Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Mice overexpressing LOX in platelets have more severe thrombosis than normal animals.
LOX expression influences platelet adhesion to collagen.
Abstract
Lysyl oxidase (LOX) is overexpressed in various pathologies associated with thrombosis, such as arterial stenosis and myeloproliferative neoplasms (MPNs). LOX is elevated in the megakaryocytic lineage of mouse models of MPNs and in patients with MPNs. To gain insight into the role of LOX in thrombosis and platelet function without compounding the influences of other pathologies, transgenic mice expressing LOX in wild-type megakaryocytes and platelets (Pf4-Loxtg/tg) were generated. Pf4-Loxtg/tg mice had a normal number of platelets; however, time to vessel occlusion after endothelial injury was significantly shorter in Pf4-Loxtg/tg mice, indicating a higher propensity for thrombus formation in vivo. Exploring underlying mechanisms, we found that Pf4-Loxtg/tg platelets adhere better to collagen and have greater aggregation response to lower doses of collagen compared with controls. Platelet activation in response to the ligand for collagen receptor glycoprotein VI (cross-linked collagen-related peptide) was unaffected. However, the higher affinity of Pf4-Loxtg/tg platelets to the collagen sequence GFOGER implies that the collagen receptor integrin α2β1 is affected by LOX. Taken together, our findings demonstrate that LOX enhances platelet activation and thrombosis.
Introduction
Lysyl oxidase (LOX) is known to convert specific lysines to aldehydes, providing stability in connective tissues through spontaneous cross-linking of collagen and elastin.1 Excessive LOX activity is associated with pathological fibrosis. Using the GATA-1low mouse model of myeloproliferative neoplasms (MPNs), our laboratory reported high expression of LOX in the bone marrow of mice that express low levels of GATA-1. 2 GATA-1 protein expression was also found to be downregulated in patients with idiopathic myelofibrosis.3 Two reports have since corroborated our findings by demonstrating higher levels of LOX in the bone marrow and sera of patients with MPNs and other types of fibrosis.4,5
Philadelphia chromosome–negative MPNs6 are a group of disorders characterized by proliferation of dysmorphic megakaryocytes and myelofibrosis in the bone marrow. In 1951, William Dameshek proposed that polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis share a common pathogenesis, based on similarities observed in clinical and bone marrow morphologic findings.7 A number of mutations have been described in this group of diseases, a highly prevalent one being the JAK2V617F hyperactivating mutation.8-11 More recently, an exon 9 mutation in the calreticulin gene12 was identified, and it was noted that calreticulin expression is restricted to megakaryocytes.13 The life expectancy of patients with PV and ET is strongly affected by hemostatic complications including thrombosis14 and, to a lesser extent, hemorrhages. The reported incidence of thrombosis ranges from 12% to 39% in PV and from 11% to 25% in ET. The pathogenesis of thrombosis in these cases is not entirely clear. Several lines of evidence suggest enhanced platelet activation as the pathogenesis of thrombosis in PV and ET.15 Markedly enhanced urinary excretion of thromboxane A2 metabolites characterizes untreated ET and PV patients.16,17 Platelet P-selectin (CD62p) expression is elevated in ET and PV patients18,19 and correlated with history of thrombosis.19 Elevated levels of soluble forms of P-selectin and CD40L were found in ET patients20-22 and correlate with occurrence of thrombosis.20,22
Considering that LOX is overexpressed in other pathologies associated with increased thrombosis, such as chronic kidney disease23,24 and arterial stenosis,25,26 we sought to determine whether this enzyme has the potential to affect platelet activation and predisposition to thrombotic events. To this end, we isolated the effect of LOX from other compounding influences of the MPN phenotype by generating and analyzing mice in which LOX is exclusively expressed in the megakaryocyte/platelet lineage. Our results show that LOX overexpression leads to increased adhesion of platelets to collagen and to elevated risk of thrombosis in vivo.
Materials and methods
See supplemental Methods, available on the Blood Web site, for procedures not described in this section.
Carotid artery injury model of vascular thrombosis
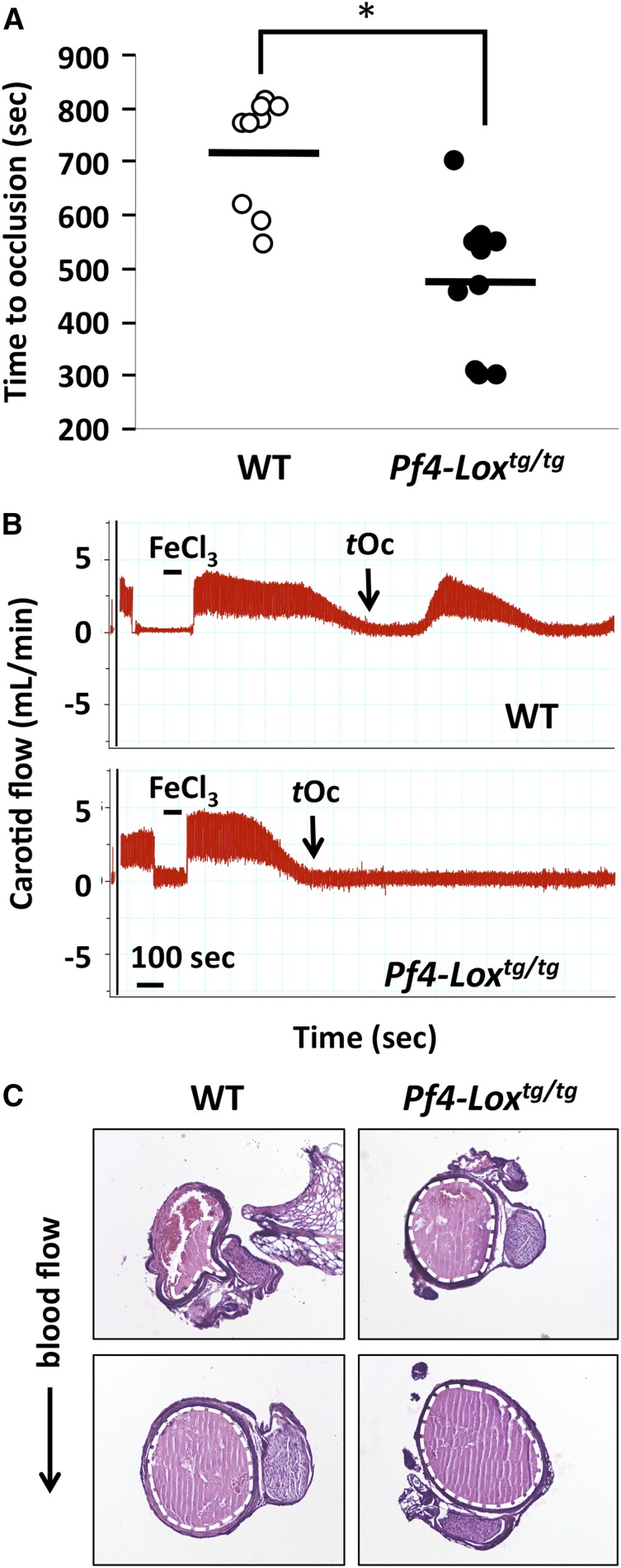
