Abstract
Recent technological advances in computed tomography (CT) technology have fulfilled the prerequisites for the cardiac application of dual-energy CT (DECT) imaging. By exploiting the unique characteristics of materials when exposed to two different x-ray energies, DECT holds great promise for the diagnosis and management of coronary artery disease. It allows for the assessment of myocardial perfusion to discern the hemodynamic significance of coronary disease and possesses high accuracy for the detection and characterization of coronary plaques, while facilitating reductions in radiation dose. As such, DECT enabled cardiac CT to advance beyond the mere detection of coronary stenosis expanding its role in the evaluation and management of coronary atherosclerosis.
Keywords: cardiac dual-energy CT, specifications of DECT, CT perfusion, plaque characterization, diagnostic accuracy, radiation dose aspects
Introduction
Coronary artery disease (CAD) is still the leading cause of death despite improvements in prevention and treatment strategies. As such, the accurate detection and diagnosis of CAD is of upmost importance. In symptomatic patients, the purpose of cardiac imaging is not only the identification of coronary atherosclerosis, but also more importantly, the depiction of myocardial ischemia.[1] Considering the role of noninvasive cardiac imaging as a gatekeeper to the catheterization laboratory,[2] ischemia rather than the mere presence of atherosclerosis should prompt referral to invasive procedures.[3] Although coronary computed tomography angiography (CCTA) is recognized as a powerful diagnostic imaging tool that is capable of ruling out obstructive disease with unequalled certainty,[4] due to its nature, conventional CCTA is unable to assess the pathophysiological consequences of a given coronary stenosis.[5,6] In comparison to conventional CT, the exploitation of two different photon energy levels by dual energy CT (DECT) holds great promise for improved tissue characterization and CT-based myocardial perfusion imaging (MPI),[7] thus overcoming the limitations of conventional cardiac CT. Rapid developments in CT hardware have fulfilled the prerequisites for the cardiac application of DECT imaging, broadening the horizons of cardiac CT and expanded its role in the evaluation and management of coronary atherosclerosis. The present review will provide an outline of the current status of cardiac DECT imaging.
Dual energy CT methods
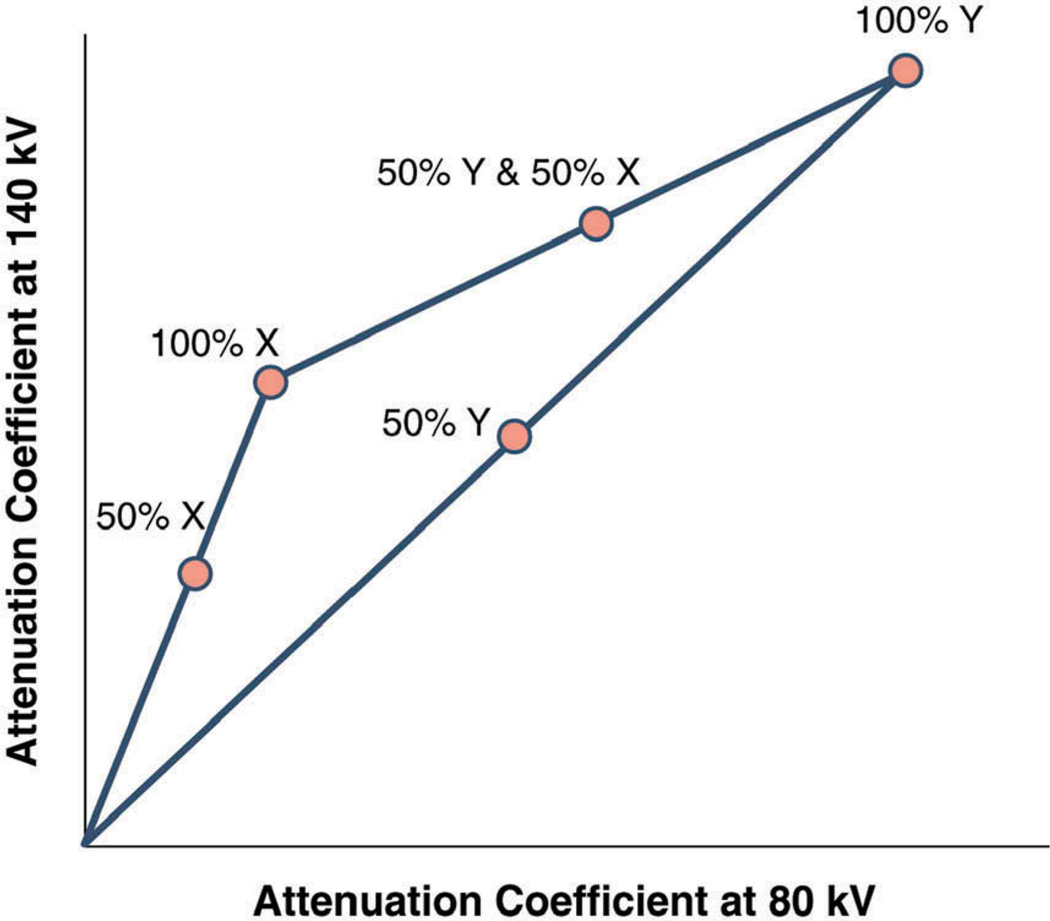
Conventional CT is performed at a fixed tube potential with polychromatic energy levels of photons set to 120 or 140 kVp, while the photon energy levels exploited by DECT are typically 80 and 140 kVp for the acquisition of low- and high-energy-dependent tissue attenuation profiles, respectively. As such, DECT allows for the acquisition of two image datasets of the same anatomical region of interest, providing information related to the change in energy-dependent attenuation of tissues when exposed to two different photon-energy levels (Figure 1), hence improving tissue characterization. Although the concept is similar, there are different manufacturer-specific approaches by which DECT images can be obtained. The exploitation of two polychromatic energy spectra by DECT can be achieved by at least three different methods: two x-ray source and detector pairs with each source operating at a different tube voltage; a single source-detector pair with an x-ray tube capable of rapidly switching between low and high tube potential or by switching tube potential between gantry positions; and an x-ray source operating at constant tube voltage with a double-layer detector capable of differentiating between low- and high-energy photons.
Figure 1. With dual-energy CT, unknown materials are reflected as a composition of basis materials X and Y allowing for improved tissue characterization compared to single-energy CT.
