Abstract
The SAGA histone acetyltransferase and TFIID complexes play key roles in eukaryotic transcription. Using hierarchical cluster analysis of mass spectrometry data to identify proteins that copurify with components of the budding yeast TFIID transcription complex, we discovered that an uncharacterized protein corresponding to the YPL047W open reading frame significantly associated with shared components of the TFIID and SAGA complexes. Using mass spectrometry and biochemical assays, we show that YPL047W (SGF11, 11-kDa SAGA-associated factor) is an integral subunit of SAGA. However, SGF11 does not appear to play a role in SAGA-mediated histone acetylation. DNA microarray analysis showed that SGF11 mediates transcription of a subset of SAGA-dependent genes, as well as SAGA-independent genes. SAGA purified from a sgf11Δ deletion strain has reduced amounts of Ubp8p, and a ubp8Δ deletion strain shows changes in transcription similar to those seen with the sgf11Δ deletion strain. Together, these data show that Sgf11p is a novel component of the yeast SAGA complex and that SGF11 regulates transcription of a subset of SAGA-regulated genes. Our data suggest that the role of SGF11 in transcription is independent of SAGA's histone acetyltransferase activity but may involve Ubp8p recruitment to or stabilization in SAGA.
A major component in the regulation of eukaryotic transcription initiation is the recruitment of transcriptional machinery to promoter elements that are arranged in tightly packed chromatin structures (21, 23, 30, 34). A number of multiprotein complexes that mediate chromatin remodeling and promote transcriptional activity have been characterized (22, 32). There are two general mechanisms by which these complexes alter chromatin structure. One class of complexes alters chromatin structure in an ATP-dependent manner (19, 20). A second class regulates the chromatin structure by covalently modifying core histone proteins (H2A, H2B, H3, and H4) (31, 45). Although several different histone modifications have been observed including phosphorylation, ubiquitination, and methylation (7, 16, 28, 47), lysine acetylation catalyzed by histone acetyltransferase (HAT) enzymes is the best-characterized. Several multiprotein HAT complexes have been characterized in yeast. These include SAGA, ADA, NuA4, and NuA3 (reviewed in reference 43).
Of the four known HAT complexes in yeast, SAGA is the best characterized. The name SAGA refers to its composition of Spt proteins (Spt3p, Spt7p, Spt8p, and Spt20p), Ada proteins (Ada1p, Ada2p, and Ada3p), and Gcn5p acetyltransferase (15). In addition to these components, SAGA also contains Tra1p and a subset of TATA-binding protein associated factors (TAFs), proteins originally identified as members of the TFIID transcription complex (13, 32). These include Taf5p, Taf6p, Taf9p, Taf10p, and Taf12p. A variant of the SAGA complex, named SALSA (SAGA altered, Spt8p absent) or SLIK (SAGA-like), has also been described (35, 42). This version of SAGA lacks Spt8p and has a truncated form of Spt7p. It has been shown that truncation of Spt7p results in loss of Spt8p from the SAGA complex (48). The functional role of SALSA/SLIK is unclear.
The function of SAGA components have been revealed through the study of SAGA-subunit-null strains. DNA microarray experiments suggest that SAGA is required for the expression of 10% of the predicted genes in S. cerevisiae (27). Furthermore, in vivo transcription experiments have pinpointed the importance of SAGA in the regulation of a specific subset of genes including GAL1 (3, 26), HIS3 (2, 42), TRP3 (2, 42), ARG1 (18, 36), and ADH1 (5, 16). Gcn5p is the HAT catalytic subunit of SAGA (12). Gcn5p alone is capable of acetylating free histones; however, acetylation of histone H3 in intact nucleosomes requires SAGA (12). Ada2p and Ada3p regulate SAGA-dependent HAT activity (1, 4, 44). Spt7p, Spt20p, and Ada1p are required for the integrity of SAGA (44, 48). Spt3p and Spt8p mediate the binding of TATA-binding protein to specific promoter regions in vitro and the activation of specific RNA polymerase II-dependent genes in vivo (2, 3). Tra1p is an essential gene and its human homolog TRRAP is a transcription regulatory protein (14, 40).
In a previous study, we described an extensive proteomic investigation of the TFIID complex in S. cerevisiae (41). Individual components of TFIID were immunoaffinity purified from cell extracts, and copurifying proteins were identified by using a high-sensitivity mass spectrometry approach termed direct analysis of large protein complexes (DALPC) (29). Affinity purification of TFIID subunits that are shared with SAGA (Taf5p, Taf6p, Taf9p, Taf10p, and Taf12p) resulted in copurification of components unique to SAGA. In addition to the previously characterized subunits of SAGA, we identified three additional proteins. These include two uncharacterized proteins corresponding to the open reading frames (ORFs) YCL010C and YGL066W (named Sgf29p and Sgf73p, respectively) and a ubiquitin-specific protease, Ubp8p. All three proteins were confirmed as novel SAGA subunits (41).
In the present study, we used a hierarchical clustering algorithm and display developed for identifying gene expression patterns from DNA microarray experiments to reanalyze our proteomic data of proteins copurifying with TFIID (8, 10). The clustering approach arranged proteins according to similarities in patterns of enrichment with the TFIID subunits. Hierarchical clustering provided an unbiased, statistical approach to identifying distinct protein complexes from a large number of proteins that were systematically immunoaffinity purified and analyzed by mass spectrometry. Based on our analysis, an uncharacterized protein corresponding to the ORF YPL047W significantly associates with shared components of TFIID and SAGA. For the present study we have focused on YPL047W (named here as SGF11, 11-kDa SAGA-associated factor) as a novel SAGA component and examined its role in SAGA structure and function. By using mass spectrometry and biochemical assays, we show that Sgf11p interacts with SAGA. We show that Sgf11p is required for the stable association of Ubp8p in purified SAGA. Sgf11p does not appear to regulate the HAT activity of SAGA. However, DNA microarray analysis shows that Sgf11p does mediate transcription of a subset of SAGA-regulated genes. Together, these results indicate that Sgf11p is a novel component of SAGA that mediates gene-specific transcription possibly through the recruitment or stabilization of Ubp8p to SAGA.
MATERIALS AND METHODS
Plasmids.
Strain construction, other genetic manipulations, and yeast medium preparation were carried out by standard methods (for a listing of yeast strains, see Table 1). To construct the Gateway cloning plasmid pENTR-SGF11, the PCR primers A-SGF11 (5′-CACCATGACCGAAGAAACTATTAC-3′) and B-SGF11 (5′-ACGTCTAGCACCCCTACTCAAAC-3′) were used to amplify a 310-bp fragment encoding the complete ORF of SGF11 from yeast genomic DNA. The PCR product was cloned into the pENTR vector (Invitrogen) to create plasmid pENTR-SGF11. The SGF11 ORF was shuttled from pENTR-SGF11 into the pYES-DEST52 yeast expression vector by using the Gateway cloning system (Invitrogen) to create plasmid pYES-SGF11.
