Key Points
Proteomic analyses and polysome profiling of developing MKs identified a striking increase in the levels of a novel protein, MARCKS, during proplatelet formation.
MARCKS deletion, inhibition, or phosphorylation inhibits proplatelet formation associated with activation of the actin-binding protein Arp2/3.
Abstract
Platelets are essential for hemostasis, and thrombocytopenia is a major clinical problem. Megakaryocytes (MKs) generate platelets by extending long processes, proplatelets, into sinusoidal blood vessels. However, very little is known about what regulates proplatelet formation. To uncover which proteins were dynamically changing during this process, we compared the proteome and transcriptome of round vs proplatelet-producing MKs by 2D difference gel electrophoresis (DIGE) and polysome profiling, respectively. Our data revealed a significant increase in a poorly-characterized MK protein, myristoylated alanine-rich C-kinase substrate (MARCKS), which was upregulated 3.4- and 5.7-fold in proplatelet-producing MKs in 2D DIGE and polysome profiling analyses, respectively. MARCKS is a protein kinase C (PKC) substrate that binds PIP2. In MKs, it localized to both the plasma and demarcation membranes. MARCKS inhibition by peptide significantly decreased proplatelet formation 53%. To examine the role of MARCKS in the PKC pathway, we treated MKs with polymethacrylate (PMA), which markedly increased MARCKS phosphorylation while significantly inhibiting proplatelet formation 84%, suggesting that MARCKS phosphorylation reduces proplatelet formation. We hypothesized that MARCKS phosphorylation promotes Arp2/3 phosphorylation, which subsequently downregulates proplatelet formation; both MARCKS and Arp2 were dephosphorylated in MKs making proplatelets, and Arp2 inhibition enhanced proplatelet formation. Finally, we used MARCKS knockout (KO) mice to probe the direct role of MARCKS in proplatelet formation; MARCKS KO MKs displayed significantly decreased proplatelet levels. MARCKS expression and signaling in primary MKs is a novel finding. We propose that MARCKS acts as a “molecular switch,” binding to and regulating PIP2 signaling to regulate processes like proplatelet extension (microtubule-driven) vs proplatelet branching (Arp2/3 and actin polymerization-driven).
Introduction
Megakaryocytes (MKs) are highly specialized cells that develop from hematopoietic stem cells that reside mainly in the bone marrow, but are also found in the yolk sac, fetal liver, and spleen in early development (reviewed in Machlus and Italiano1 and Machlus et al2). The primary purpose of MKs is to assemble and release platelets to maintain circulating platelet levels. Sustaining an appropriate platelet count is essential; thrombocytopenia (platelet counts <150 × 109/L) is a major clinical problem encountered across a number of conditions, and approximately 2.2 million apheresis-equivalent platelet units are administered yearly.3 MKs generate platelets by remodeling their cytoplasm first into proplatelets and then into preplatelets, which undergo subsequent fission events to generate circulating discoid platelets.4
Once proplatelet elongation begins, MKs undergo a dramatic transformation as they transition from round cells to proplatelet-producing and begin extending long arms into the vascular space. As such, accumulating evidence suggests that the cytoskeleton, analogous to the bones and muscle of the cell, functions as the principal machinery of proplatelet formation. Specifically, the reorganization and sliding of microtubules are essential for proplatelet formation, powering elongation in a dynein-dependent process.5,6 Likewise, the spectrin cytoskeleton is critical for both proplatelet elaboration and the proplatelet-to-platelet transition.7 Interestingly, however, the role of actin in proplatelet formation remains unclear. We previously demonstrated that F-actin is present throughout MKs and proplatelets and forms the assemblies required to bend and bifurcate proplatelets; inhibition of actin polymerization leads to formation of abnormal, nonbranched proplatelets.5,8 Although multiple studies have revealed that actin reorganization correlates well with proplatelet formation, suggesting a cause-effect relationship, the exact role actin plays in proplatelet formation remains unknown.5,8-10 Once proplatelets are released into the bloodstream, force constraints resulting from cortical microtubule band diameter and thickness determine platelet size and shape.4
Understanding the mechanisms that drive proplatelet formation is essential to control platelet production and therefore treat patients with thrombocytopenia or thrombocytosis. Despite the importance of blood platelets in human health and disease, the cellular and molecular mechanisms that trigger platelet production from MKs are poorly understood. Therefore, we took an unbiased proteomic and polysome profiling approach to identify novel regulators of proplatelet formation. In our current study, we focused on alterations in the MK proteome and transcriptome during their transition into proplatelet formation. We identify and report multiple proteins that may play a role in this process and then focus on a specific protein, myristoylated alanine-rich C-kinase substrate (MARCKS), identified in both proteomic analyses and polysome profiling.
Materials and methods
Reagents
Antibodies were obtained as described: MARCKS antibodies were kindly provided by Dr Blackshear (NIEHS, Research Triangle Park, NC), P-MARCKS and glyceraldehyde-3-phosphate dehydrogenase were from Millipore, GPIb was kindly provided by Dr Gachet (Institut National de la Santé et de la Recherche Médicale, Strasbourg, France), and Arp2 and P-Arp2 were from ECM Biosciences. Puromycin was from Invivogen, polymethacrylate (PMA) and CK-636 were from Sigma-Aldrich, Di-8-ANEPPS was from Life Technologies, C2 ceramide was from Santa Cruz Biotechnology, and okadaic acid was from Abcam. The myristoylated N-terminal sequence (MANS) and random N-terminal sequence (RNS) peptides were synthesized by Genemed Synthesis Inc. The sequences are as follows:
MANS: MA-GAQFSKTAAKGEAAAERPGEAAVAK(-fluorescean)-Amide
RNS: MA-GTAPAAEGAGAEVKRASAEAKQAFK(-fluorescean)-Amide
MK suspension cultures
Mouse fetal liver cells were collected from wild-type CD1 mice (Charles River Laboratories) on day E13.5, and cultured at 37°C and 5% CO2 in the presence of supernatant containing 70 ng/mL recombinant mouse c-Mpl ligand for 4 days.11 On day 4, round MKs were isolated by BSA gradient sedimentation and cultured for one additional day to allow for proplatelet formation, as described.12,13 Proplatelet production was quantified using day 4 MKs at 6-hour and 24-hour time points based on images generated on a Nikon TE-2000-E Microscope (Nikon, Tokyo, Japan) equipped with a 10× (N.A. = 0.3) Plan-Fluoro objective, and images were obtained using a Hamamatsu charged, coupled device camera. Images were analyzed using the Metamorph image analysis software (Molecular Devices) and ImageJ (NIH, http://rsb.info.nih.gov/ij/). Apoptosis was measured using a red caspase 3/7 detection kit (Life Technologies).
Blood collection and platelet counts
Blood collection from mice was performed with Institutional Animal Care and Use Committee approval by submandibular bleed, and complete blood counts were measured.
Incucyte
MKs were transferred to a 24-well plate and imaged using the IncuCyte HD system (Essen BioScience). Frames were captured at 1-hour intervals from 4 separate 950 × 760–μm2 regions per well using a 20× objective lens. Cultures were maintained at 37°C in an XL-3 incubation chamber (Carl Zeiss) throughout and run in quadruplicate. The extent of proplatelet production over time was measured in ImageJ Version 1.45 software using investigator-coded software as described.14 Values from all 4 regions of each well were pooled and averaged across replicates.
2D DIGE analysis
Quantitative proteomic analysis was performed by the Keck Institute at Yale University as previously described.15 Detailed methods on sample preparation, image acquisition, in-gel protein digestion, liquid chromatography coupled with tandem mass spectrometry analysis, and database searching can be found in the supplemental Methods, available on the Blood Web site.
