Abstract
Transcriptional activation of the cyclin D1 gene (CCND1) plays a pivotal role in G1-phase progression, which is thereby controlled by multiple regulatory factors, including nuclear receptors (NRs). Appropriate CCND1 gene activity is essential for normal development and physiology of the mammary gland, where it is regulated by ovarian steroids through a mechanism(s) that is not fully elucidated. We report here that CCND1 promoter activation by estrogens in human breast cancer cells is mediated by recruitment of a c-Jun/c-Fos/estrogen receptor α complex to the tetradecanoyl phorbol acetate-responsive element of the gene, together with Oct-1 to a site immediately adjacent. This process coincides with the release from the same DNA region of a transcriptional repressor complex including Yin-Yang 1 (YY1) and histone deacetylase 1 and is sufficient to induce the assembly of the basal transcription machinery on the promoter and to lead to initial cyclin D1 accumulation in the cell. Later on in estrogen stimulation, the cyclin D1/Cdk4 holoenzyme associates with the CCND1 promoter, where E2F and pRb can also be found, contributing to the long-lasting gene enhancement required to drive G1-phase completion. Interestingly, progesterone triggers similar regulatory events through its own NRs, suggesting that the gene regulation cascade described here represents a crossroad for the transcriptional control of G1-phase progression by different classes of NRs.
Mammary gland morphogenesis and development result from the interplay of genetic and epigenetic pathways, controlled by hormones, growth factors, and other signaling molecules. Derangement of one or more of these regulatory pathways results in the abnormal growth and differentiation of mammary epithelial cells, leading to breast carcinogenesis. The ovarian hormones estrogen and progesterone promote mammary gland differentiation toward the female phenotype at the onset of puberty and control breast tropism and function throughout the reproductive life by affecting epithelial cell proliferation. Mammary gland cells are endowed with high-affinity receptors for these steroids (estrogen receptor α [ERα] and ERβ and progesterone receptor A [PR-A] and PR-B, respectively), which belong to the nuclear receptor (NR) family of transcription factors (31).
Transduction of the hormonal signal to the cell genome is mainly achieved through the accumulation of steroid-receptor complexes in the nucleus, where they can affect the target gene transcription rate through several mechanisms (19). In hormone-responsive human breast cancer (hBC) cells, ligand-activated ERs and PRs regulate target gene transcription by binding as homo- or heterodimers to their DNA response elements (EREs and PREs, respectively) (6, 31, 39) or by tethering to other classes of DNA-bound trans-acting factors, such as AP-1, SP1, and NF-κB (19, 26). In both cases, transcriptional regulation involves the recruitment of coregulators that, in turn, act as building blocks of larger, multicomponent complexes, which assemble on the locus, ultimately resulting in a modification of transcriptional output (19, 33).
In mammary epithelial cells, ovarian hormones induce the recruitment of quiescent (G0) cells in the cell cycle, G1 progression, and G1/S transition through the direct transcriptional control of genes encoding key cell cycle regulators, such as c-Fos, c-Jun, or c-Myc (7, 45, 50, 61, 63), and G1 cyclin genes, in particular, CCND1, encoding cyclin D1 (4, 36, 44). This last gene, which acts as a mitogen sensor linking extracellular signaling to the cell cycle machinery (52), plays a critical role in mammary gland physiology and pathology. Indeed, CCND1−/− mice show profound defects in mammary lobular-alveolar development, more evident during pregnancy, when the breast epithelial cell compartment is unresponsive to ovarian steroids and fails to undergo the massive proliferative changes that they normally induce (18, 53). On the other hand, cyclin D1 overexpression in mouse mammary epithelial cells induces breast cancer (58), whereas CCND1 gene inactivation protects against breast cancer induced by neu and ras oncogenes (66). In humans, D1 overexpression has been reported to distinguish invasive and in situ breast carcinomas from nonmalignant lesions (60). Interestingly, 50% of breast tumors overexpress cyclin D1, even though CCND1 gene amplification can be found at a frequency of only 13 to 15% (17). These data ndicate that epigenetic mechanisms, such as promoter deregulation or aberrant hormonal signaling, contribute significantly to this cancer-specific phenotype.
The precise mechanism for CCND1 gene regulation by ovarian steroids is not fully understood to date. The effects of estrogens on cyclin D1 mRNA expression have been shown to occur predominantly at the transcriptional level in hormone-responsive hBC cells, where an estrogen-sensitive region has been mapped to positions −956 to −136 of the human CCND1 promoter (4), a DNA region that does not contain canonical DNA response elements for ovarian steroids. An estrogen-responsive region has been mapped in HeLa cells expressing ectopic ERs to a cyclic AMP response element (CRE) located 52 nucleotides upstream of the transcription start site (47), with the possible minor participation of an AP-1 site (tetradecanoyl phorbol acetate-responsive element [TRE]) located further upstream (29). Promoter regulation through the CRE, however, has been demonstrated to be indirect in hBC cells, where it requires the activation of the protein kinase A (PKA) pathway (10). For progesterone, no evidence is available to date on the mechanisms sustaining CCND1 gene regulation by this hormone in hBC cells.
We describe here the results of genetic and molecular analyses of human CCND1 gene promoter regulation by 17β-estradiol (E2) in hormone-responsive MCF-7 and ZR-75.1 hBC cells. Transcriptional activation was found to be mediated by ERα, which drives the assembly of a transcriptional enhancing complex to a composite distal promoter element by tethering to an AP-1 heterodimer complex and inducing the simultaneous displacement of transcriptional repressor Yin-Yang 1 (YY1) from the same DNA region. Subsequent to these early ERα-mediated effects, the cyclin D1 concentration reaches a threshold level in the cell, triggering the association of the cyclin D1/Cdk4 holoenzyme with the promoter, presumably through a multiprotein complex also including E2F and pRb, and thereby generating an autoregulatory loop leading to the persistent transcriptional enhancement of this gene required to drive the cell through G1. Interestingly, the PR ligand R5020 triggers the same cascade of early molecular events by interacting with PRs, suggesting the possibility that the genetic element of the CCND1 gene characterized here may represent a site for G1 regulation by multiple classes of NRs.
MATERIALS AND METHODS
Cells.
Human breast cancer MCF-7 and ZR-75.1 cells were routinely grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with phenol red, [scap]l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (50 μg/ml), insulin (6 ng/ml), hydrocortisone (3.75 ng/ml), and 5% fetal calf serum (FCS) at 37°C in a humidified atmosphere composed of 95% air and 5% CO2. Cells were provided with fresh medium every 2 to 3 days. To evaluate the effect of estrogen challenge by chromatin immunoprecipitation (ChIP) assays, both cell lines were grown in phenol red-free DMEM containing 5% dextran-charcoal-stripped FCS for 4 days; thereafter, the medium was changed to DMEM containing 0.5% dextran-charcoal-stripped FCS for a further 16 to 18 h. The cells then were shifted back to 5% dextran-charcoal-stripped FCS and stimulated with 50 nM E2. To evaluate the effect of antiestrogens, cells were treated with 50 nM E2 and 5 μM ICI 182,780.
Stable and transient transfections.
For stable transfections, 107 MCF-7 or ZR-75.1 cells were harvested in phosphate-buffered saline (PBS) prior to being treated as previously described (4). A total of 10 μg of reporter constructs with various deletion fragments from the cyclin D1 promoter cloned 5′ to the luciferase gene and 1 μg of plasmid pZipNeo were used. For transient transfections, cells were grown to 70 to 80% confluence and then processed as reported previously (4). A total of 5 μg of luciferase reporter constructs and 1 μg of plasmid NLS lacZ as an internal control were used.
In vivo footprinting.
Approximately 6 × 106 to 7 × 106 MCF-7 or ZR-75.1 cells were treated with 0.2% dimethyl sulfate (Sigma) in prewarmed medium for 2 min. The reaction was stopped by washing the plate four times with PBS, and DNA was extracted as previously described (7). Genomic DNA was treated with 1 M piperidine for 30 min at 37°C prior to be precipitated three times with ethanol.
The ligation-mediated PCR (25 cycles) was performed with 1.5 μg of modified DNA as described previously (7, 22). A total of 1.5 × 106 cpm of end-labeled primer 3 was added, and the samples were heated at 94°C for 3 min and extended at 76°C for 20 min. Naked DNA methylation in vitro was performed according to standard protocols (32). The primers used were as follows: for the coding strand—TRE-L1-TCCTTCCGTCGGGCTTC, TRE-L2-CCTACCTTGACCAGTCGGTCCTTG, TRE-L3-TCCTTGCGGGGGTCCCCAACTGCACC, CRE-L1-GCTCTCGCTTCTGCTGC, CRE-L2-GCTCTTCTGCCCCTCGCCGGAG, and CRE-L3-CCTCGCCGGAGCGTGCGGACTCTGCT; and for the noncoding strand—TRE-U1-CTGCCAGCCCCCTCAC, TRE-U2-ACGCTCACGAATTCAGTCCCAGGG, TRE-U3-CAGCGCAAATTCTAAAGGTGAAGGGACG, CRE-U1-CGCCTCAGGGATGGCTT, CRE-2-TTTGGGCTCTGCCCCTCGCTGCTC, and CRE-U3-CTCGCTGCTCCCGGCGTTTGGCGCC.
Nuclear extracts.
Nuclear extracts from quiescent or hormone-challenged cells were prepared according to standard protocols (16, 62), with minor modifications. When needed, cells were treated with 50 nM E2 in the absence or presence of 5 μM ICI 182,780 or with 50 nM R5020 for 2 h before being collected.
Electrophoretic shift mobility assays (EMSAs).
The 24-mer double-stranded synthetic oligonucleotides used as probes were end labeled with [γ-32P]dATP and T4 polynucleotide kinase. Nuclear extracts (4 to 5 μg) were incubated with approximately 1.5 fmol of 32P-labeled probe (approximately 50,000 cpm) under conditions previously reported (21, 62). In some experiments, unlabeled double-stranded oligonucleotides used as competitors were also incubated with the extracts for 20 min on ice prior to probe addition. In experiments with antibodies, nuclear extracts were incubated with the respective antibodies in the same 20-μl reaction volume for 30 min at 4°C before probe addition and processing as described above. The antibodies used were as follows. Anti-ERα antibody F3 was from D. Metzger, Strasbourg, France. Anti-c-Jun sc-45, anti-c-Fos sc-52, anti-Oct-1 sc-232, and anti-YY1 sc-1703 were from Santa Cruz Biotechnology, Santa Cruz, Calif.
