Abstract
Chemosynthetic symbiosis is one of the successful systems for adapting to a wide range of habitats including extreme environments, and the metabolic capabilities of symbionts enable host organisms to expand their habitat ranges. However, our understanding of the adaptive strategies that enable symbiotic organisms to expand their habitats is still fragmentary. Here, we report that a single-ribotype endosymbiont population in an individual of the host vent mussel, Bathymodiolus septemdierum has heterogeneous genomes with regard to the composition of key metabolic gene clusters for hydrogen oxidation and nitrate reduction. The host individual harbours heterogeneous symbiont subpopulations that either possess or lack the gene clusters encoding hydrogenase or nitrate reductase. The proportions of the different symbiont subpopulations in a host appeared to vary with the environment or with the host's development. Furthermore, the symbiont subpopulations were distributed in patches to form a mosaic pattern in the gill. Genomic heterogeneity in an endosymbiont population may enable differential utilization of diverse substrates and confer metabolic flexibility. Our findings open a new chapter in our understanding of how symbiotic organisms alter their metabolic capabilities and expand their range of habitats.
Introduction
A wide variety of animals have acquired the ability to live on inorganic carbon sources by establishing symbioses with chemoautotrophic bacteria in various different habitats from shallow water to deep sea, such as hydrothermal vents. In the deep-sea vent symbioses, the symbiotic bacteria use the chemical energy of reduced sulphur, methane or hydrogen from vent fluid (Dubilier et al., 2008; Petersen et al., 2011). These bacteria also require sources for assimilation such as carbon dioxide, methane, ammonia, nitrate and so on. The metabolic ability of the symbionts is largely affected by the geochemical conditions, whereas the physicochemical features of the hydrothermal vent environment are highly variable (Zielinski et al., 2011), and the availability of metabolic substrates is unpredictable. Due to the variable nature of hydrothermal vent environments, some host animals have multiple phylogenetically and physiologically distinct endosymbiont species allowing them to use a variety of energy sources, that is, conferring metabolic flexibility, and to occupy various habitats (Fisher et al., 1993; Distel et al., 1995; Woyke et al., 2006; Dubilier et al., 2008; Robidart et al., 2008; Beinart et al., 2012). On the other hand, intra-species subtype variations with single nucleotide substitutions have been reported in some bacterial endosymbionts (DeChaine et al., 2006; Vrijenhoek et al., 2007; Dubilier et al., 2008). However, until recently, genomic diversity on the level of gene composition within a single symbiont species has been considerably underestimated, and we have known little about the ecological importance of such genomic variations.
Here, we report a new and unique genome heterogeneity in a single thioautotrophic endosymbiont population in the deep-sea vent mussel, Bathymodiolus septemdierum. Whole-genome sequencing showed metabolically heterogeneous genomes in a B. septemdierum endosymbiont (BSEPE) population with a single type of 16S ribosomal gene in a single host individual. The symbiont population was composed of several subpopulations that possessed or lacked gene clusters for key metabolic enzymes such as hydrogenase and nitrate reductase. To confirm the generality of the heterogeneity in the BSEPE genome and to examine the proportions of the symbiont subpopulations harbouring each gene cluster within an individual host, we performed quantitative polymerase chain reaction (qPCR) in multiple hosts from two different vent sites. To investigate the distributions of the symbiont subpopulations in the host gill, we conducted in situ hybridization (ISH) analyses. In addition, to test whether the genomic variants corresponding to the symbiont subpopulations exist in the habitat, we performed PCR using DNA extracted from seawater near the Bathymodiolus mussel colony as a template. On the basis of our findings, we discuss possible scenarios for producing the heterogeneous symbiont subpopulations.
Materials and methods
Sample collection and preparation
B. septemdierum was collected from hydrothermal vents on the Myojin Knoll, at depths of 1228 m (Dive #1126), 1249 m (Dive #1127), 1303 m (Dive #1284) and 1278 m (Dive #1288), during the cruises NT10-08 (11–18 May 2010) and NT11-09 (15–26 June 2011), and from Suiyo Seamount at depths of 1386 m (Dive #0679, Dive #0680 and Dive #1286) and 1384 m (Dive #1287) m during the cruises NT07-08 (15–17 May 2007) and NT11-09 (15–26 June 2011) (see also Supplementary Table S1) with the ROV Hyper Dolphin, operated by the R/V Natsushima of the Japan Agency of Marine-Earth Science and Technology. The collected mussels were either frozen immediately in liquid nitrogen or dissected on-board. The gills were cut out using a disposable scalpel and used for DNA extraction or fixed as follows. For ISH analysis, the gills were fixed on-board in 4% paraformaldehyde in 1 × PBS for 16 h at 4 °C, followed by stepwise dehydration in an ethanol series. For RNA extraction, the gills were placed into RNAlater (Qiagen, Venlo, Netherlands), incubated for 16 h at 4 °C, and stored at –80 °C.
Genome sequencing and assembly
Genomic DNA of the symbiont was purified from the gill of a single individual of B. septemdierum from the Myojin Knoll as described previously (Kuwahara et al., 2007). Briefly, the gill was homogenized, and tissue debris was removed by filtration through nylon filters. The bacterial cells were collected by centrifugation and were subjected to DNase digestion to eliminate host DNA. Symbiont DNA was then extracted from the pelleted bacterial cells and sequenced using 454 Titanium (Roche, Basel, Switzerland) with the 3-kb paired-end library. The 454 sequence data yielded 74.5 Mb over 282 803 reads with an average read length of 264 nt. De novo genomic assembly was performed using the GS De Novo Assembler version 2.3 (Newbler, Roche). Assembly of the data resulted in 267 contigs with length >500 bp, totalling 1.5 Mb with G+C content of 36%, and ordered to 25 scaffolds. Together, all reads provided ~40 × coverage of the symbiont genome. Gaps in the genome were closed using PCR amplification and Sanger sequencing. To validate the genomic architecture and nucleotide sequence, the DNA samples were resequenced using PacBio RS (Pacific Biosciences, Menlo Park, CA, USA) and Illumina platforms (see Supplementary Methods for details). The genome sequence and annotation of BSEPE are available at GenBank/EMBL/DDBJ under accession number AP013042. The Illumina/PacBio reads for resequencing analysis are under accession number DRA002953
Construction of the pangenomic sequence of the B. septemdierum symbiont
The PCR results for gap filling suggested that there was structural heterogeneity in the BSEPE genome resulting from the presence or absence of a genomic fragment, or variation in nucleotide sequences with base substitutions and short indels. The architecture of regions with structural heterogeneity was investigated by amplifying PCR products covering the boundaries of both 5′ and 3′ sides of the heterogeneous region. In cases where gaps in the genome could not be filled by PCR because of the presence of more than two alternative architectures at the locus, cloning analysis by the TA cloning method was performed to obtain a single clone from PCR fragments. Finally, a pangenomic sequence of the B. septemdierum symbiont was constructed based on the longer gap sequences.
