Abstract
Objective
Prospective data are scarce on the relation of red meat, seafood, and poultry consumption with hypertension risk. Although red and processed meats are generally considered to have adverse cardiovascular consequences, seafood is believed to be protective and poultry's effect is controversial.
Methods
We prospectively examined the independent association of long-term intake of animal flesh with incident hypertension in three longitudinal cohort studies of non-hypertensive individuals: Nurses' Health Study I (NHS I, n=62,273 women), Nurses' Health Study II (NHS II, n=88,831 women), and Health Professionals Follow-up Study (HPFS, n=37,414 men). We used multivariable Cox proportional hazards regression to study the associations of different types of animal flesh with the risk of developing hypertension while controlling for other hypertension risk factors. We then used fixed effects meta-analysis to derive pooled estimates of effect.
Results
Compared with participants whose consumption was <1 serving/month, the pooled hazard ratios (HR) among those whose intake was ≥1 serving/day were 1.30 (95% CI: 1.23-1.39) for total meat (a combination of processed and unprocessed red meat), 1.22 (1.12-1.34) for poultry, and 1.05 (0.98-1.13) for seafood. Seafood was associated with an increased risk of hypertension in HPFS and NHS II, but not NHS I. Consumption of any animal flesh ≥1 serving/day was associated with an increased hypertension risk (pooled HR=1.30 [1.16-1.47]).
Conclusions
Long-term intake of meat and poultry were associated with increased risk of hypertension. In contrast to our hypothesis, we found a weak but significant trend towards an increased risk of hypertension with increasing seafood consumption.
Introduction
Vegetarian diets are linked to a lower prevalence of hypertension in cross-sectional studies1-3. It is not known whether these relations result from avoidance of animal flesh intake, an increased consumption of fruits, vegetables, and whole grains, or both. In addition, few prospective studies have examined the associations of specific types of animal flesh with incident hypertension4,5.
Several studies suggest that unprocessed red meat is associated with an increased risk of hypertension4,5, but conflicting data exist regarding the relations of poultry and seafood with blood pressure. Poultry intake was associated with an increased risk of hypertension in some studies, but not in others4,5. Consumption of seafood was not associated with hypertension risk in several studies4-6, despite a blood pressure lowering effect of omega-3 fatty acid supplementation in various trials7. Thus, we prospectively examined the associations of individual types of animal flesh consumption with the risk of developing hypertension in three large prospective cohorts including 188,518 individuals and more than 20 years of follow-up.
Methods
Study Population
The source population includes three large ongoing prospective cohorts, specifically the Nurses' Health Study I (NHS I, N=121,700 women, aged 30-55 at cohort inception in 1976), the Nurses' Health Study II (NHS II, N=116,430 women, aged 25-42 in 1989), and the Health Professionals Follow-up Study (HPFS, N =51,529 men, aged 40-75 in 1986). These cohort studies are discussed in details in previously published articles8,9. Information was available for >90% of the eligible person-time. Participants return a detailed questionnaire every two years documenting numerous health-related factors and medical events. Semi-quantitative food frequency questionnaires (FFQs) were answered every four years; previous work has documented the reproducibility and validity of these FFQs10,11. Participants were excluded from the current analysis if they had a diagnosis of hypertension at baseline (1984 in NHS I, 1991 in NHS II, and 1986 in HPFS), or if they did not provide dietary information, leaving study populations of 62,273 women from NHS I, 88,831 women from NHS II, and 37,414 men from HPFS available for analysis. The Brigham and Women's Institutional Review Board approved this study, including that participants provided implied consent by virtue of voluntarily returning their questionnaires.
Assessment of Hypertension
On the baseline and biennial questionnaires, participants reported if they were diagnosed with hypertension by a health professional during the preceding 2 years. Self-reported hypertension by these health professionals has been shown to be valid12-14. In 51 cases of self-reported hypertension in NHS I, for example, 77% had a pressure >160/95mmHg and 100% had a pressure >140/90mmHg12. In NHS II and HPFS, a medical record review of randomly chosen participants confirmed the diagnosis of hypertension in 94% and 100%, respectively13,14.
As this analysis is prospective, participants with prevalent hypertension were excluded. Incident cases of hypertension only included individuals who first reported this diagnosis on subsequent questionnaires, and whose year of diagnosis was after the date of the baseline questionnaire.
Assessment of Animal Flesh Intake
Starting in 1984, 1991 and 1986, an extensive FFQ was sent out to participants of NHS I, NHS II and HPFS, respectively, to assess intake of >130 foods and beverages, and similar FFQs were then administered every four years in order to update information about dietary intake. Participants reported how often, on average, they consumed each of the queried foods; nine different response categories could be selected, ranging from “never or less than once a month” to “6 or more a day”. Numerous questions on the FFQ ascertained animal flesh intake encompassing meats (processed meats, bacon, hot dogs, hamburgers, beef, pork, lamb), poultry (chicken and turkey, with or without skin), and seafood (dark meat fish, other fish, shrimp, lobster, and canned tuna).