Luc chimeric plasmids.
Constructs D1Δ-138, D1Δ-754, D1Δ-860, D1Δ-956, and D1Δ-956μ carrying fragments from the human CCND1 gene promoter were kindly provided by M. Beato (Marburg, Germany). Plasmid D1Δ-18 was obtained by digesting luciferase vector pXP20 (derived from pXP2 [kindly provided by S. K. Nordeen, Denver, Colo.] by removing the AP-1-like sequence of the plasmid backbone) with SmaI and BglII and inserting a 32-mer synthetic double-stranded oligonucleotide reproducing the sequence between −18 and + 14 of the human CCND1 gene. Constructs D1Δ-4S46 and D1Δ-4S48 were generated by digesting D1Δ-956 and D1Δ-956μ, respectively, with NaeI and BglII, followed by religation via insertion of the synthetic −18 to +14 oligonucleotide described above. Plasmids D1-AP/dE, D1-APμ/dE, D1-AP/dEμ7, and D1-APμ/dEμ7, used in transient transfection assays, were obtained by inserting synthetic 24-mer oligonucleotides with wild-type or mutant TRE sequences in plasmid D1Δ-18. Constructs T-AP/dE and M-AP/dE were obtained by inserting the D1-AP/dE oligonucleotide into the SalI sites of plasmids pT81luc and pMluc (kindly provided by S. K. Nordeen), respectively.
Chemical interference.
For methylation of guanines, the oligonucleotide used as a probe was end labeled with [γ-32P]dATP and T4 polynucleotide kinase. Approximately 50 fmol of 32P-labeled probe (2 × 106 cpm to 3 × 106 cpm) was treated with 1.5 μl of dimethyl sulfate. To detect thymines, the same amount of 32P-labeled probe was partially modified with 30 μg of KMnO4. Preparative mobility shift assays were performed as described previously (24). After autoradiography, proteins were extracted and DNA was incubated with 1 M piperidine for 30 min at 94°C. Samples were finally electrophoresed on 15% polyacrylamide gels. Sequencing reactions were performed according to standard protocols (32).
ChIP and ReIP.
A total of 106 cells were washed twice with PBS, and proteins were cross-linked to DNA by the addition of 1% formaldehyde at room temperature for 10 min. Cultures then were washed twice with ice-cold PBS containing 1 mM phenylmethylsulfonyl fluoride, 1 mg of aprotinin/ml, and 1 mg of pepstatin A/ml and collected prior to proceeding according to standard protocols, with minor modifications, as described previously (50). Cell pellets were sonicated (Ultrasonics A350G) four times for 30 s each time at the maximum setting, followed by centrifugation for 10 min. Immunoprecipitation was performed overnight at 4°C with specific antibodies, followed by the addition of 50 to 60 μl of protein A-Sepharose-salmon sperm DNA or anti-mouse immunoglobulin G (whole molecule) and further incubation at 4°C for 16 to 18 h. After washing, eluates were heated at 65°C for 5 to 6 h to reverse the formaldehyde cross-linking before DNA extraction and ethanol precipitation. For PCR, 1 to 2 μl of resuspended DNA solution (from a total of 30 μl) and 32 to 33 cycles were used. For reimmunoprecipitation (ReIP), immunocomplexes were eluted from the primary ChIP by incubation with 10 mM dithiothreitol at 37°C for 30 min and diluted 1:50 in buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl [pH 8.1]) before being reimmunoprecipitated with secondary antibodies. ReIP of supernatants was carried out in a manner similar to that of the primary ChIP.
The primers used were as follows: for CCND1—Fw(−3247) (GCTGAAACTAATTGATCTGGAG), Rv(−2931) (CCATTGTTAAGCCCTTAAGTC), Fw(−1039) (AACAAAACCAATTAGGAACCTT), Rv(−770) (ATTTCCTTCATCTTGTCCTTCT), Fw(−235) (TATGAAAACCGGACTACAGG), and Rv(−53) (CTGTTGTTAAGCAAAGATCAAAG); for pS2—Fw(−593) (CCAGGCCTACAATTTCATTAT), and Rv(−295) (AGGGATCTGAGATTCAGAAAG); and for Myc—Fw(−325) (CTCACAGGACAAGGATGCGGTTTGTCA), and Rv(−38) (TGGGCGGAGATTAGCGAGAGAGGATCT).
The antibodies used were as follows. Anti-ERα antibody 314 for the N terminus and antibody 1603 for the C terminus were from C. Abbondanza, Naples, Italy. Anti-pRb R6775 was from Sigma. Anti-c-Jun sc-1694 and sc-45, anti-c-Fos sc-52, anti-Oct-1 sc-232, anti-YY1 sc-1703, anti-pRb sc-102, anti-Cdk4 sc-260 and sc-601, anti-E2F sc-193 and sc-633, anti-polymerase II sc-899, anti-cyclin D1 sc-246 and sc-92, and anti-CREB-1 sc-58 were from Santa Cruz. Anti-phospho-CREB 06-519, anti-AcH4 Lys5 06-759 MN, and anti-AcH4 Lys8 06-760 MN were from Upstate Cell Signaling Solutions, Lake Placid, N.Y. Anti-histone deacetylase 1 (HDAC1) 210-256-C100 was from Alexis Biochemicals, San Diego, Calif. Anti-PR-A/PR-B Ab-2, Ab-3, Ab-6, and Ab-10 were from NeoMarkers, Fremont, Calif.
The PKA inhibitor H-89{N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide · 2HCl } was purchased from Calbiochem, San Diego, Calif.
Northern blot analysis.
Total RNA was isolated by the guanidinium thiocyanate-acid-phenol procedure (4). To analyze cyclin D1 mRNA levels, hormone-depleted MCF-7 cells were incubated with E2 for 30 min, 1 h, 2 h, 4 h, and 12 h prior to being harvested by scraping. RNA samples (20 μg) were resuspended in 20 μl of a denaturing solution (48% formamide, 7% formaldehyde, morpholinepropanesulfonic acid [MOPS], 5% glycerol), run on 1% agarose gels with 2% formamide, and then blotted on nylon filters. cDNA probe preparation and filter treatments were as reported earlier (4). After hybridization, filters were autoradiographed for times varying from 16 to 48 h. Normalization was accomplished by using 36B4 cDNA as a reference probe as described previously (4).
Western immunoblotting.
Immunoblotting analyses were carried out as described earlier (12). A total of 15 to 20 μg of MCF-7 or ZR-75.1 cell nuclear proteins, prepared as described above, was subjected to sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis and then transferred to cellulose nitrate filters under wet conditions. After blocking was done with 1% nonfat milk-0.1% Tween 20 in PBS for 1 h, the filters were incubated with the primary antibody diluted in 0.1% Tween 20 in PBS for 1 h. The blots then were washed and incubated with the peroxidase-conjugated secondary antibody (anti-rabbit or anti-mouse immunoglobulin-horseradish peroxidase-linked whole antibody; Amersham Life Science) for 1 h at room temperature. Protein-antibody complexes were revealed by enhanced chemiluminescence detection. The antibodies used were as follows. Anti-ERα antibody 314 was obtained from C. Abbondanza. Anti-c-Jun sc-45, anti-JunB sc-46, anti-c-Fos sc-52, anti-Oct-1 sc-232, and anti-YY1 sc-1703 were obtained from Santa Cruz.
RESULTS
Identification of an estrogen-responsive region within the CCND1 gene promoter.
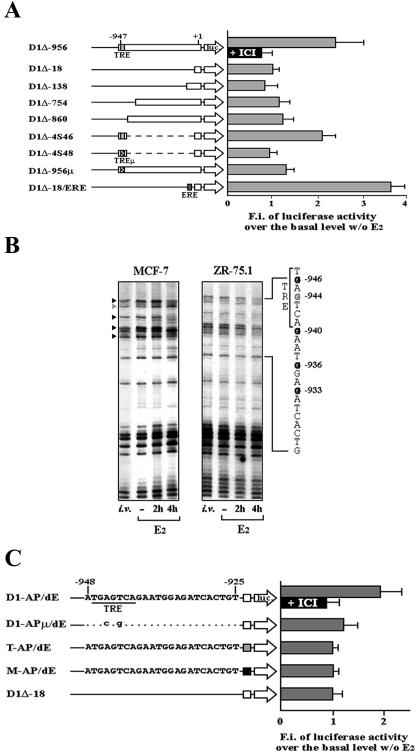
An initial analysis of cyclin D1 gene regulation by estrogens in hBC cells led to the identification of the promoter region involved in hormonal action (4). Different E2-responsive sites were later mapped within this regulatory region by various authors (10, 29, 47) using transient transfection of CCND1 promoter-based reporter genes and ER expression vectors mainly in nonmammary cell lines. In order to definitely clarify the molecular mechanism by which E2 controls cyclin D1 expression in hBC cells, we began by analyzing promoter activity in both MCF-7 and ZR-75.1 cells upon stable transfection of human CCND1 reporter genes, a condition which better mimics the promoter status in its natural chromatin environment. As shown in Fig. 1A, we identified a primary hormone-responsive site between positions −956 and −860. E2 treatment of cells harboring reporter plasmids (D1Δ-956 and D1Δ-4S46) including this region induced a 2.5-fold enhancement of transfected gene expression, comparable to that observed for the endogenous CCND1 gene under the same experimental conditions (4). Hormone responsiveness was inhibited by the pure antiestrogen ICI 182,780 (Fig. 1A), suggesting that it is mediated by the ER. Furthermore, hormonal activation was dependent upon the integrity of the AP-1 site (TRE) present in this region, as mutations of this sequence inhibited the hormone responsiveness of the test gene (D1Δ-4S48 and D1Δ-956μ). Identical results were obtained for MCF-7 cells (Fig. 1) and ZR-75.1 cells (data not shown).
FIG. 1.
