Abstract
Objective
To compare characteristics for patients scheduled for autologous chondrocyte implantation with matrix-assisted chondrocyte implantation (MACI) with those enrolled in clinical trials and to describe differences in patient selection between countries.
Design
Anonymized data from patients scheduled for MACI treatment in the knee in Europe and Australia/Asia were obtained from the Genzyme/Sanofi database. Average age, defect size, and male-female ratio were analyzed and compared by country. Clinical cohort studies and prospective comparative trials using autologous chondrocyte implantation and related treatments were identified and weighted average age, weighted defect size, and male-female ratio were analyzed and compared with data from the database.
Results
From the database 2,690 patients were included with mean age 33.7 years and male-female ratio of 67:33. Mean defect size was 5.64 cm2 and 70% of the defects were 3 to 10 cm2. There were significant differences between patients’ mean defect sizes between countries. Sixty-nine studies (57 cohorts and 12 prospective comparative trials) with a total of 5,449 patients were identified. The combined weighted mean age was 34.2 years, and the combined weighted mean defect size was 4.89 cm2. Patients scheduled for MACI had significantly larger defects that those included in clinical trials. There was no significant difference in age. No differences were found between cohorts and prospective comparative trials.
Conclusion
The vast majority of patients scheduled for autologous chondrocyte implantation with MACI have chondral defect comparable to that generally recommended, but differences exist between countries. Patients enrolled in clinical trials have significantly smaller defects than those undergoing treatment outside controlled trials.
Keywords: autologous chondrocyte implantation, articular cartilage, knee, demographics
Introduction
Matrix-assisted chondrocyte implantation (MACI) is a commercially available third-generation autologous chondrocyte implantation (ACI) technique for the treatment of articular cartilage defects. Established treatment algorithms generally agree on the use of microfracture or osteochondral autograft transfer (OAT) as first-line treatment for smaller defects (<3 cm2) of the femoral condyles. The indication for MACI and other ACI techniques has generally been limited to larger focal chondral defects (>3-4 cm2) of the knee, mostly due to the expense of these procedures. Several studies have demonstrated superiority of ACI in larger defects,1,2 confirming recommendations for the use of ACI in this population; alternatively, osteochondral allograft transplantation (OCA) can be considered but is not readily available in many countries. Despite general agreement on these algorithms, they have never been validated nor has surgical adherence to their suggestions been demonstrated.
The 4 original studies by Brittberg and Peterson described periosteum-covered autologous chondrocyte implantation (ACI-p).3-6 After these initial studies, many surgeons replaced the periosteum with a collagen type I/III membrane (ACI-c) to reduce surgical time, patient morbidity, and the risk of hypertrophy. The current third generation of ACI utilizing matrix-seeded chondrocytes (ACI-m) was introduced and made commercially available, including MACI. The indications for ACI treatments have remained consistent with the original suggestions. Because the treatment is very costly,7 much efforts have been put into ensuring that the treatment is only offered to the patients where superiority over microfracture can be expected, for example, larger defect sizes.
While evidence for using ACI and related treatment continues to expand, there have been few reports detailing patient and defect characteristics across large patient populations. It has also become evident that the strict inclusion criteria in prospective randomized clinical trials leads to patient selection that differs from that of patients actually undergoing cartilage repair treatments in clinical practice. The aim of the present study was to investigate these demographic parameters in patients receiving MACI and to compare them to the inclusion criteria for current clinical trials, essentially comparing the reality of cartilage repair with the idealized situation in a restricted trial environment. We hypothesized that patients scheduled for MACI treatment are different from those enrolled in clinical trials of chondrocyte implantation in terms of age, cartilage defect size, and number of defects, and that there are differences between countries. Secondary, we hypothesized that patients included in prospective randomized trials had smaller defects than those included in cohorts of patients treated in clinical practice, rather than a controlled trial.
Methods
Database Review
Anonymized data were obtained from the Genzyme/Sanofi database on patients scheduled for autologous chondrocyte implants with MACI between 2008 and 2013. Only data from countries with more than 10 patients treated were included. Patient demographics (age, gender) and cartilage defect characteristics (size, number of defects) were evaluated. Age and defect size were reported at the time of biopsy, rather than implantation. The database includes a large number of patients, which in the present study is used as an indicator of the characteristics of patients actually receiving ACI treatment.
Literature Review
MEDLINE and Google Scholar were reviewed for clinical cohort and comparative studies in English with unique patient data on patients treated with ACI techniques for treatment of focal cartilage defects in the knee (on April 8, 2015). Arthroscopic treatments could not be discriminated in the database, but the number of procedures is expected to be negligible. Arthroscopic approaches were excluded in the study search, due to the possible confounding of treatment indication in the comparison. The studies were evaluated based on patient age, gender, number of defects, and lesion size. Search words were “ACI,” “ACT,” “MACI,” “Autologous chondrocyte implantation,” “Autologous chondrocyte transplantation,” “Matrix-assisted chondrocyte implantation”; combined with “knee.”
Average patient age, average defect size, number of defects, and male-female ratio in the included studies were noted and compared with the database. Studies not mentioning average age and average defect size, and where these parameters could not be calculated using information in the respective articles, were excluded. Weighted average age and lesion sizes were calculated with respect to the enrolled patients in each study. Studies that presented follow-up data from patients previously published were excluded. Novel applications such as hydrogel-based administration were also excluded. Studies not directly addressing whether patients had been enrolled in previous trials were included. The included studies were grouped by country and compared by country to the database data when possible.