TABLE 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| 7503040 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0:SGF11-TAP-HIS3MX6 | OpenBiosystems |
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Research Genetics |
| BY4743 | 4741/4742 | Research Genetics |
| 32781 | sgf11 deletion in BY4743 background | Research Genetics |
| 37285 | gcn5 deletion in BY4743 background | Research Genetics |
| 33534 | ada3 deletion in BY4743 background | Research Genetics |
| 30809 | ubp8 deletion in BY4743 background | Research Genetics |
Cluster analysis of proteomics data identifying proteins copurifying with shared components of TFIID and SAGA.
Previously published mass spectrometry data from 41 TFIID immunoaffinity purification experiments and control experiments were used for the hierarchical cluster analysis (41). Relative protein abundances in each experiment were expressed as the total number of nonredundant spectra that correlated significantly to each ORF normalized to the molecular weight of the cognate protein (×104). We call this value a protein abundance factor (PAF; J. McAfee and A. Link, unpublished results). For cluster analysis, a matrix of experiments and proteins was constructed with the PAF at each protein-experiment intersection. Unsupervised average-linkage hierarchical clustering was performed with Euclidean distance as the similarity metric (8). Using these results, the cluster thumbnail, dendrogram, and area images of the clustering results were generated (10).
Affinity purification of SAGA complexes.
The SAGA complex was purified from S. cerevisiae whole-cell extracts by using two different methods. First, the SAGA complex was immunoaffinity purified from wild-type (strain BY4743) and sgf11Δ deletion (strain 32781) strains by using polyclonal antibodies against Gcn5p as previously described (41). Second, tandem affinity purification (TAP) was used to purify Sgf11p as previously described (46). Basically, the TAP-SGF11 strain (7503040) was grown in 4 liters of YPD to an optical density at 600 nm of 1.0, and cells were harvested by centrifugation (∼60 g wet weight). Cells were lysed with 0.5-mm zirconia-silica beads in lysis buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% NP-40, 1× Complete protease inhibitor [Roche]). The protein lysate was incubated with immunoglobulin G (IgG)-Sepharose beads (Pharmacia) for 1 h at 4°C and then poured into a disposable column. The beads were washed with lysis buffer, equilibrated with tobacco etch virus (TEV) cleavage buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% NP-40, 0.5 mM EDTA, 1.0 mM dithiothreitol [DTT]), and incubated with 5 U of TEV protease (Invitrogen) for 2 h at room temperature. After TEV cleavage, the IgG column eluate was incubated with calmodulin-affinity resin (Stratagene) for 1 h at 4°C, poured into a disposable column, and washed with calmodulin binding buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% NP-40, 1 mM imidazole, 2 mM CaCl2, 10 mM 2-mercaptoethanol). The bound proteins were eluted with calmodulin elution buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% NP-40, 1 mM imidazole, 20 mM EGTA, 10 mM 2-mercaptoethanol) and concentrated by trichloroacetic acid (TCA) precipitation. Purified protein complexes were reduced, alkylated, and digested with trypsin prior to analysis by mass spectrometry.
Mass spectrometry analysis of protein complexes.
The DALPC mass spectrometry approach was used to identify purified proteins as previously described (29).
Immunoaffinity copurification assays.
The sgf11Δ deletion strain (32781) was transformed with the pYES-DEST52 vector or pYES-SGF11 and cultured overnight at 30°C in 50 ml of SC-Ura medium with 2% raffinose as a carbon source. SGF11 expression was induced by addition of galactose to a final concentration of 2%, and the yeast strains were cultured for an additional 6 h at 30°C. Cells were pelleted and resuspended in 1 ml of lysis buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 5 mM 2-mercaptoethanol). Cells were lysed by using an equal volume of 0.5-mm zirconia-silica beads and a Mini-Beadbeater-8 (Biospec Products). Lysates were centrifuged at 20,000 × g for 15 min to remove particulate material. V5 epitope-tagged Sgf11p and endogenous Spt7p and Gcn5p were immunoaffinity purified from lysates by overnight incubation with either 2 μg of monoclonal anti-V5 (Invitrogen) coupled to 10 μl of protein A-Sepharose beads (Sigma) or 20 μg of affinity purified polyclonal anti-Spt7p or anti-Gcn5p antibodies (41) coupled to 10 μl of protein A-Sepharose beads. Beads were washed four times with 1 ml of wash buffer (20 mM HEPES [pH 7.9], 300 mM potassium acetate, 10% glycerol, 1 mM DTT, 1× Complete protease inhibitors). Proteins were eluted from the beads with Laemmli sodium dodecyl sulfate (SDS) sample buffer and separated by NuPAGE SDS-12% polyacrylamide gel electrophoresis (PAGE) and morpholineethanesulfonic acid buffer (Invitrogen) prior to immunoblot analysis.
Immunoblot analysis.
NuPAGE gels were transferred to nitrocellulose membranes and blocked overnight in Tris-buffered saline containing 0.05% Tween and 5% nonfat dry milk. For immunoaffinity copurification assays, membranes were probed with either affinity-purified polyclonal antibody to Spt7p or monoclonal anti-V5 (Invitrogen). For SAGA composition assays, membranes were probed with an affinity-purified polyclonal antibody to Ubp8p, Tra1p, Taf12p, Gcn5p, and Taf10p (41). For HAT assays, Gcn5p was detected by using an affinity-purified polyclonal antibody (41). Membranes were washed five times in Tris-buffered saline containing 0.05% Tween and then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Promega). Target proteins were visualized by autoradiography by using ECL Plus reagent (Amersham).
DNA microarray experiments.
Wild-type (BY4743) and sgf11Δ deletion (32781) strains were grown to an optical density at 600 nm of 1.0 in YPD at 30°C. Total RNA was extracted by using TRI Reagent and the manufacturer's suggested protocol (Molecular Research Center). Total RNA (∼100 μg) from each strain was incubated with 5 U of DNase (Promega) for 1 h at room temperature. The DNase was inactivated by incubation with 1 mM EDTA at 65°C for 10 min. The DNase-treated RNA samples were used for reverse transcription (RT) reactions. Oligo(dT)16 primer (2 μg) was added to the RNA sample, followed by incubation at 70°C for 10 min and chilling on ice for 5 min. The 30-μl samples were incubated with 30 μl of a labeling mixture (6 μl of dGTP-dATP-dCTP (each nucleotide at 1 mM), 2 μl of dTTP (1 mM), 4 μl of dUTP (1 mM), 12 μl of 5× first-strand buffer, 6 μl of DTT [0.1 M]), and 400 U of SuperScript II reverse transcriptase (Invitrogen) at 42°C for 2 h. RNA templates were removed by hydrolysis by first incubation at 95°C for 2 min and then incubation with 10 μl of 1 M NaOH and 10 μl of 0.5 M EDTA at 65°C for 30 min. After hydrolysis, 25 μl of 1 M HEPES (pH 7.5) was added, and cDNAs were purified by using a QIAquick kit (QIAGEN). The cDNAs were coupled to monofunctional NHS-ester Cy-dyes (Amersham). Wild-type cDNAs were labeled with Cy3 and cDNAs from the sgf11Δ deletion strain were labeled with Cy5. The dyes were resuspended in 10 μl of dimethyl sulfoxide, and 1.25 μl of the appropriate dye was added to each sample and incubated in the dark at room temperature for 1 h. The reactions were terminated by adding 4.5 μl of 4 M hydroxylamine (Sigma) and incubating them in the dark at room temperature for 15 min. The samples were combined, and the labeled cDNAs were purified by using a QIAquick kit. The labeled cDNAs were hybridized to DNA microarrays comprised of 6,735 yeast genes spotted in duplicate at high density on 75-by-25-mm glass slides. DNA microarrays were made by using the yeast genome oligonucleotide set (Operon) by the Vanderbilt Microarray Shared Resource Center.