Polysome profiling and analysis
MKs were prepared for ribosomal profiling using a modification of previously published methods.16,17 In brief, 100 μg/mL cycloheximide was added to the cell suspensions just before lysis. The cells were centrifuged, and the pellet was resuspended in a low-salt buffer including RNAsin and placed on ice for 5 minutes. After this incubation period, the cells were placed in a low-salt lysis buffer, mixed, and centrifuged. The cytoplasmic extracts were overlaid onto 15% to 50% linear sucrose gradients (5 mL). Extracts were resolved by centrifugation with a Beckman SW55Ti rotor at 47k rpm for 60 minutes. The gradients were passed through a continuous-flow chamber and monitored at 254 nm with a UV absorbance detector (ISCO UA-6) to obtain ribosomal profiles. Fractions were collected and lysed in Trizol LS (Invitrogen), and total RNA isolated. Preparation of poly-adenylated RNA for sequencing on the Illumina GAIIx, followed by alignment and analysis, was performed as previously described.18
Immunofluorescence microscopy
For fixed samples, MKs were purified and probed as previously described.12,13 All samples were treated with a secondary goat anti-rabbit or mouse antibody conjugated to an Alexa 488nm or 568nm fluor (Invitrogen Corporation, Molecular Probes). As background controls, slides were incubated with the appropriate secondary antibody alone, and all images were adjusted to account for non-specific binding of antibodies.
For live-cell confocal microscopy experiments, samples were examined with a Leica SP5X Laser Scanning Confocal Microscope equipped with a 63× (N.A. = 1.4) Plan-Apo oil immersion objective, and a white light laser. Images were obtained and analyzed using the Leica Applications Suite Advanced Fluorescence, version 2.6.6.
Immunogold labeling and transmission electron microscopy (TEM)
Sample processing and immunogold labeling were performed by the Harvard Medical School Electron Microscopy facility. Detailed methods on sample preparation, immunogold labeling, and TEM can be found in the supplemental Methods.
MARCKS knockout mice
Mice were generated as described previously.19 Heterozygous MARCKS mice were mated to generate homozygous MARCKS KO embryos and the date of the vaginal plug was designated as E0.5. Mouse fetal liver cells were collected on day E13.5 and prepared, isolated, and cultured as described before. Mice and embryos were genotyped by polymerase chain reaction using primer 1 (P1: 5′-CTT GTC TAC AGT GCG GCT AC-3′) and primer 2 (P2: 5′-GAC ACC CAA GAA ATG CCA AGA AC-3). These primers amplified a 472-bp endogenous wild-type allele and a 1612-bp KO allele. All mouse experiments were conducted according to the US Public Health Service policy on the humane care and use of laboratory animals. All animal procedures were approved by the National Institute of Environmental Health Sciences Institutional Animal Care and Use Committee.
Statistical analyses
For measurement of proplatelet formation using the Incucyte, values from 4 regions of each well were pooled and averaged across 4 replicates; 3 biological replicates were performed. For western blots and quantification of proplatelet formation and apoptosis, statistical differences were calculated from 3 to 6 independent studies, as indicated in the figure legends. Statistical significance of differences between groups was determined using a 2-tailed Student t test. For paired analyses of proplatelet formation with peptides, statistical significance was determined using the Holm-Sidak method, with α = 5.000%. For analysis of proplatelet formation over time, each row was analyzed individually, without assuming a consistent SD. Data are presented as mean ± SD. Significance was set at P ≤ .05.
Results
Translation of a subset of proteins is necessary for efficient proplatelet formation
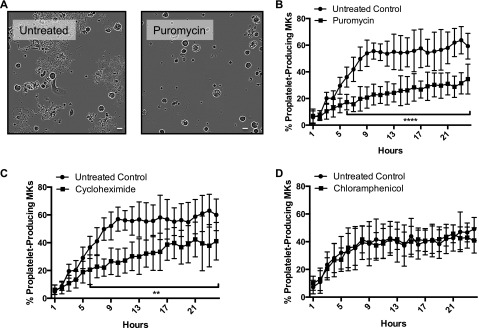
We hypothesized that translation of specific protein(s) by mature MKs was necessary for MKs to undergo the transformative task of proplatelet formation. Protein synthesis was required immediately preceding proplatelet formation. Mature murine MKs (day 4 of culture) were treated with the protein synthesis inhibitor puromycin (250 μg/mL) for 24 hours, and cell morphology was monitored using the Incucyte system as described.14 Puromycin significantly reduced the number of proplatetet-producing MKs (Figure 1A-B). These data suggest that puromycin treatment inhibits production of a protein or proteins necessary for efficient proplatelet formation. To look more specifically at nuclear vs mitochondrial protein synthesis, we used the inhibitors cycloheximide (Figure 1C) and chloramphenicol (Figure 1D), respectively. We found that cycloheximide significantly inhibited proplatelet formation, whereas chloramphenicol did not. Hence, nuclear protein synthesis late in MK maturation is important for proplatelet formation.
Figure 1.
Protein synthesis inhibition significantly reduces proplatelet formation. Mature, primary murine megakaryocytes were cultured for 24 hours in the IncuCyte system. (A) Representative images of 10-hour time point, ×20 original magnification. (B-D) Rate and extent of proplatelet production were measured in ImageJ. Cells ≥330 µm2 were categorized as (1) round-megakaryocytes (circularity ≥0.4) or (2) proplatelet-producing megakaryocytes (circularity <0.4), and objects were normalized to initial (day 4) object counts and expressed as percentage of proplatelet-producing megakaryocytes. MKs were cultured with (B) puromycin (250 μg/mL, final), (C) cycloheximide (50 μg/mL, final), or (D) chloramphenicol (250 μg/mL, final); n = 3 biological replicates. The scale bar represents 50 um. **P < .005; ****P < .0001.
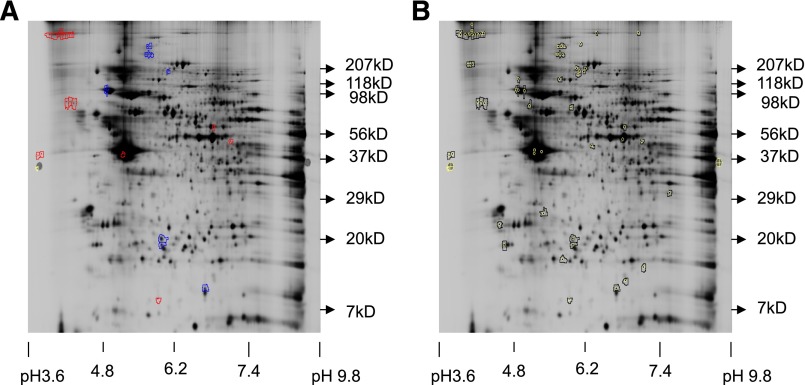
We next aimed to identify the protein or proteins that differed between round, mature (puromycin-treated), and proplatelet-producing (untreated) MKs. To analyze in detail the differences in protein expression between puromycin-treated and untreated murine MKs, quantitative proteomic analysis was performed by the Keck Institute at Yale University. MKs were incubated for 24 hours with puromycin (250 μg/mL) or vehicle control, and protein extracts were subsequently prepared for DIGE analysis, run on a 2D gel, spots picked and identified by MS/MS, and peptides analyzed and identified. Figure 2A shows a gel highlighting proteins with 1.5-fold or greater differences in spot volume ratio. Only a relatively small number of proteins showed this significant difference; 19 spots showed increased protein and 11 showed decreased protein. Spots picked for digestion are shown in Figure 2B. Of these, the seven spots with the highest fold difference were subject to MS/MS analysis; Table 1 shows select identified proteins. We focused on proteins that were increased in control MKs compared with puromycin-treated MKs, because we hypothesized that puromycin was blocking synthesis of key proteins important for proplatelet formation. Specifically increased was the protein MARCKS. a reversibly phosphorylated protein kinase C (PKC) substrate that binds to and sequesters PIP2 at the cell membrane. This membrane-PIP2 interaction is important in actin-mediated processes such as neurite outgrowth,20,21 directed cell movement,22-24 and cytoskeletal remodeling,25 making MARCKS an attractive candidate for its potential role in proplatelet formation.26
Figure 2.
