Abstract
Resveratrol (trans-3,5,4′-trihydroxystilbene), a polyphenolic compound found in plant products, including red grapes, exhibits anticancer, antioxidant, and anti-inflammatory properties. Using an animal model of multiple sclerosis (MS), we investigated the use of resveratrol for the treatment of autoimmune diseases. We observed that resveratrol treatment decreased the clinical symptoms and inflammatory responses in experimental allergic encephalomyelitis (EAE)-induced mice. Furthermore, we observed significant apoptosis in inflammatory cells in spinal cord of EAE-induced mice treated with resveratrol compared with the control mice. Resveratrol administration also led to significant down-regulation of certain cytokines and chemokines in EAE-induced mice including tumor necrosis factor-α, interferon-γ, interleukin (IL)-2, IL-9, IL-12, IL-17, macrophage inflammatory protein-1α (MIP-1α), monocyte chemoattractant protein-1 (MCP-1), regulated on activation normal T-cell expressed and secreted (RANTES), and Eotaxin. In vitro studies on the mechanism of action revealed that resveratrol triggered high levels of apoptosis in activated T cells and to a lesser extent in unactivated T cells. Moreover, resveratrol-induced apoptosis was mediated through activation of aryl hydrocarbon receptor (AhR) and estrogen receptor (ER) and correlated with up-regulation of AhR, Fas, and FasL expression. In addition, resveratrol-induced apoptosis in primary T cells correlated with cleavage of caspase-8, caspase-9, caspase-3, poly(ADP-ribose) polymerase, and release of cytochrome c. Data from the present study demonstrate, for the first time, the ability of resveratrol to trigger apoptosis in activated T cells and its potential use in the treatment of inflammatory and autoimmune diseases including, MS.
Resveratrol (trans-3,5,4′-trihydroxystilbene), a nonflavinoid polyphenolic compound found in a large number of plant products including mulberries, peanuts, and red grapes (Jang et al., 1997), is a member of the class of plant antibiotic compounds produced as a part of a plant’s defense system against fungal infection (Soleas et al., 1997). In recent years, it got attention not only for its usefulness in “French Paradox” as a phytoestrogen agent (Kopp, 1998) but also for its anticancer (Clément et al., 1998; Bernhard et al., 2000; Dörrie et al., 2001; Delmas et al., 2003; Atten et al., 2005), antioxidant (Kim et al., 2006b; Notas et al., 2006; Ovesn á et al., 2006), and an anti-inflammatory properties (Donnelly et al., 2004; de la Lastra and Villegas, 2005; Cignarella et al., 2006; Notas et al., 2006). Resveratrol is also an essential component of Ko-jo-kon, an oriental medicine used to treat diseases of the blood vessels, heart (Celotti et al., 1996; Soleas et al., 1997), and liver (Soleas et al., 1997).
Resveratrol has been the focus of recent studies of its pharmacological and beneficial properties on a wide range of diseases, including neurological, hepatic, cardiovascular, and autoimmune (Baur and Sinclair, 2006). Resveratrol has been shown to exhibit anti-inflammatory properties, and the possible mechanisms include inhibition of synthesis and release of pro-inflammatory mediators, modification of eicosanoid synthesis, and/or blockade of inducible nitric-oxide synthase and cyclo-oxygenase-2 pathways via its inhibitory effects on nuclear factor-κB or activator protein-1 (AP-1) (de la Lastra and Villegas, 2005). Resveratrol has been shown to improve health and survival of mice on a high-calorie diet and was found to mediate its effects through insulin sensitivity, reduced insulin-like growth factor-1 levels, increased AMP-activated protein kinase and peroxisome proliferator-activated receptor-γ coactivator 1α activity, increased mitochondrial number, and improved motor function (Baur et al., 2006). The beneficial effects of resveratrol have led to introduction of resveratrol as a nutritional supplement in the market and its extensive use.
Several reports demonstrate that resveratrol induces apoptosis in various cancer cells (Clément et al., 1998; Bernhard et al., 2000; Dörrie et al., 2001; Delmas et al., 2003; Atten et al., 2005). Although resveratrol has been shown to induce apoptosis in various cancer cells, the precise mechanisms and pathways involved in apoptosis of cancer cells are not similar. There are reports demonstrating that resveratrol acts as a ligand for aryl hydrocarbon receptor (AhR) and promotes translocation of AhR from cytosol to the nucleus and binding to DNA at dioxin-responsive elements of various genes (Casper et al., 1999). Resveratrol has also been shown to interact with estrogen receptors (ER) and acts as a mixed agonist-antagonist for ER (Gehm et al., 1997; Bowers et al., 2000). In addition, resveratrol has been shown to trigger CD95 signaling-dependent apoptosis in human tumor cells (Clément et al., 1998; Delmas et al., 2003). Dörrie et al. (2001) have shown that resveratrol induces apoptosis by depolarizing mitochondrial membrane and activating caspase-9 in acute lymphoblastic leukemia cells. Resveratrol may also induce FasL-related apoptosis through Cdc42 activation of ASK1/c-Jun NH2-terminal kinase-dependent signaling pathway in human leukemia HL-60 cells (Su et al., 2005).
In the current study, we demonstrate for the first time that the anti-inflammatory properties of resveratrol can be attributed, at least in part, to its ability to induce apoptosis in activated T cells. Furthermore, using a murine model of MS, we demonstrate that resveratrol can ameliorate the inflammation and clinical disease of EAE.
Materials and Methods
Mice
We purchased C57BL/6 (H-2b), AhR KO on C57BL/6 background, B6.129P2Esr1 (ER-α KO), and B6.129P2Esr2 (ER-β KO) mice from The Jackson Laboratory (Bar Harbor, ME). OT II.2a (C57BL/6-TgN (OT-II.2a)-RAG1tm1Mom) mice were purchased from Taconic Farms (Garmantown, NY). The animals were housed in University of South Carolina Animal facility. Care and maintenance of the animals were in accordance with the declaration of Helsinki and according to guide for the care and use of laboratory animals as adopted by Institutional and National Institutes of Health guidelines.
Cell Line
EL4 (mouse T lymphoma cell line) cells, maintained in complete RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 10 mM L-glutamine, 10 mM HEPES, and 100 μg/ml penicillin/streptomycin at 37°C and 5% CO2, were used in this study.
Reagents and Antibodies
RPMI 1640, L-glutamine, HEPES, Gentamicin, Dulbecco’s modified Eagle’s medium, phosphate-buffered saline (PBS), and fetal bovine serum, were purchased from Invitrogen (Carlsbad, CA). ConA was purchased from Sigma-Aldrich (St. Louis, MO). The following mAbs were purchased from BD Pharmingen (San Diego, CA): anti-mouse IgG-PE, FcBlock, CD3-PE (chain) purified anti-FasL (k-10), anti-FasL-PE (Kay-10), and anti-Fas-PE (Jo2). Inhibitors against caspase 3 (Z-DEVD), caspase 8 (Z-IETD-FMK), and caspase 9 (Z-LEHD-FMK) were purchased from R & D Systems (Minneapolis, MN). The primary Abs caspase-2, caspase-3, caspase-8, caspase-9, cytochrome c, PARP, Bax, Bad, Bcl-xl, c-FLIP (all from Cell Signaling Technologies, Danvers, MA), Bid (R & D Systems), and β-actin (Sigma-Aldrich) for Western blots were used. HRP-conjugated secondary Ab was purchased from Cell Signaling Technologies (Danvers, MA). RNeasy Mini kit and iScript cDNA synthesis kit were purchased from QIAGEN (Valencia, CA). Epicenter’s PCR premix F and Platinum Taq Polymerase kits were purchased from Invitrogen. TUNEL kits were purchased from Roche (Indianapolis, IN). α-Naphthoflavone (ANF), an antagonist for AhR, and tamoxifen, an antagonist for ER, were purchased from Sigma-Aldrich. Resveratrol was purchased from Sigma-Aldrich. Resveratrol suspended in DMSO was used in the in vitro studies. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a generous gift from Dr. K Chae (National Institute of Environmental Health Sciences, Research Triangle Park, NC). TCDD dissolved in DMSO was used in the in vitro studies.
Effect of Resveratrol on Experimental Autoimmune Encephalomyelitis (EAE) in Mice
EAE was induced in C57BL/6 mice (8–10 weeks old) by subcutaneous immunization with 100 μl of 20 mg or 150 mg of myelin oligodendrocyte glycoprotein (MOG35-55) peptide emulsified in complete Freund’s adjuvant (Difco, Detroit, MI) containing 4 mg/ml killed Mycobacterium tuberculosis (strain H37Ra; Difco). After immunization, 400 ng of pertussis toxin (Sigma-Aldrich) was i.p. injected into mice on Days 0 and 2. Next, the mice received resveratrol (100 mg/kg of body weight) on a daily basis after day 2. Resveratrol was administered as a suspension in water (0.2 ml) by oral gavage. Body weight and clinical score (0, no symptoms; 1, limp tail; 2, partial paralysis of hind limbs; 3, complete paralysis of hind limbs or partial hind and front limb paralysis; 4, tetraparalysis; 5, moribund; 6, death) were recorded on daily basis. The mean clinical score was calculated for each group every day.
Histopathological Examination of EAE
Spinal cords from control or resveratrol treated mice were collected 25 days after immunization. The spinal cord was fixed, paraffin blocks were prepared, microtome sections were generated, and tissue sections were stained using hematoxylin and eosin. The sections were examined for inflammatory cell infiltrates under a microscope.
In Situ Apoptosis in Spinal Cord of EAE Mice
Spinal cords from control or resveratrol-treated mice were collected 25 days after immunization. The spinal cord was fixed, paraffin blocks were prepared, microtome sections were generated, and in situ TUNEL assays were performed (DeadEnd Colorometric TUNEL System, Promega, Madison, WI) on tissue sections. The sections were examined for apoptosis in inflammatory cell infiltrates under a microscope.