DNA microarray data analysis.
The yeast arrays were scanned, and intensity analysis was performed by using GenePix Pro software (Axon). Gene annotation and interpretation were performed by using the program GeneTraffic (Iobion Informatics LLC, La Jolla, Calif.). The raw data were normalized by using a LOWESS sub-grid global normalization method. The following specific criteria were used to obtain a list of genes that were considered to be significantly affected by the sgf11Δ deletion. Genes were reported if there was a >2-fold change up or down from two independent experiments, the fold change was consistent between both experiments, and the effect was observed for the duplicate gene spots on each array.
RT-PCR experiments.
RT-PCR was used to validate selected microarray results. RNA isolated from wild-type (BY4743) and sgf11Δ deletion (32781) strains was reverse transcribed by using the protocol described for the microarray experiments. PCR was performed with the resulting cDNA and primer sets for CDC8 (5′-TCTGCCGCTAAGGGGACAAATG-3′ and 5′-GCGCTTCAACTTCCTGAATGCC-3′), MATα1 (5′-TTCGCAGCATCCTCCGCATTAG-3′ and 5′-ACCAATGCCAAGCTTCAGCCTC-3′), and TDH3 (5′-TCTTCCATCTTCGATGCTGCCG-3′ and 5′-AGCCTTGGCAACGTGTTCAACC-3′) (Sigma Genosys). RT-PCR products were separated by using a 6% polyacrylamide gel cast in 0.5× Tris-borate-EDTA buffer and visualized by using ethidium bromide staining. Identical experiments were also performed with RNA isolated from a ubp8Δ deletion strain (30809).
HAT assays.
The HAT Gcn5p was immunoaffinity purified from wild-type (BY4743) and deletion strains for gcn5, ada3, and sgf11 (37285, 33534, and 32781, respectively). Overnight cultures of each strain were grown in 50 ml of YPD at 30°C. Cells were pelleted and lysed in 1 ml of lysis buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, and 5 mM 2-mercaptoethanol) by using 1 ml of 0.5-mm zirconia-silica beads and a Mini-Beadbeater-8. Lysates were centrifuged at 20,000 × g for 10 min to remove particulate material. Lysates were then incubated overnight at 4°C with 20 μg of affinity-purified polyclonal anti-Gcn5p IgG covalently coupled to protein A-Sepharose beads. After incubations, beads were washed three times with 1 ml of buffer (20 mM HEPES [pH 7.9], 300 mM potassium acetate, 10% glycerol, 1 mM DTT, 1× Complete protease inhibitor) and then washed with 1 ml of HAT buffer (75 mM Tris [pH 8], 50 mM NaCl, 0.1 mM EDTA, 1 mM MgCl2, 25% glycerol, 1 mM DTT, 5 mM sodium butyrate). For the HAT reaction, the washed beads were resuspended in 30 μl of HAT buffer containing 0.25 μCi of 3H-acetyl-CoA (ICN) and 5 μg of oligonucleosomes. The oligonucleosomes were prepared by using previously described methods (33). Reaction mixtures were incubated for 1 h at 30°C. Reactions were terminated with Laemmli SDS sample buffer. Aliquots (10 μl) were used for an anti-Gcn5p immunoblot analysis, and the proteins in the remaining sample were separated by NuPAGE on 4 to 12% gradient SDS-PAGE gels. To detect the quantity and migration of histones, gels were stained with R-250 Coomassie blue. The stained gels were incubated with ENHANCE (Perkin-Elmer Life Sciences), and histone acetylation was visualized by autoradiography.
RESULTS
Identification and characterization of Sgf11p as a novel component of SAGA.
In a previous study, a combination of immunoaffinity protein purification and direct mass spectrometry analysis was used to identify components of the TFIID basal transcription complex in S. cerevisiae (41). In the present study, we used an unsupervised hierarchical cluster analysis of these proteomic data to identify groups of proteins that show similar patterns of proteins copurifying with components of TFIID (Fig. 1). A region of the cluster displayed a group of proteins copurifying with 5 of the 14 TAFs. Upon closer examination, this cluster of proteins contained all of the previously characterized components of SAGA. The SAGA components were specifically enriched in duplicate purifications of the five TAFs shared by TFIID and SAGA (Taf5p, Taf6p, Taf9p, Taf10p, and Taf12p). With the exception of TATA-binding protein (TBP), the SAGA subunits were essentially absent from all of the other TAFs not shared with SAGA. SAGA components were also absent in the control experiments used to measure the nonspecific background (Fig. 1). In addition to the known SAGA components clustering in this distinct region, an 11-kDa protein corresponding to an uncharacterized ORF, YPL047W, significantly associated with the shared TAFs (Fig. 1). These findings suggested that YPL047W was a novel component of SAGA but not of TFIID. Based on these interactions and the results below, we named the gene SGF11 (for SAGA-associated factor 11 kDa).
FIG. 1.
Cluster analysis of proteomic data identifies YPL047W as a candidate subunit of SAGA. The cluster diagram on the left shows the unsupervised hierarchical clustering of proteomic data from replicate immunoaffinity purifications of each component of TFIID and control purifications (41). The enlargement on the right expands the cluster display where a pattern of proteins copurifying with a distinct set of TAFs is observed. This cluster of proteins includes all of the previously characterized components of SAGA and a protein corresponding to the uncharacterized ORF (uORF) YPL047W. The SAGA proteins are clustering together with the five TAFs known to be shared by TFIID and SAGA. These shared TAFs are labeled with an asterisk. The estimated relative abundance of each identified protein, PAF (see Materials and Methods), is designated by a shade of red. PAFs of proteins identified in control experiments are indicated by shades of green. A square is black if the protein was not detected from the computational analysis of the experiment's mass spectrometry data. The cluster diagram on the left suggest additional groups of protein associated with specific TFIID subunits that are not addressed in this report (McAfee et al., unpublished).