Previous validation studies examined the correlations of animal flesh intake by the FFQ with intake determined by multiple one-week diet records. As an example, 173 women from NHS I completed two FFQs over a 12 month period and also recorded their food consumption in four, seven-day dietary records during this interval; deattenuated correlation coefficients between FFQs and diet records ranged from 0.38 for hamburger to 0.70 for bacon15. Another validation study was conducted in 127 participants of the HPFS; deattenuated Pearson correlation coefficients were 0.74, 0.48 and 0.59 for seafood, poultry and red meat, respectively16. In addition to validity, the reproducibility of the FFQ data has been show in both women and men[10,11,16]. Overall correlations between two FFQs administered one year apart ranged between 0.28 and 0.88. Reproducibility was generally higher for animal flesh, especially for red meats with a Pearson correlation coefficient of 0.88[16], as opposed to vegetables such as beans (Pearson correlation coefficient of 0.28)[15].
Assessment of Covariates
Every two years, we collected updated information about weight, smoking status, and physical activity. Body mass index (BMI) was calculated as the weight (in kg) divided by the height squared (in m). Physical activity, as assessed by a validated questionnaire, was estimated as metabolic equivalent tasks (METs).
Every four years, with the FFQ, we collected information about intake of alcohol and other dietary factors, such as fruits, vegetables and whole grains. The FFQ was also used to compute nutrient intake (eg, intakes of sodium, potassium, magnesium, calcium, and fiber). Questionnaire-derived information about these covariates has been validated, with correlation coefficients of 0.97 for weight, 0.74 for apples and 0.49 for broccoli 17,18.
We also ascertained the use of non-narcotic analgesics (aspirin, acetaminophen, and nonsteroidal antiinflammatory drugs) in each cohort, post-menopausal status in both female cohorts, and oral contraceptive use in NHS II. Smoking status and quantity of smoking were inquired every two years. Race was self-reported.
Statistical Methods
Each participant's person-time of follow-up was calculated from the date of return of the baseline questionnaire to the date of hypertension diagnosis, the date of death, or the end of follow-up (2010 for NHS I and HPFS, and 2011 for NHS II), whichever came first, and allocated according to exposure status. To decrease within-person variation, we used a cumulative average of an individual's flesh intake beginning with the baseline FFQ and including subsequent FFQs through the censoring event.
We analyzed the following types of flesh intake: processed meat; unprocessed red meat; meat (a combination of processed and unprocessed red meat); poultry; seafood; and all flesh (a combination of all of the types of flesh). We grouped consumption of these types of flesh intake into 5 categories: “never or less than 1 serving/month”, “1-3 servings/month”, “1-3 servings/week”, “4-6 servings/week” and “≥1 serving/day”. We then used Cox proportional hazards regression to calculate the hazard ratios (HR) and 95% confidence intervals for incident hypertension, using “never or less than 1 serving/month” as the reference group. In the primary analysis, HRs were adjusted for all of the following potential confounders including smoking status (current/missing); age; BMI; change in weight; race/ethnicity (white, African-American, Asian, Hispanic); family history of hypertension; physical activity; post-menopausal; oral contraceptive use (in NHS II); use of non-narcotic analgesic use; total energy intake (kcal/day); and intakes of alcohol (g/day), fruits and vegetables (servings/day), whole grains (servings/day), sugar-sweetened beverages and artificially-sweetened beverage (drinks/day).”
Adjusted multivariable HRs for the three cohorts were pooled using fixed effect meta-analysis, and we used the Cochrane Q statistic and the I2 statistic to examine the heterogeneity of the association among the cohorts.
A variety of secondary analyses were also performed. First, we included all types of animal flesh simultaneously in the multivariable models. Second, we analyzed types of flesh intake as continuous variables (ie, per serving) rather than in five categories. Third, we removed fruits, vegetables, and whole grains from the model and instead introduced the intakes of micronutrients, including potassium, calcium, magnesium, sodium, and fiber. Fourth, we analyzed consumption of canned tuna separately from other contributors to total seafood intake because canned tuna typically undergoes pre-package processing. Fifth, we investigated whether the associations varied significantly according to age and BMI by creating stratified models and introducing multiplicative interaction terms to our unstratified multivariable models. Sixth, after removing fruits, vegetables, whole grains and sugar-sweetened beverages from our model, we instead adjusted for the DASH diet score (a DASH score was constructed in these cohorts based on high intake of fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains, and low intake of sodium, sweetened beverages, and red and processed meats).
Finally, we performed substitution analyses to determine whether or not eating one additional serving of seafood in place of (or subtracting) one serving of total meat (or one serving of poultry) was associated with an increased or decreased risk of developing hypertension. To accomplish this from a statistical standpoint, we created the following two multivariable models: one that contained seafood, total meat, and total animal flesh as well as other covariates; and one that contained seafood, poultry, and total animal flesh as well as other covariates. In the first of these models, because both total meat and total animal flesh are held constant, the HR for seafood is in essence the “effect” of replacing a serving of poultry with a serving of seafood. Similarly, in the second model the HR for seafood is in essence the “effect” of replacing a serving to total meat with a serving of seafood.
All analyses were performed with SAS software (version 9.3; SAS Institute Inc, Cary, NC). All P values are two-sided.
Results
During 2,936,359 person-years of follow-up, 78,208 participants reported a new diagnosis of hypertension (35,777 cases/1,043,941 person-years in NHS I, 25,843 cases/1,396,062 person-years in NHS II, and 16,855 cases/564,247 person-years in HPFS). Baseline characteristics of each cohort according to the lowest and highest intake categories of meat (processed and red meat), poultry, and seafood are presented in Table 1. In all three cohorts, participants consuming ≥1 serving/day of any type of animal flesh had a higher BMI and, in general, consumed more alcohol than participants consuming animal flesh less than once per month. In each cohort, participants who ate more meat and poultry were younger, whereas participants who ate more seafood were older.
