Abstract
We have shown previously that treatment of human aortic endothelial cells (HAECs) with minimally modified low-density lipoprotein (MM-LDL) induces monocyte but not neutrophil binding. This monocyte binding was not mediated by endothelial E-selectin, P-selectin, vascular cell adhesion molecule-I, or intercellular adhesion molecule-I, suggesting an alternative monocyte-specific adhesion molecule. We now show that moncytic α4β1 integrins mediate binding to MM-LDL-treated endothelial cells. We present data suggesting that the expression of the connecting segment-1 (CS-1) domain of fibronectin (FN) is induced on the apical surface of HAEC by MM-LDL and is the endothelial α4β1 ligand in MM-LDL-treated cells. Although the levels of CS-1 mRNA and protein were not increased, we show that MM-LDL treatment causes deposition of FN on the apical surface by activation of β1integrins, particularly those associated with α5 integrins.Activation of β1 by antibody 8A2 also induced CS-1-mediated monocyte binding. Confocal microscopy demonstrated the activated β1 and CS-1colocalize in concentrated filamentous patches on the apical surface of HAEC. Both anti-CS-1 and an antibody to activated β1 showed increased staining on the luminal endothelium of human coronary lesions with active monocyte entry. These results suggest the importance of these integrin ligand interactions in human atherosclerosis.
J. Clin. Invest. 103:613–625 (1999)
Introduction
There is considerable evidence that oxidized lipoproteins play a role in the development of atherosclerosis. Early studies showed oxidized lipoproteins to be present in atherosclerotic lesions (1–3). Studies on hyperlipidemic animals demonstrated that oxidation products were increased in lesions (4). In addition, antioxidants such as probucol and butylated hydroxytoluene have been found to reduce the development and severity of lesions in a number of animal models (5, 6).
Mononuclear cell adhesion to vascular endothelium was observed in the initial steps of fatty streak formation (7, 8). Recent studies (9, 10) using mice null for monocyte chemotactic protein-1 (MCP-1) and the MCP-1 receptor have demonstrated the important role of monocytes in lesion development. Our group has used minimally modified low-density lipoprotein (MM-LDL)–stimulated aortic endothelial cells to model the development of the fatty streak (11). We have demonstrated that monocyte, but not neutrophil, adhesion and transmigration across the aortic endothelium is increased in response to MM-LDL (11). Active oxidized phospholipids isolated from MM-LDL were found to be increased in rabbit atherosclerotic lesions (12).
Previous studies from our group have identified MM-LDL–induced molecules involved in several phases of monocyte/endothelial interactions. Previous work (13, 14) suggests that in vivo entry of leukocytes into the vessel wall involves at least three steps; rolling, activation, and firm adhesion to the endothelium. The rolling step has been shown to involve the interaction of selectins on the endothelium, with their ligands on leukocytes. Studies from our group and others (15–18) suggest that P-selectin is an important rolling molecule for monocytes in atherosclerosis. Using in vitro studies, we have shown that levels of P-selectin in human aortic endothelial cells (HAEC) are increased by MM-LDL (18), whereas levels of E-selectin are decreased (19). We and others have also shown that highly oxidized low-density lipoprotein (LDL) leads to P-selectin release to the upper cell surface (18, 20). Specific cytokines and chemokines that activate monocyte adhesion ligands have been found in lesions (21–23). Prior studies (24–26) have shown that these same cytokines and chemokines are increased in vitro by treatment of endothelial cells with MM-LDL.
Upon activation, leukocytes tightly adhere, via integrin-dependent mechanisms, to various endothelial ligands (27, 28). The molecules that are involved in firm adhesion of monocytes to the endothelial cell surface in the development of atherosclerosis have not been fully identified and are the focus of the current study. The major known mononuclear-specific integrins involved in firm adhesion were α4β1 (very late antigen-4, VLA4 and α4β7; both of these integrins have been shown to bind vascular cell adhesion molecule-1 (VCAM-1; refs. 29–31). In mice and rabbits fed a high-fat diet, VCAM-1 is increased on luminal endothelium (32, 33). O’Brien et al. (34) reported that VCAM-1 was present in human neovasculature and nonendothelial cells associated with increased intimal leukocyte accumulation. In the animal studies, however, a significant number of VCAM-1–positive regions did not contain monocytes (35). In the human studies, there was no significant correlation between the expression of VCAM-1 on the luminal endothelium and areas corresponding with monocyte entry (34); and in the study by Duplaa et al. (36) in which monocyte entry was also examined, only a small number of specimens were positive for VCAM-1. Furthermore, MM-LDL, while strongly stimulating monocyte binding, was not found to increase VCAM-1 expression in human umbilical vein endothelial cells (HUVEC; ref. 37). Taken together, these studies suggested that a different endothelial ligand might play an important role in the firm adhesion of monocytes to MM-LDL–treated human endothelial cells.
It has been reported that VLA-4 on monocytes and lymphocytes can interact with a 25–amino acid sequence called connecting segment-1 (CS-1), present in an alternatively spliced form of fibronectin (FN; refs. 38–42). FN is a large extracellular matrix molecule containing three alternatively spliced regions: EIIIA, EIIIB, and the V region, where CS-1 is located (38, 39). FN can exist as a soluble form found in the plasma or in a cell-associated matrix (43, 44). Antibody inhibition studies have suggested that the interaction of extracellular FN with intracellular integrins is important for FN matrix assembly (45). Previous studies (43, 44) have reported that plasma FN does not contain either the EIIIA or EIIIB regions, whereas cellular FN can have differing amounts of these two regions. Approximately 50% of plasma FN contains the V regions, whereas almost all cellular FN contains this region (46). Thus, cellular FN is enriched in the CS-1 regions. Studies presented here demonstrate a role for a CS-1/VLA-4 interaction in monocyte binding induced by MM-LDL and demonstrate that MM-LDL increases apical CS-1 FN expression by activation of the β1 integrin, most likely associated with α5 (VLA-5), on the surface of endothelial cells.
Methods
Characterization of the reactivities of the FN antibodies used for these studies.
Because the studies presented here use FN and CS-1 antibodies for different experimental procedures, these antibodies were compared to determine the characteristics of each antibody. Four different antibodies against FN were used for these studies: (a) IgM monoclonal antibody against the CS-1 region of FN (CS-1 90.45, referred to as 90.45; Cytel Corp., San Diego, California, USA; refs. 47, 48); (b) 7E5, a second CS-1 IgM monoclonal antibody that has the same reactivity to peptides and FN as the previously reported CS-1 polyclonal antibody (Ugarova, T., personal communication; ref. 49); (c) monoclonal FN antibody (specific for a cell-binding region on the eighth type III repeat; catalog no. MAB1937; Chemicon International, Temecula, California, USA; ref. 50); and (d) polyclonal rabbit anti–human FN (IgG) antibody (catalog no. 341640; Calbiochem-Novabiochem Corp., San Diego, California, USA). Western blotting was performed testing the recognition of plasma (catalog no. FC010; Chemicon International) and endothelial cell FNs by these antibodies. For these studies, endothelial cells were solubilized with a combination of detergents and protease inhibitors (25 mM Tris-HCl [pH 8.0], 0.15 M NaCl, 1% Triton X-100, 0.5% NaDOC, 5 mM EDTA, 0.1% SDS, 1 mM PMSF, 10 μg/ml aprotinin, 1.5 μg/ml pepstatin A, and 10 μg/ml leupeptin) for 1 h at 4°C. Extracts were diluted into loading buffer (50 mM Tris-HCl [pH 6.8], 100 mM DTT, 2% SDS, 0.1% bromophenol blue, and 10% glycerol) and boiled for 5 min before being separated on a 7.5% SDS-PAGE; then the proteins were transferred onto nitrocellulose membranes. Membranes were incubated with the antibodies at a concentration of 1–2 μg/ml. Lower antibody concentrations and shorter exposure times were required for the polyclonal FN and monoclonal antibodies to the cell-binding region of FN (1 h), compared with those required for the monoclonal antibodies to CS-1 FN (overnight). Blots were developed using secondary antibodies conjugated to horseradish peroxidase (HRP) for viewing with enhanced chemiluminescence (ECL; Amersham Life Sciences Inc., Arlington Heights, Illinois, USA).
Determination of CS-1 FN mRNA levels.