The procedure was performed according to previously published protocols,27 with some modifications. Briefly, the right carotid artery was exposed in animals under isoflurane anesthesia, and basal blood flow was recorded using a 0.5PSB S-Series flowprobe connected to a TS420 perivascular transit-time flow meter (Transonic, Ithaca, NY). The probe was removed, and a piece of Whatman filter paper (1 × 10 mm) soaked in 7.5% ferric chloride (FeCl3; Sigma-Aldrich, St Louis, MO) was placed under the carotid artery for 1 minute. After washing with warm physiological saline (0.9% sodium chloride), the probe was returned, and volume of blood flow was monitored for 30 minutes starting from the placement of the filter paper. Mean, maximum, and minimum carotid flow was recorded using Powerlab Chart5 version 5.3 software, in 1-second intervals. Time to occlusion was determined as the first measurement ≤0.299 mL/min. Average basal flow volume corresponds to an average of measurements over the 30 seconds preceding placement of the filter paper. Genotype, gender, age, and weight of the animals used, and the respective average basal flow volume and time to occlusion are shown in supplemental Table 3. For histologic analysis of thrombi, carotid artery thrombi were induced with FeCl3 as described earlier, and the artery was collected by first ligating it with a 5-0 nylon suture at the level of the first rib, and then sectioning it with surgical scalpel in the most cranial position possible. The nylon suture was used as a guide to determine the direction of blood flow. For each mouse, vessels were collected upon blood flow cessation, rather than at the group’s calculated average half-time to occlusion because of the range of variability observed in individual values within each genotype (Figure 3A). The goal was to monitor the thrombus in tissue sections close to the injury site and upstream from it with respect to the direction of blood flow and injury site. The artery samples were between 3 and 5 mm long. Tissues were fixed, embedded in paraffin, and serially sectioned (10 μm) over their entire length, resulting in 100 to 300 sections per animal. Sections were stained with hematoxylin and eosin. Images were acquired using a Nikon bright field Eclipse 50i microscope and a Nikon Plan 10×/NA 0.25 objective (Nikon, Japan) equipped with a SPOT Insight2 camera and SPOT imaging software 5.0 (SPOT Imaging, Sterling Heights, MI). Genotypes, genders, ages, and results of histologic analysis of carotid arteries are shown in supplemental Table 5.
Figure 3.
Expression of LOX in platelets affects thrombosis. (A) Carotid artery injury model of vascular thrombosis in WT (n = 9) and Pf4-Loxtg/tg (n = 11) mice. Time to occlusion for each mouse is shown. *P < .001 by Student t test, difference of group means. (B) Representative flowchart of carotid artery blood flow. Interval of placement of filter paper soaked with FeCl3 and time to occlusion (tOc) are indicated. (C) Typical images from serial sections of thrombi in carotid arteries. The thrombus is delineated with a dotted white line. Top images show thrombi in regions of carotid artery upstream from the injury site. The direction of the blood flow is denoted. Bottom images represent areas closer to the site of FeCl3 injury (see “Methods”). Between 100 and 300 sections were stained and analyzed per mouse, using 11 Pf4-Loxtg/tg mice and 8 WT mice.
Platelet adhesion and spreading assay
For analysis of adhesion and spreading to different matrices, glass coverslips were coated overnight with (1) a commercially available collagen solution of 50 μg/mL of monomeric (acid-soluble) type I collagen from bovine dermis extracted by pepsin digestion and provided in 0.01 N hydrochloric acid (BD Biosciences, Bedford, MA), (2) 10 μg/mL of native fibrillar type I collagen from equine tendon (Chrono-Log, Havertown, PA), (3) 10 μg/mL of murine fibrinogen (Enzyme Research Laboratories, South Bend, IN), (4) 50 μg/mL of GFOGER (Collagen Toolkits, University of Cambridge, United Kingdom), or (5) 1 mg/mL of poly-l-lysine (Sigma-Aldrich). Coverslips were blocked with 1% fatty acid–free purified bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 2 hours at room temperature. Washed platelets were adjusted to 1 × 107 platelets per milliliter and allowed to adhere on coverslips for 30 minutes at 20°C. Nonadherent platelets were washed with modified Tyrode buffer. Coverslips were fixed with 2% paraformaldehyde in PBS, permeabilized with 0.5% Triton X-100 in PBS, blocked with 10% fetal bovine serum and 1% BSA in PBS, and stained with Alexa Fluor 568 phalloidin (Life Technologies, Carlsbad, CA) at a 1:1000 dilution in 3% BSA and 0.1 M Tris (pH 7.4), 150 mM sodium chloride, and 0.1% Triton X-100 overnight and mounted with VECTASHIELD (Vector Laboratories, Burlingame, CA). Images were acquired on an Olympus IX70 microscope with a U-Plan APO 60×/NA 0.90 objective lens, equipped with an Olympus XM10 digital camera and cellSens software (Olympus, Japan). Platelet counts were determined per field of 60× lens (0.016 mm2). Spread platelets were discriminated based on morphology. Ten to 30 fields were counted per condition per animal, using ImageJ software (version 1.48). For treatment with β-aminopropionitrile (BAPN; Sigma-Aldrich), 0.2% BAPN in drinking water was offered ad libitum for 30 days.2 For analysis of the effect of integrin α2 inhibition on binding to monomeric collagen, a previously published protocol was used, with modifications28: washed platelets were adjusted to a concentration of 3 × 107 platelets per milliliter and incubated with 10 μM indomethacin, 2 U/mL of apyrase (both from Sigma-Aldrich), and 10 μg/mL of anti-integrin α2 (CD49b, clone HMα2; eBioscience, San Diego, CA) or integrin α2 isotype control antibody (Armenian Hamster IgG; eBioscience) for 10 minutes before adhesion to coverslips coated with 50 μg/mL of monomeric collagen (BD Biosciences) and then incubated for 30 minutes at 24°C. Coverslips were washed, fixed, and stained with phalloidin as described earlier.