Table 1. Baseline characteristics of participants in the Nurses' Health Study I, Nurses' Health Study II and the Health Professionals Follow-up Study. Values are median (interquartile range) or percentages unless stated otherwise.
| Meat¥ | Poultry | Seafood | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Nurses' Health Study I† | <1 serving/month | ≥1 serving/day | <1 serving/month | ≥1 serving/day | <1 serving/month | ≥1 serving/day |
| No of participants | 868 | 23470 | 1043 | 684 | 2975 | 1435 |
| Age (years) | 52 (45-57) | 48 (43-54) | 51 (45-57) | 49 (44-56) | 50 (44-57) | 50 (45-56) |
| White (%) | 94.1 | 95.4 | 94.7 | 90.2 | 95.4 | 92.3 |
| Body Mass Index | 22.4 (20.7-24.9) | 23.7 (21.6-26.6) | 23.0 (21.2-25.8) | 24.7 (22.1-27.5) | 23.1 (21.3-25.8) | 24.2 (21.9-27.1) |
| Physical Activity (METs/w) | 11.50 (3.9-26.5) | 6.50 (2.4-16.1) | 6.5 (2.4-17.8) | 10.4 (3.4-24.8) | 6.50 (2.4-17.8) | 10.40(3.4-24.8) |
| Current Smokers (%) | 8.9 | 27.6 | 22.8 | 18.9 | 25.9 | 24.1 |
| Family History of HTN (%) | 33.8 | 42.8 | 37.4 | 40.1 | 38.9 | 39.7 |
| Alcohol Intake (g/day) | 0.00 (0.0-3.0) | 2.10 (0.0-9.5) | 0.00 (0.0-3.4) | 1.80 (0.0-6.6) | 1.80 (0.0-6.6) | 2.8 (0.0-10.8) |
|
| ||||||
| Meat¥ | Poultry | Seafood | ||||
|
| ||||||
| Nurses' Health Study II‡ | <1 serving/month | ≥1 serving/day | <1 serving/month | ≥1 serving/day | <1 serving/month | ≥1 serving/day |
|
| ||||||
| No of participants | 3431 | 22466 | 1692 | 4426 | 6682 | 1520 |
| Age (years) | 37 (33-40) | 36 (32-39) | 37 (33-40) | 36 (33-40) | 36 (32-39) | 37 (33-40) |
| White (%) | 92.4 | 92.7 | 93.1 | 89.9 | 93.2 | 86.4 |
| Body Mass Index | 21.6 (20.1-23.7) | 23.7 (21.3-27.5) | 21.9 (20.3-24.3) | 24.1 (21.6-27.9) | 22.8 (20.8-25.9) | 23.4 (21.1-26.6) |
| Physical Activity (METs/w) | 22.80(9.9-44.1) | 10.10(4.0-22.2) | 16.60(7.0-35.5) | 15.30(6.2-32.2) | 10.40(3.9-23.4) | 21.20(9.0-43.5) |
| Current Smokers (%) | 6.8 | 15.0 | 11.6 | 10.8 | 11.7 | 13.6 |
|
| ||||||
| Family History of HTN (%) | 49.1 | 50.8 | 49.1 | 52.9 | 46.6 | 52.5 |
| Alcohol Intake (g/day) | 0.90 (0.0-3.7) | 0.90 (0.00-3.0) | 0.00 (0.0-2.7) | 0.90 (0.0-3.3) | 0.0 (0.0-1.9) | 1.80 (0.0-5.5) |
|
| ||||||
| Meat¥ | Poultry | Seafood | ||||
|
| ||||||
| Health Professionals Follow-up Study§ | <1 serving/month | ≥1 serving/day | <1 serving/month | ≥1 serving/day | <1 serving/month | ≥1 serving/day |
|
| ||||||
| No of participants | 1350 | 14461 | 990 | 584 | 1678 | 1864 |
| Age (years) | 53 (45-62) | 51 (44-60) | 55 (46-63) | 50 (44-58) | 53 (44-61) | 54 (47-62) |
| White (%) | 88.6 | 92.4 | 87.9 | 89.6 | 91.7 | 87.8 |
| Body Mass Index | 23.6 (22.0-25.1) | 25.1 (23.6-27.1) | 24.4 (22.6-26.5) | 25.0 (23.3-27.0) | 25.0 (23.0-26.6) | 24.7 (23.2-26.6) |
| Physical Activity (METs/w) | 20.1(7.9-43.1) | 10.1(3.3-25.5) | 10.8(3.3-25.6) | 17.6(5.0-36.3) | 7.90(2.1-23.3) | 19.1 (7.2-37.6) |
| Current Smokers (%) | 2.0 | 12.8 | 8.9 | 5.5 | 9.3 | 5.3 |
| Family History of HTN (%) | 30.1 | 31.6 | 26.3 | 32.2 | 28.5 | 32.4 |
| Alcohol Intake (g/day) | 1.50 (0.0-7.6) | 6.60 (1.0-16.3) | 1.00 (0.0-9.7) | 3.0 (0.0-10.6) | 1.10 (0.0-8.8) | 6.2 (1.5-14.5) |
Baseline for Nurses' Health Study I is 1984.
Baseline for Nurses' Health Study II is 1991.
Baseline for Health Professionals Follow-up Study is 1986.
Unprocessed and processed red meat combined.