Reprinted with permission from the American College of Cardiology, Journal of the American College of Cardiology: Cardiovascular Imaging.[7]
DECT using two x-ray sources and two x-ray detectors
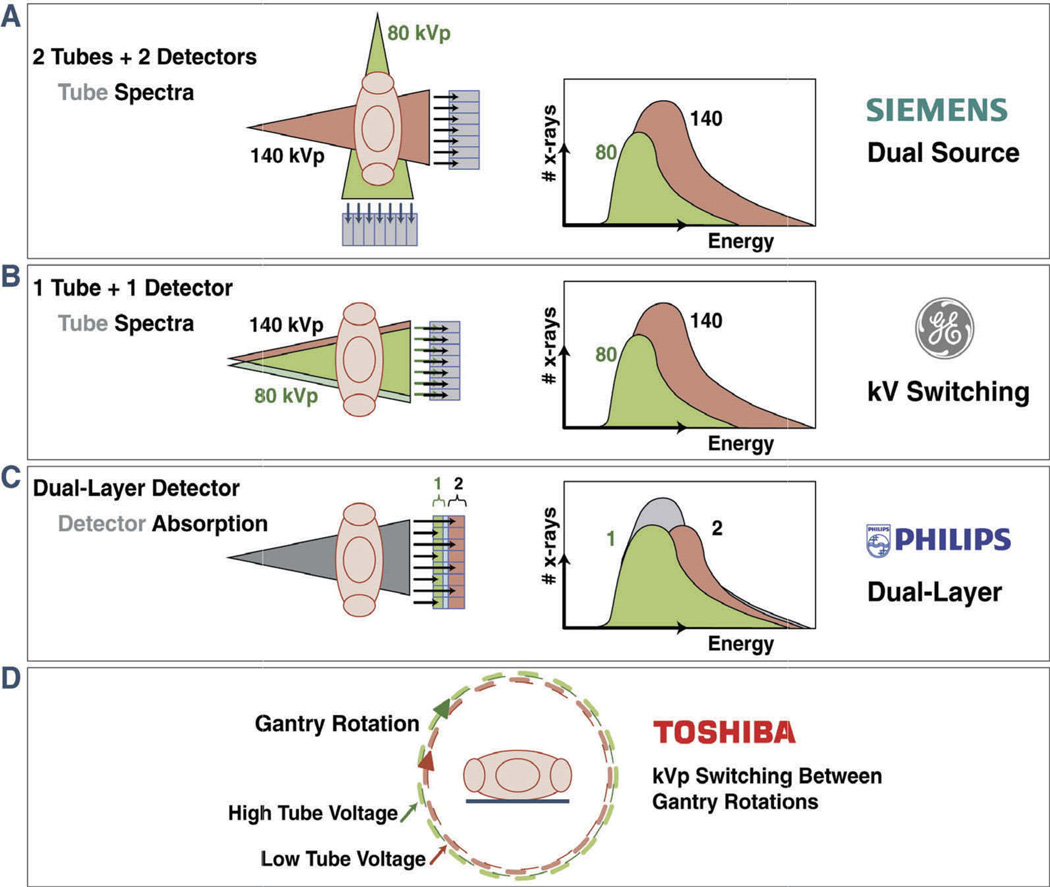
One approach for DECT acquisition is the use of a dual-source x-ray system with two corresponding detectors (Figure 2, Table 1). This system has been first implemented in the Siemens’ SOMATOM Definition Flash and now the Siemens SOMATOM Definition Force (Siemens Healthcare, Erlangen, Germany). The Siemens Definition is composed of two x-ray tube-detector pairs arranged at an angular offset of 94° on the same rotating gantry, with one tube operating at 80 kV or 100 kV and the other at 140 kV.[8–10] Due to the utilization of two sources and two detector arrays, the space in the gantry is insufficient to allow for the incorporation of two detector arrays of the same size. Therefore, the field of view for each detector is not similar and only one detector can cover the full available acquisition field of view (50 cm), while the other detector covers a smaller field of view (35 cm). However, for cardiac imaging, the smallest field of view that can be covered by one detector is sufficient for imaging the entire heart. A potential problem with this method is increased scatter radiation caused by crossing photons from the two rotating x-ray tubes (Table 2), which may result in a lower contrast-to-noise ratio as well as image artifacts. The latest dual-source CT systems measure and correct cross-scatter radiation by using specific detector elements. Moreover, movement of the scanned object (e.g., a beating heart) may impede accurate tissue characterizations due to a mismatch in projection views between low and high tube voltage projections. This may potentially lead to suboptimal correction of beam hardening artifacts. A limitation of dual-source CT is the use of two moderately broad energy spectra, restricting tissue and material discrimination.[11] However, this problem can be partly corrected for by filtering the high-energy beam.[12] An advantage of this system is the freely adjustable tube voltage. The tube current can be adjusted for each photon energy level separately (Table 2).
Figure 2. Schematic illustration of four different vendor-specific approaches for obtaining dual-energy Information.
(A) Dual source–detector pairs with each tube operating at a different voltage. Each x-ray source covers a different scan field. (B) Single source–detector pair with the source capable of rapid voltage switching (0.25 ms) in a single gantry rotation. (C) Single source–detector pair with a dual-layer detector made of two different materials capable of differentiating between low-energy (upper layer) and high-energy (bottom layer) photons, with the source operating at constant tube voltage allowing for dual-energy data during conventional CT scanning. (D) Single source–detector pair with tube voltage alternating between high and low kV with each gantry rotation. Reprinted with permission from the American College of Cardiology, Journal of the American College of Cardiology: Cardiovascular Imaging.[7] (Figures 2A, B, and C, Courtesy Philips Healthcare, Best, the Netherlands).
Table 1.
Specifications of the currently commercially available DECT devices.
| Gantry rotation time (ms) |
Temporal resolution (ms) |
Slice thickness (mm) |
Configuration | z-Axis coverage (mm) |
Dual-energy processing |
|
|---|---|---|---|---|---|---|
| Siemens Somatom Force | 250 | 66 | 0.6 | 2 · 2 · 96* × 0.60 | 57.6 | Image-space |
| GE Revolution | 280 | 140 | 0.625 | 256 × 0.625 | 160 | Projection-space |
| Philips IQon Spectral CT | 270 | 135 | 0.625 | 2 · 64* × 0.625 | 40 | Projection-space |
| Toshiba Aquilion One | 275 | 137.5 | 0.5 | 320 × 0.50 | 160 | Projection-space |
mm: millimeter; ms: milliseconds.
Flying focal spot in the z-axis doubles number of slices.
Table 2.
Characteristics of the current available dual-energy CT devices.
| Device | Advantages | Disadvantages |
|---|---|---|
| SOMATOM Definition Force (Siemens Healthcare, Erlangen, Germany) | Freely adjustable current in each tube enables optimal image quality. In single-energy mode, dual-source CT allows for high-pitch protocols and low radiation imaging by its high temporal resolution and unique geometry. Spectral separation can be improved by filtering. High temporal resolution allows for motion-free imaging and enables coronary imaging in patients with high heart rates (only in single-energy mode). |
Increased scatter radiation from the two rotating x-ray tubes. Possible mismatch in projection views between low and high tube voltage projections and therefore a suboptimal correction of beam hardening artifacts. When in dual-energy mode, imaging with regard to temporal registration is limited because the low- and high-energy datasets are obtained at slightly different time points. |
| GE Revolution (GE Healthcare, Waukesha, WI, USA) | Image acquisition of low- and high-kV-derived images is almost from the same angle. Theoretically a more accurate beam-hardening correction due to the use rapid kV-switching. |
Increased rotation time to account for acquisition of additional projections from the two tube voltages is especially challenging for fast moving organs such as the heart. Short time period between tube voltage switches impede adequate tube current adjustment, resulting in image noise. Improving spectral separation is not feasible with filtration. |
| IQon Spectral CT (Philips Healthcare, Best, The Netherlands) | Perfect alignment of low- and high-energy datasets, rendering images less prone to motion artifacts. Accurate beam-hardening correction. Provides conventional and dual-energy datasets with one single scan without adjustments of the clinical workflow. |
Less accurate separation of the different energy levels compared to the systems utilizing distinct x-ray tube voltages. Filtering cannot be applied to improve spectral separation. |
| Aquilion ONE (Toshiba America Medical Systems, Tustin, CA, USA) | Near to perfect alignment of subsequent low- and high-voltage images. Coverage of the whole heart in the diastole of one single heartbeat. |
Time difference between the two scans allows for cardiac motion to occur. Contrast material distribution may be different between sequential acquisitions, which may hamper cardiac imaging. Temporal registration is limited because the low and high datasets are obtained at two different times. |
DECT using single source x-ray and detectors with rapid switching of x-ray tube potential
A second approach for obtaining DECT images is rapid switching of tube potentials, which occurs as rapidly as 0.25 ms, based on single-source CT (Figure 2, Table 1).[13,14] This system has been incorporated by General Electric Healthcare in the Discovery CT750 HD and now GE Revolution (GE Healthcare, Waukesha, WI, USA).[15] Due to rapid switching (0.25 ms) of tube potentials, image acquisition of low- and high-kV-derived images is almost from the same angle, which allows a theoretically more accurate beam-hardening correction, rendering it less prone for potential motion artifacts (Table 2). However, a disadvantage of this technique is the increased rotation time. Gantry rotation time must be increased to account for rapid changes in tube voltage and to account for acquisition of additional projections from the two tube voltages. This is especially limiting for imaging of fast-moving organs such as the heart. Another drawback of this approach is the discrepancy in photon output between the two tube voltages, resulting in noisy images for the low tube voltage projections. In order to achieve similar photon output, the tube current should be increased during low tube voltage acquisitions. However, the short time period between tube voltage switches impede adequate tube current adjustment, resulting in image noise. Due to these limitations, application of radiation dose-reducing techniques such as tube current modulation is hampered (Table 2), resulting in relatively high radiation dose levels. In addition, since there is only one x-ray source, filtering is applied to both energy outputs. Consequently, improving spectral separation is not feasible with filtration (Table 2).