To validate the genomic architecture and nucleotide sequence, the DNA samples were resequenced using PacBio RS (Pacific Biosciences) and Illumina platforms. A 10-kb SMRT-bell library was constructed according to the manufacturer's protocol and sequenced with eight SMRT cells using C2 chemistry. The longer PacBio RS reads produced 367 131 reads totalling about 757 Mb, with a median of 1707 nt (~505 × coverage). To confirm the structural accuracy and to correct misassembled repeat regions, PacBio read mappings were performed by a BLASTN search against the pangenomic sequence. Next, paired-end Illumina sequencing (2 × 150 nt) generated 59 137 101 reads totalling ~8.87 Gb using one lane of a HiSeq2000 system. These data were mapped to the pangenomic sequence to an average depth of 2065 using CLC genomics workbench (Qiagen) with the default parameter settings. The consensus sequence of the mapped reads was created using the ‘make-consensus' module available in the AMOS package (Treangen et al., 2011).
Detection of DNA fragments from seawater
Seawater near the Bathymodiolus mussel colony on the Myojin Knoll was collected in the Dive #1645 dive of the ROV Hyper Dolphin, operated by the R/V Natsushima NT14-06 cruise of the Japan Agency of Marine-Earth Science and Technology. Microbes in ~1.2 l seawater were collected by filtration through a nitrocellulose membrane with a pore size of 0.22 μm (Millipore, Billerica, MA, USA). DNA was extracted from the collected microbes with DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer's manual. PCR was conducted with the specific primer sets for the membrane-bound uptake hydrogenase (hup) and nitrate reductase (nar) gene clusters. For PCR amplification, La Taq DNA polymerase (Takara, Shiga, Japan) was used with an initial denaturation phase of 94 °C for 1 min, followed by 35 cycles of 96 °C for 20 s, 68 °C for 3 min and 72 °C for 4 min. Symbiont genomic DNA purified from the gill was used as a positive control. Template DNA was omitted in the negative control, and no amplification was observed. The primers used in this study were as follows: Hup_5'F, 5′-TATCATTCAACCTGCCATCCGCAG-3′ Hup_5'R, 5′-AAGAAGCCGATTTGGTGCTCAGAG-3′ Hup_3'F, 5′-TGGTAAACGCATAACCATAACGACG-3′ Hup_3'R, 5′-CAATCAACACCGGAATGATGATGG-3′ Nar_5'F, 5′-TTCACGCCAAGCGATGATTATTCAG-3′ Nar_5'R, 5′-AATACTAATGTTTGGCTTGCCACACG-3′ Nar_3'F, 5′-GAATCGGATGCGAATGAGATTGATAG-3′ Nar_3'R, 5′-TCTAGGTTTCCAATCGGTGTAGCG-3′. Predicted amplicon lengths for these PCR conditions were 1203 bp for Hup_5'F and Hup_5'R, 3287 bp for Hup_3'F and Hup_3'R, 2932 bp for Hup_5'F and Hup_3'R, 1215 bp for Nar_5'F and Nar_5'R, 1496 bp for Nar_3'F and Nar_3'F, and 1895 bp for Nar_5'F and Nar_3'R. The primers were designed to be specific to the BSEPE genome sequence, and primer specificity was verified by comparison with sequence data of other known organisms via a BLASTN search against the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Furthermore, the sequence of each PCR product amplified with DNA from seawater was confirmed by DNA Sanger sequencing and found to match 99.42–100% to our BSEPE genome sequence.
ISH
To amplify the genomic fragments corresponding to the loci targeted in ISH, we designed three primer sets for Locus_D, four for Locus_H and one for Locus_N. These primers produced 5.5–10.9 kb of PCR fragments covering the target locus as a whole (Figure 1). The PCR fragments were labelled with fluorescein or digoxigenin using nick translation kits from Enzo Life Sciences or Roche, respectively (see Supplementary Methods for details). For whole-mount in situ hybridization (WISH) for genomic loci, the fixed gill filaments of two host individuals from Myojin Knoll (Supplementary Table S1) were used. The hybridization reaction with the fluorescein labelled probe was carried out overnight at 37 °C. After incubation in anti-fluorescein-AP (Roche) in PBST with blocking, hybridization was detected using NBT/BCIP solution (Roche) in TMNT buffer (see Supplementary Methods for details). For fluorescent in situ hybridization (FISH), the fixed gills of three host mussels from Myojin Knoll, different individuals from those used for WISH (Supplementary Table S1), were embedded in paraffin and sliced with a microtome into 4-μm-thick sections. Two-colour FISH of the gill sections was performed using probes labelled with fluorescein or digoxigenin and TSA Plus Cyanine 3/Fluorescein System (Perkin Elmer, Waltham, MA, USA) (see Supplementary Methods for details).
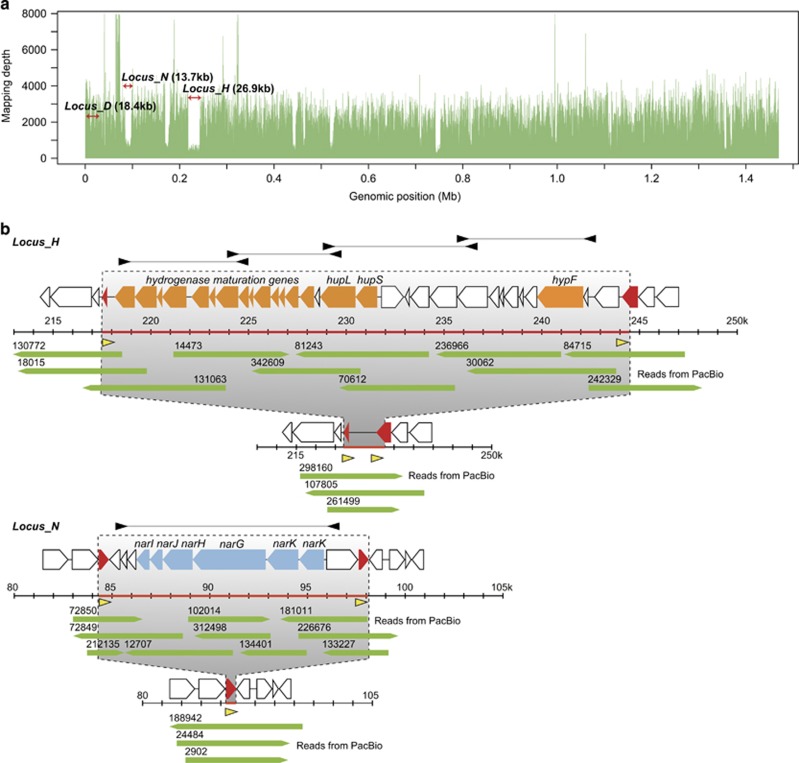
Figure 1.