In pooled analyses, higher consumption of any individual type of animal flesh was significantly and independently associated with an increased risk of incident hypertension (Table 2). The multivariable pooled HRs for intake ≥1/day as compared with <1/month were 1.04 (95% CI, 0.98-1.10; p-trend<0.001) for processed meat, 1.24 (1.17-1.31; p-trend<0.001) for unprocessed red meat, 1.30 (1.23-1.39; p-trend<0.001) for all meat (unprocessed and processed red), 1.22 (1.12-1.34; p-trend<0.001) for poultry, and 1.05 (0.98-1.13; p-trend<0.001) for seafood. Eating any animal flesh once per day or more was associated with a 30% higher risk of hypertension when compared with eating animal flesh less than once a month (HR=1.30, 1.16-1.47; p-trend<0.001).
Table 2. Pooled hazard ratios (95% confidence intervals) of hypertension for different types of flesh consumption in Nurses' Health Study I, Nurses' Health Study II and Health Professionals Follow-up Study.
| Consumption Levels (per serving) | ||||||
|---|---|---|---|---|---|---|
| <1 per month | 1-3 per month | 1-3 per week | 4-6 per week | ≥ 1 per day | Linear P Trend | |
| Processed Meat | ||||||
| Nurses' Health Study I: §* | 4353/140195 | 12142/358903 | 13630/373559 | 5105/152720 | 547/18564 | |
| Adjusted hazard ratio† | 1.00 (reference) | 1.04(1.00-1.08) | 1.08(1.04-1.12) | 1.08(1.03-1.13) | 1.03(0.94-1.13) | 0.35 |
| Nurses' Health Study II: ¶* | 3584/248867 | 9852/546000 | 8889/434223 | 3173/151478 | 345/15494 | |
| Adjusted hazard ratio† | 1.00 | 1.08(1.04-1.12) | 1.11(1.06-1.16) | 1.12(1.07-1.19) | 1.18(1.05-1.33) | <0.001 |
| Health Professional's Follow-Up Study: ¥ * | 2348/90046 | 4799/160764 | 5505/173013 | 3475/114060 | 728/26364 | |
| Adjusted hazard ratio† | 1.00 | 1.04(0.99-1.10) | 1.03(0.98-1.09) | 1.02(0.96-1.08) | 0.97(0.88-1.06) | 0.06 |
| Pooled results‡ | 1.00 | 1.05(1.03-1.08) | 1.08(1.05-1.11) | 1.07(1.04-1.11) | 1.04(0.98-1.10) | <0.001 |
| Unprocessed Red Meat | ||||||
| Nurses' Health Study I: § * | 481/19003 | 2527/77228 | 12133/334851 | 17464/507974 | 3172/104884 | |
| Adjusted hazard ratio† | 1.00 | 1.18(1.07-1.31) | 1.23(1.12-1.35) | 1.25(1.14-1.37) | 1.28(1.15-1.41) | <0.001 |
| Nurses' Health Study II: ¶* | 632/58952 | 2416/169968 | 8138/460520 | 11934/575484 | 2723/131137 | |
| Adjusted hazard ratio† | 1.00 | 1.22(1.11-1.33) | 1.26(1.16-1.37) | 1.41(1.29-1.53) | 1.40(1.28-1.54) | <0.001 |
| Health Professional's Follow-Up Study: ¥* | 584/24224 | 1802/64504 | 5008/159933 | 7024/229076 | 2437/86509 | |
| Adjusted hazard ratio† | 1.00 | 1.04(0.95-1.14) | 1.08(0.99-1.18) | 1.05(0.96-1.15) | 1.05(0.96-1.16) | 0.66 |
| Pooled results‡ | 1.00 | 1.15(1.09-1.21) | 1.19(1.13-1.25) | 1.23(1.14-1.30) | 1.24(1.17-1.31) | <0.001 |
| Total Meat (processed and unprocessed red meat) | ||||||
| Nurses' Health Study I: §* | 329/13976 | 1395/45059 | 7442/212583 | 17461/496837 | 9150/275486 | |
| Adjusted hazard ratio† | 1.00 | 1.20(1.06-1.35) | 1.26(1.13-1.41) | 1.29(1.15-1.44) | 1.33(1.18-1.49) | <0.001 |
| Nurses' Health Study II: ¶* | 434/45731 | 1413/106097 | 5586/341382 | 11888/603807 | 6522/299046 | |
| Adjusted hazard ratio† | 1.00 | 1.26(1.12-1.40) | 1.32(1.19-1.46) | 1.44(1.30-1.59) | 1.52(1.37-1.69) | <0.001 |
| Health Professional's Follow-Up Study: ¥* | 420/18817 | 1092/41427 | 3279/109548 | 6340/204696 | 5724/189758 | |
| Adjusted hazard ratio† | 1.00 | 1.07(0.95-1.19) | 1.11(0.99-1.23) | 1.09(0.99-1.21) | 1.09(0.98-1.21) | 0.9 |
| Pooled results‡ | 1.00 | 1.17(1.10-1.25) | 1.23(1.15-1.30) | 1.27(1.19-1.35) | 1.30(1.23-1.39) | <0.001 |
| Poultry | ||||||
| Nurses' Health Study I: §* | 397/14653 | 4294/130577 | 22104/655822 | 8708/234440 | 274/8449 | |
| Adjusted hazard ratio† | 1.00 | 1.14(1.03-1.26) | 1.15(1.04-1.27) | 1.15(1.03-1.27) | 1.05(0.90-1.23) | 0.89 |
| Nurses' Health Study II: ¶* | 287/24853 | 2033/126043 | 11463/621612 | 11304/584475 | 756/39079 | |
| Adjusted hazard ratio† | 1.00 | 1.23(1.08-1.39) | 1.29(1.14-1.45) | 1.32(1.17-1.48) | 1.35(1.17-1.56) | <0.001 |
| Health Professional's Follow-Up Study: ¥* | 338/13905 | 1954/65248 | 9033/312347 | 5229/163424 | 301/9322 | |