Detection of Foxp3 Expression by RT-PCR
Total RNAs from spleen harvested from EAE mice treated with vehicle or resveratrol were prepared using the RNeasy minikit (QIAGEN, Germantown, MD). First-strand cDNA synthesis was performed in a 20-μl reaction mix containing 2 μg total RNA using iScript Kit and following the protocol of the manufacturer (Bio-Rad Laboratories, Hercules, CA). After first-strand synthesis, 2 μl (10% of the reaction volume) was used as a template for PCR amplification. To detect the expression of Foxp3, forward (5′-GGG GAA GCC ATG GCA ATA GTT-3′) and reverse (5′-TGA AGT AGG CGA ACA TGC GAG TAA-3′) primers specific to mouse Foxp3 were used. PCR was performed for 35 cycles using the following conditions: 30 s at 95°C (denaturing temperature), 40 s at 56.7°C (annealing temperature), and 60 s at 72°C (extension temperature), with a final incubation at 72°C for 10 min. The PCR products, generated from mouse Foxp3 primer pairs, were normalized against PCR products generated from β-actin (427 bp) forward (5′-AAG GCC AAC CGT GAA AAG ATG ACC-3′) and reverse (5′-ACC GCT CGT TGC CAA TAG TGA TGA-3′) primers after electrophoresis on 1.5% agarose gel and visualization with UV light. The band intensity of PCR products was determined using Bio-Rad image analysis system (Bio-Rad Laboratories).
Determination of Various Cytokines/Chemokines in Serum
Blood was collected on day 25 from vehicle or resveratrol-treated EAE mice. Various cytokines/chemokines present in blood serum were determined using Bio-Plex cytokine assay kit and according to the protocol of the company (Bio-Rad).
Determination of Anti-MOG Antibody in Serum
Blood samples were collected on day 25 from control (MOG) and resveratrol-treated (MOG + resveratrol) mice and anti-MOG antibody in blood sera was determined by performing ELISA. In brief, ELISA was performed in 96-well ELISA plates (BD Biosciences, San Jose, CA). The wells were coated with MOG peptide (1 mg/ml, 50 ml/well) in coating buffer (50 mM carbonate-bicarbonate buffer, pH 9.6) for 2 h at room temperature followed by overnight at 4°C. Blocking was done using 2% BSA in PBS for 1 h at room temperature. Wells were washed three times using wash buffer (PBS/0.05% Tween 20) followed by incubation with “serum ” diluted in wash buffer containing 1% BSA for 2 h at room temperature. After washing, wells were further incubated together with secondary antibody (goat anti-mouse IgG-peroxidase conjugate) diluted 1:20,000 in wash buffer containing 1% BSA. After washing, ABTS substrate solution was added for color development and the plate was read at 405 nm using a microplate reader. Values are represented after appropriate blank corrections.
Detection of Resveratrol-Induced Apoptosis in Primary T Cells
To determine resveratrol-induced apoptosis in primary T cells, T cells from C57BL/6 mice were purified from the spleens using nylon wool column (Polysciences, Inc., Warrington, PA) followed by depletion of B cells and macrophages. The purity of T cells was more than 90% as determined by flow cytometry (Cytomics FC 500; Beckman Coulter). T cells, unactivated or activated with ConA (2.5 μg/ml) for 24 h, were treated with vehicle (DMSO) or different concentrations (1–100 μM) of resveratrol for 12 to 24 h. Apoptosis in unactivated or activated T cells after resveratrol exposure was determined by performing TUNEL assays (fluorescein isothiocyanate-dUTP nick-end labeling) using In situ Cell-Death Detection kit (Roche, Indianapolis, IN) as described previously (Camacho et al., 2004; Camacho et al., 2005) and/or using fluorescein isothiocyanate-labeled Annexin V and PI kit and following the company’s protocol (BD Pharmingen). In some experiments, purified T cells were cultured in the absence or presence of ConA and mature syngeneic dendritic cells (DCs), generated from bone marrow of C57BL/6 mice as described previously (Inaba et al., 1992) for 24 h, and apoptosis was determined 24 h after vehicle or resveratrol treatment. Furthermore, we also examined resveratrol-induced apoptosis in antigen-specific activated T cells. For analysis of ovalbumin peptide (Ova323–339: ISQAVHAAHAEINEAGR)-specific activated T cells, we used purified T cells from OT.II.2a mice and cultured them in the absence or presence of mature syngeneic DCs pulsed with agonist ovalbumin peptide for 2 days followed by treatment with vehicle or resveratrol for 24 h and analyzed for proliferation and apoptosis. To investigate the effect of resveratrol on MOG-specific activated T cells, mice were immunized into the footpads with MOG (150 μg/mice) + Freund’s complete adjuvant. Seven days after immunization, popliteal-draining lymph nodes were harvested, single-cell suspensions were prepared, and the cells (5 × 105/well) were cultured in the presence of MOG (10 μg/ml) + various doses (10–50 μM) of resveratrol or vehicle for 3 days in vitro. Proliferation was determined by incorporation of [3H]thymidine.
Reverse Transcriptase PCR to Determine the Expression of AhR, Fas, and FasL in T Cells
Total RNA was isolated from unactivated or ConA-activated primary T cells treated with vehicle (DMSO) or resveratrol using RNeasy Mini Kit and following the protocol of the company (QIAGEN). First-strand cDNA synthesis was performed in a 20-μl reaction mix containing 2 μg of total RNA using iScript Kit and following the protocol of the manufacturer (Bio-Rad Laboratories). After first-strand synthesis, 2 μl (10% of the reaction volume) was used as a template for PCR amplification. To detect the expression of mouse-specific AhR (482 bp), FasL (435 bp), and Fas (486 bp), the protocols described previously (Singh et al., 2007) were followed. The PCR products, generated from mouse AhR, Fas, and FasL primer pairs, were normalized against PCR products generated from mouse 18S forward (5′-GCC CGA GCC GCC TGG ATA C-3′) and reverse (5′-CCG GCG GGT CAT GGG AAT AAC-3′) primers after electrophoresis on 1.5% agarose gel and visualization with UV light. The band intensity of PCR products was determined using Bio-Rad Laboratories image analysis system.
Determination of the Role of AhR in Resveratrol-Induced T-Cell Apoptosis
To determine the role of AhR in resveratrol-mediated early signaling, we performed a series of in vitro assays using T cells from wild-type (C57BL/6) and AhR knock out (AhR KO) mice. In brief, purified T cells from wild-type or AhR KO mice were not activated or activated with ConA (2.5 μg) for 24 h and then treated with vehicle (DMSO) or resveratrol (5–50 μM). Apoptosis in T cells 12 and/or 24 h after resveratrol treatments was determined by performing TUNEL assays and using flow cytometry as described previously (Camacho et al., 2004, 2005). Furthermore, 1 μM/ml ANF, an antagonist for AhR, was added in the culture of wild-type T cells 1 h before resveratrol treatment. The role of AhR in resveratrol-mediated early signaling was further determined by performing luciferase assays in the presence mouse Fas or FasL promoter in an expression vector (pGL-3) as described previously (Singh et al., 2007). Luciferase expression was also determined in the absence or presence of ANF, an antagonist for AhR, in the culture. Data from three to four independent experiments were depicted as mean fluorescence units ± S.E.M.
Determine the Role of ER in Resveratrol-Induced T-Cell Apoptosis
To determine the role of ER in resveratrol-mediated early signaling, we performed a series of in vitro assays using T cells from wild-type (C57BL/6) and ER-α and ER-β knockout mice. In brief, purified T cells from wild-type and ER-α and ER-β knockout mice, unactivated or activated with ConA (2.5 μg) for 24 h, were treated with vehicle (DMSO) or resveratrol (5–50 μM). Apoptosis in T cells was determined by performing TUNEL assays and using flow cytometry 24 h after resveratrol treatments. Furthermore, 1 μM tamoxifen (TAM), an antagonist for ER, was added in the culture of wild-type T cells 1 h before resveratrol treatment and apoptosis was determined. The role of ER in resveratrol-mediated signaling was further determined by performing luciferase assays in the presence mouse Fas or FasL promoter in an expression vector (pGL-3) as described previously (Singh et al., 2007). Luciferase expression was also determined in the absence or presence of TAM. Data from three to four independent experiments were depicted as mean fluorescence units ± S.E.M.
Role of FasL in Resveratrol-Induced T-Cell Apoptosis
Primary T cells from C57BL/6 mice were cultured in the absence or presence of antibody against mouse FasL (5 μg/ml) 1 h before resveratrol treatment. Apoptosis in T cells 24 h after resveratrol treatments was determined by performing TUNEL assays as described previously (Camacho et al., 2004, 2005). At least three independent experiments were performed and the data shown represent one representative experiment. Data from three to four independent experiments were also depicted as mean fluorescence units ± S.E.M.
Analysis of Caspase 3/7, Caspase 8, and Caspase 9 Activity
Caspases 3/7, 8, and 9 were measured in T cells exposed to resveratrol using the Apo-ONE Homogeneous Caspase-3/7, caspase-8, and caspase-9 Assays according to manufacturer’s instructions (Promega, Madison, WI). In brief, ConA-activated T cells were treated with various concentrations (5–50 μM) of resveratrol or vehicle (DMSO) for 24 h at 37°C, 5% CO2. The following day, the cells were collected and used for caspase assays. A Wallac 1420 multilabel counter, Victor2 (PerkinElmer Life and Analytical Sciences, Waltham, MA) was used to measure the relative fluorescence units of each sample at an excitation wavelength of 485 nm and at an emission wavelength of 535 nm. Luminescence of caspase-8 and caspase-9 was measured using Wallac 1420 multilabel counter, Victor2 (PerkinElmer Life and Analytical Sciences). Data from three to four independent experiments were depicted as mean fluorescence units ± S.E.M.