(A) Analysis of CCND1 promoter responsiveness to estrogen upon stable transfection of the indicated luciferase reporter genes in hormone-responsive hBC cells, represented as fold induction by 50 nM E2 for 18 to 24 h (grey bars) versus the activity assayed in hormone-starved cells, arbitrarily set to 1. The black bar represents the fold induction measured in cells incubated simultaneously with 50 nM E2 and 5 μM ICI 182,780. On the left are schematically represented constructs used in transfections. TRE, TPA-responsive element; TREμ, same site mutated as previously described (22). Numbers at the top of the box mark the positions of the 5′-most nucleotides with respect to the transcription start site. Constructs D1Δ-138, D1Δ-754, D1Δ-860, D1Δ-956, and D1Δ-956μ were previously described (22). Constructs D1Δ-18, D1Δ-4S46, D1Δ-4S48, and D1Δ-18/ERE are described in Materials and Methods. F.i., fold induction. Bars indicate standard deviations. (B) In vivo footprinting of the CCND1 gene promoter region encompassing the TRE. The G residues marked by black arrowheads are specifically protected in E2-stimulated cells, whereas the G identified by a grey arrowhead is protected in the same cells depleted by E2. i.v., naked DNA methylated in vitro. (C) Estrogen responsiveness of luciferase reporter genes measured in hBC cells stably transfected with the plasmids schematically represented on the left. Synthetic wild-type or mutant 24-mer oligonucleotides reproducing the CCND1 gene sequence between residues −948 and −925 were cloned immediately upstream of the minimal promoter from the same gene (spanning residues −18 and + 14; white square), the thymidine kinase promoter (grey square; T-AP/dE), or the mouse mammary tumor virus promoter (black square; M-AP/dE). Mutant nucleotides in D1-APμ/dE are indicated. MCF-7 or ZR-75.1 cells were hormone deprived and restimulated as described above, including, where indicated, treatment with ICI 182,780. As for panel A, the results represent the means of three to five independent experiments. Luciferase activity assayed in starved cells transfected with each reporter was arbitrarily set to 1. Unless otherwise indicated, data displayed are from representative experiments carried out with MCF-7 cells; identical results were obtained with ZR-75.1 cells.
Potential in vivo protein-DNA interactions over this promoter region before and after hormone stimulation then were investigated with the same cell lines by dimethyl sulfate genomic footprinting analysis (Fig. 1B). The results showed that E2 challenge is accompanied by the binding of one or more factors to the upstream-most segment of the region identified by stable promoter transfection. This finding is revealed by protection from methylation of four G residues of the sense strand within and immediately downstream from the TRE (positions −946, −940, −936, and −933). On the other hand, the guanine at −944 appears to be protected only in quiescent cells, suggesting that different factors may bind to the CCND1 distal promoter before and after hormone treatment. Interestingly, the same DNA region is the likely target(s) of multiple mitogenic cascades, since a similar footprint was observed by Herber et al. (22) for serum-stimulated human fibroblasts, even though the TRE did not appear to be directly involved in that case. Genomic footprinting analysis of the promoter-proximal CRE region of CCND1 did not reveal any significant change in the response to E2 in hBC cells (data not shown), despite the fact that in HeLa cells this region was reported to mediate hormonal regulation of this promoter through the recruitment of a c-Jun/ATF-2 heterodimer (47). This analysis also supports the results of the promoter deletion analysis shown in Fig. 1A, which showed that deletion of the CRE region does not affect promoter responsiveness to estrogen via the upstream-most region.
To investigate whether the smaller DNA region identified in vivo was indeed functional in hBC cells, synthetic double-stranded oligonucleotides reproducing the sequence between positions −948 and −925 were cloned upstream of the cyclin D1 promoter in D1Δ-18 to generate D1-AP/dE. This construct was stably transfected into MCF-7 and ZR-75.1 cells and found to be responsive to E2 in both cell lines (Fig. 1C). The AP/dE sequence thus was named ERGE (estrogen-responsive G1 element) to signify its involvement in the hormonal regulation of CCND1 activity during G1 (see also below). Even in this case, an inactivating mutation of the TRE (D1-APμ/dE) inhibited hormone responsiveness. In addition, these results were strictly dependent on the promoter context as well, as hormone responsiveness cannot be conveyed to the heterologous herpes simplex virus thymidine kinase or mouse mammary tumor virus promoters (T-AP/dE and M-AP/dE reporters, respectively, in Fig. 1C), indicating that the trans-acting factors involved exert their activity under these conditions in combination with the “initiator ” element of the CCND1 promoter.
In vivo binding and in vitro binding of different trans-acting factors to the estrogen-responsive transcriptional enhancer of the human cyclin D1 gene.
Sequence analysis of the ERGE region, performed to identify potential cis-acting DNA elements, revealed closely spaced and partly overlapping binding sites for AP-1, YY1, and Oct-1 trans-acting factors (Fig. 2A). ChIP was used to assay the binding of each of these proteins to this DNA region in vivo. The results show that antibodies against each of these factors can indeed specifically immunoprecipitate chromatin centered around the ERGE and encompassing nucleotides −1039 to −770 of the CCND1 gene but not colinear chromatin regions from −235 to −53 (Fig. 2B, lower panel) or from −3247 to −2931 (Fig. 2C and data not shown). Comparative analyses carried out with hormone-starved versus stimulated MCF-7 and ZR-75.1 cells, however, reveal interesting differences among these factors. Indeed, while YY1 bound to the promoter can be detected only in hormone-deprived, quiescent cells, the c-Jun/c-Fos heterodimer and Oct-1 bind to this site after E2 stimulation. Of note, this transcription factor switching is accompanied by the recruitment of ERα to the same region in hormone-challenged cells (Fig. 2C), despite the fact that this DNA lacks canonical ERE sequences. A direct interaction of the receptor with the region centered around the TRE is supported by the control experiments shown in Fig. 2B, which confirmed that chromatin between the AP-1 site and the initiator was fragmented by sonication during sample preparation; for example, primers spanning the cyclin D1 region between −1039 and −53 systematically failed to amplify control (input) or immunoprecipitated DNAs (Fig. 2B, upper left panel, and data not shown). Further confirmation was provided by a lack of amplification of the region between −235 and −53 in chromatin from quiescent cells precipitated with anti-YY1, indicating that physical separation between the promoter-proximal and promoter-distal DNA regions was achieved in vitro and that we could indeed discriminate direct protein-DNA interactions occurring at either of the CCND1 promoter regions (see below).
FIG. 2.
ChIP analysis of in vivo interactions of trans-acting factors with the TRE-containing region of the CCND1 promoter. (A) Sequence of the estrogen-responsive region between residues −948 and −925. G nucleotides with black arrowheads on top represent residues protected in hormone-stimulated cells, as detected by in vivo footprinting; the G marked with a grey arrowhead is protected instead only in quiescent cells. Consensus sequences for AP-1, Oct-1, and YY1 binding sites are aligned with the homologous sequences found here. (B and C) Soluble chromatin was prepared from hBC cells before or after 30 min of treatment with 50 nM E2 and immunoprecipitated with antibodies against the indicated proteins before DNA amplification with the indicated primers. In order to obtain optimal separation of regions immediately adjacent, only DNA samples which were not amplified by PCR with primers separated by more than 500 bp, e.g., those indicated at the top left (spanning the region between −1039 and −53), were used for the ChIP assays. Input, total chromatin before immunoprecipitation; α-Mock, control, unrelated antibodies used for immunoprecipitation; pS2, estrogen-responsive region of the pS2 gene promoter. The primers used for PCR amplifications are described in Materials and Methods. Data displayed are from representative experiments carried out with ZR-75.1 cells; identical results were obtained with MCF-7 cells.
The ability of transcription factors to bind to the ERGE then was tested in vitro by EMSAs with double-stranded oligonucleotide probes reproducing the sequence of the CCND1 promoter from −948 to −925 (Fig. 3A). Analysis of the electrophoretic patterns displayed by different, independently prepared nuclear extracts revealed, besides minor species variable from one preparation to the next and probably due to protein degradation, the constant presence of three main DNA-protein complexes (c1 to c3); by use of competitor DNAs and specific antibodies, these complexes could be shown to contain Oct-1 (c1), the c-Jun/c-Fos heterodimer (c2), and YY1 (c3). A much less abundant, more slowly migrating DNA-protein complex, including both Oct-1 and c-Jun/c-Fos, could also be detected when higher concentrations of nuclear extracts were used (data not shown), indicating that all of these factors can bind simultaneously to the AP/dE site. However, they appear to interact here independently of each other, as we failed to detect cooperative binding (data not shown). Interestingly, even though the AP/dE sequence does not include canonical EREs, the labeled probe also captures ERα, as shown by supershifting with different antireceptor antibodies (Fig. 3A, lane 11, and data not shown). The receptor appears to interact with DNA by tethering only to the AP-1 complex, since inactivating mutations of the TRE but not of the Oct-1 site prevented the binding of both factors to the DNA (Fig. 3A, lanes 17 and 18), while yeast two-hybrid protein-protein interaction assays and in vitro coimmunoprecipitation experiments failed to reveal a stable interaction of ERα with Oct-1 either in solution or bound to DNA (data not shown). This evidence, together with previous results showing that c-Jun is able to bind ERα in vivo and in vitro (56), supports the possibility that the receptor is present in a subset of c2 complexes which cannot be resolved by electrophoretic analysis.
FIG. 3.
In vitro binding of transcription factors to the estrogen-responsive region of the CCND1 gene. (A) EMSAs with nuclear extracts from hormone-responsive hBC cells treated or not treated with 50 nM E2 for 2 h and challenged with 32P-labeled AP/dE double-stranded oligonucleotide; in one case (+ICI), a 100-fold molar excess of ICI 182,780 was also added to the culture media as described in Materials and Methods. In some experiments, a 100-fold molar excess of unlabeled oligonucleotide carrying consensus binding sites for the indicated factors was used as a competitor (comp.), or the nuclear extracts were pretreated with the indicated antibodies (Ab). NIS, pool of preimmune sera; ss, supershifted complexes observed with anti-ER, anti-c-Fos, and anti-Oct-1 antibodies (antibodies against c-Jun and YY1 hamper the interactions of these factors with DNA). The protein-DNA complexes indicated as c1, c2, and c3 were consistently observed with different nuclear extracts from either MCF-7 or ZR-75.1 cells and are specific, while the smaller ones represent nonspecific complexes, which were erratic or resulted from sample degradation during preparation or handling. Data displayed are representative of results obtained in multiple tests carried out with different nuclear extracts. (B) Results of EMSAs performed with equal amounts of nuclear proteins extracted from hormone-deprived (−E2) or hormone-stimulated (+E2) hBC cells and increasing amounts of AP/dE-labeled probe. Following electrophoresis and autoradiography, the intensity of the signal corresponding to complexes c1 (Oct-1), c2 (AP-1), or c3 (YY1) in each lane was measured by densitometry. Bars represent standard deviations. (C) Representative immunoblot of nuclear extracts from hBC cells treated with E2 and analyzed by Western blotting with antibodies against the represented proteins. Only the area of the filters where each protein of interest was detected is displayed. (D) Mutant analysis of in vitro transcription factor binding to the AP/dE composite element by EMSAs with nuclear extracts from hormone-treated hBC cells. In the top panel are shown the sequences tested by direct binding, with mutant residues in evidence; numbers to the right mark the corresponding lane in the autoradiogram below. Data displayed are from representative experiments carried out with MCF-7 cells; identical results were obtained with ZR-75.1 cells.