Statistical Analysis
Bartletts’s test revealed unequal variance of the patient age and defect size. Hence, t test for independent samples with unequal variances was used to test our hypotheses. Variables compared between cohort studies and comparative trials were investigated using weighted 2-sample t test.8 Register data and data in studies (age and defect size) was investigated using t test with weighting of studies based on patient number. A significance level of P < 0.05 was used.
Results
A total of 2,690 patients from 9 European countries, Australia, and Singapore were included in the evaluation. Ireland, Portugal, Qatar, United Arab Emirates, China, Hong Kong, Philippines, New Zealand, and Malaysia had less than 10 patients operated and were excluded.
Comparison of Studies and Database
In the Genzyme/Sanofi database of 2,690 patients the average age was 33.7 years (range = 11-65), and male-female ratio was 67:33. Mean defect size was 5.64 cm2 (range = 0.16-47 cm2). Single defects accounted for 81% whereas 19% were multifocal. There were no correlations between mean defect size and number of defects or patient age. On average, 18.9% of cartilage defects were small (<3 cm2) (and 63.1% of these were <2 cm2); 11% were large (>10 cm2), and the majority of defects (70%) were medium in size (3-10 cm2) (Fig. 1).
Figure 1.
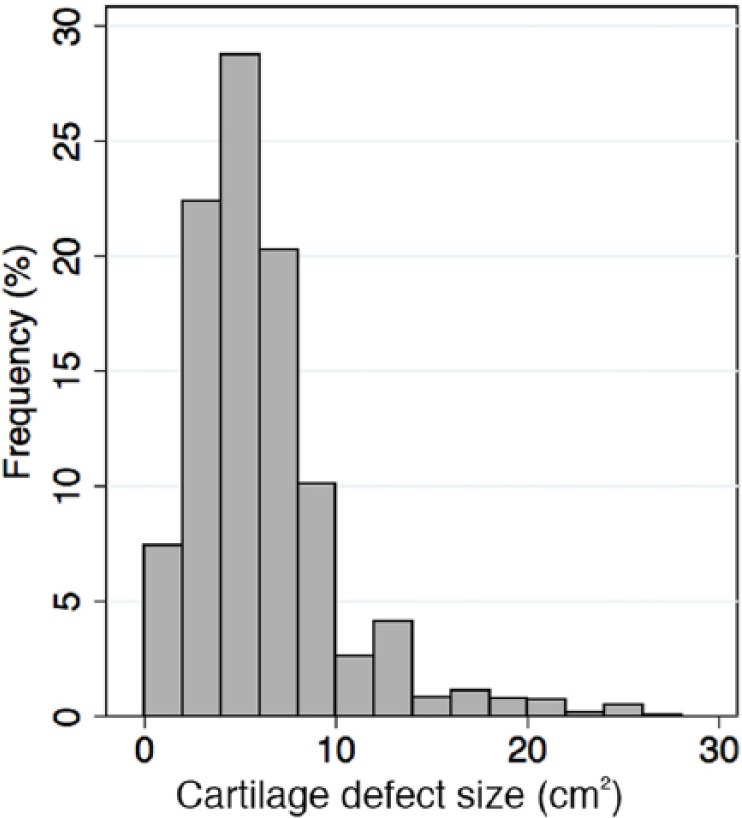
Frequency distribution of defect sizes of patients included in the database. Bars represent intervals of 2 cm2.
A total of 71 studies fulfilled the inclusion criteria with a total of 5,449 patients (Tables 1 and 2). Twelve studies were prospective randomized trials. Four randomized trials were retrospective and were assigned to the cohort group of studies, giving a total of 57 studies in the cohort group. The weighted mean age of all studies was 34.2 years (range = 8-65 years), and the combined weighted mean defect size was 4.95 cm2 (range = 0.5-36 cm2). The defect size of the patients in the database was significantly larger than that of patients included in the studies (P = 0.001). There were no difference in age between the database and the studies (P = 0.68).
Table 1.
Demographics of Patients Receiving Chondrocyte Transplantation Enrolled in Cohort Studies and Retrospective Comparative Studiesa.
