Abstract
During development, neurons progress through rapid yet stereotypical shape changes to achieve proper neuronal connectivity. This morphological progression requires carefully orchestrated plasma membrane expansion, insertion of membrane components including receptors for extracellular cues into the plasma membrane and removal and trafficking of membrane materials and proteins to specific locations. This review outlines the cellular machinery of membrane trafficking that play an integral role in neuronal cell shape change and function from initial neurite formation to pathway navigation and synaptogenesis.
1. INTRODUCTION
1.1 Neuronal Development
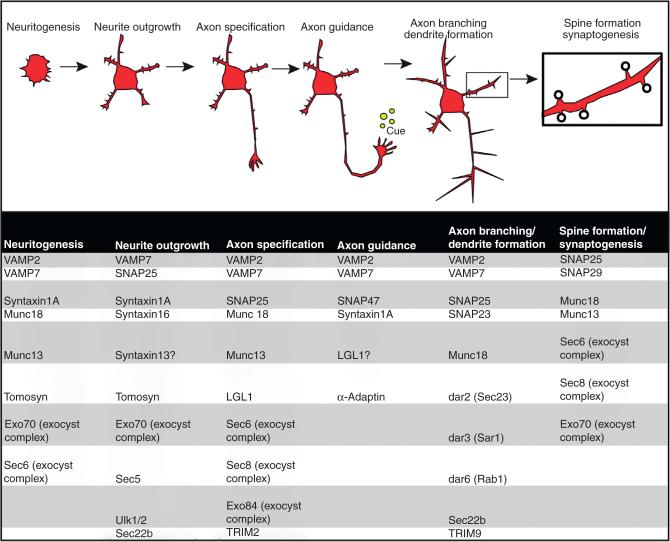
Functional connectivity of the vertebrate nervous system is established during embryonic and postnatal development and continues to adapt throughout life in response to experiences. Once generated from neural precursor cells, newly born neurons migrate from sites of neurogenesis to a specified destination (Hatten, 1999). Upon reaching this destination, neurites sprout from the postmitotic soma in a process known as neuritogenesis (Fig. 1). These neurite projections extend within the surrounding environment, probing their surroundings for guidance cues. Following initial neurite outgrowth, additional changes in neuronal polarization occur, as a single axon is specified, followed by the maturation of the remaining neurites into dendrites. At the tip of an extending axon, a specialized dynamic structure known as the growth cone (Fig. 1) senses and interprets extracellular “axon guidance” cues to guide the extending axon toward appropriate postsynaptic partners (Bark et al., 2004; Raper and Mason, 2010; Saito et al., 2000a). Upon successfully reaching a target destination, the axon tackles the next task of forming multiple synaptic connections. The cell solves this quandary by ramification of axons, known as axon branching. Many axon guidance cues such as netrin-1 also modulate axon branching, and thus regulate the ability of the axon to innervate multiple partners and increase synaptic capacity (Kennedy and Tessier-Lavigne, 1995; Tomasoni et al., 2013). Similar to axon guidance and branching (Granseth et al., 2013; Kennedy and Tessier-Lavigne, 1995; Marler et al., 2014; Schäfer and Frotscher, 2012), dendrites extend and ramify, in response to environmental cues (Danzer et al., 2002; Dijkhuizen and Ghosh, 2005; Dong et al., 2013; Kwon et al., 2011) Finally, synaptic contacts form at specialized sites between axons and dendrites. In excitatory neurons, the presynaptic region within the axon sits approximately 20 nm from the postsynaptic sites located at the tips of dendritic spines, dendritic shafts, or the soma of the postsynaptic neuron. Dendritic spines are typically mushroom-shaped dendritic projections that are malleable, changing size and shape in response to activity and external cues. Such synaptic plasticity continues into adulthood and is associated with learning and memory (Chen et al., 2012; Hamilton et al., 2012; Holtmaat and Svoboda, 2009; Nishiyama et al., 2014; Trachtenberg et al., 2002).
Figure 1.
The stages of neuronal development and associated membrane trafficking proteins. During development neurons progress through stereotypical morphological stages. First, a nascent neuron forms small protrusions during neuritogenesis. These small protrusions elongate during neurite outgrowth and one neurite takes on the characteristics of an axon during axon specification. Axons are then guided toward their synaptic partners by extracellular cues. Axon branching and dendrite formation and branching allow a single neuron to make multiple synaptic connections. Lastly, nascent synaptic spines form during synaptogenesis.
1.2 Membrane Trafficking and Neuronal Development
The progression through the stereotypical stages described earlier (Dotti et al., 1988) involves a significant increase in cell size and plasma membrane surface area. Estimates suggest that the volume of a developing vertebrate neuron can increases at a rate of 0.6% per day during axon elongation, whereas the plasma membrane surface area expansion is much greater, up to 20% in a day (Pfenninger, 2009). This dramatic increase in the plasma membrane necessitates the delivery and insertion of new plasma membrane material. In addition, due to the functional differences of axons and dendrites in transmitting and receiving synaptic transmission, distinct protein populations must differentially localize to these membrane compartments. This involves specialized trafficking to establish and maintain these compositional differences (Maeder et al., 2014). Further highlighting the physiological importance of proper neuronal trafficking, defects in membrane trafficking are implicated in a number of neurological diseases and disorders including autism spectrum and neurodegenerative disorders (Fuchs-Telem et al., 2011; Rapaport et al., 2010; Volders et al., 2011; Wang et al., 2013). Whereas the majority of the focus on vesicular trafficking in neurons has been on the exocytic fusion of synaptic vesicles and the endocytic recycling of synaptic machinery, the important role for membrane trafficking in plasma membrane expansion and neuronal polarization during neuronal development is gaining traction. Here, we highlight our understanding of how membrane trafficking contributes to establishing neuronal form and function during early stages of neuronal morphogenesis.
Understanding the functional connectivity of the nervous system and the stages of neuronal development critical to this connectivity is often referred to as the final frontier of biomedical research. This is evidenced by the announcement of the NIH BRAIN Initiative to map the structure and function of the human brain (Insel et al., 2013). While functional “connectomics” aims to provide a map of the human brain, understanding the molecular mechanisms underlying neural connectivity is an equally critical piece. However interrogating and establishing the molecular mechanisms of neuronal development necessitates a simplified model system. Luckily, the stages of neuronal development are efficiently modeled with in vitro cultures of primary embryonic neurons. Seminal work from Bradke and Dotti outlined these same stages in cultured hippocampal neurons (Fig. 1) (Bradke and Dotti, 2000; Dotti et al., 1988). Use of neuronal cultures has allowed the field to interrogate the function of specific genes and proteins in each of these stages of neuronal morphogenesis. In this review we focus on the membrane trafficking machinery critical to achieving proper neuronal morphology.
Many of the neurological and neuropsychiatric syndromes mentioned previously are associated with variation and/or mutation in genes implicated in membrane trafficking or the signaling molecules and receptors that operate upstream (Cai et al., 2009; Fujiwara, 2006; Grant et al., 2012; Rajendran and Annaert, 2012), indicating the importance of these fundamental cell biological mechanisms during neuronal development. Membrane materials and membrane-associated proteins are in flux between the endomembrane system and the plasma membrane, requiring regulated movement within this complex membranous assembly line. This interconnected network provides the synthesis, sorting, storage, secretion (exocytosis) and recycling (endocytosis) of proteins and macromolecules. For the purposes of this review, we focus on the involvement of these various phases of membrane trafficking in neuronal morphogenesis, from neuritogenesis to synaptogenesis.
2. EXOCYTOSIS
2.1 Exocytic SNARE Proteins
Exocytosis is a longstanding interest of the neuroscience community, as synaptic transmission is accomplished by the fusion of neurotransmitter-containing vesicles to the presynaptic plasma membrane. Pioneering work in budding yeast and neurons identified genes involved in secretion, including the Soluble N-ethylmaleimide-sensitive factor Attachment protein Receptors (SNARE) proteins, which comprise the SNARE complex, the minimal protein requirement for fusion of two lipid bilayers (Cotrufo et al., 2012; Hayashi et al., 1994; McMahon and Südhof, 1995; Novick et al., 1980; Söllner et al., 1993a).
Historically SNARE proteins are described as vesicle (v-SNARE) or target (t-SNAREs), although more recently they have been reclassified based on their structure and the amino acid they contribute to the assembled core SNARE complex (Dirk Fasshauer, 1998). R-SNAREs contribute an arginine, whereas Q-SNAREs contribute a glutamine. A SNARE complex contains one v-SNARE (R-SNARE) such as VAMP-2 (synaptobrevin) or VAMP-7 (Tetanus Insensitive-VAMP) and two t-SNAREs (Q-SNAREs) present at the plasma membrane such as SNAP-25 and syntaxin-1. This complex tethers and docks vesicles to the plasma membrane, bringing the two lipid bilayers in close proximity (McMahon and Südhof, 1995; McNew et al., 2000; Söllner et al., 1993b; Wilson et al., 1992). The formation of this tightly packed SNARE complex is thought to provide the energy for fusing two lipid bilayers, and the mechanisms by which this occurs is the interest of recent biophysical studies (Hernandez et al., 2012; Shi et al., 2012). Following exocytic fusion, vesicular cargoes are secreted into the extracellular space and the vesicular membrane and membrane proteins are incorporated into the plasma membrane.
SNARE-mediated fusion is critical both at the synapse and in the acquisition of neuronal morphology and neuronal membrane expansion during neuritogenesis, neurite outgrowth, axon guidance and axon branching. Thus this evolutionarily conserved process is multifaceted, allowing both signaling between neurons and promoting neuronal growth. Several SNARE proteins, the v-SNARES VAMP-2 and VAMP-7, the t-SNARES SNAP-25, SNAP-29, SNAP-47 and syntaxin and the R-SNARE, Sec22b for have been implicated in progression through the stages of neural morphology.