Single-source CT with energy-sensitive dual-layer detectors
Image reconstruction based on the detection of low- and high-kV-energy levels is another approach to obtain spectral data with a single x-ray tube. This technique has been implemented by Philips in its IQon Spectral CT (Philips Healthcare, Best, The Netherlands) and involves a detector-based approach capable of differentiating low- and high-kV-photon-energy levels with a single tube operating at a constant voltage (Figure 2, Table 1). Compared to other techniques, an energy-sensitive sandwich detector allows the simultaneous registration of low- and high-energy photons for each projection.[16,17] The principle of a dual-layer (sandwich) detector is based on a single detector composed of two different scintillating materials that differentiate between low- and high-energy photons. High-energy photons pass through the top layer and are detected by the bottom layer, whereas low-energy x-ray photons are attenuated and absorbed in the top layer.[16,17] A merit of this approach is that the acquired images are less prone to motion artifacts, since the differentiation and registration of the different x-ray energy spectra occur simultaneously for each view (Table 2). In addition, it allows material decomposition to be performed in the raw projection space domain. Furthermore, with the dual-layer detector, all information is obtained during a single scan obviating the need of two separate acquisitions. As a consequence, spectral data are always available without adjustment of scan protocols (Table 2). Furthermore, patients are not subjected to additional radiation exposure to obtain DECT datasets. A potential disadvantage, however, may be the less accurate separation of the different energy levels compared to the systems utilizing distinct x-ray tube voltages.[18] The energy overlap is larger than scanning with two different tube voltages, especially due to high-energy photons overlapping the low-energy spectrum (Table 2). Moreover, similar to the rapid-kVp-switching approach, filtering will not improve spectral separation.[12,19]
Single-source CT switching voltages between gantry rotations
The acquisition of DECT data with single-source-CT using fast tube voltage switching between gantry rotations has been implemented by Toshiba in its Aquilion ONE (Toshiba America Medical Systems, Tustin, CA, USA). The tube potential alternates between low and high for every gantry rotation (Figure 2, Table 1). The acquisition is performed with 80 and 135 kV using two gantry rotations (gantry rotation time is 275 ms) to obtain low- and high-energy datasets.[20] An advantage of this approach is perfect alignment of the subsequent images (Table 2), allowing material decomposition to be performed in the raw projection space domain. Another application of this technique is two sequential acquisitions at a low and a high tube voltage. The drawback of two sequential acquisitions, however, is the time difference between the two scans allowing for cardiac motion to occur (Table 2). Furthermore, contrast material distribution may be different between sequential acquisitions, which would be an important disadvantage for cardiac CT (Table 2).
DECT for the assessment of myocardial perfusion: static perfusion imaging
Similar to single-energy computed tomography (SECT), evaluation of myocardial perfusion by DECT is acquired by either a static or dynamic imaging approach. With regard to the static acquisition, a single snapshot of the myocardial iodine attenuation profile, which is obtained from the entire left ventricle over several heartbeats, allows for a qualitative visual estimate of myocardial perfusion. Compared with SECT, DECT may allow better tissue characterization and therefore enhanced visualization of myocardial perfusion defects, thus encouraging its use for ischemia assessment. By harnessing the unique features of DECT to allow for differentiation of iodine attenuation characteristics when it is exposed to low- and high-energy photon levels, DECT allows for the mapping of iodine distribution in the myocardium as a quantitative, albeit surrogate, marker for perfusion and blood volume based on the per-voxel amount of the iodine tracer.[21,22] Typically color-coded maps of iodine distribution are generated and superimposed on the virtual non-contrast CT data,[23] rendering the perfusion defects easier to appreciate by visual assessment. Indeed, a recent study by Koonce et al. using a phantom model demonstrated the (semi)quantitative evaluation of perfusion on static single-phase DECT imaging to exhibit a higher accuracy when compared to standard visual grading.[21] For the routine qualitative assessment, myocardial attenuation of the ischemic area is compared to that of a normal remote territory. A drawback of this approach, however, is the necessity of a normally perfused area to act as a reference standard; hence, balanced ischemia may go undetected when utilizing qualitative DECT imaging. Although there is a large body of evidence showing the clinical feasibility of a DECT myocardial perfusion protocol as a supplement to the anatomical evaluation of the coronary arteries by CCTA, the majority of these studies used a DECT device in single energy mode, disguising the potential improvement in image quality (i.e., increasing contrast conspicuity and reduction of beam hardening artifacts) by the exploitation of two energy datasets. However, static CT perfusion techniques are associated with some important limitations. Firstly, depending on the CT device and acquisition techniques, coverage of the entire left ventricle is obtained over multiple heartbeats resulting in a base to apex attenuation gradient. In addition, “snapshot” imaging possess the risk of mistiming the contrast bolus, [24] hence the peak of contrast attenuation may be missed, which may mask areas of hypoperfusion.
DECT for the assessment of myocardial perfusion: dynamic perfusion imaging
Dynamic CT MPI is the only approach allowing for a quantitative assessment of myocardial blood flow. Dynamic CT perfusion relies on time-resolved imaging of the passage of a perfusion tracer (i.e., iodine for CT-based perfusion) through the myocardium.[25] The creation of time-myocardial attenuation curves requires the coverage of the entire myocardium. A high spatial resolution is, therefore, an important prerequisite for dynamic CT perfusion imaging. In contrast to nuclear imaging techniques, ECG-gated acquisitions enable CT to reliably differentiate between sub-endocardial and subepicardial myocardial blood flow.[26] Currently, only dual-source devices meet the technical requirements (i.e., sufficiently high spatial and temporal resolution) for the DECT-based dynamic evaluation of myocardial perfusion. Third-generation dual-source systems have a higher spatial coverage of 105 mm allowing for whole-heart coverage within one cardiac cycle, whereas second-generation dual-source devices apply a “shuttle table mode” moving the table back and forward to allow for rapid coverage of the entire heart during first pass [27–30]. This will result in sufficient coverage of the heart during contraction when the length of the heart is shorter. An additional benefit of “systolic imaging” is the relatively constant length of the systolic phase, while at the same time the myocardial images are less prone to beam-hardening artifacts due to the lower amount of ventricular contrast. [31] Despite the benefits of CT-based dynamic imaging, it is associated with a substantial increase in radiation exposure compared to static imaging.