Loci with low mapping depth including the hup and nar gene clusters. (a) Illumina sequence redundancies mapped on their corresponding genomic positions. The mapping depth of some loci, represented by Locus_H and Locus_N, is lower than that of other genomic regions. (b) Predicted genomic architecture of Locus_H and Locus_N. Cases with or without each gene cluster (hup or nar) are shown. Genes related to the hydrogenase complex and nitrate reductase are indicated with orange and blue arrows, respectively. Duplicated genes (red arrows) are located at both ends of each cluster and form direct repeats (yellow arrowheads). Green arrows indicate PacBio reads. PCR primers used for template preparation of probes for ISH analysis are indicated with black arrowheads.
qPCR
Four adult B. septemdierum specimens were collected from Myojin Knoll, and two adults and six juveniles were collected from Suiyo Seamount (Supplementary Table S1). The shell lengths of the adults and juveniles were 110–120 and 2.8–5.0 mm, respectively. Genomic DNAs from the symbionts were purified from the gills of the six adult individuals, as described previously (Kuwahara et al., 2007). Total DNA was extracted from each of six whole juveniles using a DNeasy Blood and Tissue Kit (Qiagen). The qPCR analysis was performed on an Applied Biosystems 7300 Real-Time PCR System (Life Technologies, Waltham, MA, USA) with the Power SYBR Green PCR Master Mix (Life Technologies), and the threshold cycle (Cq) was determined automatically for each gene. All reactions were carried out in triplicate, including a no template control, with PCR conditions of 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min, and a dissociation stage at the end of the run from 60 °C to 95 °C. A five-fold dilution series (from 1e–5 to 1.6e–8 pmol) of the PCR fragment encompassing the region targeted by each qPCR primer set was used as a template to produce a calibration curve for absolute quantification, and the values acquired with the calibration curves were computed reflecting PCR amplification efficiency (see Supplementary Methods and Supplementary Table S2 for details). Statistical analysis was performed using Microsoft Office Excel for Mac 2011 (Microsoft, Redmond, WA, USA), in which P-values were calculated via Mann–Whitney U-tests. All qPCR analyses followed the MIQE guidelines (Bustin et al., 2009).
Results
Unique heterogeneity in the Bathymodiolus septemdierum symbiont genome
B. septemdierum, a dominant species at hydrothermal vents in the Izu-Ogasawara Arc, Japan, harbours a thiotrophic bacterium (Fujiwara et al., 2000), presumably acquired from the environment (Won et al., 2003). The BSEPE is harboured in the gill epithelial cells called bacteriocytes (Fujiwara et al., 2000), although the mechanism and timing of infection remains unclear. Our assembly of BSEPE genomic sequences yielded a circular chromosome of 1 469 434 bp containing one ribosomal RNA operon and 1471 protein-coding genes (Table 1,Supplementary Figure S1 and Supplementary Table S1). We detected no polymorphisms in the ribosomal gene operon (16S+23S) by 454 sequencing with an average read depth of 40 and Illumina sequencing with an average read depth of >1000. These data indicate that one individual B. septemdierum host harbours a single symbiont ribotype with no polymorphisms. This is in line with previous report of highly similar to identical ribotypes for nineteen 16S rRNA sequences of Bathymodiolus symbionts in 11 host individuals (two host species collected from four different vent sites) (Duperron et al., 2006). In a phylogenetic tree based on 16S rRNA genes, the thiotrophic endosymbionts of Bathymodiolus (including BSEPE) form a robust monophyletic clade with Calyptogena clam endosymbionts and their free-living relatives SUP05 (Supplementary Figure S2).
Table 1. Genomic features of the B. septemdierum symbiont, the related thioautotrophic symbionts and free-living bacteria.
| Symbol | BSEPE | eofBa | SUP05OMZ | SUP05GB1 | SUP05GB2 | Rma | Vok | eofSc |
|---|---|---|---|---|---|---|---|---|
| Organism | Endosymbiont of Bathymodiolus septemdierum str. Myojin knoll | Endosymbiont of Bathymodiolus sp. | Saanich Inlet OMZ SUP05 | Guaymas Basin SUP05 GB-1 | Guaymas Basin SUP05 GB-2 | Candidatus Ruthia magnifica str. Cm | Candidatus Vesicomyosocius okutanii HA | Scaly-foot gastropod endosymbiont |
| Host | Bathymodiolus septemdierum | Bathymodiolus sp. | Free-living | Free-living | Free-living | Calyptogena magnifica | Calyptogena okutanii | Scaly foot gastropod |
| Accession number | AP013042 | CAEB00000000 | GG729945-GG729951, GG729953-GG729965, GG729967-GG730040 | AJXC01000000a | AJXC01000000a | CP000488 | AP009247 | AP012978 |
| Genome size | 1 469 434 | >619 851 | >1 164 035 | >1 246 425 | >1 238 377 | 1 160 782 | 1 022 154 | 2 597 759 |
| GC content | 0.387 | 0.384 | 0.393 | 0.395 | 0.387 | 0.340 | 0.316 | 0.651 |
| State | Complete | Draft | Draft | Draft | Draft | Complete | Complete | Complete |
| Contigs | 1 | 78 | 90 | 100 | 120 | 1 | 1 | 1 |
| Ave. redundancy | 40 | 14.5 | 7.25 | 13.07 | 12.59 | — | — | 65 |
| Protein coding sequences | 1471 | 636 | 1282 | 1535 | 1568 | 976 | 937 | 2249 |
| Pseudogene | 52 | — | 23 | — | — | 100 | 3 | 152 |
| rRNA operon | 1 | — | 1 | 1 | 1 | 1 | 1 | 1 |
| tRNA genes | 35 | 11 | 29 | 34 | 34 | 36 | 35 | 40 |
| Coverage of genes | 0.924 | 0.889 | 0.878 | 0.933 | 0.929 | 0.799 | 0.852 | 0.826 |
| Orthologsb | — | 509 | 948 | 1066 | 1055 | 901 | 877 | 1013 |
| Maximun likelihood distance (Mode) | — | 0.18 | 0.144 | 0.162 | 0.14 | 0.404 | 0.398 | 0.707 |
| Reference | — | Petersen et al. (2011) | Walsh et al. (2009) | Anantharaman et al. (2013) | Anantharaman et al. (2013) | Newton et al. (2007) | Kuwahara et al. (2007) | Nakagawa et al. (2014) |
Accession numbers of individual genomic contigs are shown in the Supplementary Methods section.
Number of orthologs between BSEPE and the reference genome.