2.1.1 V-SNAREs: VAMP-2 and VAMP-7
VAMP2 and VAMP7 are enriched in the nervous system and have independent as well as overlapping developmental and synaptic functions. The existence of two separate routes of vesicle trafficking to the plasma membrane, one tetanus sensitive and one insensitive (Proux-Gillardeaux et al., 2005; Coco et al., 1999) may provide both redundancy and specificity in delivery. Deletion of the VAMP2 gene in mice is lethal immediately following birth, although surprisingly mice exhibit no gross neuroanatomical phenotypes (Schoch et al., 2001). In contrast, genetic deletion of the VAMP7 gene in mice (SYBL1) is not lethal, although mice exhibit increased anxiety and neuroanatomical differences including decreased brain weight and increased volume of the third ventricle (Danglot et al., 2012; Varoqueauxet al., 2002; Verhage et al., 2000). These overt neuroanatomical defects exhibited in mice correlate with aberrant behavior, which could shed some light on the etiology of neurological disorders. The presence of a G to C transversion in the regulatory region of human SYBL1 occurs at a higher frequency in patient populations with bipolar disorder, implicating altered VAMP7 function in depressive disorders (Müller et al., 2002; Saito et al., 2000b).
In contrast, studies in cultured neurons indicate that acute depletion of VAMP2 or VAMP7, or inhibition of their function via Tetanus neurotoxin (TeNT) or dominant negative approaches, respectively results in morphological defects at multiple stages of neuronal development in context-dependent fashions. For example, TeNT, which cleaves VAMP2 but not VAMP7, blocks neuritogenesis in dissociated murine cortical neurons plated on poly-D-Lysine (PDL), but not laminin (Gupton and Gertler, 2010), suggesting that VAMP2 functions in an intrinsic neuritogenesis pathway that cannot be compensated for by VAMP7 function. In contrast, expression of a NH2-terminal longin domain of VAMP7, which acts as a dominant negative by blocking endogenous VAMP7–mediated SNARE complex formation and vesicle fusion (Martinez-Arca et al., 2001), only blocks neuritogenesis stimulated by attachment to laminin but not PDL (Gupton and Gertler, 2010). This study also identified substrate-dependent changes in the dynamics and fusion frequency of these two populations of vesicles (Gupton and Gertler, 2010), indicating context dependent roles for each v-SNARE during neuritogenesis.
Although TeNT-mediated cleavage of VAMP2 blocks neuritogenesis at two days in vitro (Gupton and Gertler, 2010), TeNT treatment does not disrupt axon outgrowth in rat cortical neurons or mouse hippocampal neurons cultured for longer periods of time or when TeNT is added to the culture subsequent to neuritogenesis (Grosse et al., 1999; Osen-Sand et al., 1996). Consistent with in vivo findings, this suggests that the requirement for VAMP2 during neuritogenesis is eventually compensated by another v-SNARE, and furthermore that VAMP2 is not required for axon outgrowth once neuritogenesis has occurred. In contrast, expression of the dominant negative VAMP7 longin domain in rat hippocampal neurons dramatically reduces both axon length and dendrite outgrowth (Martinez-Arca et al., 2001), suggesting VAMP7 delivers material required for neurite outgrowth. In support of this, expression of a constitutively active form of VAMP7 enhances axon outgrowth (Martinez-Arca et al., 2001). In contrast to these dramatic effects, two groups recently generated mice lacking SYBL1, the gene encoding VAMP7. One noted a modest decrease in the length of axons in hippocampal neurons, whereas no difference was observed in the second line (Danglot et al., 2012; Sato et al., 2011). One explanation for the difference between results with acute knockdown or inhibition versus genetic deletion could be compensation for VAMP7 function by other vSNAREs. Conditional loss or simultaneous inhibition of both VAMP2 and VAMP7 during neuritogenesis and neurite outgrowth is imperative to clarify these differences. Furthermore, whether the multiple splice variants of VAMP7 (Vacca et al., 2011) differentially function in developmental exocytosis remains to be determined.
As yet, no studies implicate VAMP2 or VAMP7 function in axon specification. VAMP7 is concentrated at the tips of axonal growth cones (Coco et al., 1999), whereas VAMP2 is specifically allowed across the barrier of the axon initial segment into the axon (Song et al., 2009). These data suggest v-SNARE function may participate in establishing neuronal polarity through preferential trafficking of dendritic and axonal specific proteins (Burack et al., 2000). In an effort to further elucidate the cellular trafficking of VAMP2 during axon specification, hippocampal neurons expressing VAMP2-CFP were allowed to endocytose primary antibodies to CFP (Sampo et al., 2003). Endocytosed VAMP2 and primary antibodies are accessible to secondary antibodies only upon permeabilization. Comparison of permeabilized (total) and nonpermeabilized (surface) neurons revealed that endocytosis of VAMP2 occurs in dendrites, whereas surface localized VAMP2 was enriched in the axon. Moreover, mutation of residues within the cytoplasmic domain of VAMP2 that prevented endocytosis of VAMP2 results in decreased VAMP2 localization to the axon. (Sampo et al., 2003). Thus VAMP2 localizes to both axons and dendrites, but is selectively removed from the dendrites via endocytosis. Similarly, preferential localization and immobility of several other membrane proteins has previously been shown within the axon initial segment (Mellman et al., 1999). Taken together these data suggest the presence of a protein specific diffusion barrier at the axon initial segment, but whether this polarized trafficking plays a role in axon specification is not known.
Whereas membrane delivery is required for outgrowth, there is also evidence that asymmetrical exocytosis may be necessary or even sufficient for growth cone guidance/axon turning. Local increases in calcium activity induce either attractive or repulsive turning in response to extracellular cues (Gomez et al., 2001; Gomez and Zheng, 2006; Henley and Poo, 2004; Zheng, 2000). Using spatially-localized photolysis of caged Ca2+ on one side of a growth cone of chick dorsal root ganglion neurons (DRGs), Tojima et al., showed that on an L1-CAM substrate, localized Ca2+ release induces local, asymmetric VAMP2-mediated exocytosis and attractive turning of the growth cone toward the photolysis (Tojima et al., 2006). In this attractive paradigm, VAMP2-containing vesicles move toward and fuse within the area of photolysis, suggesting that localized exocytosis was required for growth cone turning. Indeed, attractive turning is inhibited by TeNT. In contrast, on laminin the growth cone turns away from uncaged Ca2+ and this repulsive turning was not TeNT-sensitive and did not involve changes in VAMP2-containing vesicle motility or fusion, suggesting repulsive turning may not involve changes in exocytosis.
Based on the localization of VAMP7 at the axon tip, and its requirement in neurite initiation and axon extension (Gupton and Gertler, 2010; Martinez-Arca et al., 2001, 2000), VAMP7 is situated to function during axon guidance as well. VAMP7 coimmunoprecipitates with the t-SNARE syntaxin-1 and DCC, a receptor for the axon guidance cue netrin, specifically in the embryonic brain (Cotrufo et al., 2011). Growth cones of embryonic chick spinal cord depleted of VAMP7 by dsRNAi fail to reach the floor plate or cross the midline in an open book preparation, suggesting a role for VAMP7-mediated exocytosis in axon guidance. Furthermore, embryonic mouse hippocampal explants treated with TeNT, which cleaves VAMP2 but not VAMP7, do not lose preferential attraction toward netrin-1, suggesting in this context that VAMP7 is sufficient for biased outgrowth toward an asymmetrical netrin source (Cotrufo et al., 2011). Taken together, these data suggest that neurons are capable of mediating growth cone steering through VAMP-dependent exocytic activity.
Netrin stimulation also induces axon branching in cortical neurons (Dent et al., 2004; Winkle et al., 2014). We recently showed that either VAMP2 or VAMP7 function was sufficient to provide new membrane material for axonal branching in response to netrin-1 stimulation in mouse cortical neurons. TeNT-mediated cleavage of VAMP2 or longin-mediated inhibition of VAMP7 independently were insufficient independently to decrease netrin-dependent axon branching, although simultaneous inhibition of both v-SNAREs significantly decreased branching. Total internal reflection fluorescence microscopy of neurons expressing VAMP2-pHlourin or VAMP7-pHluorin reveals that netrin-1 stimulates vesicle fusion mediated by both v-SNAREs, suggesting that VAMP2 and VAMP7 have redundant functions during netrin-stimulated axon branching (Winkle et al., 2014), although they likely deliver differential cargo to the plasma membrane during this phase of rapid plasma membrane expansion. These potential compensatory functions may explain the relative dearth of neuronal morphology defects observed in VAMP2 or VAMP7 knockouts (Danglot et al., 2012; Schoch et al., 2001).
2.1.2 SNAP Family of t-SNAREs
2.1.2.1 SNAP25
Synaptosomal associated protein 25 (SNAP25) is a highly expressed neuronal plasma membrane t-SNARE involved in VAMP2 or VAMP7-mediated exocytosis, and thus loss of SNAP25 function likely has overlapping phenotypes with loss of VAMP2 or VAMP7 function. Whereas experiments have not been published regarding a role for SNAP25 in neuritogenesis, early in the study of SNAP25, injection of SNAP25 antisense oligonucleotides into the intraocular cavity of chick embryos decreased the thickness of the inner plexiform layer of the retina, which consists predominantly of axonal and dendritic material (Osen-Sand et al., 1993). These data suggest that SNAP25 plays an important role in axon outgrowth. Immunogold labeling of SNAP25 in rat hippocampal slices indicates that SNAP25 preferentially localizes to the axonal plasma membrane, not the dendritic membrane during polarization and axon specification (Tao-Cheng et al., 2000). However, further studies are required to determine whether SNAP25 function mediates axonal specification and to identify the mechanism by which SNAP25 is restricted from dendrites.
Treatment of rat cortical neurons or mouse hippocampal neurons with botulinum neurotoxin A (BoNTA), which cleaves SNAP25 and blocks vesicle fusion, inhibits axon outgrowth, dendritic arborization, and synapse formation (Grosse et al., 1999). Acute BoNTA-mediated inhibition of SNAP25 blocks netrin-dependent axon branching, similar to inhibition of both VAMP2 and VAMP7, further supporting the hypothesis that exocytosis is required for plasma membrane expansion during axon branching (Winkle et al., 2014). As occurred with the deletion of VAMP2, SNAP25−/− mice die at birth, presumably due to an inability to produce evoked synaptic contractions of the diaphragm and consequent respiratory failure (Washbourne et al., 2001). Unexpectedly, development of the neocortex, hippocampus, thalamus and midbrain was normal as elucidated histopathologically, and cultured cortical and hippocampal neurons from SNAP25−/− embryos exhibit normal neurite outgrowth (Washbourne et al., 2001). Additionally, genetic deletion of SNAP25 did not impede the ability of thalamocortical axons to be guided to the cortex, as assessed using both in vitro explant assays and in vivo DiI tracing from dorsal thalamus to the cortex (Blakey et al., 2012; Molnar et al., 2002). Both SNAP25 and SNAP47 have been shown to contribute to the secretion of BDNF in callosal neurons (Shimojo et al., 2015). Since genetic deletion of SNAP25 does not impair neurite outgrowth and axon guidance, but acute cleavage of SNAP25 with BoNTA does, this suggests another t-SNARE compensates for long-term loss of SNAP25 function. SNAP23 is a homologous t-SNARE expressed in distinct patterns throughout the brain that specifically localizes to dendrites (Chen et al., 1999; Suh et al., 2010). The subcellular distribution of SNAP25 and SNAP23 do not overlap, thus whether SNAP23 plays a different role or compensates for the loss of SNAP25 remains to be seen.