DECT for the assessment of myocardial perfusion: diagnostic performance of DECT myocardial perfusion imaging
The majority of investigations have compared DECT MPI against a background of rest-stress single-photon emission computed tomography (SPECT), cardiac magnetic resonance or invasive coronary angiography.[32–37] Figure 3 shows the clinical feasibility of DECT MPI for evaluation of myocardial perfusion in the diagnosis and management of CAD. Weininger et al. found stress-rest first-pass myocardial perfusion DECT to detect myocardial perfusion defects on cardiac magnetic resonance with a sensitivity and specificity of 93% and 99%, respectively.[27] When compared against cardiac magnetic resonance as a reference standard, Ko et al. [35] found that stress-rest first-pass myocardial perfusion DECT could detect reversible perfusion defects with sensitivity and specificity of 89% and 78%, respectively. A recently published study by Carrascosa et al. revealed DECT, albeit using monochromatic imaging, exhibited a higher accuracy compared to SECT MPI when refereed against a background of SPECT as reflected by a higher area under the curve of 0.90 vs. 0.80 (p < 0.001), respectively.[38] Notably, beam-hardening artifacts did not seem to reduce DECT’s diagnostic performance, whereas beam hardening resulted in a substantial increase of false-positive findings for the SECT acquisition.[38] When refereed against a background of invasive coronary angiography, first-pass myocardial perfusion DECT revealed the diagnosis of significant CAD with a sensitivity and specificity of 89% and 78%, respectively.[35]
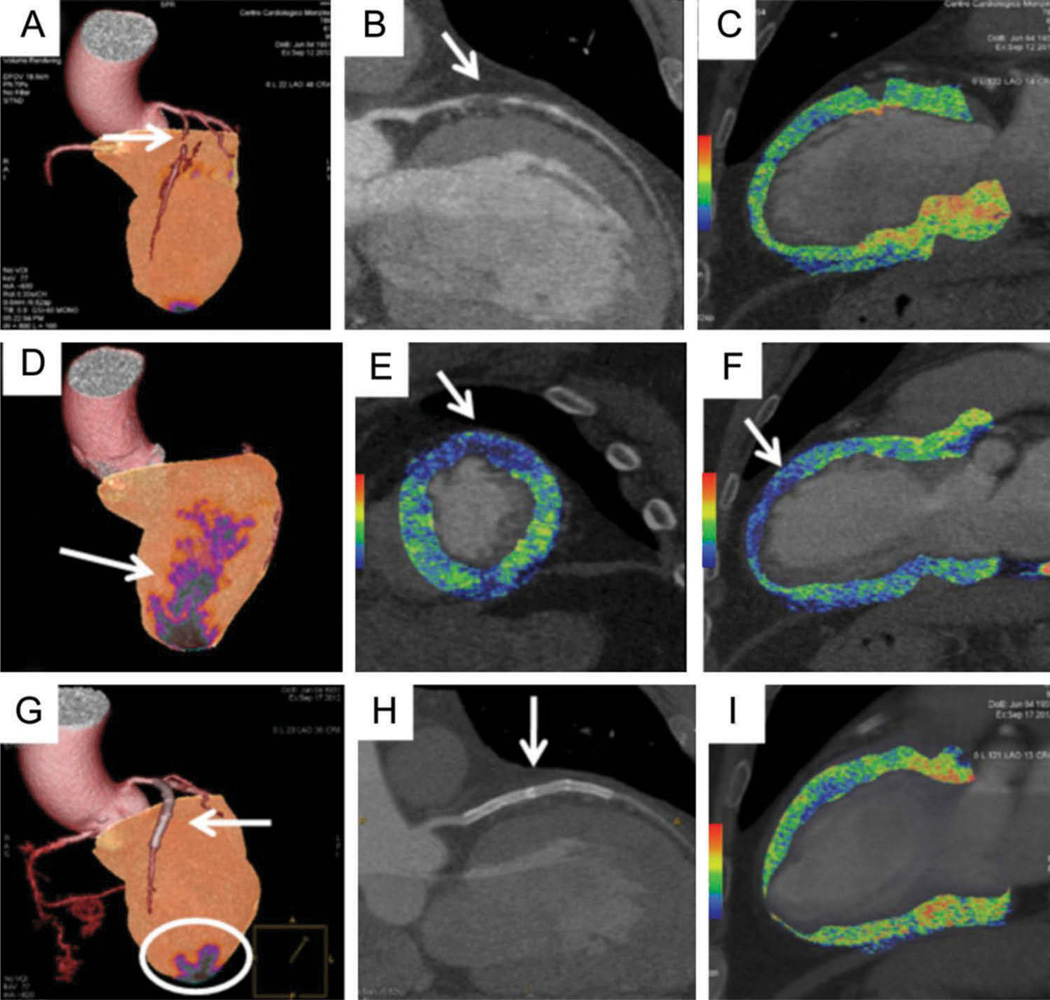
Figure 3. A 61-year-old man referred for chest pain and after an equivocal stress ECG. A rest-stress dual-energy CT (DECT) using 80–140 kVp switching was performed for the simultaneous evaluation of coronary anatomy and myocardial perfusion.
The exam showed a chronic total occlusion (CTO) of the left anterior descending (LAD) coronary artery due to a non-calcified plaque (A and B, arrows) without significant perfusion defect (A and C). After the administration of intravenous adenosine, a large perfusion defect of the anterior wall was seen during stress conditions, representing 18% of the left ventricular myocardium (D–F, arrows). The patient underwent CTO revascularization by percutaneous coronary intervention with the placement of three DES implantations. Three days later, the stress DECT study was repeated showing patency of the LAD stent (G and H, arrows) and restoration of myocardial blood flow with a reduction of the previous defect (1.8% of myocardial mass) (G, circle, and I). Reprinted with permission of Oxford University Press.[73]
DECT for the assessment of myocardial perfusion: DECT myocardial perfusion as an adjunct to coronary computed tomography angiography
It is projected that the combination of DECT perfusion with coronary CCTA will afford complementary information and augment diagnostic accuracy through improvements in specificity. To this end, several studies have documented the incremental diagnostic value of combining DECT MPI with coronary CTA when refereed against invasive angiography with SPECT and SPECT, respectively.[36,39] Ko et al. [39] demonstrated that the addition of DECT perfusion to coronary CTA resulted in improvements in sensitivity, specificity, negative predictive value and positive predictive value, as compared to invasive coronary angiography, from 91.8%, 67.7%, 87.7% and 73.6% to 93.2%, 85.5%, 91.4% and 88.3%, respectively. Whereas Wang et al. [32] observed no differences in sensitivity and negative predictive value (e.g., both remained at 100%), they did report that specificity significantly improved from 37.5% to 75.0% through the combination of DECT perfusion imaging with coronary CTA using invasive coronary angiography with a concomitant perfusion defect on SPECT as a reference standard. A recently published study confirmed the incremental value of DECT perfusion imaging as a useful adjunct to coronary CTA, which lowered the number of false-positive results on coronary CTA, as reflected by improvements in both specificity and positive predictive value (i.e. from 56% to 79% and from 55% to 71%, respectively). Subsequently, the hybrid approach improved accuracy considerably from 69% to 82%. [40] These results imply that MPI with DECT may reduce the number of false-positive results on coronary CTA, which seems fitting with the observations found for hybrid positron emission tomography and SPECT as well as coronary CTA. Despite the notion that combined physiologic-anatomic evaluation by DECT provided incremental diagnostic value, Wang et al. [32] observed a negligible effect of MPI with DECT when added to coronary CTA for measures of diagnostic accuracy, with a compromised increase in sensitivity from 82% to 90% at the loss of specificity (from 91% to 86%) for the detection of hemodynamic significant CAD as defined by a significant stenosis on ICA with a concomitant perfusion defect on SPECT. Correspondingly, a recently published pilot study documented that MPI with DECT improved the precision of coronary CTA alone, but the combination of these two tests resulted in lower performance compared with DECT perfusion imaging alone when refereed against SPECT.[41] Several important considerations of MPI with DECT have been discussed and need to be considered for the optimization of MPI protocols. Among the early studies of MPI with DECT, there has been some variability regarding when to perform the “rest” portion and “stress” portion, with many contending that vasodilator stress is important to perform first in order to reduce the likelihood of residual contrast that may confound perfusion defects. Others advocate for a rest-first protocol, thereby maintaining the significance of coronary artery evaluation by coronary CTA as the foremost information to be acquired from the study. With regard to the latter, rest DECT has been found to permit the detection of perfusion defects that are otherwise not visible on rest SPECT,[32,37] thus implying its use as a promising adjunct to traditional evaluation with coronary CTA. This finding may be due to a myriad of reasons, including the higher spatial resolution of CT, which may encourage the detection of subtle perfusion abnormalities that are not detectable on SPECT.[32,37,42] Nevertheless, a recent study by Ko et al. [40] reported stress MPI with DECT to convey higher accuracy for the detection of ischemia as compared with rest DECT perfusion imaging against a background of invasive coronary angiography with cardiac magnetic resonance imaging as a reference standard. However, in light of these early studies emphasizing the utility of DECT to provide complementary information on CAD, MPI with DECT is somewhat regarded as being in its infancy, with published studies to date limited by small sample sizes, referral bias and the lack of a suitable reference standard.