Results
A mouse model with LOX-expressing megakaryocytes
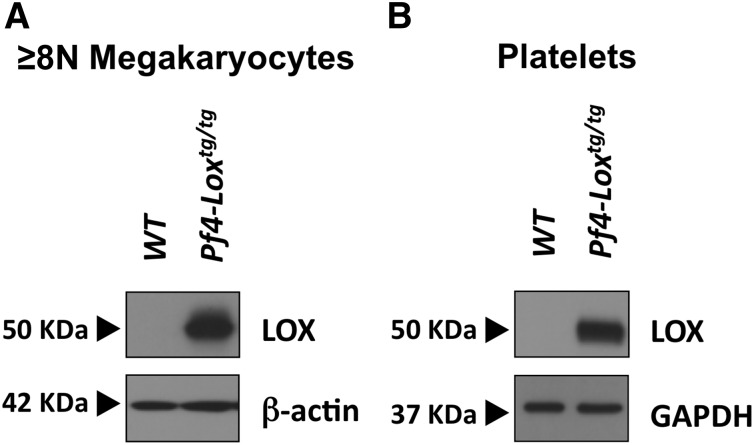
LOX is negligibly expressed in high-ploidy megakaryocytes and platelets of wild-type (WT) mice, but significantly detected in mouse and human MPN cells.2,4 To test the direct effect of LOX on platelet function, free of compounding influences of MPNs, we generated Pf4-Loxtg/tg mice in which LOX protein is expressed on a WT background, driven by the megakaryocyte lineage–specific Pf4 gene promoter, as described in numerous other studies.29,30 LOX protein expression was detected in purified megakaryocytes from Pf4-Loxtg/tg mice, but not in WT control BSA-purified megakaryocytes (Figure 1A). Our previous findings show that Lox expression is limited to immature megakaryocytes, of ploidy up to 4N2. The ploidy of BSA-purified megakaryocytes is typically 8N or higher,31 and no significant LOX expression is expected in this fraction of WT cells. Similarly, LOX protein was detected in platelets of Pf4-Loxtg/tg mice (Figure 1B). Expression was also confirmed at the messenger RNA level (supplemental Figure 1A). This line was selected for continued studies because its megakaryocytes express LOX and show LOX activity at a level similar to that which we detected in megakaryocytes of the GATA-1low MPN model2 (supplemental Figure 1B).
Figure 1.
A mouse model expressing LOX in megakaryocytes and platelets. Immunoblot analysis of LOX expression in megakaryocytes (A) and platelets (B) of Pf4-Loxtg/tg mice and matching WT mice, generated as described under “Methods.” Megakaryocytes were purified by an albumin gradient, which enriches for large megakaryocytes ≥8N megakaryocytes. β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as loading controls.
Expression of LOX affects the megakaryocyte progenitor pool
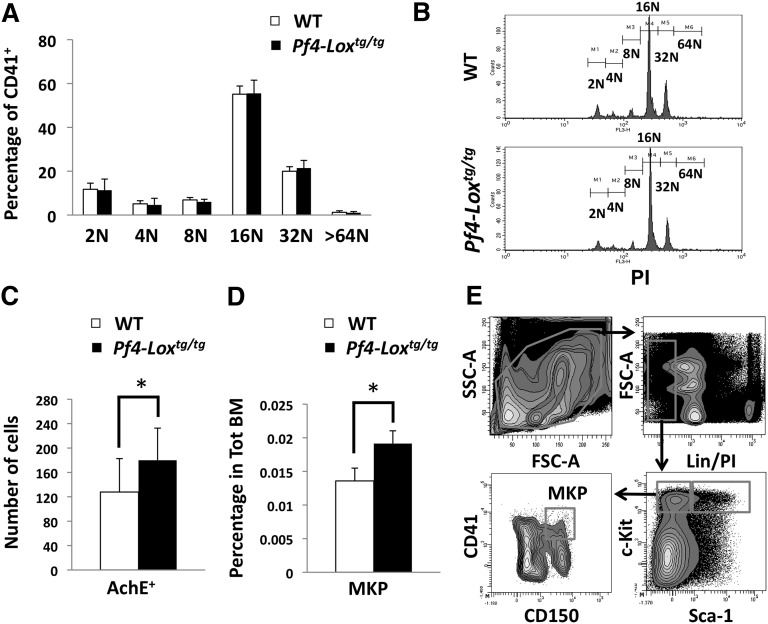
The differential expression of LOX in low-ploidy megakaryocytes2 suggests its possible involvement in megakaryocyte polyploidization and/or expansion. Indeed, our earlier studies showed a LOX-dependent increase in megakaryocytes in GATA-1low mice.2 Megakaryocyte ploidy was not affected in Pf4-Loxtg/tg mice (Figure 2A-B). The number of megakaryocytes in the bone marrow, as determined by the number of acetylcholinesterase-positive cells, was slightly increased in Pf4-Loxtg/tg mice compared with controls (Figure 2C). Because acetylcholinesterase stains both immature and mature megakaryocytes, the observed difference could originate from the expansion of immature megakaryocytes. Indeed, the pool of MKPs, as defined by Lin−c-Kit+Sca-1−CD150+CD41+ staining,32 was significantly increased in Pf4-Loxtg/tg animals compared with controls (Figure 2D-E). Flow cytometry analysis of CD41+ and CD42+ cells (mature megakaryocytes) showed a tendency toward an increase in percentage of this cell population upon LOX expression, but this increase was not statistically significant (supplemental Figure 2). Considering the very low percentage in the bone marrow and the variability in size of mature megakaryocytes, we believe that quantification by flow cytometry may have limitations when analyzing this population. It has been our experience that only large differences between experimental groups are reliably detected by this method. Similarly, the 2N/4N fraction of the ploidy profile (Figure 2A) also did not show a statistically significant difference, most likely because the MKP population is only a fraction of the CD41+ 2N/4N population.
Figure 2.
Expression of LOX transgene induces MKP expansion. (A) Flow cytometric analysis of megakaryocyte ploidy in Pf4-Loxtg/tg mice compared with matching WT mice. Average ± SD of percentage of cells in each ploidy level is shown (n = 6). (B) Representative flow cytometric analysis of megakaryocyte ploidy. Histograms represent gated CD41+PI+ regions, with CD41 staining megakaryocytes and propidium iodide (PI) staining DNA. (C) Average ± SD of number of acetylcholinesterase-positive (AchE+) cells per 2 × 105 cytospun bone marrow cells in Pf4-Loxtg/tg mice (n = 6) compared with WT mice (n = 8). *P < .01. (D) Average ± SD (n = 6, 6-week-old mice) of percentage of MKPs (Lin−c-Kit+Sca-1−CD150+CD41+) in WT and Pf4-Loxtg/tg mice. (E) Gating strategy for analysis of MKPs. FSC, forward scatter; MKP, megakaryocyte progenitor; SD, standard deviation; SSC, side scatter; Tot BM, total bone marrow.
Catalytic activity of LOX has been associated with deposition of collagen fibers in the bone marrow of GATA-1low mice.2 Reticulin staining, routinely used to detect deposition of collagen fibers in the bone marrow extracellular matrix (ECM) through silver impregnation,33 was used to evaluate the bone marrow of Pf4-Loxtg/tg animals. Myelofibrosis was not detected in these mice up to 40 weeks of age (data not shown), which was not surprising because the number of megakaryocytes in the marrow of these mice, although elevated by LOX, is still marginal compared with that seen in other MPN models. Splenomegaly, another hallmark of myeloproliferative diseases in mice, was not detected in Pf4-Loxtg/tg mice at 14 weeks of age (supplemental Figure 3).
Expression of LOX in platelets affects the propensity to thrombosis
Platelet numbers in the peripheral blood of Pf4-Loxtg/tg mice were similar as compared with controls (Table 1). A small decrease in MPV was observed, but no gross ultrastructural abnormalities were detected by electron microscopic analysis (supplemental Figure 4A). The number and shape of microtubule coils, which can affect MPV,34,35 were similar between WT and Pf4-Loxtg/tg mice (supplemental Figure 4). Immunohistochemical staining for β1-tubulin, a cytoskeletal protein necessary for microtubule formation and modulation of discoid shape in platelets, showed a similar diameter of marginal rings and staining intensity between WT and Pf4-Loxtg/tg mice (supplemental Figure 5).