SNAP25 also plays a role during synapse formation. A switch from expression of SNAP25a to the alternative isoform SNAP25b occurs post-natally, indicating that SNAP25a functions early in development, whereas SNAP25b potentially regulates later stage processes like synapse formation (Bark et al., 2004; Yamamori et al., 2011). A targeted mutation that impairs the switch between SNAP25a and SNAP25b causes lethality in a majority of mice between the ages of 3 and 5 weeks, coinciding with the time frame of SNAP25b expression and synapse formation. Moreover, presynaptic plasticity is enhanced by overexpression of SNAP25a in CA1 hippocampal synapses, suggesting that the A isoform facilitates early synaptic release in immature synapses as opposed to mature synapses (Bark et al., 2004). These results suggest that continued expression of the SNAP25a isoform impairs synaptic maturation by maintaining nascent synapses in the early stages of synaptic development. Interestingly, data suggest that hippocampal injury induced increases in SNAP25a expression, which correlates with reinnervation of hippocampal circuitry (Patanow et al., 1996), further supporting the role of SNAP25a isoform in early formation of synaptic connectivity. A reduction in SNAP25 expression via siRNA or the overexpression of GFP-SNAP25 leads to the development of immature or an overabundant formation of spines, respectively (Tomasoni et al., 2013). Together these results reveal highly specified roles for SNAP25 splice variants in forming nascent synapses and allowing proper maturation of function connections, but whether these roles are exocytosis dependent or indicate other functions for SNAP25 is not yet known.
2.1.2.2 SNAP29
Unlike SNAP25, SNAP29 is a broadly expressed t-SNARE located on multiple intracellular membranes, including the Golgi complex and synaptic vesicles (Steegmaier et al., 1998; Su et al., 2001; Wong et al., 1999). SNAP29 was identified as a syntaxin1 binding partner that blocks SNARE complex disassembly and modulates synaptic transmission (Su et al., 2001). Whereas SNAP25 and SNAP23 interact with plasma membrane specific syntaxins 1–4, SNAP29 interacts with both plasma membrane and intracellular syntaxins including syntaxin 4, 6, and 7 (Hohenstein and Roche, 2001; Steegmaier et al., 1998; Wong et al., 1999), suggesting SNAP29 may have a distinct role in membrane and protein trafficking, separate from SNAP25 and SNAP23. Whereas Wong et al initially reported SNAP29 binding specificity to syntaxin-6 at the Golgi, based on Golgi membrane preparations, Hohenstein et al, and Steegmaier et al. later showed more promiscuous syntaxin binding using purified proteins. Whether purified protein recapitulate possible physiological interactions occurring outside the Golgi remains to be clarified, and may explain the discrepancy between interactions.
A 1-bp deletion in the human SNAP29 gene results in the absence of the protein and leads to cerebral dysgenesis, neuropathy, ichthyosis, and keratoderma (CEDNIK) syndrome (Fuchs-Telem et al., 2011; Sprecher et al., 2005), which is associated with severe psychomotor retardation and skin scaling, corpus callosum dysgenesis, microcephaly, and facial dysmorphisms. Fibroblasts cultured from CEDNIK patients were characterized by accumulation of early endosomes corresponding to an impaired endocytic recycling (Fuchs-Telem et al., 2011; Rapaport et al., 2010).
2.1.2.3 SNAP47
Another novel and understudied SNAP25 family member (Qbc-SNARE), SNAP47 is also expressed in the nervous system, localizes to the plasma membrane and can substitute for SNAP25 to form SNARE complexes with syntaxin-1A and VAMP2 in cultured neurons. Not only does SNAP47 form SNARE complexes, it also participates in membrane fusion events, although less effectively than SNAP25, as evidenced by the decreased thermal stability of SNAP47 complexes relative to SNAP25 complexes (Holt et al., 2006). Given the ubiquitous expression SNARE complex formation and fusion capabilities of SNAP47, compensation by SNAP47 may account for the lack of major neuroanatomical anomalies in SNAP25−/− mice (Washbourne et al., 2001). VAMP2, SNAP25 and SNAP47 mediate the vesicular release of the neurotrophic factor BDNF from the axon. Moreover, ablation of the function of SNAP47, BDNF or the BDNF receptor TrkB disrupts callosal axon branching both in vitro and in vivo, suggesting that SNAP47 plays a role in both exocytosis and axon branching (Shimojo et al., 2015). Thus SNAP47 may have both redundant and unique functions neurologically, although more study regarding its various roles is necessary given the paucity of information on this protein.
2.1.3 Syntaxins
The t-SNARE syntaxin-1 is expressed in neurons, localizes to the plasma membranes, forms a SNARE complex with SNAP25 and VAMP2, and is involved in neurotransmitter release (Söllner et al., 1993a). As is the case with deletion of SNAP25, genetic deletion of the syntaxin1A gene (STX1A) is not associated with gross neuroanatomical differences (Fujiwara, 2006). In contrast, treatment with botulinum neurotoxin C (BoNTC1), which cleaves both SNAP25 and syntaxin1, causes growth cone collapse and inhibited neurite outgrowth in chick DRGs, and blocks biased neurite outgrowth toward an asymmetric netrin-1 source in mouse hippocampal explants (Cotrufo et al., 2012; Hayashi et al., 1994; Igarashi, 1996). Since treatment with BoNTA, which cleaves SNAP25 and leaves syntaxin intact, does not recapitulate these results, syntaxin1 is likely required for preferential outgrowth toward netrin, whereas another SNAP family member appears to compensate for loss of SNAP25 function.
Two other syntaxin family members, syntaxin 13 and syntaxin 16 have been identified in the developing brain (Chua and Tang, 2008; Hirling et al., 2000). Syntaxin13 is implicated in both exocytosis and early endosomal trafficking in axons and dendrites and is enriched in the growth cone of cortical neurons (Hirling et al., 2000; Prekeris et al., 1999). Although it is as yet unclear if syntaxin13 plays a specific role in neurite outgrowth in neurons, overexpression of syntaxin 13 in PC12 cells increases neurite outgrowth. Given the expression of syntaxin13 in the brain and specific localization to neurite processes, syntaxin13 may play a role in neuronal plasma membrane expansion by forming complexes with SNAP25 and contributing to plasma membrane expansion (Hirling et al., 2000; Sarria et al., 2002). Syntaxin 16 is enriched in neuronal dendrites at Golgi outposts. Expression of a dominant negative variant of syntaxin 16 inhibits neurite outgrowth in mouse cortical neurons (Chua and Tang, 2008), suggesting a role for endogenous syntaxin16 in neuritogenesis. Whether either syntaxin13 or 16 play redundant or unique roles to syntaxin1 is unknown.
2.1.4 Regulators of SNARE Complex
2.1.4.1 Munc Proteins
The localization and interaction of SNARE complex proteins is regulated by a host of interacting proteins. Many of these interaction partners regulate exocytic activity and endocytic recycling of various SNARE components, further complicating the molecular pathways involved in neuronal membrane trafficking. One key group of interacting proteins is the Sec1/Munc18 (SM) family, which regulates synaptic vesicle exocytosis mediated by SNARE complex proteins. There are 14 members in this family, 6 that are specific to mammals. The SM family is implicated in exocytosis and in mediating synaptic activity, and at least three of these proteins are present in neurons (Halachmi and Lev, 1996). Two family members in particular, Munc18 and Munc13 appear to play distinct roles in neuronal membrane trafficking. Munc18 binds to the n-terminus of syntaxin-1A in its closed conformation, preventing the open conformation and inhibiting SNARE complex formation (Dulubova et al., 2007; Rickman and Duncan, 2010). However, Munc18 also precipitates with assembled SNARE complexes in biochemical crosslinking studies, suggesting that although Munc18 plays an inhibitory role in initial SNARE complex formation, it may also be important for execution of exocytic fusion (Dulubova et al., 2007). Munc13 interacts with syntaxin 1A to promote the transition from the Munc18 dependent closed conformation complex to the open conformation SNARE complex (Ma et al., 2011).
Genetic deletion of either Munc18-1 or of both Munc13-1 and Munc13-2 in mice abolishes both spontaneous and evoked neurotransmitter release in neurons leading to neonatal lethality (Varoqueaux et al., 2002; Verhage et al., 2000), however, dissociated neurons from these mice can be cultured in vitro for experimental purposes. Neurons lacking Munc18-1 exhibit defects in vesicle recycling and actin cytoskeleton dynamics (Varoqueaux et al., 2002; Verhage et al., 2000), whereas deletion of either Munc18-1 and the Munc13s decreases axonal outgrowth speed and neurite number early in development, which is recovered just prior to synaptogenesis (Broeke et al., 2012). Because the lag in neurite outgrowth only occurs prior to synaptogenesis in Munc13 knockout neurons, these data suggest loss of Munc13 only delays but does not inhibit neurite outgrowth (Broeke et al., 2012). These data suggest that a later stage compensatory mechanism counteracts the impact of the genetic loss of either Munc gene. In hippocampal neurons, overexpression of Munc18 increases axon branching (Steiner et al., 2002). Thus Munc proteins function in distinct steps of vesicle fusion, including vesicle priming and SNARE complex formation and likely provide spatial and temporal specificity to membrane expansion necessary for early neuronal morphology and development. Whether they impact later stages such as synapse formation remains poorly understood.