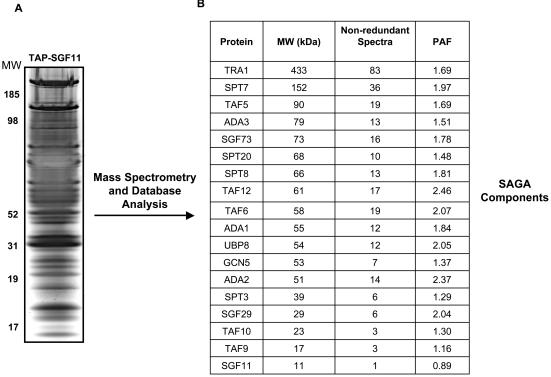
To examine whether Sgf11p is an actual component of the SAGA complex, the corresponding protein was purified from a yeast strain expressing a TAP-tagged allele of SGF11. To measure nonspecific background, control TAP experiments were performed in parallel with an isogenic yeast strain expressing the TAP sequences alone. The purified proteins from the TAP-SGF11 and TAP-control strains were identified by using DALPC mass spectrometry (29). No SAGA or TFIID components were identified in the control purifications. An aliquot of the TAP-SGF11 purification was separated by SDS-PAGE and silver stained (Fig. 2A). Mass spectrometry analysis showed that all of the previously characterized SAGA components copurified with TAP-SGF11 (Fig. 2B). No TFIID components, other than the shared TAFs and TBP, were identified from the SGF11 purification. Due to the aim of these experiments, we only show the SAGA components that copurified with Sgf11p. However, a total of 193 unique proteins were identified from the SGF11 purification. Although many of these proteins were also identified from control purifications and considered to be nonspecific background, some were unique to the SGF11 purification. (A complete list of proteins identified from TAP-SGF11 and control purifications is available at http://www.linklab.mc.vanderbilt.edu.) Our results indicate that Sgf11p associates with SAGA and not TFIID. Our findings do not, however, rule out the possibility that Sgf11p also associates with additional HAT complexes that share common components with SAGA, such as ADA, HAT-A2, and SALSA/SLIK (12, 35, 42, 43).
FIG. 2.
SAGA components copurify with TAP-Sgf11p from yeast extracts. (A) Proteins recovered after purification from a TAP-SGF11 yeast strain were separated by SDS-PAGE and silver stained. (B) The proteins purified with TAP-Sgf11p were identified by DALPC mass spectrometry. None of the listed proteins were identified from control purifications. The number of nonredundant spectra identifying each protein and a PAF (the spectra were normalized to molecular weight [MW] of the cognate protein [104]) are shown. The complete set of proteins identified by mass spectrometry is available at http://linklab.mc.vanderbilt.edu.
Immunoaffinity copurification assays were used to further validate that Sgf11p copurifies with SAGA. Copurification of Sgf11p with the SAGA subunits Gcn5p and Spt7p was examined. Lysates from yeast containing a V5-tagged SGF11 or the empty vector were incubated with anti-Gcn5p or anti-Spt7p antibodies coupled to protein A-Sepharose. Immunoaffinity-purified proteins were separated by SDS-PAGE, and copurification of V5-Sgf11p was detected by immunoblot analysis for the V5 epitope. Figure 3A shows V5-Sgf11p in the cell lysates. Sgf11p copurified with Gcn5p and Spt7p (Fig. 3B and C) but was not detected in control purifications with no antibody (Fig. 3D). In a complementary experiment, immunoaffinity purification of V5-Sgf11p also isolated Spt7p (Fig. 3E). These data, along with the mass spectrometry results, demonstrate that Sgf11p is a novel component of the yeast SAGA HAT complex.
FIG. 3.
Sgf11p copurifies with the SAGA subunits Gcn5p and Spt7p. Immunoaffinity copurification assays were performed with yeast strains containing either a plasmid expressing V5-tagged-SGF11 (pYes-SGF11) or the empty vector (pYes-DEST52). Proteins were analyzed by immunoblotting (IB) as follows. The numbers on the left of each panel represent molecular weight standards. (A) Cell lysates were probed with an anti-V5 antibody. The V5 fusion protein is seen at the expected molecular mass of 13 kDa. (B and C) Proteins immunoaffinity purified (IP) with anti-Gcn5p and anti-Spt7p antibodies, respectively, were probed with an anti-V5 antibody. V5-Sgf11p was recovered with both Gcn5p and Spt7p. (D) Proteins purified with protein A-Sepharose alone were probed with an anti-V5 antibody. No V5-Sgf11p was recovered. (E) Proteins immunoaffinity purified (IP) with an anti-V5 antibody were probed with an anti-Spt7p antibody. Spt7p was recovered with V5-Sgf11p.
Role of Sgf11p in the composition of SAGA.
To investigate a possible function for Sgf11p, we examined whether its absence affects the interaction of other proteins in the SAGA complex. SAGA was purified from wild-type and sgf11Δ deletion strains by Gcn5p immunoaffinity purification in duplicate experiments. The components were analyzed by DALPC mass spectrometry. A comparative analysis was used to identify differences in SAGA components purified from the two strains (Table 2). The SAGA components with differences >1 standard deviation (SD) from the ratio of the estimated relative abundances in wild-type and sgf11Δ deletion strains were consider significant. These proteins were further examined by immunoblot analysis. The most dramatic observation from the mass spectrometry analysis was the absence of detectable Ubp8p in SAGA purified from the sgf11Δ strain (Table 2). A separate immunoblot analysis showed essentially equal amounts of Ubp8p in wild-type and sgf11Δ lysates. However, immunoaffinity-purified SAGA complexes from the two strains showed an ∼4-fold reduction of Ubp8p in SAGA purified from the sgf11Δ strain (Fig. 4A). The mass spectrometry analysis also showed differences of >1 SD for Taf12p and Gcn5p (Table 2). Differences for Taf12p and Gcn5p, as well as smaller differences observed for Tra1p and Taf10p, were examined by immunoblot analysis. Immunoblot analysis failed to detect significant changes in the amounts of Taf12p, Gcn5p, Tra1p, and Taf10p in SAGA purified from wild-type and sgf11Δ strains (Fig. 4B). Together, these results indicate that Sgf11p does not have a substantial role in maintaining the overall composition or integrity of SAGA but appears to mediate either recruitment or stable association of Ubp8p in SAGA.
TABLE 2.
Mass spectrometry analysis of SAGA purified from wild-type and sgf11 deletion strainsa
| ORF | Protein | WT avg NPAF | sgf11Δ avg NPAF | NPAF sgf11Δ/WT ratio |
|---|---|---|---|---|
| YGR252W | TRA1 | 0.00624 | 0.00475 | 0.7613 |
| YDR145W | SPT7 | 0.00661 | 0.00621 | 0.94 |
| YLR055C | TAF5 | 0.00649 | 0.00749 | 1.1529 |
| YOL148C | ADA3 | 0.01354 | 0.01425 | 1.0523 |
| YDR448W | SGF73 | 0.00774 | 0.00961 | 1.2419 |
| YGL066W | SPT20 | 0.00599 | 0.00817 | 1.363 |
| YCL010C | SPT8 | 0.00634 | 0.00905 | 1.4278 |
| YBR198C | TAF12 | 0.0054 | 0.00935 | 1.7327 |
| YGL112C | TAF6 | 0.00607 | 0.00626 | 1.0307 |
| YDR176W | ADA1 | 0.00711 | 0.00467 | 0.6565 |
| YDR392W | UBP8 | 0.00686 | 0 | 0 |
| YBR081C | GCN5 | 0.00405 | 0.00875 | 2.1584 |
| YMR236W | ADA2 | 0.01256 | 0.01597 | 1.2718 |
| YHR099W | SPT3 | 0.00738 | 0.007 | 0.9477 |
| YPL254W | SGF29 | 0.01307 | 0.01538 | 1.1766 |
| YDR167W | TAF10 | 0.00545 | 0.00343 | 0.6288 |
| YMR223W | TAF9 | 0.00708 | 0.00587 | 0.8289 |
| YPL047W | SGF11 | 0.00458 | 0 | 0 |
Proteins immunoaffinity purified with Gcn5p from the wild type (WT) and from a sgf11Δ deletion strain were identified by DALPC mass spectrometry. To estimate the relative composition of individual components in purified SAGA, the PAF value for each subunit was normalized to the total number of nonredundant spectra identifying the entire SAGA complex (see Materials and Methods). We call this value the normalized PAF (NPAF). The table shows the average NPAF values from duplicate experiments and the ratio of these averages from the two strains. The overall average ratio ± its SD was 1.02 ± 0.527.