Proteomic analysis reveals that MARCKS is upregulated in proplatelet-producing MKS. Representative labeled spot maps of murine megakaryocytes cultured for 24 hours ± puromycin (250 μg/mL, final). (A) Gel showing 1.5× or greater differences in spot volume ratio. Blue = increase in Cy5/Cy3 (Puromycin/Untreated), red = increase in Cy3/Cy5 (Untreated/Puromycin). (B) All picked spots to date; of these, the 7 spots with the highest fold difference were subject to MS/MS analysis.
Table 1.
Proteins identified as increasing during proplatelet formation by proteomics
| Protein name (protein symbol) | Sequence coverage % | Mascot score | emPAI* |
|---|---|---|---|
| Myristoylated alanine-rich C-kinase substrate (MARCKS) | 31.1 | 131 | 0.82 |
| γ-actin (ACTG) | 20.1 | 130 | 0.55 |
| α-tubulin isotype M-α-2 (TUBA2M) | 4.2 | 74 | 0.15 |
| Apoptosis-inducing factor 1 (AIFM1) | 1.8 | 53 | 0.06 |
| Rabphilin-3A (RPH3A) | 1.3 | 35 | 0.05 |
| Pleckstrin 1 (PLEK) | 8.6 | 29 | 0.31 |
| Coagulation factor V precursor (F5) | 0.5 | 30 | 0.01 |
| Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) | 17.3 | 241 | 0.61 |
| Vacuolar protein-sorting-associated protein 36 (VPS36) | 12.4 | 92 | 0.28 |
| Platelet-activating factor acetylhydrolase IB (PAFAH1B1) | 9.3 | 81 | 0.17 |
| Cathepsin D (CTSD) | 24.9 | 369 | 1.41 |
Exponentially Modified Protein Abundance Index, relative quantitation of protein based on coverage by the peptide matches in database search result.
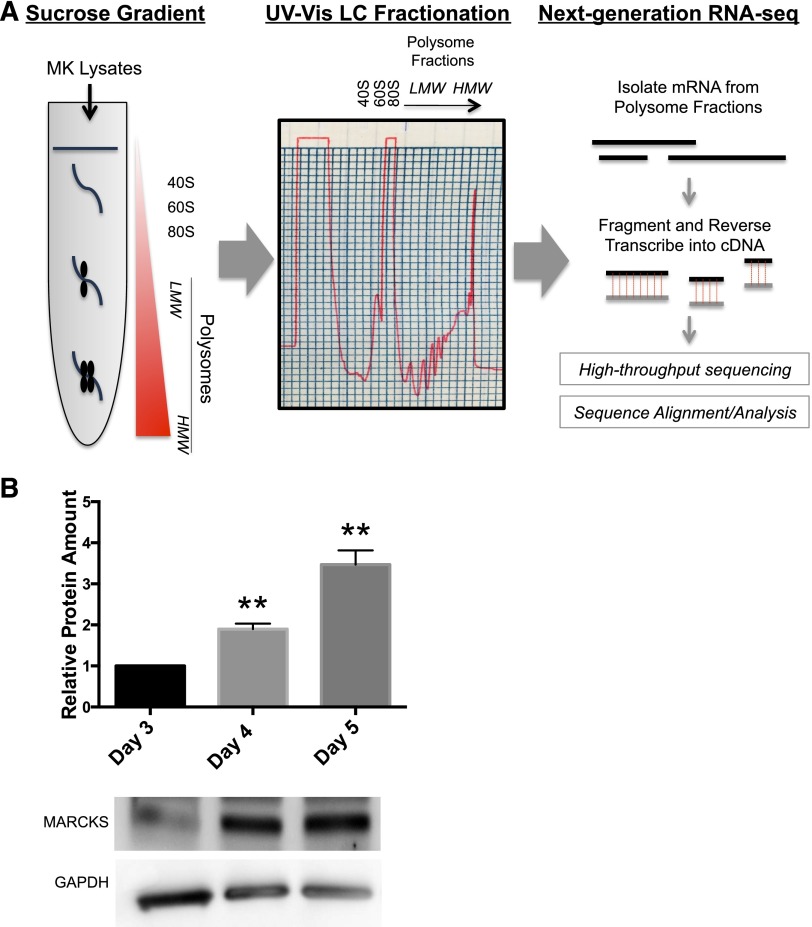
As a complementary technique to proteomic approaches and to more specifically identify transcripts that were translated during proplatelet formation, we performed polysome profiling followed by next-generation RNA sequencing of cultured murine MKs immediately preceding (day 4) and during (day 5) proplatelet formation (Figure 3A), as described.16-18 Transcripts from the polysome fraction of MKs before (day 4) and during (day 5) proplatelet formation were compared to identify transcripts enriched in proplatelet-producing MKs. Table 2 shows proteins enhanced in proplatelet-producing MK transcripts (vs day 4 MKs), which suggests they are upregulated during proplatelet formation. Interestingly, MARCKS was the only protein not previously studied in MKs that was identified in both proteomics and polysome profiling, suggesting it may play an important role in proplatelet formation.
Figure 3.
Polysome profiling and western blot show that MARCKS is enriched in proplatelet-producing MKs. (A) Lysates from either round, mature murine MKs preceding proplatelet formation (day 4) or proplatelet-producing MKs (day 5) were subject to sucrose gradient, fractionation, and RNA-seq, as described in Methods. A representative ribosomal profile used in polysome profiling is shown. (B) Murine fetal liver MKs were cultured as described in Methods and lysed at indicated times. Western blots were quantified relative to the loading control, and then normalized to amount of protein on day 3 (n = 3; **P < .005, compared with day 3).
Table 2.