Coronary atherosclerotic plaque characterization by dual-energy CT
Current strategies for the diagnosis and treatment of CAD are essentially aimed at detecting obstructive coronary stenoses and subsequent relief of symptoms. However, such a strategy often fails to identify subjects at risk for developing an acute coronary syndrome (ACS). Defects to the surface of frequent non-significant coronary plaques may eventually lead to plaque rupture that provoke acute coronary thrombosis, which is the most important mechanism underlying the sudden onset of ACS. To this end, in recent decades, tremendous efforts have been made to identify ruptureprone “vulnerable plaques”. From prior invasive and pathologic evaluations, several anatomical characteristics have been implicated as crucial to the pathogenesis of ACSs, amongst others, thin cap fibroatheroma, micro calcifications and a necrotic lipid-rich core.[43–45] Given their importance, these plaque features have been extensively investigated by SECT, given the relative ease with which SECT can reliably separate calcified and non-calcified plaques. Yet, conventional CT faces a significant challenge in differentiating the various anatomical components of non-calcified plaques (e.g., lipid-rich vs. fibrous). Identification of different atherosclerotic plaque components is rather challenging and several studies showed considerable overlap in Hounsfield units between lipid-rich and fibrous-rich non-calcified plaques attributable to the spatial resolution of CT and a variable intra-plaque uptake of iodine contrast agents. [46–48] Owing to its capability of tissue decomposition, DECT may overcome these limitations and allow for improved plaque differentiation. However, prior studies have reported ambiguous results. CT-attenuation-based characterization of non-calcified plaques using DECT has been examined by an ex vivo study of 15 human arteries, which showed discriminatory improvement with DECT over conventional SECT.[49] In contrast, a recently published study by Obaid et al. among patients undergoing virtual histology-intravascular ultrasound (VH-IVUS) and CT, reported DECT to exhibit a similar sensitivity compared to SECT (45% vs. 39%, respectively) for necrotic core detection.[50] Considering the large overlap in Hounsfield Units between the different plaque types, DECT is to date limited in the accurate detection of necrotic core plaque components when compared to VH-IVUS. [51] For the detection of thin-cap fibroatheroma, one is still reliant on invasive coronary techniques. Moreover, when using post-mortem samples wherein image quality was not governed by body habitus or motion, DECT had a similar accuracy as SECT for the attenuation-based characterization of coronary plaques.[50,52] These observed mixed findings may be attributable to an array of issues, including scanning protocols as well as DECT image visualization. By exploiting DECT’s ability to perform material basis decompositions, plaque visualization will arguably be improved. However, the precise material basis pair and/or level of monochromatic energy required to achieve a benefit in plaque characterization remains to be established in forthcoming studies.
Radiation dose aspects of dual-energy CT: radiation dose of cardiac dual-energy CT
Despite the great value of CT, a major disadvantage is the inevitable exposure to ionizing radiation, which is considered an issue of great concern. In recent years, substantial reductions in radiation doses have been achieved with the implementation of ECG-guided tube modulation, prospective-ECG gated imaging (step-and-shoot mode), and low tube voltage imaging in selected populations. Due to its use of two photon energy levels to acquire one given anatomical area, questions have been raised as to whether the use of DECT comes at a cost of increased radiation dose compared to SECT. In an earlier small clinical study, Kerl et al. reported that dual-source CT in single-energy mode (4.54 ± 1.87 mSv) and DECT (9.8 ± 4.77) delivered less radiation than regular 16-slice multi-detector CCTA (12 ± 3.59 mSv) within a routine clinical setting comprising patients with low and stable heart rates.[53] Further still, in a clinical setting, Halliburton and colleagues compared dual-source and 32-slice CT with regard to radiation exposure, and reported no differentiation in radiation doses for coronary imaging between the two modalities.[54] In a prospective randomized trial among 102 patients, coronary angiography by DECT (rapid-kV-switching) was shown to deliver comparable dosage levels as contemporary CCTA (e.g., 2.31 vs. 2.23 mSv, respectively). [55] To date, no study has investigated the radiation dose generated by double-layer technology scanners for cardiac imaging – presumably these devices will produce radiation doses similar to single-energy CT, most likely owing to their use of one-time acquisitions to obtain spectral data. Notably, to allow sufficient separation of low- and high-energy photons, data are acquired at 140 kVp, which is a relatively high tube potential for most cardiac imaging studies. Arguably, lowering tube current will stabilize the effects of high tube voltage imaging without impacting spectral separation. However, clinical studies using dual-layer technology for coronary imaging are still warranted.
Radiation dose aspects of dual-energy CT: high-pitch scan protocols
Another potential approach to lessen radiation dose is by increasing pitch value. Dual-source CT devices have fulfilled the prerequisites for the implementation of high-pitch scan protocols up to pitch values of 3.0 and higher due to their unique geometry.[56–58] The dual-source detector pair permits the acquisition of one cross-sectional image by utilizing only one-quarter of the gantry rotation. Subsequently, the use of high-pitch values will avoid overlapping radiation exposure, allow for shorter scan times and, as a consequence, reduce effective radiation dose. High-pitch protocols can cover the entire heart in the diastolic phase of one cardiac cycle.[56] Clinical feasibility studies have shown promising results, reporting radiation dosages below 1 mSv for a coronary imaging study.[56–64] Noteworthy, the temporal resolution is a major bottleneck for the use of high-pitch protocols for the assessment of coronary anatomy. Due to the fact that the image data have to be acquired during the diastasis of one heartbeat, the temporal resolution is extremely crucial to ensure low radiation exposure and sufficient image quality. Provided the length of the heart is approximately 12 cm, second-generation dual-source devices (acquisition speed 458 mm/s and temporal resolution of 75 ms at a gantry rotation time of 282 ms) typically require 250 ms for its entire coverage. Therefore, the high-pitch scan mode is only confined to patients with a low heart (<65 bpm) and regular heart rate in order to match the required long image acquisition window.[56–61,63] With the introduction of third-generation dual-source CT devices, temporal resolution has improved to 66 ms and recently published pilot studies demonstrated the clinical feasibility of high-pitch coronary imaging protocols in patients with a heart rate up to 75 bpm with sub-mSv radiation dose, without jeopardizing image quality.[64–66] Of importance, the achievement of coronary imaging below 1 mSv is not only attributable to high-pitch protocols, but is rather a combination of multiple dose-saving strategies such as prospective ECG-triggering, low tube voltages and iterative image reconstructions. The application of low-tube voltage imaging comes at a cost of increased noise.[64,65,67] Iterative reconstruction techniques mitigate image noise derived from low-photon counting, while improving image quality in order to preserve images of sufficient diagnostic quality.[64–66] A recently published study by Hell et al. in 26 patients with low heart rates reported effective radiation doses to be 0.3 mSv, albeit in a selected population with body weights <100 kg, with low tube voltage imaging (70 kVp) using a third-generation dual-source device.[64] Similarly, a small-scaled clinical study by Schuhbaeck and colleagues managed to reduce effective radiation dose below 0.1 mSv without sacrificing image quality using a high-pitch spiral acquisition mode with low tube voltage (80 kVp) in conjunction with low tube voltage and iterative image reconstructions.[65] In addition, iterative reconstructions allow for a sufficiently high diagnostic CCTA quality, despite sub-mSv dose delivery to patients, yielding CCTA to exhibit a sensitivity and specificity of 95.7% and 94.1%, respectively, while using ICA as a reference standard.[68] However, the initial experience of this type of imaging is limited to centers with high expertise and is currently not widely available. Also, highly selected patient populations were investigated who do not represent the heterogeneity of patients seen in daily clinical practice. Arguably, the inclusion of non-obese patients with low and stable heart rates might have enhanced the results, and, therefore, large clinical studies are warranted to investigate the incremental value of iterative image reconstructions in clinical patients. Finally, it should be acknowledged that all high-pitch dual-source CT studies were performed in single-energy mode, disguising the advantages of dual-energy imaging with regard to the assessment of CAD. Nevertheless, by harnessing the advantages of dual-source CT devices combined with low voltage imaging and iterative image reconstructions, a coronary CT examination can be achieved with sub-mSv radiation doses.
