Abstract
Background
TNF/TNFR superfamily members conform a group of molecular interaction pathways of essential relevance during the process of T cell activation and differentiation towards effector cells and particularly for the maintenance phase of the immune response. Specific blockade of these interacting pathways, such as CD40/CD40L, contributes to modulate the deleterious outcome of allogeneic immune responses. We postulated that antagonizing the interaction of LIGHT expression on activated T cells with its receptors, HVEM and LTβR may decrease T cell-mediated allogeneic responses.
Methods
A flow cytometry competition assay was designed to identify anti-LIGHT monoclonal antibodies capable to prevent the interaction of mouse LIGHT with its receptors expressed on transfected cells. An antibody with the desired specificity was evaluated in a short-term in vivo allogeneic cytotoxic assay and tested for its ability to detect endogenous mouse LIGHT.
Results
We provide evidence for the first time that in mice, as previously described in humans, LIGHT protein is rapidly and transiently expressed after T cell activation, and this expression was stronger on CD8 T cells than on CD4 T cells. Two anti-LIGHT antibodies prevented interactions of mouse LIGHT with its two known receptors HVEM and LTβR. In vivo administration of anti-LIGHT antibody (clone 10F12) ameliorated host anti-donor short-term cytotoxic response in WT B6 mice, although to a lesser extent than that observed in LIGHT-deficient mice.
Conclusions
The therapeutic targeting of LIGHT may contribute to achieve a better control of cytotoxic responses refractory to current immunosuppressive drugs in transplantation.
Keywords: HVEM (TNFRSF14), LIGHT (TNFSF14), LTβR (TNFRSF3), DcR3 (TNFRSF6b), co-stimulation, transplantation, alloreactivity, graft rejection, graft versus host disease, cytotoxicity
Introduction
Human LIGHT (homologous to lymphotoxin, exhibits inducible expression and competes with HSV glycoprotein D for binding to herpesvirus entry mediator, a receptor expressed on T lymphocytes) is a member of the TNF superfamily transiently detected on human T cells upon activation (1,2) and immature dendritic cells (3,4). Mouse LIGHT is a type II transmembrane protein of 239 amino acids, with an extracellular region 74% similar in amino acid sequence to human LIGHT (1,5).
LIGHT can act as a costimulatory molecule independently of CD28 (3,4), fostering T cell proliferation in the mixed lymphocyte reaction and promoting the process of DC maturation as well (6). It can even augment antitumor activity directly (7) or indirectly through enhancing CTL activity against tumor cells (4). In line with the costimulatory activity of LIGHT, constitutive transgenic expression of LIGHT under the control of a T cell-specific promoter led to chronic inflammation of mucosal tissues (8,9). In contrast, gene deletion of LIGHT results in defective CD8 T cell proliferation and acquisition of CTL effector function, which is associated with prolonged graft survival in several allogeneic mouse models of transplantation (10-13).
One of the LIGHT receptors is HVEM (TNFRSF14), which is broadly expressed on hematopoietic and non hematopoietic cells (14,15). HVEM is a type I transmembrane molecule with an extracellular portion divided into cysteine-rich domains (CRD1-4) (16-18) with distinct binding sites for its ligands. BTLA and CD160 bind to the CRD1 and part of the CRD2 of HVEM, and so does the viral protein gD of Herpes Simplex Virus (HSV) (19,20), whereas LIGHT interacts with CRD2 and CRD3 on opposite sides of the extracellular part of HVEM (21). Furthermore, membrane LIGHT can be released by the action of a metalloprotease (22) and the soluble form of LIGHT binds to BTLA/HVEM complex and strengthens the molecular interaction, whereas engagement of membrane anchored HVEM by LIGHT in cis displaces BTLA from its interaction with HVEM and allows bidirectional trans co-stimulatory contacts between HVEM and LIGHT (1,23,24).
The other well-characterized receptor of LIGHT is the LTβR, which is expressed on follicular dendritic cells (FDCs), dendritic cells (DCs), macrophages, stromal cells and high endothelial venules (HEV) (25). LTαβ CD4+CD3− inducer cells interact with LTβR on stromal organizer cells to guide lymphoid organogenesis during development and, later on, stroma-derived LTβR signaling is still essential for the maintenance of the lymphoid tissue structure (26,27). LTαβ expression on activated CD4+ helper T cells (28) and LTβR on DCs and B cells follows a similar pattern to that of CD40L and CD40 expression on T cells and antigen presenting cells respectively, suggesting that LTαβ/LTβR pathway may regulate the exchange of information between antigen presenting cells and T cells, and therefore participate in T cell activation and differentiation. LIGHT expressed on activated T cells may provide a licensing signal upon interaction with LTβR expressed on DC (6) or on stromal cells that would in turn modify the lymphoid tissue environment to achieve proper T cell priming.
So far, there have been no reagents available capable to specifically recognize conformational epitopes on the extracellular region of the mouse LIGHT, although reagents against human LIGHT are available (6), (29). In an attempt to define the therapeutic potential of targeting LIGHT in animal model systems, and to detect and follow membrane LIGHT expression, rat monoclonal antibodies against mouse LIGHT were raised and selected based on their ability to block the binding of soluble LTβR-Ig or HVEM-Ig to LIGHT-transduced cells. Their therapeutic activity was then assessed in an in vivo mouse model of alloreactivity and we demonstrated that the specific blockade of LIGHT mitigated the in vivo cytotoxic allogeneic immune response. These observations pointed out that LIGHT/HVEM/LTβR interacting pathway is an amenable therapeutic target for the immune intervention for the control of cell-mediated cytotoxic responses.
Results
Conserved cross-interactions between mouse LIGHT receptors and mouse and human LIGHT
The TNF receptor-binding domain of LIGHT interacts with CRD2 and CRD3 on one side of membrane anchored HVEM, whereas BTLA and CD160 interact with CRD1 and CRD2 on the opposite side of HVEM (2,21,30). According to molecular modeling and previous studies, the receptors HVEM, LTβR and DcR3 share widely overlapping binding sites on LIGHT (2). DcR3 is a soluble decoy receptor that is present in human but has no known counterpart in mouse. Interactions of mouse LIGHT with its receptors are not very well documented, in part due to the difficulty of preparing active soluble mouse LIGHT (31). We indeed found that either recombinant soluble mouse LIGHT fused to an IgG2a Fc fragment, or LIGHT multimerized with an isoleucine zipper, failed to bind its receptors (data not shown) (32). However, a third soluble form of mouse LIGHT, containing amino acids 72-239 linked to a Flag-Foldon tag proved to be active and efficiently reacted with mouse HVEM, mouse LTβR and human DcR3 expressed as full-length or glycolipid-anchored proteins on HEK-293T cells (Figure 1A). Human LIGHT gave a similar binding pattern, except that its binding to mouse LTβR was weaker in this particular experimental setting (Figure 1A). Similar results were obtained when cells expressing full-length mouse or human LIGHT were stained with receptor.Ig fusion proteins. In these experiments, mouse HVEM and mouse LTβR bound to mouse and human LIGHT, whereas human DcR3 gave weaker stainings (Figure 1B).