Cell treatment with E2 increases the levels of c1 and c2 complexes, and this effect can be prevented by ICI 182,780 (compare lanes 1, 2, and 3 in Fig. 3A), suggesting that hormone stimulation increases Oct-1 and AP-1 binding to their respective sites. In our opinion, the failure of E2 to reduce the level of the c3 complex does not contradict in vivo data showing that YY1 was not detected on the cyclin D1 promoter after hormone treatment, since this finding might relate to specific chromatin features at this site. Indeed, the control of chromatin structure by interactions of transcription factors with cis-acting elements is well known and is absent in vitro when naked DNA is used. Scatchard analysis (Fig. 3B) demonstrates that E2 does not promote significant changes in the Oct-1 or AP-1 binding affinity (Kd) for DNA but increases instead the binding capacity (Vmax) of these factors for their respective target sites. For AP-1, this effect can be related to consistent increases in c-Fos and c-Jun concentrations in nuclear extracts from hormone-treated cells, detectable by Western blotting (Fig. 3C) and mainly consequent to activation of their respective genes during the immediate-early cellular response to estrogen (61). On the contrary, the Oct-1 protein concentration remains unchanged following E2 treatment (Fig. 3C); this result suggests that enhanced binding of this factor to DNA is likely due here to the activation of a preexisting pool of inactive proteins, perhaps resulting from posttranslational modifications, such as those shown to control the ability of Oct-1 to bind to DNA during the cell cycle (46, 49), and/or interactions of this factor with coregulators (68). Mutational analysis of AP/dE-protein interactions in vitro confirms that while the Oct-1 and AP-1 binding sites are distinct and physically separated from each other, the YY1 binding site overlaps the octamer element (Fig. 3D).
Estrogen activates the CCND1 gene promoter through the recruitment of AP-1, Oct-1, and ERα to the ERGE and the displacement of a repressor complex containing YY1.
To investigate the dynamics and nature of transcription factor assembly to the estrogen-responsive CCND1 promoter region, sequential ChIP assays were performed with both MCF-7 and ZR-75.1 cells before and at different times after (5 to 30 min) hormonal stimulation. The results showed that YY1 can indeed be detected bound to the AP/dE site of the CCND1 gene in vivo before and for up to 10 min after E2 challenge, after which it becomes undetectable, whereas c-Jun, c-Fos, ERα, and Oct-1 are all recruited together on the same DNA (Fig. 4A). To assess whether the presence of YY1 may contribute in this situation to the maintenance of chromatin in a repressed state, we performed immunoprecipitation with antibodies against HDAC1, one of the ubiquitously expressed chromatin-modifying enzymes previously shown to interact with YY1 on promoters inhibited by this protein (15, 64). The results showed that the DNA region under study was indeed precipitated by anti-HDAC1 antibodies with kinetics similar to those for YY1 (Fig. 4A), indicating the simultaneous presence of both proteins on the ERGE and suggesting that their combined activity may contribute to CCND1 promoter trans-repression in hormone-starved quiescent cells. Gene inhibition then would be relieved upon hormonal stimulation by displacement of these repressors by an activating complex including the c-Jun/c-Fos dimer and ligand-activated ERα, cooperating with Oct-1. Indirect support for this possibility is provided by the observation that H4 histone acetylation at lysines 5 and 8 increases at this chromatin site upon cell stimulation with E2 with kinetics similar to those of the regulatory protein switch described above (Fig. 4A). Mutual exclusion between YY1 and AP-1/Oct-1 at the AP/dE site is also consistent with the results of methylation interference experiments, which supported the results of the in vitro binding assays with mutant AP/dE oligonucleotides (Fig. 3D) and which showed how the binding site for YY1 overlaps both the TRE and the octamer sites (Fig. 4B). Of note, YY1—but not the octamer factor—contacts in vitro the G residue located at −944 of the sense strand, which was found to be protected in vivo only in hormone-starved cells (Fig. 1B).
FIG. 4.
Kinetics of in vivo transcription factor interactions with the estrogen-responsive region of the CCND1 gene in hormone-treated hBC cells and assessment of the transcriptional output. (A) ChIP analysis of CCND1 gene upstream regulatory site occupancy by different transcription factors in quiescent and estrogen-stimulated cells. (B) Summary of chemical interference assays carried out with nuclear extracts from MCF-7 cells following estrogen stimulation. The TRE sequence is boxed, and nucleotides contacting Oct-1 or YY1 proteins are indicated by black (Gs) and white (Ts) dots. (C) (Left panel) Transient transfection analysis of the effects of transcription factor overexpression on the indicated reporter gene activity in hBC cells stimulated with estrogen. A total of 50 to 200 ng of the indicated expression vectors was transfected, together with the reporter gene and an internal control; luciferase activity measured in the same cells transfected with an empty expression vector was arbitrarily set to 1. (Right panel) Effects of the AP/dE sequence on basal reporter gene activity in the absence of E2; luciferase activity of the cyclin D1 promoter-based D1Δ-18 vector was arbitrarily set to 1. Transfections were performed as described in Materials and Methods. F.i., fold induction. Bars indicate standard deviations of two to four independent assays performed in duplicate. (D) Northern blot analysis of cyclin D1 mRNA expression in ZR-75.1 cells before and at the indicated times after stimulation with E2; 36B4 ribosomal protein gene mRNA was also quantitated in the same blot as a control. (E) Sequential ChIP (ReIP) analyses of transcription factor interaction with the estrogen-responsive region of the CCND1 gene between positions −1039 and −770. II°IP, results obtained following a second immunoprecipitation with the indicated antibodies. (F) Occupancy of CCND1 gene initiator-proximal region by polymerase II, as assessed by ChIP. E2, treatment of cells with E2. For details, see Results. Unless otherwise indicated, data displayed are from representative experiments carried out with ZR-75.1 cells; identical results were obtained with MCF-7 cells.
Since it was possible for us to resolve by ChIP adjacent promoter regions spaced >500 to 600 bp apart (see above and Fig. 2A), we next examined whether recruitment of the positively acting factors described above at the ERGE is followed by transcription initiation complex assembly on the promoter and, if so, whether it is possible to detect physical contacts between the upstream enhancer and the transcription machinery. To this end, we assessed the ability of the estrogen-responsive upstream region to be immunoprecipitated by antibodies against the large subunit of RNA polymerase II, which is expected instead to be found at and downstream of the transcription start site. As shown in Fig. 4A, the association of the polymerase with ERGE-bound factors can indeed be detected at 15 min after E2 challenge, in parallel with trans-acting factor switching at the AP/dE site and subsequent recruitment of ERα. A significant accumulation of CCND1 mRNA already is detectable in cells 30 min after E2 challenge (Fig. 4D), further implying that YY1 and HDAC1 displacement by the other regulatory factors upon hormone challenge is indeed immediately followed by an increase in the rate of transcription of CCND1.
To define the causal role of the transcription factors described above in mediating the cyclin D1 gene response to E2, we analyzed the effect of their overexpression on cyclin D1 promoter activity in transient transfection assays (Fig. 4C). The results of such assays carried out with both hBC cell lines under study confirmed the enhancing effect of c-Jun, c-Fos, and Oct-1 on promoter activity and the repressor role of YY1, which also counteracts the stimulatory effect of c-Jun and Oct-1. The inhibitory effect of YY1 overexpression is inhibited in the mutant reporter D1-AP1/dEμ7, which is unable to bind to this protein (Fig. 4C, left panel). This same reporter also showed a significantly higher level of basal transcriptional activity than did D1-AP1/dE (Fig. 4C, right panel). Interestingly, D1-AP1/dEμ7 activity also was unaffected by Oct-1 overexpression, despite the fact that it is still estrogen responsive. This result indicates that, in the absence of YY1 binding, Oct-1 may be dispensable for AP-1/ERα complex recruitment to the TRE. In turn, this would suggest that the main role for the octamer factor is to help AP-1 bind to ERGE by displacing YY1, at least in hBC cells after estrogen stimulation.
Focusing on ERα, we set forth to verify that the assembly of this protein to the transcription-activating complex present on the AP/dE site determines CCND1 promoter activation. To this end, we performed serial ChIP analyses by dividing chromatin from quiescent and E2-challenged cells into two aliquots, one of which was immunoprecipitated with anti-YY1 antibodies and the other of which was immunoprecipitated with anti-ERα antibodies. The complexes then were released and reimmunoprecipitated with antibodies recognizing the other key factors under study. The same procedure also was carried out with unbound supernatant fractions from the primary immunoprecipitations and, in all instances, the upstream region from −1039 to −770 was amplified by PCR. As shown in Fig. 4E, RNA polymerase II could be detected, together with c-Jun and Oct-1, in the bound chromatin pulled down by anti-ER antibodies and in the unbound supernatant fractions from samples immunoprecipitated with anti-YY1 antibodies. The exact opposite was true for HDAC1. It is worth noting that the polymerase also can be found associated with the region across the transcription start site of the gene in hormone-depleted cells (Fig. 4F), thereby justifying the basal CCND1 activity observed under these conditions (Fig. 4D). These data, when combined, demonstrate differential interactions of the polymerase with the hormone-responsive and initiator regions of the gene, confirm that ligand-activated ER assembles with the ERGE in a transcription-competent complex, and provide a direct demonstration that the CCND1 gene is a primary target of nuclear ERα in growth-stimulated hBC cells (see Discussion).
