Abstract
Memory T-cell populations in human antitumor tumor-infiltrating lymphocytes (TILs) for adoptive cell transfer have not been fully characterized. Our studies demonstrated that CD62L, CD27 and CD28 positive effector memory T-cells were present in the TIL samples from the tumor tissues of melanoma patients and T-cell expansion led to the significant loss of memory T-cells. CD27- and CD28-positive T-cells had high levels of CD44 expression. T-Cell expansion resulted in significant down-regulation of CD44 expression. Interleukin-2 (IL-2) and anti-CD3 antibody stimulation may be responsible for CD44 down-regulation on CD8+ T-cells during expansion. Furthermore, CD44 down-regulation using small interfering RNA (siRNA) on TILs dramatically reduced interferon-gamma and IL-2 release upon tumor stimulation. These results suggest that the regulation of CD44 expression in TILs may play an important role in memory T-cell maintenance and antitumor immune response.
Keywords: Memory T- cell, CD44, tumor-infiltrating lymphocytes, melanoma
Recent clinical trials have shown that immunotherapy involving adoptive cell transfer with autologous tumor-specific tumor-infiltrating lymphocytes (TILs) along with high-dose interleukin-2 (IL-2) administration following lymphodepletion chemotherapy can cause tumor regression in approximately 50% of treated patients with refractory metastatic melanoma (1, 2). Because transferred lymphocytes play an important role in mediating tumor regression after adoptive cell transfer, T-cell survival and persistence in vivo after treatment is believed to be critical for successful adoptive immunotherapy. Sequence analysis of T-cell receptor (TCR) beta chain variable gene products expressed in the administered TILs and peripheral blood lymphocytes obtained from melanoma patients after adoptive cell transfer demonstrated that there is a significant correlation between tumor regression and the degree of persistence in the peripheral blood of adoptively transferred T-cells, suggesting that inadequate T-cell persistence may represent a major factor limiting responses to adoptive immunotherapy (3–6). Further studies demonstrated that telomere length of transferred T-cells correlated with in vivo T-cell persistence and clinical responses in melanoma patients after adoptive immunotherapy (7).
It is well established that memory T-cell populations are maintained for a long time, and the anamnestic response that is mediated by memory T-cells is more rapid and aggressive than the primary response (8). Thus, transfer of cells with memory properties, including a heightened recall response and the ability to undergo self-renewal, may be superior mediators of an antitumor response (9). Recent studies indicated that memory T-lymphocytes contain distinct populations of central memory and effector memory cells characterized by distinct homing capacity and effector function (10, 11). Protective memory is mediated by effector memory T-cells that migrate to inflamed peripheral tissues and display immediate effector function, whereas long-term memory is mediated by central memory T-cells that home to T-cell areas of secondary lymphoid organs, have little or no immediate effector function, but readily proliferate and differentiate into effector cells in response to antigenic stimulation (12). Human central memory T-cells are CD45R0+ memory cells that constitutively express C-C chemokine receptor type 7 (CCR7) and L-selectin (CD62L). Following TCR triggering, these memory cells produce mainly IL-2, but after proliferation they efficiently differentiate into effector cells and produce large amounts of interferon-gamma (IFN-γ) or IL-4. On the contrary, human effector memory T-cells are memory cells that have lost the constitutive expression of CCR7, are heterogeneous for CD62L expression, and display characteristic sets of chemokine receptors and adhesion molecules that are required for homing to inflamed tissues. They are characterized by rapid effector function. CD8+ effector memory T-cells carry large amounts of perforin, and both CD4 and CD8 produce IFN-γ, IL-4 and IL-5 within hours following antigenic stimulation (10). Thus, in humans, the effector memory pool contains bona fide T-helper 1 (TH1), TH2, and cytotoxic T lymphocytes (CTL) (10).
Subsets of central and effector memory T-cells with distinct functional programs can be identified according to the expression of their surface molecules. Co-stimulatory molecules were the first markers used to dissect the heterogeneity of memory T-cells. CD27 and CD28, which are expressed on naïve T-cells, are also expressed on some memory T-cells but are absent from a subset of CD8+ memory T-cells characterized by high effector function and expression of CD45RA (13, 14). CD27 interacts with its ligand, CD70, and thus augments TCR-stimulated proliferation of CD8+ T-cells (15). Similarly, CD28, which interacts with its ligands B7.1 (CD80) and B7.2 (CD86) on antigen-presenting cells, amplifies TCR-mediated T-cell proliferation, differentiation and activation (16). It has been reported that CD27 is required for the generation and maintenance of T-cell memory (17).
CD44 is a cell surface transmembrane glycoprotein, encoded by a single gene. The human CD44 gene is located on the short arm of chromosome 11, containing at least 20 exons spanning about 50 kilobases of DNA. The gene is composed of two groups of exons. One group, comprising exons 1–5 and 16–20, are expressed together as the standard form. The ten variable exons (exons 6–15) can be alternatively spliced and included within the standard exons at an insertion site between exons 5 and 16. Transcripts for this gene undergo complex alternative splicing that results in many functionally distinct isoforms. CD44 is expressed in a variety of tissues derived from hematopoietic, epithelial, endothelial, and mesodermal origins (18). CD44 expression on tumor cells is documented to play a role in metastasis (19, 20). CD44s (the standard form) is known to be important in T-cell signaling and a variety of immune cell functions (21–23). In addition, CD44s plays a role in T- and B-cell adhesion (24), cell aggregation (25), proliferation (26) and migration (27). CD44s is also an important cytotoxic triggering molecule on CTL, double-negative T-cells and natural killer (NK) cells in mice (28). CD44 is also used to characterize T-cells in mice by the simultaneous expression of four surface markers, according to which they are phenotypically categorized as naïve, effector or memory T-cells, based on generally accepted markers (29). Currently, high expression of CD44 seems to be the most reliable marker for memory cells in mice, both for CD4+ and CD8+ cells (30, 31). Therefore, CD44 may play a role in human memory T-cell generation and maintenance.
