accords with previous findings indicating that skin rash could be used as a predictor of therapeutic response to TKIs6,11,12. Digestive tract injuries were less correlated with therapeutic responses.
Although many other SNP associations did not survive multiple testing correction, they could indicate weak associations between SNPs and the phenotypes, which could be further validated with larger sample. First, The SNPs that were associated with both therapeutic and adverse responses indicated that therapeutic and adverse responses might share common genetic basics. Secondly, we assumed that TKIs induced diarrhea might have a genetic basis different from that of skin rash and therapeutic responses. This assumption also accords with our current knowledge that TKIs induced diarrhea might result from general interference caused by TKI molecule7 and it is supported by the following evidence: the association between SNPs and digestive injury was weaker than the association between SNPs and skin injury or ADRs as a whole; TKIs induced diarrhea was less correlated with therapeutic responses than TKIs induced skin rash. In addition, previous studies in Caucasian populations found that ABCG2 were associated with diarrhea25,26 but this finding was not repeated in our study. This indicated that the genetics basic of TKIs induced diarrhea might vary with different populations. From all above, we assume it may be possible to develop other population-specific biomarkers or therapy to reduce the risk of digestive tract injury in the treatment of NSCLC driven by EGFR activating mutations.
We also analyzed multivariant interaction among the EGFR signaling pathways, drug metabolism/transport pathways and miRNA with MDR method. A four-genes model (rs884225 in EGFR 3′UTR, rs7787082 in ABCB1 intron, rs38845 in MET intron and rs3803300 in AKT1 5′UTR) was associated with TKIs induced ADRs and skin rash. The model contains 1 SNP in the drug transport pathway, 2 in the EGFR signaling pathway and 1 in a gene that influences the EGFR pathway. In support for the fidelity of this model, some of the SNPs in this model were associated with other drug responses and oncogenesis. The genotype of rs7787082 in ABCB1 was mildly associated with risk of ADRs to erlotinib in this study (unadjusted p value = 0.0356). Allele rs7787082 G was associated with non-response to clozapine in Korean schizophrenia patients30. Rs3803300 was associated with risk of schizophrenia and therapeutic response31,32 and risk of oral squamous cell carcinoma33 and survival of early stage NSCLC34. This multivariant model indicated that ADRs to TKIs might result from gene interaction among multiple pathways.
In conclusion, we found a strong association between SNP rs884225 and ADR to erlotinib. The multivariant model also indicated that ADRs to TKIs might be regulated by multivariate interactions. These positive results are potential biomarkers for predicting ADRs to TKIs. Other predictions made from our study (e.g. the SNPs that were associated with both therapeutic and adverse responses indicated that therapeutic and adverse responses might share common genetic basis) could serve as guideline for further validation and more in-depth biomarker research. Our study helped to implement personalized medication for Chinse NSCLC patients in terms of both theory and application.
Subjects and Methods
Patient recruitment
We recruited 226 NSCLC patients who underwent EGFR TKIs erlotinib, gefitinib and icotinib hydrochloride therapy through our clinical network in Shanghai. We collected their blood sample and clinical records including their gender, age at presentation, cancer family, history, smoking record, cancer diagnosis, pathologic type, stage, medication administration record of adverse reaction and progression free survival (PFS) and blood test results etc.
We gained the patients’ informed consent for their participation. The Ethic Committee of Shanghai Ethical Committee of Human Genetic Resources approved this study. Patient recruiting, blood sample collection, clinical information collection and usage were performed according to the guideline and regulation of the committee.
Genotyping
We genotyped 48 SNP sites in EGFR, AKT1, CMET, CYP1A1, CYP1A2, CYP2C9, CYP2C19, CYP3A4, CYP3A5, UGT1A1, miRNA, ABCB1 and ABCG2. SNP selection were based on the literature review. We predicted the miRNA which possibly influenced the expression of EGFR based on the microRNA database miRBase35. Germline genomic DNA was extracted from blood sample with Axygen Blood Genomic DNA Extraction Mini Kit. Genotyping was first performed with MassArray system (Sequenom, CA, USA). The genotyping was designed with Assay Design Suite 2.0 Software. 10–20 ng of genomic DNA was amplified with Gene Amp® PCR system 9700. The PCR product was then processed with iPLEX Gold assay and MassArray System (Sequenom, CA, USA). The SNP sites that were rejected by Assay Design Suite 2.0 were genotyped with ViiA™ 7 System (life Technologies, Carlsbad, California) using TaqMan®. The genotyping probes were provided by the Applied Biosystems service. The PCR was performed with TaqMan Universal PCR Master Mix reagent kits in 5ul system (Foster City, California, USA) as the product guideline dictated.
Data analysis and statistics
The SNPs with success rate <90%, MAF <1% or homogeneous among all the samples were excluded in the following analyses. 40 SNPs were further analyzed (as shown in detail in supplementary file 1).
To reveal the genetic factors that were potentially responsible for different responses to target drugs to NSCLC, we used Response Evaluation Criteria in Solid Tumors (RECIST) system to evaluate the clinical response. We analyzed the association between the patients’ genotypes and objective response to any of the drugs or specific drug (rated “partially response” versus “stable disease” and “progressive disease” in the first month of medication).