ERα has been shown to be able to activate the CCND1 promoter in HeLa cells via a CRE site located near the initiator through a PKA-independent mechanism (29, 47), suggesting that this DNA element may contribute to cyclin D1 promoter regulation in some cellular backgrounds. On the other hand, Castro-Rivera et al. (10) reported that CREB phosphorylation by PKA is instead an essential requirement for CCND1 promoter activation via the CRE in estrogen-treated ZR-75.1 cells. Although the results of our stable transfection assays allow us to exclude the possibility that the CCND1 CRE represents a primary site of action of estrogen when the gene is endowed in hBC cell chromatin (4) (Fig. 1A), we cannot exclude the possibility that cross talk may indeed exist between the CRE and the ERGE, perhaps contributing to the fine regulation of CCND1 activity in specific physiological conditions and/or cell types. CREB activation by PKA, for example, could be required to facilitate receptor assembly to the CCND1 promoter (10). For this reason, we set out to verify to what extent ERα recruitment to the AP/dE site depends upon the phosphorylation of CRE-bound CREB by PKA. To this end, chromatin from hBC cells stimulated with E2 in the absence or in the presence of the PKA inhibitor H89 was subjected to immunoprecipitation with anti-ERα antibodies, and the precipitated fractions were subjected to ReIP with antibodies against the large subunit of RNA polymerase II to verify the presence of protein-protein interactions indicative of promoter activation via the distal hormone-responsive site (see above). The results reported in Fig. 5A demonstrate that the receptor and the polymerase can be recruited together to this promoter after E2 treatment, even in the presence of PKA blockade by H89, which instead greatly affects the phosphorylation of CREB bound to its CRE site. These results, in conjunction with data from the ReIP assays shown in Fig. 4E and the stable transfections shown in Fig. 1A, indicate the ability of ERα bound to the ERGE to promote CCND1 transcription independently of CREB activation by PKA.
FIG. 5.
(A) PKA inhibition does not prevent ERα recruitment to the estrogen-responsive region of the CCND1 gene promoter. Sequential ChIP (ReIP) analyses of the TRE-centered region from the CCND1 promoter. Chromatin prepared from cells treated or not treated with 50 nM E2 in the absence or presence of the PKA inhibitor H89 was subjected to the ChIP procedure with an antibody against ERα (α-ER: I°IP) and then again with anti-RNA polymerase II antibodies (α-Pol II: II°IP). In some experiments, 5 μM H89 was added to the cells 30 min before E2. Input, total chromatin before the first immunoprecipitation. As a control, antibodies against total (α-CREB) or phosphorylated (αP-CREB) CREB were used to immunoprecipitate the CRE-containing proximal region of the CCND1 promoter from hormone-deprived (−E2) and hormone-stimulated (+E2) cells. (B) In vitro binding of cyclin D1 and pRb to the upstream regulatory region of the CCND1 gene. Soluble chromatin was prepared from hormone-responsive hBC cells before and at the indicated times after treatment with 50 nM E2 and immunoprecipitated with antibodies against the indicated proteins before PCR amplification. Input, total chromatin before immunoprecipitation; α-Mock, control, unrelated antibodies used for immunoprecipitation. The presence of the E2F-containing regulatory region of the c-Myc proto-oncogene was investigated with the same immunoprecipitated samples as described in Materials and Methods. (C) Sequential ChIP (ReIP) analyses of the AP/dE region from the CCND1 promoter. Chromatin prepared from cells treated or not treated with 50 nM E2 for the indicated times was subjected to the ChIP procedure with an antibody against cyclin D1 (α-D1: I°IP) and then again with anti-pRb antibodies (α-pRb: II°IP). Input, total chromatin before the first immunoprecipitation. (D) Assays like those in panel A were carried out with chromatin extracted from hBC cells stimulated at time zero with 50 nM E2 followed, where indicated (ICI), by the addition after 60 min of 5 μM ICI 182,780 to the cell culture media. The times indicated when cells were collected for analysis. Results shown are representative of multiple independent experiments. Unless otherwise indicated, data displayed are from experiments carried out with ZR-75.1 cells; identical results were obtained with MCF-7 cells.
Cyclin D1 associates with a trans-acting complex present on its own gene promoter.
All of the gene responses described so far relate to early events, occurring within the first 30 min of cell stimulation by E2. The rate of CCND1 gene transcription and cyclin D1 mRNA levels, however, keep increasing steadily for at least 4 h of E2 stimulation and are sustained for several hours thereafter (Fig. 1B and 4D), despite the fact that AP-1 activation by estrogen in hBC cells is transient and rapidly reversible (26, 61) (Fig. 3C). We speculated that these results might be the consequence of stable interactions with the ERGE of the nuclear proteins identified above, possibly coupled to the recruitment of additional regulatory factors in the CCND1 promoter. The persistent association of both c-Jun and ERα can indeed be detected for up to 4 h of estrogen exposure (Fig. 5B), whereas Oct-1 cannot be revealed anymore by ChIP after 2 h. Although we cannot exclude the possibility of epitope masking in this last example, presumably due to further chromatin modifications (see below), the failure of different polyclonal antibodies against this protein to detect it at the Ap/dE site after longer cell exposure to E2 (data not shown) strongly supports the possibility that the binding of Oct-1 to this site may indeed be transient.
Among all of the possibilities investigated, ChIP analysis showed that E2F and its functional partner, pRb (59), can be found associated with the CCND1 promoter in quiescent cells (Fig. 5B), presumably at an E2F binding site located approximately 200 nucleotides downstream from ERGE (22). It was reported that E2 promotes in hBC cells the assembly and activation of the cyclin D1/Cdk4 complex and pRb phosphorylation within 1 to 3 h of stimulation (4, 12), conditions which may promote the activation of genes actively trans-repressed by E2F/pRb complexes. We thus speculated that estrogen might induce cyclin D1/Cdk4 holoenzyme interactions with the E2F/pRb complexes bound to CCND1, thereby providing further and long-lasting enhancement of CCND1 gene transcription. Interestingly, we found that both cyclin D1 and Cdk4 proteins assemble to the chromatin region under study, where they start to be detectable 60 min after E2 treatment and increase progressively thereafter, to reach a maximum after 4 h (Fig. 5B). Cdk2, which does not form an active holoenzyme with cyclin D1 (4), could not be detected on the CCND1 promoter by ChIP (data not shown). Sequential ChIP analyses with anti-cyclin D1 and anti-pRb antibodies indicated that the two proteins are closely associated with each other on the CCND1 promoter after estrogen exposure (Fig. 5C), suggesting that pRb likely is phosphorylated by cyclin-associated Cdk4 under these conditions.
Cyclin D1 can physically interact with ERα and thereby act as a transcriptional coregulator of the receptor (38, 69). It is thus possible that cyclin D1 detected by ChIP on the CCND1 gene in estrogen-stimulated cells is, all or in part, associated with ERα. To test this possibility, we carried out ChIP analyses with cells exposed to an excess of ICI 182,780, the antiestrogen that renders the receptor unable to bind to DNA, 60 min after E2 exposure, i.e., after the initial assembly of the multiprotein complexes described above on the CCND1 promoter. As shown in Fig. 5D, receptor blockade with ICI 182,780 causes the rapid release of ERα but not cyclin D1 from the CCND1 promoter, indicating that it seems unlikely that the cyclin interacts with chromatin via ERα. c-Jun binding, on the other hand, is less readily inhibited by estrogen blockade and appears to be independent from ERα disassembly. Chromatin returns then to its prestimulation level after 90 min of hormonal blockade, with a decline in c-Jun binding and the reappearance of YY1 and HDAC1; these activities suggest a “clearance ” process that allows chromatin to return to a prestimulation level permissive for reactivation, such as that recently described by Metivier et al. (34). Interestingly, the kinetic study with the antiestrogen displayed in Fig. 5D also revealed that after 60 min of cell stimulation by estrogen, the interaction of RNA polymerase II with the upstream regulatory region of the CCND1 gene was no longer linked to the presence of ERα or c-Jun on the AP/dE site, as it decreased instead with the release of cyclin D1 from chromatin. These results suggest that, while during the initial phase of response to the hormone the factors that assemble on ERGE play a pivotal role in transcriptional enhancement of this gene, later on during cell cycle progression this role could be assumed by additional trans-acting complexes, such as cyclin D1. It has been shown that the inhibition of protein synthesis in MCF-7 cells prevents the maximal accumulation of cyclin D1 mRNA in response to estrogen, suggesting that the synthesis of one or more proteins likely is required to achieve the full stimulation of CCND1 gene expression by estrogen (4, 43). On the other hand, we show here that cyclin D1 functionally interacts with its own gene promoter in vivo. Taken together, these results lead us to propose that cyclin D1 regulates the transcription of its own gene during a delayed phase of G1 control by E2, characterizing CCND1 as the first example of both primary and secondary estrogen-responsive genes.
Surprisingly, pRb can be detected bound to the CCND1 promoter in both estrogen-starved and estrogen-stimulated cells (Fig. 5B), even after hormone-induced cyclin D1/Cdk4 complex activation and recruitment to the promoter, where presumably it induces pRb phosphorylation with the consequent release from E2F as it occurs, under the same conditions, on the “canonical ” E2F site of the c-Myc gene (Fig. 5B). We can exclude the possibility that the persistence of pRb on the CCND1 promoter in this study is due to an experimental artifact during immunoprecipitation, because different antibodies, both monoclonal and polyclonal, directed against different epitopes of the protein yielded the same results (data not shown). Furthermore, pRb could not be detected, in the same immunoprecipitated samples, in a far-upstream, unrelated region of the CCND1 gene (between positions −3247 and −2931) (data not shown). On the other hand, because pRb can bind to several transcription factors and other regulatory chromatin components, including HDACs, chromatin-remodeling factors, and general transcription factors (2, 14, 51, 67), it is possible that this protein was present in this study in more than one complex in quiescent cells, only one of which (and which also includes E2F) was disrupted by Cdk4 after estrogen stimulation, while others were stable even in cycling cells. Alternatively, pRb could be part of different multiprotein complexes in quiescent and stimulated cells, conditions that would be indistinguishable by ChIP. Indeed, while pRb can be found stably associated with the CCND1 promoter in quiescent cells, the blockade of cell cycle progression by ICI 182,780 after Cdk4 recruitment to the same promoter causes the release of pRb (Fig. 5D), indicating that hormone stimulation of the cell somehow modifies the interaction of pRb with chromatin at this site.
The estrogen-responsive region upstream of the CCND1 gene also mediates activation by progesterone in hBC cells and binds in vivo the PR.
Progesterone (P4) induces cyclin D1 mRNA and protein expression during mitogenic stimulation of mammary epithelial cells (36, 48) and, in this respect, its hormonal effects are comparable to those of estrogen. In hBC cells, CCND1 gene activation is induced by both PR-A and PR-B isoforms (44), but the genetic mechanisms involved are unknown, because the promoter region of this gene does not include canonical PREs. Interestingly, the AP-1 complex interacts with multiple members of the NR superfamily of transcription factors, including PRs (5), tethering each of them to responsive gene promoters. These data led us to consider the possibility that the AP/dE element of the CCND1 gene acts as a regulatory crossroad to convey multiple NRs to the cyclin D1 promoter, in which case we should have been able to observe an effect of P4 on its activity. As shown in Fig. 6A, the luciferase reporter construct D1Δ-956 could be activated by the PR agonist R5020 when transfected stably into hBC cells. The same was true for reporter construct D1-AP/dE, which includes the estrogen-responsive region of the gene (Fig. 1), but not for D1-APμ/dE, in which the TRE is inactivated and unable to bind to the AP-1 complex (Fig. 6A).