Recent success in adoptive transfer therapy of tumor-specific T-cells for the treatment of melanoma in humans and in animal models raises the possibility that this strategy may be explored for other tumor types as well. Various parameters, however, require optimization for successful adoptive immunotherapy of cancer patients. Manipulations of memory T-cell generation and maintenance in vitro may improve adoptive immunotherapy. Understanding the role of CD44 in generating and maintaining tumor-reactive memory T-cells for adoptive cell transfer could lead to improved immunotherapy for patients with cancer. Thus, the objective of the present study was to characterize memory T-cells in TILs from melanoma patients, determine the factors leading to the loss of memory phenotype T-cells after T-cell rapid expansion for adoptive cell transfer therapy, and understand the role of CD44 in memory T-cell maintenance and antitumor immune response.
Materials and Methods
TIL samples and their expansion
This study was approved by the University of South Carolina Institute Review Board (HSA3671) and Palmetto Health Institute Review Board (#2006-130 and #2010-88). All patients in this study signed an Institutional Review Board-approved consent. The techniques of TIL growth have already been described in detail (32). Briefly, explants of small (2 mm3) tumor fragments or 1×106 viable cells of tumor tissue digests were used to initiate TIL cultures (denoted as IL-2-TIL) in 2 ml of RPMI-1640-based medium (Invitrogen Life Technologies, Carlsbad, CA, USA) containing 10% human serum and 6000 IU/ml IL-2 (Chiron, Emeryville, CA, USA). After 2–4 weeks of culture, usually several million TILs were obtained and screened by IFN secretion assay (PeproTech, Rocky Hill, NY, USA) for recognition of tumor cells. Antitumor TIL cultures were further expanded in AIM V medium (Invitrogen Life Technologies) supplemented with irradiated allogeneic feeder cells, anti-CD3 antibody (OKT3; Ortho Biotech, Bridgewater, NJ, USA), and 6000 IU/ml IL-2 (Chiron) (Rapid Expansion Protocol) (32). This expansion protocol typically resulted in 1000-fold expansion of cells by the time of administration, 14–15 days after initiation of the expansion. All of the TILs (denoted as anti-CD3+IL-2-TILs) secreted >100 pg/ml IFN-γ and at least twice the background in co-cultures with autologous melanoma cells, HLA-matched melanoma cell lines, or T2 cells pulsed with the dominant HLA-A2-restricted peptide epitopes MART-1:27–35 or gp100:209–217 (Abgent, San Diego, CA, USA). IL-2-TILs and anti-CD3+IL-2-TILs were further used in characterizing T-cells with memory phenotype.
Peripheral blood mononuclear cell (PBMC) samples were isolated from whole blood using Lymphocyte Separation Medium (Cambrex, Walkersville, MD, USA) and used as allogeneic feeder cells. Whole blood samples from healthy donors were purchased from the Gulf Coast Regional Blood Center (Houston, TX, USA).
Fluorescence-activated cell sorter (FACS) analysis of memory phenotypic marker expression
TIL samples were co-stained with fluorescein isothiocyanate (FITC) or peridinin-chlorophyll-protein (PerCP)-conjugated anti-human CD8 monoclonal antibody (mAb) and R-phycoerythrin (PE)-conjugated mAbs against CCR7, CD62L, CD27 and CD28. They were co-stained with PE-conjugated anti-human CD4 or CD8 mAb and FITC-conjugated anti-human CD44 mAb. They were also co-stained with FITC-conjugated anti-human CD44 mAb and PE-conjugated mAbs against CCR7, CD62L, CD27 and CD28. Antibodies against human CCR7, CD4, CD8, CD27, CD28, CD44 and CD62L were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Phenotypic marker expression was analyzed using a Cytomics FC500 instrument (Beckman Coulter, Indianapolis, IN, USA).
IL-2 and anti-CD3 antibody stimulation
All experiments were performed in X-VIVO 15 medium (Cambrex) containing 10% fetal calf serum. Due to the low availability, only anti-CD3+IL-2-TIL samples were used in these experiments. A total of 1×106 cells per well from anti-CD3+IL-2-TIL samples were cultured in 24-well plates with 1 ml culture medium. Then, 0, 50, 250, 500 and 1000 CU/ml IL-2, or 0, 5, 30, 100 and 250 ng/ml OKT3, or 1000 CU/ml IL-2 in combination with different concentrations of OKT3, or 30 ng/ml OKT3 in combination with different concentrations of IL-2 were added to the culture medium to stimulate T-cells. After culture for two days in a humidified incubator at 37°C with 5% CO2, T-cells were co-stained with PE-conjugated anti-human CD4 or CD8 mAb and FITC-conjugated anti-human CD44 mAb for determination of regulation of CD44 expression on T-cells by FACS analysis.
Anti-CD27 and CD28 antibody stimulation
Twenty-four-well plates were coated with 0, 10 or 1000 ng/ml purified anti-CD27 or anti-CD28 mAb (BD Biosciences) in 1 ml of PBS per well at 4°C overnight. After the plates were washed with X-VIVO 15 medium twice, 1×106 cells per well from anti-CD3+IL-2-TIL samples were cultured in the antibody-coated plates with 1 ml culture medium of X-VIVO 15 medium (Cambrex) containing 10% fetal calf serum. After culture for two days in a humidified incubator at 37°C with 5% CO2, T-cells were co-stained with PE-conjugated anti-human CD4 or CD8 mAb and FITC-conjugated anti-human CD44 mAb for determination of regulation of CD44 expression on T-cells by FACS analysis.
CD44 down-regulation by siRNA
Human CD44 siRNA and negative control siRNA were purchased from Dharmacon (Chicago, IL, USA). SiRNA (0, 40, 200 and 1000 nM) was introduced into lymphocytes in TIL samples by electroporation using an ECM803 electroporation System (BTX, Holliston, MA, USA) at 400 V for 500 μs. After electroporation, 1×106 T-cells were transferred into a 24-well plate with 1 ml of X-VIVO 15 medium containing 10% fetal calf serum in each well and incubated at 37°C with 5% CO2. After culture for two days, CD44 down-regulation in T-cells was monitored by FACS analysis. Meanwhile, 1×106 antigen-specific and HLA-matched melanoma tumor cells were added to each well. After culture for an additional day, IFN-γ and IL-2 release in the culture supernatant were determined by ELISA.