FIG. 4.
Association of Ubp8p with SAGA is reduced in a sgf11 deletion strain. (A) Proteins immunoaffinity purified (IP) with an anti-Gcn5p antibody from wild-type and sgf11Δ deletion strains were probed with an anti-Ubp8p antibody. The recovery of Ubp8p was reduced in the purification from the sgf11 deletion strain. The input of Ubp8p in cell lysates is also shown. (B) Proteins immunoaffinity purified (IP) with an anti-Gcn5p antibody from wild-type and sgf11 deletion strains were also probed with antibodies for Taf12p, Gcn5p, Tra1p, and Taf10p.
Role of Sgf11p in SAGA-mediated histone acetylation.
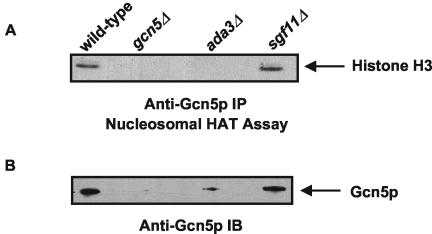
HAT activity is a major function of SAGA in transcriptional regulation (15). Thus, we examined the role of SGF11 in SAGA-mediated HAT activity. SAGA is required for Gcn5p-mediated acetylation of histone H3 in intact nucleosomes (12, 38), and this HAT activity is regulated by the SAGA component ADA3 (1). Therefore, SAGA was recovered from wild-type and gcn5Δ, ada3Δ, and sgf11Δ deletion mutants by Gcn5p immunoaffinity purification. Purified complexes were incubated with purified oligonucleosomes and 3H-acetyl-CoA. After these reactions, histones were separated by SDS-PAGE and Coomassie blue stained, and acetylation was visualized with autoradiography. The primary band detected by autoradiography corresponds to the expected migration of histone H3 (Fig. 5). Immunoblot analysis was used to measure the amount of Gcn5p purified from each strain. As expected, Gcn5p was required for histone H3 acetylation. Gcn5p purification was less robust from the ada3Δ strain, and a reduced histone H3 acetylation was observed. These results are consistent with the requirement of Ada3p for SAGA-mediated HAT activity (1). In three independent experiments, Gcn5p purification and histone acetylation of the sgf11Δ deletion were similar to that of the wild-type strain. Together, these results suggest that the histone acetylation observed in our experiments is both Gcn5p/SAGA-dependent and Sgf11p independent.
FIG. 5.
Sgf11p is not required for SAGA's HAT activity. SAGA complexes were immunoaffinity purified from the indicated strains with an anti-Gcn5p antibody and used in HAT assays containing nucleosomal histones and 3H-acetyl-CoA. (A) Aliquots from each HAT assay were subjected to SDS-PAGE, followed by autoradiography to reveal labeled histones. (B) Aliquots of the same reactions were subjected to immunoblotting (IB) with the anti-Gcn5p antibody to show recovery of Gcn5p.
Role of Sgf11p in transcriptional regulation.
The association of Sgf11p with the SAGA complex suggests that Sgf11p is important for transcription initiation. To investigate the role of Sgf11p in transcription, we used DNA microarray experiments to determine mRNA levels for 6,735 genes in a sgf11Δ deletion strain and an isogenic wild-type strain. Genes were reported if there was a >2-fold change up or down from two independent experiments and the fold change was consistent between both experiments. The sgf11Δ deletion caused a >2-fold downregulation in the transcription of 37 genes and a >2-fold upregulation of 17 genes relative to the wild type (Tables 3 and 4, respectively). The values are averages from duplicate experiments. The raw data are available at http://www.linklab.mc.vanderbilt.edu. Although direct or indirect effects cannot be discriminated from these studies, comparing the effects of the sgf11 deletion to a previous microarray study for deletions of other SAGA components (27) suggests that SGF11 regulates the transcription of a subset of genes previously scored as SAGA dependent, as well as SAGA-independent genes.
TABLE 3.
Transcripts reduced >2-fold by sgf11 deletiona
| ORFb | Gene | Fold change | Molecular function |
|---|---|---|---|
| YER170Wbf | MUP3 | −17.4 | Adenylate kinase |
| YPL187Wa | MATα1 | −15.2 | Pheromone |
| YAR070C | −10.5 | Unknown | |
| YBR116Cce | −9.1 | Unknown | |
| YPL205Cd | −6.2 | Unknown | |
| YKL202Wbf | −5.6 | Unknown | |
| YPR142Cbf | −5.5 | Unknown | |
| YNL078Wa | NIS1 | −4.1 | Unknown |
| YAR066Wa | SEN34 | −4.0 | tRNA-intron endonuclease |
| YPL189Wd | GUP2 | −3.5 | Glycerol uptake |
| YJL169W | −3.3 | Unknown | |
| YBR099Cc | −3.1 | Unknown | |
| YPL163Cac | SVS1 | −3.0 | Protein required for vanadate resistance |
| YPR130C | −3.0 | Unknown | |
| YDL177C | −2.8 | Unknown | |
| YBL040Ca | ERD2 | −2.6 | HDEL sequence binding |
| YOL135C | MED7 | −2.6 | RNA polymerase II transcription mediator |
| YOR129C | −2.6 | Unknown | |
| YBL016Wa | FUS3 | −2.5 | Mitogen-activated protein kinase |
| YOR394Wa | −2.5 | Unknown | |
| YPL056C | −2.5 | Unknown | |
| YPR141Cbc | KAR3 | −2.5 | Microtubule motor |
| YPL058C | PDR12 | −2.4 | Xenobiotic-transporting ATPase |
| YOR032Cae | HMS1 | −2.4 | Transcription factor |
| YNL172W | APC1 | −2.4 | Protein binding |
| YBR013Cb | −2.4 | Unknown | |
| YHR005Cac | GPA1 | −2.3 | Heterotrimeric G-protein GTPase |
| YML027W | YOX1 | −2.3 | DNA binding |
| YIL054Wa | −2.3 | Unknown | |
| YIL123Wa | SIM1 | −2.3 | Regulation of cell cycle |
| YKR022C | −2.3 | Unknown | |
| YIL036W | CST6 | −2.2 | Specific RNA polymerase II transcription factor |
| YOR338Wae | −2.2 | Unknown | |
| YOR366W | −2.2 | Unknown | |
| YOL152W | FRE7 | −2.1 | Ferric-chelate reductase |
| YJL158Ca | CIS3 | −2.0 | Structural constituent of cell wall |
The microarray results for the sgf11 deletion were compared to those previously reported for deletion of the SAGA components SPT20, GCN5, and SPT3 (27).