| Author | Year | Country | Treatment | n | Age (Years) | Defect size (cm2) |
|---|---|---|---|---|---|---|
| Brittberg et al.3 | 1994 | Sweden | ACI-p | 23 | 27.0 (14-48) | 3.1 (1.6-6.5) |
| Peterson et al.6 | 2000 | Sweden | ACI-p | 101 | 29.4 (15-51) | 4.4 (1.3-12.0) |
| Minas et al.17 | 2001 | USA | ACI-p | 169 | 36.2 (13-58) | 7.3 (—) |
| Micheli et al.18 | 2001 | USA | ACI-p | 50 | 31.0 (19-53) | 4.2 (0.4-20) |
| Peterson et al.4 | 2002 | Sweden | ACI-p | 61 | 28.4 (—) | 4.1 (1.3-12.0) |
| Peterson et al.5 | 2003 | Sweden | ACI-p | 58 | 26.4 (14-52) | 5.7 (1.5-12.0) |
| Cherubino et al.19 | 2003 | Italy | ACI-p | 13 | 35.0 (18-49) | 3.5 (2.0-4.5) |
| Haddo et al.20 | 2004 | England | ACI-c | 30 | 31.0 (15-51) | 2.9 (1-7) |
| Minas et al.21 | 2005 | USA | ACI-p | 45 | 36.9 (15-54) | 10.54 (—) |
| Dozin et al.22 | 2005 | Italy | ACI-p | 22 | 29.6 (—) | 1.97 (—) |
| Browne et al.23 | 2005 | USA | ACI-p | 100 | 37.0 (14-55) | 4.9 (0.84-23.5) |
| Fu et al.24 | 2005 | USA | ACI-p | 58 | 36.9 (—) | 5.1 (—) |
| Marcacci et al.25 | 2005 | Italy | ACI-m | 192 | 37.6 (—) | 3.5 (—) |
| Behrens et al.26 | 2006 | Germany | ACI-m | 38 | 35.0 (18-58) | 4.1 (0.64-17.75) |
| Gobbi et al.27 | 2006 | Italy | ACI-m | 32 | 30.5 (15-55) | 4.7 (0.8-12) |
| Ossendorf et al.28 | 2007 | Germany | ACI-m | 40 | 36 (17-64) | 4.6 (2-15) |
| Steinwachs et al.29 | 2007 | Germany | ACI-c | 63 | 34.3 (18-50) | 5.9 (3-16) |
| Mandelbaum et al.30 | 2007 | USA | ACI-p | 40 | 37.0 (16-48) | 4.5 (1-14) |
| Kreuz et al.31 | 2007 | Germany | ACI-m | 118 | 35.0 (18-50) | 6.5 (3-16) |
| Niemeyer et al.32 | 2008 | Germany | ACI-p/c/m | 309 | 35.2 (—) | 4.6 (—) |
| Niemeyer et al.33 | 2008 | Germany | ACI-c | 70 | 34.3 (—) | 4.41 (—) |
| Nehrer et al.34 | 2008 | Austria | ACI-m | 8 | 30 (19-40) | 4.61 (1.8-7.9) |
| Rosenberger et al.35 | 2008 | USA | ACI-p | 56 | 48.6 (45-60) | 4.7 (1-15) |
| Ebert et al.36 | 2008 | Australia | ACI-m | 62 | 38.3 (16-62) | 3.3 (0.65-10) |
| Rue et al.37 | 2008 | USA | ACI-p | 16 | 23.4 (13-38) | 3.9 (1.8-7.5) |
| Gomoll et al.38 | 2009 | USA | ACI-p/c | 401 | 32.0 (13-56) | 7.2 (0.5-36) |
| Zaslav et al.39 | 2009 | USA | ACI-p | 154 | 34.5 (—) | 4.6 (1-30) |
| McNickie et al.40 | 2009 | USA | ACI-p | 137 | 30.3 (13-49) | 5.2 (0.8-26.6) |
| Gobbi et al.41 | 2009 | Italy | ACI-m | 34 | 31.2 (15-55) | 4.45 (3-12) |
| Kreuz et al.42 | 2009 | Germany | ACI-m | 19 | 35.0 (25-50) | 4.0 (2-6) |
| Niemeyer et al.43 | 2010 | Germany | ACI-m | 59 | 37.0 (21-57) | 4.64 (1-8) |
| Niemeyer et al.44 | 2010 | Germany | ACI-m | 67 | 37.4 (—) | 4.3 (—) |
| Erggelet et al.45 | 2010 | Germany | ACI-m | 82 | 35.0 (16-63) | 5.51 (2-17.5) |
| Macmull et al.46 | 2011 | England | ACI-p/m | 31 | 16.3 (14-18) | 5.3 (0.96-15.75) |
| Ebert et al.47 | 2011 | Australia | ACI-m | 41 | 38.5 (13-65) | 3.0 (1.9) |
| Ossendorf et al.48 | 2011 | Germany | ACI-p | 51 | 36 (13-61) | 7.25 (3-17.5) |
| Dhollander et al.49 | 2012 | Belgium | ACI-c | 32 | 29.8 (—) | 3.1 (—) |
| Filardo et al.16 | 2013 | Italy | ACI-m | 250 | 31.3 (—) | 2.98 (—) |
| Bode et al.50 | 2013 | Germany | ACI-p | 43 | 39.1 (—) | 4.6 (—) |
| Gomoll et al.51 | 2014 | USA | ACI-p | 110 | 33 (15-55) | 5.4 (1-13.2) |
| Minas et al.52 | 2014 | USA | ACI-p | 210 | 35.8 (8-57) | 8.4 (—) |
| Meyerkort et al.53 | 2014 | Australia | ACI-m | 25 | 42.3 (—) | 3.5 (—) |
| Aldrian et al.54 | 2014 | Austria | ACI-m | 16 | 33.3 (19-44) | 3.80 (1.2-6.7) |
| Pachowsky et al.55 | 2014 | Austria | ACI-m | 40 | 35.2 (—) | 4.34 (—) |
| Nawaz et al.56 | 2014 | England | ACI-p/c/m | 827 | 34.0 (14-56) | 4.09 (0.64-20.75) |
| Niemeyer et al.57 | 2014 | Germany | ACI-p/c | 23 | 31.7 (—) | 5.1 (—) |
| Zhang et al.58 | 2014 | China | ACI-m | 15 | 33.9 (14-57) | 4.0 (0.5-12) |
| Pestka et al.59 | 2014 | Germany | ACI-m | 80 | 37.9 (17-57) | 4.6 (1-8.8) |
| Salzman et al.60 | 2014 | Germany | ACI-p | 70 | 33.3 (—) | 6.5 (—) |
| Zak et al.61 | 2014 | Austria | ACI-m | 23 | 30.8 (22-46) | 4.1 (1.8-10) |
| Ebert et al.62 | 2014 | Australia | ACI-m | 56 | 39 (18-60) | 2.3 (1-9) |
| Ebert et al.63 | 2014 | Australia | ACI-m | 83 | 38.9 (13-62) | 3.30 (1-9) |
| Ebert et al.64 | 2015 | Australia | ACI-m | 47 | 37.4 (20-61) | 3.3 (1-7.2) |
| Niethammer et al.65 | 2015 | Germany | ACI-m | 30 | 36.4 (12-51) | 5.40 (2-12) |
| Wondrasch et al.66 | 2015 | Austria | ACI-m | 31 | 31.0 (18-55) | 4.86 (1.1-8.1) |
| Bode et al.67 | 2015 | Germany | ACI-p | 40 | 37.6 (—) | 4.4 (—) |
ACI-p = periosteum-covered autologous chondrocyte implantation; ACI-m = matrix-seeded autologous chondrocyte implantation; ACI-c = collagen type I/III membrane autologous chondrocyte implantation.