Radiation dose aspects of dual-energy CT: virtual unenhanced imaging
Another way of reducing radiation exposure is through the generation of virtual unenhanced images (VUE) using post-imaging reconstructions that are unique to DECT. As such, VUE may substitute for real unenhanced images, thus reducing image acquisition time and effective radiation dose. [12,69] For example, a coronary artery calcium (CAC) scan may be derived from a CCTA can, obviating the need for a separate non-contrast CAC-scoring CT. A point of concern is whether the amount of coronary calcium based on VUE coincides with the true CAC score as determined on true unenhanced images considering the large overlap in Hounsfield units between these two materials. Pursuant to this, several studies demonstrated the feasibility of CAC-scoring on VUE by showing a good agreement of the CAC score derived from VUE with true non-contrast CAC-score scans.[70,71] Interestingly, Yamada et al. revealed that DECT coronary angiography using VUE for calcium scoring resulted in a substantial radiation dose reduction of 20% compared to conventional CCTA with a prior separate non-contrast CAC scan,[71] while in a recently published study, an average dose reduction of 51% was seen by replacing separate CAC-score scans by VUE imaging for quantification of CAC score.[72] Although promising, the technique is still in its infancy and further validation and more sophisticated correction algorithms are warranted to avoid underestimation of CAC burden by incorrect subtraction of calcium content mimicking iodine contrast agents.
Conclusions
Recent improvements in CT hardware have fulfilled the prerequisites for the clinical application of DECT imaging. By exploiting the attenuation profile of materials when exposed to two energy spectra, DECT enables the evaluation of both coronary anatomy and myocardial perfusion in one imaging session. As such, DECT may become a hybrid tool in its own right. To date, beam-hardening artifacts are hampering the diagnostic performance of DECT MPI. However, with the continuous technical developments, sophisticated correction algorithms will be developed, facilitating its clinical application. In addition, DECT possess the potential to improve coronary plaque characterizations and differentiate more accurately between plaque components. Altogether, DECT technology has broadened the horizons of cardiac CT; however, its cardiac application is still in its infancy and substantial barriers need to be overcome before DECT is fully embraced in clinical practice.
Expert commentary
The potential to evaluate coronary anatomy and myocardial perfusion during one single scan session has advanced cardiac CT beyond the mere detection of CAD. Static CT perfusion imaging by conventional CT also enables perfusion assessment; however, DECT allows for the mapping of iodine distribution providing a semi-quantitative measure of myocardial perfusion, which arguably improves detection of subtle perfusion defects. Although studies showed DECT to exhibit high accuracy for the detection of myocardial ischemia, studies using fractional flow reserve (FFR) as a reference standard are so far lacking. DECT perfusion imaging will be confined to the sideline of the clinical arena if its performance is not refereed by FFR. It is anticipated that the Dual Energy CT for Ischemia Determination Compared to “Gold Standard” Non-Invasive and Invasive Techniques (DECIDE-Gold) study will provide important evidence with regard to the accuracy of DECT MPI for the depiction of lesion-specific ischemia. Also, the characterization of coronary plaque composition by dual-energy technology appears to accurately fail to delineate necrotic core tissue. However, the full capabilities of DECT have not been exploited yet. In this regard, material basis pair images may allow for a more sophisticated lesion characterization. To date, the exact energy and/or material basis pair that optimizes plaque visualization has not been systematically evaluated. Future studies will be required to determine the exact material basis pair for plaque characterization by this emerging technology.
Five-year view
Dual-energy CT will be commonly used in clinical practice, since all modern CT devices have the ability to acquire dual-energy datasets. However, quantitative K-edge imaging, also referred to as spectral CT, will become commercially available and find clinical application. Every manufacturer has a spectral CT device in development and the clinical application of spectral CT will be a game-changer in the field of cardiology, providing an unprecedented noninvasive characterization of coronary plaque composition, resembling virtual histology. Although the use of iodinated contrast for K-edge imaging is challenging, new techniques integrating contrast injectors in the CT will overcome some of these limitations.
Key issues.
Dual-energy computed tomography (DECT) allows for the acquisition of two image datasets of the same anatomical region of interest, providing information related to the change in energy-dependent attenuation of tissues when exposed to two different photon-energy levels, hence improving tissue characterization.
DECT imaging can be achieved by at least three different methods: two x-ray source and detector pairs; rapid switching of tube potentials based on single-source CT or by switching tube potential between gantry positions; and one x-ray source operating at constant tube voltage with a double-layer detector.
DECT allows for the mapping of iodine distribution in the myocardium as a quantitative, albeit surrogate, marker for perfusion.
Myocardial perfusion imaging by DECT exhibits high diagnostic accuracy when refereed against single-photon emission computed tomography, magnetic resonance imaging and invasive coronary angiography.
Evaluation of myocardial perfusion by DECT reduced the rate of false-positive coronary computed tomography angiography scans, hence improving diagnostic accuracy.
By exploiting DECT’s ability to perform material basis decompositions, plaque visualization will arguably be improved enabling the accurate assessment of high-risk coronary plaque features.
DECT is not associated with increased radiation exposure to patients, but DECT rather facilitates further reductions in radiation dose due to its unique characteristics.
Acknowledgments
Financial & competing interests disclosure
This manuscript was funded, in part, by grants from the National Institutes of Health and the National Heart Lung and Blood Institute (R01 HL111141, R01 HL115150 and R01 HL118019), as well as from a generous gift from the Dalio Foundation. J. K. Min has served on the medical advisory boards of GE Healthcare, Arineta, Astra Zeneca and Bristol-Myers Squibb; on the Speakers Bureau of GE Healthcare; received research support from GE Healthcare, Vital Images and Phillips Healthcare; serves as a consultant to Astra Zeneca, Abbott Vascular, HeartFlow, NeoGraft Technologies, MyoKardia and CardioDx.