The mapping depth of short reads obtained from next-generation sequencing of the genome of BSEPE was obviously lower in some genomic loci than in others (Figure 1a and Supplementary Table S3). The largest of these loci (Locus_H) occupied 26.9 kb and included a cluster of genes encoding key enzymes for hydrogen oxidation (Figures 1a and b and Supplementary Table S3). In the BSEPE genome, we found a membrane-bound uptake hydrogenase (hup) (Vignais and Billoud, 2007) gene cluster consisting of the key structural genes hupL and hupS and other accessory genes (Figure 1b and Supplementary Table S3), as has been reported for other bacteria (Hansel et al., 2001; Brito et al., 2005). The second-largest locus with a low mapping depth (Locus_N, 13.7 kb) included a cluster of genes that encode enzymes involved in dissimilatory nitrate reduction, nitrate reductase (Nar) (Figures 1a and b and Supplementary Table S3). In the BSEPE genome, narG, -H, -J and -I are present in this cluster (Figure 1b and Supplementary Table S3), as in some other bacteria (Blasco et al., 1990; Hartig et al., 1999). Our PCR analysis using primer sets covering these two loci (Figure 2) and the long sequencing reads obtained from a PacBio RS sequencer showed the occurrence of two genomic architectures: one with and the other without these gene clusters (Figure 1b). In our genome sequence data, we did not observe any 16S rRNA sequence likely derived from the close relatives of the symbiont. Furthermore, in BLASTN searches with Locus_H and Locus_N (Figure 1 and Supplementary Table S3), no homologous sequences (coverage >60% and identity >80%) were found in sequence data of other known organisms. These results indicated the presence of genomic heterogeneity in BSEPE within a single host mussel, that is, the BSEPE genome is not uniform, but rather is heterogeneous within a symbiont population with a single type of 16S ribosomal gene. Genomic regions that include the hup or nar gene clusters are absent in some symbiont subpopulations in a population living in one host mussel.
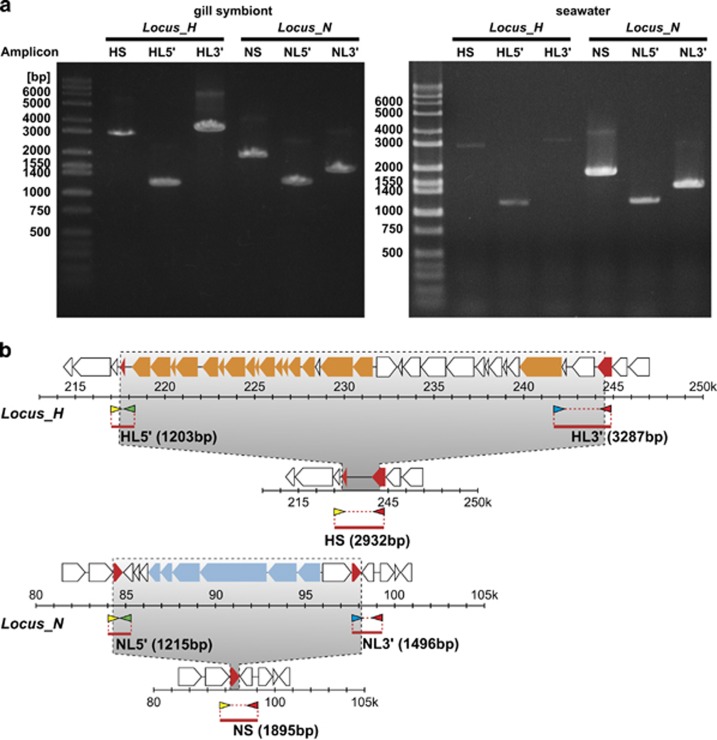
Figure 2.
Detection of DNA fragments that possess or lack the symbiont's hup and nar gene clusters, from seawater near a Bathymodiolus mussel colony. (a) Left, result of PCR using symbiont genomic DNA purified from the gill. Right, result of PCR using DNA extracted from seawater. Detection of amplicon HS or NS indicates lack of the hup or nar gene cluster, respectively, and detection of amplicon HL5′/ HL3′ or NL5′/ NL3′ indicates presence of each cluster. To detect the presence of the hup or nar gene cluster, both the 5′ and 3′ terminal ends of each gene cluster were amplified. In our PCR conditions using Hup_5′F or Nar_5'F primers (represented by yellow arrowheads in (b) and Hup_3′R or Nar_3'R (represented by red arrowheads in (b), an amplicon containing the hup or nar gene cluster was not detected because estimated lengths of these amplicons from our bioinformatic data were too long at 27 865 and 15 178 bp, respectively. (b) Genomic positions of amplicons and specific primers. Genomic architectures of Locus_H and Locus_N with or without each gene cluster (hup or nar) are shown with the original genomic positions. The hup and nar gene clusters are indicated in orange and blue, respectively. Duplicated genes are indicated in red. Primer sets of amplicons HS and NS are indicated with yellow (Hup_5′F or Nar_5′F primers) and red (Hup_3′R or Nar_3′R primers) arrowheads. Primer sets of amplicon HL5′ and NL5′ are indicated with yellow and green (Hup_5′R or Nar_5'R primers) arrowheads, and primer sets of amplicon HL3′ and NL3′ are indicated with blue (Hup_3′F or Nar_3′F primers) and red arrowheads. Lengths of amplicons estimated from our bioinformatic data are shown in the respective parentheses.
Composition and distribution of the symbiont subpopulations
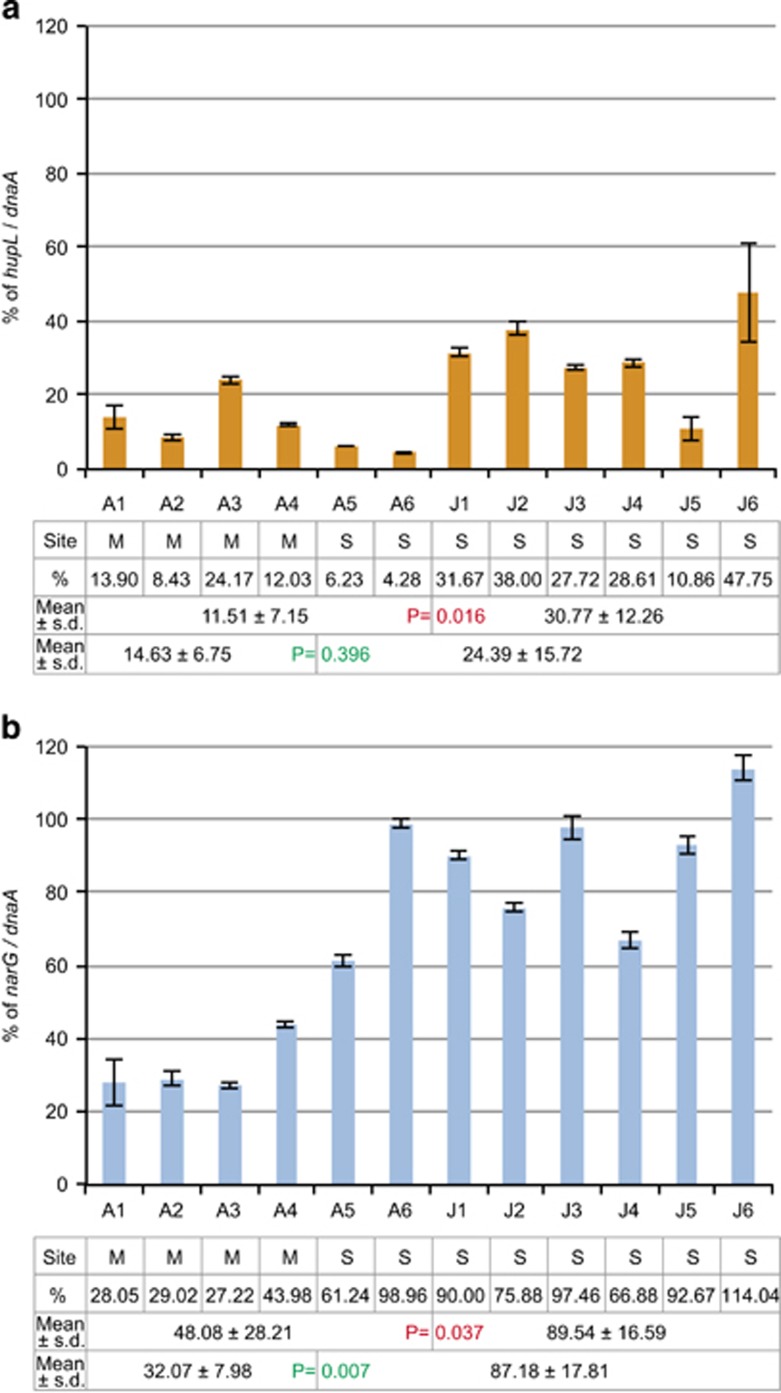