A lysate RNase assay kit (United States Biochemical, Cleveland, Ohio, USA) was used to determine the message levels of HAEC either unstimulated or stimulated with 250 μg/ml MM-LDL for 4 h at37°C. Cells were centrifuged, the supernatant was removed, and the cell pellets were dissolved in guanidine thiocyanate lysis solution (107 cells/ml). 32P-labeled RNA probes complementary to FN cDNA regions 2619–2978 (HepII; 350 nucleotides) and 3081–3154 (CS-1; 74 nucleotides) (47) were used for the RNase protection analysis along with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control probe that was provided with the kit. The labeled probes were diluted in lysis solution to 105–106 cpm/5 μl. Hybridization of a single probe to control cell lysate or a combination of probes either to control or to MM-LDL–treated cell lysates was performed at 50°C overnight. The reaction solution was incubated with RNase at 37°C for 30 min followed by incubation with protease for 30 min at 37°C. Isopropanol was added and samples were centrifuged for 10 min. Resulting pellets were air dried for 20 min before resuspending each sample into 10 μl gel buffer. The samples were heated to 95°C for 5 min and then put onto ice before being loaded on a 4%–6% polyacrylamide/7 M urea gel. Once the bromophenol blue dye marker had migrated to the bottom of the gel, it was removed and exposed to Kodak film(Eastman Kodak Co., Rochester, New York, USA) for 24 h.
Testing of agents that block monocyte/endothelial cell interactions in vitro.
The basic methods for isolation and culture of HAEC and preparation of MM-LDL have been described previously (37). For all of the following experiments, HAEC grown in a 48-well dish (catalog no. 3548; Corning-Costar Corp., Acton, Massachusetts, USA) were either untreated or treated for 4 h at 37°C with MM-LDL (250 μg/ml) in 5% FBS (catalog no. A-1111-L; HyClone Laboratories, Logan, Utah, USA) in 5% Medium 199 (catalog no. 1902, Irvine Scientific, Santa Ana, California, USA). Human peripheral blood monocytes were isolated, and adhesion was tested as described previously (51). Three types of inhibition experiments were performed: (a) treatment of monocytes with antibody or peptide; (b) treatment of HAEC with MM-LDL or 8A2 β1-activating antibody followed by treatment with blocking antibodies; and (c) treatment of HAEC with blocking antibodies before the addition of MM-LDL. For all inhibition experiments, a range of antibody concentrations were tested (data not shown), with 5 μg/ml yielding maximum inhibition. For the first type of blocking experiments, monocytes were pretreated for 30 min at room temperature with antibodies to either α4 (L25, catalog no. 550019; Becton Dickinson Immunocytometry Systems, San Jose, California, USA), β1 (PSD2; gift of E. Wayner, University of Minnesota Medical School, Minneapolis, Minnesota, USA; ref. 52), β2 (TS/18; American Type Culture Collection, Rockville, Maryland, USA), or β7 (gift of A. Lazarovits, University of Western Ontario, Ontario, Canada; ref. 53); or with either 500 μg/ml CS-1 active (peptidomimetic of the CS-1 region of FN) or 500 μg/ml scrambled control peptide (Cytel Corp.; ref. 48) before addition to the endothelial cells for 15 min. In the second type of experiments, HAEC were incubated for 4 h as untreated control, or separately, with 250 μg/ml MM-LDL and 3 μg/ ml of 8A2, or with 2 ng/ml of LPS as a positive control. After the incubation, the endothelial cells were treated for 30 min with a 5 μg/ml blocking concentration of either VCAM-1 (BBA-S; BRI, Oxford, United Kingdom), 90.45, 7E5, or polyclonal FN antibody before the addition of monocytes. A monocyte binding assay was performed, and unattached cells were rinsed off and fixed as described earlier here. For the last set of inhibition experiments, HAEC were pretreated with anti-α3 (P1B5), anti-α5 (MAB 16), anti-αv (MAB 1980; Chemicon International), anti-β1 (MAB 13, catalog no. 550034;Becton Dickinson), or anti-β3 (catalog no. LM609; Chemicon International) be fore the addition of MM-LDL for 4 h at 37°C (anti-α5 treatment was also performed 90 min after the addition of MM-LDL). After the incubation, monocytes were added to the monolayers. Unattached monocytes were removed by rinsing. The cells were then fixed with 1% glutaraldehyde in 1× PBS. Rabbit IgG Fc (catalog no. 31194; Pierce Chemical Co., Rockford, Illinois, USA), mouse IgG1 (catalog no. X931; DAKO Corp., Carpinteria, California, USA), and irrelevant IgM (mouse anti–human B lymphocyte CD20, catalog no. AHS2001; BioSource International Inc., Camarillo, California, USA) were used as control antibodies for these experiments. In all experiments in which endothelial cells were exposed to antibody, the cells were thoroughly washed to remove excess antibody before adding untreated monocytes to the wells.
For each inhibition study, four separate experiments were performed. In each experiment, four fields from each of four wells were visually counted for adherent monocytes under an inverted microscope with an eyepiece grid.
ELISAs.
Cells in 96-well dishes (catalog no. 3595; Corning-Costar Corp.) were incubated for 4 h at 37°C without or with MM-LDL diluted to 250 μg/ml, 2 ng/ml LPS, 3 μg/ml 8A2 (β1-activating antibody; provided by N.L. Kovach, University of Washington, Seattle, Washington, USA; ref. 54), or 1.5–3 mM MnCl2 for the times indicated in each experiment. For some experiments, cells were treated with 200 ng/ml of cycloheximide (CHX) for 30 min before the treatments listed earlier here. After incubation of HAEC with various activators, the wells were rinsed with 1× PBS containing Ca/Mg before being fixed in 4% paraformaldehyde for 20 min at room temperature. After the fixative, the cells were thoroughly rinsed with 1× PBS + 0.1% BSA (0.1% APBS). To block nonspecific binding, the cells were incubated in 3% BSA + 1× PBS (3% APBS) for 1 h at room temperature. Cells were then incubated overnight at 4°C with antibodies to either CS-1 (1 μg/ml) or VCAM-1 (1 μg/ml) (catalog no. 1229.27-1; Biomedical Alternatives International, Raleigh, North Carolina, USA) in 3% APBS. Other cells were incubated for 1 h with monoclonal FN antibody (0.5 μg/ml) or with HUTS-21 (to detect activated β1; provided by C. Cabanas: ref. 55) for 2 h (2 μg/ ml). In a separate ELISA, the following primary antibodies were used at 1 μg/ml for 2 h: anti-α3, anti-α5, anti-αv, anti-β1, and anti-β3. After the incubation with primary antibody, the wells were rinsed and blocked thoroughly before they were exposed to a HRP-conjugated secondary antibody. After the incubation, cells were rinsed again with 1× PBS followed by double distilled H2O. The peroxidase color reaction was developed in the dark using OPD (catalog no. P8787; Sigma Chemical Co., St. Louis, Missouri, USA). The plate was then read on a Molecular Devices (Menlo Park, California, USA) kinetic microtiter plate reader using a Softmax 881 L-1 end-point program(Molecular Devices) at an OD of 450 nm. The antibody concentration and incubation times were optimized to ensure testing in the linear range.
Immunofluorescence confocal microscopy:
For these studies, glass coverslips (catalog no. 12-545-100; Fisher Scientific Co., Pittsburgh, Pennsylvania, USA) placed into a 12-well dish (catalog no. 3512; Corning-Costar Corp.) were used. The procedure of coating the coverslips with Cell Tak (catalog no. 40240; Becton Dickenson, Bedford, Massachusetts, USA) and Vitrogen 100 (catalog no. 0701-1N; Collagen Asthetics, Palo Alto, California, USA) promoted HAEC adhesion without changing the characteristics of the cells. Cell Tak was mixed 1:1 with 2 M Na2CO3, and 5 μl was placed into the center of each coverslip. The Cell Tak/Na2CO3 mixture was then spread evenly over the entire surface of the coverslip by using a sterile plastic cell scraper. Vitrogen (1 ml/well of a 12-well dish diluted 1:50 into sterile 1× PBS) was then added to the wells, and coverslips were incubated overnight at 37°C before plating cells. For immunofluorescence, cells were fixed with 4% paraformaldehyde and processed as described earlier. For permeabilization of the membrane, some cells were incubated with 0.5% Triton X-100 for 5 min at room temperature. The 90.45, 7E5, HUTS-21, and monoclonal FN primary antibodies were used. For single immunofluorescence using 7E5, cells were incubated overnight at 4°C. The antibody was then viewed using a secondary antibody conjugated to CY-3 (red). For double immunofluorescence between 90.45 and the monoclonal FN antibodies, cells were first incubated overnight at 4°C with 90.45 antibody, and the next day, with the monoclonal FN antibody for 2 h at room temperature. Antibody was removed and cells rinsed before adding secondary antibodies for both 90.45 [αIgM F(ab′)2 CY3; red] and monoclonal FN antibody [αIgG F(ab′)2 FITC; green] (catalog no. 115-095-006; Lampire Biological Pipersville, Pennsylvania, USA) for 1 h at room temperature. For double immunofluorescence between 90.45 and HUTS-21, the cells were incubated with the 90.45 antibody overnight at 4°C. The next day, the 90.45 antibody was rinsed off and the cells were incubated with HUTS-21 for 2 h at room temperature. The 90.45 was recognized first using αIgM FITC (green)–conjugated secondary (catalog no. F0205; DAKO Corp.). Afterward, the HUTS-21 was detected using CY3 (red)–conjugated IgG secondary antibody (catalog no. 115-166-003; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). Control experiments using the secondary antibodies in the reverse order did not affect the overall results (data not shown). For double immunofluorescence between CS-1 recognized by the 90.45 antibody and wheat germ agglutinin (WGA), a well-characterized membrane marker that recognizes N-glycosaminoglycans, cells were incubated with 90.45 and IgM CY3 secondary antibody as already described here. The cells were rinsed and then incubated for 2 h at room temperature with WGA conjugated to FITC (catalog no. FL-1021; Vector Laboratories, Burlingame, California, USA). After rinsing and mounting with Vectashield immunofluorescent solution (catalog no. H1000; Vector Laboratories), the edges of the coverslip were sealed using clear nail polish. Confocal microscopy was performed on the single- and double-labeled immunofluorescent samples to determine the conformation and relative locations of the 7E5, WGA, 90.45 with argon ion (488 nm excitation), and helium neon green lasers (543 nm excitation), and it was used to make optical sections of the cell samples. A Z series made up of several consecutive 0.5-μm-thick sections were taken starting at the apical side and ending at the basal side for analysis of the preparation.