ELISA assay of IFN-γ and IL-2 production
ELISA kits for IFN-γ and IL-2 production analysis were purchased from PeproTech (Rocky Hill, NY, USA). ELISA assay was performed according to the manufacturer’s instructions. The culture supernatants from in vitro tumor stimulation of lymphocytes in the TIL samples were used for IFN-γ and IL-2 ELISA assay
Statistical analysis
Data were analyzed by Mann-Whitney U-test, and differences were considered significant at p<0.05.
Results
Loss of T-cells with memory phenotype after expansion using anti-CD3+IL-2
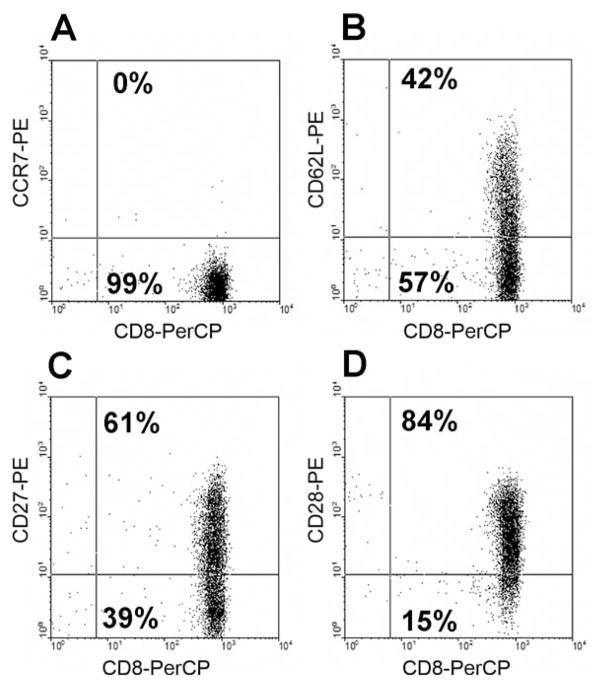
Because immune memory has a critical role in mediating protection against infections as well as potentiating allergic and autoimmune diseases, we determined if memory T-cells are present in the IL-2-TIL microculture samples from melanoma patients. Flow cytometric analysis indicated that the CD8+ T-cells in the TIL samples did not express CCR7, but a significant proportion of cells expressed CD62L, CD27 and CD28. Representative data are shown in Figure 1 and data from multiple samples are depicted in Figure 2. Analysis of multiple TIL samples showed that CD8+ T-cells in the initial IL-2-TIL microcultures generated from the tumor tissues of melanoma patients did not express CCR7. However, 35±5% of the CD8+ T-cells expressed CD62L, 67±5% expressed CD27 and 70±6% expressed CD28, suggesting that effector memory T-cells could be identified in the TIL samples from melanoma patients for adoptive cell transfer (Figure 2). As shown in Figure 1, almost all T-cells in IL-2-TIL microculture samples were CD8+ T-cells, and thus we focused on the analysis of memory phenotype marker expression on CD8+ T-cells in TIL samples.
Figure 1.
Representative profile of memory phenotype marker expression on CD8+ T-cells in an IL-2-TIL sample from a melanoma patient. The IL-2-TIL sample was initiated from a melanoma patient in the presence of high-dose interleukin-2 (IL-2), and the expression of memory phenotype markers on T-cells was determined by flow cytometric analysis after staining with mAbs against human CCR7, CD27, CD28, CD62L and CD8. The x-axis represents the fluorescence staining with anti-CD8-PerCP, and the y-axis represents staining with anti-CCR7-PE (A), anti-CD62L-PE (B), anti-CD27-PE (C), and anti-CD28-PE (D), respectively.
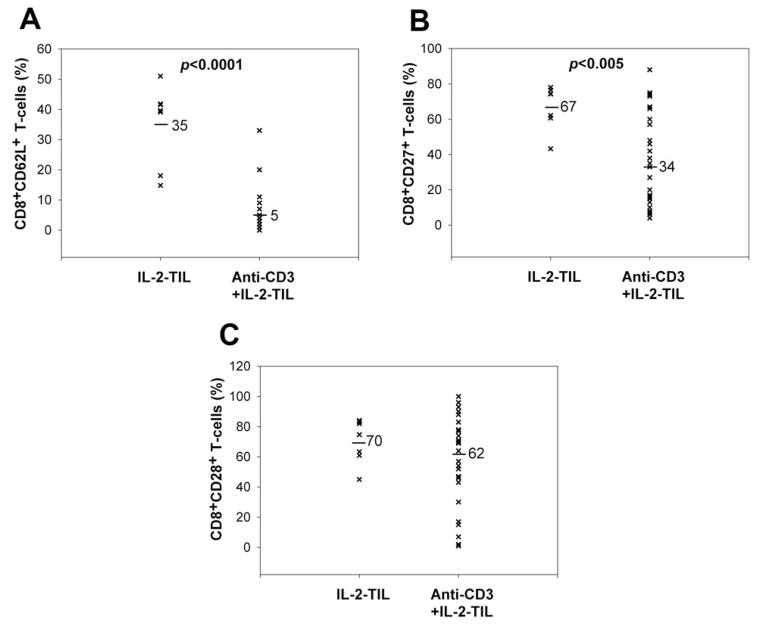
Figure 2.
Comparison of memory phenotype marker expression on CD8+ T-cells between IL-2-TIL microcultures and anti-CD3+IL-2-TIL samples from melanoma patients. IL-2-TIL microcultures represent TIL samples which were initiated from the tumor specimens resected from melanoma patients in the presence of high-dose interleukin-2 (IL-2) for two or three weeks. Anti-CD3+IL-2-TIL samples refer to the TIL samples for adoptive cell transfer after using the rapid expansion protocol in the presence of high-dose IL-2, anti-CD3 antibody and allogeneic peripheral blood mononuclear cells for two weeks. T-Cells from both IL-2-TIL microculture samples and anti-CD3-IL-2-TIL samples were co-stained with R-phycoerythrin-conjugated anti-human CD27, CD28, CD62L and fluorescein isothiocyanate-conjugated anti-human CD8 for FACS analysis. The percentage of CD8+CD62L+ (A), CD8+CD27+ (B) and CD8+CD28+ (C) T-cells from both IL-2-TIL microculture samples and anti-CD3+IL-2-TIL samples were calculated and compared.