Table 1.
Peripheral blood complete blood count
| Platelet count, 103/μL | MPV, fL | WBC, 103/μL | Hb level, g/dL | |
|---|---|---|---|---|
| WT (n = 25) | 637.5 ± 111.7 | 4.1 ± 0.2 | 5.8 ± 2.6 | 9.7 ± 2.4 |
| Pf4-Loxtg/tg (n = 38) | 626.7 ± 174.8 | 3.9 ± 0.2 | 6.6 ± 3.0 | 9.6 ± 2.6 |
| P | .74 | <.001 | .16 | .94 |
Values are mean ± SD.
Hb, hemoglobin; MPV, mean platelet volume; WBC, white blood cell count.
The carotid artery injury model was used to analyze thrombus formation in Pf4-Loxtg/tg mice. FeCl3 was used to induce endothelial injury, and time to occlusion of the artery was monitored as a read-out for time for thrombus formation.36 The mean time to occlusion in Pf4-Loxtg/tg mice was 4.1 minutes shorter than in WT mice (2-tailed Student t test, 95% confidence interval, 2.18-6.03; P < .001) (Figure 3A-B; supplemental Table 3). To assess whether other variables might account for this observed difference, we also performed a multivariable regression analysis, controlling for gender, age, weight, and average basal flow. This model also showed a significant difference between the experimental groups: time to occlusion for Pf4-Loxtg/tg mice was 3.82 ± 1.25 minutes shorter than for WT mice (P = .00851) (supplemental Table 4). Moreover, at this dose of FeCl3, which is relatively mild compared with previously published protocols,37 transient resumption of blood flow was observed in 8 of 9 WT animals (Figure 3B), whereas flow resumption was observed only in 1 of 11 Pf4-Loxtg/tg mice.
In repeated experimental sets, histologic analysis of transverse sections of the artery showed that in both WT and Pf4-Loxtg/tg mice, thrombi occupied the whole internal area of the vessel near the FeCl3-induced injury site. However, genotypes differed considerably in the size of the thrombus in sections of the carotid artery upstream from the injury site. Sections from 8 of 11 Pf4-Loxtg/tg animals showed complete occlusion, whereas occlusion was observed only in 4 of 8 WT animals, suggesting different dynamics of thrombus formation (see also Figure 3C; supplemental Table 5). In each genotype, the average time to occlusion in animals that displayed complete occlusion of the vessel upstream from the injury site was shorter than in animals that showed partial occlusion. Consistent with results shown in Figure 3A, the average time to occlusion in all Pf4-Loxtg/tg animals analyzed by histology was shorter by ∼35% than in all WT animals (data not shown). Collectively, these results suggest that LOX increases the propensity for thrombosis in vivo.
Pf4-Loxtg/tg platelets have enhanced activation in response to collagen
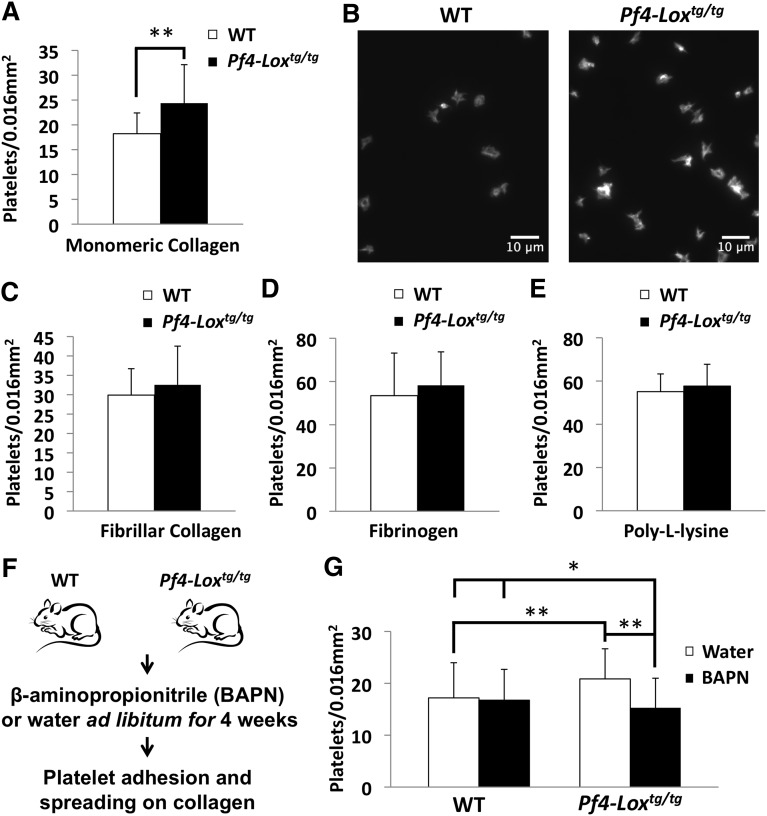
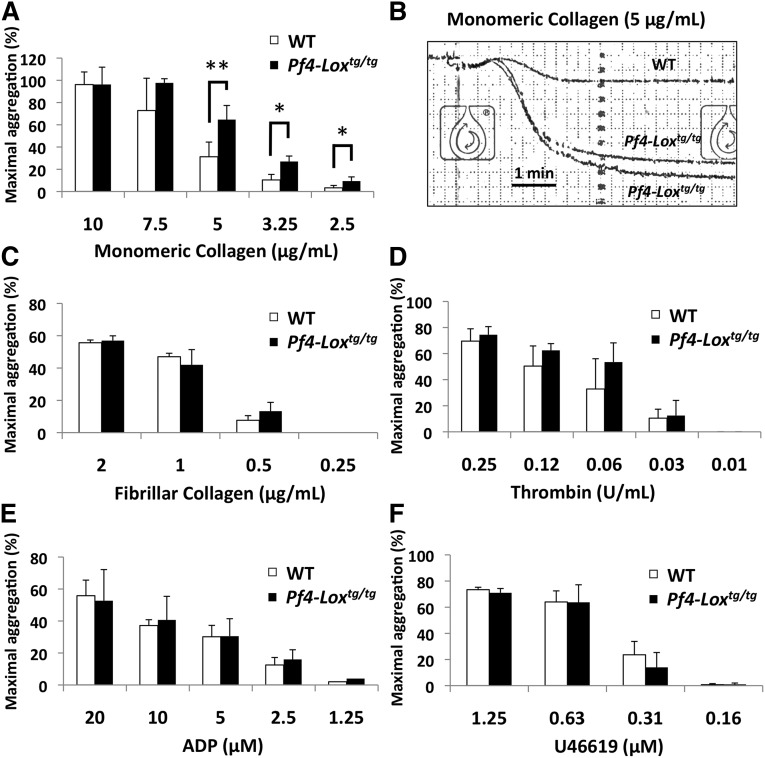
The initial event in platelet-mediated hemostasis and thrombosis is attachment of platelets to exposed collagen in subendothelial ECM.38 Following up our in vivo thrombosis data described earlier, we tested whether LOX-expressing platelets have greater potential to adhere to collagen. Indeed, a higher number of Pf4-Loxtg/tg platelets adhered to and spread on monomeric collagen compared with WT platelets in the same period of time (Figure 4A-B). This effect was not observed in fibrillar collagen (Figure 4C). Adhesion to fibrinogen, a ligand for integrin αIIbβ3, and to poly-l-lysine was not affected (Figures 4D-E). Treatment of animals with the LOX inhibitor BAPN in vivo (Figure 4F) restored the adhesion of Pf4-Loxtg/tg platelets to monomeric collagen to WT levels, indicating that LOX activity, not other factors, is necessary for this effect (Figure 4G). Platelet aggregation to monomeric collagen was enhanced at intermediate and lower doses (from 5 to 2.5 μg/mL) in Pf4-Loxtg/tg platelets (Figure 5A-B). However, the maximal aggregation response at 10 μg/mL was not affected. Platelet aggregation responses to fibrillar collagen (Figure 5C) also showed a tendency toward enhanced aggregation in Pf4-Loxtg/tg platelets compared with controls at 0.5 μg/mL, but the difference was not statistically significant (P = .26). Platelet aggregation to thrombin (Figure 5D), adenosine 5′-diphosphate (Figure 5E), and U46619, a thromboxane A2 analog (Figure 5F), were similar between WT and Pf4-Loxtg/tg animals. These results suggest that interaction with monomeric collagen results in enhanced activation of LOX-expressing platelets.