Footnotes
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Fihn SD, Blankenship JC, Alexander KP, et al. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. 2014;130(19):1749–1767. doi: 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 2.Marwick TH, Cho I, Ó Hartaigh B, et al. Finding the gatekeeper to the cardiac catheterization laboratory: coronary CT angiography or stress testing? J Am Coll Cardiol. 2015;65(25):2747–2756. doi: 10.1016/j.jacc.2015.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (fractional flow reserve versus angiography for multivessel evaluation) study. J Am Coll Cardiol. 2010;56(3):177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Jiang B, Wang J, Lv X, et al. Dual-source CT versus single-source 64-section CT angiography for coronary artery disease: A meta-analysis. Clin Radiol. 2014;69(8):861–869. doi: 10.1016/j.crad.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Chen WJ, Danad I, Raijmakers PG, et al. Effect of type 2 diabetes mellitus on epicardial adipose tissue volume and coronary vasomotor function. Am J Cardiol. 2014;113(1):90–97. doi: 10.1016/j.amjcard.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Gaemperli O, Schepis T, Valenta I, et al. Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology. 2008;248(2):414–423. doi: 10.1148/radiol.2482071307. [DOI] [PubMed] [Google Scholar]
- 7.Danad I, Fayad ZA, Willemink MJ, et al. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. JACC: Cardiovasc Imaging. 2015;8(6):710–723. doi: 10.1016/j.jcmg.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flohr TG, McCollough CH, Bruder H, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16(2):256–268. doi: 10.1007/s00330-005-2919-2. [DOI] [PubMed] [Google Scholar]
- 9.Arnoldi E, Lee YS, Ruzsics B, et al. CT detection of myocardial blood volume deficits: dual-energy CT compared with single-energy CT spectra. J Cardiovasc Comput Tomogr. 2011;5(6):421–429. doi: 10.1016/j.jcct.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Petersilka M, Bruder H, Krauss B, et al. Technical principles of dual source CT. Eur J Radiol. 2008;68(3):362–368. doi: 10.1016/j.ejrad.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Anderson NG, Butler AP. Clinical applications of spectral molecular imaging: potential and challenges. Contrast Media Mol Imaging. 2014;9(1):3–12. doi: 10.1002/cmmi.1550. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann S, Sauter A, Spira D, et al. Tin-filter enhanced dual-energy-CT: image quality and accuracy of CT numbers in virtual noncontrast imaging. Acad Radiol. 2013;20(5):596–603. doi: 10.1016/j.acra.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Vetter JR, Perman WH, Kalender WA, et al. Evaluation of a prototype dual-energy computed tomographic apparatus. II. Determination of vertebral bone mineral content. Med Phys. 1986;13(3):340–343. doi: 10.1118/1.595951. [DOI] [PubMed] [Google Scholar]
- 14.Kalender WA, Perman WH, Vetter JR, et al. Evaluation of a prototype dual-energy computed tomographic apparatus. I. Phantom studies. Med Phys. 1986;13(3):334–339. doi: 10.1118/1.595958. [DOI] [PubMed] [Google Scholar]
- 15.So A, Hsieh J, Imai Y, et al. Prospectively ECG-triggered rapid kV-switching dual-energy CT for quantitative imaging of myocardial perfusion. JACC: Cardiovasc Imaging. 2012;5(8):829–836. doi: 10.1016/j.jcmg.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Roessl E, Herrmann C, Kraft E, et al. A comparative study of a dual-energy-like imaging technique based on counting-integrating readout. Med Phys. 2011;38(12):6416–6428. doi: 10.1118/1.3651643. [DOI] [PubMed] [Google Scholar]
- 17.Gabbai M, Leichter I, Mahgerefteh S, et al. Spectral material characterization with dual-energy CT: comparison of commercial and investigative technologies in phantoms. Acta Radiol. 2014;56(8):960–969. doi: 10.1177/0284185114545150. [DOI] [PubMed] [Google Scholar]
- 18.Bornefalk H, Danielsson M. Photon-counting spectral computed tomography using silicon strip detectors: a feasibility study. Phys Med Biol. 2010;55(7):1999–2022. doi: 10.1088/0031-9155/55/7/014. [DOI] [PubMed] [Google Scholar]
- 19.Saito M. Optimized low-kV spectrum of dual-energy CT equipped with high-kV tin filtration for electron density measurements. Med Phys. 2011;38(6):2850–2858. doi: 10.1118/1.3584200. [DOI] [PubMed] [Google Scholar]
- 20.Rossi A, Merkus D, Klotz E, et al. Stress myocardial perfusion: imaging with multidetector CT. Radiology. 2014;270(1):25–46. doi: 10.1148/radiol.13112739. [DOI] [PubMed] [Google Scholar]
- 21.Koonce JD, Vliegenthart R, Schoepf UJ, et al. Accuracy of dual-energy computed tomography for the measurement of iodine concentration using cardiac CT protocols: validation in a phantom model. Eur Radiol. 2014;24(2):512–518. doi: 10.1007/s00330-013-3040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So A, Hsieh J, Narayanan S, et al. Dual-energy CT and its potential use for quantitative myocardial CT perfusion. J Cardiovasc Comput Tomogr. 2012;6(5):308–317. doi: 10.1016/j.jcct.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Kang DK, Schoepf UJ, Bastarrika G, et al. Dual-energy computed tomography for integrative imaging of coronary artery disease: principles and clinical applications. Semin Ultrasound CT MR. 2010;31(4):276–291. doi: 10.1053/j.sult.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Otton J, Morton G, Schuster A, et al. A direct comparison of the sensitivity of CT and MR cardiac perfusion using a myocardial perfusion phantom. J Cardiovasc Comput Tomogr. 2013;7(2):117–124. doi: 10.1016/j.jcct.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bamberg F, Klotz E, Flohr T, et al. Dynamic myocardial stress perfusion imaging using fast dual-source CT with alternating table positions: initial experience. Eur Radiol. 2010;20(5):1168–1173. doi: 10.1007/s00330-010-1715-9. [DOI] [PubMed] [Google Scholar]
- 26.George RT, Arbab-Zadeh A, Miller JM, et al. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ Cardiovasc Imaging. 2012;5(3):333–340. doi: 10.1161/CIRCIMAGING.111.969303. [DOI] [PubMed] [Google Scholar]
- 27.Weininger M, Schoepf UJ, Ramachandra A, et al. Adenosine-stress dynamic real-time myocardial perfusion CT and adenosine-stress first-pass dual-energy myocardial perfusion CT for the assessment of acute chest pain: initial results. Eur J Radiol. 2012;81(12):3703–3710. doi: 10.1016/j.ejrad.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Bastarrika G, Ramos-Duran L, Rosenblum MA, et al. Adenosine-stress dynamic myocardial CT perfusion imaging: initial clinical experience. Invest Radiol. 2010;45(6):306–313. doi: 10.1097/RLI.0b013e3181dfa2f2. [DOI] [PubMed] [Google Scholar]
- 29.Ko BS, Cameron JD, Defrance T, et al. CT stress myocardial perfusion imaging using multidetector CT–A review. J Cardiovasc Comput Tomogr. 2011;5(6):345–356. doi: 10.1016/j.jcct.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Bamberg F, Hinkel R, Schwarz F, et al. Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Invest Radiol. 2012;47(1):71–77. doi: 10.1097/RLI.0b013e31823fd42b. [DOI] [PubMed] [Google Scholar]
- 31.Mahnken AH, Klotz E, Pietsch H, et al. Quantitative whole heart stress perfusion CT imaging as noninvasive assessment of hemodynamics in coronary artery stenosis: preliminary animal experience. Invest Radiol. 2010;45(6):298–305. doi: 10.1097/RLI.0b013e3181dfa3cf. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Yu W, Wang Y, et al. Incremental value of dual-energy CT to coronary CT angiography for the detection of significant coronary stenosis: comparison with quantitative coronary angiography and single photon emission computed tomography. Int J Cardiovasc Imaging. 2011;27(5):647–656. doi: 10.1007/s10554-011-9881-7. [DOI] [PubMed] [Google Scholar]
- 33.Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009;54(12):1072–1084. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Meyer M, Nance JW, Jr, Schoepf UJ, et al. Cost-effectiveness of substituting dual-energy CT for SPECT in the assessment of myocardial perfusion for the workup of coronary artery disease. Eur J Radiol. 2012;81(12):3719–3725. doi: 10.1016/j.ejrad.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 35.Ko SM, Choi JW, Song MG, et al. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur Radiol. 2011;21(1):26–35. doi: 10.1007/s00330-010-1897-1. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Qin L, Shi X, et al. Adenosine-stress dynamic myocardial perfusion imaging with second-generation dual-source CT: comparison with conventional catheter coronary angiography and SPECT nuclear myocardial perfusion imaging. AJR. 2012;198(3):521–529. doi: 10.2214/AJR.11.7830. [DOI] [PubMed] [Google Scholar]