As the numbers of T-cells generated during an initial culture in IL-2 are not generally deemed sufficient for treatment, those cells are generally expanded in a rapid expansion protocol using irradiated feeder cells to obtain sufficient T-cells for treatment (32). Experimental data showed that 85±4% of T-cells in anti-CD3+IL-2-TIL samples were CD8+ T-cells, suggesting that a small proportion of the cells may have down-regulated CD8 expression. Furthermore, the CD8+ T-cells in the anti-CD3+IL-2-TIL samples, as compared with the initial IL-2-TIL microcultures before using the rapid expansion protocol, had significantly lower levels of CD62L and CD27 expression, reduced from 35±5% down to 5±1% and from 67±5% down to 34±5% respectively (Figure 2). CD28 expression, however, did not change dramatically after the large-scale expansion (70±6% before expansion and 62±5% after expansion). The effect of rapid expansion on CCR7 expression on T-cells was not evaluated due to there being a very low level of CCR7 expression on T-cells in the IL-2-TIL microculture samples. These data demonstrated that rapid T-cell expansion led to the significant loss of effector memory T-cells.
Association between CD44 expression and memory phenotype marker expression on T-cells
Inasmuch as CD44 is a multifunctional glycoprotein, we were interested in determining the level of expression of CD44 on CD8+ T-cells in TILs in order to understand its role in T-cell activity. Flow cytometric analysis indicated that CD8+ T-cells in the anti-CD3+IL-2-TILs from melanoma patients expressed CD44 heterogeneously and the majority of T-cells in anti-CD3+IL-2-TIL samples were CD44 positive (Figure 3).
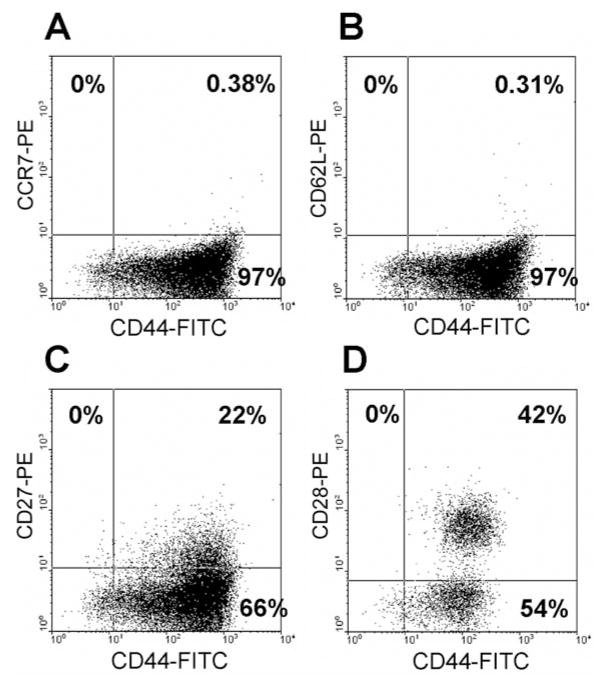
Figure 3.
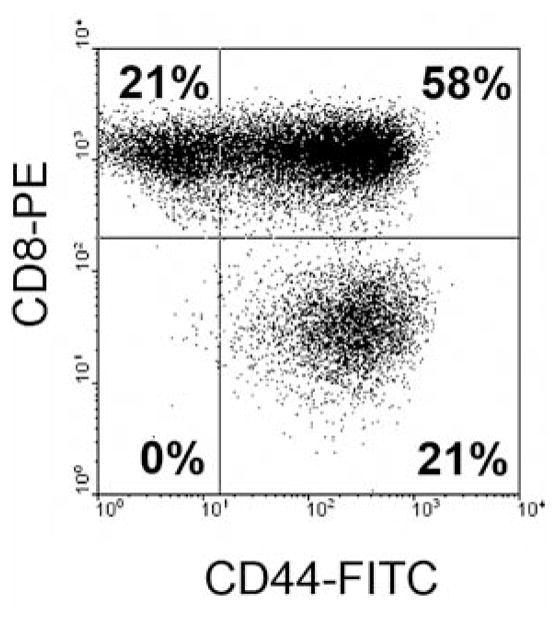
CD44 expression on CD8+ T-cells in the anti-CD3+IL-2-TILs from melanoma patients for adoptive cell transfer. T-Cells from anti-CD3+IL-2-TIL samples were stained with R-phycoerythrin (PE)-conjugated anti-human CD8 antibody and fluorescein isothiocyanate (FITC)-conjugated anti-human CD44 antibody and then FACS analysis was performed to determine CD44 expression on T-cells. CD44 expression on CD8+ T-cells from a representative TIL sample derived from a melanoma patient is shown. The x-axis represents CD44 staining and the y-axis CD8 staining.
High expression of CD44 seems to be the most reliable marker for memory cells in mice, both for CD4+ and CD8+ cells. Thus, we examined if CD44 expression is associated with memory T-cells in human lymphocytes. FACS analysis indicated that all CD27+ and CD28+ T-cells in the TIL sample from a melanoma patient expressed high levels of CD44 (Figure 4), whereas the CD27−, CD28−, CD62L− and CCR7−T-cells were heterogeneous for the level of expression of CD44. The results suggested that CD44 may represent a good marker for defining memory T-cells in human lymphocytes and possibly may play an important role in the generation and maintenance of memory T-cells in the TIL samples from melanoma patients for adoptive immunotherapy.
Figure 4.
Coexpression of CD44 and the memory phenotype markers on T-cells in a representative anti-CD3+IL-2-TIL sample from a melanoma patient. T-Cells from anti-CD3+IL-2-TIL sample were stained with R-phycoerythrin (PE)-conjugated anti-human CCR7, CD62L, CD27, CD28 monoclonal antibodies and fluorescein isothiocyanate (FITC)-conjugated anti-human CD44 monoclonal antibody and then flow cytometric analysis was performed to determine the association between CD44 expression and the memory phenotype marker expression on T-cells. Coexpression of CD44 and the memory phenotype markers on T-cells from a representative TIL sample derived from a melanoma patient is shown. The x-axis represents the fluorescence staining of CD44, and the y-axis represents CCR7 (A), CD62L (B), CD27 (C) and CD28 (D) staining, respectively.