Figure 1. Cross-interaction binding assays of soluble TNFRSF and TNFSF recombinant proteins of the LIGHT/HVEM/LTβR/DcR3 system.
(A) The complete gene encoding mouse HVEM molecule fused to GFP, mouse LTβR-GPI or human DcR3-GPI were transfected into HEK-293T cells (red solid lines) and stained with FF-mouse LIGHT (upper panel) or Flag-tagged human LIGHT (lower panel). HEK-293T cells transfected with the empty plasmid were used as controls (black dotted lines). Receptor/ligand interactions were detected with biotinylated anti-Flag mAb (clone M2) plus allophycoerythrin-coupled streptavidin.
(B) 2 × 105 NIH-3T3 cells transduced with pMIG-mouse LIGHT-IRES-GFP (left panel, red solid lines) or HEK-293T cells transfected with human LIGHT bound to GFP (right panel, red solid lines) were incubated with mHVEM-Ig, mLTbR-Ig or hDcR3-Ig. As negative controls, NIH-3T3 or HEK-293T cells were transduced or transfected with the empty expression vector (black dotted lines). The ligand/receptor interactions were detected using biotinylated anti-mouse IgG2a or anti-human IgG1 followed by allophycocyanin-coupled streptavidin.
Taken together, the binding results indicate that in the mouse system, LIGHT indeed binds the relevant receptors HVEM and LTβR, which is in agreement with and extends conclusions of previous reports describing the binding interactions between LIGHT and its natural receptors (7,31).
Differential competition of soluble forms of LTβR and HVEM for binding to LIGHT
HVEM delivers costimulatory signals to T cells when engaged by LIGHT, while LIGHT/ LTβR functionally modulates dendritic cells and stromal cells to promote an adequate environment for T cell priming (33,34). Since both LTβR and HVEM bind to LIGHT, when both receptors are simultaneously expressed in cis (on the same cell) or in trans (in different cells), the advantageous competition of one of the receptors over the other would displace the less competitive receptor from interacting with LIGHT (2). Under those circumstances, binding of the receptor with the highest affinity may dominate a particular signaling pathway. Saturation LIGHT binding curves were established for mHVEM.IgG2a (HVEM-Ig) and for mLTβR.IgG1 (LTβR-Ig). Interestingly, concentrations of receptors required to achieve saturation binding was about 20-fold higher for HVEM-Ig than for LTβR-Ig (Figure 2A). LIGHT-transduced cells preincubated with a saturated amount of soluble HVEM-Ig did not prevent LTβR-Ig from binding to LIGHT (Figure 2B, left lower panel). In contrast, preincubation of LIGHT transduced cells with LTβR-Ig completely abrogated the binding of HVEM-Ig to LIGHT-transduced cells (Figure 2B, right lower panel).
Figure 2. LTβR competes with HVEM for binding to the TNFR binding site of LIGHT.
(A) LIGHT-transduced NIH-3T3 (2 × 105) cells were incubated with graded concentrations of purified sHVEM.IgG2a (left panel) or LTβR.hIgG1 (right panel). The binding of HVEM and LTβR to membrane LIGHT was evaluated by flow cytometry with appropriate anti-mouse and anti-human secondary antibodies. The mean fluorescence intensity (MFI) binding of LIGHT receptors to membrane-bound LIGHT was calculated and plotted against graded concentrations of HVEM.mIgG2a (left side panel) and LTβR.hIgG1 (left side panel).
(B) To test whether mouse HVEM-Ig and LTβR-Ig bind to the same or overlapping regions on mouse LIGHT, 2 × 105 NIH-3T3 transduced cells expressing mouse LIGHT on the cell surface were incubated with sHVEM.mIgG2a at 50 μg/ml (left panel) or LTβR.hIgG1 at 10 μg/ml (right panel) (red solid lines) or with the controls mIgG2a or hIgG1 (black dotted lines). In the presence of the inhibitors, either LTβR.hIgG1 at 10 μg/ml (left side panel) or sHVEM.mIgG2a at 50 μg/ml (right side panel) was added to the reaction and detected with biotinylated anti-human IgG or anti-mouse IgG2a followed by SA-APC.
These experiments suggest that HVEM, at least in its recombinant form, has a lower affinity for mouse LIGHT than for mouse LTβR and confirm that HVEM and LTβR binding sites on LIGHT overlap.