FIG. 6.
(A) Analysis of CCND1 promoter responsiveness to progesterone upon stable transfection of the indicated luciferase reporter genes in MCF-7 cells, reported as fold induction by 50 nM R5020 for 18 to 24 h versus the activity assayed in hormone-starved cells transfected with the same construct, arbitrarily set to 1. Plasmids used for transfections are named as shown in Fig. 1. MMTV-luc, progesterone-responsive reporter gene including the mouse mammary tumor virus long terminal repeat. Results reported represent the means of three to five independent experiments carried out several times. F.i., fold induction. Bars indicate standard deviations. (B) EMSAs with nuclear extracts from hormone-responsive hBC cells treated, where indicated (+), with 50 nM E2 or R5020 for 2 h and challenged with 32P-labeled AP-1/dE oligonucleotide. Data are representative of multiple experiments carried out with at least two different nuclear extracts from either MCF-7 or ZR 75.1 cells. (C) ChIP analysis of in vivo binding of progesterone receptors and other transcription regulatory factors to the upstream element of the CCND1 gene in ZR-75.1 cells. Input, total chromatin before immunoprecipitation. Data are representative of three experiments, one of which was carried out with MCF-7 cells.
EMSAs showed that the stimulation of hBC cells with a mitogenic dose of R5020 activated AP-1 to about 50 to 75% the level achieved by E2 under the same conditions (Fig. 6B). ChIP assays with hBC cells before and after stimulation with R5020 showed PR recruitment to the distal promoter region of the gene, as well as the same trans-acting factor switch as that observed following promoter activation by E2, including the physical proximity of RNA polymerase II to this upstream regulatory site (Fig. 6C). These results demonstrate that receptors for both ovarian hormones, which belong to distinct NR subfamilies, indeed can functionally and physically interact with the AP/dE element of the CCND1 gene promoter.
DISCUSSION
The most relevant function for estrogens in the mammary gland and in breast cancer is promotion of cell proliferation, where the CCND1 gene plays an essential role highlighted by several lines of evidence. Estrogens induce cyclin D1 gene in hBC cells (4) and treatment of these cells with cyclin D1 antisense oligonucleotides blocks hormone-dependent cell proliferation and decreases cyclin E-CDK2 activity, a downstream effect of E2 through D1 (9). Furthermore, E2 fails to stimulate G1/S transition in hBC cells upon microinjection of either antibodies against cyclin D1 or the CDK4-specific inhibitor p16INK4A (30), whereas ectopic cyclin D1 is sufficient to recapitulate the hormonal effects on cell cycle progression (38, 42).
CCND1 gene regulation by estrogens appears complex, involving both primary and secondary events that have not yet been fully defined. We thus set forth here to analyze in detail CCND1 gene promoter regulation by estrogens and their receptors in hBC cells. This was carried out by insertion of recombinant CCND1 gene promoter constructs in hormone-responsive cells by stable transfection, under conditions that reproduced as much as possible endogenous gene responses to the hormone. It has been demonstrated, in fact, that enhancement of transcription from estrogen regulated promoters is better analyzed when chromatin is used as a template (25, 34), indicating that chromatin organization of promoters greatly affects NRs activity on target genes. This approach led us to identify a 24bp composite regulatory element, located about 940 nucleotides upstream of CCND1 transcription start site, which acts as a main determinant for estrogen regulation. This composite regulatory site, which we operatively named ERGE, includes an AP-1 element (TRE), which binds the c-Jun/c-Fos heterodimer in hBC cells, and overlapping binding sites for two additional trans-acting proteins: Oct-1 and YY1 (Fig. 3 and 7).
FIG. 7.
Schematic representation of estrogen-dependent transcription factor assembly on the CCND1 gene promoter in hormone-responsive hBC cells and their interactions with each other, the distal regulatory sites, and the basal promoter. In hormone-deprived, quiescent cells, HDAC1 is present in a complex with YY1 on the ERGE. Upon estrogen (or progesterone) stimulation, these trans-repressors are displaced from DNA by a composite AP-1/ER (or PR) complex and Oct-1, inducing physical interactions of ERGE-bound complexes with the basal transcriptional machinery. At a later time (>1 h), the cyclin D1 (Cyc D1)/Cdk4 holoenzyme associates with the complex, presumably relieving the promoter from trans-repression and thereby inducing a second, longer-lasting enhancement of transcription. This event may be accompanied by Oct-1 release from ERGE. Ac, acetylated histone tails.
In hormone-starved, quiescent cells, where CCND1 transcription is at its minimum, ERGE is occupied in vivo by a complex comprising YY1 and HDAC1. YY1 is a zinc finger protein that shows a dual behavior on gene transcription, depending upon cell type-specific factors and the promoter context (57), and regulates the expression of pivotal cell cycle genes, generally inhibiting their transcription (20), and has been suggested to play a role in tumor suppression (28). Inhibition of transcription by YY1 relates to its ability to form trans-repressing complexes with histone-modifying enzymes, in particular, HDAC1 (15, 64). The repressor role of YY1 on key regulatory elements is often linked to competition for binding of positive acting factors to the same DNA, including among others the AP-1 complex (65) and Oct-1 (35). Interestingly, trans-repression by YY1 can be prevented by its interaction with nucleophosmin/B23, which is much more abundant in tumor or proliferating cells (11, 23) and is activated by estrogens in MCF-7 and ZR-75.1 cells (13, 54). Based on these data and the observations reported here that the YY1 binding site of the CCND1 promoter overlaps with those for AP-1 and Oct-1 in the ERGE, that its overexpression in the cell abolishes hormone-mediated activation of the promoter and the high basal level of promoter activity driven by a sequence unable to bind YY1 (Fig. 4C) and, finally, that its presence is mutually exclusive with that of RNA polymerase II on the promoter (Fig. 4A and E), we propose that YY1 inhibits CCND1 gene expression in hormone-starved cells, contributing to growth quiescence under these conditions.
Therefore, relieving cyclin D1 from YY1-mediated inhibition is most likely a key event in hormone-stimulated cells that affects early G1-phase progression (Fig. 7). Indeed, at 10 to 15 min after cell stimulation with E2, the YY1/HDAC1 complex detaches from the ERGE, to be replaced by AP-1 and Oct-1 which, in turn, interact with transcriptionally competent RNA polymerase II. The transcription factor exchange at the CCND1 ERGE, with the enhancement of gene expression that follows, results from increased concentrations of the c-Fos and c-Jun proteins in the nucleus consequent to activation of the corresponding genes by the hormone during the immediate-early cell cycle phase and, possibly, posttranslational modifications which affect their dimerization and binding to DNA. This is accompanied by increased binding of Oct-1 to its octamer-like sequence located immediately downstream from the TRE in the ERGE. Oct-1 belongs to the POU-homeo-domain family of trans-activators and binds to the octamer sequence ATGCAAAT (55). Depending upon the cell type and functional status, DNA binding by Oct-1 is favored by homodimerization and interaction with coregulators and can be inhibited by phosphorylation of its POU domain, which occurs during mitosis and is reversed as cells reenter G1 (49). It is possible that specific phosphoprotein phosphatases are activated upon recruitment of quiescent cells in the cell cycle by E2, resulting in reactivation of the ability of Oct-1 to bind to its target DNA, even though intervention of a cofactor interacting with Oct-1 and affecting its binding to DNA cannot be excluded. Binding of Oct-1 to the ERGE might exert different functions.
First, it may help in displacing YY1, thereby favoring recruitment of AP-1, as close association with an Oct-1 site has already been shown to be of crucial importance for AP-1 binding to a noncanonical TRE of the interleukin-2 gene (40). Second, the presence of Oct-1 may help establish cooperative interactions between the ERGE and trans-activators bound to different sites in CCND1, as this transcription factor has already been shown to enhance CRE-driven activation of this promoter in MCF-7 cells by mechanisms involving its ability to interact with phospho-CREB (8). Third, DNA looping and protein bridges that connect ERGE to the basal transcriptional machinery assembled at the transcription start site might be reinforced by Oct-1, if a protein-interacting surface of the DNA-bound factor remains available for general transcription factors. It has been already reported, in fact, that Oct-1 is able to interact directly with components of the basal transcription machinery (37), enhancing transcription in the absence of coactivators. According to this hypothesis, binding of transcriptional coactivators to the ERGE would involve primarily AP-1, whose presence is central for transcriptional induction, while Oct-1 roles could be both to assist the YY1 to AP-1 exchange and to promote a stable interaction of the latter factor with the transcription initiation complex.
The increased binding of both AP-1 and Oct-1 to ERGE in estrogen-stimulated cells is accompanied by recruitment of ERα to the promoter (Fig. 7). Concerning the possible role of the receptor, it is possible to assume that in the context of the CCND1 promoter it is required to act as a transcriptional coregulator for the AP-1 complex. The TRE, in fact, has been shown to play only a minor role in CCND1 promoter activation by serum in WI-38 human fibroblasts (22), despite the known stimulatory effects of serum mitogens on AP-1. The c-Jun/c-Fos heterodimer was shown to enhance CCND1 transcription in JEG-3 human trophoblasts through interaction with the p300 coactivator (3). However, this effect was observed under test conditions leading to strong activation of the trans-activating potential of AP-1, which is not the case for estrogen-stimulated hBC cells, where AP-1 activity is instead only moderately enhanced (62). It is therefore conceivable that in hormone-stimulated hBC cells, ERGE-bound AP-1 is inefficient in recruiting coactivators to the CCND1 promoter, and ERα plays a determinant role in helping recruit specific coactivators to the AP-1 complex. Indeed, it has been shown that AIB-1, a member of the p160 family of coactivators whose gene is found overexpressed in breast cancer and that acts as a limiting factor for hormone-dependent hBC cell growth, enhances estrogen-mediated induction of the CCND1 promoter upon transfection in keratinoytes and associates in vivo with this promoter, together with ERα, in E2-treated MCF-7 cells (41). Interestingly, the same was not observed with other ER coactivators, including CBP/p300, GRIP, and SRC-1 (41). These data support the notion that direct ERα interaction with the CCND1 promoter mediates estrogen activation of this gene in G1, highlighting the central role of the nuclear ER pathway in promotion of hormone-responsive hBC cell growth.