Loss of CD44 expression during expansion with anti-CD3+IL-2
Our analysis indicated that CD44 expression might be a good marker for memory T-cells in human TILs (Figure 4), and T-cell expansion may cause the loss of T-cells with memory phenotype (Figure 2). It is valuable to understand if rapid expansion also reduces the CD44 expression on T-cells. Our results indicated that T-cell expansion stimulated by high-dose IL-2, anti-CD3 antibody and allogeneic PBMCs led to significant loss of CD44 expression on T-cells in TIL samples (Figure 5). Thus, CD44 may represent a reliable marker in identifying memory T-cells in human lymphocytes and modulation of CD44 expression on T-cells may maintain memory T-cells during rapid expansion of TIL samples for adoptive cell transfer therapy.
Figure 5.
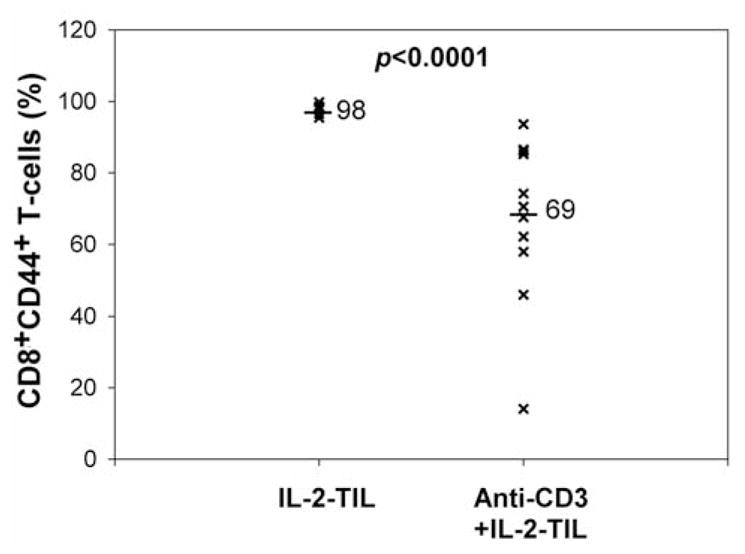
Comparison of CD44 expression on T-cells between IL-2-TIL microcultures and anti-CD3+IL-2-TIL samples from melanoma patients. The IL-2-TIL microcultures refer to the TIL samples which were initiated from the tumor specimens resected from melanoma patients in the presence of high-dose interleukin-2 (IL-2) for two or three weeks. Anti-CD3+IL-2-TIL samples refer to the TIL samples after using the rapid expansion protocol in the presence of high-dose interleukin-2 (IL-2), anti-CD3 antibody and allogeneic peripheral blood monocytes for two weeks. T-Cells from both IL-2-TIL microculture samples and anti-CD3+IL-2-TIL samples were co-stained with R-phycoerythrin (PE)-conjugated anti-human CD44 and fluorescein isothiocyanate (FITC)-conjugated anti-human CD8 for flow cytometric analysis. The percentage of CD8+CD44+T-cells from both IL-2-TIL microculture samples and anti-CD3+IL-2-TIL samples were calculated and compared.
Factors regulating CD44 expression during expansion with anti-CD3+IL-2
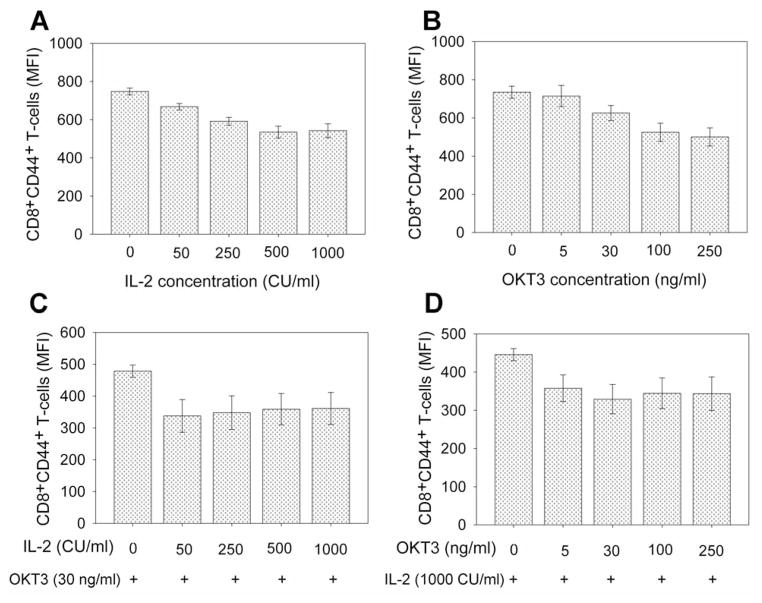
Because T-cell expansion stimulated by high-dose IL-2, anti-CD3 antibody and allogeneic peripheral lymphocytes led to significant loss of CD44 expression on T-cells in TILs (Figure 5), we next determined which factors are associated with CD44 down-regulation in T-cells. The results showed that stimulation with >50 CU/ml IL-2 or >30 ng/ml anti-CD3 antibody (OKT3) significantly reduced CD44 expression on CD8+ T-cells (p<0.05) (Figure 6A and 6B). Stimulation by the combination of OKT3 and IL-2, even at low concentrations, significantly reduced CD44 expression on CD8+ T-cells (p<0.01) (Figure 6C and 6D). Stimulation of CD27 and CD28 co-stimulatory molecules, however, did not significantly affect CD44 expression on CD8+ T-cells during rapid expansion (p=0.33–0.67) (Figure 7). Thus, OKT3 and IL-2 stimulation may be factors which caused the loss of CD44 expression on T-cells in TILs during rapid expansion of T-cells.
Figure 6.
Effect of interleukin-2 (IL-2) and anti-CD3 antibody stimulation on CD44 expression on CD8+ T-cells in TILs. T-Cells in TIL samples were stimulated with different concentrations of IL-2 (A), anti-CD3 antibody (OKT3) (B), or combination of IL-2 and OKT3 (C and D) in X-VIVO 15 culture medium for two days. T-Cells were then stained with R-phycoerythrin-conjugated anti-human CD44 and fluorescein isothiocyanate-conjugated anti-human CD8 for flow cytometric analysis. The mean fluorescence intensity (MFI) of CD8+CD44+ T-cells from TIL samples were calculated and compared at various concentrations of IL-2, anti-CD3 antibody, or combinations of IL-2 and anti-CD3 antibody.
