Abstract
Glycogen serves as a repository of glucose in many mammalian tissues. Mice lacking this glucose reserve in muscle, heart, and several other tissues were generated by disruption of the GYS1 gene, which encodes an isoform of glycogen synthase. Crossing mice heterozygous for the GYS1 disruption resulted in a significant underrepresentation of GYS1-null mice in the offspring. Timed matings established that Mendelian inheritance was followed for up to 18.5 days postcoitum (dpc) and that ∼90% of GYS1-null animals died soon after birth due to impaired cardiac function. Defects in cardiac development began between 11.5 and 14.5 dpc. At 18.5 dpc, the hearts were significantly smaller, with reduced ventricular chamber size and enlarged atria. Consistent with impaired cardiac function, edema, pooling of blood, and hemorrhagic liver were seen. Glycogen synthase and glycogen were undetectable in cardiac muscle and skeletal muscle from the surviving null mice, and the hearts showed normal morphology and function. Congenital heart disease is one of the most common birth defects in humans, at up to 1 in 50 live births. The results provide the first direct evidence that the ability to synthesize glycogen in cardiac muscle is critical for normal heart development and hence that its impairment could be a significant contributor to congenital heart defects.
Glycogen is a high-molecular-weight polysaccharide that serves as a repository of glucose units for utilization in times of metabolic need. The synthesis of glycogen requires several reactions. After a specialized initiation step mediated by glycogenin (EC 2.4.1.186), glycogen synthase (EC 2.4.1.11) catalyzes the addition of glucose residues to the growing glycogen molecule through the formation of α-1,4-glycosidic linkages, the basic polymerizing linkages of the polysaccharide. The branch points, introduced by the branching enzyme (EC 2.4.1.18), are formed by α-1,6-glycosidic linkages.
Mammals express two isoforms of glycogen synthase, encoded by the GYS1 and GYS2 genes. GYS1, encoding the muscle isoform of glycogen synthase, is expressed in skeletal muscle, cardiac muscle, adipose tissue, kidneys, and brain (29). GYS2 expression has been found only in liver (30). Many cells can synthesize glycogen but, in absolute amounts, the major stores are in the liver and skeletal muscle. Although its basic role as an energy reserve is common to all cells, there are differences in glycogen function between tissues. Liver glycogen contributes primarily to blood glucose homeostasis, being synthesized during periods of nutritional sufficiency and subsequently converted to glucose, which is released into the bloodstream to combat hypoglycemia (34). In the fed state, glycogen is also synthesized in muscle, where it functions as an energy reserve to fuel contraction (34). Glycogen provides a portion of the glucose utilized by the aerobic working adult heart (16) and is preferentially oxidized compared to exogenous glucose (24, 26). Although long-chain fatty acids are normally the major substrates for the adult heart, when blood glucose and insulin concentrations are high, such as after a meal, glucose becomes a more prominent energy source (41). Various stresses also provoke greater reliance on glycogen. When the workload of the heart is increased, such as after stimulation by epinephrine (12, 23) and/or increased exercise (24, 26), glycogen provides a readily accessible source of additional energy. In ischemia, glucose assumes a more important role as a fuel for the heart, since fatty acid oxidation is restricted and glycogen supplies glucose for anaerobic glycolysis (16). An analogous protective role may occur in hibernating animals, which exhibit dramatically increased cardiac glycogen levels (19) similar to those seen in the fetal heart (16).
The ontogeny of glycogen accumulation and glycogen synthase activity have been examined in a number of mammals. Relatively low levels of glycogen are present in fertilized mouse eggs (38); however, there is evidence of high glycogen synthase activity in one-cell mouse embryos (8), and by the two-cell stage, significant glycogen accumulation can be measured (39). Several tissues from a variety of mammals have been analyzed for the appearance of glycogen and glycogen synthase activity in later stages of embryonic development (7, 15, 25). In the developing lung, the glycogen level is low early in gestation and high from midgestation until term, when it declines (7, 14, 15). Liver glycogen is present at a low level until late gestation (7, 14, 15, 25), consistent with a role in providing glucose for the newborn prior to the development by the liver of the capacity to produce glucose by gluconeogenesis (22). The concentration of fetal skeletal muscle glycogen has already reached the adult level when glycogen accumulation is just beginning in the liver. By late gestation, the level of muscle glycogen is higher than that in adult muscle (15). Cardiac glycogen is present at high levels during early to midgestation before falling to low levels at the time of birth (7, 14, 15, 25). The facts that glycogen is present and that its level changes during the embryonic development of several tissues suggest that it may have a specific role, but few studies have provided definitive evidence regarding this point.
In the present work, we analyzed a mouse model (muscle glycogen synthase knockout [MGSKO] mice) in which the GYS1 gene has been disrupted, thereby eliminating the ability to store glycogen in skeletal muscle and cardiac muscle. About 90% of the homozygous null offspring from crosses of heterozygotes died perinatally due to abnormal cardiac development at between 11.5 days postcoitum (dpc) and 18.5 dpc. Congenital heart disease is one of the most common birth defects in newborns. Reports of the incidence of moderate to severe defects range from 6 to 19 per 1,000 live births (11, 28). The present work suggests that glycogen plays a critical role as an energy source during cardiogenesis and that impaired glycogen synthesis may contribute to congenital heart disease.
MATERIALS AND METHODS
Generation of MGSKO mice.