Polysome-enriched transcripts identified as increasing in megakaryocytes during proplatelet formation by polysome profiling
| Proteins that correspond to isolated and sequenced mRNA transcripts (protein symbol) | Round MK (polysome) | Proplt MK (polysome) | Ratio (proplt/round) |
|---|---|---|---|
| Myristoylated alanine-rich C-kinase substrate (MARCKS) | 4.33 | 24.58 | 5.68 |
| Tubulin, β 2B class IIb (TUBB2B) | 33.60 | 139.47 | 4.15 |
| BCL2-like 11 (BCL2l11) | 17.54 | 84.12 | 4.80 |
| BCL2 binding component 3 (BBC3) | 105.51 | 504.99 | 4.97 |
| Kruppel-like factor 10 (KLF10) | 4.99 | 24.33 | 4.87 |
| Interleukin 15 (IL15) | 13.12 | 72.22 | 5.50 |
| Vascular endothelial growth factor C (VEGFC) | 5.98 | 35.51 | 5.93 |
| Chemokine (C-C motif) ligand 5/RANTES (CCL5) | 4.40 | 88.57 | 20.12 |
| Active BCR-related (ABR) | 3.69 | 19.46 | 5.27 |
| Histone cluster 1, H4h (HIST1H4H) | 16.21 | 155.65 | 9.60 |
| Growth arrest and DNA-damage-inducible (GADD45B) | 13.20 | 197.18 | 14.93 |
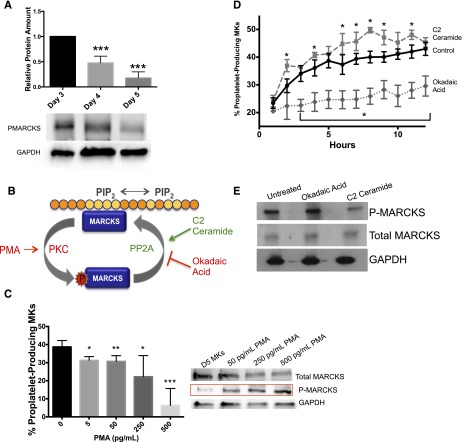
MARCKS is upregulated during MK maturation and proplatelet formation
MARCKS has not previously been studied in primary MKs. To confirm its existence and the differential expression identified by proteomic and polysome profiling data, western blots were performed on MKs during maturation (day 3), before proplatelet formation (day 4), and during proplatelet formation (day 5). Consistent with proteomic and polysome profiling results, western blots confirmed that MARCKS increases 3.4-fold during MK maturation and proplatelet formation (Figure 3B).
MANS peptide binds to MARCKS, reveals demarcation membrane system (DMS) localization, and inhibits proplatelet formation
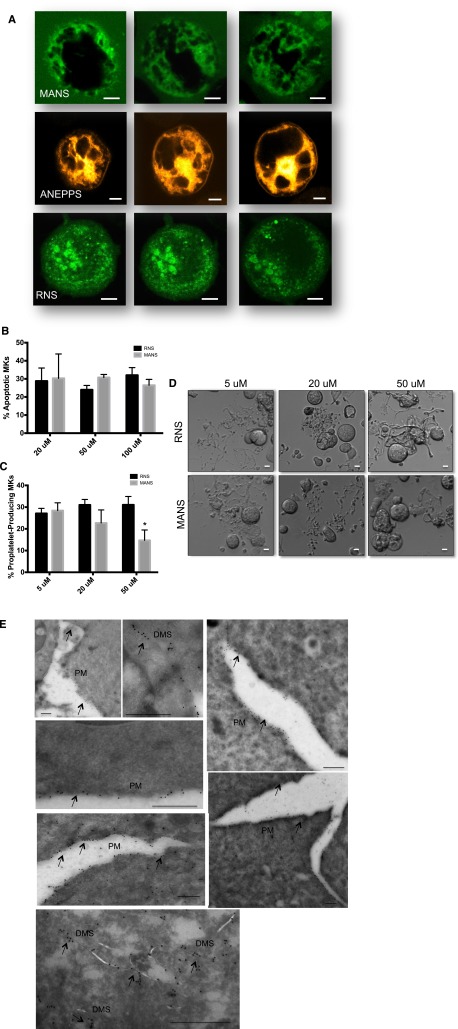
To further characterize MARCKS function in MKs, we synthesized a membrane-soluble green fluorescent protein–linked peptide (MANS) and scrambled control peptide (RNS) commonly used to disrupt MARCKS function.27-30 Because of its membrane permeability and fluorescence, we used MANS to visualize MARCKS localization in living MKs using confocal microscopy. Di-8-Anepps was used to highlight the DMS staining pattern in MKs, as described.31 MANS fluorescence revealed that MARCKS seems to be localized to the DMS in mature MKs. MANS staining pattern was similar to that of Di-8-Anepps, both revealing an intertwined tubular membrane network. In contrast, the control RNS peptide showed only nonspecific, punctate staining. Representative images are presented in Figure 4A and confocal stacks are shown in supplemental Figure 1. In addition, fluorescent staining of both round and proplatelet-producing fixed MKs with both MARCKS and GPIb antibodies showed a strikingly similar pattern (supplemental Figure 2), consistent with localization to the DMS. We also performed immunogold labeling using a MARCKS-specific antibody, followed by TEM (Figure 4E). TEM analysis revealed MARCKS localization restricted to the plasma membrane and throughout the membrane invaginations of the DMS (Figure 4E). This confirmed the immunofluorescence data, showing MARCKS localization to the DMS. These data are consistent with MARCKS binding to PIP2, which is located throughout the plasma membrane and DMS in MKs.
Figure 4.
MANS peptide binds to MARCKS and inhibits proplatelet formation. (A) Live MKs at day 4 were treated with either MANS peptide specific for MARCKS (100 μg/mL) RNS control peptide (100 μg/mL) or Di-8-Anepps to highlight the demarcation membrane. Confocal microscopy was performed with a Leica SP5X Laser Scanning Confocal Microscope equipped with a 63Χ (N.A. = 1.4) Plan-Apo oil immersion objective, and a white light laser. Images were obtained and analyzed using the Leica Applications Suite Advanced Fluorescence, version 2.6.6. MANS and ANEPPS are localized to DMS, whereas the control peptide, RNS, is nonspecific and punctated throughout the MK. (B) Apoptosis and (C) Proplatelet formation after treatment with MANS and RNS peptides (n = 5; *P < .01). (D) Representative images; scale bar represents 10 μm. (E) Immunogold transmission electron microscopy showing MARCKS labeling along the plasma membrane (PM) and demarcation membrane invaginations (DMS). Scale bars represent 500 nm.
Because MARCKS is upregulated during proplatelet formation, we tested whether its function is important for this process. MANS was used to inhibit MARCKS in live cells, as previously reported.27-30 Treatment with MANS or the control peptide, RNS, did not affect cell viability (Figure 4B). MANS dose-dependently, significantly diminished proplatelet formation up to 53% (Figure 4C-D). These data provide evidence that MARCKS activity is necessary for efficient proplatelet formation.
MARCKS phosphorylation inhibits proplatelet formation
MARCKS binds PIP2 in the plasma membrane through its effector domain.32-35 MARCKS can also be phosphorylated (P-MARCKS) by PKC, disrupting membrane binding and causing translocation to the cytoplasm.27 Therefore, we probed the phosphorylation status of MARCKS over time. Figure 5A reveals that despite an increase in total MARCKS (Figure 3B), the amount of P-MARCKS decreased 1.8-fold as MKs maturated and proplatelets were formed (Figure 5A), suggesting that MARCKS phosphorylation may be detrimental to proplatelet formation. Because PMA is a potent PKC activator that leads to specific and preferential MARCKS phosphorylation,37,38 we used it to induce MARCKS phosphorylation (schematic in Figure 5B). Treatment of MKs with PMA significantly, dose-dependently decreased the number of proplatelet-producing MKs but increased the amount of P-MARCKS (Figure 5C). Our data indicate that P-MARCKS decreased over the course of proplatelet formation, suggesting that P-MARCKS is dephosphorylated. PP2A has been shown to be the main phosphatase responsible for MARCKS dephosphorylation.20,38,39 Therefore, we treated MKs with the specific PP2A activator C2 ceramide and PP2A inhibitor okadaic acid (Figure 5B). Consistent with our model where MARCKS dephosphorylation is necessary for proplatelet formation, C2 ceramide significantly enhanced proplatelet formation, whereas okadaic acid significantly inhibited proplatelet formation (Figure 5D). Importantly, C2 ceramide and okadaic acid treatment resulted in decreased and increased MARKCS phosphorylation, respectively (Figure 5E). At the dosages used, PMA, okadaic acid, and C2 ceramide did not affect MK viability (data not shown).