Figure 7.
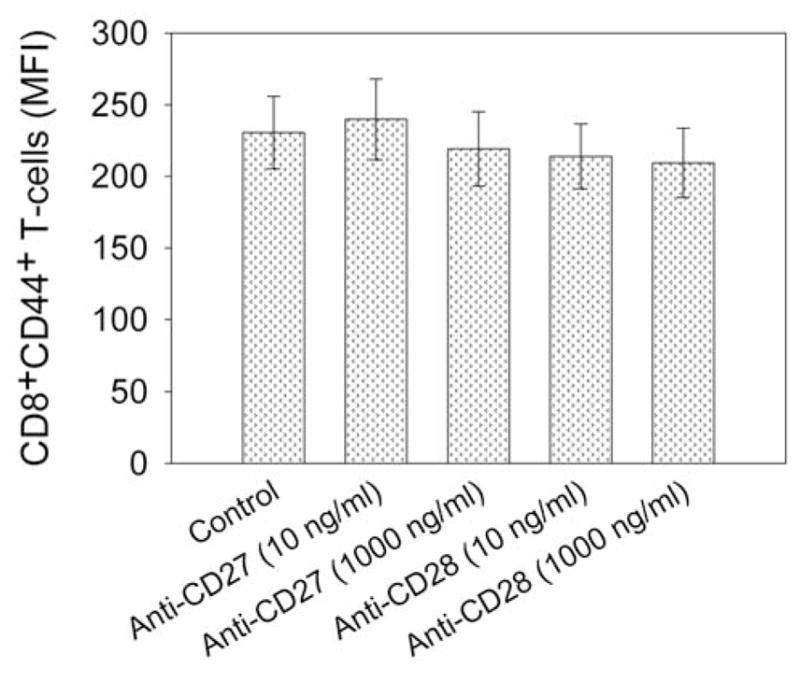
Effect of co-stimulatory molecule stimulation on CD44 expression on CD8+ T-cells in TILs. T-Cells in TIL samples were stimulated with plate-coated purified anti-human CD27 or anti-human CD28 in X-VIVO 15 culture medium for two days. Then T-cells were stained with R-phycoerythrin-conjugated anti-human CD44 and fluorescein isothiocyanate-conjugated anti-human CD8 for flow cytometric analysis. The mean fluorescence intensity (MFI) of CD8+CD44+ T-cells from anti-CD3+IL-2-TIL samples were calculated and compared.
Inhibition of antitumor immune response by CD44 down-regulation on TILs
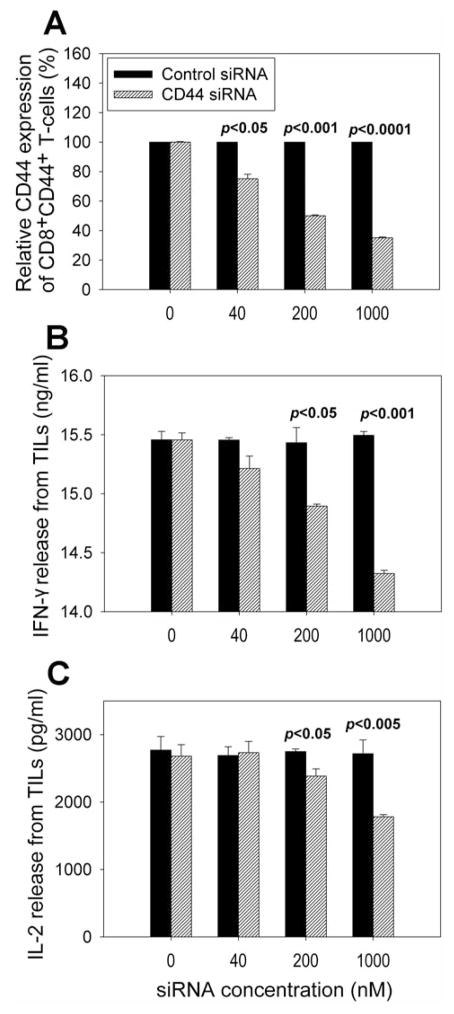
We next addressed if CD44 down-regulation plays a role in T-cell functions in human antitumor immunity. Our studies demonstrated that CD44 expression on TIL samples from melanoma patients could be dramatically down-regulated by use of siRNA (p<0.0001) (Figure 8A). Down-regulation of CD44 expression led to significant inhibition of IL-2 and IFNγ release from TIL samples upon tumor stimulation (p<0.001) (Figure 8B and 8C), suggesting that CD44 may be involved in antitumor immune response.
Figure 8.
Effects of CD44 down-regulation by siRNA on cytokine release from TIL samples upon tumor stimulation. Down-regulation of CD44 expression on TILs was carried out using CD44 siRNA and negative control siRNA (A). CD44 siRNA was introduced into TIL samples by electroporation; cells were then cultured for two days, and CD44 expression was determined by flow cytometric analysis. Meanwhile, TIL samples were stimulated with antigen-specific and HLA-matched melanoma tumor cells. After culture for an additional day, interferon-γ (IFN-γ) (B) and interleukin-2 (IL-2) (C) release from TILs were measured by ELISA.
Discussion
Recent success in the use of in vitro activation followed by in vivo re-infusion of tumor-specific T-cells for the treatment of melanoma in humans (1, 33) and in animal models (34) raises the possibility that this strategy may also be explored for other tumor types. It has been documented that immune memory has a critical role in mediating protection against infections, as well as potentiating allergic and autoimmune diseases (35–37). Mouse model studies demonstrated that transfer of cells with memory properties may be superior mediators of an antitumor response (38). Our current analysis indicated that CCR7−CD62L+CD27+CD28+ effector memory T-cells were present in TIL samples for adoptive cell transfer therapy of melanoma patients (Figure 1). These effector memory T-cells may play an important role in tumor regression mediated by transferred T-cells in melanoma patients after adoptive cell transfer therapy.