Age and defect size are presented as mean and range. The number of patients (n) reflects that of patients receiving chondrocyte transplantation. (—) range was not obtainable.
Table 2.
Demographics of Patients Receiving Chondrocyte Transplantation Enrolled in Prospective Clinical Comparative Trialsa.
| Author | Year | Country | Treatment | n | Age (Years) | Defect Size (cm2) |
|---|---|---|---|---|---|---|
| Bentley et al.68 | 2003 | England | ACI-c | 58 | 31.3 (16-49) | 4.66 (1-12.2) |
| Horas et al.69 | 2003 | Germany | ACI-p | 20 | 33.4 (18-44) | 3.75 (3.2-5.6) |
| Bartlett et al.70 | 2005 | England | ACI-c/m | 91 | 33.6 (15-49) | 6.05 (1-22) |
| Dozin et al.22 | 2005 | Italy | ACI-p | 22 | 29.6 (16-40) | 1.97 (—) |
| Gooding et al.71 | 2006 | England | ACI-p/c | 68 | 30.5 (15-52) | 4.54 (1-12) |
| Knutsen et al.72 | 2007 | Norway | ACI-p | 40 | 32.2 (—) | 4.8 (2-10) |
| Saris et al.73 | 2008 | Netherlands | ACI-p | 57 | 33.9 (18-50) | 2.6 (1-5) |
| Zeifang et al.74 | 2010 | Germany | ACI-p/m | 21 | 29.3 (—) | 4.1 (—) |
| Ebert et al.75 | 2012 | Australia | ACI-m | 63 | 38.2 (16-63) | 3.27 (0.65-10) |
| Lim et al.76 | 2012 | South Korea | ACI-p | 18 | 25.1 (18-32) | 5.2 (3.0-7.2) |
| Saris et al.2 | 2014 | Multicenter | ACI-m | 72 | 34.8 (—) | 5.8 (—) |
| Akgun et al.77 | 2015 | Turkey | ACI-m | 7 | 32.7 (18-46) | 3 (2.3-4.3) |
| Gobbi et al.78 | 2014 | Italy | ACI-m | 19 | 43.1 (—) | 9.73 (—) |
ACI-p = periosteum-covered autologous chondrocyte implantation; ACI-m = matrix-seeded autologous chondrocyte implantation; ACI-c = collagen type I/III membrane autologous chondrocyte implantation.
Age and defect size are presented as mean and range. The number of patients (n) reflects that of patients receiving chondrocyte transplantation. (—) range was not obtainable.
The weighted mean ages in cohort studies and randomized trials were 34.3 years and 32.9 years, respectively, and this difference of 1.6 years was not statistically significant (P = 0.91). Weighted mean defect size was equal for the 2 groups of studies (4.94 cm2 vs. 4.37 cm2; P = 0.93). Male-female ratios in the 2 groups of studies were also similar (60%), which was lower than that in the database (67%). Prospective comparative studies included only patients with single lesions while 19% of the treated patients in the register had multifocal lesions.
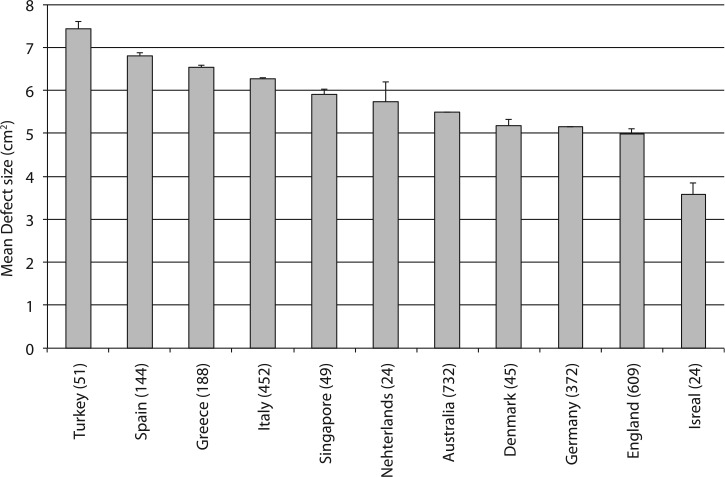
International Comparisons
Significant differences in patient age and cartilage defects size in the database were observed between countries (Fig. 2). Defect sizes are presented in descending order: Turkey 7.4 cm2 [6.3; 8.5]; Spain 6.8 cm2 [6.0; 7.6]; Greece 6.5 cm2 [5.9; 7.2]; Italy 6.3 cm2 [5.8; 6.8]; Singapore 5.9 cm2 [5.1; 6.8]; Netherlands 5.7 cm2 [3.4; 8.1]; Australia 5.5 cm2 [5.1; 5.8]; Denmark 5.2 cm2 [4.3; 6.1]; Germany 5.1 cm2 [4.8; 5.5]; England 5.0 cm2 [4.7; 5.2]; Israel 3.6 cm2 [2.2; 4.9].