Immunohistochemistry on human endothelial lesions.
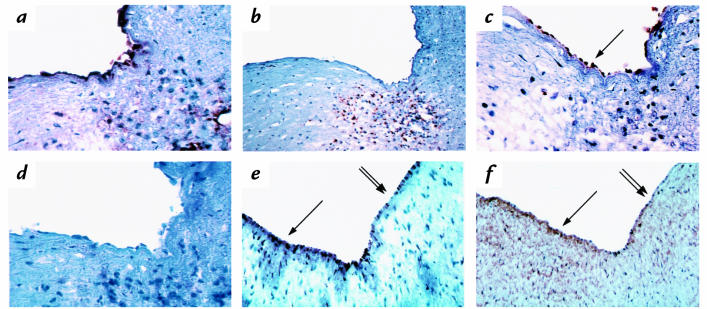
Frozen sections of human aortic endothelium were stained for the presence of 90.45, HUTS-21, VCAM-1, CD 31 (catalog no. M0823; DAKO Corp.) or HAM 56 (catalog no. EAB-935; ENZO New York, New York, USA) in lesions. The tissues were fixed with acetone for 5 min at 4°C, and slides were blocked for 1 h at room temperature with serum of the same species as the secondary antibody. Sections were then incubated for various times with the primary antibody (diluted to 1–2 μg/ml). After the antibody incubation (see Fig. 10 a–d), the sections were rinsed and stained with biotinylated secondary antibody. Endogenous peroxidase activity was blocked with a 20-min incubation of 0.3% H2O2/MeOH solution. Antibodies were viewed using ABC (catalog no. PK6100; Vector Laboratories) and AEC (catalog no. SO1; BiomedaFoster City, California, USA) kits. For Figure 10, e and f, diaminobenzidine was used to view the antibodies.
Figure 10.
Human coronary lesions stained for CD 31, HAM 56, VCAM-1, 90.45, and HUTS-21. Human coronary lesions were stained with CD 31 (a), HAM 56 (b), 90.45 (c), or VCAM-1 (d). Antibodies in a–d were viewed with ABC and AEC. These four panels show that sections containing macrophages display endothelial CS-1 as detected by the 90.45 antibody but not VCAM-1 staining. In a separate study, the luminal endothelium of coronary vessels was stained for 90.45 (e) and HUTS-21 (f). Antibodies in e and f were viewed with DAB. Areas that stained most positively for 90.45 wer e mirrored by HUTS-21 (arrow), whereas areas of lesser staining were also parallel between the two antibodies (double arrow). ×1000. DAB, diaminobenzidine; ABC, avidin/biotinylated horseradish peroxide macromolecular complex; AEC, amino-9-ethyl carbazole.
Statistical analysis.
For all experiments described here, data were analyzed using the Statview 4.5 program(Abacus Concepts Inc., Berkeley, California, USA). All P values were calculated using ANOVA and Fisher's protected least significant difference test.
Results
Characterization of the FN antibodies used for these studies.
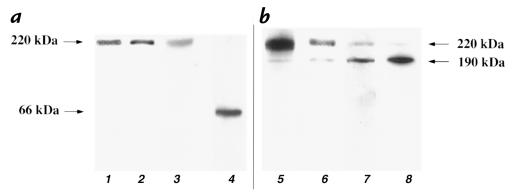
A previous study (49) has shown that a polyclonal antibody to the CS-1 peptide reacted more strongly with fragmented than intact plasma FN. We used Western blotting to characterize the reactivity of the CS-1 monoclonals used in this study with plasma and cellular FN (Fig. 1). The polyclonal FN (lane 1), monoclonal FN antibody (lane 2), and CS-1 90.45 (lane 3) all detected a single band at ∼220 kDa in plasma FN (5 μg/lane) (Fig. 1a). As expected, the FN monoclonal antibody displayed much stronger recognition of intact plasma FN (5 μg/lane). A chymotryptic digest of FN (5 μg) showed that the CS-1 90.45 antibody recognized a band at ∼66 kDa (lane 4) and that the intensity of the band was much higher when compared with intact plasma FN (Fig. 1a, compare lanes 3 and 4). The antibody recognition of cellular FN was then examined using lysates of endothelial cultures (Fig. 1b). FN polyclonal antibody (lane 5), FN monoclonal antibody (lane 6), CS-1 monoclonal antibody 90.45 (lane 7), and CS-1 monoclonal antibody (lane 8) all recognized two bands from whole cell lysates, one at ∼190 kDa. Others have shown that FN antibodies can recognize molecules of 220 kDa and 190 kDa (49, 56), the latter being formed by fragmentation with certain proteases. As expected, the CS-1 monoclonals reacted more strongly with the neoepitope present in the 190-kDa band, which is likely exposed by fragmentation of the 220-kDa FN. The FN antibodies reacted more strongly with the larger molecule. These results in HAEC contrast with observations in astrocytes, where the 90.45 antibody recognized a 300-kDa band not recognized by a FN antibody (57). Taken together, these studies demonstrate that CS-1 monoclonals used in these studies specifically detect cellular FN and react most strongly with 190-kDa form of cellular FN.
Figure 1.
Western blot analysis of 90.45 and FN antibody binding to plasma FN and cell lysates. Plasma FN (5 μg/lane) was electrophoresed and transferred onto nitrocellulose. Membranes were probed with polyclonal FN (lane 1), monoclonal FN (lane 2), and 90.45 (lane 3) antibodies. All three antibodies recognized, to varying intensities, pFN to be a single band at ∼220 kDa (a). In a chymotryptic digest of pFN (5 μg/ lane), the 90.45 antibody recognized a band at ∼66 kDa (lane 4) with an intensity greater than its recognition of intact pFN (compare lanes 3 and 4) (a). To compare the pattern of antibodies, whole cell lysates prepared in RIPA lysis buffer were separated and transferred onto nitrocellulose. The polyclonal FN (lane 5), monoclonal FN (lane 6), 90.45 (lane 7), and 7E5 antibodies (lane 8) detected two bands of 220 and 190 kDa. For the monoclonal and polyclonal FN antibodies, the 220-kDa band stained with a greater intensity (lanes 5 and 6) than the 190-kDa band, whereas the CS-1 and 7E5 antibodies recognized the 190-kDa band better than the 220-kDa band (lanes 7 and 8) (b). FN, fibronectin; RIPA, radioimmunoprecipitation assay.
Effect of integrin antibodies, CS-1 FN antibodies, and active CS-1 peptide on MM-LDL– induced monocyte binding.
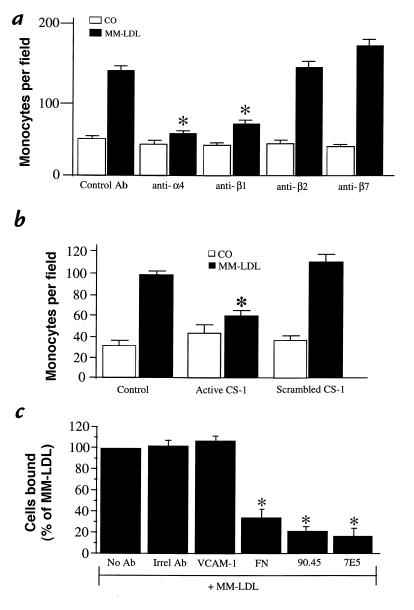
Studies to determine the monocyte integrins responsible for the adhesion to MM-LDL–treated HAEC were performed by incubating monocytes, before their addition to the HAEC, with either control antibody or with antibodies against α4, β1, β2, and β7 integrin subunits. Control antibody was not effective at reducing levels of MM-LDL–induced monocyte binding (Fig. 2a). However, the level of monocyte binding to MM-LDL–treated cells was reduced by incubation of monocytes with antibodies against α4 and β1 but not with antibodies against β2 and β7 (P < 0.0005) (Fig. 2a). Further confirmation for a role for VLA-4 was obtained by exposing monocytes to a peptidomimetic of the CS-1 region of FN, a known inhibitor of VLA-4 interaction with its ligands (58). The active peptide reduced the number of adherent monocytes by ∼76% (after subtraction of control), whereas the scrambled control peptide did not reduce the number bound (P < 0.0005) (Fig. 2b). These results indicated that the VLA-4 integrin present on the surface of monocytes was involved in MM-LDL–induced monocyte adhesion to the endothelial cell surface.