Figure 4.
Platelets from Pf4-Loxtg/tg mice have enhanced adhesion and spreading to monomeric collagen. (A) Platelet adhesion and spreading assay of WT and Pf4-Loxtg/tg platelets (15-week-old male mice, n = 4 per group) to monomeric collagen. **P < .001. (B) Representative image of platelet adhesion and spreading assay on monomeric collagen. (C-E) Platelet adhesion and spreading assay of WT and Pf4-Loxtg/tg platelets (15-week-old male mice, n = 4 per group) to fibrillar collagen (C), fibrinogen (D), and poly-l-lysine (E). Data in panels A,C-E are representative results of 3 independent experiments. Similar results were observed in female mice. Data represent the average ± SD of platelet counts of 25 fields per animal, except for poly-l-lysine, in which 5 fields were counted. (F) WT and Pf4-Loxtg/tg mice were pretreated with the LOX inhibitor BAPN or water before platelet adhesion and spreading assay on monomeric collagen (n = 4 for WT and Pf4-Loxtg/tg water-treated groups, n = 5 for the BAPN-treated Pf4-Loxtg/tg group, and n = 6 for the BAPN-treated WT group; all mice were 14- to 15-week-old females at the start of BAPN treatment). (G) Data represent the average ± SD of platelet counts of 25 fields per animal. *P < .05, **P < .001. No statistically significant difference was observed in WT animals treated with water or BAPN, whereas BAPN treatment reduced the upregulated platelet adhesion observed in LOX-expressing platelets compared with WT (P < .001).
Figure 5.
Platelets from Pf4-Loxtg/tg mice have enhanced aggregation to monomeric collagen. (A) Platelet aggregation in response to monomeric collagen in WT (n = 8) and Pf4-Loxtg/tg platelets (n = 6). *P < .01, **P < .001. (B) Representative aggregation trace at 5 μg/mL of monomeric collagen. (C-F) Platelet aggregation in response to fibrillar collagen in WT and Pf4-Loxtg/tg platelets (n = 3 per genotype) (C), to thrombin in WT (n = 7) and Pf4-Loxtg/tg platelets (n = 6) (D), to adenosine 5′-diphosphate (ADP) in WT (n = 7) and Pf4-Loxtg/tg platelets (n = 6) (E), and to U46619 in WT and Pf4-Loxtg/tg platelets (n = 5 per genotype) (F). Data represented as average ± SD.
Enhanced activation by collagen in Pf4-Loxtg/tg platelets is mediated by integrin α2β1
The 2 major receptors for collagen in platelets are the immunoglobulin superfamily member glycoprotein (GP)VI and the integrin α2β1. Surface expression levels of GPVI, integrin α2, and integrin β1 were similar between Pf4-Loxtg/tg and WT platelets in a resting state (Table 2), demonstrating that the increase in expression of receptors was not a cause of the observed phenotype.
Table 2.
Analysis of collagen receptor expression on platelets
| GPVI, au | Integrin α2, au | Integrin β1, au | |
|---|---|---|---|
| WT (n = 3) | 739.3 ± 111.53 | 1503.7 ± 35.8 | 14 646 ± 335.8 |
| Pf4-Loxtg/tg (n = 3) | 802.7 ± 77.9 | 1318.3 ± 205.7 | 14 670.7 ± 202.2 |
| P | .55 | .28 | .94 |
Values are mean fluorescence intensity ± SD.
Au, arbitrary units.
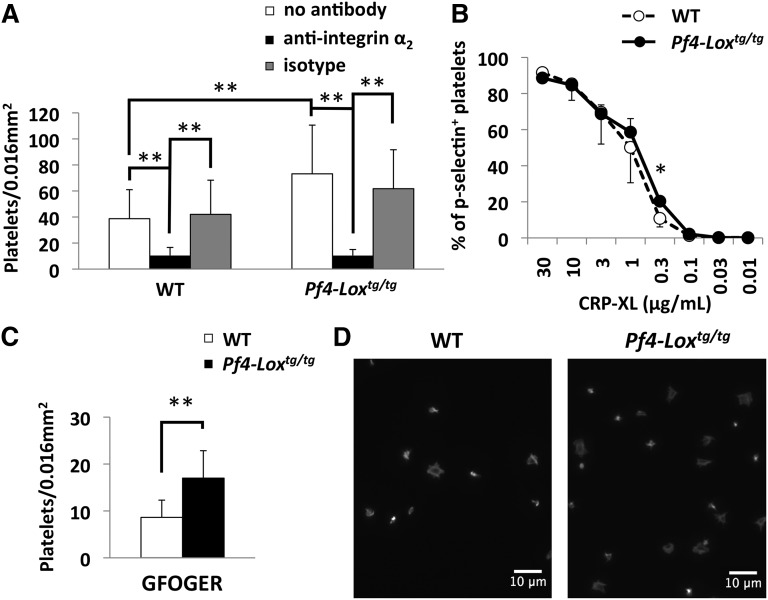
GPVI alone is not sufficient for interaction with monomeric collagen, as shown by the lack of aggregation and adhesion to monomeric collagen in integrin α2–deficient animals.39,40 On the other hand, integrin α2β1 is not necessary for aggregation and adhesion to fibrillar collagen.40 As described earlier, platelets from Pf4-Loxtg/tg mice showed enhanced adhesion (Figure 4A) and aggregation (Figure 5A) to monomeric collagen, but not to fibrillar collagen (Figures 4C and 5C), which suggests that integrin α2β1, not GPVI, may be a target of LOX. To confirm the role of integrin α2β1 in binding to monomeric collagen in Pf4-Loxtg/tg platelets, platelet adhesion assays were performed after preincubation with an antibody against integrin α2, as described earlier.41 Inhibition of integrin α2β1 decreased the adhesion of platelets to monomeric collagen in both WT and Pf4-Loxtg/tg platelets (Figure 6A). This result suggests that adhesion to monomeric collagen in Pf4-Loxtg/tg, as in WT platelets, mostly happens through this integrin. This effect was not observed using anti-α5 as control (supplemental Figure 6).