Anti-LIGHT antibodies that efficiently recognize and block mouse LIGHT
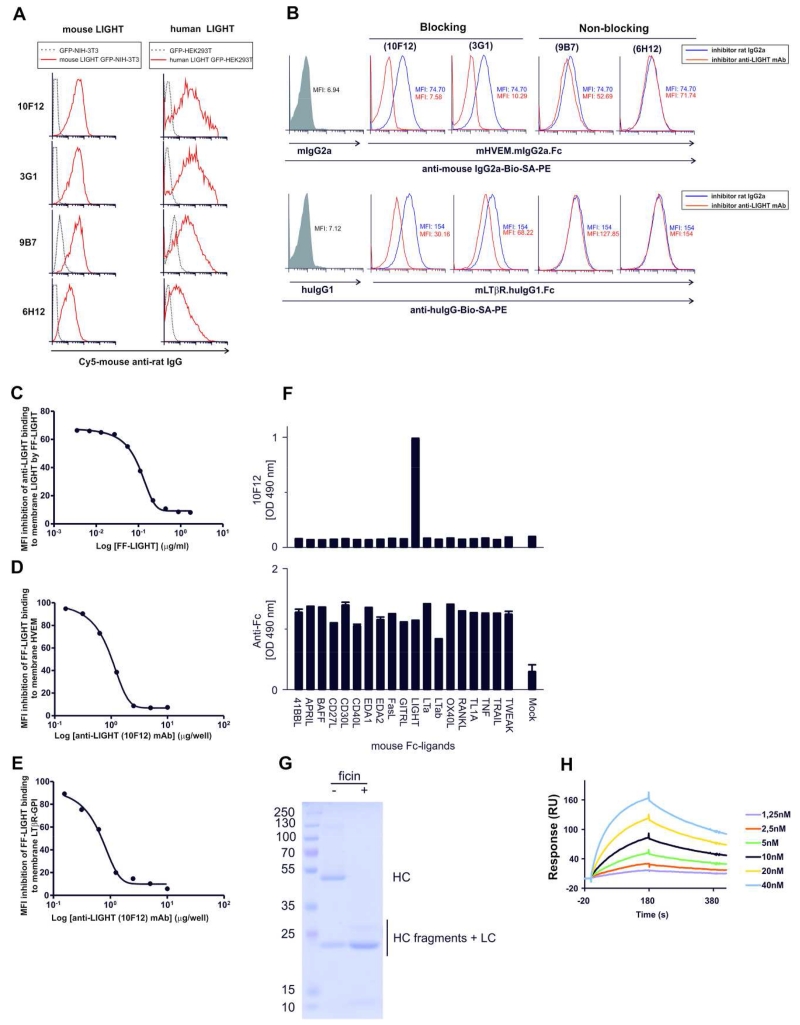
A LIGHT-transduced NIH-3T3 cell line coexpressing eGFP (clone 2B7) was obtained by limiting dilution cloning and used as immunogen in rats to produce anti-mouse LIGHT hybridomas. Hybridoma supernatants were screened for their recognition of mouse LIGHT by flow cytometry using LIGHT-GFP- or control GFP-transduced NIH-3T3 cell lines. Four rat IgG2a anti-LIGHT antibodies (10F12, 3G1, 6H12 and 9B7) specifically recognized an epitope located at the extracellular region of mouse LIGHT (Figure 3A, left panels). Of note, all four antibodies cross-reacted with human LIGHT expressed on transiently transfected HEK-293T cells (Figure 3A, right panels).
Figure 3. Specificity, cross-reactivity and antagonist activity of anti-LIGHT mAbs.
(A, left panels) NIH-3T3 cells (2 × 105) were transduced with either pMIG-mouse LIGHT-IRES-GFP (red solid lines) or control plasmid pMIG-IRES-GFPs (black dotted lines) and were incubated with the indicated anti-LIGHT mAbs followed by Cy5-labeled mouse anti-rat IgG secondary antibody.
(A, right panels) Cross-reactivity of anti-mouse LIGHT mAbs with human LIGHT was tested with HEK-293T cells (2 × 105) cotransfected with either pcDNA3.1 plasmid harboring human LIGHT fused to GFP (red solid lines) or empty plasmid pcDNA3.1 - GFP (black dotted lines). Binding was detected with Cy5-labeled mouse anti-rat IgG secondary antibody.
(B) Mouse LIGHT-transduced NIH-3T3 cells were incubated for 1 h at 37°C with anti-LIGHT mAbs (clones 10F12, 3G1, 9B7 and 6H12, red solid lines) or isotype-matched control rat IgG2a (black dotted lines). Grey shaded histograms represent LIGHT-transduced cells incubated with either mIgG2a or hIgG1 as control for the recombinant proteins. In the presence of saturating amounts of anti-LIGHT antibodies, the reaction was incubated with HVEM-Ig (upper panel) or LTβR-Ig (lower panel) and the binding of receptor.Ig was detected with appropriate biotinylated secondary antibodies followed by phycoerythrin-coupled streptavidin. The reduction of the mean fluorescence intensity (MFI) shown in each plot indicates the antagonist functional activity of anti-LIGHT antibodies.
(C) Graded concentrations of soluble recombinant mouse LIGHT were preincubated with a saturated amount of anti-mouse LIGHT monoclonal antibody (clone 10F12) for 1 hour at 37 °C. The antigen/antibody complex was added to LIGHT-transduced cells for 30 minutes at 37°C, then the reaction was washed and developed with biotinylated mouse anti-rat IgG2a and SA-PE. The MFI inhibition of anti-LIGHT binding to membrane LIGHT in the presence of graded concentrations of FF-LIGHT is shown.
Graded concentrations of anti-LIGHT mAb were preincubated for 1 hour at 37 °C with an amount of mouse FF-mouse LIGHT recombinant protein sufficient to saturate binding to membrane-expressed receptors. Then, the antigen/antibody complex was added to HVEM-transfected CHO cells (D) or LTβR-GPI transfected HEK293T (E) cells for 30 minutes at 37°C. The reaction was washed and developed with biotinylated mouse anti-Flag mAb (anti-Flag, clone BioM2) and SA-PE. The MFI inhibition of soluble FF-mouse LIGHT binding to HVEM transfected cells or LTβR transfected cells is plotted.
F) Nineteen mouse TNF family ligands fused to the Fc portion of human IgG1 were captured in an ELISA plate and revealed with biotinylated 10F12 anti-mLIGHT mAb (top panel) or with an anti-human IgG antibody to reveal the presence of the various ligands (bottom panel).
G) Preparation of anti-LIGHT (10F12) Fab fragments. 10 μg of 10F12 mAb digested with or without immobilized ficin was analyzed by SDS-PAGE and Coomassie blue staining. HC: heavy chain. LC: light chain.
H) The Fab fragment of 10F12 mAb at the indicated concentrations was analyzed by surface plasmon resonance onto immobilized FF-mouse LIGHT. Fab solutions were applied for 180 s, and subsequently washed with buffer. The equilibrium dissociation constant KD was 5.8×10−9 M.
Anti-LIGHT antibodies were screened for their ability to prevent the binding of HVEM-Ig and LTβR-Ig to LIGHT-transduced cells. Two anti-LIGHT antibodies (10F12 and 3G1) fully prevented the binding of HVEM-Ig to mouse LIGHT-expressing cells in a FACS-based assay, whereas antibodies 9B7 and 6H12 did not (Figure 3B). Anti-LIGHT, clone 10F12 was the most potent antibody to inhibit the binding of LTβR-Ig to mouse LIGHT, although inhibition did not reach baseline, whereas 3G1 only partially inhibited this interaction (Figure 3B).