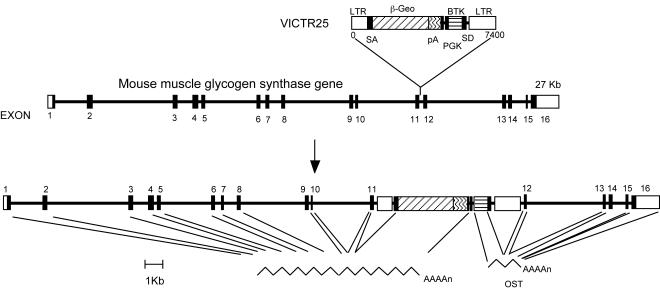
Mice heterozygous for the muscle glycogen synthase gene (GYS1) were obtained from Lexicon Genetics Incorporated. Embryonic stem (ES) cells (129/SvEvBrd) from the Lexicon Genetics Omnibank library of gene-trapped mouse ES cell clones were infected with retroviral gene trap vector VICTR25 (44, 45), which was integrated upstream of exon 12 of the muscle glycogen synthase gene (Fig. 1). The karyotype of targeted ES cells was found to be normal. ES cells with GYS1 disrupted were injected into C57BL/6J blastocysts, which were implanted in pseudopregnant mothers. The resulting pups were crossed with C57BL/6J × 129/SvJ mice. Southern analysis was used to confirm the genotype of heterozygous animals which were supplied to us. Heterozygous mice were crossed with C57BL/6J mice to generate heterozygous breeders which were 75% C57BL/6J. These GYS1−/+ heterozygotes were crossed with each other to generate the wild-type, heterozygous, and homozygous knockout mice used in the studies presented here. All comparisons were among littermates. Mice were maintained in temperature- and humidity-controlled conditions with a 12-h light-12-h dark cycle and were allowed food and water ad libitum in the American Association for the Accreditation of Laboratory Animal Care-approved facility at the Indiana University School of Medicine. All procedures were approved by the Indiana University School of Medicine Animal Care and Use Committee.
FIG. 1.
Disruption of the GYS1 gene. LTR, long terminal repeat; β-Geo, β-galactosidase-neomycin phosphotransferase fusion gene (45); pA, polyadenylation sequence; SA, splice acceptor sequence; SD, splice donor sequence; PGK, phosphoglycerate kinase 1 promoter; BTK, Bruton's tyrosine kinase; OST, Omnibank sequence tag.
Preparation of samples for biochemical analyses.
Mice were sacrificed by cervical dislocation, and muscle and heart tissues were rapidly excised, quick-frozen in liquid nitrogen, and stored at −80°C. Frozen heart or muscle was powdered, and samples (∼30 mg) were homogenized in 10 or 30 volumes, respectively, of buffer containing 50 mM Tris-HCl (pH 7.8), 10 mM EDTA, 2 mM EGTA, 0.1 mM N-p-tosyl-l-lysine chloromethyl ketone, 2 mM benzamidine, 0.5 mM phenylmethylsulfonyl fluoride, 50 mM mercaptoethanol, and 10 μg of leupeptin/ml with a Tissue Tearor model 285-370 (Biospec Products, Inc.) at the maximum setting (35,000 rpm) for 20 s. Tissue homogenates were used for Western analysis and assays of glycogen synthase and phosphorylase activities as described below. Protein content was determined by the method of Bradford (9).
Western analysis.
For Western analysis, a homogenate (25 μg of protein) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes and incubated with antibodies raised against rabbit muscle glycogen synthase (4). Detection was done with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. Levels of protein expression were quantitated by densitometric scanning of autoradiograms.
Enzyme activities.
Glycogen synthase activity in tissue homogenates was determined by measuring the incorporation of glucose from UDP-[U-14C]glucose into glycogen in the presence or absence of glucose-6-phosphate by the method of Thomas et al. (43) as described by Suzuki et al. (40). Reactions were carried out at 30°C typically for 10 to 15 min. Phosphorylase activity in tissue homogenates diluted 1:4 (heart) or 1:6 (muscle) was determined by monitoring the reverse reaction, in which the incorporation of [14C]glucose from [14C]glucose-1-phosphate (21) into glycogen in the absence or presence of AMP is measured as described by Suzuki et al. (40). The activity ratio is defined as the enzyme activity measured in the absence of an allosteric effector divided by that determined in the presence of 7.2 mM glucose-6-phosphate for glycogen synthase or 3 mM AMP for phosphorylase under standard conditions. Rabbit liver glycogen (Sigma-Aldrich) was deionized by passage through an MBD-22 (Resintech, Inc.) mixed-bed exchanger prior to use.
Determination of glycogen content.
Glycogen content was estimated by measuring glucose released by amyloglucosidase digestion of ethanol-precipitable material from muscle or liver tissue (40). Samples of frozen muscle and heart tissue (∼30 to 60 mg) were boiled in 200 μl of 30% (wt/vol) KOH for 30 min with occasional shaking. After cooling, 67 μl of 1 M Na2SO4 and 535 μl of ethanol were added. Next, samples were heated at 100°C for 2 min and centrifuged at 17,500 × g for 20 min at 4°C to collect glycogen. The glycogen pellet was suspended in water (100 μl), 200 μl of ethanol was added, and centrifugation as described above was used to harvest glycogen. This ethanol precipitation step was repeated, and the glycogen pellet was dried in a Speed-Vac. Dried glycogen pellets were suspended in 100 μl of amyloglucosidase (0.3 mg/ml in 0.2 M sodium acetate [pH 4.8]) and incubated at 37°C for 3 h to digest glycogen. To determine the glucose concentration in the samples, an aliquot (50 μl) of digested glycogen was added to 950 μl of a solution containing 0.3 M triethanolamine (pH 7.6), 0.4 mM MgCl2, 0.9 mM NADP, 1 mM ATP, and 1 μg of glucose-6-phosphate dehydrogenase/ml. The absorbance at 340 nm was read before and after the addition of 1 μg of hexokinase (5).
Timed matings.
For timed matings, breeding pairs or trios were established by introducing female mice into cages with male mice in the late afternoon. On the following morning, females were checked for vaginal plugs indicative of mating. The date when a plug was found was designated embryonic day 0.5.
Histological analysis.
Pregnant mice were sacrificed at various stages of gestation by cervical dislocation. Embryos were harvested, and either whole embryos or isolated hearts were fixed in 10% neutral buffered formalin or formalin-ethanol-acetic acid solution by standard methods. Adult hearts were isolated from mice sacrificed by cervical dislocation. Fixed samples were embedded in paraffin, sectioned (5 μm), and stained with hematoxylin-eosin to examine organ morphology.
Thymidine labeling of cardiomyocytes.
To analyze the proliferative activity of the developing heart, timed-mated females were injected intraperitoneally with 200 μCi of [3H]thymidine (28 Ci/mmol; 1 mCi/ml; Amersham Biosciences Corp.). Embryos were harvested after a 3-h labeling period, followed by fixation (10% neutral buffered formalin) and paraffin sectioning. Deparaffined sections were stained with Hoechst stain in phosphate-buffered saline to identify the cell nucleus. The Hoechst-stained slides were coated with photographic emulsion (Polysciences, Inc., Warrington, Pa.) and further processed for autoradiography. The [3H]thymidine labeling index was defined as the percentage of labeled nuclei out of the total number of nuclei.