Superscript letters indicate transcript types as follows: a, transcripts regulated in the same direction by spt20 deletion; b, transcripts regulated in the opposite direction by spt20 deletion; c, transcripts regulated in the same direction by gcn5 deletion; d, transcripts regulated in the opposite direction by gcn5 deletion; e, transcripts regulated in the same direction by spt3 deletion; f, transcripts regulated in the opposite direction by spt3 deletion.
TABLE 4.
Transcripts increased more than twofold by sgf11 deletiona
| ORFb | Gene | Fold change | Molecular function |
|---|---|---|---|
| YJR057W | CDC8 | 6.8 | Thymidylate kinase |
| YGL231Cb | 6.6 | Unknown | |
| YOR254C | SEC63 | 6.2 | Protein transporter |
| YJR135W-A | TIM8 | 3.7 | Protein transporter |
| YJR108Wbc | ABM1 | 3.1 | Microtubule cytoskeleton organization and biogenesis |
| YHR077C | NMD2 | 3.0 | Protein binding |
| YLR449W | FPR4 | 2.7 | Peptidyl-prolyl cis-trans isomerase |
| YDL092W | SRP14 | 2.5 | Signal sequence binding |
| YEL045Cac | 2.4 | Unknown | |
| YJL104Wa | MIA1 | 2.3 | Protein transport |
| YIL016W | SNL1 | 2.2 | Interacts with the nuclear pore complex |
| YMR101C | SRT1 | 2.2 | Prenyltransferase |
| YNL212W | VID27 | 2.2 | Vacuole import and degradation |
| YGR128Ca | UTP8 | 2.1 | snoRNA binding |
| YNR057C | BIO4 | 2.1 | Dethiobiotin synthase |
| YIL069Ca | RPS24B | 2.0 | Structural constituent of ribosome |
| YMR251W-Aa | HOR7 | 2.0 | Responsiveness to hyperosmolarity |
The microarray results for the sgf11 deletion were compared to those previously reported for deletion of the SAGA components SPT20, GCN5, and SPT3 (27).
Superscript letters indicate transcript types as follows: a, transcripts regulated in the opposite direction by spt20 deletion; b, transcripts regulated in the opposite direction by gcn5 deletion; c, transcripts regulated in the opposite direction by spt3 deletion.
RT-PCR experiments were used to validate a subset of our DNA microarray data. We chose CDC8, which was upregulated and MATα1, which was downregulated by the sgf11Δ deletion. MATα1, but not CDC8, was previously reported to be a SAGA-regulated gene (27). RNA was prepared from wild-type and sgf11Δ mutants independently from the samples used in the microarray experiments. The RT-PCR results for both genes were consistent with the DNA microarray experiments (Fig. 6). Based on these results for CDC8 and MATα1, we postulate that the genes listed in Tables 3 and 4 represent probable targets of Sgf11p-mediated transcription.
FIG. 6.
SGF11 and UBP8 regulate transcription. RNA isolated from wild-type and mutant strains with sgf11 or ubp8 deletions was used as the template in RT-PCRs. The products from cycle 15 were separated on a polyacrylamide gel and stained with ethidium bromide. The numbers of the left indicate base pair standards. Changes in the expression of MATα1 and CDC8 were detected in the mutant strains. Amplified products of TDH3 were used as a loading control.
Because the sgf11 deletion resulted in the reduced association of Ubp8p with purified SAGA (Table 2 and Fig. 4A), we predicted that a ubp8Δ deletion strain would have a pattern of gene transcription similar to the sgf11Δ deletion strain. To test this, we used RT-PCR to compare the mRNA expression levels of MATα1 in wild-type and a ubp8Δ deletion strain. Like the sgf11 deletion, deletion of ubp8 resulted in decreased expression of MATα1 mRNA relative to that of wild-type (Fig. 6). An increase in CDC8 expression in the ubp8Δ deletion strain was not observed (data not shown). Our results are consistent with a model in which SAGA-dependent transcriptional activation is regulated by Sgf11p through fostering an association of Ubp8p with SAGA.
DISCUSSION
We present the discovery and characterization of a novel subunit in the Saccharomyces cerevisiae SAGA HAT complex. We previously reported an extensive proteomic investigation of proteins that associate with immunoaffinity-purified components of the TFIID complex (41). A statistical scoring system was developed to identify proteins that significantly associate with individual shared components of TFIID and SAGA. This approach identified three novel SAGA subunits Sgf29p, Sgf73p, and Ubp8p. In the present study, we found by using hierarchical cluster analysis of the mass spectrometry data that an additional protein termed Sgf11p significantly associates with only the shared components of TFIID and SAGA. A more thorough description of the clustering approach will be presented elsewhere (K. J. McAfee, D. Duncan, and A. J. Link, unpublished data). Using a high-sensitivity mass spectrometry approach and conventional biochemical experiments, we validated that Sgf11p associates with SAGA and not TFIID. Our results do not, however, preclude the possibility that Sgf11p is also a component of additional HAT complexes that share common components with SAGA. A number of reports have demonstrated the utility of using mass spectrometry-based proteomics to identify novel components of purified protein complexes (9, 11, 17, 29, 41). Our findings from the analysis of TF11D and SAGA complexes shows that hierarchical clustering of mass spectrometry data is an effective approach to identify novel protein associations from systematically purified protein complexes.
Role of Sgf11p in SAGA complex composition and HAT activity.
Reports have shown that the SAGA subunits Ada1p, Spt7p, and Spt20p function as scaffold or adaptor proteins and are required for the integrity of the SAGA complex (44, 48). Null mutants for these genes grow slowly when grown on low-quality carbon sources or when exposed to various transcription inhibitors (35, 44, 48). The generally robust growth of the sgf11Δ mutant is consistent with the fact that the SAGA complex is largely intact in the absence of Sgf11p. Moreover, SAGA composition analysis indicates that Sgf11p does not appear to have a substantial role in maintaining the integrity of SAGA but may be important for recruiting or retaining Ubp8p to SAGA.
An important aspect of SAGA function is its HAT activity. Studies with gcn5Δ-null strains have demonstrated that GCN5-mediated histone acetylation regulates gene transcription in vivo (24, 25, 38). Other components of SAGA (Ada2p and Ada3p) are required for Gcn5p-mediated acetylation of nucleosomes in vivo (1) and for Gcn5p HAT activity in vitro (12). In contrast, Sgf11p is not required for Gcn5p-mediated acetylation of nucleosomal histone H3. By extension, we postulate that SAGA's HAT activity does not require Ubp8p, since this component is reduced in SAGA purified from the sgf11Δ deletion strain. In support of this postulation, it was recently reported that a ubp8Δ deletion does not alter SAGA-mediated HAT activity (6, 16).
Role of Sgf11p in SAGA-regulated transcription.