Figure 2.
Agents that block monocyte/endothelial interactions in vitro. HAEC were stimulated with 250 μg/ml MM-LDL at 37°C for 4 h. Human monocytes were incubated with 5 μg/ml anti-α4, β1, β2, or β7 for 30 min before addition to the endothelial cell layer. Unbound monocytes were rinsed off and the cells fixed with 1% gluteraldehyde in 1× PBS. Anti-α4 and anti-β1 both significantly reduced the number of monocytes bound to the endothelial cell layer. Neither anti-β2 nor anti-β7 demonstrated an effect on the level of monocytes bound (a). In a separate study, HAEC were treated as just described and monocytes were incubated with either active CS-1 (500 μg) or scrambled peptide (500 μg) for 20 min at room temperature. A binding assay was performed as just described. The active CS-1 peptide significantly reduced the levels of bound monocytes, whereas the scrambled peptide did not affect the levels bound (b). In the last experiment, HAEC were treated with MM-LDL, as described, before being incubated with blocking antibodies to VCAM-1 (4B9), polyclonal FN, 90.45, or 7E5 at 5 μg/ml for 30 min. Untreated monocytes were used to perform the binding assay. The antibody against VCAM-1 did not reduce the number of monocytes bound to MM-LDL–stimulated HAEC. However, FN, 90.45, and 7E5 antibodies all significantly reduced monocyte adhesion (c). For each condition, the number of monocytes was determined by visually counting four fields per well in four separate wells. Additionally, all experiments are representative of four separate studies. Values represent mean ± SD (n = 12). *P < 0.0005. CS-1, connecting segment-1; HAEC, human aortic endothelial cells; MM-LDL, minimally modified low-density lipoprotein; VCAM-1, vascular cell adhesion molecule-1; APBS, PBS + BSA; GNS, goat normal serum.
To identify the endothelial ligand for monocyte VLA-4, the MM-LDL– treated endothelial cells were exposed to antibodies against VCAM-1 and FN, the two known alternative ligands for VLA-4. Endothelial cells were treated for 4 h with MM-LDL and then exposed for 30 minutes to antibody. The antibodies were washed off, and the monocytes were added to the treated HAEC. A monoclonal blocking antibody against VCAM-1 (4B9) significantly blocked lipopolysaccharide (LPS)–induced (P < 0.0005; data not shown), but not MM-LDL–induced, monocyte binding (Fig. 2c). To determine the role of CS-1 in MM-LDL–induced binding, MM-LDL–treated HAEC were exposed to two different monoclonal antibodies against the 25–amino acid CS-1 sequence, as well as a polyclonal antibody to FN (Fig. 2c). MM-LDL–stimulated endothelial cells that were treated with either the 90.45 or 7E5 antibody before the addition of monocytes showed a significant reduction (70%–75% reduction) in the level of monocyte binding (P < 0.0005), whereas irrelevant antibody did not reduce levels of binding. In addition, a polyclonal antibody to FN was also effective at reducing levels of MM-LDL–induced monocyte binding (61% reduction). The antibodies did not have an effect on monocyte binding to untreated cells (data not shown). These data strongly suggest that MM-LDL induces monocyte binding by the interaction of monocyte VLA-4 with CS-1 FN on the surface of endothelial cells.
MM-LDL stimulates CS-1 apical surface expression in HAEC.
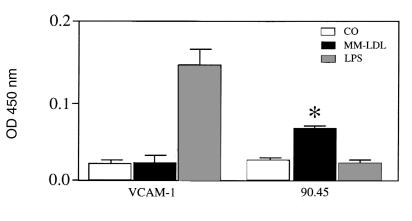
We next examined the ability of MM-LDL to increase CS-1 at the level of mRNA, protein and cell-surface expression. RNase protection assay did not detect an increase in the levels of CS-1 message after MM-LDL treatment (CO = 1.76, MM = 1.70; data expressed as CS-1/GAPDH ratio). In addition, Western blotting with radioimmunoprecipitation assay (RIPA) lysis buffer demonstrated that levels of CS-1 FN total protein (matrix plus cell-associated) were not increased in MM-LDL–treated cells (CO = 0.4, MM = 0.3; data expressed as CS-1/tubulin ratio). However, treatment of endothelial cells with MM-LDL, but not LPS, significantly increased apical surface expression of CS-1 as determined by enzyme-linked immunosorbent assay (ELISA) on nonpermeabilized attached cells using the 90.45 antibody (Fig. 3). (The confocal studies described later here showed that antibody bound only to the apical surface.) Levels of surface expression were increased ∼1.7–3-fold over a series of several experiments. Similar results were seen with 7E5 (data not shown). Levels of VCAM-1 were not increased by MM-LDL on these HAEC (Fig. 3). Previous studies by our group (37) demonstrated the ability of CHX to reduce monocyte adhesion to MM-LDL–stimulated cells. Cell-surface ELISA demonstrated that the addition of CHX significantly reduced the increase in CS-1 surface expression after MM-LDL stimulation (60% reduction, P = 0.0175; data not shown). These series of experiments demonstrates that MM-LDL increases the expression of FN on the apical surface without increasing the levels of total FN message or protein and that this process depends on protein synthesis.
Figure 3.
MM-LDL stimulates surface expression of CS-1 but not VCAM-1. HAEC were plated into 96 wells and then stimulated with 250 μg/ml of MM-LDL or 2 ng/ml LPS for 4 h at 37°C. After the treatment, cells were rinsed and then fixed with 4% paraformaldehyde for 20 min at room temperature. Cells were rinsed and then blocked for 1 h with 3% APBS. Primary antibodies were diluted to 1 μg/ml in the blocking solution and then incubated overnight at 4°C. Cells were then rinsed and blocked with GNS before incubation with either the IgM (90.45) or IgG (VCAM-1) secondary antibodies conjugated to HRP. Development was with o-phenylenediamine dihydrochloride in a phosphate citrate buffer. The plate was read on a microtiter plate reader at an OD of 450 nm. A representative experiment from four separate studies is shown. Values represent mean ± SD (n = 4). *P < 0.0001. HRP, horseradish peroxidase.
Characterization of CS-1 localization by immunofluorescence confocal microscopy.
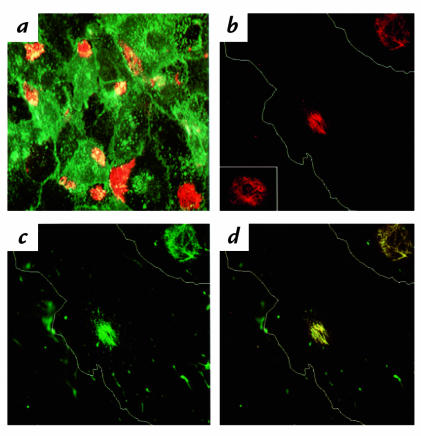
Immunofluorescence confocal microscopy was used to determine the location of the FN recognized by the 90.45, 7E5, and monoclonal FN antibodies. Evidence for CS-1 localization to the apical surface was obtained by double immunofluorescence using the 90.45 antibody and WGA, a well-characterized membrane marker. Fluorescein isothiocyanate (FITC)–labeled WGA was found to colocalize with CS-1 on the apical surface by confocal microscopy (Fig. 4a). The distribution of FN localized by the 90.45 and monoclonal FN antibodies in MM-LDL–treated cells was examined under higher magnification (Fig. 4, b–d). Thin optical sections were made starting at the apical surface where CS-1 staining was most prominent. To provide better orientation, the cell is outlined in white on the figure. At the apical surface, the 90.45 antibody stained a patchlike configuration located adjacent to the nucleus (Fig. 4b). This type of staining pattern was also observed using 7E5 (Fig. 4b, inset). The monoclonal FN antibody also stained the filamentous patch on the apical surface (Fig. 4c) in addition to binding to fibrillar strands on other parts of the apical surface and on the cell periphery (data not shown). There was clear colocalization of the monoclonal FN antibody within the CS-1 patch, which is seen by the yellow color of the combined fluorochromes (Fig. 4d). The colocalization was not due to an artifact of double immunofluorescence, because the fluorochromes used have emissions that are sufficiently separated from one another to avoid overlap in detection (FN; FITC 492 nm and CS-1; CY3 550 nm). Colocalization, therefore, can be attributed to the recognition of the same molecule by both antibodies. Permeabilization of cells with Triton X-100 further confirmed that patches were localized to the apical surface. In permeabilized cells, the 90.45 antibody demonstrated cytoplasmic staining of intracellular FN (data not shown). The patterns of antibody staining described here were observed in cells cultured in serum containing FN, as well as in FN-free serum (data not shown).