Echocardiography and blood pressure.
To assess left ventricle function and dimensions, mice were subjected to echocardiography (35) at the Vanderbilt University School of Medicine Mouse Metabolic Phenotyping Center. Conscious animals were imaged by use of a 15-Mhz linear transducer; optimal parasternal long- and short-axis views were achieved by adjusting gain settings for visualizing endocardial and epicardial walls. Two-dimensional targeted M-Mode echocardiographic images were taken at the level of the papillary muscles. Measurements represent the average of three to five beats with the leading-edge technique. A trained echocardiographer reviewed and interpreted data from each mouse.
Blood pressure was also measured at the Vanderbilt University School of Medicine Mouse Metabolic Phenotyping Center; the tail cuff method was used (31). Mice were acclimated to the cuff apparatus on three successive days. Systolic pressure was measured twice for each mouse.
RESULTS
Perinatal mortality of mice lacking GSY1.
Mice homozygous for a disruption of GYS1 (MGSKO mice) were generated by mating GYS1−/+ mice. It was immediately apparent that the number of MGSKO pups being produced was far short of what would be expected from Mendelian inheritance (Table 1). This number correlated with an average litter size (5.5) smaller than that of other mouse colonies that we maintain (average, 7 to 9 pups). To test whether the loss of the GYS1 gene caused embryonic lethality, timed matings were performed and embryos were examined at 11.5, 14.5, 17.5, and 18.5 dpc. Up to 18.5 dpc, close to the expected number of GYS1−/− pups was observed (Table 1), with an average litter size of 7.5. Pups recovered from mothers at 18.5 dpc were kept warm and gently rubbed with a finger. Most pups started breathing and became more pink. Some pups, later genotyped as GYS1−/−, did not respond and died without taking a breath. Morphological examination of the lungs of GYS1−/− embryos was consistent with their failure to inflate (Fig. 2c). We conclude that MGSKO pups die shortly after birth.
TABLE 1.
Muscle glycogen synthase is important for pup survival
Pups were genotyped at a minimum of 2 weeks of age.
Timed matings were established, and embryos were collected at between 11.5 and 18.5 dpc.
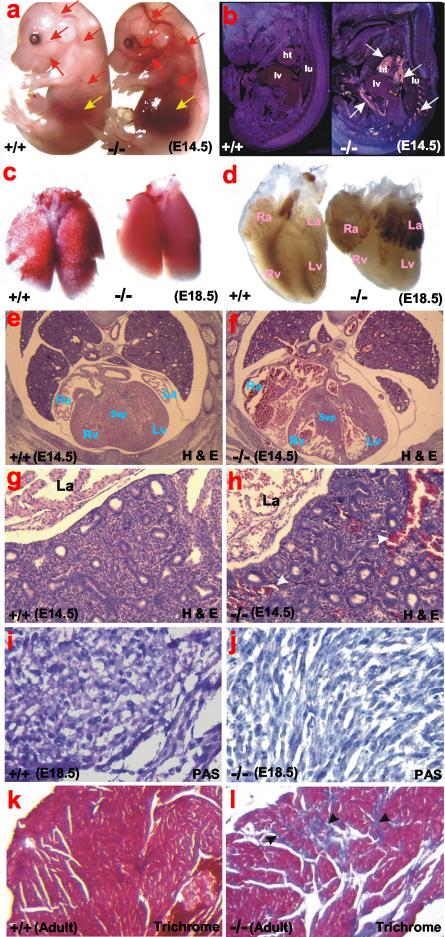
FIG. 2.
Morphological and histological analyses of GYS1-deficient mice. (a) At E14.5, GYS1-deficient embryos have prominent vessels congested with blood and hemorrhagic livers. Red arrows indicate congested blood vessels; yellow arrows indicate livers. (b) Dark-field images of sagittal sections of E14.5 embryos. White arrows indicate the autofluorescence of red blood cells pooled in the hearts and blood vessels throughout GYS1-deficient embryos. ht, heart; lv, liver; lu, lung. (c) The majority of GYS1-deficient mice fail to inflate the lungs at the time of birth and die within 5 to 10 min after birth. (d) At E18.5, GYS1-deficient mice have significantly smaller ventricles but dilated atria. Ra, right atrium; La, left atrium; Rv, right ventricle; Lv, left ventricle. (e and f) Transverse sections of wild-type and GYS1-deficient embryos at E14.5. Mutant hearts have thin ventricular walls and dilated atria and show abnormal formation of the ventricular septum. Note the huge amount of pooled blood in mutant atria and blood vessels, suggesting very poor cardiac function. Sep, septum. (g and h) Histological sections of wild-type and GYS1-deficient lungs (left lobes). Note severe pulmonary congestion in the mutant lungs. White arrows indicate pooled blood in pulmonary vessels. (i and j) Periodic acid-Schiff (PAS) staining of heart sections from wild-type and GYS1-deficient embryos at 18.5 dpc. Staining was done to visualize the level of glycogen content in cardiomyocytes. Mutants do not appear to have glycogen in cardiomyocytes. (k and l) Trichrome (Masson) staining. Staining was done to visualize abnormal fibrosis (arrows) in aged GYS1-deficient adult ventricles compared to those in age-matched littermate controls.
Heart defects in MGSKO embryos.