Figure 5.
MARCKS is differentially expressed and phosphorylated during maturation and proplatelet formation in primary murine MKs. (A) MKs were cultured as described and lysed at indicated times. Western blots were quantified relative to the loading control, and then normalized to amount of protein on day 3 (n = 4; ***P < .0001, compared with day 3). (B) Schematic of MARCKS phosphorylation/internalization and dephosphorylation. (C) MKs at day 4 were treated with PMA (PKC activator) at indicated dosages. Percent proplatelet formation was quantified manually and western blots were done to show differential MARCKS phosphorylation status with PMA treatments (n = 4). (D) MKs on day 4 were treated with C2 ceramide (0.1 μM, PP2A activator) or okadaic acid (0.1 μM, PP2A inhibitor). Percent proplatelet formation over time was quantified using the Incucyte imaging system and (E) western blots were done to show differential MARCKS phosphorylation status with treatments (n = 3; *P < .05, **P < .005, ***P < .0001, compared with control).
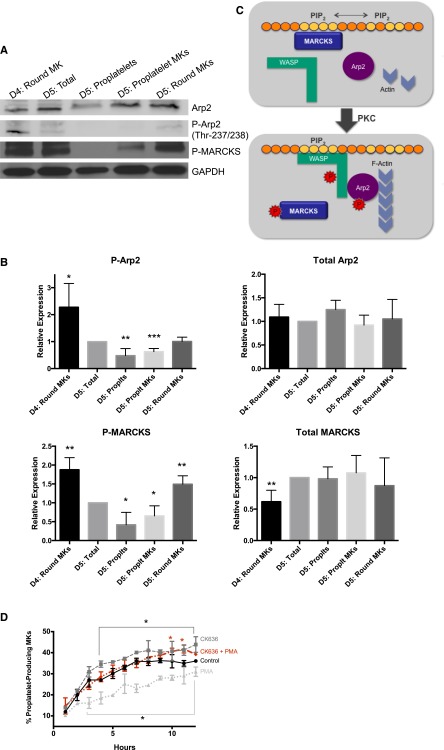
MARCKS phosphorylation results in its displacement from the membrane, thereby uncovering PIP2 for signaling.26,37,40,41 PIP2 binding by WASP protein family members can result in Arp2/3 phosphorylation and actin polymerization. A previous study in endothelial cells reported that after MARCKS phosphorylation and translocation, PIP2 was released and bound to N-WASP to promote its phosphorylation and interaction with Arp2/3, thereby leading to Arp2/3-dependent actin polymerization and cytoskeletal remodeling.23 Importantly, we and others have found that actin polymerization is not necessary for proplatelet elongation,5,8 and, in fact, inhibiting actin polymerization enhances proplatelet formation.42,43 Therefore, we hypothesized that the interaction between MARCKS and PIP2 may prevent Arp2/3 phosphorylation and actin polymerization. To interrogate this mechanism, we analyzed MARCKS and Arp2 phosphorylation over the course of proplatelet formation (Figure 6A). We found that both MARCKS and Arp2 phophorylation were decreased during proplatelet formation (Figure 6B). In fact, although total MARCKS and Arp remained relatively unchanged, P-MARCKS and P-Arp2 were almost completely absent from proplatelet-producing MKs and released proplatelets. The majority of residual P-MARCKS and P-Arp2 in MK fractions on day 5 could be attributed to round MKs that had failed to undergo proplatelet formation (Figure 6A-B). These data suggest that MARCKS is dephosphorylated during proplatelet formation, preventing Arp2 phosphorylation.
Figure 6.
Phosphorylated MARCKS and Arp2 are downregulated in proplatelet-producing MKs. Murine fetal liver MKs were cultured as described in Methods and lysed at indicated times. Cell fractions were separated by BSA gradient as described in Methods. (A) Representative western blot. (B) Western blots were quantified relative to the loading control, and then normalized to the total amount of protein at day 5 (n = 4; *P < .05, **P < .01, ***P < .005, compared with D5:total). (C) Proposed model of the role of MARCKS vs P-MARCKS in proplatelet formation. (D) MKs on day 4 were treated with PMA (500 pg/mL, PKC activator), CK636 (1 μM, Arp2 inhibitor), or both simultaneously, and percent proplatelet formation over time was quantified using the Incucyte imaging system (n = 3; *P < .05, compared with control).
Figure 6C shows a schematic of the role of MARCKS in proplatelet formation. We propose that MARCKS binding PIP2 shields it from binding other proteins. MARCKS phosphorylation releases it from PIP2, and PIP2 is immediately available to signal. In this way, the MARCKS/PIP2 interaction may work like a molecular switch to tightly regulate processes like proplatelet extension (microtubule driven) vs proplatelet branching (actin polymerization driven).
As per our proposed model, MARCKS phosphorylation is detrimental to proplatelet formation because it results in Arp2/3 activation. Therefore, we treated MKs with the specific Arp2 inhibitor CK636 and found that it significantly enhanced proplatelet formation (Figure 6D), consistent with our model. We then treated cells with the PKC activator PMA, which induced MARCKS phosphorylation and decreased proplatelet formation (Figures 5C and 6D). If MARCKS phosphorylation results in decreased proplatelet formation through Arp2/3 activation, we would expect treatment of CK636 to decrease the inhibitory effect of PMA. In fact, treatment of MKs with both CK636 and PMA resulted in a recovery in proplatelet formation (Figure 6D), suggesting the mechanism by which MARCKS drives proplatelet formation is through inhibition of the Arp2/3 pathway.
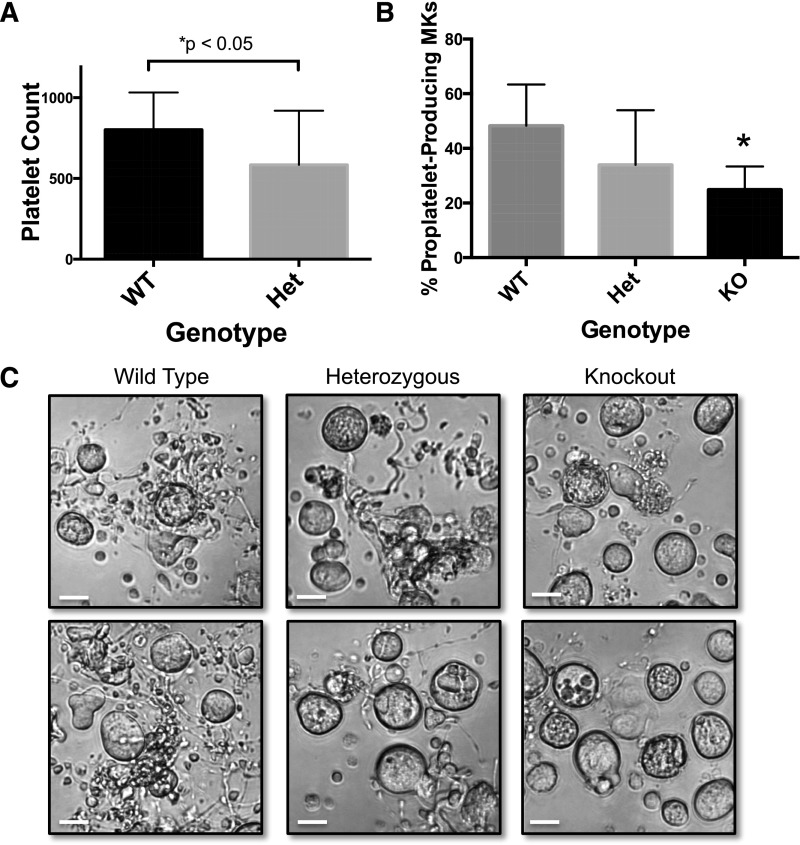
As per our proposed model, the role of MARCKS in proplatelet formation is to regulate an inhibitory signaling pathway. To verify that MARCKS plays a direct role in platelet formation in vivo, we studied MARCKS heterozygous mice, generously provided by Dr Perry Blackshear. We measured platelet counts in male wild-type and heterozygous mice and found a significant decrease in MARCKS heterozygous mice (Figure 7A). We next studied MKs from MARCKS knockout mice. Homozygous mice with targeted deletions of the Marcks gene die before or within a few hours of birth because of abnormalities in central nervous system morphology.19 We took advantage of our model system and bred heterozygotes, collected and genotyped embryos, and isolated fetal liver cells from each individual embryo to generate MARCKS KO MKs (Figure 7A). There were no significant differences in the size or number of MKs derived from the KO or heterozygous embryos compared with wild-type (Figure 7C and supplemental Figure 3). However, MKs from KO embryos had impaired proplatelet formation compared with their wild-type controls (Figure 7B-C and supplemental Figure 3). Specifically, we quantified the percentage of total MKs that were producing proplatelets on day 5 of culture. Although only a small percentage of KO MKs produced proplatelets, their proplatelet morphology was grossly altered. Accurate quantification was not possible because of the small number of MKs obtained from each liver, and the even smaller number of MKs that produced proplatelets in heterozygous and KO MKs. However, we observed that most proplatelets from KO MKs were short and less elaborate/branched than the proplatelets from wild-type MKs (supplemental Figure 3). This is consistent with our hypothesis that MARCKS regulates proplatelet branching. These data reveal that expression of MARCKS is important for proplatelet formation.