Figure 4.
Confocal series for WGA, 90.45, 7E5, and monoclonal FN antibodies on nonpermeabilized cells. HAEC were treated with MM-LDL for 4 h at 37°C before being fixed and processed as nonpermeabilized cells. Optical sections were taken starting at the apical surface where 90.45 staining was most prominent. Cells were double labeled with 90.45 viewed by CY3 (red) and with the membrane marker WGA conjugated to FITC (green). The levels of both 90.45 and WGA were most intense on the apical side of the cell (×500) (a). A higher magnification of the MM-LDL–treated cells revealed the morphology of the CS-1 patch viewed with 90.45 to be a filamentous pattern with interwoven filaments. For better orientation, the cell is outlined in white (b, inset). The monoclonal FN antibody stained areas within the CS-1 patch in addition to individual fibrils spread along the apical surface (c). Colocalization at the level of the CS-1 patch between the monoclonal FN and 90.45 antibodies appear as yellow (d). b–d: ×1200. WGA, wheat germ agglutinin.
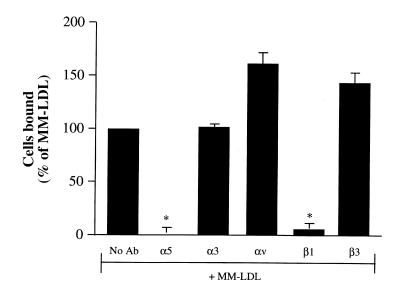
Treatment of HAEC with anti-α5 and anti-β1, but not anti-α3, anti-αv, or anti-β3, before incubation with MM-LDL, inhibited monocyte binding and CS-1 expression. We next examined the ability of MM-LDL to increase the retention of CS-1 FN on the apical surface by activation of FN-binding integrins. HAEC were pretreated with blocking antibodies to integrins previously shown to mediate FN binding to the cell surface: VLA-5, α3β1, and αvβ3. HAEC were pretreated for 30 minutes with 5 μg/ ml of blocking anti-integrin antibodies to α5, α3, αv, β1, and β3 integrins and then incubated with antibody and MM-LDL for four hours. The cells were washed thoroughly to remove the antibodies, and then a monocyte binding assay was performed. The MM-LDL–induced increase in monocyte binding was significantly inhibited (back to control levels) by antibodies to α5 and β1, but not by anti-α3, αv, or β3 integrins (Fig. 5). In fact, both αv and β3 antibodies actually stimulated binding to MM-LDL–treated cells. Treatment of control cells with the anti-α5 or anti-β1 antibodies did not decrease monocyte binding (data not shown). Addition of anti-α5 to the cells 90 minutes after MM-LDL addition was ineffective at inhibiting binding (data not shown). Treatment of HAEC with anti-α5 and anti-β1 antibodies before MM-LDL stimulation also significantly reduced the surface expression of CS-1 as determined by ELISA on nonpermeabilized HAEC (MM-LDL = 82.9 ± 6.78; α5 + MM = 21 ± 6.21; and β1 + MM = 18.5 ± 4.80, expressed as percent increase in OD above untreated cells ± SD). These findings suggest that functional α5 and β1 integrins comprising VLA-5 are important for the MM-LDL–induced CS-1 expression on HAEC.
Figure 5.
Pretreatment of HAEC with antibodies against α5 and β1, but not α3, αv, or β3, inhibited MM-LDL–mediated monocyte binding. HAEC were pretreated for 30 min with 5 μg/ml of blocking antibodies to α5, α3, αv, β1, and β3 before addition of MM-LDL for 4 h at 37°C (in the continuing presence of antibody). The cells were rinsed extensively, and a monocyte binding assay was performed. The MM-LDL–induced increase in monocyte binding was significantly blocked back to control levels by antibodies to α5 and β1 but not α3, αv, or β3. The data presented are representative of four experiments. Values represent mean ± SD (n = 12). *P < 0.001.
MM-LDL increased activation of β1 integrins but not the total amount of α5β1 on the apical surface.
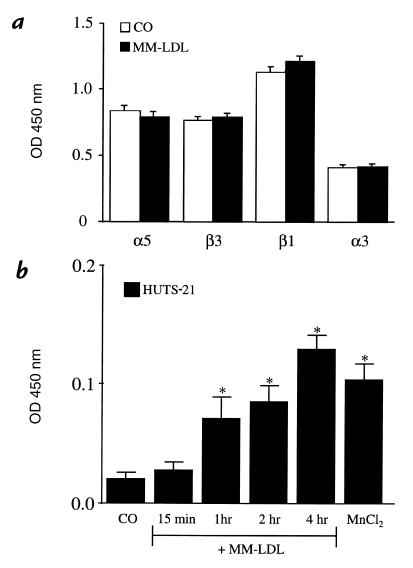
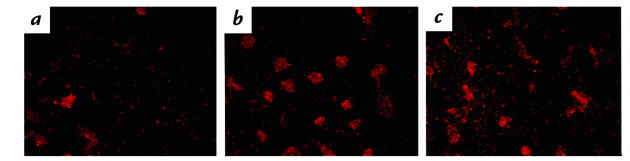
The induction of CS-1 expression by MM-LDL could be mediated by either increased expression or activation of VLA-5 (28). An ELISA performed using antibodies that have equal affinity for both the activated and the nonactivated state of the integrins detected no increase in the levels of α5, β1, α3, or β3 in response to MM-LDL (Fig. 6a). Several antibodies have been described recently (55)that specifically detect the activated conformation of β1 integrins. Another ELISA was performed using the HUTS-21 antibody (55) to detect apical surface expression of the activated β1 epitope. A time-dependent increase in the expression of the activated β1 epitope on the MM-LDL–treated cells was observed (Fig. 6b). The maximum expression (three- to fourfold increase) of this epitope was seen at four hours and remained elevated for at least six hours (data not shown). Cells treated with MnCl2 for 15 minutes were used as a positive control because MnCl2 has been shown to activate β1 integrins rapidly (Fig. 6b). This time course of expression of activated β1 is similar to that observed for monocyte binding as published previously (11). An assay using a different β1 activation detection antibody, 15/7 (59), gave similar results (data not shown). Confocal immunofluorescent studies were performed using HUTS-21 antibody to detect the distribution of activated β1 integrin on the apical surface. In control cells, there was a low level of staining where a few clusters of activated β1 were observed (Fig. 7a). Cells treated with MM-LDL (Fig. 7b) demonstrated a marked increase in the number of β1 clusters with usually only one cluster per cell. This image is a visual representation of the significant increase in β1 activation quantitated by ELISA (Fig. 6b). The cells treated with MnCl2 showed a diffuse increase in staining of activated β1 integrins with fewer and smaller clusters (Fig. 7c) Currently, there is no antibody available to detect the activated form of α5. However, because we were able to inhibit MM-LDL–induced monocyte adhesion by pretreatment with antibodies against α5 and β1 and not other anti-integrin antibodies, it is likely that the activated β1 that mediates FN binding in response to MM-LDL is associated with α5.
Figure 6.
MM-LDL increased the activation of β1 without increasing the total amounts of α5β1 on the apical surface. HAEC were either untreated or treated with MM-LDL for 4 h. ELISA was performed on nonpermeabilized cells to detect the apical expression of α5, α3, β3, and β1. MM-LDL did not increase the total levels of integrins on the surface of the HAEC (a). Values represent mean ± SD (n = 4). Using HUTS-21 to detect the activated form of the β1 integrin, ELISA demonstrated that MM-LDL increased the amount of β1 activation in a time-dependent fashion (b). Values represent mean ± SD (n = 4). *P < 0.0001. Each experiment is representative of four separate studies.
Figure 7.
Confocal microscopy of HAEC stained for HUTS-21. HAEC were either untreated (a), or treated with MM-LDL for 4 h (b) or with 1.5 mM MnCl2 for 15 min (c). Binding of the HUTS-21 antibody was viewed using CY3-conjugated secondary antibody (red). Confocal microscopy determined the β1 staining to be restricted to the apical surface. The control cells displayed a few clusters of activated β1, whereas cells stimulated with MM-LDL demonstrated an increase in these clusters. MnCl2 served as positive control, and a diffuse increase in staining with fewer and smaller clusters can be seen. ×500.
Treatment of endothelial cells with an antibody that activates β1 integrins increased monocyte binding and CS-1 expression.