Initial examination of the mutant embryos suggested defects in cardiac development and function. We first noticed a striking difference in the appearance of embryos at 14.5 dpc. Mutant embryos showed symptoms of a congested venous system throughout and severely hemorrhagic livers (Fig. 2a and b). Morphological and histological analyses of hearts at 14.5 and 18.5 dpc revealed abnormal morphology in GYS1−/− animals. The mutant ventricles were significantly smaller than those from wild-type embryos (Fig. 2d to f), while both atria of mutant hearts were dilated and, in most cases, had extensive pooling of blood (Fig. 2d to f). This finding was consistent with our later histological observations that, in fact, mutant embryos suffered from venous and pulmonary congestion (Fig. 2g and h), which suggested that poorly developed and functioning hearts contributed to this abnormality. At 14.5 dpc, the hearts had a thin ventricular wall, an abnormal ventricular septum, reduced trabecular structure and, in some cases, increased pericardial space (Fig. 2e and f). Labeling of cardiomyocytes from 14.5 dpc embryos with [3H]thymidine indicated that the thin wall of the ventricle was due to a decrease in cell proliferation rather than a decrease in cell size (12% of cells labeled for the wild type versus 9% for GYS1−/−). Examination of 11.5 dpc embryos revealed no obvious cardiac or other abnormalities (data not shown). The defective cardiac development therefore began some time after 11.5 dpc and was evident at 14.5 dpc, consistent with the early observation that higher glycogen levels in developing hearts occur at midgestation during normal cardiac development (7, 15, 25).
Heart morphology in surviving adult MGSKO mice.
Approximately 10% of the MGSKO mice survived, without an overt phenotype. Histological examination of hearts from 8- to 9-month-old mice revealed no large differences in heart morphology between wild-type and GYS1−/− adult mice (data not shown). Mice were subjected to both electrocardiography and echocardiography. Electrocardiography of 4- to 7-month-old male mice indicated no gross abnormalities in the hearts of null mice (data not shown). Mice at 5 months of age were examined by echocardiography, and no major differences in left ventricular dimensions or fractional shortening velocity were detected (Table 2). A trend toward a larger left ventricle mass was observed for GYS1−/− mice (Table 2), consistent with the significantly higher weights of GYS1−/− hearts (5.49 ± 0.18 [mean and standard error of the mean] and 4.42 ± 0.08 mg of heart/g of body weight for −/− mice and +/+ mice, respectively) (P = 0.0003). Tail cuff blood pressure measurements indicated no differences in systolic blood pressures (131 ± 5 and 121 ± 4 mm Hg for −/− mice and +/+ mice, respectively; n = 8 to 9). Heart rates measured at the same time (622 ± 23 and 643 ± 25 min−1 for −/− mice and +/+ mice, respectively) were also not significantly different. However, histological examination of the hearts of older male mice (12 to 16 months of age) revealed the presence of significant fibrosis in the GYS1−/− animals compared to the wild-type animals (Fig. 2k and l).
TABLE 2.
Echocardiography measurementsa
| Animals | Mean ± SEM
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diastolic dimension
|
Systolic dimension
|
% Fractional shortening | |||||||
| Intraventricular septum (cm) | LV (cm) | LV posterior wall (cm) | LV mass (mg) | Intraventricular septum (cm) | LV (cm) | LV posterior wall (cm)b | LV mass (mg) | ||
| +/+ | 0.098 ± 0.003 | 0.296 ± 0.008 | 0.103 ± 0.004 | 102.1 ± 5.4 | 0.176 ± 0.005 | 0.134 ± 0.007 | 0.138 ± 0.004 | 93.3 ± 6.0 | 54.6 ± 1.6 |
| −/− | 0.099 ± 0.003 | 0.301 ± 0.013 | 0.109 ± 0.006 | 112.0 ± 9.7 | 0.178 ± 0.006 | 0.142 ± 0.009 | 0.152 ± 0.005 | 109.6 ± 10.4 | 53.0 ± 1.5 |
LV, left ventricle.
The P value for a comparison of the two groups of animals was 0.049.
Glycogen metabolism.
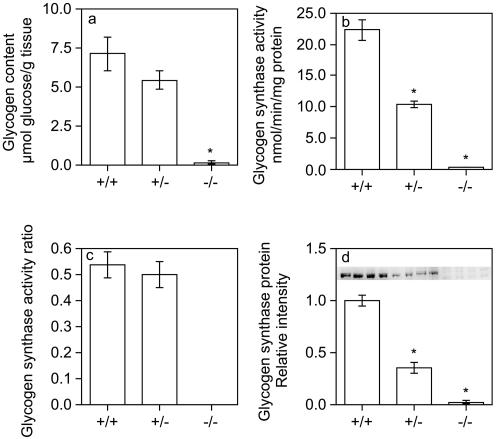
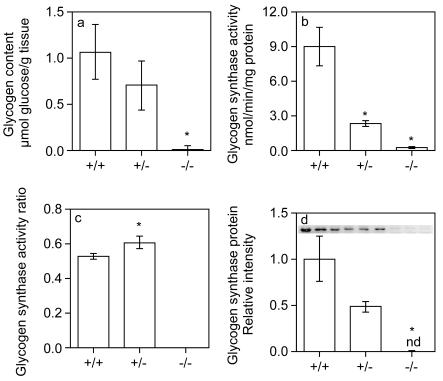
Because of the pattern of expression of GYS1, the absence of this glycogen synthase isoform should eliminate glycogen synthesis in various tissues, including skeletal muscle and heart. Amyloglucosidase digestion (see Materials and Methods) of skeletal muscle (Fig. 3a) or heart (Fig. 4a) samples from GYS1−/− mice liberated levels of glucose near the limit of detection of the assay, indicating that these tissues lacked glycogen. Muscle and heart samples from heterozygotes had glycogen levels that tended to be lower than those of wild-type animals (Fig. 3a and 4a). Consistent with this observation, periodic acid-Schiff staining also showed that mutant hearts were glycogen deficient (Fig. 2i and j). Glycogen synthase activity was reduced ∼50% in skeletal muscle or heart samples from heterozygous mice and was at the limit of detection of the assay in samples from GYS1−/− mice (Fig. 3b and 4b). The glycogen synthase activity ratio in skeletal muscle from heterozygous mice was not different from that in wild-type mice (Fig. 3c), but there was a small but significant increase in hearts from heterozygous mice relative to those from wild-type mice (Fig. 4c). The level of glycogen synthase protein, as judged by Western analysis, correlated with glycogen synthase activity (Fig. 3d and 4d).
FIG. 3.
Glycogen biosynthesis in skeletal muscle. Glycogen content (a), glycogen synthase activity (b), glycogen synthase activity ratio (c), and glycogen synthase protein expression (d) were determined for skeletal muscle from GYS1 wild-type, heterozygous, and homozygous null 7- to 9-month-old male mice as described in Materials and Methods. Values are reported as means and standard errors of the mean (n = 5 to 9). An asterisk indicates a P value of <0.0005 for comparisons with wild-type measurements.