Figure 7.
MARCKS heterozygous mice and KO MKs have impaired platelet formation. (A) Platelet counts were measured from adult, male MARCKS heterozygous (Het) and wild-type (WT) mice, as described (n = 15 mice per group; *P < .05, compared with WT). (B) Primary murine MKs were cultured as previously described after being isolated from MARCKS KO mice. Proplatelet formation was quantified 24 hours after MK gradient isolation (n = 5-6 fetal livers per condition; *P < .05, compared with WT). (C) Representative images of 24-hour time-point (original magnification ×20); scale bar represents 50 μm.
Discussion
Because the proteins that regulate proplatelet formation are poorly defined, we performed proteomic analyses and polysome profiling to identify novel proteins that are important for proplatelet production. These techniques yielded many proteins that fell into a variety of categories, including apoptosis, the cell cycle, protein degradation, and cytoskeletal maintenance (Tables 1 and 2). Many of the identified proteins are both novel and intriguing, and certainly warrant future study. However, we focused on a protein related to cytoskeletal rearrangement, hypothesizing that the dramatic morphologic changes that occur when MKs make proplatelets require cytoskeletal reorganization.
MARCKS was the sole protein identified in both proteomics and polysome profiling not studied in MKs. MARCKS is a PKC substrate that binds to and sequesters PIP2 at the membrane in its dephosphorylated form. Upon phosphorylation, P-MARCKS relocates from the plasma membrane to an internal membrane structure.27 Conversely, P-MARCKS dephosphorylation results in its relocation to the plasma membrane and restoration of PIP2 binding. Our data suggest that MARCKS acts as a “molecular switch,” binding to and regulating PIP2 signaling to tightly regulate processes like proplatelet extension (microtubule-driven) vs proplatelet branching (actin polymerization–driven). In addition, the data from proteomics, polysome profiling, and western blotting indicated that MARCKS expression is upregulated during proplatelet formation. Therefore, it suggests that a critical mass of MARCKS is necessary for this process to initiate. One could speculate that once this critical mass is reached, MARCKS binds enough PIP2 to downregulate WASP/Arp2 signaling and therefore allows proplatelet formation to begin.
In our study, proplatelet formation is inhibited by PKC-induced MARCKS phosphorylation and enhanced by C2 ceramide-induced P-MARCKS dephosphorylation. These data are consistent with the work of Williams et al showing that PKCα activation inhibits proplatelet formation.45 In this study, PKCα-null MKs showed enhanced proplatelet formation, as well as a shift to greater ploidy. In vivo, platelet production was enhanced in response to experimentally induced immune thrombocytopenia. In sum, these data indicate that PKCα and its downstream targets are negative regulators of proplatelet formation.
PKC activation may inhibit proplatelet formation by phosphorylating MARCKS, thus exposing PIP2 and activating the Arp2/3 complex. The Arp2/3 complex is active when phosphorylated, and controls nucleation of actin polymerization and branching of actin filaments. Phosphorylation of Arp2/3 occurs through WASP protein family members such as WASP, N-WASP, or WAVE. This suggests that WASP signaling through Arp2/3 may not be necessary for proplatelet formation. In fact, treatment of MKs with the Arp2 inhibitor CK636 was not detrimental to proplatelet formation, and even enhanced it at later time points. It is interesting to note that patients with Wiskott-Aldrich syndrome, a disease caused by a mutation in the WAS gene, have microthrombocytopenia. However, the mechanism of this thrombocytopenia is still not fully understood. In addition, multiple studies in WASP KO mice suggest that the cause of the thrombocytopenia is not failure of proplatelet formation, but rather that MKs prematurely release proplatelets into the bone marrow space.45-48 In fact, both murine and human WASP KO MKs in culture display proplatelet formation.45-48 Therefore, these data are consistent with our model, suggesting WASP is not essential for (pro)platelet formation.
Our model suggests that MARCKS-mediated Arp2 phosphorylation inhibits proplatelet formation. In MKs, the Arp2/3 complex has previously been shown to play a role in both DMS formation and podosome formation.49,50 However, to our knowledge, the direct role of Arp2/3 phosphorylation in proplatelet formation has not been studied. Interestingly, podosome formation by MKs has recently been proposed as a mechanism by which MKs degrade matrix and, in turn, cross basement membranes and release proplatelets into the bloodstream.50 Schachtner et al found that MKs generate classical podosomes with F-actin cores and vinculin rings. Importantly, this process was associated with actin polymerization induced by Arp2/3 phosphorylation. In this model, like ours, Arp2/3 phosphorylation and actin polymerization do not promote proplatelet formation. Rather, these processes promote matrix degradation, a response that needs to occur distinctly from and before proplatelet initiation and release. When dysregulated, proplatelets may be released into the bone marrow space, as seen in Wiskott-Aldrich syndrome. Therefore, it is tempting to speculate that MARCKS could also play a role in regulating the Arp2/3 phosphorylation “switch” that controls the distinct processes of podosome formation vs proplatelet formation. In fact, past localization studies using light and electron microscopy show that MARCKS colocalizes with vinculin, talin, and PKC in focal contacts where the actin cytoskeleton adjoins the plasma membrane.51 This hypothesis warrants further study.
Intriguingly, MARCKS has also been shown to play an important role in neurons, where it is essential for neuronal growth and elongation, a process that looks strikingly similar to proplatelet formation.20,21,41,52 In fact, overexpression and dephosphorylation of MARCKS have been shown to accelerate neurite outgrowth.20,21,41,52 In one study, overexpression of unphosphorylated MARCKS in cultured neurons caused elongation of dendritic spines, whereas overexpression of phosphorylated MARCKS caused retraction of the spines. These morphologic alterations are attributable to reorganization of the F-actin cytoskeleton structure.52 These studies in neurons closely parallel our data, showing that dephosphorylated MARCKS is important for both neuronal and proplatelet outgrowth.