Figure 2.
Mean defect size by country of patients included in the database. Values in parentheses are number of patients included by country. Bars are standard error of mean.
Stratification by country of the included studies showed that defect size in patients receiving chondrocyte transplantation was up to 1.5 to 2.5 times larger than that of the patients enrolled in their clinical trials.
In Swedish3-6 studies, average defect size was 4.5 cm2, in Austria34,53,54,60,65 3.2 cm2 (n = 154), whereas patients in the United Stated17,18,21,23,24,30,35,37-40,50,51 had the largest average defect size of 6.5 cm2 (n = 1,591). Notably, most studies included operated patients that were younger and had larger defects than their respective trials (Table 3).
Table 3.
Defect Sizes and Age of Patients Included in Studies Compared with Patients in the Database Scheduled for Chondrocyte Transplantation Treatment Stratified by Country.
| Country | n | Defect Size—Studies (cm2) | Defect Size—Database (cm2) | Index | Age—Studies (Years) | Age—Database (Years) | Index |
|---|---|---|---|---|---|---|---|
| Germany | 1173 | 5.1 | 5.1 | 100 | 35.6 | 30.9 | 87 |
| England | 1044 | 4.3 | 5.0 | 116 | 33.6 | 35.5 | 106 |
| Italy | 584 | 3.5 | 6.3 | 178 | 33.7 | 36.3 | 108 |
| Australia | 377 | 3.1 | 5.5 | 178 | 38.7 | 34.4 | 89 |
| Netherlands | 72 | 2.6 | 5.7 | 219 | 34.8 | 28.9 | 83 |
| Turkey | 7 | 3 | 7.4 | 247 | 32.7 | 28.5 | 87 |
Discussion
In the present study, we compared 2,690 patients assigned for treatment with MACI for cartilage defects in the knee with 5,449 patients enrolled in cohort studies and prospective clinical trials. The majority of the patients scheduled for MACI treatment were comparable in terms of age and defect size, to those included in cohort and prospective comparative studies instructing current treatment guidelines. Comparing average defect sizes, however, the defects were larger in the database than the average defect size in the cohort studies. We further discovered that the size of the cartilage defects in patients assigned for MACI varied significantly between countries.
The differences in cartilage defect sizes between patients enrolled in trials and those scheduled for ACI with MACI may be multifactorial. Obviously, strict inclusion criteria for studies in terms of limiting population sizes for sufficient power may be partly responsible for this difference. However, due to the significant cost of the treatment compared to other modalities, public health care systems and private insurance companies may be reluctant to offer this treatment to patients with defect size in the lower end of the recommended interval.
Engen et al. previously addressed the issue of differences between patients enrolled in cartilage repair trials and those seen in their clinic with respect to all different surgical cartilage repair modalities.9 They found that of 137 patients referred to their clinic with cartilage defects only 4.4% were eligible for inclusion in all randomized controlled trials ranging between 7% and 80% for the individual studies. The main contributor in their review was defect size, while age and additional joint injuries such as meniscal tears were also important. The database applied in our comparison did not contain information of joint comorbidities.
Treatment selection for focal articular cartilage lesions requires several patient-specific considerations as well as attention to additional joint pathologies.
Out of the various factors predicting outcome of ACI procedures for cartilage repair, age and defect size are often addressed. While some authors find age to be a factor influencing outcomes, convincing evidence of the role of defect size is still absent.10,11 Ebert et al. reviewed patients from 2 of their trials for factors predicting 5-year outcome after MACI treatment and found that while preoperative physical and mental scores in the SF-36 questionnaire contributed significantly to the 5-year KOOS value, cartilage defect size and preoperative duration of symptoms were only predictors of outcome on magnetic resonance imaging evaluation.12 Behery et al. recently reviewed the evidence of different patient-specific parameters and their effect on outcome after cartilage repair in 13 studies. They found that neither patient age nor defect size were independent factors related to the clinical outcome.13 Similar results were found by Smith et al., in an analysis of 284 patient data sets, and by Jungmann et al., investigating risk factors for revision surgery after ACI.14,15
Other factors for consideration in patient selection include alignment, ligamentous and meniscal injuries, and amount of degenerative changes. In the present article, we only address 2 specific characteristics, namely, age and defect size. Unfortunately, the database was inconsistent in the reporting of anatomical location of the defect and these data therefore were not included in our study. Females are less likely to receive ACI treatment as seen in the database compared with the studies. The role of gender in focal cartilage damage and outcome after ACI has been investigated previously and some controversy exists. While Jungmann et al. found the female gender to be negatively related to outcome, Filardo et al. showed in a match-pair analysis that while females generally had more complex cartilage injuries, all other factors equal, the female gender did not predict worse outcome after ACI-m.15,16
There are no clear explanation for the international differences observed in patient inclusion for MACI treatment. Cultural aspects may play a role but different health care and reimbursement systems may also be important. The database does not provide any information on whether patients were treated in private or public hospitals. Notably, studies carried out in the United States had the highest average defect size of the study population, but it still remains unclear how this compares to the patient population receiving chondrocyte implantation in that country.