To confirm the generality of this finding, we performed qPCR in multiple host individuals from two different vent sites, Myojin Knoll and Suiyo Seamount. In adult specimens (A1–4 from Myojin Knoll and A5 and 6 from Suiyo Seamount in Figure 3 and Supplementary Table S1), the relative amount of hupL and narG DNA compared with a reference single-copy gene (dnaA) present with average mapping depth (2486 of mapping depth) (Figure 1a) was low (4.28–24.17% for hupL and 27.22–61.24% for narG in Figures 3a and b, respectively), except for narG in one specimen from Suiyo Seamount (98.96%, A6 in Figure 3b). Therefore, genome heterogeneity in BSEPE was common in two different B. septemdierum colonies from geographically separated sites. Furthermore, the relative amount of hupL or narG compared with dnaA in juveniles (from Suiyo Seamount) was higher than that in adult specimens (from Myojin Knoll and Suiyo Seamount) (P-value=0.016 in hupL and 0.037 in narG, Figure 3 and Supplementary Table S1). Also, the relative amount of narG compared with dnaA in specimens from Suiyo Seamount (including adults and juveniles) was higher than that in specimens from Myojin Knoll (adults only) (P-value=0.007 in narG, Figure 3 and Supplementary Table S1). In addition, the sums of the proportions of hupL to dnaA and narG to dnaA in some specimens were >100% (Figure 3), indicating that Locus_H and Locus_N coexist in some symbiont genomes.
Figure 3.
The proportions of the subpopulation harbouring hupL or narG. (a, b) Proportion (%) of symbiont subpopulations with hupL (a) or narG (b) in each host individual. The amounts of DNA of hupL and narG genes relative to DNA of the reference single-copy gene, dnaA on a locus with average mapping depth, were measured by qPCR in six adult individuals (A1–A6) and six juveniles (J1–J6). Error bars indicate the s.d. for three experimental replicates. The table below the chart indicates the vent sites (M, Myojin Knoll and S, Suiyo Seamount), proportions of hupL or narG to dnaA, mean proportions±s.d. in adults and juveniles, and mean proportions±s.d. in two vent sites. P-values by Mann–Whitney U-test between adults and juveniles, and between Myojin Knoll and Suiyo Seamount are shown in red and green, respectively.
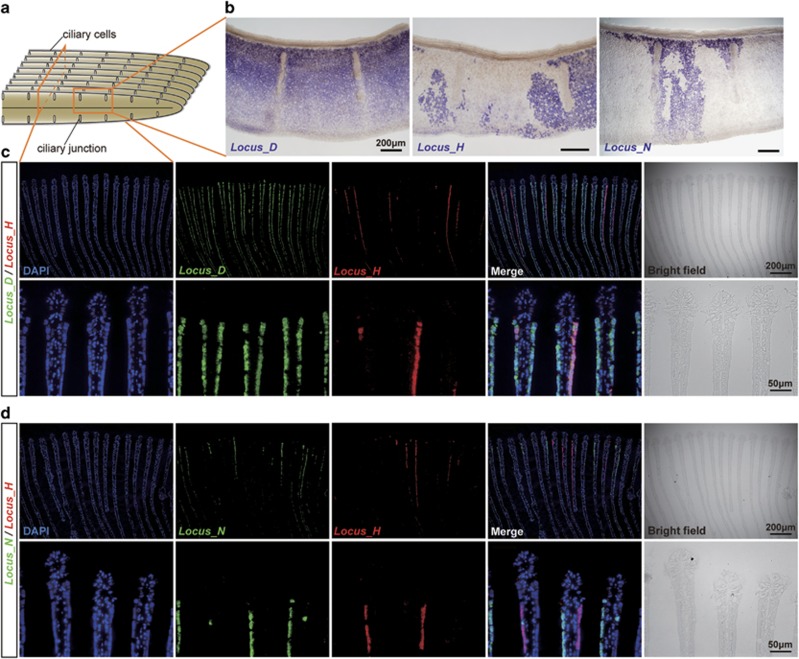
To confirm the occurrence of genomic heterogeneity in the symbiont harboured in the gill, and to investigate the distribution of symbiotic bacteria possessing the hup and nar gene clusters in the gill, we conducted ISH analysis for BSEPE genomic DNA. We used probes for Locus_H, Locus_N and Locus_D (which includes the reference dnaA gene) (Figure 1a). Locus_H and Locus_N were detected in patches in the whole-mount gill filaments and the gill sections, whereas Locus_D was detected in all the bacteriocytes (Figure 4 and Supplementary Table S1). The proportions of the area of fluorescence signals for Locus_H and Locus_N compared with that of Locus_D were comparable to the results obtained by our qPCR (Figure 3 and Supplementary Figure S3A, B). Double staining of Locus_H and Locus_N showed that symbiont subpopulations containing these loci did not make up the entire population of the gill symbiont (Figure 4d). Therefore, symbiotic bacteria that lack both Locus_H and Locus_N are common throughout the bacteriocytes.
Figure 4.
Distribution of symbiont subpopulations harbouring Locus_H or Locus_N in the gill. (a) Schematic drawing of the gill filaments. (b) Whole-mount in situ hybridization was performed for Locus_D, Locus_H and Locus_N (purple-blue signals). (c, d) Cross sections of gill filaments stained using fluorescence in situ hybridization and counterstained with DAPI staining (blue). (c) Double detection of Locus_D (green) and Locus_H (red). Lower panels show higher magnification. (d) Double detection of Locus_N (green) and Locus_H (red). Lower panels show higher magnification. For all panels in (b–d), frontal is to the top.