The pivotal role of AP-1 in CCND1 gene regulation by ERα suggested to us the possibility that the CCND1 TRE might represent a target for multiple NRs that, by accessing the promoter through the ubiquitous AP-1 complex, may modulate transcription of this gene in different cell types. Indeed, it has already been shown that ERβ can counteract, via AP-1, the positive effects of ERα on CCND1 transcription (29). Furthermore, compelling evidence indicates that cyclin D1 expression is regulated, either positively or negatively, by several NR ligands, including for example AhR and PPARγ ligands, androgens, thyroid hormones, retinoids, vitamin D, and progesterone (36, 44), although CCND1 lacks canonical NR response elements. We were able to demonstrate here that progesterone can activate the cyclin D1 gene promoter in hBC cells by inducing direct binding of c-Jun and PRs to ERGE, providing proof that the TRE might indeed act, at least under certain conditions, as a gateway to the CCND1 gene for multiple NRs, which may thereby gain access to this key cell cycle regulatory genetic switch.
During analysis of the CCND1 estrogen-responsive region, we observed that activation of promoter constructs including only the ERGE was always weaker and declining as the length of hormone stimulation increased; more important, however, the activity of the longer CCND1-Luc reporter genes could be slightly enhanced by forced expression of cyclin D1 (data not shown). This finding suggested to us that cyclin D1 may be involved somehow in the regulation of its own gene. Having found that cyclin D1 assembles on the CCND1 distal promoter, we first assumed that what we observed could be mediated by ER/cyclin D1 complex formation at the ERGE, as this cyclin can act as a coregulator of the ER in hBC cells (38, 69). However, timed ChIP analysis of hormone-stimulated cells treated with an antihormone clearly showed that it is possible to detect cyclin D1 bound to the promoter even in the absence of ERα. Furthermore, the presence on the same region of E2F, pRb and, in hormone-stimulated cells only, Cdk4 suggests the formation of a multimeric complex which includes a cyclin D1/Cdk4 holoenzyme. For these reasons, we presently exclude the possibility that the cyclin interacts with its promoter via the ER, although it is possible that it may help bridge the multiprotein complexes bound at the ERGE and other sites. This could be true, for example, with the E2F site, which the cyclin could bridge with the ERGE by interacting with both Cdk4/pRb and ER via distinct protein domains (Fig. 7). This hypothesis is consistent with the data from the ReIP tests, in which pRb and cyclin D1 could be shown to be present together on the same allele (Fig. 5C).
It has also been reported that cyclin D1 may associate with other transcription factors, such as cEBPβ, regulating in this way expression of several target genes (27). This, however, seems unlikely in this case, since cEBPβ binding sites are absent from the CCND1 promoter, including the region studied here (27) (data not shown). In addition, the association between this cyclin and cEBPβ is Cdk4 independent, while all data provided here indicate the presence of this kinase in the complex containing the cyclin (Fig. 5B and C).
Concerning pRb association with the CCND1 promoter and its involvement in hormone-dependent gene regulation, it is conceivable that this occurs, at least in quiescent cells, via E2F, according to the well-described molecular models for gene trans-repression by this antioncogene product (59). However, pRb can still be found stably associated with this promoter even after up to 4 h of mitogenic stimulation, when it is conceivable that its ability to interact with DNA-bound E2F factors is lost subsequent to hyperphosphorylation by Cdk2 (2). Based on the experimental data reported here, we propose that pRb can interact with the CCND1 promoter in a dynamic fashion, at least during early G1-phase progression, via the formation of different protein complexes. Identification of the binding partners of pRb in this context goes beyond the aims of this study. However, it has been shown that pRb can form trimeric complexes with ERα and the retinoblastoma-interacting zinc finger protein RIZ in estrogen-stimulated hBC cells, a result that suggested to us the possibility that RIZ and pRb proteins thereby could be involved with ERα in cell proliferation control by estrogen (1). Furthermore, pRb has been shown also to interact with other chromatin factors (2, 51, 68), some of which could be involved in either positive or negative control of CCND1 promoter activity.
The autoregulatory activity of cyclin D1 on its own gene may account for CCND1 gene hyperactivity under certain pathological conditions, as observed in human tumors. Furthermore, derangements of the regulatory pathway described here may be envisioned as possible pathogenic mechanisms for breast carcinogenesis and for tumor progression to a hormone-independent phenotype resistant to endocrine therapy.
Acknowledgments
We thank M. Beato, M. J. Birrer, W. Herr, J. M. Lüsker, S. K. Nordeen, and B. Wasylyk for kindly providing expression vectors and other recombinant DNAs; M. Truss for suggestions and scientific assistance; C. Abbondanza and D. Metzger for monoclonal antibodies; F. Matarese and Anna Cuomo for technical assistance; and W. Basile for help with artwork.
This research was supported by AIRC (grants 2001 to 2003), MIUR (PRIN 2004; FIRB RBNE0157EH), the European Commission (contracts QLG1-CT-2000-01935 and QLK3-CT-2002-02029), and Regione Campania (L. 5). C. Scafoglio is a Ph.D. candidate at Dottorato di Ricerca in Oncologia Medica e Chirurgica ed Immunologia Clinica (XVIII Ciclo), Seconda Università degli Studi di Napoli.
REFERENCES
- 1.Abbondanza, C., N. Medici, V. Nigro, V. Rossi, L. Gallo, G. Piluso, A. Belsito, A. Roscigno, P. Bontempo, A. A. Puca, A. M. Molinari, B. Moncharmont, and G. A. Puca. 2000. The retinoblastoma-interacting zinc-finger protein RIZ is a downstream effector of estrogen action. Proc. Natl. Acad. Sci. USA 97:3130-3135. [DOI] [PMC free article] [PubMed]
- 2.Adams, P. D. 2001. Regulation of the retinoblastoma tumor suppressor protein by cyclin cdks. Biochim. Biophys. Acta 1471:123-133. [DOI] [PubMed] [Google Scholar]
- 3.Albanese, C., M. D'Amico, A. T. Reutens, M. Fu, G. Watanabe, R. J. Lee, R. N. Kitsis, B. Henglein, M. Avantaggiati, K. Somasundaram, B. Thimmapaya, and R. G. Pestell. 1999. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274:34186-34195. [DOI] [PubMed] [Google Scholar]
- 4.Altucci, L., R. Addeo, L. Cicatiello, S. Dauvois, M. G. Parker, M. Truss, M. Beato, V. Sica, F. Bresciani, and A. Weisz. 1996. 17β-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G1-arrested human breast cancer cells. Oncogene 12:2315-2324. [PubMed] [Google Scholar]
- 5.Bamberger, A. M., C. M. Bamberger, B. Gellersen, and H. M. Schulte. 1996. Modulation of AP-1 activity by the human progesterone receptor in endometrial carcinoma cells. Proc. Natl. Acad. Sci. USA 93:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beato, M. 1989. Gene regulation by steroid hormones. Cell 56:335-344. [DOI] [PubMed] [Google Scholar]
- 7.Bonapace, I. M., R. Addeo, L. Altucci, L. Cicatiello, M. Bifulco, C. Laezza, S. Salzano, V. Sica, F. Bresciani, and A. Weisz. 1996. 17 beta-Estradiol overcomes a G1 block induced by HMG-CoA reductase inhibitors and fosters cell cycle progression without inducing ERK-1 and -2 MAP kinase activation. Oncogene 12:753-763. [PubMed] [Google Scholar]
- 8.Boulon, S., J. C. Dantonel, V. Binet, A. Vié, J. M. Blanchard, R. A. Hipskind, and A. Philips. 2002. Oct-1 potentiates CREB-driven cyclin D1 promoter activation via a phospho-CREB- and CREB binding protein-independent mechanism. Mol. Cell. Biol. 22:7769-7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, J. S., O. W. Prall, E. A. Musgrove, and R. L. Sutherland. 2000. A pure estrogen antagonist inhibits cyclin E-Cdk2 activity in MCF-7 breast cancer cells and induces accumulation of p130-E2F4 complexes characteristic of quiescence. J. Biol. Chem. 275:38221-38229. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Rivera, E., I. Samudio, and S. Safe. 2001. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J. Biol. Chem. 276:30853-30861. [DOI] [PubMed] [Google Scholar]
- 11.Chan, P. K., F. Y. Chan, S. W. Morris, and Z. Xie. 1997. Isolation and characterization of the human nucleophosmin/B23 (NPM) gene: identification of the YY1 binding site at the 5′ enhancer region. Nucleic Acids Res. 25:1225-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicatiello, L., R. Addeo, L. Altucci, V. Belsito Petrizzi, V. Boccia, M. Cancemi, D. Germano, C. Pacilio, S. Salzano, F. Bresciani, and A. Weisz. 2000. The antiestrogen ICI 182,780 inhibits proliferation of human breast cancer cells by interfering with multiple, sequential estrogen-regulated processes required for cell cycle completion. Mol. Cell. Endocrinol. 165:199-209. [DOI] [PubMed] [Google Scholar]
- 13.Cicatiello, L., G. Natoli, C. Scafoglio, L. Altucci, M. Cancemi, A. Facchiano, R. Calogero, G. Iazzetti, M. De Bortoli, C. Sfiligoi, P. Sismondi, N. Biglia, F. Bresciani, and A. Weisz. The gene expression program activated by estrogen in hormone responsive human breast cancer cells. J. Mol. Endocrinol. 2004. 32:719-775. [DOI] [PubMed] [Google Scholar]
- 14.Coqueret, O. 2002. Linking cyclins to transcriptional control. Gene 299:35-55. [DOI] [PubMed] [Google Scholar]
- 15.Coull, J. J., F. Romerio, J. M. Sun, J. L. Volker, K. M. Galvinh, J. R. Davie, Y. Shi, U. Hansen, and D. M. Margolis. 2000. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J. Virol. 74:6790-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantl, V., M. A. Richard, R. Smith, G. A. Lammie, G. Johnstone, D. Allen, W. Gregory, G. Peters, C. Dickson, and D. M. Barnes. 1990. Gene amplification on chromosome band 11q13 and oestrogen receptor status in breast cancer. Eur. J. Cancer 26:423-429. [DOI] [PubMed] [Google Scholar]
- 18.Fantl, V., G. Stamp, A. Andrews, I. Rosewell, and C. Dickson. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9:2364-2372. [DOI] [PubMed] [Google Scholar]