Caspase Blocking Assays to Determine the Role of Various Caspases in Resveratrol-Induced T-Cell Apoptosis
To investigate the role and participation of caspases in resveratrol-induced apoptosis in primary T cells, we performed in vitro assays as described above (under Detection of Resveratrol-Induced Apoptosis in Primary T Cells) with inhibitors specific to mouse caspase-3 (Z-DEVD), caspase-8 (Z-IETD-FMK), and caspase-9 (Z-LEHD-FMK) at a concentration of 20 μM. The cells were incubated with various caspase inhibitors for at least 1 h before resveratrol treatment. T cells were harvested 24 h after vehicle or resveratrol treatment, and TUNEL assays were performed to determine apoptosis as described previously (Camacho et al., 2004, 2005). At least three independent experiments were performed and the data shown represent one of the experiments.
Immunoblot Analysis
Immunoblotting was performed as described previously (Camacho et al., 2005). The source of antibodies was as follows (all from Cell Signaling Technologies unless otherwise specified): caspase-2 (1:1000; Alexis Laboratories, San Diego, CA), caspase-3, caspase-8, and caspase-9 (1:2000), Bid, (1:1000; R & D Systems), cytochrome c (1:2000), PARP (1:2000), Bax (1:2000), Bad (1:2000; Cell Signaling), Bcl-xl (1:2000), Smac (1:2000), c-FLIP (1: 2000), and β-actin (1:50,000; Sigma-Aldrich). HRP-conjugated secondary Ab was used at 1:4000 dilution (Cell Signaling Technologies). Lysates from resveratrol-treated cells were prepared by freezing and thawing, and the protein concentration was measured using standard Bradford assay (Bio-Rad Laboratories). The proteins were fractionated in SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes using a dry-blot apparatus (Bio-Rad Laboratories). The membrane was incubated in blocking buffer for 1 h at room temperature, followed by incubation in primary antibody at 4°C overnight. The membrane was then washed three times (10–15 min) with washing buffer (PBS + 0.2% Tween 20) and incubated for 1 h in HRP-conjugated secondary antibody (Cell Signaling Technology) in blocking buffer. The membranes were then washed several times and incubated in developing solution (equal volume of solution A and B; ECL Western blotting detection reagents; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) and signal was detected using ChemiDoc System (Bio-Rad Laboratories). Densitometric analyses of the Western blots were performed using ChemiDoc software (Bio-Rad Laboratories).
Analysis of Mitochondrial Membrane Potential
Mitochondrial membrane potential (Δψm) of T cells after vehicle (DMSO) or resveratrol treatment was determined using 3,3′-dihexyloxacarbocyanine iodide (DiOC6) dye as described previously (Camacho et al., 2004, 2005). Propidium iodide (PI) was used to differentiate the dead cells. At least three independent experiments were performed.
Statistical Analysis
Results presented here represent at least three independent experiments and are presented as the mean ± S.E.M. Statistical analyses were performed using Student’s t test or two-factor ANOVA as appropriate, with a P value of ≤ .05 considered to be statistically significant. For EAE, significant difference between control and experimental groups was determined using the Mann-Whitney U test (*, p < 0.01).
Results
Resveratrol Suppressed the Development of EAE in Mice
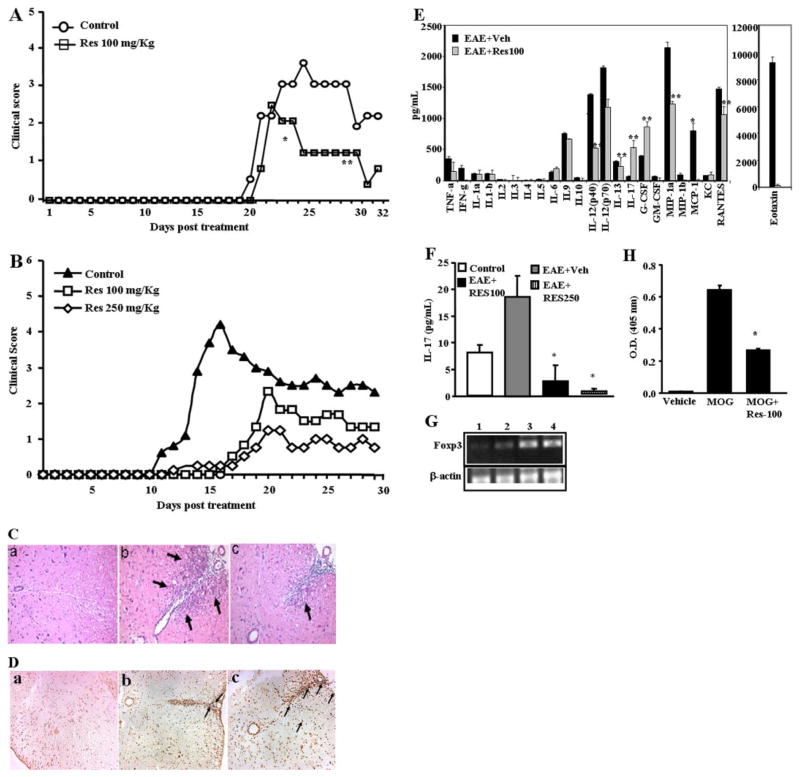
Resveratrol is known to exhibit anti-inflammatory properties. In the current study, therefore, we investigated whether resveratrol would be effective in the tretament of EAE, a model for human multiple sclerosis. To this end, EAE was induced in C57BL/6 mice (8–10 weeks of age) by subcutaneous immunization with 100 μl of 20 μg MOG35-55 peptide emulsified in complete Freund’s adjuvant containing 4 mg/ml killed M. tuberculosis. Next, mice were injected i.p. with 400 ng of pertussis toxin on days 0 and 2. These mice received resveratrol (100 mg/kg) daily from day 2 until the termination of experiment. Clinical scores of disease were recorded daily. A mean clinical score was calculated for each group for each day. As seen from Fig. 1A, with 20 μg of MOG, the symptoms started to develop around day 19 and resveratrol at 100 mg/kg caused significant suppression of clinical symptoms of EAE (p < 0.001; by Mann-Whitney U test). For example, in resveratrol-treated mice, the clinical scores never reached above 2, whereas in control mice, the score reached a peak of 4. The clinical score correlated well with histopathological data showing that there was a dose-dependent decrease in inflammatory response seen in the spinal cord of EAE-induced mice that were administered resveratrol (Fig. 1C). Next, we tested whether resveratrol would be effective against EAE induced with a higher dose of MOG, which triggers an early onset of clinical disease. To this end, we repeated experiments described above except that we injected a higher dose of MOG (150 μg) and tested the effect of resveratrol at two doses (100 and 250 mg/kg body weight). In these experiments we noted that the vehicle-treated mice showed symptoms of paralysis much earlier, by day 10 (Fig. 1B). It is noteworthy that when similar groups of MOG-injected mice were treated with resveratrol, there was not only a delay in the onset of the symptoms (> 6 days) but also a dramatic, dose-dependent reduction in the clinical disease (Fig. 1B; p = 0.0094 by Mann-Whitney U test). Resveratrol was more effective in delaying the onset of clinical disease when EAE was induced using a higher dose of MOG. This might be because the anti-inflammatory properties of resveratrol may be more effective at early stages of administration, an aspect that needs further investigation.
Fig. 1.
Resveratrol treatment diminishes clinical symptoms of EAE. A and B, C57BL/6 mice were immunized with 20 μg (A) or 150 μg (B) of MOG35-55 peptide emulsified in complete Freund’s adjuvant and 400 ng of pertussis toxin. These immunized mice received 100 mg/kg (A and B) or 250 mg/kg (B) of resveratrol daily from day 2 until the termination of the experiment. C, images from immunohistopathological examination of spinal cord of EAE-induced mice. Spinal cords were collected from: a, normal mice; b, EAE-induced vehicle-treated mice; c, EAE-induced resveratrol-treated mice. Arrows indicate infiltration of polymorphonuclear cells. D, in situ TUNEL assays for apoptosis in spinal cord of EAE-induced mice. Spinal cords from normal mice (a), EAE-induced vehicle-treated mice (b), EAE-induced resveratrol-treated mice (c) were collected and in situ apoptosis was determined using DeadEnd colometric TUNEL system. E, expression of various cytokines and chemokines in serum of EAE-induced mice on day 25 after immunization that were treated with vehicle or resveratrol. F, expression of cytokine IL-17 in serum of EAE-induced mice on day 9. G, expression of Foxp3 in splenocytes harvested from EAE induced mice (C57BL/6) treated with vehicle or resveratrol on day 25 after immunization. Lane 1, normal spleen; lane 2, MOG + Vehicle; lane 3, MOG + resveratrol (100 mg/kg), and lane 4: MOG + resveratrol (500 mg/kg). β-Actin was used as a positive control. H, presence of anti-MOG antibody in the sera of EAE induced mice (C57BL/6) treated with vehicle or resveratrol on day 25 after immunization. Data represent mean ± S.E.M. of six animals and asterisks (*) represent significant differences between resveratrol-treated groups compared with vehicle controls.
Resveratrol-Mediated Apoptosis Reduced Inflammatory Cells in Spinal Cord
Histological studies revealed that resveratrol treatment significantly inhibited the inflammation induced by MOG (Fig. 1C, compare b and c). Next, we performed in situ TUNEL assays to determine apoptosis in migrating inflammatory cells in spinal cord as described under Materials and Methods. When we counted the number of apoptotic cells at sites of inflammation at 20 different fields using a microscope, we noted six apoptotic cells in untreated mice, 17 in MOG + vehicle-treated mice and 49 in MOG + resveratrol-injected mice (Fig. 1D). These data suggested that presence of less inflammation in spinal cord of resveratrol-treated EAE mice compared with vehicle-treated EAE mice might result from resveratrol-mediated apoptosis in inflammatory cells.