The importance of SAGA in regulating S. cerevisiae mRNA levels was demonstrated in a previous microarray study (27). By comparing transcript levels in wild-type and deletion mutants, the study showed that SPT20, GCN5, and SPT3 regulate 10, 4, and 3%, respectively, of the tested genes. Although some transcripts were affected by mutations in each of the three genes, others were affected by deletion of only specific, individual genes. The authors also propose that since other reports have shown deletion of spt20 results in a SAGA-null molecular phenotype (44), the genes regulated by spt20Δ deletion represent the totality of SAGA-dependent genes. Using DNA microarray analysis with similar growth conditions and applying similar statistical criteria, we show that deletion of sgf11 also affects transcription of a small subset of yeast genes (17 upregulated and 37 downregulated, ∼1% of the genes tested). Comparative analysis showed that 23 of 54 (∼43%) of the genes regulated by SGF11 were also regulated by SPT20 (Tables 3 and 4). Thus, SGF11 has a role not only in SAGA-dependent but also SAGA-independent transcription. Of the 268 reported GCN5 target genes, 9 are shared with SGF11, and only three of these are SPT20 regulated. Such a limited overlap of SGF11 and GCN5 target genes is expected, since sgf11 deletion has no apparent effect on Gcn5p association with SAGA or Gcn5p-mediated HAT activity. Of the common targets of SPT20 and SGF11, those that are regulated in the same direction are all reduced in the mutants. This suggests that Sgf11p largely promotes SAGA-mediated gene transcription. Ubp8p, whose association with SAGA is reduced in an sgf11Δ deletion strain, also regulates SAGA-activated genes (6, 16). We found that one of the genes affected by both SGF11 and SPT20, MATα1, is similarly affected by UBP8. Although the interaction of Sgf11p and Ubp8p was not examined further, these results suggest that the differences in SAGA-dependent gene activation in the sgf11Δ mutant are related to reduced association of Ubp8p with SAGA.
In addition to the core SAGA components, several other transcription regulators were also identified at low levels from the Sgf11p purification (data are available at http://www.linklab.mc.vanderbilt.edu). Although these interactions were not investigated further, interaction of Sgf11p with non-SAGA transcription regulators suggest a possible means by which SGF11 regulates SAGA-independent transcription. One identified protein of particular importance to SAGA-dependent transcription is TBP. Genetic studies have demonstrated that the SAGA components Spt3p and Spt8p regulate the binding of TBP to the TATA box of specific promoter and the activation of specific RNA polymerase II-dependent genes (2, 3). These data suggest a physical interaction between SAGA and TBP. In support of this, our clustering analysis indicates that SAGA and Sgf11p physically associate with TBP. Others have also shown that TBP copurifies from cell extracts with the SAGA components Ada3p and Spt20p (37, 39). From comparative analysis of our cDNA microarray results with those previously reported (27), we found that SPT3 and SGF11 share eight common gene targets, and six of these genes are also regulated by SPT20. Although further investigation is required, it is intriguing to speculate that common gene regulation by SPT20, SPT3, and SGF11 is a function of their interaction with and regulation of TBP.
Acknowledgments
We are grateful to Tracey Fleischer, Vince Gerbasi, Elizabeth Link, and Jay Kirchner for experimental advice throughout this study or for discussions and comments during the preparation of the manuscript. We also thank Jay Kirchner for the SAGA antibodies. We thank Jerry Workman for the purified nucleosomes. Lastly, we thank all of the members of the Vanderbilt Microarray Shared Resource Center for their assistance with microarray protocols and data analysis.
This study was supported by an NIH grant ES11993 and NCI SPORE Lung Cancer Pilot Project Initiative awarded to A.J.L. (1 P50 CA90949). D.W.P was supported by NIH training grant T32-HL69765. C.M.W. and J.L.J. are supported by NIH grants GM64779 and HL68744. K.J.M. is supported by NIH grants ES11993, GM64779, NS43952, and GM68900. P.A.W. is supported by NIH grants ES11993 and GM52461. A.J.L. is supported by NIH grants ES11993, GM64779, HL68744, NS43952, and CA098131.
REFERENCES
- 1.Balasubramanian, R., M. G. Pray-Grant, W. Selleck, P. A. Grant, and S. Tan. 2002. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 277:7989-7995. [DOI] [PubMed] [Google Scholar]
- 2.Belotserkovskaya, R., D. E. Sterner, M. Deng, M. H. Sayre, P. M. Lieberman, and S. L. Berger. 2000. Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol. Cell. Biol. 20:634-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaumik, S. R., and M. R. Green. 2002. Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol. Cell. Biol. 22:7365-7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candau, R., J. X. Zhou, C. D. Allis, and S. L. Berger. 1997. Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 16:555-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang, Y. C., P. Komarnitsky, D. Chase, and C. L. Denis. 1996. ADR1 activation domains contact the histone acetyltransferase GCN5 and the core transcriptional factor TFIIB. J. Biol. Chem. 271:32359-32365. [DOI] [PubMed] [Google Scholar]
- 6.Daniel, J. A., M. S. Torok, Z. W. Sun, D. Schieltz, C. D. Allis, J. R. Yates III, and P. A. Grant. 2004. Deubiquitination of histone H2B by a yeast acetyltransferase complex regulates transcription. J. Biol. Chem. 279:1867-1871. [DOI] [PubMed] [Google Scholar]
- 7.Davie, J. R., and L. C. Murphy. 1994. Inhibition of transcription selectively reduces the level of ubiquitinated histone H2B in chromatin. Biochem. Biophys. Res. Commun. 203:344-350. [DOI] [PubMed] [Google Scholar]
- 8.De Hoon, M. J., S. Imoto, J. Nolan, and S. Miyano. 2004. Open source clustering software. Bioinformatics 20:1453-1454. [DOI] [PubMed]
- 9.Deshaies, R. J., J. H. Seol, W. H. McDonald, G. Cope, S. Lyapina, A. Shevchenko, R. Verma, and J. R. Yates III. 2002. Charting the protein complexome in yeast by mass spectrometry. Mol. Cell Proteomics 1:3-10. [DOI] [PubMed] [Google Scholar]
- 10.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 12.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11:1640-1650. [DOI] [PubMed] [Google Scholar]
- 13.Grant, P. A., D. Schieltz, M. G. Pray-Grant, D. J. Steger, J. C. Reese, J. R. Yates III, and J. L. Workman. 1998. A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell 94:45-53. [DOI] [PubMed] [Google Scholar]
- 14.Grant, P. A., D. Schieltz, M. G. Pray-Grant, J. R. Yates III, and J. L. Workman. 1998. The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol. Cell 2:863-867. [DOI] [PubMed] [Google Scholar]