- 19.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 20.Gualberto, A., D. LePage, G. Pons, S. L. Mader, K. Park, M. L. Atchison, and K. Walsh. 1992. Functional antagonism between YY1 and the serum response factor. Mol. Cell. Biol. 12:4209-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennighausen, L., and H. Lubon. 1987. Interaction of protein with DNA in vitro. Methods Enzymol. 152:721-735. [DOI] [PubMed] [Google Scholar]
- 22.Herber, B., M. Truss, M. Beato, and R. Muller. 1994. Inducible regulatory elements in human cyclin D1 promoter. Oncogene 9:1295-1304. [PubMed] [Google Scholar]
- 23.Inouye, C. J., and E. Seto. 1994. Relief of YY1-induced transcriptional repression by protein-protein interaction with the nucleolar phosphoprotein B23. J. Biol. Chem. 269:6506-6510. [PubMed] [Google Scholar]
- 24.Klug, J., S. Knapp, I. Castro, and M. Beato. 1994. Two distinct factors bind to the rabbit uteroglobin TATA-box region and are required for efficient transcription. Mol. Cell. Biol. 14:6208-6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraus, W. L., and J. T. Kadonaga. 1998. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 12:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushner, P. J., D. A. Agard, G. L. Greene, T. S. Scanlan, A. K. Shiau, R. M. Uht, and P. Webb. 2000. Estrogen receptor pathways to AP-1. J. Steroid Biochem. Mol. Biol. 74:311-317. [DOI] [PubMed] [Google Scholar]
- 27.Lamb, J., S. Ramaswamy, H. L. Ford, B. Contreras, R. V. Martinez, F. S. Kittrell, C. A. Zahnow, N. Patterson, T. R. Golub, and M. E. Ewen. 2003. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114:323-334. [DOI] [PubMed] [Google Scholar]
- 28.Lichy, J. H., M. Majidi, J. Elbaum, and M. M. Tsai. 1996. Differential expression of the human ST5 gene in HeLa-fibroblast hybrid cell lines mediated by YY1: evidence that YY1 plays a part in tumor suppression. Nucleic Acids Res. 24:4700-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, M. M., C. Albanese, C. M. Anderson, K. Hilty, P. Webb, R. M. Uht, R. H. Price, Jr., R. G. Pestell, and P. J. Kushner. 2002. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J. Biol. Chem. 277:24353-24360. [DOI] [PubMed] [Google Scholar]
- 30.Lukas, J., J. Bartkova, and J. Bartek. 1996. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol. Cell. Biol. 16:6917-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. E. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxam, A. M., and W. Gilbert. 1977. A new method for sequencing DNA. Proc. Natl. Acad. Sci. USA 74:560-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenna, N. J., and B. W. O'Malley. 2002. Minireview: nuclear receptor coactivators—an update. Endocrinology 143:2461-2465. [DOI] [PubMed] [Google Scholar]
- 34.Metivier, R., G. Penot, M. R. Hubner, G. Reid, H. Brand, M. Kos, and F. Gannon. 2003. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751-763. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno, D., Y. Takahashi, T. Hiroi, S. Imaoka, T. Kamataki, and Y. Funae. 2003. A novel transcriptional element which regulates expression of the CYP2D4 gene by Oct-1 and YY-1 binding. Biochim. Biophys. Acta 1627:121-128. [DOI] [PubMed] [Google Scholar]
- 36.Musgrove, E. A., J. A. Hamilton, C. S. Lee, K. J. Sweeney, C. K. Watts, and R. L. Sutherland. 1993. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol. Cell. Biol. 13:3577-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakshatri, H., P. Nakshatri, and R. A. Currie. 1995. Interaction of Oct-1 with TFIIB. Implications for a novel response elicited through the proximal octamer site of the lipoprotein lipase promoter. J. Biol. Chem. 270:19613-19623. [DOI] [PubMed] [Google Scholar]
- 38.Pacilio, C., D. Germano, R. Addeo, L. Altucci, V. B. Petrizzi, M. Cancemi, L. Cicatiello, S. Salzano, F. Lallemand, R. J. Michalides, F. Bresciani, and A. Weisz. 1998. Constitutive overexpression of cyclin D1 does not prevent inhibition of hormone-responsive human breast cancer cell growth by antiestrogens. Cancer Res. 58:871-876. [PubMed] [Google Scholar]
- 39.Perillo, B., A. Sasso, C. Abbondanza, and G. Palumbo. 2000. 17β-Estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol. Cell. Biol. 20:2890-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeuffer, I., S. Klein-Hessling, A. Heinfling, S. Chuvpilo, C. Escher, T. Brabletz, B. Hentsch, H. Schwarznenbach, P. Matthias, and E. Serfling. 1994. Octamer factors exert a dual effect on the IL-2 and IL-4 promoters. J. Immunol. 153:5572-5585. [PubMed] [Google Scholar]
- 41.Planas-Silva, M. D., Y. Shang, J. L. Donaher, M. Brown, and R. A. Weinberg. 2001. AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res. 61:3858-3862. [PubMed] [Google Scholar]
- 42.Prall, O. W., E. M. Rogan, E. A. Musgrove, C. K. Watts, and R. L. Sutherland. 1998. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol. Cell. Biol. 18:4499-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prall, O. W., B. Sarcevic, E. A. Musgrove, C. K. Watts, and R. L. Sutherland. 1997. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J. Biol. Chem. 272:10882-10894. [DOI] [PubMed] [Google Scholar]
- 44.Richer, J. K., B. M. Jacobsen, N. G. Manning, M. G. Abel, D. M. Wolf, and K. B. Horwitz. 2002. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 277:5209-5218. [DOI] [PubMed] [Google Scholar]
- 45.Richer, J. K., C. A. Lange, N. G. Manning, G. Owen, R. Powell, and K. B. Horwitz. 1998. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J. Biol. Chem. 273:31317-31326. [DOI] [PubMed] [Google Scholar]
- 46.Roberts, S. B., N. Segil, and N. Heintz. 1991. Differential phosphorylation of the transcription factor Oct1 during the cell cycle. Science 253:1022-1026. [DOI] [PubMed] [Google Scholar]
- 47.Sabbah, M., D. Courilleau, J. Mester, and G. Redeuilh. 1999. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc. Natl. Acad. Sci. USA 96:11217-11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Said, T. K., O. M. Conneely, D. Medina, B. W. O'Malley, and J. P. Lydon. 1997. Progesterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell in vivo. Endocrinology 138:3933-3939. [DOI] [PubMed] [Google Scholar]
- 49.Segil, N., S. B. Roberts, and N. Heintz. 1991. Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science 254:1814-1816. [DOI] [PubMed] [Google Scholar]
- 50.Shang, Y., X. Hu, J. DiRenzo, M. A. Lazar, and M. Brown. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843-852. [DOI] [PubMed] [Google Scholar]
- 51.Shao, Z., J. L. Siegert, S. Ruppert, and P. D. Robbins. 1997. Rb interacts with TAF(II)250/TFIID through multiple domains. Oncogene 15:385-392. [DOI] [PubMed] [Google Scholar]
- 52.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 53.Sicinski, P., J. L. Donaher, S. B. Parker, T. Li, A. Fazeli, H. Gardner, S. Z. Haslam, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621-630. [DOI] [PubMed] [Google Scholar]
- 54.Skaar, T. C., S. C. Prasad, S. Sharareh, M. E. Lippman, N. Brunner, and R. Clarke. 1998. Two-dimensional gel electrophoresis analyses identify nucleophosmin as an estrogen regulated protein associated with acquired estrogen-independence in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 67:391-402. [DOI] [PubMed] [Google Scholar]
- 55.Sturm, R. A., G. Das, and W. Herr. 1988. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 2:1582-1599. [DOI] [PubMed] [Google Scholar]
- 56.Teyssier, C., K. Belguise, F. Galtier, and D. Chalbos. 2001. Characterization of the physical interaction between estrogen receptor α and JUN proteins. J. Biol. Chem. 276:36361-36369. [DOI] [PubMed] [Google Scholar]
- 57.Thomas, M. J., and E. Seto. 1999. Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene 236:197-208. [DOI] [PubMed] [Google Scholar]
- 58.Wang, T. C., R. D. Cardiff, L. Zukerberg, E. Lees, A. Arnold, and E. V. Schmidt. 1994. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369:669-671. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg, R. A. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323-330. [DOI] [PubMed] [Google Scholar]
- 60.Weinstat-Saslow, D., M. J. Merino, R. E., Manrow, J. A. Lawrence, R. F. Bluth, K. D. Wittenbel, J. F. Simpson, D. L. Page, and P. S. Steeg. 1995. Overexpression of cyclin D1 mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat. Med. 1:1257-1260. [DOI] [PubMed] [Google Scholar]
- 61.Weisz, A., and F. Bresciani. 1993. Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit. Rev. Oncog. 4:361-388. [PubMed] [Google Scholar]
- 62.Weisz, A., and R. Rosales. 1990. Identification of an estrogen response element upstream of the human c-fos gene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res. 18:5097-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanagisawa, J., H. Kitagawa, M. Yanagida, O. Wada, S. Ogawa, M. Nakagomi, H. Oishi, Y. Yamamoto, H. Nagasawa, S. B. McMahon, M. D. Cole, L. Tora, N. Takahashi, and S. Kato. 2002. Nuclear receptor function requires a TFTC-type histone acetyl transferase complex. Mol. Cell 93:553-562. [DOI] [PubMed] [Google Scholar]
- 64.Yao, Y. L., W. M. Yang, and E. Seto. 2001. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol. Cell. Biol. 21:5979-5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye, J., M. Cippitelli, L. Dorman, J. R. Ortaldo, and H. A. Young. 1996. The nuclear factor YY1 suppresses the human gamma interferon promoter through two mechanisms: inhibition of AP1 binding and activation of a silencer element. Mol. Cell. Biol. 16:4744-4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu, Q., Y. Geng, and P. Sicinski. 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017-1021. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, H. S., M. Gavin, A. Dahiya, A. A. Postigo, D. Ma, R. X. Luo, J. W. Harbour, and D. C. Dean. 2000. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101:79-89. [DOI] [PubMed] [Google Scholar]
- 68.Zheng, L., R. G. Roeder, and Y. Luo. 2003. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114:255-266. [DOI] [PubMed] [Google Scholar]
- 69.Zwijsen, R. M., E. Wientjens, R. Klompmaker, J. van der Sman, R. Bernards, and R. J. Michalides. 1997. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88:405-415. [DOI] [PubMed] [Google Scholar]