| Adjusted hazard ratio† | 1.00 | 1.12(0.99-1.26) | 1.13(1.00-1.26) | 1.18(1.05-1.32) | 1.26(1.07-1.48) | 0.001 |
| Pooled results‡ | 1.00 | 1.15(1.08-1.23) | 1.18(1.11-1.26) | 1.20(1.13-1.28) | 1.22(1.12-1.34) | <0.001 |
| Seafood | ||||||
| Nurses' Health Study I: §* | 1472/43922 | 10343/312274 | 17464/501282 | 6139/173810 | 6139/12653 | |
| Adjusted hazard ratio† | 1.00 | 0.99(0.93-1.04) | 0.99(0.93-1.04) | 1.04(0.98-1.10) | 0.91(0.81-1.02) | 0.05 |
| Nurses' Health Study II: ¶* | 2173/120436 | 10288/571193 | 10481/547269 | 2706/145855 | 195/11310 | |
| Adjusted hazard ratio† | 1.00 | 0.99(0.95-1.04) | 1.04(0.99-1.09) | 1.07(1.00-1.13) | 1.13(0.97-1.31) | 0.003 |
| Health Professional's Follow-Up Study: ¥* | 653/23849 | 4322/151055 | 7325/241332 | 4005/129626 | 550/18384 | |
| Adjusted hazard ratio† | 1.00 | 1.01(0.93-1.10) | 1.04(0.96-1.13) | 1.12(1.03-1.22) | 1.16(1.03-1.31) | <0.001 |
| Pooled results‡ | 1.00 | 0.99(0.96-1.03) | 1.03(0.99-1.06) | 1.06(1.02-1.10) | 1.05(0.98-1.13) | <0.001 |
| Animal Flesh$ | ||||||
| Nurses' Health Study I: §* | 92/4020 | 57/2769 | 651/21519 | 8853/265062 | 26124/750572 | |
| Adjusted hazard ratio† | 1.00 | 0.96(0.69-1.34) | 1.32(1.05-1.64) | 1.32(1.07-1.63) | 1.38(1.12-1.70) | <0.001 |
| Nurses' Health Study II: ¶* | 34/4748 | 67/7323 | 480/42580 | 4741/306896 | 20521/1034516 | |
| Adjusted hazard ratio† | 1.00 | 0.92(0.68-1.24) | 1.11(0.89-1.38) | 1.31(1.06-1.60) | 1.46(1.19-1.79) | <0.001 |
| Health Professional's Follow-Up Study: ¥* | 104/4696 | 42/2039 | 294/11646 | 3287/116429 | 13128/429437 | |
| Adjusted hazard ratio† | 1.00 | 0.91(0.63-1.31) | 1.03(0.82-1.29) | 1.06(0.87-1.30) | 1.11(0.90-1.35) | 0.04 |
| Pooled results‡ | 1.00 | 0.93(0.77-1.13) | 1.15(1.01-1.30) | 1.22(1.08-1.37) | 1.30(1.16-1.47) | <0.001 |
Follow-up in Nurses' Health Study I was from 1984 to 2010.
Follow-up in Nurses' Health Study II was from 1991-2011.
Follow-up in Health Professionals Follow-up study was from 1986 to 2010.
Cases/person-years observation.
Adjusted for age (years), race/ethnicity (white, African-American, Asian/Hispanic), body mass index, current smoking status, physical activity, weight change per Food Frequency Questionnaire cycle, post-menopausal (yes or no), alcohol intake, current oral contraceptive use (yes or no, for Nurses' Health Study II), family history of hypertension (yes or no), total energy intake (kcal/day), total fruits, vegetables and whole grains (quintiles), sugar-sweetened beverage intake (per day), artificially-sweetened diet beverage intake (per day) and analgesic use.
Pooled hazard ratios of the three cohorts using a fixed effects model.
Animal flesh is a combination of unprocessed and processed red meat, poultry and seafood.
However, there were some inconsistencies among the three individual cohorts. Specifically, in young women (NHS II), higher intake of any type of animal flesh was independently related to an increased risk of hypertension. However, in NHS I, higher intakes of unprocessed red meat and total meat were associated with an increased risk of hypertension, but processed meat, poultry and seafood were not associated with hypertension. In men (HPFS), greater poultry consumption and greater seafood consumption were independently related with increased hypertension risk, and there was no association with higher consumption of processed meat (Table 2).
The associations of animal flesh intake with hypertension were overall similar when all types of flesh intake were included simultaneously in the same multivariable model. Specifically, comparing the highest (≥1 serving/day) with the lowest (<1 serving/month) categories of intake, the pooled HRs were 1.03 (0.97-1.09) for processed meat, 1.21 (1.14-1.29) for unprocessed red meat, 1.12 (1.02-1.23) for poultry, and 1.05 (0.98-1.14) for seafood. When the intake of various types of flesh were analyzed as continuous variables, the pooled HRs for hypertension associated with one additional serving per day were 1.04 (1.01-1.07) for processed meat, 1.09 (1.06-1.11) for unprocessed red meat, 1.07 (1.03-1.10) for poultry, and 1.09 (1.06-1.13) for seafood.