Effect of Resveratrol on Cytokine/Chemokine Profile and Foxp3 Expression in EAE-Induced Mice
Next, we examined the presence of various cytokines in the sera of EAE-induced mice and observed significant reduction in certain cytokines [tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), interleukin (IL)-2, IL-9, IL-12, IL-17, macrophage inflammatory protein-1α (MIP-1α), monocyte chemoattractant protein-1 (MCP-1), regulated on activation normal T-cell expressed and secreted (RANTES)] and a modest increase in other cytokines (IL-6, IL-17, and granulocyte cell-stimulating factor) in the serum of mice treated with resveratrol (Fig. 1E), compared with vehicle-treated mice with EAE, demonstrating that resveratrol treatment decreased the induction of a majority of cytokines screened. Of particular interest was the demonstration that vehicle-treated mice with EAE exhibited very high levels of eotaxin, which was dramatically decreased after resveratrol treatment. Recent studies have shown that IL-17 plays a critical role in EAE. Thus, we were surprised at the low levels of IL-17 seen during EAE in the serum and the fact that resveratrol did not decrease the levels. This may be because the serum was analyzed for cytokines on day 25 of EAE. It is noteworthy that when we measured the levels of IL-17 on day 9 of EAE, we found that IL-17 levels were increased in vehicle-treated EAE mice and that resveratrol treatment caused a significant decrease in IL-17 levels (Fig. 1F). Furthermore, we also examined the effect of resveratrol on induction of Foxp3, an important transcription factor of Treg cells during EAE development. We observed significant up-regulation of Foxp3 gene in splenocytes of mice treated with resveratrol, compared with control mice (Fig. 1G).
Effect of Resveratrol on Production of Anti-MOG Antibody in EAE Mice
We determined whether resveratrol had any effect on the production of antibody against MOG peptides in EAE-developing mice. Upon analysis of anti-MOG antibody production, we observed significantly less production of anti-MOG antibody in resveratrol-treated EAE mice compared with vehicle-treated EAE mice (Fig. 1H). These data demonstrated that resveratrol effectively blocked the production of anti-MOG antibody in EAE mice.
Effect of Resveratrol on Unactivated and Activated Primary T Cells
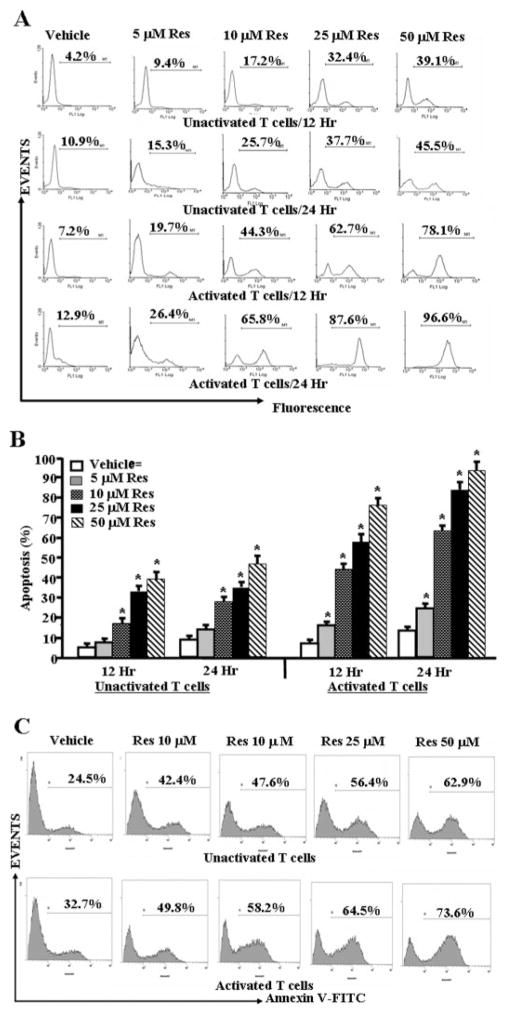
We next performed in-depth analysis of the mode of action of resveratrol using in vitro assays. To this end, purified T cells from C57BL/6 mice were either unactivated or activated with ConA for 24 h and treated with various concentrations of resveratrol (5–100 μM). These concentrations were selected based on pilot studies in which we noted that resveratrol at <5 μM concentrations did not induce significant apoptosis in T cells. First, we determined the survival of primary T cells after resveratrol or vehicle treatments using trypan blue dye and observing the cells under an inverted phase contrast microscope. We observed a statistically significant effect of resveratrol on survival of T cells (data not shown); the total number of viable unactivated or ConA-activated primary T cells was reduced in a dose-dependent manner. However, we observed significantly higher reduction in survival of ConA-activated primary T cells after resveratrol treatment at all doses (data not shown). The data obtained from TUNEL assays demonstrated that primary T cells unactivated or activated with ConA and cultured in the presence of resveratrol underwent apoptosis in a dose-dependent fashion (Fig. 2, A and B). The induction of apoptosis by resveratrol was also confirmed using Annexin V/PI staining (Fig. 2C). We observed 5 to 6% necrotic (PI positive) cells (data not shown). These data demonstrated that resveratrol can induce apoptosis in primary T cells and that activated T cells are more sensitive to resveratrol-induced apoptosis compared with unactivated T cells.
Fig. 2.
Resveratrol triggers apoptosis in primary T cells. Purified primary T cells from C57BL/6 mice were activated or not with ConA for 24 h followed by treatment with resveratrol or vehicle for 12 to 24 h (A–C). T cells were analyzed for apoptosis by TUNEL assays (A and B) and Annexin V/PI (C). A demonstrates representative experiment for apoptosis and B shows mean ± S.E.M. of four independent experiments. C demonstrates representative experiment on apoptosis determined using Annexin V/PI. Cells stained for Annexin but negative for PI alone have been depicted. We observed 5 to 6% dead (PI-positive) cells (data not shown). Asterisks (*) indicate statistically significant difference between resveratrol-treated groups compared with vehicle controls.
Resveratrol Induced Apoptosis in Antigen-Specific Activated T Cells
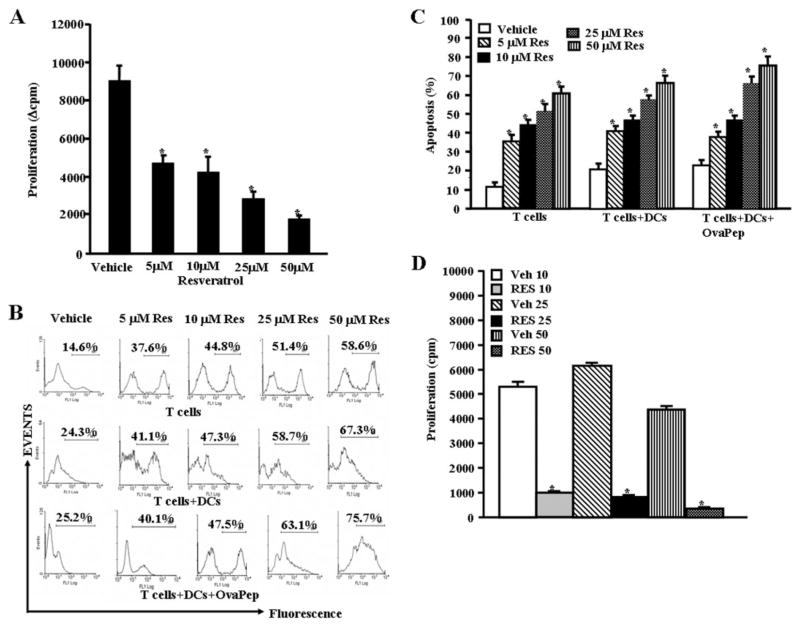
Next, we investigated whether resveratrol would induce apoptosis in antigen-specific T cells activated by DCs. To this end, we used purified T cells from OT.II.2a mice, which recognize specific ovalbumin peptides, cultured them alone or in the presence of syngeneic mature DCs or DCs pulsed with agonist ovalbumin peptides (Ova323–339: ISQAVHAAHAEINEAGR) for 3 days and then treated with various concentrations (5–50 μM) of resveratrol for 24 h. The T cell proliferation was measured by thymidine (3H) incorporation and apoptosis in T cells was determined by gating CD3 positive T cells and detecting TUNEL positive T cells. We noted a dose-dependent decrease in antigen-specific T cell proliferation upon resveratrol treatment (Fig. 3A). Using TUNEL analysis, we noted dose-dependent apoptosis in unactivated T cells and in T cells cultured with DCs. Moreover, significant apoptosis was also noted in T cells cultured with DCs + ovalbumin peptide (Fig. 3, B and C). We noted that the levels of apoptosis, particularly at higher concentrations of resveratrol, were slightly higher in cultures consisting of T + DCs + ova compared with T cells alone. However, these differences in susceptibility of antigen-activated T cells versus unactivated T cells were less dramatic than ConA-activated T cells. This may be because in the ova experiments, the T cells had been cultured in vitro for 2 days before resveratrol treatment. Furthermore, upon examination of the effect of resveratrol on MOG-specific activated T cells, we observed significant reduction in proliferation of MOG-specific activated T cells in the presence of resveratrol, compared with vehicle treatment (Fig. 3D). Together, these data demonstrated that antigen-specific activated T cells became more susceptible to resveratrol-induced apoptosis.
Fig. 3.