MARCKS inhibition by either the MANS peptide or by genetic deletion did not fully inhibit proplatelet formation. This may be the result of a variety of factors. First, in addition to MARCKS, there is another member of the MARCKS family of proteins, MARCKS-Like 1 (ML1 or Mac-MARCKS). ML1 shares the same 3 highly conserved domains, a membrane-binding domain, an MH2 domain, and an effector domain, and functions very similarly to MARCKS in vitro.53,54 Importantly, ML1 can also bind to and aggregate PIP2.34,53-55 Therefore, it is reasonable to hypothesize that ML1 may be playing a compensatory role after MARCKS knockdown or inhibition. Deletion of both MARCKS and ML1 may result in more complete inhibition of proplatelet formation. In addition, MARCKS is necessary for the PIP2 sequestration that facilitates PIP2 availability and signaling.33,34,41,56-58 Therefore, it is possible that the absence of MARCKS could lead to diminished PIP2 signaling. If this is true, deleting MARCKS could result in a paradoxical increase in proplatelet formation in some circumstances because of a lack of WASP signaling (similar to the WASP KO mouse46). The complexity of this system makes it likely that both of these possibilities contribute to residual proplatelet formation. However, the deletion of MARCKS undoubtedly has a detrimental effect on proplatelet formation.
We used the unbiased approaches of proteomic analyses and polysome profiling to identify a novel cytoskeletal regulatory pathway that controls proplatelet formation. This is especially important because of the lack of information in the MK and platelet field about mechanisms that control this complex process. Importantly, results from the studies proposed could have a significant impact on the treatment of thrombocytopenia: the proteins and corresponding pathways may become therapeutic targets to directly stimulate an “autotransfusion” of platelets from existing bone marrow MKs in thrombocytopenic patients. This would result in an immediate increase in platelet count and represent a significant advantage over current standard-of-care drugs, thrombopoietin mimetics, which take 5 to 12 days to reach their maximal efficacy.
Acknowledgments
Confocal images were collected at the Dana Farber Confocal and Light Microscopy Core with the help of Dr Lisa Cameron. We would like to thank Dr Elisabeth Battinelli, Dr Jonathan Thon, and Dr Douglas Medvetz for their insightful conversations and feedback.
This work was supported in part by grants from the National Institutes of Health National Heart, Lung, and Blood Institute R01Hl68130 (J.E.I.), 5F32HL118865-02 (K.R.M.), PO1 HL48743 (T.M.), P01 HL120846 (W.B.), and R01 HL126547 and U54 HL112311 (A.S.W.); and an American Heart Association Postdoctoral Fellowship (H.K.). J.E.I. is an American Society of Hematology Junior Faculty Scholar.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.R.M. designed and performed experiments, and wrote the manuscript; S.K.W., D.J.S., T.S.S., D.S.P., and R.A.C. designed and performed experiments; A.S.W., P.J.B., W.B., T.M., H.K., and J.H.H. contributed scientific expertise and reagents, helped design experiments, and edited the manuscript; and J.E.I. oversaw all studies, designed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: J.E.I. has financial interest in and is a founder of Platelet BioGenesis, a company that aims to produce donor-independent human platelets from human-induced pluripotent stem cells at scale. J.E.I. is an inventor on this patent. The interests of J.E.I. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. The remaining authors declare no competing financial interests.
Correspondence: Joseph E. Italiano, Brigham and Women’s Hospital, 1 Blackfan Circle, Karp 5, Boston, MA 02115; e-mail: jitaliano@partners.org.
References
- 1.Machlus KR, Italiano JE., Jr The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201(6):785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machlus KR, Thon JN, Italiano JE., Jr Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol. 2014;165(2):227–236. doi: 10.1111/bjh.12758. [DOI] [PubMed] [Google Scholar]
- 3.DHHS. The 2011 national blood collection and utilization survey report. Department of Health and Human Services; 2013. [Google Scholar]
- 4.Thon JN, Macleod H, Begonja AJ, et al. Microtubule and cortical forces determine platelet size during vascular platelet production. Nat Commun. 2012;3:852. doi: 10.1038/ncomms1838. [DOI] [PubMed] [Google Scholar]
- 5.Patel SR, Richardson JL, Schulze H, et al. Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood. 2005;106(13):4076–4085. doi: 10.1182/blood-2005-06-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender M, Thon JN, Ehrlicher AJ, et al. Microtubule sliding drives proplatelet elongation and is dependent on cytoplasmic dynein. Blood. 2015;125(5):860–868. doi: 10.1182/blood-2014-09-600858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel-Hett S, Wang H, Begonja AJ, et al. The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood. 2011;118(6):1641–1652. doi: 10.1182/blood-2011-01-330688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147(6):1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handagama PJ, Feldman BF, Jain NC, Farver TB, Kono CS. In vitro platelet release by rat megakaryocytes: effect of metabolic inhibitors and cytoskeletal disrupting agents. Am J Vet Res. 1987;48(7):1142–1146. [PubMed] [Google Scholar]
- 10.Rojnuckarin P, Kaushansky K. Actin reorganization and proplatelet formation in murine megakaryocytes: the role of protein kinase calpha. Blood. 2001;97(1):154–161. doi: 10.1182/blood.v97.1.154. [DOI] [PubMed] [Google Scholar]
- 11.Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost. 2009;7(6):911–918. doi: 10.1111/j.1538-7836.2009.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thon JN, Montalvo A, Patel-Hett S, et al. Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191(4):861–874. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel-Hett S, Richardson JL, Schulze H, et al. Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood. 2008;111(9):4605–4616. doi: 10.1182/blood-2007-10-118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thon JN, Devine MT, Jurak Begonja A, Tibbitts J, Italiano JE., Jr High-content live-cell imaging assay used to establish mechanism of trastuzumab emtansine (T-DM1)--mediated inhibition of platelet production. Blood. 2012;120(10):1975–1984. doi: 10.1182/blood-2012-04-420968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu TL. Two-dimensional difference gel electrophoresis. Methods Mol Biol. 2006;328:71–95. doi: 10.1385/1-59745-026-X:71. [DOI] [PubMed] [Google Scholar]
- 16.Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154(3):485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyrich AS, Denis MM, Kuhlmann-Eyre JR, et al. Dipyridamole selectively inhibits inflammatory gene expression in platelet-monocyte aggregates. Circulation. 2005;111(5):633–642. doi: 10.1161/01.CIR.0000154607.90506.45. [DOI] [PubMed] [Google Scholar]
- 18.Rowley JW, Oler AJ, Tolley ND, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118(14):e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stumpo DJ, Bock CB, Tuttle JS, Blackshear PJ. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci USA. 1995;92(4):944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanabe A, Shiraishi M, Negishi M, Saito N, Tanabe M, Sasaki Y. MARCKS dephosphorylation is involved in bradykinin-induced neurite outgrowth in neuroblastoma SH-SY5Y cells. J Cell Physiol. 2012;227(2):618–629. doi: 10.1002/jcp.22763. [DOI] [PubMed] [Google Scholar]
- 21.Shiraishi M, Tanabe A, Saito N, Sasaki Y. Unphosphorylated MARCKS is involved in neurite initiation induced by insulin-like growth factor-I in SH-SY5Y cells. J Cell Physiol. 2006;209(3):1029–1038. doi: 10.1002/jcp.20814. [DOI] [PubMed] [Google Scholar]