2.1.4.2 TRIM9
The E3 ubiquitin ligase TRIM9 was identified as a SNAP25 binding partner that competes with VAMP2 for SNAP25 binding (Li et al., 2001). Following genetic deletion of TRIM9, cortical neurons exhibit elevated SNARE complex formation and enhanced frequency of vesicle fusion mediated by either VAMP2 or VAMP7 (Winkle et al., 2014). These increases in exocytosis were correlated with increased axon branching both in vitro and in vivo, and increased branching could be reduced by BoNTA- dependent cleavage of SNAP25. TRIM9−/− mice also exhibit a significantly thicker corpus callosum with increased axon branching. By regulating SNARE-mediated exocytosis and membrane delivery, TRIM9 appears to spatially and temporally regulate axon branching in vitro and in vivo. There are ~ 70 mammalian TRIM ligases, that have also been implicated in number of cellular processes, including autophagy (Mandell et al., 2014). A comprehensive analysis of TRIM function in cellular morphogenesis may be warranted.
2.2 Vesicle Tethering Proteins
2.2.1 LGL Family Proteins
The evolutionarily conserved lethal giant larvae (LGL) family of proteins regulates the tethering of exocytic vesicles to the plasma membrane. These proteins are conserved from yeast to mammals and regulate a variety of polarization processes (Mechler et al., 1985; Fujita et al., 1998; Lehman et al., 1999). The mammalian orthologs, Lgl1 and Tomosyn, are enriched in brain (Fujita et al., 1998; Klezovitch et al., 2004). Deletion of LGL1 in mice causes severe hydrocephalis, as neuroprogenitors fail to polarize and differentiate into neurons, and instead continue to proliferate and die by apoptosis (Klezovitch et al., 2004). Lgl1 regulates the activation of Rab10, a key regulator of membrane trafficking of membrane proteins by directly interacting with and promoting the membrane attachment of Rab10. In cultured hippocampal neurons Lgl1 localizes to the tips of extending axons (Wang et al., 2011). Overexpression of Lgl1 promotes axon outgrowth and vesicle fusion events in hippocampal neurons, whereas RNAi-mediated depletion of Lgl1 reduces axon length and vesicle fusion frequency. These data suggest Lgl1 promotes exocytosis, axon outgrowth and polarization via downstream activation of Rab10.
Tomosyn is an LGL family member originally identified as a binding partner of syntaxin-1 (Fujita et al., 1998). Overexpression of tomosyn in rat hippocampal neurons reduces neurite number and length and strongly increases the number of cells exhibiting no neurite formation. RNAi-mediated tomosyn knockdown in a neuroblastoma cell line increases the number of neurites and secondary branching (Sakisaka et al., 2004). Overexpression of tomosyn in a neuroblastoma cell line inhibits vesicle trafficking to the cell surface and decreases interaction between VAMP2 and syntaxin1, as observed with immunocytochemistry studies and coimmunoprecipitation, suggesting tomosyn negatively regulates neurite outgrowth by preventing the delivery and tethering of vesicles (Sakisaka et al., 2004).
While the literature regarding the involvement of SNARE complexes and their regulators in neuronal development is expansive, it should be noted that there are still areas that are not well understood. For instance, although distinct vesicles populations are likely to carry specific cargoes, the identity of specific cargoes carried by either a VAMP2 or VAMP7 positive vesicle and how a neuron differentiates between vesicle populations is understudied. The involvement of SNAREs in recycling of surface receptors back to the plasma membrane after endocytosis is another question that, with the advent of super resolution imaging, may be an area of great interest as well.
2.2.2 Exocyst Complex
The exocyst is an evolutionarily conserved octomeric protein complex that localizes at sites of polarized membrane growth (TerBush et al., 1996). Components of the complex were first identified in a screen for genes essential to polarized growth in budding yeast (Novick et al., 1980). The complex comprises eight subunits: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84, which assemble into a Y-shaped structure (Hsu et al., 1998). Each subunit mediates unique interactions providing specialized roles in neuronal trafficking (Heider and Munson, 2012). A number of biochemical and microscopy-based studies have demonstrated that the exocyst complex is involved in both transporting and tethering vesicles to sites of fusion at the plasma membrane, prior to the formation of SNARE complexes and vesicle fusion (Grote et al., 2000; Whyte and Munro, 2002). Disruption of the exocyst in mice results in severe defects and embryonic lethality, suggesting that the exocyst may play a key role during development.
In neurons, the exocyst complex localizes to the growth cone periphery in sprouting neurites and synaptogenic regions (Hazuka et al., 1999; Vega and Hsu, 2001). Overexpression of the Exo70 subunit can induce filopodia and increase secretion (Gupton and Gertler, 2010; Wang et al., 2004). In embryonic mouse cortical neurons, Exo70 overexpression promotes neuritogenesis, supporting an important role for exocytosis and the exocyst complex in developing neurons (Fujita et al., 2013; Gupton and Gertler, 2010; Vega and Hsu, 2001). Several groups have shown various exocyst components at the tips of extending neurites, further supporting their role in neuritogenesis and neurite extension (Hazuka et al., 1999; Lalli and Hall, 2005; Mehta et al., 2005). Expression levels of the exocyst subunit Sec6 correlate temporally with the occurrence of neuritogenesis (Chin et al., 2000). During axon specification, the exocyst complex is integral in both vesicle targeting to the axon (Fujita et al., 2013) and the regulation of cell polarity (He and Guo, 2009). Lalli reported that siRNA depletion of exocyst subunits Sec6, Sec8, or Exo84 inhibits axon specification in rat cortical neurons, as no neurites become positive for the axon marker tau. These three exocyst subunits colocalize and coimmunoprecipitate with par-3, a key polarity related protein (Lalli and Hall, 2005). Taken together, these data suggest that the exocyst complex may be necessary for the proper specification of axons and dendrites.
Expression levels of the exocyst subunit Sec6 correlate temporally with the occurrence of synaptogenesis (Chin et al., 2000). As the nascent synapse forms, the signaling receptors AMPA and NMDA are trafficked to the structure. During synaptogenesis, complex components Sec8 and Exo70 mediate the targeting and insertion of AMPA receptors in hippocampal cells through Sec8 interactions with the synaptic scaffolding proteins PSD95 and SAP102 (Sans et al., 2003). Overexpression of a tagged variant of the Sec8 C-terminus acts as a dominant negative and inhibits endogenous Sec8 in CA1 hippocampal cells. This inhibition reduces both AMPAR and NMDAR synaptic responses (Riefler et al., 2003; Sans et al., 2003), suggesting that the exocyst is necessary for trafficking of receptors to newly formed synapses.
In Drosophila, mutation of the Sec5 exocyst subunit, which produces null alleles and effectively depletes Sec5, results in a decrease in the percentage of cells exhibiting neurite outgrowth and impaired membrane trafficking but does not impact synaptic vesicle fusion (Murthy et al., 2003), suggesting that the exocyst is required for membrane protein localization but not synaptic vesicle tethering. The authors suggest that although the exocyst is essential for exocytosis in yeast, it may be dispensable in synaptic vesicle fusion. It is also possible that tethering LGL family members may offer redundant functions, or that synaptic vesicles are tethered to the plasma membrane by trans SNARE complexes, and thus readily releasable (Kavalali, 2002; Whyte and Munro, 2002; Zhang et al., 2005). Further, whereas these results reinforce the view that different exocyst complex subunits can perform individual functions during exocytosis (Heider and Munson, 2012), it remains unclear whether these individual functions rely on conformational changes within subunits, or are specific to the cell specific membrane proteins that recruit the exocyst to the plasma membrane. While centriolin targets the exocyst complex and SNARE components to the midbody in HeLa cells during cytokinesis (Gromley et al., 2005), it is unknown whether a similar targeting to neuronal growth processes is necessary during development and how this targeting might be controlled spatially and temporally. Further work to address these questions is necessary to improve or understanding to the role of the exocyst in neuronal development.
3. ENDOCYTOSIS
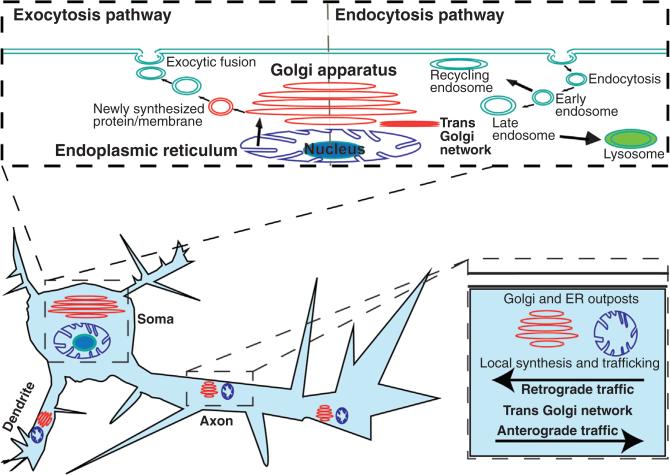
Neurons use exocytic fusion to secrete extracellular signals, add receptors to the plasma membrane and contribute membrane material at sites of growth. Retrieval of membrane components and extracellular cue via endocytosis allows for plasma membrane remodeling during neuronal morphogenesis as well as activation of intracellular signaling pathways that further enhance neuronal morphology. Seminal work by Heuser and Reese in the early 1970s identified the coated pits responsible for vesicle recycling at the synapse of frog neuromuscular junctions (Heuser and Reese, 1973), revealing a new field of inquiry. Receptor internalization can activate downstream signaling cascades to modulate cell morphology, dynamics, and function (Winckler and Yap, 2011). Endocytosis can be divided between clathrin-dependent and clathrin-independent mechanisms. In clathrin-mediated endocytosis, adaptor proteins and clathrin are recruited to specific docking sites and activated in a manner independent of receptor signaling (Santini et al., 1998). Unlike the clathrin-dependent internalization route of endocytosis, which is sensitive to drugs that block the formation of clathrin coated pits, clathrin-independent methods such as caveolin dependent endocytosis are sensitive to cholesterol depletion, suggesting that they are lipid raft dependent (Stern and Mermelstein, 2010). Clathrin independent endocytosis allows neurons to intake other nonligand substances to adapt to their environment. The endocytic pathway incorporates the early endosome, the late endosome, recycling endosome, and the lysosome (Fig. 2) (Grant and Donaldson, 2009). The early endosomes localize to the cell periphery and are composed of vesicles that were recently invaginated and pinched off from the plasma membrane. The late endosomes act as receiving stations of internalized materials prior to fusion to lysosomes, where degradation and recycling of materials occurs (Grant and Donaldson, 2009). Much of what we know about endocytosis in other cell types applies to neurons.
Figure 2.