Resveratrol causes apoptosis in antigen-specific activated T cells in vitro. Purified primary T cells from OT.II.2a mice were activated with ovalbumin-specific peptide (ISQAVHAAHAEINEAGR) by coculturing T cells and peptide-pulsed mature DCs for 2 days and then treated with various doses of resveratrol (5–50 μM; A–C). Proliferation of T cells was determined by [3H]thymidine incorporation (A) 24 h after resveratrol or vehicle exposure and apoptosis as determined by TUNEL assays (B and C). A represents mean ± S.E.M. of triplicate cultures and data were expressed as change in counts per minute. Asterisks (*) represent significant difference in proliferation of antigen-specific (ova-peptides) activated OT.II.2a T cells treated with resveratrol compared with vehicle controls. B is a representative of three independent TUNEL assays, and C represents mean ± S.E.M. of three independent TUNEL experiments and asterisks (*) represent significant difference in apoptosis between resveratrol-treated groups compared with vehicle controls. D, popliteal draining lymph nodes were harvested from mice immunized with MOG (150 μg/mice) on day 7 and cultured in the presence of MOG (10 μg/ml) and vehicle or resveratrol (10–50 μM) for 3 days. Proliferation of lymph node cells was determined by [3H]thymidine incorporation (D). D represents mean ± S.E.M. of triplicate cultures. Asterisks (*) represent significant difference in proliferation of MOG-specific activated lymph node cells treated with resveratrol compared with vehicle controls.
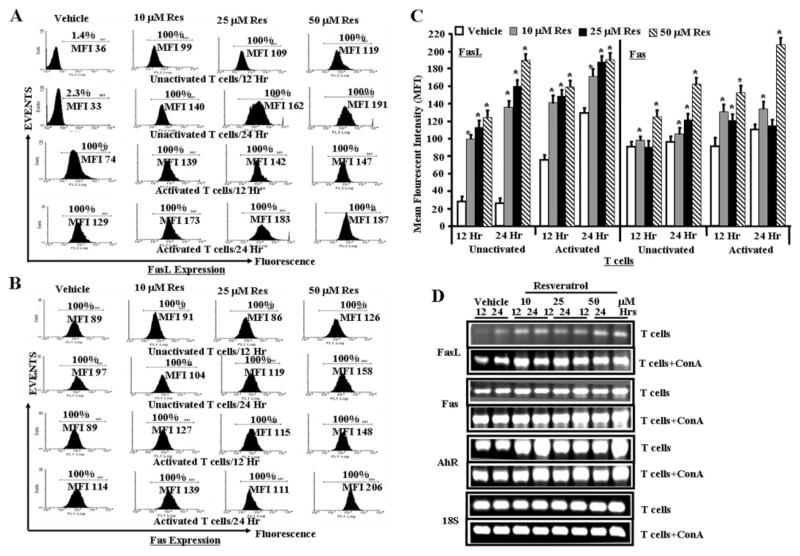
Resveratrol Up-Regulated AhR, Fas, and FasL Expression in T Cells
Next, we determined whether resveratrol regulated the expression of AhR, Fas, and FasL in T cells in vitro by staining the cells with mouse-specific anti-Fas and FasL antibodies, respectively, and performing RT-PCR. Vehicle-exposed activated but not unactivated T cells expressed significant levels of FasL (Figs. 4A and C). Furthermore, resveratrol treatment caused significant and dose-dependent increase in FasL in both unactivated and activated T cells (Fig. 4, A and C). We also observed a significant increase in expression of Fas in both unactivated and activated upon resveratrol treatment (Fig. 4, B and C). Data obtained from RT-PCRs for the expression of Fas, and FasL in T cells after reseveratrol/vehicle treatments corroborated flow cytometry data (Fig. 4D). In addition, resveratrol treatment caused a significant increase in expression of AhR in both unactivated and activated T cells, compared with vehicle (DMSO)-treated T cells as determined by RT-PCR (Fig. 4D). We observed similar 18S (a house keeping gene) expression between resveratrol-and vehicle-treated T cells (Fig. 4D).
Fig. 4.
Role of Fas and FasL in resveratrol-mediated apoptosis in T cells. Expression of Fas and FasL in unactivated or ConA-activated T cells was determined by staining the cells with anti-mouse Fas-PE and anti-mouse FasL-PE antibodies and analyzed by Flow cytometry and RT-PCR. A, expression of FasL in unactivated or ConA-activated T cells 12 and 24 h after resveratrol or vehicle treatment (flow cytometry). B, expression of Fas in unactivated or ConA-activated T cells 12 and 24 h after resveratrol or vehicle treatment. C, mean fluorescent intensity of FasL and Fas expression after resveratrol or vehicle treatment. C represents mean ± S.E.M. of three independent experiments and asterisks (*) represent significant difference between resveratrol-treated groups compared with vehicle controls. D, expression of AhR, Fas, and FasL in unactivated T cells and activated T cells (T cells + ConA) using RT-PCR. 18S, a housekeeping gene, was used as a positive control.
FasL Played a Significant Role in Initiating Death-Receptor Pathway during Resveratrol-Induced T-Cell Apoptosis
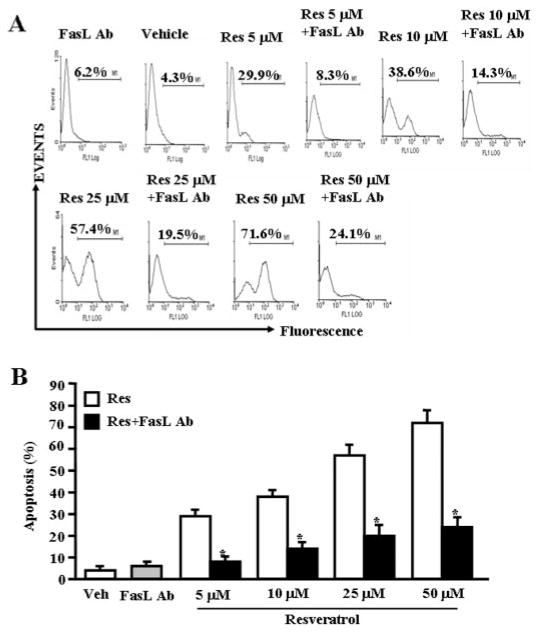
To test the role of FasL in resveratrol-induced apoptosis in T cells, we performed in vitro assays using primary T cells from C57BL/6 mice. Purified T cells were activated with ConA for 24 h followed by culture in the absence or presence of anti-mouse FasL mAb (5 μg/ml). We observed significant reduction in resveratrol-induced T-cell apoptosis when Abs against FasL mAb was added to the culture (Figs. 5A and B). Addition of isotype control Abs failed to exhibit any significant effect on resveratrol-induced apoptosis (data not shown). The data suggested a role for FasL in initiating resveratrol-mediated death-receptor pathway.
Fig. 5.
FasL plays critical role in induction of death-receptor pathway of apoptosis by resveratrol. T cells purified from C57BL/6 (wild-type) mice were activated with ConA and cultured in the presence of vehicle or resveratrol (5–50 μM) and incubated in the absence or presence of mouse-specific anti-FasL Ab. Apoptosis in T cells was determined by TUNEL assays. The data presented in A are representative of three independent experiments. B represents mean of three independent experiments, and asterisks (*) represent significant reduction in resveratrol-induced apoptosis of T cells cultured in the presence of FasL Ab compared with the controls.
Role of AhR In Resveratrol-Induced Apoptosis in T Cells
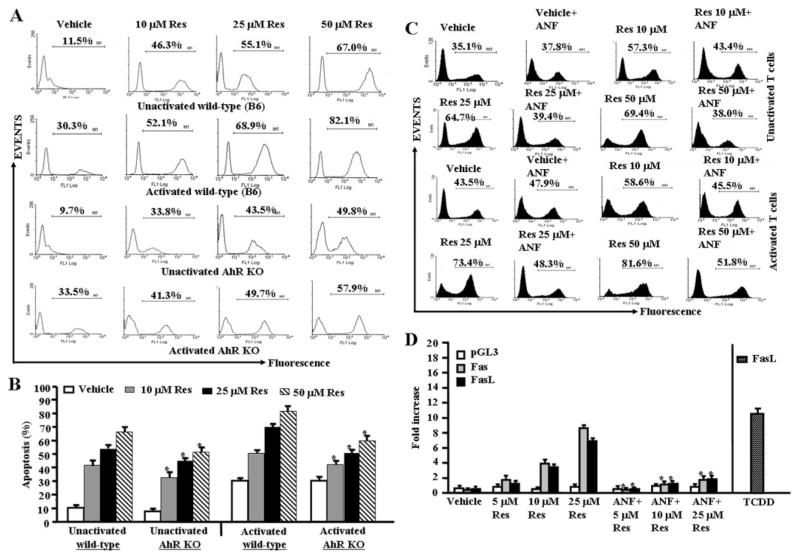
We tested the role of AhR in resveratrol-induced apoptosis in T cells. To this end, we performed a series of in vitro assays using primary T cells from wild-type (C57BL/6) or AhR KO mice. We observed significantly less resveratrol-induced apoptosis in T cells from AhR KO mice compared with AhR wild-type mice at all resveratrol doses tested (Fig. 6, A and B). Likewise, we observed significant reduction in resveratrol-induced apoptosis in T cells from wild-type mice in the presence of ANF, an antagonist for AhR compared with T cells that were cultured in the absence of ANF (Fig. 5C). These data together demonstrated that AhR plays an important role in initiating early signals leading to resveratrol-induced T-cell apoptosis. Furthermore, upon examination of luciferase expression under the control of mouse Fas or FasL promoter after resveratrol treatments, we observed up-regulation of luciferase expression under the control of Fas or FasL promoter (Fig. 6D), but the expression of luciferase was significantly reduced in the presence of ANF (Fig. 6D) thereby suggesting that AhR activation may regulate the expression of Fas and FasL by resveratrol. In these experiments, we used TCDD as a positive control because our previous studies demonstrated that it up-regulates FasL expression (Singh et al., 2007). It should be noted that T cells from AhR KO mice did exhibit decreased apoptosis at all doses of resveratrol (Fig. 6, A and B), and ANF was only partially able to block resveratrol-induced apoptosis. These data suggested that in addition to AhR, other molecules might also play a role in initiating resveratrol-induced apoptosis.
Fig. 6.