The present study used nonstandardized surgeon assessment of defect size, which could potentially confound the data. If all surgeons overestimated or underestimated the defect size during arthroscopy for the database compared with a postdebridement measurement in the studies, this may be a potential source of bias. It is, however, a measurement method similar to that most commonly used in clinical studies. For example, the largest combined defect size in the register is estimated to be 47 cm2, which likely represents an outlier. The high number of included patients and surgeons performing the evaluations limit the role of this potential confounder as well as the influence of the very few statistical outliers. As the database contained age at the time of biopsy, the actual age of the patients at the time of surgery will be higher, but the actual age at the time of surgery is unknown. However, it could be argued that since the biopsy is taken at the time of indication for chondrocyte transplantation, this may be the more correct measure to use, as we do not look at the outcome in relation to age. The Genzyme/Sanofi database did not contain information on body mass index and the reproducible information on anatomical location of the defect was insufficient to allow for analysis. There were also no data on additional knee injury such as meniscal and ligament tears. All these factors are however also important in considering the correct patient selection for treatment of cartilage injuries with autologous chondrocyte implantation.
We compared patients scheduled for a commercially available third-generation ACI treatment—MACI. This was compared with patients scheduled for many different types of ACI-related treatments. In the comparison made in the present study, emphasis is put on indications, removing potential confounding since the indications are similar for all types of ACI treatments regardless of generation or commercialization.
In our comparison there is overlap in patient data between the database and the studies, since patients receiving MACI in the studies are also present in the database. This could impair the validity in terms of potential bias in the country-stratification comparison if countries with no or little difference between defect size in the database and reported studies (e.g., Germany and England) were only using MACI in the reported studies. This is however not the case and the risk of bias is presumed to be of small in this comparison.
Conclusion
This study shows that the vast majority of patients scheduled for ACI with MACI have articular cartilage defect sizes that are within the range of what is generally recommend for this procedure, although patients enrolled in clinical trials have significantly smaller defects than those scheduled for treatment outside a trial environment. This study also shows that patients receiving MACI treatment in 9 European countries, Australia, and Singapore have different cartilage defect sizes, and in some countries the difference between patients enrolled in trials and generally assigned for surgery differs significantly.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: No ethical approval was required for the completion of this work.
References
- 1. Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519-27. [DOI] [PubMed] [Google Scholar]
- 2. Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42:1384-94. [DOI] [PubMed] [Google Scholar]
- 3. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 4. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 5. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(Suppl 2):17-24. [DOI] [PubMed] [Google Scholar]
- 6. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. [DOI] [PubMed] [Google Scholar]
- 7. Samuelson EM, Brown DE. Cost-effectiveness analysis of autologous chondrocyte implantation: a comparison of periosteal patch versus type I/III collagen membrane. Am J Sports Med. 2012;40:1252-8. [DOI] [PubMed] [Google Scholar]
- 8. Bland JM, Kerry SM. Statistics notes. Weighted comparison of means. BMJ. 1998;316:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engen CN, Engebretsen L, Årøen A. Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage. 2010;1:312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Windt TS, Bekkers JE, Creemers LB, Dhert WJ, Saris DB. Patient profiling in cartilage regeneration: prognostic factors determining success of treatment for cartilage defects. Am J Sports Med. 2009;37(Suppl 1):58S-62S. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, et al. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88:61-4. [DOI] [PubMed] [Google Scholar]
- 12. Ebert JR, Smith A, Edwards PK, Hambly K, Wood DJ, Ackland TR. Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint. Am J Sports Med. 2013;41:1245-54. [DOI] [PubMed] [Google Scholar]
- 13. Behery OA, Harris JD, Karnes JM, Siston RA, Flanigan DC. Factors influencing the outcome of autologous chondrocyte implantation: a systematic review. J Knee Surg. 2013;26:203-11. [DOI] [PubMed] [Google Scholar]
- 14. Smith GD, Jones P, Ashton JB, Richardson JB. Identification of factors which affect clinical outcome of autologous chondrocyte implantation using z-transformation and multiple regression analysis. J Bone Joint Surg Br. 2006;88. [Google Scholar]
- 15. Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P. Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med. 2012;40:58-67. [DOI] [PubMed] [Google Scholar]
- 16. Filardo G, Kon E, Andriolo L, Vannini F, Buda R, Ferruzzi A, et al. Does patient sex influence cartilage surgery outcome? Analysis of results at 5-year follow-up in a large cohort of patients treated with matrix-assisted autologous chondrocyte transplantation. Am J Sports Med. 2013;41:1827-34. [DOI] [PubMed] [Google Scholar]