- 37.Ruzsics B, Schwarz F, Schoepf UJ, et al. Comparison of dual-energy computed tomography of the heart with single photon emission computed tomography for assessment of coronary artery stenosis and of the myocardial blood supply. Am J Cardiol. 2009;104(3):318–326. doi: 10.1016/j.amjcard.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 38.Carrascosa PM, Cury RC, Deviggiano A, et al. Comparison of myocardial perfusion evaluation with single versus dual-energy CT and effect of beam-hardening artifacts. Acad Radiol. 2015;22(5):591–599. doi: 10.1016/j.acra.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Ko SM, Choi JW, Hwang HK, et al. Diagnostic performance of combined noninvasive anatomic and functional assessment with dual-source CT and adenosine-induced stress dual-energy CT for detection of significant coronary stenosis. AJR. 2012;198(3):512–520. doi: 10.2214/AJR.11.7029. [DOI] [PubMed] [Google Scholar]
- 40.Ko SM, Park JH, Hwang HK, et al. Direct comparison of stress- and rest-dual-energy computed tomography for detection of myocardial perfusion defect. Int J Cardiovasc Imaging. 2014;30(Suppl 1):41–53. doi: 10.1007/s10554-014-0410-3. [DOI] [PubMed] [Google Scholar]
- 41.Carrascosa PM, Deviggiano A, Capunay C, et al. Incremental value of myocardial perfusion over coronary angiography by spectral computed tomography in patients with intermediate to high likelihood of coronary artery disease. Eur J Radiol. 2015;84(4):637–642. doi: 10.1016/j.ejrad.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Meinel FG, De Cecco CN, Schoepf UJ, et al. First-arterial-pass dual-energy CT for assessment of myocardial blood supply: do we need rest, stress, and delayed acquisition? Comparison with SPECT. Radiology. 2014;270(3):708–716. doi: 10.1148/radiol.13131183. [DOI] [PubMed] [Google Scholar]
- 43.Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30(7):1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 44.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 45.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 46.Petranovic M, Soni A, Bezzera H, et al. Assessment of nonstenotic coronary lesions by 64-slice multidetector computed tomography in comparison to intravascular ultrasound: evaluation of nonculprit coronary lesions. J Cardiovasc Comput Tomogr. 2009;3(1):24–31. doi: 10.1016/j.jcct.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43(7):1241–1247. doi: 10.1016/j.jacc.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 48.Pohle K, Achenbach S, Macneill B, et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS. Atherosclerosis. 2007;190(1):174–180. doi: 10.1016/j.atherosclerosis.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Tanami Y, Ikeda E, Jinzaki M, et al. Computed tomographic attenuation value of coronary atherosclerotic plaques with different tube voltage: an ex vivo study. J Comput Assist Tomogr. 2010;34(1):58–63. doi: 10.1097/RCT.0b013e3181b66c41. [DOI] [PubMed] [Google Scholar]
- 50.Obaid DR, Calvert PA, Gopalan D, et al. Dual-energy computed tomography imaging to determine atherosclerotic plaque composition: a prospective study with tissue validation. J Cardiovasc Comput Tomogr. 2014;8(3):230–237. doi: 10.1016/j.jcct.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obaid DR, Calvert PA, Gopalan D, et al. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6(5):655–664. doi: 10.1161/CIRCIMAGING.112.000250. [DOI] [PubMed] [Google Scholar]
- 52.Henzler T, Porubsky S, Kayed H, et al. Attenuation-based characterization of coronary atherosclerotic plaque: comparison of dual source and dual energy CT with single-source CT and histopathology. Eur J Radiol. 2011;80(1):54–59. doi: 10.1016/j.ejrad.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Kerl JM, Bauer RW, Maurer TB, et al. Dose levels at coronary CT angiography–a comparison of Dual Energy-, Dual Source- and 16-slice CT. Eur Radiol. 2011;21(3):530–537. doi: 10.1007/s00330-010-1954-9. [DOI] [PubMed] [Google Scholar]
- 54.Halliburton SS, Sola S, Kuzmiak SA, et al. Effect of dual-source cardiac computed tomography on patient radiation dose in a clinical setting: comparison to single-source imaging. J Cardiovasc Comput Tomogr. 2008;2(6):392–400. doi: 10.1016/j.jcct.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Raju R, Thompson AG, Lee K, et al. Reduced iodine load with CT coronary angiography using dual-energy imaging: a prospective randomized trial compared with standard coronary CT angiography. J Cardiovasc Comput Tomogr. 2014;8(4):282–288. doi: 10.1016/j.jcct.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Achenbach S, Marwan M, Schepis T, et al. High-pitch spiral acquisition: a new scan mode for coronary CT angiography. J Cardiovasc Comput Tomogr. 2009;3(2):117–121. doi: 10.1016/j.jcct.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Lell M, Marwan M, Schepis T, et al. Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: technique and initial experience. Eur Radiol. 2009;19(11):2576–2583. doi: 10.1007/s00330-009-1558-4. [DOI] [PubMed] [Google Scholar]
- 58.Achenbach S, Goroll T, Seltmann M, et al. Detection of coronary artery stenoses by low-dose, prospectively ECG-triggered, high-pitch spiral coronary CT angiography. JACC: Cardiovasc Imaging. 2011;4(4):328–337. doi: 10.1016/j.jcmg.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31(3):340–346. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]
- 60.Alkadhi H, Stolzmann P, Desbiolles L, et al. Low-dose, 128-slice, dual-source CT coronary angiography: accuracy and radiation dose of the high-pitch and the step-and-shoot mode. Heart. 2010;96(12):933–938. doi: 10.1136/hrt.2009.189100. [DOI] [PubMed] [Google Scholar]
- 61.Gordic S, Husarik DB, Desbiolles L, et al. High-pitch coronary CT angiography with third generation dual-source CT: limits of heart rate. Int J Cardiovasc Imaging. 2014;30(6):1173–1179. doi: 10.1007/s10554-014-0445-5. [DOI] [PubMed] [Google Scholar]
- 62.Morsbach F, Gordic S, Desbiolles L, et al. Performance of turbo high-pitch dual-source CT for coronary CT angiography: first ex vivo and patient experience. Eur Radiol. 2014;24(8):1889–1895. doi: 10.1007/s00330-014-3209-7. [DOI] [PubMed] [Google Scholar]
- 63.Leschka S, Stolzmann P, Desbiolles L, et al. Diagnostic accuracy of high-pitch dual-source CT for the assessment of coronary stenoses: first experience. Eur Radiol. 2009;19(12):2896–2903. doi: 10.1007/s00330-009-1618-9. [DOI] [PubMed] [Google Scholar]
- 64.Hell MM, Bittner D, Schuhbaeck A, et al. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: feasibility, image quality, radiation dose, and effect of iterative reconstruction. J Cardiovasc Comput Tomogr. 2014;8(6):418–425. doi: 10.1016/j.jcct.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Schuhbaeck A, Achenbach S, Layritz C, et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur Radiol. 2013;23(3):597–606. doi: 10.1007/s00330-012-2656-2. [DOI] [PubMed] [Google Scholar]
- 66.Yin WH, Lu B, Li N, et al. Iterative reconstruction to preserve image quality and diagnostic accuracy at reduced radiation dose in coronary CT angiography: an intraindividual comparison. JACC: Cardiovasc Imaging. 2013;6(12):1239–1249. doi: 10.1016/j.jcmg.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Menke J, Kowalski J. Diagnostic accuracy and utility of coronary CT angiography with consideration of unevaluable results: a systematic review and multivariate Bayesian random-effects meta-analysis with intention to diagnose. Eur Radiol. 2015 doi: 10.1007/s00330-015-3831-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Yin WH, Lu B, Hou ZH, et al. Detection of coronary artery stenosis with sub-milliSievert radiation dose by prospectively ECG-triggered high-pitch spiral CT angiography and iterative reconstruction. Eur Radiol. 2013;23(11):2927–2933. doi: 10.1007/s00330-013-2920-0. [DOI] [PubMed] [Google Scholar]
- 69.Numburi UD, Schoenhagen P, Flamm SD, et al. Feasibility of dual-energy CT in the arterial phase: imaging after endovascular aortic repair. AJR. 2010;195(2):486–493. doi: 10.2214/AJR.09.3872. [DOI] [PubMed] [Google Scholar]
- 70.Schwarz F, Nance JW, Jr, Ruzsics B, et al. Quantification of coronary artery calcium on the basis of dual-energy coronary CT angiography. Radiology. 2012;264(3):700–707. doi: 10.1148/radiol.12112455. [DOI] [PubMed] [Google Scholar]
- 71.Yamada Y, Jinzaki M, Okamura T, et al. Feasibility of coronary artery calcium scoring on virtual unenhanced images derived from single-source fast kVp-switching dual-energy coronary CT angiography. J Cardiovasc Comput Tomogr. 2014;8(5):391–400. doi: 10.1016/j.jcct.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Fuchs TA, Stehli J, Dougoud S, et al. Coronary artery calcium quantification from contrast enhanced CT using gemstone spectral imaging and material decomposition. Int J Cardiovasc Imaging. 2014;30(7):1399–1405. doi: 10.1007/s10554-014-0474-0. [DOI] [PubMed] [Google Scholar]
- 73.Pontone G, Grancini L, Andreini D, et al. Myocardial perfusion imaging using dual-energy computed tomography: a clinical case. Eur Heart J Cardiovasc Imaging. 2013;14(8):835. doi: 10.1093/ehjci/jet034. [DOI] [PubMed] [Google Scholar]