Role of AhR in resveratrol-induced apoptosis in T cells. A, unactivated or ConA-activated purified T cells from AhR wild-type or AhR KO mice were treated with vehicle or resveratrol, and apoptosis was determined by performing TUNEL assays as described in Fig. 2. B, mean ± S.E.M. of three independent experiments; asterisks (*) represent significant difference of resveratrol-induced apoptosis in AhR KO T cells compared with wild-type T cells. C, unactivated or ConA-activated T cells were cultured with resveratrol (10–50 μM) or vehicle (DMSO) for 24 h in the absence or presence of ANF in the culture and TUNEL assays were performed. The data presented are representative for 3 independent assays. D, ConA-activated EL4 cells were transfected with pGL-3-Fas or pGL-3-FasL promoter, cultured in the presence of resveratrol (5–25 μM) or vehicle for 24 h in the absence or presence of ANF in the culture, and luciferase assays were performed. Data represent mean of three independent experiments, and asterisks (*) indicate significant down-regulation of luciferase expression in the presence of ANF compared with cultures that did not receive ANF. Expression of luciferase in the presence of FasL promoter and treated with TCDD represents positive control.
Resveratrol Also Used Estrogen Receptor (ER) to Initiate Early Signaling Leading to Apoptosis
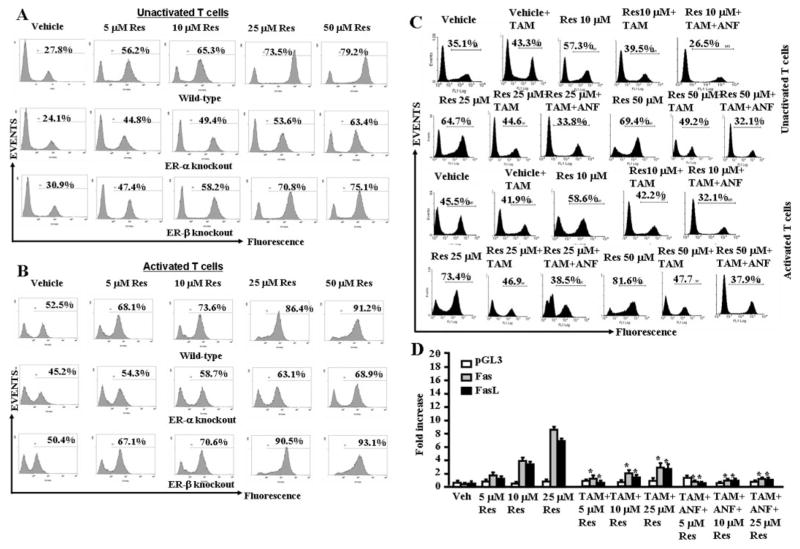
To examine the role of ER in resveratrol-induced early signaling, we purified T cells from wild-type (C57BL/6), ER-α, and ER-β KO mice and cultured them in the absence or presence of TAM and treated the cells with vehicle or various concentrations (5–50 μM) of resveratrol. We observed significantly lower resveratrol-induced apoptosis in T cells from ER-α KO, compared with wild-type mice (Figs. 7, A and B). However, we observed no difference in resveratrol-induced apoptosis in T cells from wild-type (Fig. 7A) and ER-β KO mice (Fig. 7B). These data demonstrated that ER-α plays an important role in resveratrol-induced apoptosis. Furthermore, when T cells from wild-type mice were cultured in the presence of TAM and treated the cells with vehicle or various concentrations of resveratrol (10–50 μM), we observed significant reduction in T-cell apoptosis in the presence of TAM (Fig. 7C), further demonstrating the role of ER in resveratrol-induced apoptosis. Because addition of ANF (Fig. 6C) or TAM (Fig. 7C) separately to cultures had partially blocked resveratrol-induced apoptosis, we further investigated the effect of a combination of ANF + TAM (Fig. 7C). The data demonstrated that combination treatment with TAM + ANF completely reversed the resveratrol-induced apoptosis in T cells and brought the apoptosis to the level seen with the vehicle-treated groups. Upon examination of luciferase expression under the control of mouse Fas or FasL promoter after resveratrol treatments, we observed up-regulation of luciferase expression under the control of Fas or FasL promoter (Fig. 7D), but the expression of luciferase was significantly reduced in the presence of TAM (Fig. 7D) demonstrating the role of ER in activation of Fas and FasL. Furthermore, a combination of TAM + ANF (Fig. 7D) was further able to decrease the luciferase activity, thereby corroborating the involvement of both ER and AhR in the regulation Fas and FasL by resveratrol.
Fig. 7.
Role of ER in resveratrol-induced apoptosis in T cells. A, unactivated T cells from wild-type (C57BL/6), ER-α knockout, ER-β knockout mice were cultured in the presence of various concentration (5–50 μM) of resveratrol (Res) or vehicle (DMSO) for 24 h, and TUNEL assays were performed 24 h after treatment. The data presented are representative of three independent assays. B, ConA-activated T cells from wild-type (C57BL/6), ER-α knockout, and ER-β knockout mice were cultured in the presence of various concentration (5–50 μM) of resveratrol (Res) or vehicle (DMSO) for 24 h, and TUNEL assays were performed 24 h after treatment. The data presented are representative of three independent assays. C, unactivated or ConA-activated T cells were cultured with resveratrol (10–50 μM) or vehicle (DMSO) for 24 h in the absence or presence of TAM alone or in combination with ANF (TAM+ANF) in the culture and TUNEL assays were performed. The data presented are representative for three independent assays. D, ConA-activated EL4 cells were transfected with pGL-3-Fas or pGL-3-FasL promoter, cultured in the presence of resveratrol (5–25 μM) or vehicle for 24 h in the absence or presence of TAM alone or in combination of ANF (TAM+ANF) in the culture, and luciferase assays were performed. D, mean ± S.E.M. of three independent experiments; asterisks (*) represent significant down-regulation of luciferase expression compared with controls.
Resveratrol Triggered Both Death-Receptor (Extrinsic) and Mitochondrial (Intrinsic) Pathways to Cause Apoptosis in T Cells
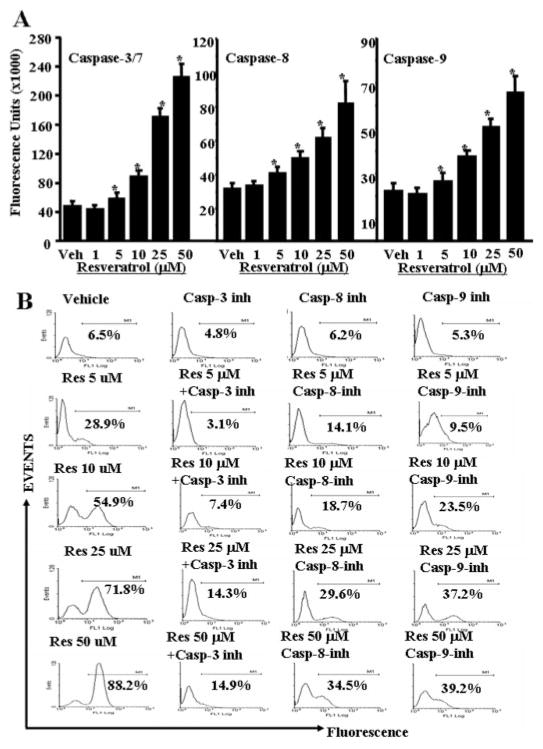
To investigate the role of various apoptotic pathways in resveratrol-induced T-cell apoptosis, we determined the role of various caspases (caspase-3/7, caspase-8, and caspase-9). Standard enzymatic assays using caspase assays reagents from Promega and following the company’s protocols were performed after vehicle or resveratrol exposure of T cells for 24 h. We observed significantly increased caspase enzymatic activities for all three caspases examined in ConA-activated, resveratrol-treated T cells compared with vehicle-treated T cells (Fig. 8A). The role of various caspases was further confirmed by blocking caspase activity using various caspase inhibitors (caspase-3, Z-DEVD; caspase-8, Z-IETD-FMK; and caspase-9; Z-LEHD-FMK). TUNEL assays performed on ConA-activated T cells that were cultured in the presence of various caspase inhibitors and treated with reveratrol (10–50 μM) demonstrated almost complete blocking (~90%) of resveratrol-induced T-cell apoptosis in the presence of caspase-3 inhibitor (Fig. 8B), significant blocking (~65%) in the presence of caspase-8 inhibitor (Fig. 8B), and partial blocking (~50%) in the presence of caspase-9 inhibitor (Fig. 8B). These data demonstrated that resveratrol-induced apoptosis in T cells was caspase-dependent and that caspases-3, -8, and -9 participated in resveratrol-induced T-cell apoptosis.
Fig. 8.
Resveratrol activates and cleaves various caspases to cause apoptosis in primary T cells. Enzymatic activities of caspase-3/7, -8, and -9 were determined in ConA-activated primary T cells 24 h after resveratrol treatment. A, caspase-3/7, -8, and -9 activities, and the data represent mean of three independent experiments. Vertical bars represent S.E. of three experiments. Asterisks (*) represent statistically significant (p < 0.02) increase in enzymatic activities of caspase-3, -8, and -9 in resveratrol-treated groups compared with vehicle-treated T cells. B, ConA-activated T cells were cultured with vehicle or resveratrol in the presence or absence of inhibitors of caspase-3, -8, and -9. The data presented are representative of three independent experiments.