For ADRs we divided the patients in case and control group according to their clinical record on adverse drug reactions. The ADRs in our study were either skin injuries (mainly skin rash except one case of paronychia), digestive tract injuries (mainly diarrhea except one case of nausea and one case of nausea and diarrhea), or both.
The discrepancies of allele and genotype frequency of case and control, odds ratios (ORs) and their 95% confidence intervals (CIs), SNP case-control association analysis and Hardy-Weinberg equilibrium were calculated with SHEsis (http://analysis.bio-x.cn/myAnalysis.php). Haplotype block construction was run by Haploview36 . The haplotype case-control association study was performed with SHEsis.
Multivariant interaction analyses were performed by multifactor dimensionality reduction (MDR) software37. The threshold of statistical significance was p value <0.05 derived from 1000 permutations. The correlation between objective response to TKIs and ADR were calculated with SPSS (http://www-01.ibm.com/software/analytics/spss/).
Additional Information
How to cite this article: Ruan, Y. et al. Genetic Association of Curative and Adverse Reactions to Tyrosine Kinase Inhibitors in Chinese advanced Non-Small Cell Lung Cancer patients. Sci. Rep. 6, 23368; doi: 10.1038/srep23368 (2016).
Supplementary Material
Acknowledgments
We sincerely thank all the patients who agreed to participate in the study and all the staff who helped with sample collection and lab work. We also sincerely thank our colleagues in King’s College London for their great help and inspiration during the manuscript preparation. This work was supported by grants from the 863 Program (2012AA02A515, 2012AA021802), the 973 Program (2010CB529600), the National Key Technology R&D Program (2012BAI01B09), the National Nature Science Foundation of China (81121001, 81273596, J1210047,30900799, 30972823), Shanghai Municipal Commission of Science and Technology Program (11DZ1950300, 09DJ1400601), the Public Health Key Disciplines in Shanghai-Health Microbiology (12GWZX0801), Public Science and Technology Research Funds (201210056), the Shanghai Jiao Tong University Interdisciplinary Research fund, and the Shanghai Leading Academic Discipline Project (B205).
Footnotes
Author Contributions Conception and design: S.Q. Sample process: X.Z., J.J., Y.R., M.L., H.D. and C.M. Analysis: Y.R., J.J., M.L., L.H. and H.D. Interpretation of data: Y.R., J.J., L.G., S.L., H.H. and Y.L. Drafting the article: Y.R., J.J. and S.Q. Critical revision: all authors. Final approval: all authors.
References
- National Cancer Institute. General Information About Non-Small Cell Lung Cancer (NSCLC), http://www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/HealthProfessional/page1 (published in 2014) Date of access 09/05/2015.
- .Bardelli A. et al. Mutational Analysis of the Tyrosine Kinome in Colorectal Cancers. Science 300, 949, doi: 10.1126/science.1082596 (2003). [DOI] [PubMed] [Google Scholar]
- Sawyers C. L. Opportunities and challenges in the development of kinase inhibitor therapy for cancer. Genes Dev 17, 2998–3010, doi: 10.1101/gad.1152403 (2003). [DOI] [PubMed] [Google Scholar]
- Jorissen R. N. et al. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 284, 31–53 (2003). [DOI] [PubMed] [Google Scholar]
- Mayo C. et al. Pharmacogenetics of EGFR in lung cancer: perspectives and clinical applications. Pharmacogenomics 13, 789–802, doi: 10.2217/pgs.12.54 (2012). [DOI] [PubMed] [Google Scholar]
- Rosell R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology 13, 239–246, doi: 10.1016/S1470-2045(11)70393-X (2012). [DOI] [PubMed] [Google Scholar]
- Liu S. & Kurzrock R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treatment Reviews 40, 883–891, doi: 10.1016/j.ctrv.2014.05.003 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The Lancet Oncology 12, 735–742, doi: 10.1016/S1470-2045(11)70184-X (2011). [DOI] [PubMed] [Google Scholar]
- Riely G. J. et al. Clinical Course of Patients with Non–Small Cell Lung Cancer and Epidermal Growth Factor Receptor Exon 19 and Exon 21 Mutations Treated with Gefitinib or Erlotinib. Clinical Cancer Research 12, 839–844 (2006). [DOI] [PubMed] [Google Scholar]
- Mitsudomi T. & Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer science 98, 1817–1824 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist L. V., Bell D. W., Lynch T. J. & Haber D. A. Molecular Predictors of Response to Epidermal Growth Factor Receptor Antagonists in Non–Small-Cell Lung Cancer. Journal of Clinical Oncology 25, 587–595 (2007). [DOI] [PubMed] [Google Scholar]
- Liu H. B. et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 8, e55128. doi: 55110.51371/journal.pone.0055128. Epub 0052013 Jan 0055130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli F., Borgonovo K., Cabiddu M., Lonati V. & Barni S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: A literature-based meta-analysis of 24 trials. Lung Cancer 78, 8–15, doi: 10.1016/j.lungcan.2012.06.009 (2012). [DOI] [PubMed] [Google Scholar]