FIG. 4.
Glycogen biosynthesis in cardiac muscle. Hearts from GYS1 wild-type, heterozygous, and homozygous null 5- to 9-month-old male mice were analyzed for glycogen content (a), glycogen synthase activity (b), glycogen synthase activity ratio (c), and glycogen synthase protein expression (d) as described in Materials and Methods. Values are reported as means and standard errors of the mean (n = 3 to 6). An asterisk indicates a P value of <0.05 for comparisons with wild-type measurements. nd, not detectable.
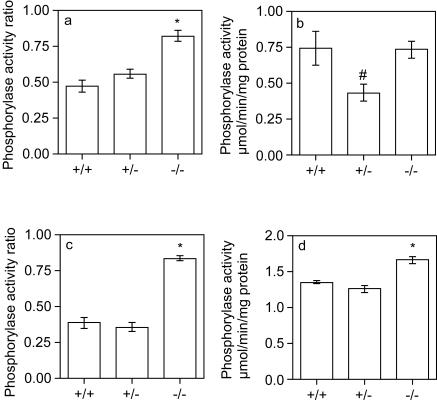
Glycogen phosphorylase (EC 2.4.1.1) is the enzyme that degrades glycogen in the cytosol (20). It is regulated by phosphorylation, and its degree of activation can be assessed by measuring activity in the presence or in the absence of the allosteric activator AMP. The glycogen phosphorylase activity ratio was significantly increased in hearts from GYS1−/− mice (Fig. 5a), with no increase in total phosphorylase activity (Fig. 5b), indicating activation of the enzyme. A similar increase in the phosphorylase activity ratio was observed in skeletal muscle (Fig. 5c). There was a modest but significant increase in total phosphorylase activity in skeletal muscle (Fig. 5d). It is teleologically counterintuitive that the absence of glycogen correlates with the activation of an enzyme whose function is to break down this polymer. One possibility is that the enzyme is less accessible to the glycogen-associated protein phosphatases that normally inactivate phosphorylase.
FIG. 5.
Glycogen phosphorylase activity in skeletal muscle and cardiac muscle. Glycogen phosphorylase activity ratio (a and c) and total activity (b and d) were determined for cardiac muscle (a and b) and skeletal muscle (c and d) from 5- to 9-month-old male mice as described in Materials and Methods. Values are reported as means and standard errors of the mean (n = 3 to 6). An asterisk indicates a P value of <0.0005 for comparisons with wild-type measurements; a number sign indicates a P value of 0.02 for comparisons with −/− measurements.
Rescue of MGSKO lethality by transgenic overexpression of glycogen synthase.
In previous work, Manchester et al. had generated mice that overexpress a mutant form of rabbit muscle glycogen synthase under the control of the muscle creatine kinase promoter (32). This mutant glycogen synthase has two important phosphorylation sites (2 and 3a) mutated to alanine, preventing the enzyme from being fully inactivated by phosphorylation (37). Three independent mouse lines that differed in the level of glycogen synthase expression were created. The GSL25 line has about 2-fold overexpression in skeletal muscle, with no change in the heart (9.5 ± 1.1 and 9.0 ± 1.0 μmol/min/mg of protein for the wild-type and for GSL25, respectively), whereas the GSL30 line has a greater-than-10-fold increase in muscle glycogen synthase expression and an approximately 4-fold increase in heart glycogen synthase expression (9.5 ± 1.1 and 39.7 ± 3.5 μmol/min/mg of protein for the wild-type and for GSL30, respectively) (P = 0.0008). GSL30 has a sevenfold increase in cardiac glycogen accumulation (12.6 ± 3.0 and 1.8 ± 1.0 μmol of glucose/g of tissue for GSL30 and the wild type, respectively) (P = 0.01).
GSL25 and GSL30 mice were crossed with MGSKO mice to generate progeny heterozygous for endogenous GYS1 but expressing the transgene (MGSKO+/−/L25 and MGSKO+/−/L30, respectively). These mice then were mated with MGSKO+/− and MGSKO−/− animals to generate mice null for endogenous muscle glycogen synthase but expressing the transgene (MGSKO−/−/L25 and MGSKO−/−/L30, respectively). Crossing of GSL25 mice with MGSKO mice resulted in a threefold increase in the survival of pups null for endogenous GYS1 but carrying the transgene, compared to null mice not carrying the transgene (Table 3). Crossing MGSKO mice with GSL30 mice, which have much higher glycogen synthase expression levels, completely rescued the lethality associated with the null disruption of GYS1, so that the number of observed MGSKO−/−/L30 mice was that expected for Mendelian inheritance (Table 3).
TABLE 3.
Rescue of perinatal lethality
| Mating | Genotype | No. of mice
|
|
|---|---|---|---|
| Actuala | Expectedb | ||
| MGSKO × MGSKO+/−/L25 | MGSKO−/− | 6 | 70 |
| MGSKO−/−/L25 | 19 | 70 | |
| MGSKO × MGSKO+/−/L30 | MGSKO−/− | 6 | 29 |
| MGSKO−/−/L30 | 28 | 29 | |
Number of mice which survived to genotyping age (2 weeks of age).
Number of pups predicted in the absence of lethality associated with the gene disruption.
DISCUSSION
An immediate and important outcome of the present study is the definitive demonstration that tissues believed to express GYS1 are devoid of glycogen in the MGSKO mice. These tissues include skeletal and cardiac muscle, as well as brain and lung (data not shown). This finding suggests first that GYS1 encodes the sole glycogen synthase isoform expressed in these tissues and that the other isoform, coded by GYS2, is not upregulated by an adaptive response in the developing MGSKO mice. The results also provide compelling evidence that there is no alternative and hitherto unknown pathway for glycogen synthesis in these tissues and provide strong genetic support for the absolute requirement of glycogen synthase in the synthesis of glycogen. The most striking phenotype associated with the MGSKO mice is their poor survival rate, with only ∼10% surviving birth. The viable mice, however, were for the most part normal, indicating that life is possible in the absence of glycogen in a variety of important organs.