- 15.Grant, P. A., D. E. Sterner, L. J. Duggan, J. L. Workman, and S. L. Berger. 1998. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 8:193-197. [DOI] [PubMed] [Google Scholar]
- 16.Henry, K. W., A. Wyce, W. S. Lo, L. J. Duggan, N. C. Emre, C. F. Kao, L. Pillus, A. Shilatifard, M. A. Osley, and S. L. Berger. 2003. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 17:2648-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, Y., A. Gruhler, A. Heilbut, G. D. Bader, L. Moore, S. L. Adams, A. Millar, P. Taylor, K. Bennett, K. Boutilier, L. Yang, C. Wolting, I. Donaldson, S. Schandorff, J. Shewnarane, M. Vo, J. Taggart, M. Goudreault, B. Muskat, C. Alfarano, D. Dewar, Z. Lin, K. Michalickova, A. R. Willems, H. Sassi, P. A. Nielsen, K. J. Rasmussen, J. R. Andersen, L. E. Johansen, L. H. Hansen, H. Jespersen, A. Podtelejnikov, E. Nielsen, J. Crawford, V. Poulsen, B. D. Sorensen, J. Matthiesen, R. C. Hendrickson, F. Gleeson, T. Pawson, M. F. Moran, D. Durocher, M. Mann, C. W. Hogue, D. Figeys, and M. Tyers. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415:180-183. [DOI] [PubMed] [Google Scholar]
- 18.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 19.Imbalzano, A. N. 1998. Energy-dependent chromatin remodelers: complex complexes and their components. Crit. Rev. Eukaryot. Gene Expr. 8:225-255. [DOI] [PubMed] [Google Scholar]
- 20.Imbalzano, A. N. 1998. SWI/SNF complexes and facilitation of TATA binding protein-nucleosome interactions. Methods 15:303-314. [DOI] [PubMed] [Google Scholar]
- 21.Kornberg, R. D., and Y. Lorch. 1992. Chromatin structure and transcription. Annu. Rev. Cell Biol. 8:563-587. [DOI] [PubMed] [Google Scholar]
- 22.Kornberg, R. D., and Y. Lorch. 1999. Chromatin-modifying and -remodeling complexes. Curr. Opin. Genet. Dev. 9:148-151. [DOI] [PubMed] [Google Scholar]
- 23.Kornberg, R. D., and Y. Lorch. 1999. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98:285-294. [DOI] [PubMed] [Google Scholar]
- 24.Kuo, M. H., J. E. Brownell, R. E. Sobel, T. A. Ranalli, R. G. Cook, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature 383:269-272. [DOI] [PubMed] [Google Scholar]
- 25.Kuo, M. H., J. Zhou, P. Jambeck, M. E. Churchill, and C. D. Allis. 1998. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12:627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larschan, E., and F. Winston. 2001. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 15:1946-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, T. I., H. C. Causton, F. C. Holstege, W. C. Shen, N. Hannett, E. G. Jennings, F. Winston, M. R. Green, and R. A. Young. 2000. Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 405:701-704. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., Q. Lin, H. G. Yoon, Z. Q. Huang, B. D. Strahl, C. D. Allis, and J. Wong. 2002. Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol. Cell. Biol. 22:5688-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Link, A. J., J. Eng, D. M. Schieltz, E. Carmack, G. J. Mize, D. R. Morris, B. M. Garvik, and J. R. Yates III. 1999. Direct analysis of protein complexes using mass spectrometry. Nat. Biotechnol. 17:676-682. [DOI] [PubMed] [Google Scholar]
- 30.Lorch, Y., J. W. LaPointe, and R. D. Kornberg. 1987. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell 49:203-210. [DOI] [PubMed] [Google Scholar]
- 31.Mizzen, C., M. H. Kuo, E. Smith, J. Brownell, J. Zhou, R. Ohba, Y. Wei, L. Monaco, P. Sassone-Corsi, and C. D. Allis. 1998. Signaling to chromatin through histone modifications: how clear is the signal? Cold Spring Harbor Symp. Quant. Biol. 63:469-481. [DOI] [PubMed] [Google Scholar]
- 32.Narlikar, G. J., H. Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475-487. [DOI] [PubMed] [Google Scholar]
- 33.Owen-Hughes, T., R. T. Utley, D. J. Steger, J. M. West, S. John, J. Cote, K. M. Havas, and J. L. Workman. 1999. Analysis of nucleosome disruption by ATP-driven chromatin remodeling complexes. Methods Mol. Biol. 119:319-331. [DOI] [PubMed] [Google Scholar]
- 34.Owen-Hughes, T., and J. L. Workman. 1994. Experimental analysis of chromatin function in transcription control. Crit. Rev. Eukaryot. Gene Expr. 4:403-441. [PubMed] [Google Scholar]
- 35.Pray-Grant, M. G., D. Schieltz, S. J. McMahon, J. M. Wood, E. L. Kennedy, R. G. Cook, J. L. Workman, J. R. Yates III, and P. A. Grant. 2002. The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol. Cell. Biol. 22:8774-8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci, A. R., J. Genereaux, and C. J. Brandl. 2002. Components of the SAGA histone acetyltransferase complex are required for repressed transcription of ARG1 in rich medium. Mol. Cell. Biol. 22:4033-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts, S. M., and F. Winston. 1997. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics 147:451-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Garcia, A. B., R. Sendra, M. Pamblanco, and V. Tordera. 1997. Gcn5p is involved in the acetylation of histone H3 in nucleosomes. FEBS Lett. 403:186-190. [DOI] [PubMed] [Google Scholar]
- 39.Saleh, A., V. Lang, R. Cook, and C. J. Brandl. 1997. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J. Biol. Chem. 272:5571-5578. [DOI] [PubMed] [Google Scholar]
- 40.Saleh, A., D. Schieltz, N. Ting, S. B. McMahon, D. W. Litchfield, J. R. Yates III, S. P. Lees-Miller, M. D. Cole, and C. J. Brandl. 1998. Tra1p is a component of the yeast Ada.Spt transcriptional regulatory complexes. J. Biol. Chem. 273:26559-26565. [DOI] [PubMed] [Google Scholar]
- 41.Sanders, S. L., J. Jennings, A. Canutescu, A. J. Link, and P. A. Weil. 2002. Proteomics of the eukaryotic transcription machinery: identification of proteins associated with components of yeast TFIID by multidimensional mass spectrometry. Mol. Cell. Biol. 22:4723-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterner, D. E., R. Belotserkovskaya, and S. L. Berger. 2002. SALSA, a variant of yeast SAGA, contains truncated Spt7, which correlates with activated transcription. Proc. Natl. Acad. Sci. USA 99:11622-11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterner, D. E., P. A. Grant, S. M. Roberts, L. J. Duggan, R. Belotserkovskaya, L. A. Pacella, F. Winston, J. L. Workman, and S. L. Berger. 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell. Biol. 19:86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 46.Tasto, J. J., R. H. Carnahan, W. H. McDonald, and K. L. Gould. 2001. Vectors and gene targeting modules for tandem affinity purification in Schizosaccharomyces pombe. Yeast 18:657-662. [DOI] [PubMed] [Google Scholar]
- 47.Wang, H., Z. Q. Huang, L. Xia, Q. Feng, H. Erdjument-Bromage, B. D. Strahl, S. D. Briggs, C. D. Allis, J. Wong, P. Tempst, and Y. Zhang. 2001. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293:853-857. [DOI] [PubMed] [Google Scholar]
- 48.Wu, P. Y., and F. Winston. 2002. Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol. Cell. Biol. 22:5367-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]