The emergence of memory T-cells, their maintenance, and their re-activation upon re-exposure to antigen are very important factors in cancer immunology. Various parameters are required for optimization of memory T-cell generation in vitro, as well as for optimization of active immunization with antitumor memory T-cell responses in vivo. The flexibility in manipulation of T-cells in vitro may facilitate generating optimal antitumor immunity, including long-lasting memory T-cells, which would not be possible with purely in vivo immunization strategies. T-Cells for in vitro stimulation and expansion remove tumor cells from the in vivo activation environment. This eliminates the adverse possibility of recreating patient’s own ineffective in vivo antitumor T-cell priming process. Instead, in the absence of tumor cells, the immune response may be skewed toward desirable generation of memory T-cells. Therefore, it is critical to determine if memory T-cells are maintained during in vitro activation and expansion. Studies in a TCR transgenic mouse model indicated that after multiple in vitro stimulations with specific antigen and IL-2, naïve pmel-1 cells (gp100-reactive T-cells) differentiated into fully mature effector CD8+ T-cells, as indicated by the progressive down-regulation of CD62L, CCR7 and CD27, and the acquisition of an effector phenotype was associated with an increase in cytolytic activity. Acquisition of full effector function in vitro, however, paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T-cells (39). Our results demonstrated that in vitro activation and large-scale expansion by the rapid expansion protocol using high-dose IL-2, anti-CD3 antibody and allogeneic peripheral blood lymphocytes significantly reduced the population of CD27+ and CD62L+ CD8+ T-cells in the TIL samples (Figure 2), suggesting that the rapid expansion protocol led to a significant loss of memory T-cells. Large-scale T-cell expansion in vitro is an essential step to obtain enough antitumor lymphocytes for adoptive cell transfer immunotherapy. Thus, loss of memory T-cells during T-cell expansion may represent a limiting factor for successful adoptive T-cell transfer therapy for cancer.
Classic T-cell memory, which is long lived in that it can be recalled long after the initial exposure to antigen, is carried out by small resting lymphocytes rather than ones with the characteristics of activated effectors. In the mouse, naïve T-cells are CD44 low, and memory T-cells are CD44 high, and low in the expression of activation markers such as CD25 and CD69 (40, 41). Currently, high expression of CD44 seems to be the most reliable marker for memory cells in mice, both for CD4+ and CD8+ cells (30, 41). Therefore, CD44 may also play a role in human memory T-cell generation and maintenance. Moreover, in mature lymphocytes, CD44 is up-regulated in response to antigenic stimuli and may participate in the effector stage of immunological responses (28, 42). Thus, CD44 expression on T-cells in TIL samples for adoptive cell transfer was examined. The results showed that T-cells in TILs expressed CD44 heterogeneously and the majority of T-cells were CD44+ (Figure 3), suggesting that CD44 may play a role in the regulation of T-cell functions in antitumor immunity (Figure 8). It has been reported that CD44 plays a role in hematopoiesis (43), T-cell development (44), T-cell adhesion (24), activation (45), proliferation (26), migration (27) and CD4+CD25+ regulatory T-cell function (46). Animal studies discovered that CD44-deficient T-cells exhibited a specific defect in interstitial migration and were thus impaired in their tumor rejection (47). Therefore, CD44 may play a role in antitumor immune response. Our further studies demonstrated that there was a close association between CD44 expression and the other memory phenotypic marker expression (Figure 4). CD44 expression on CD8+ T-cells in TILs was significantly down-regulated after in vitro stimulation and expansion with high-dose IL-2, anti-CD3 antibody and allogeneic peripheral blood lymphocytes (Figure 5). These data indicated that CD44 appears to be a good marker in defining memory T-cells in human and probably plays a role in the generation and maintenance of memory T-cells for adoptive cell transfer therapy for melanoma patients.
Although the effect of cytokines on CD44 expression and binding capacity has been investigated in different cell types (18), regulation of CD44 expression on T-cells has not yet been elucidated. The results presented here showed that IL-2 and anti-CD3 antibody (OKT3) stimulation significantly reduced CD44 expression on CD8+ T-cells (Figure 6). Stimulation of co-stimulatory molecules using anti-CD27 and anti-CD28 antibodies, however, did not significantly affect CD44 expression on CD8+ T-cells during rapid expansion (Figure 7). Thus, we provide evidence for the first time that OKT3 and IL-2 stimulation may be factors which caused the loss of CD44 expression on T-cells in TILs during rapid expansion. It has been reported that in vitro stimulation of tumor-reactive cytotoxic T lymphocytes by anti-CD3 and anti-CD28 antibodies in the presence of IL-2 reduced the expression of memory marker CD62L substantially (48) and transcriptional repressors of IL-2 signaling increased the memory CD8+ T-cell response (49). It is important to determine which factors may maintain or up-regulate CD44 expression on T-cells during T-cell expansion and it is expected that these may maintain memory T-cells during T-cell expansion.
In summary, this study shows that effector memory T-cells were present in IL-2-TIL samples. Rapid expansion of T-cells resulting from culturing cells with OKT3 Ab, IL-2 and irradiated allogeneic cells significantly led to a loss of T-cells with CD44-associated memory phenotype in TIL samples for adoptive cell transfer therapy. Down-regulation of CD44 expression in TILs dramatically reduced IFN-γ and IL-2 release from T-cells upon tumor stimulation. Our data demonstrated that the regulation of CD44 expression in TILs may play an important role in memory T-cell maintenance and antitumor immune response. It is expected that the maintenance and up-regulation of CD44 expression on T-cells to enhance memory T-cells in TIL samples may lead to improved adoptive cell transfer immunotherapy for cancer patients.