Anti-LIGHT mAb 10F12 was further characterized in a binding assay between membrane-bound and soluble LIGHT, in which 10F12 binding to membrane-bound LIGHT is competed by soluble Flag-Foldon (FF)-LIGHT (Figure 3C). At the EC50, 10F12 and FF-LIGHT were at equimolar concentrations, indicating no obvious bias of 10F12 to recognize membrane-bound LIGHT, and therefore validating the quality of soluble recombinant FF-LIGHT. The ability of 10F12 to prevent the binding of FF-LIGHT to membrane-bound HVEM and LTβR was then investigated (Figures 3D, E). 10F12 inhibited the binding of FF-LIGHT to both full-length HVEM and GPI-anchored LTβR with a similarly good efficiency: at EC50, the ratio of antibody binding sites to FF-LIGHT epitopes was stoichiometric, and a five-fold molar excess of antibody to LIGHT totally abrogated LIGHT binding to both receptors (Figures 3D, E). Of the nineteen mouse TNF family members, 10F12 only recognized LIGHT in a sandwich ELISA assay, validating its binding specificity (Figure 3F). The affinity of the interaction of FF-LIGHT with a monomeric Fab fragment of 10F12 prepared by ficin digestion (Fig. 3G) was measured by surface plasmon resonance. The association rate constant (ka) was 4.2×105 M−1 s−1, the dissociation rate constant (kd)) was 2.3×10−3 s−1 and the affinity (KD) was 5.4 nM (Rmax: 176, Chi2: 3.4) (Figure 3H). For comparison, a panel of Fab from agonist anti-EDAR antibodies with in vivo activity had kd ranging from 9.6 to 0.24 ×10−3 s−1 (the smallest the number, the better the antibody sticks to its antigen once bound) and affinities from 0.5 - 40 nM (35).
In summary, we have identified both blocking and non-blocking monoclonal antibodies recognizing surface-exposed mouse LIGHT that cross-reacted with human LIGHT. One of these antibodies had decent binding parameters and showed efficient and specific blockade of the interaction of LIGHT with its two receptors, making it possible for the first time to monitor the expression of mouse LIGHT at protein level and to conduct LIGHT-blocking therapeutic experiments in mice.
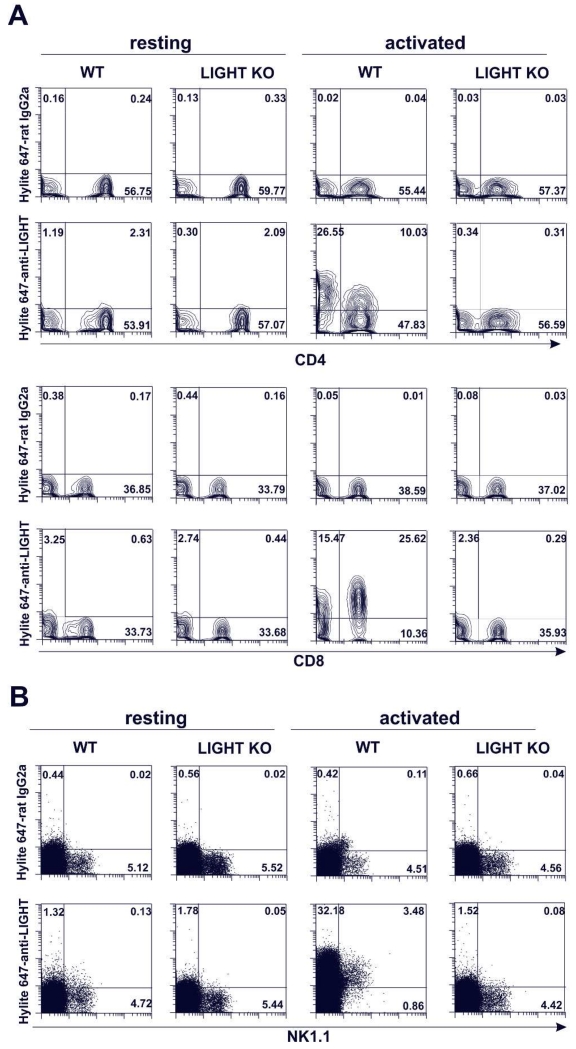
Mouse LIGHT is transiently expressed on activated CD8 T cells and NK cells, and to a lesser extent in CD4 T cells
Anti-LIGHT antibodies were tested for their ability to detect endogenous LIGHT. Considering that in humans, LIGHT protein is not detectable on resting T cells, fresh mouse B6 WT and B6 LIGHT KO splenocytes were stimulated in vitro with PMA plus ionomycin for 5 h in the presence of either HiLyte-647 labeled anti-LIGHT (10F12) or HiLyte-647-labeled rat IgG2a isotype control. This strong polyclonal T cell activation prompted a transient expression of LIGHT that was readily detected in virtually all CD8 T cells and to a lower extent in a subset of CD4 T cells of WT B6 mice, but not in LIGHT-deficient T cells (Figure 4A), in line with the previous description of LIGHT expression in human T cells (29), (1). Polyclonal activation with PMA plus ionomycin also induced transient expression of LIGHT on NK cells, but not in similarly stimulated LIGHT KO NK1.1 cells (Figure 4B). Because of the transient expression of LIGHT in activated T cells, it is noteworthy to mention that the labeled antibody needs to be present during the course of activation in order to achieve successful detection.
Figure 4. Mouse LIGHT is expressed upon activation more prominently on CD8 T cells than on CD4 T cells and it is also expressed on activated NK cells.
C57BL/6 WT or LIGHT-deficient splenocytes were plated at 2 × 105 cells per well in 96-well plates and were left untreated or stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 5 h. Hylite 647-labeled anti-LIGHT mAb (clone 10F12) or Hylite 647-labeled isotype control (rat IgG2a) were added to the cells during the incubation. The expression of mouse LIGHT was analyzed on living lineage-negative cells for CD19, CD11b and CD11c resting and activated CD4+ (upper panel) and CD8+ (lower panel) T cells (A) and on resting and activated NK cells (NKT cells and non-T NK cells) of WT and LIGHT KO after gating out CD19+ and CD11c+ cells (B).