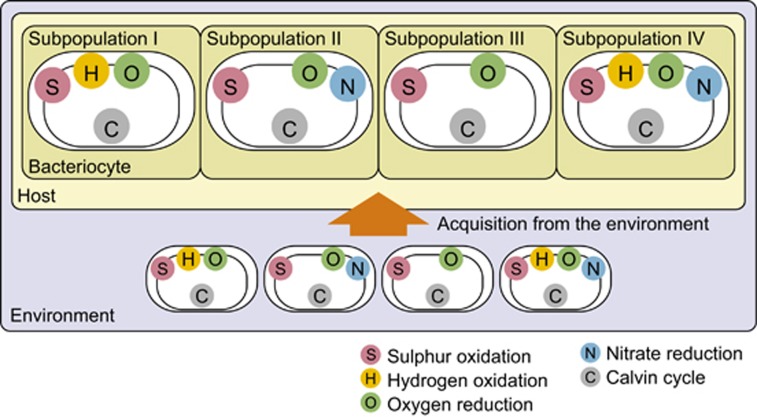
We conclude that each B. septemdierum individual harbours heterogeneous subpopulations of the sulphur-oxidizing symbiont with different sets of genes encoding key metabolic enzymes such as Hup and Nar. There are at least four subpopulations: subpopulation I with hup genes but without nar genes, subpopulation II with nar genes but without hup genes, subpopulation III without either gene and subpopulation IV with both genes (Figure 5). In addition, the finding that some other loci had low-mapping depths suggests the presence of other subpopulations with different genetic compositions (Supplementary Table S3 and Supplementary Figure S4). Thus, the genome sequence of BSEPE we report here is a pan-genome from a number of subpopulations with a single ribotype.
Figure 5.
Model for production of heterogeneous symbiont subpopulations in a host individual. Genomic variants of the symbiont corresponding to subpopulations I–IV (or more) exist in the environment and are acquired from the environment in a host individual and localized in patches in the gill by unknown mechanisms.
Coexisting genomic variants corresponding to the symbiont subpopulations in the surrounding seawater
If B. septemdierum acquires its symbionts from the environment, similarly to other Bathymodiolus species (Won et al., 2003), the heterogeneous symbiont subpopulations within an individual host may reflect coexisting genomic variants corresponding to subpopulations I–IV (or others) in the habitat. We performed a PCR analysis of seawater near a Bathymodiolus mussel colony and detected DNA fragments that either possessed or lacked the symbiont's hup and nar gene clusters (Figure 2). Therefore, symbiont variations corresponding to subpopulations I–IV likely exist in the surrounding seawater. The amount of DNA recovered from seawater near the Bathymodiolus mussel colony was too small to conduct qPCR using it; therefore, the relative proportion of the symbiont variations in the environment remained unclear.
Discussion
Possible scenarios for producing the heterogeneous subpopulations
Here we have shown that a symbiont population was composed of several subpopulations possessing one of the heterogeneous genomes, each of which had or lacked gene clusters for key metabolic enzymes such as hydrogenase and nitrate reductase. Petersen et al. (2011) reported that hydrogen could be used as an energy source by thiotrophic symbionts of Bathymodiolus mussels from the Mid-Atlantic Ridge. Nitrate respiration has been reported in some symbiotic bacteria (Hentschel and Felbeck, 1993; Hentschel et al., 1996), and it has been recently shown that nitrate reductase is also used for nitrate assimilation by chemoautotrophic symbioses (Liao et al., 2014).
The different symbiont variants might have been produced by the gain or loss of gene clusters in specific symbiont populations. Recently, Kleiner et al. (2012) proposed that the ancestor of Bathymodiolus symbionts acquired hupL through lateral gene transfer. Our phylogenetic analysis of hupL gene products supports this idea. The hupL gene might have been laterally transferred into the lineage of the clade that includes Bathymodiolus symbionts and SUP05 (Supplementary Figure S5A). As the genomic synteny of the hup gene clusters was relatively well conserved in this clade (Supplementary Figure S6A), hup genes probably moved intergenomically as a cluster. The most parsimonious explanation for the phylogenetic relationship between the core genomes of chemoautotrophic sulphur-oxidizing symbiotic and free-living bacteria and their hydrogen oxidation cluster (Supplementary Figure S6B) is that the shared ancestor of the Bathymodiolus symbionts and free-living SUP05, as well as the Calyptogena symbiont, already harboured the hup gene cluster, which might have been gained by lateral gene transfer into the ancestral lineage. The hup gene cluster was not found in one metagenomic datum of SUP05 (Walsh et al., 2009) or Calyptogena symbiont lineages (Kuwahara et al., 2007; Newton et al., 2007). These situations can be accounted for by loss of hup genes from these lineages. Similarly, the hup gene cluster was probably lost from some BSEPE populations. Thus, at least for hup genes, the genomic variations in BSEPE could have been produced by the loss of gene clusters in specific symbiont populations. In contrast to HupL, NarG from BSEPE, a SUP05 and a Calyptogena symbiont did not form a monophyletic cluster (Supplementary Figure S5B). However, because only three NarG sequences are currently available in these taxa and their close relatives, the evolutionary history of nar genes is difficult to estimate in the lineage into BSEPE from this limited data set.
Our PCR data indicate that genomic variants of the symbiont also exist in the environment (Figure 2). Then, the genomic variants of the symbiont in the environment might be horizontally transmitted in a host individual likely being unable to discriminate among the multiple metabolic subtypes (Figure 5). In our qPCR, the proportion of the symbiont subpopulation harbouring hup or nar genes in juveniles (from Suiyo Seamount) was higher than that in adult specimens (from Myojin Knoll and Suiyo Seamount) (Figure 3 and Supplementary Table S1). Also, the qPCR results indicated that the proportion of the symbiont subpopulation harbouring nar genes in specimens from Suiyo Seamount (including adults and juveniles) was higher than that in specimens from Myojin Knoll (adults only) (Figure 3 and Supplementary Table S1). These results suggest that the composition of symbiont subpopulations varies depending on the environment (difference within a vent site over time or between two different vent sites) or host developmental stage. Symbiont localization in Bathymodiolus mussels has been reported to shift from a wide range of epithelia in early life stages (⩽9 mm) to only the gills in later life stages (Wentrup et al., 2013). Therefore, certain subpopulations may be selected in the process of establishing a symbiotic relationship in the gill epithelium during development. Alternatively, multiple infections of the symbiont over time may affect the composition of symbiont subpopulations. The cause of the variations in the composition of symbiont subpopulations remains to be determined.
It is also not clear how the patchy distribution patterns of subpopulations are produced, and each bacteriocyte contains one or more symbiont subpopulations. Further investigations into the infection and distribution of symbiont subpopulations throughout host ontogeny, the proliferation of bacteriocytes and symbionts, and single-cell genome sequencing will improve our understanding of these questions.