The neuronal pathways of exocytosis and endocytosis. During exocytosis newly synthesized proteins are packages at the Golgi apparatus, and delivered to the plasma membrane where vesicles fuse, adding new membrane material and releasing their cargo. In endocytosis, a vesicle buds, encapsulating its cargo. The endocytic vesicles are cycled through the endosome and either delivered to be degraded (lysosome) or to be recycled (Recycling endosome). Golgi and ER outposts in the dendrites, axon and growth cones of developing neurons allow for local synthesis and trafficking of proteins and act as way stations within the trans-Golgi network.
3.1 Receptor Endocytosis in Neurite Outgrowth
During development the endocytosis of receptors and their ligands is an important signaling mechanism, which promotes neuronal differentiation, migration, neuritogenesis and outgrowth, and axon guidance (Itofusa, 2011; Kawauchi, 2012; Tian et al., 2012; Zhang et al., 2000). Pharmacological inhibition of endocytosis increases surface localization of FGFR1 and increases FGF2-dependent axon branching, suggesting that basal endocytosis of the receptor reduces FGF dependent axon branching (Hausott et al., 2011). In mouse spinal neurons, RNAi knockdown of key Rab5 endocytic pathway regulators Unc-51-like kinases 1/2 (Ulk1/2), also decreases NGF receptor TrkA endocytosis resulting in increased arborization with stunted axon extensions (Zhou et al., 2007). These data suggest that the endocytosis of these receptors is particularly important in axonal outgrowth signaling.
Endocytosis of receptors may also play a key role in axon guidance, especially in the case of cell adhesion. Polarized substrate adhesion at the growth cone is necessary for axon pathfinding (Kamiguchi, 2007). For instance, a growth cone extending on an L1-CAM (L1 Cell adhesion molecule) substrate maintains strong adhesion at the leading edge and weaker adhesion at the central domain, providing the necessary traction for directional growth (Kamiguchi and Yoshihara, 2001). In chick and rat embryonic dorsal root ganglia grown on L1-CAM, axonal outgrowth correlated with an increase in growth cone specific endocytosis of L1-CAM.
L1-CAM is preferentially endocytosed at the central domain of the growth cone and reinserted at the leading edge, leading to polarized adhesion to L1-CAM. Inhibition of clathrin-mediated endocytosis with a function-blocking antibody against α-adaptin (a subunit of the AP2 adaptor necessary for clathrin pit formation) decreases endocytosis of L1-CAM, which attenuates polarized L1-CAM substrate adhesion and decreases growth cone migration (Kamiguchi and Yoshihara, 2001). These results are an example of how spatial control of receptor endocytosis promotes axon guidance and substrate adhesion.
Spatial specificity in endocytosis is not only important for polarized substrate adhesion, but can promote directional membrane retraction during axon guidance as well. Asymmetric application of the repulsive cue sema3A to axonal growth cones increases clathrin coated pit formation nearest to the sema3A source (Tojima et al., 2010). Inducing asymmetric endocytosis on one side causes local retraction and growth cone turning toward the side of lower endocytosis. These data indicate that manipulation of membrane trafficking is sufficient to induce axon turning (Tojima et al., 2010; 2014)
Endocytosis not only modulates plasma membrane expansion and retraction, but is required for receptor mediated signaling pathway activation as well. The extracellular guidance cue netrin-1 induces endocytosis and degradation of the receptor DCC in embryonic mouse cortical neurons (Kim et al., 2005). The endocytosis of the receptor activates downstream signaling pathways involved in axon guidance, though whether the receptor is degraded or recycled is poorly understood (Kim et al., 2005; Li et al., 2004). In Xenopuslaevis retinal growth cone adaptation to netrin or sema3A signaling by endocytosis of their receptors DCC, Neuropilin-1 and plexin1A allows the axons to follow a gradient of these chemotropic cues to their synaptic destinations. This adaptation is dependent upon two processes; an acute endocytosis-dependent desensitization to the ligand, followed by a slower protein synthesis dependent resensitization, wherein new receptors are inserted into the plasma membrane (Piper et al., 2005). Cultured retinal explants pretreated with PAO, an inhibitor of all modes of endocytosis, or MDC, an inhibitor specific to clathrin-mediated endocytosis, are unable to desensitize the axonal growth cone to netrin or Sema3A, as evident by increased occurrences of growth cone collapse, and an inability to reestablish the growth cone some minutes after pretreatment (Piper et al., 2005). Thus receptor endocytosis in response to extracellular cues is required for axonal outgrowth and guidance.
Tojima et al. recently illustrated that spatial control of asymmetric calcium signals shifts the spatial balance between endocytosis and exocytosis in chick DRG growth cones. Growth cone turning assays and TIRF microscopy-based imaging of markers for endo- and exocytosis, show that attractive Ca++ signals suppress endocytosis and increase exocytosis on the opposing side of the growth cone (Tojima et al., 2014; 2010). Clathrin coated pit formation occurs asymmetrically in response to the repulsive cue Semaphorin3A. Pharmacological inhibition of calcium signaling, exocytosis or endocytosis significantly altered growth cone responses to extracellular cues (Tojima et al., 2010, 2014). Taken together these data indicate that regulated endocytosis is likely involved in proper axon guidance.
3.2 Compartmentalization of Neuronal Endosomes
Unlike cells with simpler morphologies, neurons are unique in their surface area to volume ratio and face an interesting challenge of having portions of the cell such as the tips of axons and dendrites and synaptic spines localized at great distances from the soma. This necessitates a vast and compartmentalized endosomal system with both general and location specific regulation (Winckler and Yap, 2011). This compartmentalization of endosomes throughout the neuron allows for polarized endocytosis and recycling of material to and from distinct cell compartments. For example, TrkA receptors on the somal surface of rat sympathetic neurons are endocytosed and reinserted into the axonal plasma membrane in a process called ‘transcytosis’(Ascano et al., 2009). Whereas the concept of a compartmentalized system is relatively new, many researchers are currently working on its structure and function, as well as adaptors that may differentiate endosomes at different locations throughout the cells. Two recent reviews cover these topics in depth, and thus will not be covered here (Chan Choo Yap, 2015; Yap and Winckler, 2012).
Taken together, these data suggest that carefully orchestrated and specifically localized endocytosis of signaling receptors, coupled with the location specific addition of new plasma membrane via exocytosis likely accounts for a large portion of directional axon guidance. Although these findings are intriguing, there is some conjecture regarding their applicability regarding endocytic signaling in vivo, where multiple guidance factors are in play and gene expression is wildly heterogeneous across neuronal populations. The idea that a growth cone increases exocytosis on the side closest to an attractive cue or the side furthest from a repulsive cue in order to respond accordingly during axon guidance is likely too simple with many questions left unanswered. For instance, we know that exocytosis inserts receptors, while endocytosis removes them from the plasma membrane but little is understood regarding potential signaling between these two processes. Moreover, this field of study is further complicated by the fact that vesicle materials as well as receptors are either degraded, recycled or some combination of both, and the fate of these materials can vary spatiotemporally, and on a receptor-by-receptor basis. Lastly, defining how the endosome at the dendrite differs from the endosome at the soma will be integral to our understanding of endosomal activity in neuronal development, necessitating further investigation.
4. ER AND GOLGI
Though much of what we know about membrane trafficking in neurons is regarding the exocytic and endocytic machinery at the plasma membrane, the secretory pathway also includes the endoplasmic reticulum (ER), and Golgi complex. Since the surface area of developing neurons increases rapidly, recycling of membrane material would be insufficient to increase plasma membrane surface area (Pfenninger, 2009), thus newly synthesized material is synthesized in axonal and dendritic structures that are functionally equivalent to ER and Golgi in the soma, called ER and Golgi outposts (Merianda et al., 2009; Ori-McKenney et al., 2012).
4.1 ER and Golgi Outposts
One theory posits that the preferential presence of Golgi outposts in dendrites may play a role in differential development of axons and dendrites (Ye et al., 2007). Indeed axons and dendrites in class IV dopaminergic Drosophila neurons are differentially impacted by loss of dar2, dar3, or dar 6; drosophila homologs of Sec23, Sar1, and Rab1 respectively. The loss of any of these three proteins, which regulate ER to Golgi transport by the COPII vesicles, decreases dendritic arbors, while leaving axonal outgrowth intact. Golgi-mosaic analysis with a repressible cell marker experiments revealed defective somal Golgi and Golgi outpost shape in mutants of a critical ER to Golgi transport mediator, Dar3 (Sar1) (Ye et al., 2007). Moreover, laser ablation of Golgi outposts in dendrites reduced extension and retraction of branches, resulting in distinct rearrangements of the arbor in comparison to nonilluminated or illuminated regions that did not contain Golgi outposts. siRNA knockdown of Sar1 in mouse embryonic hippocampal neurons resulted in similar defects in both dendritic arborization and Golgi morphology, suggesting a conserved role for this protein (Ye et al., 2007). These data suggest that dendritic arborization is heavily dependent upon ER to Golgi transport and Golgi outpost activity. More recent evidence suggests that Golgi outposts manifest these changes in dendritic arborization by directly nucleating microtubules within the arbor, a process which is essential for the stabilization of nascent branches (Ori-McKenney et al., 2012). In Drosophila class IV da neurons, Golgi outposts are located at 47% of all dendritic branch points and colocalize with EB1 (a marker of growing microtubule plus ends) in vivo. Furthermore Golgi vesicles promote the nucleation of microtubules, which promotes branches stability or extension instead of retraction, indicating microtubules and associated Golgi vesicles are integral to dendritic arbor maintenance (Ori-McKenney et al., 2012).
Delivery of material from the ER to the Golgi is also critical for axon guidance. Axons of cultured Xenopus retinal ganglion cells stain positive for the ER associated protein calreticulin, and rat DRG neurons treated with ER-tracker dye also show ER signals in axonal processes (Merianda et al., 2009). Pharmacological inhibition of ER to Golgi transport with brefeldin-A decreases repulsive growth cone turning in response to the guidance cue Engrailed-2 in Xenopus retinal ganglion cells. In cultured rat DRG neurons, axons cleaved from their somal bodies express newly synthesized membrane proteins and traffic them to the cell surface, suggesting that local protein synthesis separate from somal synthesis is occurring in the axon (Merianda et al., 2009). These data indicate that ER to Golgi trafficking is necessary for proper axon guidance.