In vivo allogeneic cytotoxic activity is significantly reduced after antibody-mediated blockade of the LIGHT/HVEM/LTβR pathway, although to a lesser extent than in LIGHT-deficient mice
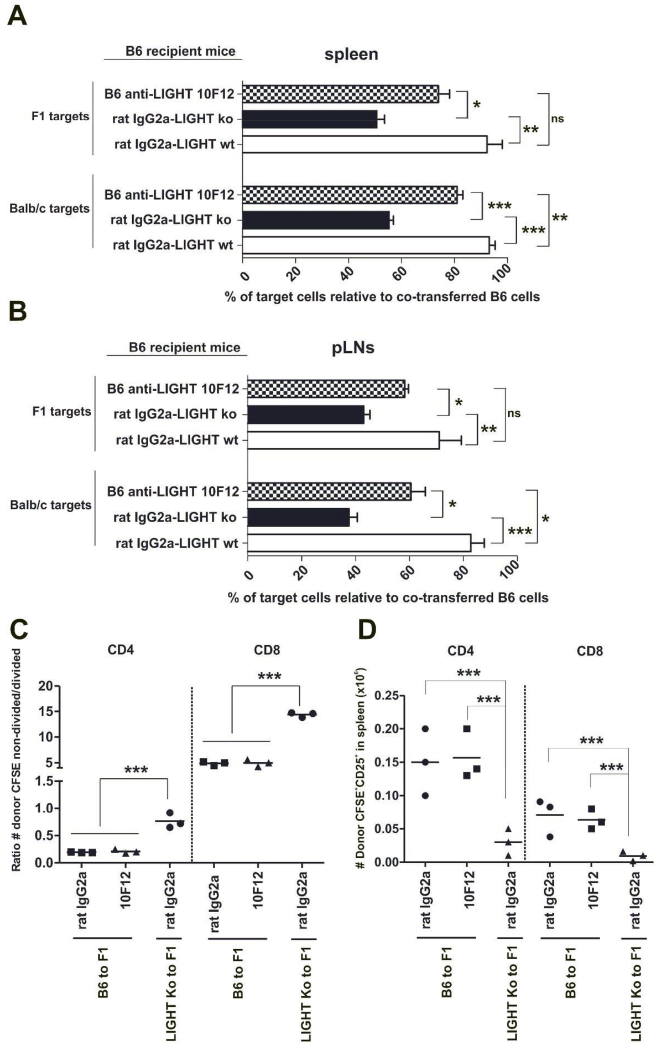
To elucidate whether antibody blockade of the LIGHT/HVEM/LTβR pathway could modulate allogeneic cytotoxic responses, B6 recipient mice were injected with an identical number of B6, BALB/c and F1 target cells labeled with different amounts of CFSE, as mentioned in the Material and Methods section. As shown in figure 5, the percentage of killing of allogeneic BALB/c and F1 target cells in host B6 spleen (Figure 5A) and peripheral lymph nodes (Figure 5B) was significantly reduced in LIGHT-deficient mice compared to B6 WT mice (BALB/c target cells: spleen and pLNs, p<0.0005 and F1 target cells: spleen and pLNs, p<0.005). In line with these results, antagonist anti-LIGHT monoclonal antibody 10F12 significantly mitigated host anti-donor cytotoxic responses against BALB/c target cells (spleen, p<0.005 and pLNs, p<0.05), and showed a trend towards protection of F1 targets that however did not reach statistical significance (Figure 5A and 5B).
Figure 5. Decreased host anti-donor short-term cytotoxic response in vivo after antibody-mediated blockade of LIGHT.
Isotype control (white bars) and anti-LIGHT-treated (shaded striped bars) WT B6 mice and isotype control-treated B6 LIGHT-deficient mice (black bars) received 30 × 106 splenocytes of each B6, BALB/c and F1 differentially labeled with CFSE. The percentage of specific lysis of the BALB/c and F1 populations relative to the B6 population in spleen (A) and peripheral lymph nodes (pLNs) (inguinal plus axillar) (B) was monitored 72 h after cell transfer. Data are representative of two independent experiments with three to four mice per group. Bars indicate mean ± SEM and t test was used to compare differences between groups.
70×106 splenocytes from B6 WT or LIGHT-deficient mice labeled with 5 μM CFSE were injected into F1 recipients, which were treated with isotype control or anti-LIGHT 10F12 mAb. Three days later, the ratio of absolute number of donor CFSE labeled non-divided/divided CD4 and CD8 T cells (C) as well as the absolute number of donor CD4 and CD8 T cells expressing CD25 was calculated (D). Statistical significance is indicated as follows: *, p< 0.05; **, p< 0.005; ***, p< 0.0005 and ns, non-significant.
As the cytolytic response was diminished in LIGHT KO mice and also to a certain extent in anti-LIGHT-treated WT mice, we evaluated whether donor alloreactive T cell proliferation would be altered in semiallogeneic F1 recipients. Donor LIGHT KO CD4 and CD8 T cells proliferated significantly less efficiently than isotype control or anti-LIGHT treated donor WT T cells in the spleen of adoptively transferred F1 recipients (Figure 5C). Moreover, this observation correlated with a diminished frequency of LIGHT KO donor alloreactive CD4 T cells and CD8 T cells (Figure 5D) expressing IL-2Rα when compared to isotype- or anti-LIGHT treated WT donor T cells adoptively transferred into semiallogeneic F1 recipients.
Therefore, this short-term in vivo cytotoxic assay reveals that in vivo administration of anti-LIGHT mAb ameliorated the host anti-donor short-term cytotoxic response in WT B6 mice although to a lesser extent than that observed in LIGHT-deficient mice.
Discussion
The development of biologics aimed at preventing receptor / ligand interactions between TNF/TNFR molecules are alternative therapeutic arms of interest to conventional immunosuppression for the control of alloreactivity (14), (36). One of such therapeutic targets is the process of T cell activation and differentiation that drives the acquisition of effector T cell function. The molecules involved in the exchange of information between DC/T, B/T and T/T cell interactions belong to two major families of proteins, the Immunoglobulin (Ig) superfamily and the Tumor Necrosis Factor / Tumor Necrosis Family Receptor Superfamily (TNF/TNFRSF), the latter exhibiting cysteine-rich domains in the extracellular region of the molecule (31,37).
In this work, we provide for the first time a monoclonal antibody capable to detect LIGHT expression on mouse T cells and NK cells, and that additionally blocks the receptor binding site of LIGHT. We also demonstrated that therapeutic intervention with this antagonist anti-LIGHT antibody protected to some extent against rejection, although it did not fully recapitulate the attenuated cytotoxic immune response seen in LIGHT-deficient mice.
Although numerous reports in mouse models of inflammatory diseases have provided indirect experimental evidence that LIGHT may be involved in the pathogenesis of these immune-related diseases (10,38-42), this information contrasts with the lack of appropriate reagents for the detection of endogenous mouse LIGHT. The reason for this gap was the difficulty to engineer a genetic construct that produced a bioactive mouse LIGHT molecule with binding affinity for membrane-bound LIGHT receptors. We and others have evaluated a classical approach of fusing the extracellular region of mouse LIGHT either to Flag, the Fc fragment of the immunoglobulin heavy chain, or to an isoleucine zipper for the multimerization of the molecule (32), but none of these engineered genetic constructs succeeded in generating a protein with detectable binding affinity for mouse HVEM and LTβR. This was possible through a strategy that consisted of fusing the extracellular region of mouse LIGHT to a Flag-Foldon tag at the C-terminal site of this type II transmembrane protein (7).