Genome heterogeneity may be beneficial for utilizing diverse energy substrates
The lack of hydrogenase genes may be associated with the geochemical and geographical characteristics of the studied fields. Three decades of investigation into global hydrothermal fields have revealed a clear relationship between H2 concentration of high-temperature hydrothermal fluid and tectonic background (Nakamura and Takai, 2014). The H2 concentration of the fluid venting at the Western Pacific Arc/Bac-Arc setting, including the Izu-Ogasawara Arc, is generally one or two orders of magnitude lower than at the Mid-Ocean Ridge setting, including the Mid-Atlantic Ridge and Eastern Pacific Rise, where the ability to use hydrogen as an energy source in symbioses is proposed to be widespread (Petersen et al., 2011). Moreover, compilation of calculations of bioavailable energy based on fluid chemistry and vent-endemic microbiological investigations (Nakamura and Takai, 2014) indicates that the structure of chemolithotrophic microbial communities in hydrothermal environments is controlled primarily by the concentration of H2 in the fluid. When the H2 concentration is low, thiotrophic metabolism is favoured, whereas hydrogenotrophic metabolism is favoured when H2 is high. Thus, in the Western Pacific region, where sulphur oxidation is more preferable, the BSEPE is likely to have a tendency to lose the hydrogenase. The loss of non-essential genes accompanying a size reduction in the bacterial genome is often attributed to minimization of the material costs of cellular replication, and to the genetic drift (Mira et al., 2001). In experimental bacterial populations, it has been proposed that loss of specific gene(s) is beneficial and driven by selection (Lee and Marx, 2012).
One drawback to the loss of genes that encode non-essential metabolic pathways is reduced flexibility in occupying variable geochemical regimes. In the BSEPE genome, all of the genes necessary for thiotrophy and aerobic respiration occur on loci with average mapping depth, and thus are present in all symbiont subpopulations, whereas genes for hydrogen oxidation and nitrate respiration are not essential for autotrophic growth. However, in the expression analysis, two structural hup genes (hupL and -S) and four structural nar genes (narG, -H, -I, and -J) were transcribed in the host gill, suggesting that these genes are functional in the symbiosis (Supplementary Figure S3C and Supplementary Table S1), although actual activities of the enzymes need to be assayed to draw conclusion. In the hydrothermal vent environment occupied by Bathymodiolus mussels, sulphide and dissolved oxygen regimes appear to be unstable (Zielinski et al., 2011); therefore, the retention of variation for energy acquisition potentially increases the opportunities to colonize different and variable geochemical conditions. Thus, hup (and possibly nar) genes may be conserved in specific subpopulations of BSEPE with functional activity, based on a subtle balance between the tendency to lose unnecessary genes and the tendency to retain flexibility to occupy a variable environment.
The genome heterogeneity in BSEPE may permit the host-symbiont association to utilize diverse metabolic substrates. In ocean microorganisms, metabolic capability is deeply correlated with the organism's local acclimation and niche acquisition (Kashtan et al., 2014). It has been proposed that some host animals have physiologically distinct multiple endosymbiont species allowing them to use a variety of energy sources, which may confer metabolic flexibility and enable the organism to occupy a range of habitats (Fisher et al., 1993; Distel et al., 1995; Woyke et al., 2006; Dubilier et al., 2008; Robidart et al., 2008; Beinart et al., 2012). The genomic heterogeneity at the sub-species level we found here may also enable differential utilization of diverse substrates, although this model remains to be validated. In horizontally transmitted endosymbioses, the acquisition of symbionts from a (potentially) diverse free-living population is expected to result in multiple symbiont subtypes coexisting within a single host individual (Stewart and Cavanaugh, 2009). In line with this expectation, intra-species subtype variations in a host have been reported in some horizontally transmitted bacterial endosymbionts (DeChaine et al., 2006; Vrijenhoek et al., 2007). However, until recently, little was known about the ecological significance of the genomic diversity within a symbiotic population. The findings presented here advance our understanding of metabolic acclimation and genomic evolution in symbiotic bacteria.
Conclusions
Here, we have shown that a symbiont population with a single ribotype in an individual B. septemdierum host is composed of several heterogeneous subpopulations that differ in gene sets for key metabolic enzymes. Recently, genomic diversity in single nucleotide polymorphisms, short insertions/deletions and structural variants within microbial species has been also discovered in free-living populations and human gut microbes (Gonzaga et al., 2012; Grote et al., 2012; Schloissnig et al., 2013; Kashtan et al., 2014). Our findings shed light on the ecological significance of the genomic diversity not only within symbiotic bacterial species, but also natural free-living or other animal-associated microbial communities.
Acknowledgments
The authors thank Dr Hiroyuki Kimura from Shizuoka University for permission to use the frozen B. septemdierum samples, Dr Kiyotaka Takishita from JAMSTEC for valuable comments on this study, and the captains and crew of the R/V Natsushima and ROV Hyper Dolphin for sample collection. This work was supported by JSPS KAKENHI Grant Number 24570252 and the Okinawa Intellectual Cluster Program.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Anantharaman K, Breier JA, Sheik CS, Dick GJ. (2013). Evidence for hydrogen oxidation and metabolic plasticity in widespread deep-sea sulfur-oxidizing bacteria. Proc Natl Acad Sci USA 110: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinart RA, Sanders JG, Faure B, Sylva SP, Lee RW, Becker EL et al. (2012). Evidence for the role of endosymbionts in regional-scale habitat partitioning by hydrothermal vent symbioses. Proc Natl Acad Sci USA 109: E3241–E3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco F, Iobbi C, Ratouchniak J, Bonnefoy V, Chippaux M. (1990). Nitrate reductases of Escherichia coli: sequence of the second nitrate reductase and comparison with that encoded by the narGHJI operon. Mol Gen Genet 222: 104–111. [DOI] [PubMed] [Google Scholar]
- Brito B, Baginsky C, Palacios JM, Cabrera E, Ruiz-Argueso T, Imperial J. (2005). Biodiversity of uptake hydrogenase systems from legume endosymbiotic bacteria. Biochem Soc Trans 33: 33–35. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- DeChaine EG, Bates AE, Shank TM, Cavanaugh CM. (2006). Off-axis symbiosis found: characterization and biogeography of bacterial symbionts of Bathymodiolus mussels from Lost City hydrothermal vents. Environ Microbiol 8: 1902–1912. [DOI] [PubMed] [Google Scholar]