- 17. Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res. 2001;(391 Suppl):S349-61. [DOI] [PubMed] [Google Scholar]
- 18. Micheli LJ, Browne JE, Erggelet C, Fu F, Mandelbaum B, Moseley JB, et al. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med. 2001;11:223-8. [DOI] [PubMed] [Google Scholar]
- 19. Cherubino P, Grassi FA, Bulgheroni P, Ronga M. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong). 2003;11:10-5. [DOI] [PubMed] [Google Scholar]
- 20. Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, et al. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11:51-5. [DOI] [PubMed] [Google Scholar]
- 21. Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005;(436):30-9. [DOI] [PubMed] [Google Scholar]
- 22. Dozin B, Malpeli M, Cancedda R, Bruzzi P, Calcagno S, Molfetta L, et al. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15:220-6. [DOI] [PubMed] [Google Scholar]
- 23. Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB, Jr, Micheli LJ, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;(436):237-45. [DOI] [PubMed] [Google Scholar]
- 24. Fu FH, Zurakowski D, Browne JE, Mandelbaum B, Erggelet C, Moseley JB, Jr, et al. Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: an observational cohort study with 3-year follow-up. Am J Sports Med. 2005;33:1658-66. [DOI] [PubMed] [Google Scholar]
- 25. Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;(435):96-105. [DOI] [PubMed] [Google Scholar]
- 26. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13:194-202. [DOI] [PubMed] [Google Scholar]
- 27. Gobbi A, Kon E, Berruto M, Francisco R, Filardo G, Marcacci M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34:1763-73. [DOI] [PubMed] [Google Scholar]
- 28. Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23:381-7. [DOI] [PubMed] [Google Scholar]
- 30. Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB, Jr, Erggelet C, et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35:915-21. [DOI] [PubMed] [Google Scholar]
- 31. Kreuz PC, Steinwachs M, Erggelet C, Lahm A, Krause S, Ossendorf C, et al. Importance of sports in cartilage regeneration after autologous chondrocyte implantation: a prospective study with a 3-year follow-up. Am J Sports Med. 2007;35:1261-8. [DOI] [PubMed] [Google Scholar]
- 32. Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36:2091-9. [DOI] [PubMed] [Google Scholar]
- 33. Niemeyer P, Steinwachs M, Erggelet C, Kreuz PC, Kraft N, Kostler W, et al. Autologous chondrocyte implantation for the treatment of retropatellar cartilage defects: clinical results referred to defect localisation. Arch Orthop Trauma Surg. 2008;128:1223-31. [DOI] [PubMed] [Google Scholar]
- 34. Nehrer S, Chiari C, Domayer S, Barkay H, Yayon A. Results of chondrocyte implantation with a fibrin-hyaluronan matrix: a preliminary study. Clin Orthop Relat Res. 2008;(466):1849-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36:2336-44. [DOI] [PubMed] [Google Scholar]
- 36. Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T. Traditional vs accelerated approaches to post-operative rehabilitation following matrix-induced autologous chondrocyte implantation (MACI): comparison of clinical, biomechanical and radiographic outcomes. Osteoarthritis Cartilage. 2008;16:1131-40. [DOI] [PubMed] [Google Scholar]
- 37. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36:1770-8. [DOI] [PubMed] [Google Scholar]
- 38. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902-8. [DOI] [PubMed] [Google Scholar]
- 39. Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37:42-55. [DOI] [PubMed] [Google Scholar]
- 40. McNickle AG, L’Heureux DR, Yanke AB, Cole BJ. Outcomes of autologous chondrocyte implantation in a diverse patient population. Am J Sports Med. 2009;37:1344-50. [DOI] [PubMed] [Google Scholar]
- 41. Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37:1083-92. [DOI] [PubMed] [Google Scholar]
- 42. Kreuz PC, Muller S, Ossendorf C, Kaps C, Erggelet C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Res Ther. 2009;11:R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niemeyer P, Lenz P, Kreuz PC, Salzmann GM, Sudkamp NP, Schmal H, et al. Chondrocyte-seeded type I/III collagen membrane for autologous chondrocyte transplantation: prospective 2-year results in patients with cartilage defects of the knee joint. Arthroscopy. 2010;26:1074-82. [DOI] [PubMed] [Google Scholar]
- 44. Niemeyer P, Salzmann G, Steinwachs M, Sudkamp NP, Schmal H, Lenz P, et al. Presence of subchondral bone marrow edema at the time of treatment represents a negative prognostic factor for early outcome after autologous chondrocyte implantation. Arch Orthop Trauma Surg. 2010;130:977-83. [DOI] [PubMed] [Google Scholar]
- 45. Erggelet C, Kreuz PC, Mrosek EH, Schagemann JC, Lahm A, Ducommun PP, et al. Autologous chondrocyte implantation versus ACI using 3D-bioresorbable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch Orthop Trauma Surg. 2009;130:957-64. [DOI] [PubMed] [Google Scholar]
- 46. Macmull S, Parratt MT, Bentley G, Skinner JA, Carrington RW, Morris T, et al. Autologous chondrocyte implantation in the adolescent knee. Am J Sports Med. 2011;39:1723-30. [DOI] [PubMed] [Google Scholar]
- 47. Ebert JR, Robertson WB, Woodhouse J, Fallon M, Zheng MH, Ackland T, et al. . Clinical and magnetic resonance imaging-based outcomes to 5 years after matrix-induced autologous chondrocyte implantation to address articular cartilage defects in the knee. Am J Sports Med. 2011;39:753-63. [DOI] [PubMed] [Google Scholar]
- 48. Ossendorf C, Steinwachs MR, Kreuz PC, Osterhoff G, Lahm A, Ducommun PP, et al. Autologous chondrocyte implantation (ACI) for the treatment of large and complex cartilage lesions of the knee. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dhollander AA, Verdonk PC, Lambrecht S, Almqvist KF, Elewaut D, Verbruggen G, et al. The combination of microfracture and a cell-free polymer-based implant immersed with autologous serum for cartilage defect coverage. Knee Surg Sports Traumatol Arthrosc. 2012;20:1773-80 [DOI] [PubMed] [Google Scholar]