Figure 6.
Enhanced activation by collagen in Pf4-Loxtg/tg platelets is mediated by integrin α2β1. (A) Platelet adhesion and spreading assay of WT and Pf4-Loxtg/tg platelets preincubated with antibodies against integrin α2 or isotype control (10 μg/mL). Results are the average ± SD of 3 independent experiments, with 1 mouse per group in each experiment. No statistically significant difference was detected between “no antibody” and “isotype” samples in either the WT or the Pf4-Loxtg/tg group. (B) Flow cytometric analysis of P-selectin (CD62p) expression after stimulation with CRP-XL in platelets from WT (n = 4) and Pf4-Loxtg/tg (n = 4) mice. Data are represented as average ± SD. (C) Platelet adhesion and spreading assay on GFOGER (50 μg/mL); the average ± SD of the number of adhered and spread platelets is shown. (D) Representative image of platelet adhesion and spreading assay on GFOGER. *P < .05, **P < .001.
To discriminate the effects of GPVI and α2β1 receptors and to precisely determine which receptor is affected in Pf4-Loxtg/tg platelets, functional assays using ligands specific for each receptor were performed. To examine the possibility that the GPVI receptor is involved in the enhanced response to collagen in Pf4-Loxtg/tg platelets, the GPVI-specific ligand peptide CRP-XL42 was used. Expression of P-selectin, a marker of platelet activation, was generally similar between Pf4-Loxtg/tg and WT platelets after stimulation with CRP-XL (Figure 6B), although a statistically significant difference was detected at the dose of 0.3 μg/mL.
The GFOGER peptide, which specifically binds to integrin α2β1, was used to evaluate the function of this integrin in Pf4-Loxtg/tg platelets.43 Because GFOGER does not induce platelet aggregation (supplemental Figure 7A)42 or cell surface expression of P-selectin (supplemental Figure 7B), an adhesion assay was used to evaluate the effect of the peptide. In the platelet adhesion assay, Pf4-Loxtg/tg platelets bound to and spread more avidly on GFOGER than WT platelets in the same period of time (Figure 6C-D), demonstrating the involvement of integrin α2β1 in enhanced binding of Pf4-Loxtg/tg platelets to collagen. Collectively, data from functional studies indicate that integrin α2β1 is the most likely substrate of LOX in platelets.
Discussion
In this study, we report a previously uncharacterized role of LOX in platelet activation. In a previous study, we uncovered the role of LOX in myelofibrosis using the GATA-1low mouse model of MPNs.2 Analyses of MPN patient samples corroborated our findings, reporting elevated levels of LOX in sera and megakaryocytes as compared with matching control samples.4 In analyzing published gene profiling arrays,44 we noted LOX as upregulated by 2.6- to 2.9-fold in human primary myelofibrosis samples compared with normal samples, depending on the LOX probes used in the microarrays. MPNs are associated with a tendency for activated platelets and thrombosis, the mechanisms of which have remained elusive.14 LOX was also found to be upregulated in cases of thrombosis associated with other organ pathologies (such as chronic kidney disease) and elevated plasma levels of uric acid23,24 and arterial stenosis,25,26 thus broadening the scope of our inquiry and findings. To test the impact of LOX on thrombosis, we generated mice expressing LOX in a WT background in order to isolate the effect of LOX from other compounding influences of the MPN phenotype and other pathologies.
Lox gene expression is higher in low ploidy (<8N) compared with high ploidy (≥8N) megakaryocytes.2 Analysis of MKPs indicates that LOX-induced expansion of immature megakaryocytes, although not sufficient to induce a myeloproliferative phenotype in Pf4-Loxtg/tg mice, may contribute to megakaryocyte expansion observed in MPNs. This finding is in accordance with our earlier reports that LOX affects the platelet-derived growth factor (PDGF) receptor and amplifies its proliferative signaling in smooth muscle cells and in megakaryocytes.2,45 Because LOX amplifies PDGF receptor signaling, and because inhibition of this signaling is important for transition of common myeloid progenitors to megakaryocytes,46 LOX inhibition may be helpful in halting megakaryocyte proliferation in MPNs. Yet, we do not rule out the possibility that LOX influences the MKP pool also via mechanisms independent of PDGF, such as its newly identified influence on collagen receptors. This possible mechanism will require further investigation.
Pf4-Loxtg/tg mice showed shorter time to occlusion in a carotid artery thrombosis model. Although thrombi structures seemed essentially similar in the WT and Pf4-Loxtg/tg mice, the genotypes differed in thrombus size in sections upstream from the injury site. Platelets “roll” on surfaces before stable adhesion and thrombus formation. We suspect that if Pf4-Loxtg/tg platelets are more prone to activation and adhesion, then the duration and distance of rolling may be shorter.47,48 Another factor that could account for a shorter time to occlusion is the stability of thrombus binding to the vessel wall. The higher frequency of transient resumption of blood flow in WT animals compared with Pf4-Loxtg/tg animals suggests that transgenic animals’ thrombi were less likely to detach and were, therefore, likely more stable. Future access to intravital microscopy will allow quantification of dynamic aspects of thrombus formation in this model.
The first event in thrombus formation is the adhesion of platelets to the exposed subendothelial ECM, in which collagen represents up to 40% of the total protein. Collagen is the only matrix protein that supports both platelet adhesion and complete activation.49 Although LOX is known to cross-link collagen, no studies to date have examined the relationship between the degree of collagen cross-linking in blood vessels and platelet adhesion to collagen in vivo. Here, we showed that LOX-expressing platelets have greater ability to adhere to commercially available collagen in vitro, thus focusing our attention on a possible direct effect on collagen receptors, rather than on the degree of collagen cross-linking.
It has been proposed that of the 2 major collagen receptors, GPVI and α2β1, GPVI is the central receptor in collagen-induced platelet activation. Activation of integrin α2β1 requires a conformation change that induces an increase in affinity to the substrate, and “inside-out” signaling by GPVI is thought to be essential for this process.39 However, studies of platelet adhesion in a flow chamber show that platelets can adhere to collagen in the absence of GPVI50 and that thrombus formation in vivo can occur in GPVI-deficient animals.27,37 Several studies reported a GPVI-independent pathway of integrin α2β1 activation, and adhesion to collagen, in particular, seems to occur independently of GPVI signals.42,51,52
The presence of integrin α2β1 is essential for platelet adhesion and aggregation to monomeric collagen,40 but not to fibrillar collagen, although initiation of aggregation is somewhat delayed in the absence of integrin α2.40 Adhesion and aggregation to monomeric collagen, but not to fibrillar collagen, is enhanced in Pf4-Loxtg/tg platelets. Importantly, this effect is ablated upon in vivo administration of a LOX pharmacologic inhibitor. Using CRP-XL, a GPVI-specific ligand, we established that platelet activation in response to GPVI-ligand binding is not affected in Pf4-Loxtg/tg platelets. However, Pf4-Loxtg/tg platelets adhered to and spread more avidly on GFOGER than WT platelets, indicating a gain of function of integrin α2β1 receptor–mediated adhesion in Pf4-Loxtg/tg platelets.