The presence of glycogen in various tissues during embryonic development was recognized almost 150 years ago (6). Studies of rat and rabbit (7) indicated a large accumulation of glycogen in developing heart. Approximately 2% of the cell volume of an adult cardiomyocyte is occupied by glycogen, while in fetal heart cells, glycogen can comprise up to 30% of the cell volume (36). In the rabbit (7), glycogen begins to accumulate in the heart on day 18 of embryonic development, corresponding to day 16 dpc in the mouse (10, 17a, 42). The maximum accumulation occurs between days 22 and 24, at which time the heart contains approximately 40 times the amount of glycogen found in the adult heart (7). In rat heart, massive amounts of glycogen are found in both 14.5 and 18.5 dpc embryos, corresponding to 13 and 17 dpc mouse embryos (1, 10, 17a, 42). Glycogen accumulation in the mouse embryo seems to have been analyzed only in preimplantation embryos. While there may be species differences in the profile of glycogen accumulation, rat and rabbit embryos have a high heart glycogen content at a stage of embryonic development approximately equivalent to when heart abnormalities are first observed in GYS1-null mice. Dawes et al. (14, 15) hypothesized that cardiac glycogen may function to allow embryos and newborn pups to survive anoxia. Our results indicate that the 10% of MGSKO pups that survive do so in the absence of cardiac glycogen. The more important role of cardiac glycogen appears to be during embryonic development.
At the stage of embryonic development where we observed a transition from normal to abnormal morphology in the hearts of GYS1-null embryos (i.e., 11.5 to 14.5 dpc), there appear to be no reports in the literature characterizing cardiac glycogen metabolism. The only study in this time frame was in rabbits at the stage corresponding to 13 dpc in mouse. At this stage heart had accumulated high levels of glycogen and exhibited glycogen synthase activity. Interestingly, during this period cardiac growth is an important event in response to the rapid increase of hemodynamic load and maintaining normal proliferative activity in cardiomyocytes is critical to producing a normal heart. The demonstration that the absence of glycogen led to abnormalities suggests that glycogen is required to maintain normal cardiomyocyte growth and chamber maturation at midgestation. Smaller hearts, less proliferative activity, and poor cardiac function in the GYS1-null embryos suggest that glycogen is an important energy source for developing cardiomyocytes to proliferate and to support adequate circulation for the developing embryos.
Why can ∼10% of embryos pass through this stage without cardiac glycogen? There are several possible explanations for this strong but not completely obligate requirement for glycogen. First, there is the issue of the genetic background. The mice analyzed have mixed 129/SvJ and C57BL/6J (75% C57BL/6J and 25% 129/SvJ) backgrounds, and there could be some other genetic factors, even in littermates, that combine with the absence of GSY1 to cause the observed pathology. We consider this possibility unlikely. The MGSKO mice have now been backcrossed six times into the SvJ background and eight times into the C57 background. Breeding heterozygotes of these F6 and F8 mice is not resulting in any increase in the proportion of GYS1−/− survivors (unpublished data). A second possibility is if the embryos that survive the critical stage in development have undergone an adaptive response. This reasoning is proposed by Meeson et al. (33) to explain their observation of partial embryonic lethality in embryos null for myoglobin. The majority of mice lacking myoglobin die at midgestation, but a small number survive by mounting an adaptive response via reprogramming gene expression (33). A third possibility is that glycogen is an energy source for completion of a certain developmental phase, as discussed above, but there is enough variability that statistically some embryos are successful even in its absence.
Our immediate thought, given the defects in cardiac development and signs of impaired cardiac function in the affected embryos, is that the death of the MGSKO mice is attributable to defects in the heart. After switching at birth to a circulation completely independent of the mother, the defective heart would fail in the face of the increased load and perhaps contribute to the failure of the lungs to inflate (13, 27). Some support for lack of cardiac glycogen causing perinatal mortality came from expressing the glycogen synthase transgene of the GSL mice in the MGSKO background. In hearts from GSL25 mice, the glycogen synthase activity and glycogen levels are indistinguishable from wild-type but in the GSL30 line there is a sevenfold increase in cardiac glycogen. The observation that the glycogen synthase transgene from the GSL30 line completely restored viability to mice lacking a functional GYS1 gene while that from GSL25 does not, supports the hypothesis that the need for glycogen in cardiac development is the cause of death in pups unable to synthesize this polymer. A more definitive demonstration should come from expression of glycogen synthase driven by a heart specific promoter in the MGSKO background to see if this restores viability to the offspring. We are currently crossing transgenic mice expressing glycogen synthase under the control of the atrial natriuretic factor promoter with MGSKO mice.
Adult hearts from MGSKO mice less than 1 year old were normal by our analyses, except for their lack of glycogen and glycogen synthase activity and a larger cardiac mass. Other than by size, the hearts were normal morphologically. Analysis by echocardiography revealed no major alterations in cardiac dimensions in male MGSKO mice, except for a trend toward increased left ventricular mass, consistent with overall heart weight. Functionally, no abnormalities were observed. Likewise, electrocardiograms were not anomalous. However, we cannot exclude the possibility that the hearts might have behaved abnormally in response to specific stresses, such as ischemia or β-adrenergic stimulation. The only cardiac anomaly detected was in older MGSKO mice, more than 1 year old, in which fibrosis became apparent. How this observation is related to cardiac glycogen is not readily apparent but could be linked to reduced ability of the myocytes to cope with stresses as the animals grow older. Most glycogen-based aberrations in hearts have been linked to the hyperaccumulation of glycogen, such as in patients with various glycogen storage diseases (reviewed in references 17 and 18) or Wolff-Parkinson-White syndrome (2). A mouse model involving overexpression of a mutant form of the γ2 subunit of AMP-activated protein kinase causes glycogen hyperaccumulation (30-fold above that in the wild type) in the heart (3). These mice suffer from ventricular hypertrophy and electrophysical abnormalities. It will be worthwhile to study more extensively hearts from MGSKO mice in terms of stress and cardiac function upon aging.