A test of heterogeneity among the three cohorts was significant for the associations of poultry (P for heterogeneity of 0.03), total meat (P<0.001) and animal flesh (P<0.001).
Adjusting for sodium intake revealed similar results, so did the inclusion of other micronutrients into our model, such as potassium, calcium, magnesium and fiber. There were no consistent interactions between any type of flesh intake and either age or BMI as pertains to hypertension risk. Adjusting for the DASH diet score did not materially alter our findings (data not shown).
To further investigate the association of seafood with hypertension, a secondary analyses reported an increased risk of hypertension with canned tuna and dark meat fish (which could include canned salmon and sardines), but not other types of seafood (Supplementary Table 1). As examples, the pooled multivariable HRs comparing intake 4-6 times per week with <1/month were 1.13 (1.06-1.20; p-trend<0.001) for canned tuna and 1.24 (1.06-1.46; p-trend=0.01) for dark meat fish. In contrast, other contributors to total seafood intake were not associated with hypertension. As an example, a similar comparison for shrimp, lobster or scallops intake yielded a pooled multivariable HR of 0.92 (0.67-1.26, p-trend=0.15) for 4-6 servings/week as compared to <1/month.
The results of our substitution analyses are summarized in Figure 1. Replacing one serving of total (processed and unprocessed) meat or poultry with one serving seafood was associated with an increased risk of developing hypertension in HPFS (HR=1.12 [1.05-1.18] and HR= 1.09 [1.00-.1.20], respectively). Replacing one serving of total (processed and unprocessed) meat with one serving of seafood was not associated with hypertension in the female cohorts, and no other substitution analyses yielded significant findings.
Figure 1.
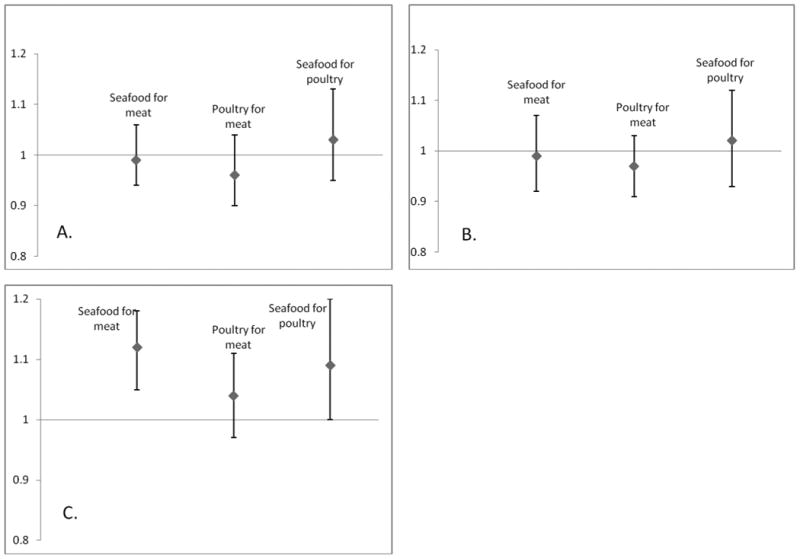
Pooled multivariable adjusted hazard ratios and 95% confidence intervals (error bars) of hypertension associated with substitution of one serving of an animal flesh product with another in Nurses' Health Study I (A), Nurses' Health Study II (B) and Health Professionals Follow-up Study (C).
Discussion
In three prospective cohorts of US men and women, we found that animal flesh intake was significantly associated with an increased risk of hypertension, independent of other factors, including intake of fruits, vegetables, and whole grains. The relation was consistent for total meat (processed and red meat) in all three cohorts and, in two of the three cohorts, higher consumption of poultry and fish were associated with a higher risk of hypertension. To our knowledge, this study is the largest prospective examination of animal flesh intake and incident hypertension, and has the longest duration of follow-up.
Our finding that more meat intake was associated with increased hypertension risk is broadly consistent with prior studies. As an example, in a prospective cohort of 28,766 females aged ≥45 years, red meat intake ≥1.5 servings/day was associated with a 35% higher risk of hypertension as compared with women who consumed meat less than once a month4. Similar results were reported in a smaller prospective cohort of 1,709 men; men who consumed more than 20 servings of a 120gram portion of red meat per month had a 6mmHg greater increase in systolic blood pressure (SBP) after 7 years of follow-up when compared with men who consumed <8 servings of red meat per month5. In a more recent French prospective study, women who consumed ≥5 servings of processed meat per week had a 17% higher risk of hypertension than women whose consumption was <1 serving/week19. Thus, our study, in conjunction with past research, suggests that long-term avoidance of meat may reduce the risk of developing hypertension.
We also found that a greater consumption of poultry was independently associated with increased hypertension risk. Few comparative data are available. In the Chicago Western Electric Study, for example, men who consumed >20 servings of poultry (120gram portion) per month had a 3.9mmHg higher SBP than those whose consumption was less than 4 servings per month (p= 0.003)5. In contrast, there was no association between poultry risk and blood pressure among older women in the Women's Health Initiative (multivariable relative risk of 1.03 for the highest quintile of poultry intake)4.