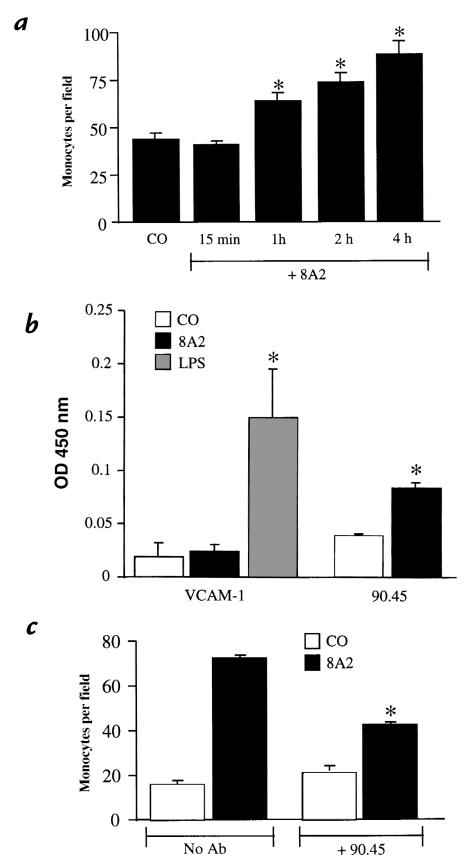
To determine whether an alternative method of β1 activation could also lead to increased CS-1 expression and monocyte binding, an antibody that activates β1 integrins (8A2) was used (54). HAEC were treated with 3 μg/ml of 8A2 antibody for periods of 15 minutes to four hours. After treatment, the cells were thoroughly rinsed to remove the antibody, and a monocyte binding assay was performed using human monocytes. HAEC treatment with 8A2 induced monocyte binding in a time-dependent manner, with a maximum increase (about three- to fourfold) seen at the end of four hours (Fig. 8a). That monocyte binding was not increased after treatment for 15 and 30 minutes indicates that rinses to remove the activating antibody from HAEC were sufficient to avoid direct activation of monocytes by 8A2. In a separate set of experiments, 8A2 did not induce neutrophil binding (data not shown). The 8A2-induced monocyte binding at four hours was completely inhibited by treatment of monocytes with anti-α4 antibody but not by a nonspecific IgG (8A2= 122 ± 7.1; 8A2 + α4 = 34 ± 6.0; 8A2 + IgG = 128 ± 6.5 monocytes per field). An ELISA on nonpermeabilized cells demonstrated that at four hours of treatment, 8A2 did not significantly increase VCAM-1 expression, whereas there was a significant increase in the apical surface expression of CS-1 (Fig. 8b). To confirm further a role for CS-1 in 8A2-mediated monocyte binding, the effect of the 90.45 antibody was examined. Cells were treated for four hours with 8A2, exposed to the 90.45 antibody, and rinsed, and monocyte binding was measured. The 90.45 antibody inhibited monocyte binding of monocytes to 8A2 treated by 55% but caused no significant reduction in monocyte binding to untreated cells (Fig. 8c). From these studies, we conclude that, like MM-LDL, activation of β1 integrin by antibody increases monocyte binding by inducing retention of CS-1 FN on the apical cell surface.
Figure 8.
The β1-activating antibody 8A2 induces monocyte binding and surface expression of CS-1 on HAEC. HAEC were untreated or treated with 3 μg/ml 8A2-activating antibody for various times (15 min–4 h) before performing a monocyte binding assay. Treatment with 8A2 induced monocyte binding in a time-dependent manner with a maximal increase at 4 h (a). Values represent mean ± SD (n = 12) (*P < 0.001). In a separate study, an ELISA was done on HAEC treated with 8A2 (b). The β1-activating antibody significantly increased the levels of CS-1 but not of VCAM-1. Values represent mean ± SD (n = 4) (*P < 0.001). To determine the involvement of CS-1 in 8A2-induced monocyte adhesion, HAEC were treated with either 8A2 only or with 8A2 for 4 h and then incubation with the 90.45 antibody (c). The 90.45 antibody was able to effectively reduce the number of monocytes bound to 8A2-treated HAEC. Values represent mean ± SD (n = 12). *P < 0.001.
CS-1 and activated β1 integrin colocalize on the apical surface of HAEC.
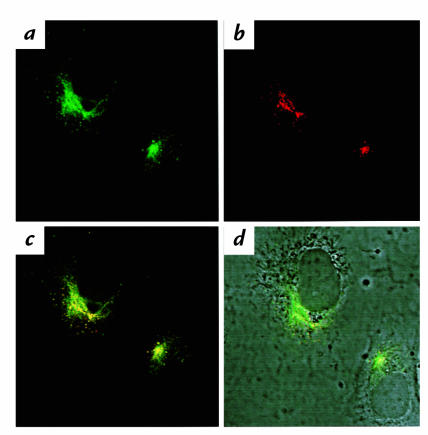
Confocal microscopy on double immunofluorescence was performed on HAEC stained for both CS-1 (monoclonal antibody IgM 90.45, FITC green–conjugated secondary) and activated β1 (monoclonal antibody IgG HUTS-21, CY3 red–conjugated secondary). Both CS-1 (Fig. 9a) and β1 (Fig. 9b) were found on the upper cell surface in a patchlike and cluster configuration, as seen in Figs. 4b and 7b. CS-1 and activated β1 were found to colocalize together on the cell surface and appear as yellow (Fig. 9c). Patches were most frequently found on the apical surface near the nuclear periphery (Fig. 9d), although some patches overlapped the nuclear surface.
Figure 9.
Confocal microscopy on CS-1 and activated β1 colocalization on the apical surface of HAEC. HAEC grown to confluence on coverslips were treated with MM-LDL for 4 h at 37°C before being fixed. The cells were double-labeled with the 90.45 antibody, viewed by FITC (green) and activated β1 (HUTS-21), viewed by CY3 (red). The staining with both 90.45 (a) and HUTS-21 (b) was most intense on the apical surface of cells as determined by optical sections taken starting at the basal surface of the cells. A double-immunofluorescent image of 90.45 and HUTS-21 together shows areas of yellow, which indicates colocalization (c). A phase micrograph of the same cells is shown with an overlay of the double-immunofluorescent staining for comparison and orientation. ×1800.
Immunohistochemistry of human coronary lesions.
Studies were performed on 15 different coronary lesions taken from five different individuals to compare 90.45, VCAM-1, and HUTS-21 staining on luminal endothelium. All sections examined stained positively for CD 31, which detected the presence of endothelium (Fig. 10a); some regions also stained positively for HAM 56, which detected the presence of macrophages (Fig. 10b). In 13 of 15 vessels, in areas where staining was positive for both CD 31 and HAM 56, staining for 90.45 was observed on the luminal endothelium (Fig. 10c). In areas lacking HAM 56, the luminal endothelium on only two vessels was positively stained. VCAM-1 staining was not seen on the luminal endothelium in 13 of 15 vessel areas with HAM 56–positive staining (Fig. 10d) but was seen in one area that stained negative for HAM 56. Sections incubated with the 90.45 antibody preabsorbed to the active CS-1 peptidiomimetic did not yield any observable staining (data not shown), A separate study was performed on seven different coronary vessels, one each from four different individuals and three from a fifth individual, to determine whether similar areas demonstrated staining of luminal endothelium for HUTS-21 (Fig. 10f) and 90.45 (Fig. 10e). HUTS-21 staining was generally more extensive than 90.45, going slightly deeper into the intima. However, in six of the seven vessels, areas of highest intensity of 90.45 and HUTS-21 were very similar but not completely identical (Fig. 10, e and f, arrow and double arrow). We noted that the endothelial cells in areas that were positive for HUTS-21 and 90.45 were taller and exhibited more nuclei per square centimeter, which suggested activation. However, it was not possible to determine by immunohistochemistry whether both the CS-1 and HUTS-21 staining were localized to the apical surface of the endothelium. These studies do suggest that staining of luminal endothelium in areas where monocyte/ macrophages are present is higher for 90.45 than VCAM-1 and that most 90.45 staining occurs in areas of staining for activated β1 integrin.
Discussion
The goal of our studies was to determine the ligands on the monocyte and endothelium responsible for enhanced binding to endothelial cells treated with MM-LDL. Monocytes express a variety of surface integrins that they use to bind to the endothelial cell layer (60). Using blocking antibodies, we demonstrated that VLA-4 was the major monocyte integrin responsible for MM-LDL–induced binding (Fig. 2a). We present several lines of evidence that MM-LDL induces monocyte binding to endothelial CS-1 FN, one of the two major ligands previously identified for VLA-4. Treatment of endothelial cells with MM-LDL induced the apical expression of CS-1 FN (Fig. 3). Exposure of monocytes to an active but not scrambled CS-1 peptide strongly inhibited binding to MM-LDL–treated endothelial cells (Fig. 2b). However, this result is not definitive because VCAM-1 binding is also blocked by the CS-1 peptide, although to a lesser extent (48). More importantly, treatment of endothelial cells with two different antibodies to the CS-1 region of FN and with a polyclonal antibody to FN strongly inhibited the binding of monocytes to MM-LDL–treated cells (Fig. 2c). In the present studies, immunofluorescence on nonpermeabilized HAEC demonstrated that different CS-1 monoclonal antibodies recognized filamentous patches on the upper cell surface located adjacent to the nucleus (Fig. 4b, inset). These patches were also recognized by a monoclonal FN antibody (Fig. 4c). Additionally, these patches correspond to regions where most monocytes have been found to bind in MM-LDL–treated cells (data not shown). It has been demonstrated that binding of VLA-4 to CS-1, like the binding of VCAM-1 to VLA-4, represents firm adhesion and requires that VLA-4 be either fully or partially activated (61). Studies by Weber and colleagues (62) demonstrated that CS-1 is able to function as a firm adhesion molecule under flow conditions. Furthermore, the adhesive strength of VLA-4 on isolated human monocytes for a CS-1 containing FN fragment was rapidly increased and then reduced by MCP-1 (62). Previous studies by our group (24–26) have demonstrated that MCP-1 and other monocyte activators are increased by MM-LDL treatment of endothelial cells. We hypothesize that although a low level of CS-1 is expressed by untreated HAEC, it is not involved in monocyte binding to these cells owing to lack of monocytic VLA-4 activators. Thus our data suggest that the firm adhesion molecule induced by MM-LDL on endothelial cells is CS-1 FN; this molecule causes binding to the activated monocyte VLA-4 ligand.