Human LIGHT is constitutively expressed on intestinal mucosal CD8 T cells, CD4 T cells and NK cells and this expression is inducible by CD2-mediated signaling, which correlates with the typical activated state of resident lymphoid cells populating the intestinal mucosa (43), (44). In contrast to mucosal sites, in peripheral blood, LIGHT is inducible after exposure to PMA/ionomycin in CD8 T cells and to a similar extent in CD4+/CD45RO memory T cells and in CD4 Th1 IFN-γ producer cells and this expression was higher than that detected on naive CD4 T cells (44). The differential expression of LIGHT in humans, which is constitutive on lymphocytes of mucosal tissues and inducible on peripheral blood lymphocytes, indicates that regulation of LIGHT expression is associated with an activation and responsive status of the lymphoid cell. These results are in agreement with the observed transient expression of LIGHT on mouse peripheral lymphoid cells upon exposure to PMA/Ionomycin for 5 hours, indicating a similar regulation of inducible expression on peripheral blood of both human and mouse. This suggests that mice models of disease may contribute to unravel the physiology of LIGHT and its role during the course and in the context of an allogeneic response.
LIGHT (TNFSF14) and CD40L (also named CD154, TNFSF5) are both members of the TNF ligand superfamily. CD40L expression is not detectable on naive T cells (45) and the same holds true for the expression of human LIGHT (29), which is agreement with the observation in our work that LIGHT expression is neither detectable on naïve T cells and NK cells and was only seen transiently upon T cell activation with a strong polyclonal stimulus. As a matter of fact, mouse LIGHT protein detection needed the presence of the fluorescently-conjugated antibody against LIGHT during in vitro stimulation of T cells and NK cells. This is probably due to the fact that LIGHT is rapidly internalized after transient surface exposure. Contrary to CD40L, which is mainly expressed on CD4 T cells, mouse LIGHT presents a more pronounced expression on CD8 T cells than on CD4 T cells, suggesting a more predominant functional role on this T cell subset.
TcR recognition of a foreign peptide in the context of MHC along with costimulation drives T cell activation, IL-2 secretion and up-regulation of IL-2Rα chain (CD25), which associates with beta and gamma chain of IL-2 to configure the high affinity IL-2R. This permits IL-2-mediated autocrine CD4 T cell proliferation and clonal expansion and provides help for CD8 T cell clonal expansion and differentiation to effector CD8 T cells. In vitro studies with LIGHT KO CD4 T cells evidenced a deficiency in IL-2 secretion compared to WT CD4 T cells in response to polyclonal activation with anti-CD3/CD28 (11), which was also noticeable in the mixed lymphocyte reaction (12). The lack of secreted IL-2 likely contributes to the impaired CD8 T cell proliferation and differentiation to effector T cells, which also express less CD25 and therefore would proliferate less efficiently in response to IL-2 (12), (11). In agreement with this defective in vitro functional activity, our data also reflected similar defects in vivo such as a lower frequency of donor alloreactive CD4 and CD8 T cells expressing the IL-2Rα chain and decreased proliferative rate observed in F1 recipients receiving LIGHT KO semiallogeneic splenocytes compared to F1 recipients receiving semiallogeneic B6 WT splenocytes either treated with isotype control or with anti-LIGHT mAb.
The treatment of mice with soluble decoy receptors administered as recombinant fusion proteins disrupts various ligand/receptor interactions simultaneously. Despite the indirect evidences collected from the use of these decoy receptors on different mouse models of allogeneic transplantation, these approaches do not provide clear evidence on whether LIGHT could be a potential target for immune intervention. For example, the administration of decoy receptors, such as HVEM-Ig, LTβR-Ig or sDcR3-Ig attenuates alloreactivity in murine models of disease, but it is difficult to conclude whether the observed outcome is the result of inhibiting LIGHT interaction with its receptors and to which extent the observed effects might be due in part to inhibition of other ligands such as lymphotoxin or FasL (4,46-48). Therefore, assignment of the most significant ligand/receptor pathway responsible for the observed in vivo effect is inherently difficult. Another consideration is that most of the decoy receptors used in preclinical rodent models of transplantation are composed of the extracellular region of the receptor bound to human IgG1 Fc fragment. It is well known that human IgG1 binds efficiently to mouse FcγRIV, the main receptor in mice involved in ADCC-mediated depletion by myeloid cells and NK cells (49). This means that many claims in the literature stating that these decoy receptors function as blocking reagents can be biased if depletion of ligand-expressing cells indeed may occur.
In contrast to recombinant fusion proteins, selective antibody-based approaches targeting one particular ligand/receptor interaction will likely provide more relevant information than the use of soluble decoy receptors. The selective antibody-mediated blockade of the LIGHT/HVEM/LTβR pathway prevented the in vivo host anti-donor cytotoxic alloresponse, although to a lesser extent than that seen in LIGHT-deficient mice. The relative lack of efficacy of the antibody in vivo was unexpected given the stoichiometric inhibition of LIGHT by the antibody in vitro and the favorable antibody to ligand ratio that can be achieved in vivo. Perhaps recombinant and over-expressed proteins used in vitro underestimated the binding affinity of LIGHT for its receptors in vivo, and 10F12 only partially blocked endogenous LIGHT. In this case, we predict that residual signaling may be preferentially delivered through LTβR, for which LIGHT has a higher affinity. Alternatively, LIGHT may engage its receptors in vivo in the immunological synapses established between cells that may exclude extracellular medium containing the antibody. Finally, it is also conceivable that, because of a life-long deficiency of LIGHT, LIGHT-ko mice are intrinsically hyporesponsive, a phenotype that could not be reproduced by an acute, even full inhibition of LIGHT. Although LIGHT deficiency has been associated with impaired lymphocyte migration to lymph nodes, this only occurred under strong inflammatory conditions, which are not present in our experimental in vivo cytotoxic setting. This rules out the possibility that the observed reduction of cytotoxicity in LIGHT deficient mice or anti-LIGHT treated mice was a consequence of reduced migration of the target cells to these secondary lymphoid organs (50).
In summary, we report for the first time specific blocking and non-blocking monoclonal antibodies against mouse LIGHT as new reagents in the field of TNF/TNFR interactions to follow LIGHT protein expression and explore the preclinical consequences of interrupting LIGHT interactions with its receptors. We also demonstrated that targeting LIGHT may offer novel avenues for the control of cytotoxic responses in the setting of transplantation.