- Distel DL, Lee HK, Cavanaugh CM. (1995). Intracellular coexistence of methano- and thioautotrophic bacteria in a hydrothermal vent mussel. Proc Natl Acad Sci USA 92: 9598–9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubilier N, Bergin C, Lott C. (2008). Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat Rev Microbiol 6: 725–740. [DOI] [PubMed] [Google Scholar]
- Duperron S, Bergin C, Zielinski F, Blazejak A, Pernthaler A, McKiness ZP et al. (2006). A dual symbiosis shared by two mussel species, Bathymodiolus azoricus and Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), from hydrothermal vents along the northern Mid-Atlantic Ridge. Environ Microbiol 8: 1441–1447. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Brooks JM, Vodenichar JS, Zande M, Childress JJ, Burke RA Jr. (1993). The co-occurrence of methanotrophic and chemoautotrophic sulfur-oxidizing bacterial symbionts in a deep-sea mussel. Mar Ecol 14: 277–289. [Google Scholar]
- Fujiwara Y, Takai K, Uematsu K, Tsuchida S, Hunt JC, Hashimoto J. (2000). Phylogenetic characterization of endosymbionts in three hydrothermal vent mussels: influence on host distributions. Mar Ecol Prog Ser 208: 147–155. [Google Scholar]
- Gonzaga A, Martin-Cuadrado AB, Lopez-Perez M, Megumi Mizuno C, Garcia-Heredia I, Kimes NE et al. (2012). Polyclonality of concurrent natural populations of Alteromonas macleodii. Genome Biol Evol 4: 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote J, Thrash JC, Huggett MJ, Landry ZC, Carini P, Giovannoni SJ et al. (2012). Streamlining and core genome conservation among highly divergent members of the SAR11 clade. mBio 3: e00252–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel A, Axelsson R, Lindberg P, Troshina OY, Wunschiers R, Lindblad P. (2001). Cloning and characterisation of a hyp gene cluster in the filamentous cyanobacterium Nostoc sp. strain PCC 73102. FEMS Microbiol Lett 201: 59–64. [DOI] [PubMed] [Google Scholar]
- Hartig E, Schiek U, Vollack KU, Zumft WG. (1999). Nitrate and nitrite control of respiratory nitrate reduction in denitrifying Pseudomonas stutzeri by a two-component regulatory system homologous to NarXL of Escherichia coli. J Bacteriol 181: 3658–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel U, Felbeck H. (1993). Nitrate respiration in the hydrothermal vent tubeworm Riftia pachyptila. Nature 366: 338–340. [Google Scholar]
- Hentschel U, Hand S, Felbeck H. (1996). The contribution of nitrate respiration to the energy budget of the symbiont-containing clam Lucinoma aequizonata: a calorimetric study. J Exp Biol 199: 427–433. [DOI] [PubMed] [Google Scholar]
- Kashtan N, Roggensack SE, Rodrigue S, Thompson JW, Biller SJ, Coe A et al. (2014). Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science 344: 416–420. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Petersen JM, Dubilier N. (2012). Convergent and divergent evolution of metabolism in sulfur-oxidizing symbionts and the role of horizontal gene transfer. Curr Opin Microbiol 15: 621–631. [DOI] [PubMed] [Google Scholar]
- Kuwahara H, Yoshida T, Takaki Y, Shimamura S, Nishi S, Harada M et al. (2007). Reduced genome of the thioautotrophic intracellular symbiont in a deep-sea clam, Calyptogena okutanii. Curr Biol 17: 881–886. [DOI] [PubMed] [Google Scholar]
- Lee MC, Marx CJ. (2012). Repeated, selection-driven genome reduction of accessory genes in experimental populations. PLoS Genet 8: e1002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Wankel SD, Wu M, Cavanaugh CM, Girguis PR. (2014). Characterizing the plasticity of nitrogen metabolism by the host and symbionts of the hydrothermal vent chemoautotrophic symbioses Ridgeia piscesae. Mol Ecol 23: 1544–1557. [DOI] [PubMed] [Google Scholar]
- Mira A, Ochman H, Moran NA. (2001). Deletional bias and the evolution of bacterial genomes. Trends Genet 17: 589–596. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Shimumura S, Takaki Y, Suzuki Y, Murakami S, Watanabe T et al. (2014). Allying with armored snails: the complete genome of gammaproteobacterial endosymbiont. ISMEJ 8: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Takai K. (2014). Theoretical constraints of physical and chemical properties of hydrothermal fluids on variations in chemolithotrophic microbial communities in seafloor hydrothermal systems. Prog Earth Planet Sci 1: 5. [Google Scholar]
- Newton IL, Woyke T, Auchtung TA, Dilly GF, Dutton RJ, Fisher MC et al. (2007). The Calyptogena magnifica chemoautotrophic symbiont genome. Science 315: 998–1000. [DOI] [PubMed] [Google Scholar]
- Petersen JM, Zielinski FU, Pape T, Seifert R, Moraru C, Amann R et al. (2011). Hydrogen is an energy source for hydrothermal vent symbioses. Nature 476: 176–180. [DOI] [PubMed] [Google Scholar]
- Robidart JC, Bench SR, Feldman RA, Novoradovsky A, Podell SB, Gaasterland T et al. (2008). Metabolic versatility of the Riftia pachyptila endosymbiont revealed through metagenomics. Environ Microbiol 10: 727–737. [DOI] [PubMed] [Google Scholar]
- Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A et al. (2013). Genomic variation landscape of the human gut microbiome. Nature 493: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FJ, Cavanaugh CM. (2009). Pyrosequencing analysis of endosymbiont population structure: co-occurrence of divergent symbiont lineages in a single vesicomyid host clam. Environ Microbiol 11: 2136–2147. [DOI] [PubMed] [Google Scholar]
- Treangen TJ, Sommer DD, Angly FE, Koren S, Pop M. (2011). Next generation sequence assembly with AMOS. Curr Protoc Bioinformatics Chapter 11: Unit 11. [DOI] [PMC free article] [PubMed]
- Vignais PM, Billoud B. (2007). Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev 107: 4206–4272. [DOI] [PubMed] [Google Scholar]
- Vrijenhoek RC, Duhaime M, Jones WJ. (2007). Subtype variation among bacterial endosymbionts of tubeworms (Annelida: Siboglinidae) from the Gulf of California. Biol Bull 212: 180–184. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Zaikova E, Howes CG, Song YC, Wright JJ, Tringe SG et al. (2009). Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326: 578–582. [DOI] [PubMed] [Google Scholar]
- Wentrup C, Wendeberg A, Huang JY, Borowski C, Dubilier N. (2013). Shift from widespread symbiont infection of host tissues to specific colonization of gills in juvenile deep-sea mussels. ISME J 7: 1244–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won YJ, Hallam SJ, O'Mullan GD, Pan IL, Buck KR, Vrijenhoek RC. (2003). Environmental acquisition of thiotrophic endosymbionts by deep-sea mussels of the genus Bathymodiolus. Appl Environ Microbiol 69: 6785–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyke T, Teeling H, Ivanova NN, Huntemann M, Richter M, Gloeckner FO et al. (2006). Symbiosis insights through metagenomic analysis of a microbial consortium. Nature 443: 950–955. [DOI] [PubMed] [Google Scholar]
- Zielinski FU, Gennerich HH, Borowski C, Wenzhofer F, Dubilier N. (2011). In situ measurements of hydrogen sulfide, oxygen, and temperature in diffuse fluids of an ultramafic-hosted hydrothermal vent field (Logatchev, 14 degrees 45 'N, Mid-Atlantic Ridge): implications for chemosymbiotic bathymodiolin mussels. Geochem Geophys Geosys 12: Q0AE04. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.