The mechanism by which MM-LDL induces apical expression of FN was examined in a series of experiments. MM-LDL did not increase alternative splicing of FN, nor did it increase levels of total FN (cell-associated plus matrix-associated) as seen by RNase protection assay and Western blots of RIPA lysates. Rather, we provide extensive evidence that MM-LDL increases retention of FN on the apical surface of the cell. Peters and colleagues (63) have shown that endothelial cells secrete FN constitutively along the apical and the basal surface in culture. The process of FN binding to cells has been extensively studied in fibroblasts (64), and several cell-surface integrins have been shown to mediate FN assembly (45, 64–67). Our results demonstrate that treatment of endothelial cells with blocking antibodies to endothelial integrins α5 and β1, but not α3, αv, or β3, before stimulation with MM-LDL, blocked the induction of monocyte binding and CS-1 expression (Fig. 5; see Results) indicating that VLA-5 mediated FN binding. Binding of FN to VLA-5 has been shown to occur via the RGD-containing cell-binding domain of FN (68). Our results in endothelial cells are different from an earlier report (69), which noted that native LDL and very-low-density lipoprotein (VLDL) increased FN binding to MG-63 osteosarcoma cells by threefold via exposure of the FN–FN association sites. MM-LDL treatment led to increased expression of activated β1 integrin (Fig. 6b) without increasing the total amount of either α5 or β1 (Fig. 6a), which are constitutively expressed on the apical (luminal) surface of endothelial cells (67). The finding of increased β1 activation was confirmed using two different anti-β1 antibodies (15/7 and HUTS-21; refs. 55, 59) and both ELISA and immunofluorescence techniques (Fig. 6b and Fig. 7, a and b). Furthermore, there was colocalization between HUTS-21 and 90.45 antibodies on the apical surface of MM-LDL–treated endothelial cells (Fig. 9c). Further confirmation that activation of β1 can lead to apical expression of CS-1 FN in endothelial cells was provided by our studies with 8A2, a β1 integrin–activating antibody (54). We observed that treatment of endothelial cells with 8A2 increased monocyte-specific binding (Fig. 8a) and CS-1 but not VCAM-1 expression (Fig. 8b). Most definitively, 8A2-induced monocyte adhesion could be inhibited by treatment of monocytes with blocking VLA-4 antibodies (see Results) or by treatment of HAEC with the 90.45 antibody against CS-1 (Fig. 8c). These results with 8A2 strengthen our conclusion that increased CS-1 expression on the apical surface of MM-LDL–treated HAEC is due to increased deposition of apical FN by the activation of β1 integrin.
The present studies provide evidence that MM-LDL–mediated β1 activation and the formation of stable VLA-5/CS-1 complexes that bind monocytes involve several steps. Activation of integrins requires a conformational change that can be brought about directly by an activating antibody or indirectly by activation of G proteins, protein kinases, phosphatidyl inositol turnover, and possibly protein synthesis (28). The present studies suggest that MM-LDL–induced activation of β1 is indirect. MM-LDL treatment of HAEC caused a gradual increase in the expression of the activated β1 epitope over the course of four hours (Fig. 6b). This is in contrast to HAEC treated with MnCl2, in which the expression of the HUTS-21 epitope occurred within 15 minutes. Our observations suggest that activation of β1 is necessary but not sufficient to cause monocyte binding. It is known that 8A2 activates β1 integrins, presumably by bringing about a conformational change, within 15 minutes (54). However, the induction of monocyte binding in response to 8A2 antibody took at least one hour (Fig. 8a). This suggests that some postreceptor events are involved in the process of monocyte binding in response to 8A2 treatment of HAEC. Ishida and colleagues (70) have shown that 8A2-induced FAK tyrosine phosphorylation in HAEC begins after at least 30 minutes and then progressively increases over the next two hours. Additionally, FAK phosphorylation leads to integrin clustering. It is known that the clustering of integrins involves the interaction of the cytoplasmic tails of integrins with several cytoskeletal proteins and extracellular matrix proteins on the outer side of the cells to constitute focal adhesions (71). Immunofluorescence confocal microscopy revealed a clustering of the activated β1 integrins on the apical surface in response to MM-LDL (Fig. 7b). We hypothesize that MM-LDL–induced integrin clustering is due to cytoskeletal interactions that lead to the patchlike distribution of CS-1. We have shown that treatment of endothelial cells with CHX before MM-LDL stimulation inhibited monocyte binding (37) and CS-1 expression (see Results). Thus, at least one step in the process leading to CS-1 expression and monocyte binding is mediated by new protein synthesis. The slow time course of β1 activation by MM-LDL and the slow induction of monocyte binding, may be explained by both the time required for inside-out activation and the timerequired for integrin clustering that facilitates FN deposition.
The in vivo relevance of CS-1 to human atherosclerosis is suggested by the presence of CS-1 in the endothelium of human coronary lesions, although the exact location within the endothelium is not demonstrated by these studies (Fig. 10, c and e). A previous study (76) suggests a role for CS-1 FN in a number of other chronic inflammatory states. An increase in the amount of FN that is recognized by the 90.45 antibody has been identified in rheumatoid arthritic synovium, on the lumen of rheumatoid arthritic endothelium (47, 48, 58, 77), and in transplant coronary artery disease (48). Studies of chronic kidney inflammation have indicated that FN may also be involved in the inflammatory process (78–80). Antibodies to oxidized phospholipids are increased in animals and patients with atherosclerosis and a number of other chronic inflammatory conditions (81–83). On the basis of these observations, we hypothesize that oxidized phospholipids may contribute to chronic inflammation by activation of β1 dimerized to α5, leading to increased expression on the endothelial cell surface of CS-1 FN, a monocyte-specific ligand.
Acknowledgments
The authors thank Matthew Schibler, David Allen, and Giuliano Mottino for their major contributions to the confocal studies; Thomas A. Drake for providing the human coronary lesions; Edward F. Plow for valuable discussions and assistance; Michael J. Giese, Deborah Schwartz, and Shirley Wang for expert technical assistance; and Rick Grambo of UCLA Biomedical Technology and Research Imaging Project (BTRIP)for excellent imaging assistance. D.K. Vora was a Sam Nassi Fellow in Cardiology at the UCLA School of Medicine during the course of these studies. This research was supported by US Public Health Service grant HL-30568 and funds from the Laubisch Foundation.