4.2 R-SNARE Sec22
Contacts between the ER and plasma membrane that are dependent on extended synaptotagmins have been identified in yeast, HeLa cells and mammalian neurons (Fernández-Busnadiego et al., 2015; Giordano et al., 2013; Spacek and Harris, 1997). A recent study showed that the nonfusogenic SNARE protein Sec22b resides in the ER and the ER to Golgi intermediate compartment, particularly at sites where the ER is in close apposition to syntaxin 1 at the plasma membrane (Petkovic et al., 2014). Sec22b can form a trans SNARE complex with syntaxin that was suggested to bridge the ER directly to plasma membrane expansion through nonvesicular delivery of membrane components. This was supported by the observation that an extended Sec22b mutant increases the distance between the ER and the plasma membrane and decreases the length of neurites. Expression of a dominant negative longin domain of Sec22b in embryonic mouse cortical neurons also significantly decreases both dendrite and axon outgrowth (Petkovic et al., 2014). Future studies to further investigate these novel contact sites between ER and the neuronal plasma membrane will be an exciting area of research.
5. OTHER REGULATORS OF MEMBRANE TRAFFIC
The large family of Rab and ARF GTPases regulate the transport, tethering, and fusion of vesicles, and thus are implicated in neuritogenesis, outgrowth, polarization, and the differential trafficking of axonal and dendritic components. In neurons specifically, GTPases are known to function in protrusion, migration, establishing polarity, and trafficking between cellular compartments, making these small proteins an integral part of neuronal development. The literature regarding GTPase function in neuronal development is expansive, and we direct readers to a recent review regarding these functions (Villarroel-Campos et al., 2014).
6. CONCLUSION AND FUTURE DIRECTIONS
The unique morphology of neurons, including their long extensions, axonal and dendritic compartmentalization, and the specialized shape and structure of the synapse are achieved through highly regulated neuronal membrane trafficking. In this review, we have focused on exocytic and endocytic machinery and the role the Golgi and ER play in controlling plasma membrane expansion and receptor dynamics. Recent studies have identified possible molecules and mechanisms which may bridge these cell biological functions including TRIM9, PACSINS, JIP1, and cytoskeletal motor proteins which transport vesicles (Deng et al., 2014; Kessels, 2004; Winkle et al., 2014; Zhang et al., 2004; Zhu et al., 2007). This is an area awaiting further investigation.
In addition, membrane trafficking is differentially regulated downstream of extracellular cues, including axon guidance cues and extracellular matrix components. Specificity of vesicular cargo and membrane trafficking pathways likely allows axons to extend over long ranges as they respond to intermediate cues and subsequently distal cues. For example, during neuritogenesis VAMP2 functions in an intrinsic pathway, whereas the activity of VAMP7 and Arp2/3 are required during neuritogenesis promoted by integrin activation by laminin (Gupton and Gertler, 2010). In contrast, during axon branching, both VAMP2 and VAMP7 participate in axon branching in response to Netrin-1 stimulation (Winkle et al., 2014). Delineation of the distinct signaling pathways that operate in these stages of neuronal development is critical.
Moreover, the consequences of using distinct vesicle machinery or cyto-skeletal regulatory networks and cytoskeletal architectures are unknown, but are likely critical in providing specificity to neuronal development. For example, SNAP25 controls spine formation through the adaptor protein p140Cap, which regulates the actin cytoskeleton in rat hippocampal neurons in an exocytosis independent manner (Tomasoni et al., 2013), but whether other exocytic proteins have similar roles in cytoskeletal dynamics during neuronal development remains to be seen.
Now that we are within the “omics” era of scientific investigation, bridging the information gathered from genomic and proteomic approaches to that of nervous system connectomics is an obvious next step. Understanding the cellular functions of genes associated with neurological diseases is an incredible jumping off point for this. As we identify genetic variations and mutations associated with neurological conditions, and how their loss of function is associated with connectivity changes in animal models, neuronal culture models should be employed to define the cell biological mechanisms of their action. One such critical example of this is mutations in the SNARE component SNAP29 that cause CEDNIK syndrome (Fuchs-Telem et al., 2011; Rapaport et al., 2010; Sprecher et al., 2005). Relatively little is known about the cellular functions of SNAP29, but its importance in human health is clear. Coupling recent technological developments such as super resolution imaging and optogenetic manipulation with more standard pharmacological perturbations and genetic manipulations will continue to advance our understanding of how normal and impaired neuronal development are intimately linked to membrane trafficking in vivo.
ACKNOWLEDGMENTS
Stephanie Gupton was supported by the National Institutes of Health (R01GM108970) and Cortney Winkle was supported by the National Institutes of Health (F31NS087837).
REFERENCES
- Ascano M, Richmond A, Borden P, Kuruvilla R. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J. Neurosci. 2009;29:11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark C, Bellinger FP, Kaushal A, Mathews JR, Partridge LD, Wilson MC. Developmentally regulated switch in alternatively spliced SNAP-25 isoforms alters facilitation of synaptic transmission. J. Neurosci. 2004;24:8796–8805. doi: 10.1523/JNEUROSCI.1940-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakey D, Wilson MC, Molnár Z. Termination and initial branch formation of SNAP-25-deficient thalamocortical fibres in heterochronic organotypic co-cultures. Eur. J. Neurosci. 2012;35(10):1586–1594. doi: 10.1111/j.1460-9568.2012.08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol. 2000;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Broeke JHP, Roelandse M, Luteijn MJ, Boiko T, Matus A, Toonen RF, Verhage M. Munc18 and Munc13 regulate early neurite outgrowth. Bio. Cell. 2012;102:479–488. doi: 10.1042/BC20100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J. Biol. Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Choo Yap BW. Adapting for endocytosis: roles for endocytic sorting adaptors in directing neural development. Front. Cell. Neurosci. 2015;9:119. doi: 10.3389/fncel.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Minger SL, Honer WG, Whiteheart SW. Organization of the secretory machinery in the rodent brain: distribution of the t-SNAREs, SNAP-25 and SNAP-23. Brain Res. 1999;831:11–24. doi: 10.1016/s0006-8993(99)01371-2. [DOI] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PTC, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin LS, Weigel C, Li L. Transcriptional regulation of gene expression of Sec6, a component of mammalian exocyst complex at the synapse. Brain Res. Mol. Brain Res. 2000;79:127–137. doi: 10.1016/s0169-328x(00)00110-8. [DOI] [PubMed] [Google Scholar]
- Chua CEL, Tang BL. Syntaxin 16 is enriched in neuronal dendrites and may have a role in neurite outgrowth. Mol. Membr. Biol. 2008;25:35–45. doi: 10.1080/09687680701504649. [DOI] [PubMed] [Google Scholar]
- Coco S, Raposo G, Martinez S, Fontaine JJ, Takamori S, Zahraoui A, Jahn R, Matteoli M, Louvard D, Galli T. Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J. Neurosci. 1999;19(22):9803–9812. doi: 10.1523/JNEUROSCI.19-22-09803.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrufo T, Andrés RM, Ros O, Pérez-Brangulí F, Muhaisen A, Fuschini G, Martínez R, Pascual M, Comella JX, Soriano E. Syntaxin 1 is required for DCC/Netrin-1-dependent chemoattraction of migrating neurons from the lower rhombic lip. Eur. J. Neurosci. 2012;36:3152–3164. doi: 10.1111/j.1460-9568.2012.08259.x. [DOI] [PubMed] [Google Scholar]
- Cotrufo T, Perez-Branguli F, Muhaisen A, Ros O, Andres R, Baeriswyl T, Fuschini G, Tarrago T, Pascual M, Urena J, Blasi J, Giralt E, Stoeckli ET, Soriano E. A signaling mechanism coupling Netrin-1/deleted in colorectal cancer chemoattraction to SNARE-mediated exocytosis in axonal growth cones. J. Neurosci. 2011;31:14463–14480. doi: 10.1523/JNEUROSCI.3018-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Zylbersztejn K, Petkovic M, Gauberti M, Meziane H, Combe R, Champy MF, Birling MC, Pavlovic G, Bizot JC, Trovero F, Ragione, Della F, Proux-Gillardeaux V, Sorg T, Vivien D, D'Esposito M, Galli T. Absence of TI-VAMP/Vamp7 leads to increased anxiety in mice. J. Neurosci. 2012;32:1962–1968. doi: 10.1523/JNEUROSCI.4436-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, Crooks KRC, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J. Neurosci. 2002;22:9754–9763. doi: 10.1523/JNEUROSCI.22-22-09754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C-Y, Lei W-L, Xu X-H, Ju X-C, Liu Y, Luo Z-G. JIP1 mediates anterograde transport of Rab10 cargos during neuronal polarization. J. Neurosci. 2014;34:1710–1723. doi: 10.1523/JNEUROSCI.4496-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Barnes AM, Tang F. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J. Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen PA, Ghosh A. BDNF regulates primary dendrite formation in cortical neurons via the PI3-kinase and MAP kinase signaling pathways. J. Neurobiol. 2005;62:278–288. doi: 10.1002/neu.20100. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen PA, Ghosh A. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Liu OW, Howell AS, Shen K. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell. 2013;155:296–307. doi: 10.1016/j.cell.2013.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippo-campal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Khvotchev M, Liu S, Huryeva I, Südhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Saheki Y, De Camilli P. Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum–plasma membrane contact sites. Proc. Natl. Acad. Sci. 2015;112:E2004–E2013. doi: 10.1073/pnas.1503191112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Telem D, Stewart H, Rapaport D, Nousbeck J, Gat A, Gini M, Lugassy Y, Emmert S, Eckl K, Hennies HC, Sarig O, Goldsher D, Meilik B, Ishida-Yamamoto A, Horowitz M, Sprecher E. CEDNIK syndrome results from loss-of-function mutations in SNAP29. Br. J. Dermatol. 2011;164(3):610–616. doi: 10.1111/j.1365-2133.2010.10133.x. [DOI] [PubMed] [Google Scholar]