- 22.Green TD, Park J, Yin Q, et al. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. J Leukoc Biol. 2012;92(3):633–639. doi: 10.1189/jlb.1211604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalwa H, Michel T. The MARCKS protein plays a critical role in phosphatidylinositol 4,5-bisphosphate metabolism and directed cell movement in vascular endothelial cells. J Biol Chem. 2011;286(3):2320–2330. doi: 10.1074/jbc.M110.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Jang E, Kim JH, Kim JH, Lee WH, Suk K. Lipocalin-type prostaglandin D2 synthase protein regulates glial cell migration and morphology through myristoylated alanine-rich C-kinase substrate: prostaglandin D2-independent effects. J Biol Chem. 2012;287(12):9414–9428. doi: 10.1074/jbc.M111.330662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myat MM, Anderson S, Allen LA, Aderem A. MARCKS regulates membrane ruffling and cell spreading. Curr Biol. 1997;7(8):611–614. doi: 10.1016/s0960-9822(06)00262-4. [DOI] [PubMed] [Google Scholar]
- 26.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 27.Takashi S, Park J, Fang S, Koyama S, Parikh I, Adler KB. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am J Respir Cell Mol Biol. 2006;34(6):647–652. doi: 10.1165/rcmb.2006-0030RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott LE, Sung EJ, Melvin AT, et al. Fibroblast Migration Is Regulated by Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) Protein. PLoS One. 2013;8(6):e66512. doi: 10.1371/journal.pone.0066512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer M, Martin LD, Vargaftig BB, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10(2):193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 30.Lee SM, Suk K, Lee WH. Myristoylated alanine-rich C kinase substrate (MARCKS) regulates the expression of proinflammatory cytokines in macrophages through activation of p38/JNK MAPK and NF-κB. Cell Immunol. 2015;296(2):115–121. doi: 10.1016/j.cellimm.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Mahaut-Smith MP, Thomas D, Higham AB, et al. Properties of the demarcation membrane system in living rat megakaryocytes. Biophys J. 2003;84(4):2646–2654. doi: 10.1016/S0006-3495(03)75070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swierczynski SL, Blackshear PJ. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein. Mutational analysis provides evidence for complex interactions. J Biol Chem. 1995;270(22):13436–13445. doi: 10.1074/jbc.270.22.13436. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Arbuzova A, Hangyás-Mihályné G, McLaughlin S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2001;276(7):5012–5019. doi: 10.1074/jbc.M008355200. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Gambhir A, Hangyás-Mihályné G, Murray D, Golebiewska U, McLaughlin S. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J Biol Chem. 2002;277(37):34401–34412. doi: 10.1074/jbc.M203954200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Crocker E, McLaughlin S, Smith SO. Binding of peptides with basic and aromatic residues to bilayer membranes: phenylalanine in the myristoylated alanine-rich C kinase substrate effector domain penetrates into the hydrophobic core of the bilayer. J Biol Chem. 2003;278(24):21459–21466. doi: 10.1074/jbc.M301652200. [DOI] [PubMed] [Google Scholar]
- 36.Robinson PJ. Differential stimulation of protein kinase C activity by phorbol ester or calcium/phosphatidylserine in vitro and in intact synaptosomes. J Biol Chem. 1992;267(30):21637–21644. [PubMed] [Google Scholar]
- 37.Allen LA, Aderem A. Protein kinase C regulates MARCKS cycling between the plasma membrane and lysosomes in fibroblasts. EMBO J. 1995;14(6):1109–1121. doi: 10.1002/j.1460-2075.1995.tb07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toledo A, Zolessi FR, Arruti C. A novel effect of MARCKS phosphorylation by activated PKC: the dephosphorylation of its serine 25 in chick neuroblasts. PLoS One. 2013;8(4):e62863. doi: 10.1371/journal.pone.0062863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke PR, Siddhanti SR, Cohen P, Blackshear PJ. Okadaic acid-sensitive protein phosphatases dephosphorylate MARCKS, a major protein kinase C substrate. FEBS Lett. 1993;336(1):37–42. doi: 10.1016/0014-5793(93)81604-x. [DOI] [PubMed] [Google Scholar]
- 40.Verghese GM, Johnson JD, Vasulka C, Haupt DM, Stumpo DJ, Blackshear PJ. Protein kinase C-mediated phosphorylation and calmodulin binding of recombinant myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein. J Biol Chem. 1994;269(12):9361–9367. [PubMed] [Google Scholar]
- 41.Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149(7):1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avanzi MP, Izak M, Oluwadara OE, Mitchell WB. Actin inhibition increases megakaryocyte proplatelet formation through an apoptosis-dependent mechanism. PLoS One. 2015;10(4):e0125057. doi: 10.1371/journal.pone.0125057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tablin F, Castro M, Leven RM. Blood platelet formation in vitro. The role of the cytoskeleton in megakaryocyte fragmentation. J Cell Sci. 1990;97(Pt 1):59–70. doi: 10.1242/jcs.97.1.59. [DOI] [PubMed] [Google Scholar]
- 44.Williams CM, Harper MT, Poole AW. PKCalpha negatively regulates in vitro proplatelet formation and in vivo platelet production in mice. Platelets. 2014;25(1):62–68. doi: 10.3109/09537104.2012.761686. [DOI] [PubMed] [Google Scholar]
- 45.Haddad E, Cramer E, Rivière C, et al. The thrombocytopenia of Wiskott Aldrich syndrome is not related to a defect in proplatelet formation. Blood. 1999;94(2):509–518. [PubMed] [Google Scholar]
- 46.Sabri S, Foudi A, Boukour S, et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108(1):134–140. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Aardema J, Kale S, et al. Loss of the F-BAR protein CIP4 reduces platelet production by impairing membrane-cytoskeleton remodeling. Blood. 2013;122(10):1695–1706. doi: 10.1182/blood-2013-03-484550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender M, Stritt S, Nurden P, et al. Megakaryocyte-specific Profilin1-deficiency alters microtubule stability and causes a Wiskott-Aldrich syndrome-like platelet defect. Nat Commun. 2014;5:4746. doi: 10.1038/ncomms5746. [DOI] [PubMed] [Google Scholar]
- 49.Schulze H, Korpal M, Hurov J, et al. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107(10):3868–3875. doi: 10.1182/blood-2005-07-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schachtner H, Calaminus SD, Sinclair A, et al. Megakaryocytes assemble podosomes that degrade matrix and protrude through basement membrane. Blood. 2013;121(13):2542–2552. doi: 10.1182/blood-2012-07-443457. [DOI] [PubMed] [Google Scholar]
- 51.Rosen A, Keenan KF, Thelen M, Nairn AC, Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med. 1990;172(4):1211–1215. doi: 10.1084/jem.172.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48(1):77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- 54.Sundaram M, Cook HW, Byers DM. The MARCKS family of phospholipid binding proteins: regulation of phospholipase D and other cellular components. Biochem Cell Biol. 2004;82(1):191–200. doi: 10.1139/o03-087. [DOI] [PubMed] [Google Scholar]
- 55.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 56.Arbuzova A, Schmitz AA, Vergères G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362(Pt 1):1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arbuzova A, Wang L, Wang J, et al. Membrane binding of peptides containing both basic and aromatic residues. Experimental studies with peptides corresponding to the scaffolding region of caveolin and the effector region of MARCKS. Biochemistry. 2000;39(33):10330–10339. doi: 10.1021/bi001039j. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi H, Shiraishi M, Fukami K, et al. MARCKS regulates lamellipodia formation induced by IGF-I via association with PIP2 and beta-actin at membrane microdomains. J Cell Physiol. 2009;220(3):748–755. doi: 10.1002/jcp.21822. [DOI] [PubMed] [Google Scholar]