Like with poultry, long-term prospective information about the association of fish or seafood intake with the risk of hypertension is limited to two studies. Longitudinal follow-up of white individuals aged 21-74 years enrolled into the First National Health and Nutrition Survey (NHANES I) found that fish consumption was not related to hypertension6. Similarly, fish intake and hypertension were not associated in the Chicago Western Electric Study5. On the other hand, short-term trials of omega-3 fatty acid supplementation (which is present in large amounts in oily fish) have suggested a blood pressure lowering effect, although trials of fish intake (rather than supplementation) are scarce20,21. We observed a weak increased risk of hypertension with increasing fish consumption overall, an association found principally in younger women and men, but not in older women. Furthermore, the increased risk of hypertension with greater seafood consumption might be limited to certain types of seafood, specifically canned tuna and dark meat fish (eg, salmon [including canned salmon], swordfish, and bluefish).
The mechanisms by which meat, poultry, and seafood may influence the risk of hypertension remain hypothetical and controversial. One hypothesis relates to the formation of Maillard Reaction Products (MRPs) in cooked food, particularly cooked flesh22. Through a complex network of chemical reactions, MRPs such as heterocyclic amines (HAAs), acrylamides, and advanced glycoxidation end-products (AGEs) are produced, especially at higher cooking temperatures23. As examples, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), a HAA, is produced in high concentrations in barbecued chicken, fried chicken, and fried salmon24,25. In addition, ɛN-carboxymethyl-lysine (CML), an AGE, is generated in fried and roasted chicken at levels of 9,000 kU and 4,300 kU per 90 gram serving, respectively26. In contrast, AGEs are found in substantially smaller quantities in fruits, vegetables, and carbohydrates26. Both HAAs and AGEs increase oxidative stress and inflammation, both potential factors in the development of hypertension27,28 . AGEs act on receptors found in vascular tissue (called RAGEs). RAGEs modulate vascular function and blood pressure homeostasis by increasing pro-inflammatory cytokines and oxidative stress28-30HYPERLINK \l "_ENREF_24" \o "Baumann, 2012 #149".
Another recently proposed mechanism relates to the conversion of L-carnitine, principally found in red meat, to trimethylamine (TMA) by gut microflora31,32. TMA is then absorbed and transported to the liver where it is converted to trimethylamine-N-oxide (TMAO). TMAO, which is also present at large quantities in salt-water fish, is pro-atherogenic in animals and humans31,33. TMAO increases two pro-atherogenic scavenger receptors, specifically CD36 and scavenger receptor A (SRA)34-36. CD36 pathways have been associated with endothelial dysfunction, inflammation and oxidative stress, which are all potentially important mechanisms in the development of hypertension37-40.
In our study, a secondary analysis showed that moderate intakes of canned tuna and dark meat fish (4-6 servings a week) were independently associated with a higher risk of hypertension. To our knowledge, no other study has analyzed the different types of seafood and the risk of hypertension. Although speculative, these findings could be related to processing methods of canned tuna and salmon (information not obtained in the FFQ), and potentially high levels of MRPs in cooked salmon24-26,41.
There are limitations to this study. First, the diagnosis of hypertension was self-reported and we did not directly measure the participants' blood pressures. However, the participants in the three cohorts are health professionals and this method of hypertension diagnosis was previously found to be valid12-14. Second, categorization of food intake could have been misclassified due imperfect ascertainment of dietary intake using the FFQ, as well as to seasonal changes in diet that may not be captured by the FFQ. However, this type of error would likely be random and therefore result in an underestimate of the true association. Third, as in any observational study, we cannot rule out the presence of residual confounding. However, we carefully controlled for numerous hypertension risk factors. Fourth, our findings did suggest some degree of heterogeneity between the three different cohorts; including fish poultry and processed red meat. This heterogeneity could be partly explained by the differences among the cohorts such years of follow-up (26, 20 and 24 years for NHS I, NHS II and HPFS, respectively), age, sex and food choices made by the participants. Finally, participants were mostly non-Hispanic white men and women, and these associations should be examined in other populations.
One of the strengths of our study lies in the age composition of our three different cohorts; despite some overlap, ages varied from 25 to 75 years, increasing the generalizability of the study.
In conclusion, we found an independent and significant association between higher intake of animal flesh and a greater risk of developing hypertension. Given the increasing prevalence of hypertension in the United States and around the world, these data have important public health implications. Future studies are needed to further assess the potential mechanisms underlying these associations.
Supplementary Material
Acknowledgments
Borgi, Curhan, and Forman contributed to the conception and design of the study. All authors were involved in the analysis and interpretation of the data. Borgi and Forman designed and conducted the statistical analysis. Borgi, Curhan and Forman worked on the drafting of the manuscript, which was thoroughly reviewed and approved by all authors.
Funding: the first author was funded by the American Heart Association (AHA) grant (14POST20380070).
This manuscript was funded by the following grants: Nurses' Health Study (NHS; P01 CA87969), the Nurses' Health Study II (NHS II; UM1 CA176726) and the Health Professional Health Study (HPFS; UM1 CA167552).
Footnotes
Conflict of Interest Disclosures: None reported.