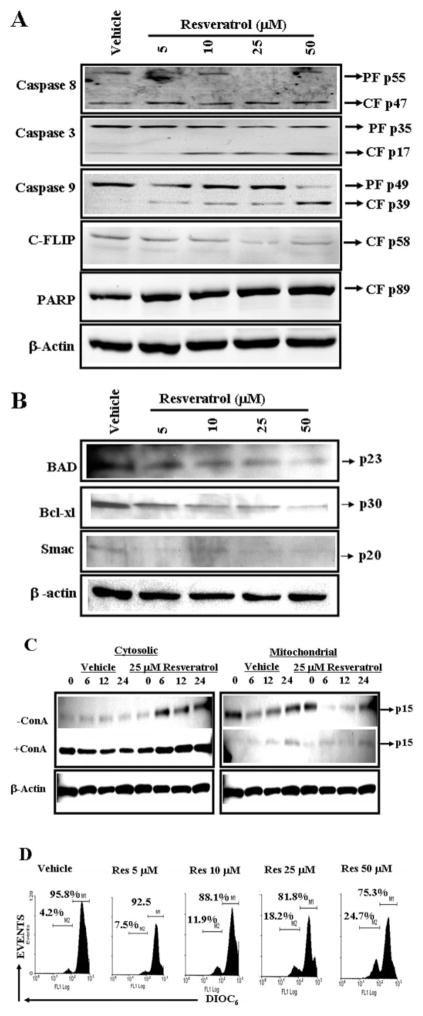
To further corroborate the role of caspases, Western blot analysis for various caspases was performed. The data demonstrated that resveratrol treatment caused cleavage of caspases-8, -3, and -9 (Fig. 9A). Resveratrol treatment also caused a decrease in c-FLIP and cleavage of PARP (Fig. 9A).
Fig. 9.
Resveratrol activates members of death-receptor and mitochondrial pathways. Cell lysates prepared from resveratrol-treated (24 h) unactivated primary T cells (A and B) and unactivated (−ConA) or activated primary T cells (+ConA) (C) were resolved by SDS-polyacrylamide gel electrophoresis and probed with primary Abs against various appropriate molecules. A and B, cell lysates prepared from unactivated and resveratrol-treated T cells were probed with caspase-8, caspase-3, caspase-9, caspase-2, c-FLIP, PARP (A), Bad, Bcl-xl, and Smac (B) antibodies. C, cell lysates prepared from unactivated and ConA-activated and resveratrol-treated T cells were probed with cytochrome c antibodies. β-Actin was used as a control. D, effect of resveratrol on Δψm in primary T cells. Unactivated primary T cells were treated with vehicle (DMSO) or resveratrol (5–50 μM) and stained with DIOC6 to evaluate Δψm. Results are representative of at least three independent experiments.
We also examined the expression of BAD, Bcl-xl, and Smac in resveratrol-treated activated T cells and observed significant reductions after resveratrol exposure. These data demonstrated that resveratrol caused decrease in expression of antiapoptotic genes such as Bcl-xl and Smac and thus allowed apoptosis in T cells. As shown in Fig. 9C, cytochrome c was released from mitochondria to cytoplasm in resveratrol-treated T cells, thus demonstrating the role of the mitochondrial pathway in resveratrol-induced T cell apoptosis.
To further corroborate the role of mitochondrial pathway, we examined mitochondrial membrane potential loss (Δψm) using DiOC6 dye after resveratrol or vehicle treatment. We observed significant reduction in Δψm in resveratrol-treated T cells compared with vehicle-treated T cells (Fig. 9D). However even at a high dose of 50 μM resveratrol, we noted moderate levels of reduction in Δψm (in ~24% cells), suggesting that mitochondrial pathway plays only a partial role in resveratrol-mediated apoptosis.
Discussion
Multiple sclerosis is an autoimmune disease that affects the central nervous systems of patients and is triggered by myelin-specific T cells that cause inflammation, resulting in demyelinated plaques and neurological symptoms (Sospedra and Martin, 2005). In the current study, we investigated the effect of resveratrol on EAE by triggering the clinical disease in C57BL/6 mice through injection of MOG. We noted that resveratrol treatment had the following beneficial effects on EAE compared with vehicle-treated control mice: 1) Significant decrease in severity and delayed onset of clinical disease (Fig. 1, A and B), 2) presence of less number of inflammatory cells in the spinal cord (Fig. 1C), 3) increased numbers of apoptotic inflammatory cells in the spinal cord (Fig. 1D), 4) down-regulation of a majority of cytokines tested (Fig. 1E) and IL-17 (Fig. 1F), 5) up-regulation of Foxp3 (Fig. 1G), and 6) Reduced anti-MOG antibody production (Fig. 1H). The presence of fewer inflammatory cells in spinal cords of resveratrol-treated EAE-induced mice correlated with increased apoptosis, thereby suggesting that resveratrol may act in vivo through induction of apoptosis in activated T cells. Up-regulation of Foxp3 in resveratrol-treated mice demonstrates an increase in the regulatory T cell activity, which has been shown to play important role in immunosuppression (Humrich and Riemekasten, 2006; Jiang et al., 2006a,b; Kim et al., 2006a; Seo et al., 2007).
We noted significant reduction in certain cytokines (TNF, IFN-γ, IL-2, IL-9, IL-12, MIP-1α, MCP-1, and RANTES) and a modest increase in other cytokines (IL-6, IL-17, and granulocyte cell-stimulating factor) in serum of EAE-induced mice treated with resveratrol (Fig. 1E). These data are consistent with reports demonstrating resveratrol-mediated suppression of various cytokines such as IFN-γ (Wirleitner et al., 2005), TNF-α, IL-1β, IL-2, and IL-6 (Marier et al., 2005; Schroecksnadel et al., 2005; Wirleitner et al., 2005). Recent studies have shown that IL-17 plays a critical role in induction of EAE (Lock et al., 2002; Iwakura and Ishigame, 2006). It is noteworthy that EAE-bearing mice had low levels of IL-17 and, in fact, resveratrol treatment caused a modest increase in IL-17. This may be because we measured IL-17 much later in the disease course. When we measured the levels of IL-17 on day 9 of EAE, we found that IL-17 levels were increased in vehicle-treated EAE mice and resveratrol treatment caused a significant decrease in IL-17 levels (Fig. 1F). In the central nervous systems of EAE mice, mRNAs for RANTES, eotaxin, MIP-1α, MIP-1β, MIP-2, inducible protein-10, and MCP-1 were detected at disease onset, increased as disease progressed, and fell as clinical signs improved (Rajan et al., 2000). The decrease in cytokines may result from inhibitory effects on nuclear factor-κB and AP-1 (de la Lastra and Villegas, 2005) or induction of apoptosis in T cells, as seen in the current study. Eotaxin, a recently described chemotactic factor belonging to the CC chemokine family, has been implicated in animal and human eosinophilic inflammatory states (Rothenberg et al., 1996; Fukuyama et al., 2000). In the present study, we observed a robust induction of eotaxin in animals exhibiting EAE and a dramatic reduction of eotaxin upon resveratrol treatment (Fig. 1D). At present, we do not know the precise role played by eotaxin in the development of EAE in mice; additional studies are clearly necessary.
Resveratrol has been previously identified as an AhR mixed agonist/antagonist (de Medina et al., 2005). In addition, we have earlier shown that activation of AhR induces Fas and FasL expression and consequent apoptosis in T cells (Camacho et al., 2005). Resveratrol treatment caused significant and dose-dependent increase in FasL in both unactivated and activated T cells (Fig. 4, A and C). We also observed a significant increase in expression of Fas in both unactivated and activated T cells upon resveratrol treatment (Fig. 4, B and C). Data obtained from RT-PCR for the expression of FasL and Fas in T cells after reseveratrol/vehicle treatment corroborated flow cytometry data (Fig. 4D). In addition, resveratrol treatment caused a significant increase in expression of AhR in both unactivated and activated T cells, compared with vehicle-treated T cells as determined by RT-PCR (Fig. 4D). Next, we tested whether up-regulation of Fas and FasL was directly involved in resveratrol-induced apoptosis. We observed significant reduction in resveratrol-induced apoptosis in T cells in the presence of Abs against FasL (Fig. 5, A and B), whereas isotype control Abs failed to mediate an effect. The data suggested a role for FasL in initiating resveratrol-mediated death-receptor pathway of apoptosis.
In the current study, we noted that AhR played a significant role in resveratrol-induced apoptosis in T cells. However, it should be noted that T cells from AhR KO mice did exhibit decreased apoptosis at all doses of resveratrol (Fig. 6, A and B), and ANF was able to block resveratrol-induced apoptosis only partially, suggesting that other molecules in addition to AhR might also be involved in initiating resveratrol-induced apoptosis.
Resveratrol is a phytoestrogen that exhibits variable degrees of estrogen receptor agonism in different test systems (Gehm et al., 1997). We therefore examined the role of ER in resveratrol-induced apoptosis using T cells from wild-type, ER-α, and ER-β KO mice in the absence or presence of TAM, an ER antagonist. We observed significantly lower resveratrol-induced apoptosis in T cells from ER-α KO compared with wild-type mice (Fig. 7, A–C). However, we observed no significant difference in resveratrol-induced apoptosis in T cells from wild-type and ER-β KO mice (Fig. 7C). These data demonstrated that ER-α plays an important role in resveratrol-induced apoptosis.
Mor et al. (2003) showed that estrogen treatment increased FasL expression in monocytes through the binding of ER to the estrogen responsive elements and AP-1 motifs present on FasL promoter. To test whether resveratrol regulated Fas and FasL promoter activity through AhR and/or ER, we performed luciferase assays in the presence mouse Fas or FasL promoter in an expression vector (pGL-3). We observed that resveratrol induced luciferase expression under the control of Fas or FasL promoter (Figs. 6D and 7D), and the expression of luciferase was significantly reduced in the presence of ANF (Fig. 6D) and TAM (Fig. 7D). In these experiments, we used TCDD, a potent AhR agonist as a positive control shown to up-regulate FasL expression (Singh et al., 2007). Furthermore, a combination of TAM + ANF (Fig. 7D) was further able to decrease the luciferase activity, thereby corroborating the involvement of both AhR and ER in the regulation Fas and FasL by resveratrol.