The functional significance of an observed minute decrease in MPV induced by LOX (Table 1) is unclear, although MPV has been linked to cardiovascular disease.53 Analysis of the effect of MPV on thrombus formation using computational modeling showed that a higher MPV is favorable to thrombus formation54; accordingly, we do not expect that the lower MPV observed in Pf4-Loxtg/tg platelets accounts for the propensity of thrombus formation in this model. The decrease in MPV cannot be attributed to changes in megakaryocyte ploidy levels because LOX did not affect ploidy in the current model.
The integrin α2β1 is not a reported substrate of LOX. Integrin β3 and integrin β4 were reported to mediate effects of LOX on phosphorylation of v-src sarcoma viral oncogene homolog and on metastatic cell proliferation.55 Modification of the cell surface receptors for PDGF45 and transforming growth factor β156 by LOX was reported to modulate receptor binding to its ligand. The definitive answer as to whether and where integrin α2β1 is modified by LOX will depend on future proteomic approaches to examining LOX-mediated modification of lysine residues.
The thrombotic phenotype of MPNs has been studied in mouse models of the most prevalent mutation in this pathology, the JAK2V617F mutation. Platelets from JAK2V617F knock-in mice showed increased activation in response to CRP-XL, thrombin, collagen, and fibrinogen.57 In another model transplanted with JAK2V617F knock-in cells, time to occlusion after FeCl3 treatment was decreased as compared with controls, but formed thrombi were unstable, which was attributed to reduced levels of GPVI.58 Analysis of thrombosis in vivo in the GATA-1low mouse model is precluded by the extreme thrombocytopenia observed in those animals.59 One interesting prospect of future studies will be to analyze the contribution of LOX to the thrombotic phenotype observed in JAK2V617F mouse models.
An effect of LOX expression enhancing platelet adhesion onto collagen through integrin α2β1 is described here for the first time. Accordingly, we propose LOX as a possible target in antithrombotic therapy in MPNs and other pathologies associated with LOX overexpression.
Acknowledgments
The authors thank Hervé Falet from the Brigham and Women's Hospital at Harvard Medical School for providing anti-GPVI antibody and CRP-XL and for valuable suggestions, Thomas G. Christensen at t2he Department of Pathology and Laboratory Medicine at Boston University School of Medicine for electron microscopy analysis, Gregory Martin from the Transgenic Core at the Boston University School of Medicine for transgenic expertise, Kimberly J. Bayer from the Laboratory Animal Science Center at the Boston University School of Medicine for training on in vivo thrombosis studies, and Jonathan Bloch for statistical analysis and editorial review of the manuscript.
This study was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grant HL080442 (K.R.). K.R. is an Established Investigator with the American Heart Association.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.M. designed and performed most of the experiments, analyzed and interpreted the data, and cowrote the manuscript; R.M. assisted in the carotid artery thrombosis experiments; A.E. and N.P. generated the Pf4-Loxtg/tg mice and performed the initial colony analyses; S.P. assisted in the histologic analysis; J.T. and J.E.I. performed the β1-tubulin staining and analyzed and interpreted these data; P.C.T. advised on the LOX assays and reviewed the manuscript; M.K. participated in the platelet function studies; P.T. performed the electron microscopy experiments and analyzed and interpreted the data; and K.R. designed the research, analyzed and interpreted the data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katya Ravid, Boston University School of Medicine, 700 Albany St, W-601, Boston, MA 02118; e-mail: kravid@bu.edu.
References
- 1.Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5(3):206–210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 2.Eliades A, Papadantonakis N, Bhupatiraju A, et al. Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J Biol Chem. 2011;286(31):27630–27638. doi: 10.1074/jbc.M111.243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vannucchi AM, Pancrazzi A, Guglielmelli P, et al. Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis. Am J Pathol. 2005;167(3):849–858. doi: 10.1016/S0002-9440(10)62056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tadmor T, Bejar J, Attias D, et al. The expression of lysyl-oxidase gene family members in myeloproliferative neoplasms. Am J Hematol. 2013;88(5):355–358. doi: 10.1002/ajh.23409. [DOI] [PubMed] [Google Scholar]
- 5.Rimar D, Rosner I, Nov Y, et al. Brief report: lysyl oxidase is a potential biomarker of fibrosis in systemic sclerosis. Arthritis Rheumatol. 2014;66(3):726–730. doi: 10.1002/art.38277. [DOI] [PubMed] [Google Scholar]
- 6.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 7.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372–375. [PubMed] [Google Scholar]
- 8.Tefferi A, Vainchenker W. Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011;29(5):573–582. doi: 10.1200/JCO.2010.29.8711. [DOI] [PubMed] [Google Scholar]
- 9.James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 10.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 11.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280(24):22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vannucchi AM, Rotunno G, Bartalucci N, et al. Calreticulin mutation-specific immunostaining in myeloproliferative neoplasms: pathogenetic insight and diagnostic value. Leukemia. 2014;28(9):1811–1818. doi: 10.1038/leu.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falanga A, Marchetti M. Thrombosis in myeloproliferative neoplasms. Semin Thromb Hemost. 2014;40(03):348–358. doi: 10.1055/s-0034-1370794. [DOI] [PubMed] [Google Scholar]
- 15.Patrono C, Rocca B, De Stefano V. Platelet activation and inhibition in polycythemia vera and essential thrombocythemia. Blood. 2013;121(10):1701–1711. doi: 10.1182/blood-2012-10-429134. [DOI] [PubMed] [Google Scholar]
- 16.Rocca B, Ciabattoni G, Tartaglione R, et al. Increased thromboxane biosynthesis in essential thrombocythemia. Thromb Haemost. 1995;74(5):1225–1230. [PubMed] [Google Scholar]
- 17.Landolfi R, Ciabattoni G, Patrignani P, et al. Increased thromboxane biosynthesis in patients with polycythemia vera: evidence for aspirin-suppressible platelet activation in vivo. Blood. 1992;80(8):1965–1971. [PubMed] [Google Scholar]
- 18.Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol. 2005;33(5):523–530. doi: 10.1016/j.exphem.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Arellano-Rodrigo E, Alvarez-Larrán A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91(2):169–175. [PubMed] [Google Scholar]
- 20.Arellano-Rodrigo E, Alvarez-Larrán A, Reverter JC, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2009;84(2):102–108. doi: 10.1002/ajh.21338. [DOI] [PubMed] [Google Scholar]
- 21.Viallard JF, Solanilla A, Gauthier B, et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99(7):2612–2614. doi: 10.1182/blood.v99.7.2612. [DOI] [PubMed] [Google Scholar]
- 22.Musolino C, Alonci A, Bellomo G, et al. Myeloproliferative disease: markers of endothelial and platelet status in patients with essential thrombocythemia and polycythemia vera. Hematology. 2000;4(5):397–402. [PubMed] [Google Scholar]