References
- 1.Pettersen BJ, Anousheh R, Fan J, Jaceldo-Siegl K, Fraser GE. Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2) Public health nutrition. 2012 Oct;15(10):1909–1916. doi: 10.1017/S1368980011003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleby PN, Davey GK, Key TJ. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public health nutrition. 2002 Oct;5(5):645–654. doi: 10.1079/PHN2002332. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama Y, Nishimura K, Barnard ND, et al. Vegetarian diets and blood pressure: a meta-analysis. JAMA internal medicine. 2014 Apr;174(4):577–587. doi: 10.1001/jamainternmed.2013.14547. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Manson JE, Buring JE, Sesso HD. Meat intake and the risk of hypertension in middle-aged and older women. Journal of hypertension. 2008 Feb;26(2):215–222. doi: 10.1097/HJH.0b013e3282f283dc. [DOI] [PubMed] [Google Scholar]
- 5.Miura K. Relation of Vegetable, Fruit, and Meat Intake to 7-Year Blood Pressure Change in Middle-aged Men: The Chicago Western Electric Study. American journal of epidemiology. 2004;159(6):572–580. doi: 10.1093/aje/kwh085. [DOI] [PubMed] [Google Scholar]
- 6.Gillum RF, Mussolino ME, Madans JH. Fish consumption and hypertension incidence in African Americans and whites: the NHANES I Epidemiologic Follow-up Study. Journal of the National Medical Association. 2001 Apr;93(4):124–128. [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. American journal of hypertension. 2014 Jul;27(7):885–896. doi: 10.1093/ajh/hpu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. Journal of women's health / the official publication of the Society for the Advancement of Women's Health Research. 1997 Feb;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 9.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of Type 2 diabetes in younger and middle-aged women. Diabetologia. 2003 Nov;46(11):1465–1473. doi: 10.1007/s00125-003-1220-7. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. American journal of epidemiology. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology. 1992 May 15;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. American journal of epidemiology. 1986 May;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 13.Ascherio A, Rimm EB, Giovannucci EL, et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992 Nov;86(5):1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 14.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008 Nov;52(5):828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International journal of epidemiology. 1989 Dec;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. The American journal of clinical nutrition. 1999 Feb;69(2):243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology (Cambridge, Mass) 1990 Nov;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994 Oct;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 19.Lajous M, Bijon A, Fagherazzi G, Rossignol E, Boutron-Ruault MC, Clavel-Chapelon F. Processed and unprocessed red meat consumption and hypertension in women. The American journal of clinical nutrition. 2014 Sep;100(3):948–952. doi: 10.3945/ajcn.113.080598. [DOI] [PubMed] [Google Scholar]
- 20.Radack K, Deck C, Huster G. The effects of low doses of n-3 fatty acid supplementation on blood pressure in hypertensive subjects. A randomized controlled trial. Archives of internal medicine. 1991 Jun;151(6):1173–1180. [PubMed] [Google Scholar]
- 21.Bonaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromso study. The New England journal of medicine. 1990 Mar 22;322(12):795–801. doi: 10.1056/NEJM199003223221202. [DOI] [PubMed] [Google Scholar]
- 22.Tessier FJ, Birlouez-Aragon I. Health effects of dietary Maillard reaction products: the results of ICARE and other studies. Amino acids. 2012 Apr;42(4):1119–1131. doi: 10.1007/s00726-010-0776-z. [DOI] [PubMed] [Google Scholar]
- 23.Ames JM. Dietary Maillard Reaction Products: Implications for Human Health and Disease. Czech J Food Sci. 2009;27 [Google Scholar]
- 24.Thomson B. Heterocyclic amine levels in cooked meat and the implication for New Zealanders. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 1999 Jul;8(3):201–206. doi: 10.1097/00008469-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Puangsombat K, Gadgil P, Houser TA, Hunt MC, Smith JS. Occurrence of heterocyclic amines in cooked meat products. Meat science. 2012 Mar;90(3):739–746. doi: 10.1016/j.meatsci.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg T, Cai W, Peppa M, et al. Advanced glycoxidation end products in commonly consumed foods. Journal of the American Dietetic Association. 2004 Aug;104(8):1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 27.Maeda H, Sato K, Akaike T. Superoxide radical generation from heterocyclic amines. Princess Takamatsu symposia. 1995;23:103–112. [PubMed] [Google Scholar]
- 28.Baumann M. Role of advanced glycation end products in hypertension and cardiovascular risk: human studies. Journal of the American Society of Hypertension : JASH. 2012 Nov-Dec;6(6):427–435. doi: 10.1016/j.jash.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Farmer DG, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacology & therapeutics. 2009 Nov;124(2):185–194. doi: 10.1016/j.pharmthera.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Negrean M, Stirban A, Stratmann B, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. The American journal of clinical nutrition. 2007 May;85(5):1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 31.Ussher JR, Lopaschuk GD, Arduini A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis. 2013 Dec;231(2):456–461. doi: 10.1016/j.atherosclerosis.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine. 2013 May;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012 Sep 13;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 34.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. The Journal of clinical investigation. 2000 Apr;105(8):1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997 Mar 20;386(6622):292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011 Apr 7;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho S. CD36 as a therapeutic target for endothelial dysfunction in stroke. Current pharmaceutical design. 2012;18(25):3721–3730. doi: 10.2174/138161212802002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landmesser U, Drexler H. Endothelial function and hypertension. Current opinion in cardiology. 2007 Jul;22(4):316–320. doi: 10.1097/HCO.0b013e3281ca710d. [DOI] [PubMed] [Google Scholar]
- 39.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes care. 2008 Feb;31(Suppl 2):S170–180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The Immunological Basis of Hypertension. American journal of hypertension. 2014 Aug 23;27(11):1327–37. doi: 10.1093/ajh/hpu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen G, Scott Smith J. Determination of advanced glycation endproducts in cooked meat products. Food chemistry. 2015 Feb 1;168:190–195. doi: 10.1016/j.foodchem.2014.06.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