In summary, from the analysis of genetically engineered mice, we have established that animals can exist, ostensibly normally, in the absence of the storage polymer glycogen in several important tissues, including skeletal muscle and heart. However, we found that lack of glycogen had a severe impact on cardiac development, resulting in perinatal mortality. It is interesting that congenital heart disease in humans is extremely common, resulting in a variety of abnormalities of differing severity (11, 28). As many as 1 in 50 live births may involve a moderate to serious cardiac deformity, the cause of which is often not known. Many of these defects likely result from genetic or environmental challenges to the programs governing gene expression patterns, cell fate determination, and tissue formation. Analysis of MGSKO mice, however, has demonstrated that a simple metabolic defect can also have grave consequences for cardiac development and it is not impossible that impaired glycogen metabolism contributes to congenital heart disease in humans. Mice lacking cardiac glycogen should provide valuable insights into the role of glycogen in heart development and function.
Acknowledgments
This work was supported in part by National Institutes of Health grant DK27221. B.A.P. was supported by a mentor-based postdoctoral research award from the American Diabetes Association (to P.J.R.). Blood pressure and echocardiography measurements were made at the Vanderbilt University Mouse Metabolic Phenotyping Center, which is supported in part by National Institutes of Health grant U24 DK59637.
We thank David Wasserman and Owen McGuiness of Vanderbilt University and Beth Thurberg of Genzyme Corporation, Framingham, Mass., for helpful advice and discussions. We are indebted to Cheryl Bock of the Comprehensive Cancer Center, Duke University Medical Center, for karyotyping ES cells. Carlie R. Cope provided invaluable technical assistance.
REFERENCES
- 1.Altman, P. L., and D. S. Dittmer. 1962. Growth including reproduction and morphological development. Federation of American Societies for Experimental Biology, Washington, D.C.
- 2.Arad, M., D. W. Benson, A. R. Perez-Atayde, W. J. McKenna, E. A. Sparks, R. J. Kanter, K. McGarry, J. G. Seidman, and C. E. Seidman. 2002. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J. Clin. Investig. 109:357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arad, M., I. P. Moskowitz, V. V. Patel, F. Ahmad, A. R. Perez-Atayde, D. B. Sawyer, M. Walter, G. H. Li, P. G. Burgon, C. T. Maguire, D. Stapleton, J. P. Schmitt, X. X. Guo, A. Pizard, S. Kupershmidt, D. M. Roden, C. I. Berul, C. E. Seidman, and J. G. Seidman. 2003. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation 107:2850-2856. [DOI] [PubMed] [Google Scholar]
- 4.Azpiazu, I., A. R. Saltiel, A. A. DePaoli-Roach, and J. C. Lawrence. 1996. Regulation of both glycogen synthase and PHAS-I by insulin in rat skeletal muscle involves mitogen-activated protein kinase-independent and rapamycin-sensitive pathways. J. Biol. Chem. 271:5033-5039. [DOI] [PubMed] [Google Scholar]
- 5.Bergmeyer, H. U., E. Berndt, F. Schmidt, and H. Stork. 1974. Glucose determination with hexokinase and glucose-6-phosphate dehydrogenase, p. 1196-1201. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis, 2nd ed., vol. 3. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 6.Bernard, C. 1859. De la matiere glycogene consideree comme condition de developpement de certains tissues chez le foetus avant l'apparition de la fonction glycogenique de foie. C. R. Acad. Sci. 48:673-684. [Google Scholar]
- 7.Bhavnani, B. R. 1983. Ontogeny of some enzymes of glycogen metabolism in rabbit fetal heart, lungs, and liver. Can. J. Biochem. Cell Biol. 61:191-197. [DOI] [PubMed] [Google Scholar]
- 8.Biggers, J. D., and S. Stern. 1973. Metabolism of the preimplantation mammalian embryo. Adv. Reprod. Physiol. 6:1-59. [PubMed] [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 10.Butler, H., and B. H. J. Juurlink. 1987. An atlas for staging mammalian and chick embryos. CRC Press, Inc., Boca Raton, Fla.
- 11.Clark, E. B. 2001. Etiology of congenital cardiovascular malformation: epidemiology and genetics, p. 2-9. In H. D. Allen, H. G. Gutgesell, E. B. Clark, and D. J. Driscoll (ed.), Moss and Adams' heart disease in infants, children, and adolescents: including the fetus and young adult, 6th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 12.Collins-Nakai, R. L., D. Noseworthy, and G. D. Lopaschuk. 1994. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am. J. Physiol. 267:H1862-H1871. [DOI] [PubMed] [Google Scholar]
- 13.Crystal, R. G. 1997. The lung: scientific foundations, 2nd ed. Lippincott-Raven, Philadelphia, Pa.
- 14.Dawes, G. S., J. C. Mott, and H. J. Shelley. 1959. The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia. J. Physiol. (London) 146:516-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawes, G. S., and H. J. Shelley. 1968. Physiological aspects of carbohydrate metabolism in the foetus and newborn, p. 87-121. In F. Dickens, P. J. Randle, and W. S. Whelan (ed.), Carbohydrate metabolism and its disorders, vol. 2. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 16.Depre, C., J. L. Vanoverschelde, and H. Taegtmeyer. 1999. Glucose for the heart. Circulation 99:578-588. [DOI] [PubMed] [Google Scholar]
- 17.DiMauro, S., and C. Lamperti. 2001. Muscle glycogenoses. Muscle Nerve 24:984-999. [DOI] [PubMed] [Google Scholar]
- 17a.Edwards, J. A. 1968. The external development of the rabbit and rat embryo. Adv. Teratol. 3:239-262. [Google Scholar]
- 18.Elpeleg, O. N. 1999. The molecular background of glycogen metabolism disorders. J. Pediatr. Endocrinol. Metab. 12:363-379. [DOI] [PubMed] [Google Scholar]
- 19.Elsasser, A., M. Schlepper, W. P. Klovekorn, W. J. Cai, R. Zimmermann, K. D. Muller, R. Strasser, S. Kostin, C. Gagel, B. Munkel, W. Schaper, and J. Schaper. 1997. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation 96:2920-2931. [DOI] [PubMed] [Google Scholar]
- 20.Fletterick, R. J., and N. B. Madsen. 1980. The structures and related functions of phosphorylase a. Annu. Rev. Biochem. 49:31-61. [DOI] [PubMed] [Google Scholar]
- 21.Gilboe, D. P., K. L. Larson, and F. Q. Nuttall. 1972. Radioactive method for the assay of glycogen phosphorylases. Anal. Biochem. 47:20-27. [DOI] [PubMed] [Google Scholar]
- 22.Girard, J., P. Ferre, J. P. Pegorier, and P. H. Duee. 1992. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol. Rev. 72:507-562. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin, G. W., F. Ahmad, T. Doenst, and H. Taegtmeyer. 1998. Energy provision from glycogen, glucose, and fatty acids on adrenergic stimulation of isolated working rat hearts. Am. J. Physiol. 274:H1239-H1247. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin, G. W., F. Ahmad, and H. Taegtmeyer. 1996. Preferential oxidation of glycogen in isolated working rat heart. J. Clin. Investig. 97:1409-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez-Correa, J., M. Hod, J. V. Passoneau, and N. Freinkel. 1991. Glycogen and enzymes of glycogen metabolism in rat embryos and fetal organs. Biol. Neonate 59:294-302. [DOI] [PubMed] [Google Scholar]
- 26.Henning, S. L., R. B. Wambolt, B. O. Schonekess, G. D. Lopaschuk, and M. F. Allard. 1996. Contribution of glycogen to aerobic myocardial glucose utilization. Circulation 93:1549-1555. [DOI] [PubMed] [Google Scholar]
- 27.Hlastala, M. P., and A. J. Berger. 1996. Physiology of respiration. Oxford University Press, New York, N.Y.