- 50. Bode G, Schmal H, Pestka JM, Ogon P, Sudkamp NP, Niemeyer P. A non-randomized controlled clinical trial on autologous chondrocyte implantation (ACI) in cartilage defects of the medial femoral condyle with or without high tibial osteotomy in patients with varus deformity of less than 5 degrees. Arch Orthop Trauma Surg. 2013;133:43-9. [DOI] [PubMed] [Google Scholar]
- 51. Gomoll AH, Gillogly SD, Cole BJ, Farr J, Arnold R, Hussey K, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42:1074-81. [DOI] [PubMed] [Google Scholar]
- 52. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meyerkort D, Ebert JR, Ackland TR, Robertson WB, Fallon M, Zheng MH, et al. Matrix-induced autologous chondrocyte implantation (MACI) for chondral defects in the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:2522-30. [DOI] [PubMed] [Google Scholar]
- 54. Aldrian S, Zak L, Wondrasch B, Albrecht C, Stelzeneder B, Binder H, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42:2680-8. [DOI] [PubMed] [Google Scholar]
- 55. Pachowsky ML, Werner S, Marlovits S, Stelzeneder D, Renner N, Trattnig S, et al. 3D-isotropic high-resolution morphological imaging and quantitative T2 mapping as biomarkers for gender related differences after matrix-associated autologous chondrocyte transplantation (MACT). J Orthop Res. 2014;32:1341-8. [DOI] [PubMed] [Google Scholar]
- 56. Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96:824-30. [DOI] [PubMed] [Google Scholar]
- 57. Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, et al. First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop. 2014;38:2065-70. [DOI] [PubMed] [Google Scholar]
- 58. Zhang Z, Zhong X, Ji H, Tang Z, Bai J, Yao M, et al. Matrix-induced autologous chondrocyte implantation for the treatment of chondral defects of the knees in Chinese patients. Drug Des Dev Ther. 2014;8:2439-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pestka JM, Bode G, Salzmann G, Steinwachs M, Schmal H, Sudkamp NP, et al. Clinical outcomes after cell-seeded autologous chondrocyte implantation of the knee: when can success or failure be predicted? Am J Sports Med. 2014;42:208-15. [DOI] [PubMed] [Google Scholar]
- 60. Salzmann GM, Erdle B, Porichis S, Uhl M, Ghanem N, Schmal H, et al. Long-term T2 and qualitative MRI morphology after first-generation knee autologous chondrocyte implantation: cartilage ultrastructure is not correlated to clinical or qualitative MRI outcome. Am J Sports Med. 2014;42:1832-40. [DOI] [PubMed] [Google Scholar]
- 61. Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, et al. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med. 2014;42:1618-27. [DOI] [PubMed] [Google Scholar]
- 62. Ebert JR, Smith A, Fallon M, Wood DJ, Ackland TR. Degree of preoperative subchondral bone edema is not associated with pain and graft outcomes after matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2014;42:2689-98. [DOI] [PubMed] [Google Scholar]
- 63. Ebert JR, Smith A, Fallon M, Wood DJ, Ackland TR. Correlation between clinical and radiological outcomes after matrix-induced autologous chondrocyte implantation in the femoral condyles. Am J Sports Med. 2014;42:1857-64. [DOI] [PubMed] [Google Scholar]
- 64. Ebert JR, Fallon M, Smith A, Janes GC, Wood DJ. Prospective clinical and radiologic evaluation of patellofemoral matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2015;43:1362-72. [DOI] [PubMed] [Google Scholar]
- 65. Niethammer TR, Valentin S, Gulecyuz MF, Rossbach BP, Ficklscherer A, Pietschmann MF, et al. Bone marrow edema in the knee and its influence on clinical outcome after matrix-based autologous chondrocyte implantation: results after 3-year follow-up. Am J Sports Med. 2015;43:1172-9. [DOI] [PubMed] [Google Scholar]
- 66. Wondrasch B, Risberg MA, Zak L, Marlovits S, Aldrian S. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: a prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am J Sports Med. 2015;43:146-53. [DOI] [PubMed] [Google Scholar]
- 67. Bode G, Ogon P, Pestka J, Zwingmann J, Feucht M, Sudkamp N, et al. Clinical outcome and return to work following single-stage combined autologous chondrocyte implantation and high tibial osteotomy. Int Orthop. 2015;39:689-96. [DOI] [PubMed] [Google Scholar]
- 68. Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-30. [DOI] [PubMed] [Google Scholar]
- 69. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85:185-92. [DOI] [PubMed] [Google Scholar]
- 70. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 71. Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13:203-10. [DOI] [PubMed] [Google Scholar]
- 72. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-12. [DOI] [PubMed] [Google Scholar]
- 73. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-46. [DOI] [PubMed] [Google Scholar]
- 74. Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924-33. [DOI] [PubMed] [Google Scholar]
- 75. Ebert JR, Fallon M, Zheng MH, Wood DJ, Ackland TR. A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Am J Sports Med. 2012;40:1527-37. [DOI] [PubMed] [Google Scholar]
- 76. Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470:2261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Akgun I, Unlu MC, Erdal OA, Ogut T, Erturk M, Ovali E, et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135:251-63. [DOI] [PubMed] [Google Scholar]
- 78. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-Induced Autologous Chondrocyte Implantation versus Multipotent Stem Cells for the Treatment of Large Patellofemoral Chondral Lesions: A Nonrandomized Prospective Trial. Cartilage. 2015. April;6(2):82-97. doi: 10.1177/1947603514563597. [DOI] [PMC free article] [PubMed] [Google Scholar]