References
- 1.Dudley ME. Adoptive cell therapy for patients with melanoma. J Cancer. 2011;2:360–362. doi: 10.7150/jca.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Selective growth, in vitro and in vivo, of individual T-cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J Immunol. 2004;173:7622–7629. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, Figini M, June CH, Coukos G, Powell DJ., Jr In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T-cells is enhanced by co-stimulatory signaling through CD137 (4–1BB) Cancer Res. 2011;71:4617–4627. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker JM, Slifka MK. Longevity of T-cell memory following acute viral infection. Adv Exp Med Biol. 2010;684:96–107. doi: 10.1007/978-1-4419-6451-9_8. [DOI] [PubMed] [Google Scholar]
- 9.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T-cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T-cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 11.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T-cells. Nat Immunol. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzavecchia A, Sallusto F. Dynamics of T-lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 13.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T-cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 14.Mojumdar K, Vajpayee M, Chauhan NK, Singh A, Singh R, Kurapati S. Altered T-cell differentiation associated with loss of CD27 and CD28 in HIV infected Indian individuals. Cytometry B Clin Cytom. 2011 doi: 10.1002/cyto.b.20610. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol. 2011;89:195–203. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 16.Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H, Flies D, Luo L, Wang S, Chen L. B7-h2 is a co-stimulatory ligand for CD28 in human. Immunity. 2011;34:729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai H, Chen J, Shao W, Wang F, Xu S, Peng Y, Lin Y, Xia J, Ekberg H, Wang X, Qi Z. Blockade of CD27/CD70 pathway to reduce the generation of memory T-cells and markedly prolong the survival of heart allografts in presensitized mice. Transpl Immunol. 2011;24:195–202. doi: 10.1016/j.trim.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Gee K, Kryworuchko M, Kumar A. Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2004;52:13–26. [PubMed] [Google Scholar]
- 19.Saya H, Nagano O. Role of CD44-mediated signal in cancer invasion and metastasis. Tanpakushitsu Kakusan Koso. 2008;53:1225–1230. [PubMed] [Google Scholar]
- 20.Ween MP, Oehler MK, Ricciardelli C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int J Mol Sci. 2011;12:1009–1029. doi: 10.3390/ijms12021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marhaba R, Bourouba M, Zoller M. CD44v6 promotes proliferation by persisting activation of MAP kinases. Cell Signal. 2005;17:961–973. doi: 10.1016/j.cellsig.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Mielgo A, Brondani V, Landmann L, Glaser-Ruhm A, Erb P, Stupack D, Gunthert U. The CD44 standard/ezrin complex regulates Fas-mediated apoptosis in Jurkat cells. Apoptosis. 2007;12:2051–2061. doi: 10.1007/s10495-007-0115-3. [DOI] [PubMed] [Google Scholar]
- 23.Rajasagi M, Vitacolonna M, Benjak B, Marhaba R, Zoller M. CD44 promotes progenitor homing into the thymus and T-cell maturation. J Leukoc Biol. 2009;85:251–261. doi: 10.1189/jlb.0608389. [DOI] [PubMed] [Google Scholar]
- 24.Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T-cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- 25.Bourguignon LY, Zhu D, Zhu H. CD44 isoform – cytoskeleton interaction in oncogenic signaling and tumor progression. Front Biosci. 1998;3:d637–649. doi: 10.2741/a308. [DOI] [PubMed] [Google Scholar]
- 26.Foger N, Marhaba R, Zoller M. CD44 supports T-cell proliferation and apoptosis by apposition of protein kinases. Eur J Immunol. 2000;30:2888–2899. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Fanning A, Volkov Y, Freeley M, Kelleher D, Long A. CD44 cross-linking induces protein kinase C-regulated migration of human T-lymphocytes. Int Immunol. 2005;17:449–458. doi: 10.1093/intimm/dxh225. [DOI] [PubMed] [Google Scholar]
- 28.Rafi-Janajreh AQ, Nagarkatti PS, Nagarkatti M. Role of CD44 in CTL and NK cell activity. Front Biosci. 1998;3:d665–671. doi: 10.2741/a311. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann M, Brinkmann V, Zerwes HG. FTY720 preferentially depletes naive T-cells from peripheral and lymphoid organs. Int Immunopharmacol. 2006;6:1902–1910. doi: 10.1016/j.intimp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Sprent J, Surh CD. T-Cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 31.Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity. 2011;32:104–115. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T-cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bevan MJ. Memory T-cells as an occupying force. Eur J Immunol. 2011;41:1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanetti M, Castiglioni P, Ingulli E. Principles of memory CD8 T-cells generation in relation to protective immunity. Adv Exp Med Biol. 2010;684:108–125. doi: 10.1007/978-1-4419-6451-9_9. [DOI] [PubMed] [Google Scholar]
- 37.Epstein MM. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol Ther. 2006;109:107–136. doi: 10.1016/j.pharmthera.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T-cells confer superior antitumor immunity compared with effector memory T-cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T-cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutton RW, Bradley LM, Swain SL. T-Cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 41.Dutt S, Baker J, Kohrt HE, Kambham N, Sanyal M, Negrin RS, Strober S. CD8+CD44(hi) but not CD4+CD44(hi) memory T-cells mediate potent graft antilymphoma activity without GVHD. Blood. 2011;117:3230–3239. doi: 10.1182/blood-2010-10-312751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Law GP, McKallip RJ. Role of CD44 in lymphokine-activated killer cell-mediated killing of melanoma. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1105-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sackstein R. The biology of CD44 and HCELL in hematopoiesis: the ‘step 2-bypass pathway’ and other emerging perspectives. Curr Opin Hematol. 2011;18:239–248. doi: 10.1097/MOH.0b013e3283476140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham VA, Marzo AL, Tough DF. A role for CD44 in T-cell development and function during direct competition between CD44+ and CD44− cells. Eur J Immunol. 2007;37:925–934. doi: 10.1002/eji.200635882. [DOI] [PubMed] [Google Scholar]
- 45.Hegde VL, Singh NP, Nagarkatti PS, Nagarkatti M. CD44 mobilization in allogeneic dendritic cell – T-cell immunological synapse plays a key role in T-cell activation. J Leukoc Biol. 2008;84:134–142. doi: 10.1189/jlb.1107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 co-stimulation promotes FoxP3+ regulatory T-cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol. 2009;183:2232–2241. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T-cells and enables efficient antitumor immunity. Immunity. 2008;29:971–985. doi: 10.1016/j.immuni.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teschner D, Wenzel G, Distler E, Schnurer E, Theobald M, Neurauter AA, Schjetne K, Herr W. In vitro stimulation and expansion of human tumor-reactive CD8+ cytotoxic T-lymphocytes by anti-CD3/CD28/CD137 magnetic beads. Scand J Immunol. 2011;74:155–164. doi: 10.1111/j.1365-3083.2011.02564.x. [DOI] [PubMed] [Google Scholar]
- 49.Fearon DT, Carr JM, Telaranta A, Carrasco MJ, Thaventhiran JE. The rationale for the IL-2-independent generation of the self-renewing central memory CD8+ T-cells. Immunol Rev. 2006;211:104–118. doi: 10.1111/j.0105-2896.2006.00390.x. [DOI] [PubMed] [Google Scholar]