- 23.Yang Z, Xiaohua W, Lei J, et al. Uric acid increases fibronectin synthesis through upregulation of lysyl oxidase expression in rat renal tubular epithelial cells. Am J Physiol Renal Physiol. 2010;299(2):F336–F346. doi: 10.1152/ajprenal.00053.2010. [DOI] [PubMed] [Google Scholar]
- 24.Jain N, Hedayati SS, Sarode R, Banerjee S, Reilly RF. Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: what is the evidence? Clin J Am Soc Nephrol. 2013;8(4):665–674. doi: 10.2215/CJN.06790712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez M, Pascual G, Cifuentes A, Perez-Köhler B, Bellón JM, Buján J. Role of lysyl oxidases in neointima development in vascular allografts. J Vasc Res. 2011;48(1):43–51. doi: 10.1159/000317399. [DOI] [PubMed] [Google Scholar]
- 26.Nuthakki VK, Fleser PS, Malinzak LE, et al. Lysyl oxidase expression in a rat model of arterial balloon injury. J Vasc Surg. 2004;40(1):123–129. doi: 10.1016/j.jvs.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 27.Konstantinides S, Ware J, Marchese P, Almus-Jacobs F, Loskutoff DJ, Ruggeri ZM. Distinct antithrombotic consequences of platelet glycoprotein Ibalpha and VI deficiency in a mouse model of arterial thrombosis. J Thromb Haemost. 2006;4(9):2014–2021. doi: 10.1111/j.1538-7836.2006.02086.x. [DOI] [PubMed] [Google Scholar]
- 28.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160(5):769–780. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravid K, Beeler DL, Rabin MS, Ruley HE, Rosenberg RD. Selective targeting of gene products with the megakaryocyte platelet factor 4 promoter. Proc Natl Acad Sci USA. 1991;88(4):1521–1525. doi: 10.1073/pnas.88.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 31.Sher N, Von Stetina JR, Bell GW, Matsuura S, Ravid K, Orr-Weaver TL. Fundamental differences in endoreplication in mammals and Drosophila revealed by analysis of endocycling and endomitotic cells. Proc Natl Acad Sci USA. 2013;110(23):9368–9373. doi: 10.1073/pnas.1304889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pronk CJ, Rossi DJ, Månsson R, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1(4):428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Vannucchi AM, Bianchi L, Cellai C, et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood. 2002;100(4):1123–1132. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- 34.Davis B, Toivio-Kinnucan M, Schuller S, Boudreaux MK. Mutation in beta1-tubulin correlates with macrothrombocytopenia in Cavalier King Charles Spaniels. J Vet Intern Med. 2008;22(3):540–545. doi: 10.1111/j.1939-1676.2008.0085.x. [DOI] [PubMed] [Google Scholar]
- 35.Schwer HD, Lecine P, Tiwari S, Italiano JE, Jr, Hartwig JH, Shivdasani RA. A lineage-restricted and divergent beta-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr Biol. 2001;11(8):579–586. doi: 10.1016/s0960-9822(01)00153-1. [DOI] [PubMed] [Google Scholar]
- 36.Li W, McIntyre TM, Silverstein RL. Ferric chloride-induced murine carotid arterial injury: A model of redox pathology. Redox Biol. 2013;1(1):50–55. doi: 10.1016/j.redox.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107(10):3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera J, Lozano ML, Navarro-Núñez L, Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94(5):700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieswandt B, Brakebusch C, Bergmeier W, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20(9):2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtkötter O, Nieswandt B, Smyth N, et al. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277(13):10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Holly SP, Larson MK, et al. Rap1b is critical for glycoprotein VI-mediated but not ADP receptor-mediated alpha2beta1 activation. J Thromb Haemost. 2009;7(4):693–700. doi: 10.1111/j.1538-7836.2009.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight CG, Morton LF, Onley DJ, et al. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc Res. 1999;41(2):450–457. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 43.Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin alpha2beta1. Cell. 2000;101(1):47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 44.Kleppe M, Kwak M, Koppikar P, et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 2015;5(3):316–331. doi: 10.1158/2159-8290.CD-14-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucero HA, Ravid K, Grimsby JL, et al. Lysyl oxidase oxidizes cell membrane proteins and enhances the chemotactic response of vascular smooth muscle cells. J Biol Chem. 2008;283(35):24103–24117. doi: 10.1074/jbc.M709897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boitano AE, de Lichtervelde L, Snead JL, Cooke MP, Schultz PG. An image-based screen identifies a small molecule regulator of megakaryopoiesis. Proc Natl Acad Sci USA. 2012;109(35):14019–14023. doi: 10.1073/pnas.1212545109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrett NE, Holbrook L, Jones S, et al. Future innovations in anti-platelet therapies. Br J Pharmacol. 2008;154(5):918–939. doi: 10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmaier AA, Zou Z, Kazlauskas A, et al. Molecular priming of Lyn by GPVI enables an immune receptor to adopt a hemostatic role. Proc Natl Acad Sci USA. 2009;106(50):21167–21172. doi: 10.1073/pnas.0906436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2(4):561–573. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 50.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer DA, Kahn ML. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106(4):1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecut C, Schoolmeester A, Kuijpers MJ, et al. Principal role of glycoprotein VI in alpha2beta1 and alphaIIbbeta3 activation during collagen-induced thrombus formation. Arterioscler Thromb Vasc Biol. 2004;24(9):1727–1733. doi: 10.1161/01.ATV.0000137974.85068.93. [DOI] [PubMed] [Google Scholar]
- 52.Mazzucato M, Cozzi MR, Battiston M, et al. Distinct spatio-temporal Ca2+ signaling elicited by integrin alpha2beta1 and glycoprotein VI under flow. Blood. 2009;114(13):2793–2801. doi: 10.1182/blood-2008-12-193490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavil Y, Sen N, Yazici H, et al. Coronary heart disease is associated with mean platelet volume in type 2 diabetic patients. Platelets. 2010;21(5):368–372. doi: 10.3109/09537101003628421. [DOI] [PubMed] [Google Scholar]
- 54.Chesnutt JK, Han HC. Platelet size and density affect shear-induced thrombus formation in tortuous arterioles. Phys Biol. 2013;10(5):056003. doi: 10.1088/1478-3975/10/5/056003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker AM, Cox TR, Bird D, et al. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103(5):407–424. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 56.Atsawasuwan P, Mochida Y, Katafuchi M, et al. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J Biol Chem. 2008;283(49):34229–34240. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hobbs CM, Manning H, Bennett C, et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013;122(23):3787–3797. doi: 10.1182/blood-2013-06-501452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamrani L, Lacout C, Ollivier V, et al. Hemostatic disorders in a JAK2V617F-driven mouse model of myeloproliferative neoplasm. Blood. 2014;124(7):1136–1145. doi: 10.1182/blood-2013-10-530832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16(13):3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]