References
- 1.Morton RE, West GA, Hoff HF. A low density lipoprotein–sized particle isolated from human atherosclerotic lesions is internalized by macrophages via a non–scavenger-receptor mechanism. J Lipid Res. 1986;27:1124–1134. [PubMed] [Google Scholar]
- 2.Hoff HF, Gaubatz JW. Isolation, purification, and characterization of a lipoprotein containing Apo B from the human aorta. Atherosclerosis. 1982;42:273–297. doi: 10.1016/0021-9150(82)90157-5. [DOI] [PubMed] [Google Scholar]
- 3.Yla-Herttuala S, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palinski W, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carew TE, Schwenke DC, Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci USA. 1987;84:7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kita T, et al. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerrity RG. The role of the monocyte in atherogenesis. I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 8.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 9.Gu L, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis low density lipoprotein receptor–deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 10.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2–/– mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 11.Berliner JA, et al. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson AD, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 13.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 14.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 15.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin–deficient mice. Cell. 1993;74:541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RC, et al. Absence of P-selectin delays fatty streak formation in mice. J Clin Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144:952–961. [PMC free article] [PubMed] [Google Scholar]
- 18.Vora DK, et al. Induction of P-selectin by oxidized lipoproteins. Separate effects on synthesis and surface expression. Circ Res. 1997;80:810–818. doi: 10.1161/01.res.80.6.810. [DOI] [PubMed] [Google Scholar]
- 19.Parhami F, et al. Minimally modified low density lipopro-tein–induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993;92:471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebuhrer V, Murphy JF, Bordet JC, Reck MP, McGregor JL. Oxidized low-density lipoprotein induces the expression of P-selectin (GMP140/PADGEM/CD62) on human endothelial cells. Biochem J. 1995;306:293–298. doi: 10.1042/bj3060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yla-Herttuala S, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinton SK, et al. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- 23.Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor–deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cushing SD, et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz D, et al. Role of the GRO family of chemokines in monocyte adhesion to MM-LDL–stimulated endothelium. J Clin Invest. 1994;94:1968–1973. doi: 10.1172/JCI117548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajavashisth TB, et al. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990;344:254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- 27.Haas TA, Plow EF. Integrin–ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 28.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 29.Chan BM, Elices MJ, Murphy E, Hemler ME. Adhesion to vascular cell adhesion molecule 1 and fibronectin. Comparison of alpha 4 beta 1 (VLA-4) and alpha 4 beta 7 on the human B cell line JY. J Biol Chem. 1992;267:8366–8370. [PubMed] [Google Scholar]
- 30.Elices M J, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 31.Osborn L, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 32.Qiao JH, et al. Pathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb. 1994;14:1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- 33.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien KD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 36.Duplaa C, et al. Monocyte/macrophage recruitment and expression of endothelial adhesion proteins in human atherosclerotic lesions. Atherosclerosis. 1996;121:253–266. doi: 10.1016/0021-9150(95)05729-3. [DOI] [PubMed] [Google Scholar]
- 37.Kim JA, et al. Partial characterization of leukocyte binding molecules on endothelial cells induced by minimally oxidized LDL. Arterioscler Thromb. 1994;14:427–433. doi: 10.1161/01.atv.14.3.427. [DOI] [PubMed] [Google Scholar]
- 38.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 39.Wayner EA, Garcia-Pardo MJ, Humphries A, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphries MJ, Komoriya A, Akiyama SK, Olden K, Yamada KM. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987;262:6886–6892. [PubMed] [Google Scholar]
- 41.Lobb RR, Hemler ME. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mould AP, et al. Integrin alpha 4 beta 1–mediated melanoma cell adhesion and migration on vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced IIICS region of fibronectin. J Biol Chem. 1994;269:27224–27230. [PubMed] [Google Scholar]
- 43.Mosher, D.F. 1989. Fibronectin. Academic Press Publishers. San Diego, CA. 474 pp.
- 44.Hynes, R.O. 1990. Fibronectins. Springer-Verlag. New York, NY. 576 pp.
- 45.Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarzbauer J. Selective secretion of alternatively spliced fibronectin variants. J Cell Biol. 1989;109:3445–3453. doi: 10.1083/jcb.109.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elices MJ, et al. Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest. 1994;93:405–416. doi: 10.1172/JCI116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molossi S, et al. Blockade of very late antigen-4 integrin binding to fibronectin with connecting segment-1 peptide reduces accelerated coronary arteriopathy in rabbit cardiac allografts. J Clin Invest. 1995;95:2601–2610. doi: 10.1172/JCI117962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ugarova TP, Ljubimov AV, Deng L, Plow EF. Proteolysis regulates exposure of the IIICS-1 adhesive sequence in plasma fibronectin. Biochemistry. 1996;35:10913–10921. doi: 10.1021/bi960717s. [DOI] [PubMed] [Google Scholar]
- 50.Ljubimov AV, et al. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- 51.Fogelman AM, et al. Modification of the Recalde method for the isolation of human monocytes. J Lipid Res. 1988;29:1243–1247. [PubMed] [Google Scholar]
- 52.Wayner EA, Kovach NL. Activation-dependent recognition by hematopoietic cells of the LDV sequence in the V region of fibronectin. J Cell Biol. 1992;116:489–497. doi: 10.1083/jcb.116.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazarovits AI, Karsh J. Differential expression in rheumatoid synovium and synovial fluid of alpha 4 beta 7 integrin. A novel receptor for fibronectin and vascular cell adhesion molecule-1. J Immunol. 1993;151:6482–6489. [PubMed] [Google Scholar]
- 54.Kovach N L, Carlos TM, Yee E, Harlan JM. A monoclonal antibody to beta 1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luque A, et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 56.Thoumine O, Nerem RM, Girard PR. Changes in organization and composition of the extracellular matrix underlying cultured endothelial cells exposed to laminar steady shear stress. Lab Invest. 1995;73:565–576. [PubMed] [Google Scholar]
- 57.van der Laan L J, De Groot CJ, Elices MJ, Dijkstra CD. Extracellular matrix proteins expressed by human adult astrocytes in vivo and in vitro: an astrocyte surface protein containing the CS1 domain contributes to binding of lymphoblasts. J Neurosci Res. 1997;50:539–48. doi: 10.1002/(SICI)1097-4547(19971115)50:4<539::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 58.Wahl SM, et al. Synthetic fibronectin peptides suppress arthritis in rats by interrupting leukocyte adhesion and recruitment. J Clin Invest. 1994;94:655–662. doi: 10.1172/JCI117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yednock TA, et al. Alpha 4 beta 1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J Biol Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]
- 60.Pawlowski NA, Abraham EL, Pontier S, Scott WA, Cohn ZA. Human monocyte-endothelial cell interaction in vitro. Proc Natl Acad Sci USA. 1985;82:8208–8212. doi: 10.1073/pnas.82.23.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masumoto A, Hemler ME. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993;268:228–234. [PubMed] [Google Scholar]
- 62.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of α4b1 and α5b1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters JH, Sporn LA, Ginsberg MH, Wagner DD. Human endothelial cells synthesize, process, and secrete fibronectin molecules bearing an alternatively spliced type III homology (ED1) Blood. 1990;75:1801–1808. [PubMed] [Google Scholar]
- 64.Ichihara-Tanaka K, Titani K, Sekiguchi K. Role of the carboxyl-terminal Fib2 domain in fibronectin matrix assembly. J Cell Sci. 1995;108:907–915. doi: 10.1242/jcs.108.3.907. [DOI] [PubMed] [Google Scholar]
- 65.Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 66.Potts JR, Campbell ID. Structure and function of fibronectin modules. Matrix Biol. 1996;15:313–320. doi: 10.1016/s0945-053x(96)90133-x. [DOI] [PubMed] [Google Scholar]
- 67.Conforti G, et al. Human endothelial cells express integrin receptors on the luminal aspect of their membrane. Blood. 1992;80:437–446. [PubMed] [Google Scholar]
- 68.McDonald JA. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- 69.Checovich WJ, Mosher DF. Lysophosphatidic acid enhances fibronectin binding to adherent cells. Arterioscler Thromb. 1993;13:1662–1667. doi: 10.1161/01.atv.13.11.1662. [DOI] [PubMed] [Google Scholar]
- 70.Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells. Role of beta 1 integrins and tyrosine kinases. Circ Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 71.Ylanne J, et al. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sligh JE, Jr, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McEvoy LM, et al. Novel vascular molecule involved in monocyte adhesion to aortic endothelium in models of atherogenesis. J Exp Med. 1997;185:2069–2077. doi: 10.1084/jem.185.12.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calderon TM, Factor SM, Hatcher VB, Berliner JA, Berman JW. An endothelial cell adhesion protein for monocytes recognized by monoclonal antibody IG9. Expression in vivo in inflamed human vessels and atherosclerotic human and Watanabe rabbit vessels. Lab Invest. 1994;70:836–849. [PubMed] [Google Scholar]
- 75.Nageh MF, et al. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17:1517–1520. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- 76.Elices MJ. The integrin alpha 4 beta 1 (VLA-4) as a therapeutic target. Ciba Found Symp. 1995;189:79–85. [PubMed] [Google Scholar]
- 77.Laffon A, et al. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991;88:546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rabb H, Rosen R, Ramirez G. VLA-4 and its ligands: relevance to kidney diseases. Springer Semin Immunopathol. 1995;16:417–425. doi: 10.1007/BF00196097. [DOI] [PubMed] [Google Scholar]
- 79.Tamaki K, et al. TGF-beta 1 in glomerulosclerosis and interstitial fibrosis of adriamycin nephropathy. Kidney Int. 1994;45:525–536. doi: 10.1038/ki.1994.68. [DOI] [PubMed] [Google Scholar]
- 80.Sharma K, Ziyadeh FN. The emerging role of transforming growth factor-beta in kidney diseases [editorial] Am J Physiol. 1994;266:F829–F842. doi: 10.1152/ajprenal.1994.266.6.F829. [DOI] [PubMed] [Google Scholar]
- 81.Palinski W, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E–deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horkko S, et al. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids: recognition of cardiolipin by monoclonal antibodies to epitopes to oxidized low density lipoprotein. J Clin Invest. 1996;98:815–825. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fichorova R, Nakov L, Baleva M, Nikolov K, Gegova I. Sperm, nuclear, phospholipid, and red blood cell antibodies and isotype RF in infertile couples and patients with autoimmune rheumatic diseases. Am J Reprod Immunol. 1996;36:309–316. doi: 10.1111/j.1600-0897.1996.tb00181.x. [DOI] [PubMed] [Google Scholar]