Supplementary Material
Acknowledgements
We are particularly grateful to animal technicians for outstanding animal husbandry. We also thank Dr. Angel Corbi (Center for Biological Research, CSIC, Madrid) for suggestions and critical reading of the manuscript.
This work has been supported by grants of the Spanish Ministry of Health (Fondo de Investigaciones Sanitarias, PI10/01039), Department of Education of Castilla and Leon Regional Government (Grant# LE007A10-2) and Mutua Madrileña Foundation (Basic research grants 2012) to J.I.R.B.; by Miguel Servet National Grant (Health National Organization Research Programme) CP12/03063 to M.L.R.G.; by the Swiss National Science Foundation to PS.
List of abbreviations
- LIGHT
homologous to lymphotoxin, exhibits inducible expression and competes with HSV glycoprotein D for binding to herpesvirus entry mediator, a receptor expressed on T lymphocytes
- mAb
monoclonal antibody
- GvHR
Graft versus host reaction
- CD
Cluster of differentiation
- HSV
Herpesvirus
- HVEM
Herpesvirus entry mediator
- CRD
Cysteine rich domain
- APC
Antigen presenting cells
- FDC
Follicular dendritic cell
- TNF
Tumor necrosis factor
- LTβR
Lymphotoxin beta receptor
- DC
Dendritic cell
- CTL
Cytotoxic T lymphocyte
- NK
Natural killer
- WT
Wild type
- KO
Knock-out
- mGFP
monster green fluorescent protein
- eGFP
enhanced green fluorescent protein
- APC
Allophycocyanin
- PE
Phycoerythrin
- MHC
Major Histocompatibility Complex
- GPI
Glycosylphosphatidylinositol
- PMA
Phorbol myristate acetate
- CFSE
Carboxyfluorescein succinimidyl ester
- Flag-shLIGHT
Flag-tagged soluble human LIGHT
- FF-LIGHT
Flag-Foldon-tagged soluble mouse LIGHT
- HVEM-Ig: HVEM.mIgG2a.Fc
Herpesvirus entry mediator bound to mouse IgG2a Fc fragment.
- LTβR-Ig: LTβR.huIgG1.Fc
Lymphotoxin beta receptor bound to human IgG1 Fc fragment
Footnotes
M.L.R. performed experiments, analyzed data, and wrote the paper. C.F.R. analyzed data and discussed results. S.S., K.F. and Y.S. provided critical reagents. M.K. gave advice and constructively discussed results. P.S. provided reagents, contributed to design the research, performed experiments and revised the manuscript. O.C. performed surface plasmon resonance analysis, and J.I.R.B. designed the research, analyzed data and wrote the manuscript. All authors contributed to the editing of the final draft.
Disclosure of Conflicts of Interest
The authors declare no conflicting financial interests.
Reference List
- 1.Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 2.Del Rio ML, Schneider P, Fernandez-Renedo C, Perez-Simon JA, Rodriguez-Barbosa JI. LIGHT/HVEM/LTbetaR Interaction as a Target for the Modulation of the Allogeneic Immune Response in Transplantation. Am J Transplant. 2013;13:541–551. doi: 10.1111/ajt.12089. [DOI] [PubMed] [Google Scholar]
- 3.Tamada K, Shimozaki K, Chapoval AI, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164:4105–4110. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 4.Tamada K, Shimozaki K, Chapoval AI, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6:283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 5.Harrop JA, McDonnell PC, Brigham-Burke M, et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273:27548–27556. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 6.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479–2486. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Iwamoto K, Tsuji I, et al. Trimerization of murine TNF ligand family member LIGHT increases the cytotoxic activity against the FM3A mammary carcinoma cell line. Appl Microbiol Biotechnol. 2011;90:1691–1699. doi: 10.1007/s00253-011-3168-8. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh RB, Santee S, Granger SW, et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167:6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg MW, Shui JW, Ware CF, Kronenberg M. Regulating the mucosal immune system: the contrasting roles of LIGHT, HVEM, and their various partners. Semin Immunopathol. 2009;31:207–221. doi: 10.1007/s00281-009-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Q, Fraser CC, Gao W, et al. Modulation of LIGHT-HVEM costimulation prolongs cardiac allograft survival. J Exp Med. 2002;195:795–800. doi: 10.1084/jem.20012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Schmidt CS, Zhao F, et al. LIGHT-deficiency impairs CD8+ T cell expansion, but not effector function. Int Immunol. 2003;15:861–870. doi: 10.1093/intimm/dxg082. [DOI] [PubMed] [Google Scholar]
- 12.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Flies AS, Flies DB, et al. Selective targeting of the LIGHT-HVEM costimulatory system for the treatment of graft-versus-host disease. Blood. 2007;109:4097–4104. doi: 10.1182/blood-2006-09-047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware CF. Targeting the LIGHT-HVEM Pathway. Adv Exp Med Biol. 2009;647:146–155. doi: 10.1007/978-0-387-89520-8_10. [DOI] [PubMed] [Google Scholar]
- 15.Ware CF, Sedy JR. TNF Superfamily Networks: bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14) Curr Opin Immunol. 2011;23:627–631. doi: 10.1016/j.coi.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu H, Solovyev I, Colombero A, Elliott R, Kelley M, Boyle WJ. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem. 1997;272:13471–13474. doi: 10.1074/jbc.272.21.13471. [DOI] [PubMed] [Google Scholar]
- 17.Compaan DM, Gonzalez LC, Tom I, Loyet KM, Eaton D, Hymowitz SG. Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. J Biol Chem. 2005;280:39553–39561. doi: 10.1074/jbc.M507629200. [DOI] [PubMed] [Google Scholar]
- 18.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 19.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez LC, Loyet KM, Calemine-Fenaux J, et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung TC, Humphreys IR, Potter KG, et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc Natl Acad Sci U S A. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF. Genomic characterization of LIGHT reveals linkage to an immune response locus on chromosome 19p13.3 and distinct isoforms generated by alternate splicing or proteolysis. J Immunol. 2001;167:5122–5128. doi: 10.4049/jimmunol.167.9.5122. [DOI] [PubMed] [Google Scholar]
- 23.Cheung TC, Steinberg MW, Oborne LM, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106:6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy TL, Murphy KM. Slow Down and Survive: Enigmatic Immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 25.Ehlers S, Holscher C, Scheu S, et al. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J Immunol. 2003;170:5210–5218. doi: 10.4049/jimmunol.170.10.5210. [DOI] [PubMed] [Google Scholar]