- Fujita A, Koinuma S, Yasuda S, Nagai H, Kamiguchi H, Wada N, Nakamura T. GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PLoS ONE. 2013a;8:e79689. doi: 10.1371/journal.pone.0079689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Koinuma S, Yasuda S, Nagai H, Kamiguchi H, Wada N, Nakamura T. GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PLoS ONE. 2013b;8:e79689. doi: 10.1371/journal.pone.0079689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara T. Analysis of knock-out mice to determine the role of HPC-1/syntaxin 1A in expressing synaptic plasticity. J. Neurosci. 2006;26(21):5767–5776. doi: 10.1523/JNEUROSCI.0289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P2-dependent and Ca2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon path-finding. Nat. Rev. Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Granseth B, Fukushima Y, Sugo N, Lagnado L, Yamamoto N. Regulation of thalamocortical axon branching by BDNF and synaptic vesicle cycling. Front. Neural. Circuits. 2013;7:202. doi: 10.3389/fncir.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Fathalli F, Rouleau G, Joober R, Flores C. Association between schizophrenia and genetic variation in DCC: a case–control study. Schizophr. Res. 2012;137:26–31. doi: 10.1016/j.schres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen C-T, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE Complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Grosse G, Grosse J, Tapp R, Kuchinke J, Gorsleben M, Fetter I, Höhne-Zell B, Gratzl M, Bergmann M. SNAP-25 requirement for dendritic growth of hippo-campal neurons. J. Neurosci. Res. 1999;56:539–546. doi: 10.1002/(SICI)1097-4547(19990601)56:5<539::AID-JNR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J. Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev. Cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi N, Lev Z. The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J. Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- Hamilton AM, Oh WC, Vega-Ramirez H, Stein IS, Hell JW, Patrick GN, Zito K. Activity-dependent growth of new dendritic spines is regulated by the protea-some. Neuron. 2012;74:1023–1030. doi: 10.1016/j.neuron.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu. Rev. Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hausott B, Rietzler A, Vallant N, Auer M, Haller I, Perkhofer S, Klimaschewski L. Inhibition of fibroblast growth factor receptor 1 endocytosis promotes axonal branching of adult sensory neurons. Neuroscience. 2011;188:13–22. doi: 10.1016/j.neuroscience.2011.04.064. [DOI] [PubMed] [Google Scholar]
- Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuka CD, Foletti DL, Hsu SC, Kee Y, Hopf FW, Scheller RH. The Sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley J, Poo M-M. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JM, Stein A, Behrmann E, Riedel D, Cypionka A, Farsi Z, Walla PJ, Raunser S, Jahn R. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science. 2012;336:1581–1584. doi: 10.1126/science.1221976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H, Steiner P, Chaperon C, Marsault R, Regazzi R, Catsicas S. Syntaxin 13 is a developmentally regulated SNARE involved in neurite outgrowth and endosomal trafficking. Eur. J. Neurosci. 2000;12:1913–1923. doi: 10.1046/j.1460-9568.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- Hohenstein AC, Roche PA. SNAP-29 is a promiscuous syntaxin-binding SNARE. Biochem. Biophys. Res. Commun. 2001;285:167–171. doi: 10.1006/bbrc.2001.5141. [DOI] [PubMed] [Google Scholar]
- Holt M, Varoqueaux F, Wiederhold K, Takamori S, Urlaub H, Fasshauer D, Jahn R. Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J. Biol. Chem. 2006;281:17076–17083. doi: 10.1074/jbc.M513838200. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hsu S-C, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition protein interactions, and structures of the mammalian brain Sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Igarashi M. Growth cone collapse and inhibition of neurite growth by Botulinum neurotoxin C1: a t-SNARE is involved in axonal growth. J. Cell Biol. 1996;134(1):205–215. doi: 10.1083/jcb.134.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Landis SC, Collins FS. The NIH BRAIN Initiative. Science. 2013;340:687–688. doi: 10.1126/science.1239276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itofusa R. Polarizing membrane dynamics adhesion for growth cone navigation. Mol. Cell Neurosci. 2011;48(4):332–338. doi: 10.1016/j.mcn.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H. Advances in Experimental Medicine and Biology. Springer; New York, NY: 2007. The role of cell adhesion molecules in axon growth and guidance; pp. 95–102. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Yoshihara F. The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones. J. Neurosci. 2001;21:9194–9203. doi: 10.1523/JNEUROSCI.21-23-09194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET. SNARE interactions in membrane trafficking: a perspective from mammalian central synapses. Bioessays. 2002;24(10):926–936. doi: 10.1002/bies.10165. [DOI] [PubMed] [Google Scholar]
- Kawauchi T. Cell adhesion and its endocytic regulation in cell migration during neural development and cancer metastasis. IJMS. 2012;13:4564. doi: 10.3390/ijms13044564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TE, Tessier-Lavigne M. Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Curr. Opin. Neurobiol. 1995;5:83–90. doi: 10.1016/0959-4388(95)80091-3. [DOI] [PubMed] [Google Scholar]
- Kessels MM. The syndapin protein family: linking membrane trafficking with the cytoskeleton. J. Cell. Sci. 2004;117:3077–3086. doi: 10.1242/jcs.01290. [DOI] [PubMed] [Google Scholar]
- Kim T-H, Lee HK, Seo IA, Bae HR, Suh DJ, Wu J, Rao Y, Hwang K-G, Park HT. Netrin induces down-regulation of its receptor, deleted in colorectal cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron. J. Neurochem. 2005;95:1–8. doi: 10.1111/j.1471-4159.2005.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M, Fernández JR, Zegarek GF, Lo SB, Firestein BL. BDNF-promoted increases in proximal dendrites occur via CREB-dependent transcriptional regulation of cypin. J. Neurosci. 2011;31:9735–9745. doi: 10.1523/JNEUROSCI.6785-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G, Hall A. Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol. 2005;171:857–869. doi: 10.1083/jcb.200507061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman K, Rossi G, Adamo JE, Brennwald P. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell. Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, Lee S-H, Liu G, Aurandt J, Shen T-L, Fearon ER, Guan J-L, Han M, Rao Y, Hong K, Guan K-L. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chin LS, Weigel C, Li L. Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis. J Biol Chem. 2001;276:40824–40833. doi: 10.1074/jbc.M106141200. [DOI] [PubMed] [Google Scholar]
- Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin–Munc18 complex to the SNARE complex. Nat Struct Mol Biol. 2011;18:542–549. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder CI, Shen K, Hoogenraad CC. Axon and dendritic trafficking. Curr Opin Neurobiol. 2014;27:165–170. doi: 10.1016/j.conb.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Mandell MA, Jain A, Arko-Mensah J, Chauhan S, Kimura T, Dinkins C, Silvestri G, Münch J, Kirchhoff F, Simonsen A, Wei Y, Levine B, Johansen T, Deretic V. TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev Cell. 2014;30:394–409. doi: 10.1016/j.devcel.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler KJ, Suetterlin P, Dopplapudi A, Rubikaite A, Adnan J, Maiorano NA, Lowe AS, Thompson ID, Pathania M, Bordey A, Fulga T, Van Vactor DL, Hindges R, Drescher U. BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP. J Neurosci. 2014;34:969–979. doi: 10.1523/JNEUROSCI.1910-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 2000;149:889–899. doi: 10.1083/jcb.149.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Coco S, Mainguy G, Schenk U, Alberts P, Bouillé P, Mezzina M, Prochiantz A, Matteoli M, Louvard D, Galli T. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J Neurosci. 2001;21:3830–3838. doi: 10.1523/JNEUROSCI.21-11-03830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Südhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J. Biol. Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- McNew JA, Weber T, Parlati F, Johnston RJ, Melia TJ, Söllner TH, Rothman JE. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell. Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler BM, McGinnis W, Gehring WJ. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophilamelanogaster. EMBO J. 1985;4(6):1551–1557. doi: 10.1002/j.1460-2075.1985.tb03816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SQ, Hiesinger PR, Beronja S, Zhai RG, Schulze KL, Verstreken P, Cao Y, Zhou Y, Tepass U, Crair MC, Bellen HJ. Mutations in Drosophila Sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 2005;46:219–232. doi: 10.1016/j.neuron.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Mellman I, Winckler B, Forscher P. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JSY, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol. Cell. Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar ZN, López-Bendito G, Small J, Partridge LD, Blakemore C, Wilson MC. Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity. J. Neurosci. 2002;22:10313–10323. doi: 10.1523/JNEUROSCI.22-23-10313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Müller DJ, Schulze TG, Jahnes E, Cichon S, Krauss H, Kesper K, Held T, Maier W, Propping P, Nöthen MM, Rietschel M. Association between a polymorphism in the pseudoautosomal X-linked gene SYBL1 and bipolar affective disorder. Am. J. Med. Genet. 2002;114:74–78. doi: 10.1002/ajmg.10115. [DOI] [PubMed] [Google Scholar]
- Nishiyama N, Colonna J, Shen E, Carrillo J, Nishiyama H. Long-term in vivo time-lapse imaging of synapse development and plasticity in the cerebellum. J. Neurophysiol. 2014;111:208–216. doi: 10.1152/jn.00588.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ori-McKenney KM, Jan LY, Jan Y-N. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen-Sand A, Staple JK, Naldi E, Schiavo G, Rossetto O, Petitpierre S, Malgaroli A, Montecucco C, Catsicas S. Common and distinct fusion proteins in axonal growth and transmitter release. J. Comp. Neurol. 1996;367:222–234. doi: 10.1002/(SICI)1096-9861(19960401)367:2<222::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, Grenningloh G, Catsicas S. Inhibition of axonal growth by Snap-25 antisense oligonucleotides in-vitro and in-vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Patanow CM, Day JR, Billingsley ML. Alterations in hippocampal expression of SNAP-25, GAP-43, stannin and glial fibrillary acidic protein following mechanical and trimethyltin-induced injury in the rat. Neuroscience. 1996;76:187–202. doi: 10.1016/s0306-4522(96)00335-1. [DOI] [PubMed] [Google Scholar]
- Petkovic M, Jemaiel A, Daste F, Specht CG, Izeddin I, Vorkel D, Verbavatz J-M, Darzacq X, Triller A, Pfenninger KH, Tareste D, Jackson CL, Galli T. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat. Cell Biol. 2014;16:434–444. doi: 10.1038/ncb2937. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nat Rev. Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- Piper M, Salih S, Weinl C, Holt CE, Harris WA. Endocytosis-dependent desensitization and protein synthesis–dependent resensitization in retinal growth cone adaptation. Nat. Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Foletti DL, Scheller RH. Dynamics of tubulovesicular recycling endosomes in hippocampal neurons. J. Neurosci. 1999;19:10324–10337. doi: 10.1523/JNEUROSCI.19-23-10324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proux-Gillardeaux V, Rudge R, Galli T. The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways? Traffic. 2005;6:366–373. doi: 10.1111/j.1600-0854.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Annaert W. Membrane trafficking pathways in Alzheimer's disease. Traffic. 2012;13:759–770. doi: 10.1111/j.1600-0854.2012.01332.x. [DOI] [PubMed] [Google Scholar]