- 28.Hoffman, J. I., and S. Kaplan. 2002. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 39:1890-1900. [DOI] [PubMed] [Google Scholar]
- 29.Kaslow, H. R., and D. D. Lesikar. 1984. Isozymes of glycogen synthase. FEBS Lett. 172:294-298. [DOI] [PubMed] [Google Scholar]
- 30.Kaslow, H. R., D. D. Lesikar, D. Antwi, and A. W. Tan. 1985. L-type glycogen synthase. Tissue distribution and electrophoretic mobility. J. Biol. Chem. 260:9953-9956. [PubMed] [Google Scholar]
- 31.Krege, J. H., J. B. Hodgin, J. R. Hagaman, and O. Smithies. 1995. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25:1111-1115. [DOI] [PubMed] [Google Scholar]
- 32.Manchester, J., A. V. Skurat, P. Roach, S. D. Hauschka, and J. C. Lawrence, Jr. 1996. Increased glycogen accumulation in transgenic mice overexpressing glycogen synthase in skeletal muscle. Proc. Natl. Acad. Sci. USA 93:10707-10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meeson, A. P., N. Radford, J. M. Shelton, P. P. Mammen, J. M. DiMaio, K. Hutcheson, Y. Kong, J. Elterman, R. S. Williams, and D. J. Garry. 2001. Adaptive mechanisms that preserve cardiac function in mice without myoglobin. Circ. Res. 88:713-720. [DOI] [PubMed] [Google Scholar]
- 34.Roach, P. J., A. V. Skurat, and R. A. Harris. 2001. Regulation of glycogen metabolism, p. 609-647. In A. D. Cherrington and L. S. Jefferson (ed.), The endocrine pancreas and regulation of metabolism, vol. 2. Oxford University Press, New York, N.Y. [Google Scholar]
- 35.Rottman, J. N., G. Ni, M. Khoo, Z. Wang, W. Zhang, M. E. Anderson, and E. C. Madu. 2003. Temporal changes in ventricular function assessed echocardiographically in conscious and anesthetized mice. J. Am. Soc. Echocardiogr. 16:1150-1157. [DOI] [PubMed] [Google Scholar]
- 36.Shelley, H. J. 1961. Cardiac glycogen in different species before and after birth. Br. Med. Bull. 17:137-156. [Google Scholar]
- 37.Skurat, A. V., H. L. Peng, H. Y. Chang, J. F. Cannon, and P. J. Roach. 1996. Rate-determining steps in the biosynthesis of glycogen in COS cells. Arch. Biochem. Biophys. 328:283-288. [DOI] [PubMed] [Google Scholar]
- 38.Snyder, T. E., H. M. Weitlauf, and S. R. Nelson. 1971. Comparison of the glycogen content of eggs in the uteri and oviducts of intact and hypophysectomized mice. Biol. Reprod. 5:314-318. [DOI] [PubMed] [Google Scholar]
- 39.Stern, S., and J. D. Biggers. 1968. Enzymatic estimation of glycogen in the cleaving mouse embryo. J. Exp. Zool. 168:61-66. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki, Y., C. Lanner, J. H. Kim, P. G. Vilardo, H. Zhang, J. Yang, L. D. Cooper, M. Steele, A. Kennedy, C. B. Bock, A. Scrimgeour, J. C. Lawrence, Jr., and A. A. DePaoli-Roach. 2001. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Mol. Cell. Biol. 21:2683-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taegtmeyer, H. 1994. Energy metabolism of the heart: from basic concepts to clinical applications. Curr. Probl. Cardiol. 19:59-113. [DOI] [PubMed] [Google Scholar]
- 42.Theiler, K. 1972. The house mouse: development and normal stages from fertilization to 4 weeks of age. Springer-Verlag KG, Berlin, Germany.
- 43.Thomas, J. A., K. K. Schlender, and J. Larner. 1968. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal. Biochem. 25:486-499. [DOI] [PubMed] [Google Scholar]
- 44.Zambrowicz, B. P., A. Abuin, R. Ramirez-Solis, L. J. Richter, J. Piggott, H. BeltrandelRio, E. C. Buxton, J. Edwards, R. A. Finch, C. J. Friddle, A. Gupta, G. Hansen, Y. Hu, W. Huang, C. Jaing, B. W. Key, Jr., P. Kipp, B. Kohlhauff, Z. Q. Ma, D. Markesich, R. Payne, D. G. Potter, N. Qian, J. Shaw, J. Schrick, Z. Z. Shi, M. J. Sparks, I. Van Sligtenhorst, P. Vogel, W. Walke, N. Xu, Q. Zhu, C. Person, and A. T. Sands. 2003. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc. Natl. Acad. Sci. USA 100:14109-14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zambrowicz, B. P., G. A. Friedrich, E. C. Buxton, S. L. Lilleberg, C. Person, and A. T. Sands. 1998. Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells. Nature 392:608-611. [DOI] [PubMed] [Google Scholar]