- Shah D. R., Shah R. R. & Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf 36, 413–426, doi: 10.1007/s40264-013-0050-x (2013). [DOI] [PubMed] [Google Scholar]
- Xu Y., Tan L. J., Grachtchouk V., Voorhees J. J. & Fisher G. J. Receptor-type protein-tyrosine phosphatase-kappa regulates epidermal growth factor receptor function. J Biol Chem. 280, 42694–42700. Epub 42005 Oct 42631 (2005). [DOI] [PubMed] [Google Scholar]
- Mattila E. et al. Negative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat Cell Biol. 7, 78–85. Epub 2004 Dec 2012 (2005). [DOI] [PubMed] [Google Scholar]
- Liu W. et al. A functional common polymorphism in a Sp1 recognition site of the epidermal growth factor receptor gene promoter. Cancer Res 65, 46–53 (2005). [PubMed] [Google Scholar]
- Brandt B. et al. Modification of breast cancer risk in young women by a polymorphic sequence in the egfr gene. Cancer Res 64, 7–12 (2004). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. Epidermal growth factor receptor polymorphisms and clinical outcomes in non-small-cell lung cancer patients treated with gefitinib. Pharmacogenomics J 8, 129–138, doi: 10.1038/sj.tpj.6500444 (2008). [DOI] [PubMed] [Google Scholar]
- Rudin C. M. et al. Pharmacogenomic and Pharmacokinetic Determinants of Erlotinib Toxicity. Journal of Clinical Oncology 26, 1119–1127, doi: 10.1200/jco.2007.13.1128 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardelli A. et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer discovery 3, 658–673, doi: 10.1158/2159-8290.cd-12-0558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B. M., Robles A. I. & Harris C. C. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 10, 389–402. doi: 310.1038/nrc2867. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirl-Carrillo M. et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92, 414–417, doi: 10.1038/clpt.2012.96 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C. J., Liu D. Y., Jiang J. & Hu P. Effect of the CYP2C19 genotype on the pharmacokinetics of icotinib in healthy male volunteers. Eur J Clin Pharmacol 68, 1677–1680, doi: 10.1007/s00228-012-1288-4 (2012). [DOI] [PubMed] [Google Scholar]
- Lemos C. et al. Impact of ABCG2 polymorphisms on the clinical outcome and toxicity of gefitinib in non-small-cell lung cancer patients. Pharmacogenomics. 12, 159–170. doi: 110.2217/pgs.2210.2172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusatis G. et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst. 98, 1739–1742 (2006). [DOI] [PubMed] [Google Scholar]
- Suzumura T. et al. Reduced CYP2D6 function is associated with gefitinib-induced rash in patients with non-small cell lung cancer. BMC Cancer 12, 568, doi: 10.1186/1471-2407-12-568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. et al. Relationship Among Gefitinib Exposure, Polymorphisms of Its Metabolizing Enzymes and Transporters, and Side Effects in Japanese Patients With Non-Small-Cell Lung Cancer. Clin Lung Cancer, doi: 10.1016/j.cllc.2014.12.004 (2014). [DOI] [PubMed] [Google Scholar]
- Chu H. et al. EGFR 3′UTR 774T > C polymorphism contributes to bladder cancer risk. Mutagenesis 28, 49–55, doi: 10.1093/mutage/ges051 (2013). [DOI] [PubMed] [Google Scholar]
- Lee S. T. et al. Association study of 27 annotated genes for clozapine pharmacogenetics: validation of preexisting studies and identification of a new candidate gene, ABCB1, for treatment response. J Clin Psychopharmacol. 32, 441–448. doi: 410.1097/JCP.1090b1013e31825ac31835c (2012). [DOI] [PubMed] [Google Scholar]
- Xu M. Q. et al. Association of AKT1 gene polymorphisms with risk of schizophrenia and with response to antipsychotics in the Chinese population. J Clin Psychiatry. 68, 1358–1367 (2007). [DOI] [PubMed] [Google Scholar]
- Ikeda M. et al. Variants of dopamine and serotonin candidate genes as predictors of response to risperidone treatment in first-episode schizophrenia. Pharmacogenomics. 9, 1437–1443. doi: 1410.2217/14622416.14622419.14622410.14621437 (2008). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Genetic variants in AKT1 gene were associated with risk and survival of OSCC in Chinese Han Population. J Oral Pathol Med. 44, 45–50. doi: 10.1111/jop.12211. Epub 12014 Jul 12224 (2015). [DOI] [PubMed] [Google Scholar]
- Kim M. J. et al. AKT1 polymorphisms and survival of early stage non-small cell lung cancer. J Surg Oncol. 105, 167–174. doi: 110.1002/jso.22071. Epub 22011 Aug 22012 (2012). [DOI] [PubMed] [Google Scholar]
- Kozomara A. & Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68–73. doi: 10.1093/nar/gkt1181. Epub 2013 Nov 1025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J. & Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005). [DOI] [PubMed] [Google Scholar]
- Hahn L. W., Ritchie M. D. & Moore J. H. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 19, 376–382 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