- Rapaport D, Lugassy Y, Sprecher E, Horowitz M. Loss of SNAP29 impairs endocytic recycling and cell motility. PLoS ONE. 2010;5:e9759. doi: 10.1371/journal.pone.0009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb. Perspect. Biol. 2010;2:a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman C, Duncan RR. Munc18/Syntaxin interaction kinetics control secretory vesicle dynamics. J. Biol. Chem. 2010;285:3965–3972. doi: 10.1074/jbc.M109.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler GM, Balasingam G, Lucas KG, Wang S, Hsu S-C, Firestein BL. Exocyst complex subunit sec8 binds to postsynaptic density protein-95 (PSD-95): a novel interaction regulated by cypin (cytosolic PSD-95 interactor). Biochem. J. 2003;373:49. doi: 10.1042/BJ20021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Parsia S, Papolos DF, Lachman HM. Analysis of the pseudoautosomal X-linked geneSYBL1in bipolar affective disorder: description of a new candidate allele for psychiatric disorders. Am. J. Med. Genet. 2000a;96:317–323. doi: 10.1002/1096-8628(20000612)96:3<317::aid-ajmg17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Saito T, Parsia S, Papolos DF, Lachman HM. Analysis of the pseudoautosomal X-linked gene SYBL1in bipolar affective disorder: description of a new candidate allele for psychiatric disorders. Am. J. Med. Genet. 2000b;96:317–323. doi: 10.1002/1096-8628(20000612)96:3<317::aid-ajmg17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Baba T, Tanaka S, Izumi G, Yasumi M, Takai Y. Regulation of SNAREs by tomosyn and ROCK: implication in extension and retraction of neurites. J. Cell Biol. 2004;166:17–25. doi: 10.1083/jcb.200405002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Sans N, Prybylowski K, Petralia RS, Chang K, Wang Y-X, Racca C, Vicini S, Wenthold RJ. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- Santini F, Marks MS, Keen JH. Endocytic clathrin-coated pit formation is independent of receptor internalization signal levels. Mol. Biol. Cell. 1998;9:1177–1194. doi: 10.1091/mbc.9.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria F, Hirling H, Hornung J-P, Catsicas S. Developmental and spatial expression pattern of syntaxin 13 in the mouse central nervous system. Cell Tissue Res. 2002;309:209–218. doi: 10.1007/s00441-002-0600-5. [DOI] [PubMed] [Google Scholar]
- Sato M, Yoshimura S, Hirai R, Goto A, Kunii M, Atik N, Sato T, Sato K, Harada R, Shimada J, Hatabu T, Yorifuji H, Harada A. The Role of VAMP7/TIVAMP in cell polarity and lysosomal exocytosis in vivo. Traffic. 2011;12:1383–1393. doi: 10.1111/j.1600-0854.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- Schäfer MKE, Frotscher M. Role of L1CAM for axon sprouting and branching. Cell Tissue Res. 2012;349:39–48. doi: 10.1007/s00441-012-1345-4. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deák F, Königstorfer A, Mozhayeva M, Sara Y, Südhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Shi L, Shen QT, Kiel A, Wang J, Wang HW, Melia TJ, Rothman JE, Pincet F. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo M, Courchet J, Pieraut S, Torabi-Rander N, Sando RI, Polleux F, Maximov A. SNAREs controlling vesicular release of BDNF and development of callosal axons. Cell Reports. 2015;11:1054–1066. doi: 10.1016/j.celrep.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A-H, Wang D, Chen G, Li Y, Luo J, Duan S, Poo M-M. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher E, Ishida-Yamamoto A, Mizrahi-Koren M, Rapaport D, Goldsher D, Indelman M, Topaz O, Chefetz I, Keren H, O'brien TJ, Bercovich D, Shalev S, Geiger D, Bergman R, Horowitz M, Mandel H. A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neuro-cutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am. J. Human Genet. 2005;77:242–251. doi: 10.1086/432556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, Luo Y, Scheller RH. Three novel proteins of the syntaxin/SNAP-25 family. J Biol Chem. 1998;273:34171–34179. doi: 10.1074/jbc.273.51.34171. [DOI] [PubMed] [Google Scholar]
- Steiner P, Sarria JCF, Huni B, Marsault R, Catsicas S, Hirling H. Overexpression of neuronal Sec1 enhances axonal branching in hippocampal neurons. Neuroscience. 2002;113:893–905. doi: 10.1016/s0306-4522(02)00225-7. [DOI] [PubMed] [Google Scholar]
- Stern CM, Mermelstein PG. Caveolin regulation of neuronal intracellular signaling. Cell Mol. Life Sci. 2010;67:3785–3795. doi: 10.1007/s00018-010-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Mochida S, Tian JH, Mehta R, Sheng ZH. SNAP-29: a general SNARE protein that inhibits SNARE disassembly and is implicated in synaptic transmission. Proc. Natl. Acad. Sci. 2001;98:14038–14043. doi: 10.1073/pnas.251532398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JTR, Roche KW, Roche PA. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat. Neurosci. 2010;13:338–343. doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Du J, McBain CJ. Snap-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons. J. Neurocytol. 2000;29:67–77. doi: 10.1023/a:1007168231323. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15(23):6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Tian N, Leshchyns'ka I, Welch JH, Diakowski W, Yang H, Schachner M, Sytnyk V. Lipid raft-dependent endocytosis of close homolog of adhesion molecule L1 (CHL1) promotes neuritogenesis. J. Biol. Chem. 2012;287:44447–44463. doi: 10.1074/jbc.M112.394973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat. Neurosci. 2006;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- Tojima T, Itofusa R, Kamiguchi H. Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron. 2010;66:370–377. doi: 10.1016/j.neuron.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Tojima T, Itofusa R, Kamiguchi H. Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. J. Neurosci. 2014;34:7165–7178. doi: 10.1523/JNEUROSCI.5261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni R, Repetto D, Morini R, Elia C, Gardoni F, Di Luca M, Turco E, Defilippi P, Matteoli M. SNAP-25 regulates spine formation through postsynaptic binding to p140Cap. Nat. Commun. 2013;4:2136. doi: 10.1038/ncomms3136. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Vacca M, Albania L, Ragione F, Carpi A, Rossi V, Strazzullo M, et al. Alternative splicing of the human gene SYBL1 modulates protein domain architecture of longin VAMP7/TI-VAMP, showing both non-SNARE and synaptobrevin-like isoforms. BMC Molecular Biology. 2011;12(1):26. doi: 10.1186/1471-2199-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. PNAS. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. 2001;21:3839–3848. doi: 10.1523/JNEUROSCI.21-11-03839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Südhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Villarroel-Campos D, Gastaldi L, Conde C, Cáceres A, González-Billault C. Rab-mediated trafficking role in neurite formation. J. Neurochem. 2014;129:240–248. doi: 10.1111/jnc.12676. [DOI] [PubMed] [Google Scholar]
- Volders K, Nuytens K, Creemers JWM. The autism candidate gene neurobeachin encodes a scaffolding protein implicated in membrane trafficking and signaling. Curr. Mol. Med. 2011;11:204–217. doi: 10.2174/156652411795243432. [DOI] [PubMed] [Google Scholar]
- Wang D, Chan C-C, Cherry S, Hiesinger PR. Membrane trafficking in neuronal maintenance and degeneration. Cell Mol. Life Sci. 2013;70:2919–2934. doi: 10.1007/s00018-012-1201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liu Y, Adamson CL, Valdez G, Guo W, Hsu SC. The mammalian exocyst, a complex required for exocytosis inhibits tubulin polymerization. J. Biol. Chem. 2004;279:35958–35966. doi: 10.1074/jbc.M313778200. [DOI] [PubMed] [Google Scholar]
- Wang T, Liu Y, Xu X-H, Deng C-Y, Wu K-Y, Zhu J, Fu X-Q, He M, Luo Z-G. Lgl1 activation of Rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev. Cell. 2011;21:431–444. doi: 10.1016/j.devcel.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, oacute GL-B, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nature Neuroscience. 2001;5(1):19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Whyte JRC, Munro S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- Wilson DW, Whiteheart SW, Wiedmann M, Brunner M, Rothman JE. A multisubunit particle implicated in membrane fusion. 1992;117:531–538. doi: 10.1083/jcb.117.3.531. jcb.rupress.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Yap CC. Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors. Traffic. 2011;12:1099–1108. doi: 10.1111/j.1600-0854.2011.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, McClain LM, Valtschanoff JG, Park CS, Maglione C, Gupton SL. A novel Netrin-1-sensitive mechanism promotes local SNARE-mediated exocytosis during axon branching. J. Cell Biol. 2014;205:217–232. doi: 10.1083/jcb.201311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Griffiths G, Lowe SL, Subramaniam VN, Seow KT, Hong W. GS32, a novel Golgi SNARE of 32 kDa, interacts preferentially with syntaxin 6. Mol. Biol. Cell. 1999;10:119–134. doi: 10.1091/mbc.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori S, Itakura M, Sugaya D, Katsumata O, Sakagami H, Takahashi M. Differential expression of SNAP-25 family proteins in the mouse brain. J. Comp. Neurol. 2011;519:916–932. doi: 10.1002/cne.22558. [DOI] [PubMed] [Google Scholar]
- Yap CC, Winckler B. Harnessing the power of the endosome to regulate neural development. Neuron. 2012;74:440–451. doi: 10.1016/j.neuron.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan Y-N. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Berg JS, Li Z, Wang Y, Lång P, Sousa AD, Bhaskar A, Cheney RE, Strömblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol. 2004;6:523–531. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang P, Gangar A, Zhang J, Brennwald P, TerBush D, Guo W. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J Cell Biol. 2005;170:273–283. doi: 10.1083/jcb.200502055. doi:10.1083/jcb.200502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ. Turning of nerve growth cones induced by localized increases in intra-cellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- Zhou X, Babu JR, da Silva S, Shu Q, Graef IA, Oliver T, Tomoda T, Tani T, Wooten MW, Wang F. Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons. Proc. Natl. Acad. Sci. 2007;104:5842–5847. doi: 10.1073/pnas.0701402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X-J, Wang C-Z, Dai P-G, Xie Y, Song N-N, Liu Y, Du Q-S, Mei L, Ding Y-Q, Xiong W-C. Myosin X regulates netrin receptors and functions in axonal path-finding. Nature. 2007;9:184–192. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]