- 26.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 27.Gommerman JL, Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat Rev Immunol. 2003;3:642–655. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- 28.Gramaglia I, Mauri DN, Miner KT, Ware CF, Croft M. Lymphotoxin alphabeta is expressed on recently activated naive and Th1-like CD4 cells but is down-regulated by IL-4 during Th2 differentiation. J Immunol. 1999;162:1333–1338. [PubMed] [Google Scholar]
- 29.Morel Y, Schiano de Colella JM, Harrop J, et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165:4397–4404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- 30.Watts TH, Gommerman JL. The LIGHT and DARC sides of herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102:13365–13366. doi: 10.1073/pnas.0506707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossen C, Ingold K, Tardivel A, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 32.Stark S, Flaig RM, Sandusky M, Watzl C. The use of trimeric isoleucine-zipper fusion proteins to study surface-receptor-ligand interactions in natural killer cells. J Immunol Methods. 2005;296:149–158. doi: 10.1016/j.jim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 33.De TC, Ware CF. The TNF receptor and Ig superfamily members form an integrated signaling circuit controlling dendritic cell homeostasis. Cytokine Growth Factor Rev. 2008;19:277–284. doi: 10.1016/j.cytogfr.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev. 2011;244:169–187. doi: 10.1111/j.1600-065X.2011.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowalczyk C, Dunkel N, Willen L, et al. Molecular and therapeutic characterization of anti-ectodysplasin A receptor (EDAR) agonist monoclonal antibodies. J Biol Chem. 2011;286:30769–30779. doi: 10.1074/jbc.M111.267997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ware CF. Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev. 2008;223:186–201. doi: 10.1111/j.1600-065X.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27:19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 38.Jungbeck M, Daller B, Federhofer J, et al. Neutralization of LIGHT ameliorates acute dextran sodium sulphate-induced intestinal inflammation. Immunology. 2009;128:451–458. doi: 10.1111/j.1365-2567.2009.03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Lo JC, Foster A, et al. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest. 2001;108:1771–1780. doi: 10.1172/JCI13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anand S, Wang P, Yoshimura K, et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest. 2006;116:1045–1051. doi: 10.1172/JCI27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown GR, Lane GW, Whittington BJ. Disparate Role of LIGHT in Organ-specific Donor T Cells Activation and Effector Molecules in MHC Class II Disparate GVHD. J Clin Immunol. 2009 doi: 10.1007/s10875-009-9337-1. [DOI] [PubMed] [Google Scholar]
- 42.Lee WH, Kim SH, Lee Y, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–2010. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 43.Cohavy O, Zhou J, Ware CF, Targan SR. LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J Immunol. 2005;174:646–653. doi: 10.4049/jimmunol.174.2.646. [DOI] [PubMed] [Google Scholar]
- 44.Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR. LIGHT expression by mucosal T cells may regulate IFN-gamma expression in the intestine. J Immunol. 2004;173:251–258. doi: 10.4049/jimmunol.173.1.251. [DOI] [PubMed] [Google Scholar]
- 45.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 46.Brown GR, Lee EL, El-Hayek J, Kintner K, Luck C. IL-12-independent LIGHT signaling enhances MHC class II disparate CD4+ T cell alloproliferation, IFN-gamma responses, and intestinal graft-versus-host disease. J Immunol. 2005;174:4688–4695. doi: 10.4049/jimmunol.174.8.4688. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Salcedo TW, Wan X, et al. Modulation of T-cell responses to alloantigens by TR6/DcR3. J Clin Invest. 2001;107:1459–1468. doi: 10.1172/JCI12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Q, Fu YX, Sontheimer RD. Blockade of lymphotoxin signaling inhibits the clinical expression of murine graft-versus-host skin disease. J Immunol. 2004;172:1630–1636. doi: 10.4049/jimmunol.172.3.1630. [DOI] [PubMed] [Google Scholar]
- 49.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 50.Zhu M, Yang Y, Wang Y, Wang Z, Fu YX. LIGHT regulates inflamed draining lymph node hypertrophy. J Immunol. 2011;186:7156–7163. doi: 10.4049/jimmunol.1002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider P. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 2000;322:325–345. doi: 10.1016/s0076-6879(00)22031-4. [DOI] [PubMed] [Google Scholar]
- 52.Lin WW, Hsieh SL. Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer. Biochem Pharmacol. 2011;81:838–847. doi: 10.1016/j.bcp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Del Rio ML, Jones ND, Buhler L, et al. Selective blockade of herpesvirus entry mediator-B and T lymphocyte attenuator pathway ameliorates acute graft-versus-host reaction. J Immunol. 2012;188:4885–4896. doi: 10.4049/jimmunol.1103698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitamura T, Koshino Y, Shibata F, et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- 55.Katsel PL, Greenstein RJ. Eukaryotic gene transfer with liposomes: effect of differences in lipid structure. Biotechnol Annu Rev. 2000;5:197–220. doi: 10.1016/s1387-2656(00)05036-5. [DOI] [PubMed] [Google Scholar]
- 56.Del Rio ML, Penuelas-Rivas G, Dominguez-Perles R, Ramirez P, Parrilla P, Rodriguez-Barbosa JI. Antibody-mediated signaling through PD-1 costimulates T cells and enhances CD28-dependent proliferation. Eur J Immunol. 2005;35:3545–3560. doi: 10.1002/eji.200535232. [DOI] [PubMed] [Google Scholar]
- 57.Del Rio ML, Kaye J, Rodriguez-Barbosa JI. Detection of protein on BTLA(low) cells and in vivo antibody-mediated down-modulation of BTLA on lymphoid and myeloid cells of C57BL/6 and BALB/c BTLA allelic variants. Immunobiology. 2010;215:570–578. doi: 10.1016/j.imbio.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Oehen S, Brduscha-Riem K, Oxenius A, Odermatt B. A simple method for evaluating the rejection of grafted spleen cells by flow cytometry and tracing adoptively transferred cells by light microscopy. J Immunol Methods. 1997;207:33–42. doi: 10.1016/s0022-1759(97)00089-6. [DOI] [PubMed] [Google Scholar]
- 59.Brehm MA, Daniels KA, Ortaldo JR, Welsh RM. Rapid conversion of effector mechanisms from NK to T cells during virus-induced lysis of allogeneic implants in vivo. J Immunol. 2005;174:6663–6671. doi: 10.4049/jimmunol.174.11.6663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.