Upon examination of apoptotic pathways, we noted that both death-receptor (Fas/FasL-mediated) and mitochondrial pathways are involved in resveratrol-induced T-cell apoptosis. However, only a small proportion of apoptotic cells showed loss of mitochondrial membrane potential, and Abs against FasL could almost completely block resveratrol-mediated apoptosis in T cells, both of which suggest that the death receptor pathway may play more critical role and that the mitochondrial pathway may be activated through cross-talk via Bid.
In the current study, we used resveratrol dosed of 10, 100, and 500 mg/kg administered by oral gavage, similar to the doses used in other studies (Gao et al., 2002). Previous in vivo studies to treat cancer suggested that low doses such as 40 mg/kg are not effective, whereas higher doses, such as 80 mg/kg were partially effective (Gao et al., 2002). In another study, resveratrol was shown to inhibit tumor growth in BALB/c mice at 500, 1000 and 1500 mg/kg in a dose-dependent manner when administered for 10 days (Liu et al., 2003). In addition, in a rat model, 100 mg/kg of resveratrol was very effective in delaying tumorigenesis (Bhat et al., 2001). It is feasible to achieve in vivo 25 to 50 μM, a dose that we found to be effective in vitro. Thus, in vivo, doses greater than 100 mg/kg may be necessary to study the efficacy of resveratrol. In addition, in humans, a dose of 1 g of resveratrol has been tested (Zhou et al., 2005).
Use of resveratrol to treat inflammatory and anti-immune diseases may hold significant promise because resveratrol was more effective in inducing apoptosis in activated T cells. Thus, resveratrol may be less toxic to normal immune cells. Data from the current study demonstrate that the anti-inflammatory properties of resveratrol can be attributed, at least in part, to its ability to trigger apoptosis in activated T cells. The current study demonstrates that resveratrol may serve as a novel therapeutic agent against multiple sclerosis.
Acknowledgments
We thank Daniel Sisco and Shweta Hegde for technical help.
This work was funded in part by National Institutes of Health grants P01-AT003961, R01-ES09098, R01-DA016545, R01-AI053703, R01-HL058641, R21-DA014885, and F31-ES11562, by A. D. Williams Trust Funds (to N.P.S.), and by an American Cancer Society Institutional Grant (to N.P.S.).
ABBREVIATIONS
- resveratrol
trans-3,5,4′-trihydroxystilbene
- AP-1
activator protein-1
- AhR
aryl hydrocarbon receptor
- ER
estrogen receptor
- ConA
concanavalin A
- mAb
monoclonal antibody
- TNF-α
tumor necrosis factor-α
- INF-γ
interferon-γ
- IL
interleukin
- RANTES
regulated on activation normal T-cell expressed and secreted
- MIP
macrophage inflammatory protein
- MCP
monocyte chemoattractant protein
- Z-
N-benzyloxycarbonyl-
- FMK
fluoromethyl ketone
- PARP
poly(ADP-ribose) polymerase
- HRP
horseradish peroxidase
- Ab
antibody
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
- ANF
α-naphthoflavone
- DMSO
dimethyl sulfoxide
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- MOG
myelin oligodendrocyte glycoprotein
- PCR
polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- BSA
bovine serum albumin
- DC
dendritic cell
- bp
base pair(s)
- TAM
tamoxifen
- DiOC6
3,3′-dihexyloxacarbocyanine iodide
- PI
propidium iodide
- KO
knockout
- Δψm
mitochondrial membrane potential
References
- Atten MJ, Godoy-Romero E, Attar BM, Milson T, Zopel M, Holian O. Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest New Drugs. 2005;23:111–119. doi: 10.1007/s10637-005-5855-8. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bernhard D, Tinhofer I, Tonko M, Hubl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A. Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ. 2000;7:834–842. doi: 10.1038/sj.cdd.4400719. [DOI] [PubMed] [Google Scholar]
- Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS. Evidence for induction of apoptosis in T cells from murine fetal thymus following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2004;78:96–106. doi: 10.1093/toxsci/kfh048. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol. 2005;175:90–103. doi: 10.4049/jimmunol.175.1.90. [DOI] [PubMed] [Google Scholar]
- Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- Celotti E, Ferrarini R, Zironi R, Conte LS. Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J Chromatogr A. 1996;730:47–52. doi: 10.1016/0021-9673(95)00962-0. [DOI] [PubMed] [Google Scholar]
- Cignarella A, Minici C, Bolego C, Pinna C, Sanvito P, Gaion RM, Puglisi L. Potential pro-inflammatory action of resveratrol in vascular smooth muscle cells from normal and diabetic rats. Nutr Metab Cardiovasc Dis. 2006;16:322–329. doi: 10.1016/j.numecd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- de Medina P, Casper R, Savouret JF, Poirot M. Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J Med Chem. 2005;48:287–291. doi: 10.1021/jm0498194. [DOI] [PubMed] [Google Scholar]
- Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, Gambert P, Cherkaoui-Malki M, Jannin B, Dubrez-Daloz L, Latruffe N, et al. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem. 2003;278:41482–41490. doi: 10.1074/jbc.M304896200. [DOI] [PubMed] [Google Scholar]
- Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, Russell RE, Barnes PJ. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–L783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- Dörrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001;61:4731–4739. [PubMed] [Google Scholar]
- Fukuyama S, Inoue H, Aizawa H, Oike M, Kitaura M, Yoshie O, Hara N. Effect of eotaxin and platelet-activating factor on airway inflammation and hyperresponsiveness in guinea pigs in vivo. Am J Respir Crit Care Med. 2000;161:1844–1849. doi: 10.1164/ajrccm.161.6.9905039. [DOI] [PubMed] [Google Scholar]
- Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–2081. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humrich J, Riemekasten G. Regulatory T cells in rheumatic diseases. Dtsch Med Wochenschr. 2006;131:2288–2291. doi: 10.1055/s-2006-951367. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jiang S, Lechler RI, He XS, Huang JF. CD4+CD25+ regulatory T cell therapy for the induction of donor-specific clinical transplantation tolerance. Expert Opin Biol Ther. 2006a;6:1003–1009. doi: 10.1517/14712598.6.10.1003. [DOI] [PubMed] [Google Scholar]
- Jiang S, Lechler RI, He XS, Huang JF. Regulatory T cells and transplantation tolerance. Hum Immunol. 2006b;67:765–776. doi: 10.1016/j.humimm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006a;119:254–264. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YA, Lim SY, Rhee SH, Park KY, Kim CH, Choi BT, Lee SJ, Park YM, Choi YH. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in beta-amyloid-treated C6 glioma cells. Int J Mol Med. 2006b;17:1069–1075. [PubMed] [Google Scholar]
- Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur J Endocrinol. 1998;138:619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- Liu HS, Pan CE, Yang W, Liu XM. Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. World J Gastroenterol. 2003;9:1474–1476. doi: 10.3748/wjg.v9.i7.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Marier JF, Chen K, Prince P, Scott G, del Castillo JR, Vachon P. Production of ex vivo lipopolysaccharide-induced tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6 is suppressed by trans-resveratrol in a concentration-dependent manner. Can J Vet Res. 2005;69:151–154. [PMC free article] [PubMed] [Google Scholar]
- Mor G, Sapi E, Abrahams VM, Rutherford T, Song J, Hao XY, Muzaffar S, Kohen F. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J Immunol. 2003;170:114–122. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- Notas G, Nifli AP, Kampa M, Vercauteren J, Kouroumalis E, Castanas E. Resveratrol exerts its antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest, and NOS activation. Biochim Biophys Acta. 2006;1760:1657–1666. doi: 10.1016/j.bbagen.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ovesn á Z, Kozics K, Bader Y, Saiko P, Handler N, Erker T, Szekeres T. Antioxidant activity of resveratrol, piceatannol and 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncol Rep. 2006;16:617–624. [PubMed] [Google Scholar]
- Rajan AJ, Asensio VC, Campbell IL, Brosnan CF. Experimental autoimmune encephalomyelitis on the SJL mouse: effect of gamma delta T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J Immunol. 2000;164:2120–2130. doi: 10.4049/jimmunol.164.4.2120. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Ownbey R, Mehlhop PD, Loiselle PM, van de Rijn M, Bonventre JV, Oettgen HC, Leder P, Luster AD. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- Schroecksnadel K, Winkler C, Wirleitner B, Schennach H, Weiss G, Fuchs D. Anti-inflammatory compound resveratrol suppresses homocysteine formation in stimulated human peripheral blood mononuclear cells in vitro. Clin Chem Lab Med. 2005;43:1084–1088. doi: 10.1515/CCLM.2005.189. [DOI] [PubMed] [Google Scholar]
- Seo KS, Lee SU, Park YH, Davis WC, Fox LK, Bohach GA. Long-term staphylococcal enterotoxin C1 exposure induces soluble factor mediated immunosuppression by bovine CD4+ and CD8+ T cells. Infect Immun. 2007;75:260–269. doi: 10.1128/IAI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Nagarkatti M, Nagarkatti PS. Role of dioxin response element and nuclear factor-κB motifs in 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated regulation of Fas and Fas ligand expression. Mol Pharmacol. 2007;71:145–157. doi: 10.1124/mol.106.028365. [DOI] [PubMed] [Google Scholar]
- Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997;11:287–313. doi: 10.1002/(SICI)1098-2825(1997)11:5<287::AID-JCLA6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Su JL, Lin MT, Hong CC, Chang CC, Shiah SG, Wu CW, Chen ST, Chau YP, Kuo ML. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis. 2005;26:1–10. doi: 10.1093/carcin/bgh220. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Schroecksnadel K, Winkler C, Schennach H, Fuchs D. Resveratrol suppresses interferon-gamma-induced biochemical pathways in human peripheral blood mononuclear cells in vitro. Immunol Lett. 2005;100:159–163. doi: 10.1016/j.imlet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol. 2005;11:280–284. doi: 10.3748/wjg.v11.i2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]