Abstract
Background
Due to selective breeding, domesticated and wild Atlantic salmon are genetically diverged, which raises concerns about farmed escapees having the potential to alter the genetic composition of wild populations and thereby disrupting local adaptation. Documenting transcriptional differences between wild and domesticated stocks under controlled conditions is one way to explore the consequences of domestication and selection. We compared the transcriptomes of wild and domesticated Atlantic salmon embryos, by using a custom 44k oligonucleotide microarray to identify perturbed gene pathways between the two stocks, and to document the inheritance patterns of differentially-expressed genes by examining gene expression in their reciprocal hybrids.
Results
Data from 24 array interrogations were analysed: four reciprocal cross types (W♀ × W♂, D♀ × W♂; W♀ × D♂, D♀ × D♂) × six biological replicates. A common set of 31,491 features on the microarrays passed quality control, of which about 62 % were assigned a KEGG Orthology number. A total of 6037 distinct genes were identified for gene-set enrichment/pathway analysis. The most highly enriched functional groups that were perturbed between the two stocks were cellular signalling and immune system, ribosome and RNA transport, and focal adhesion and gap junction pathways, relating to cell communication and cell adhesion molecules. Most transcripts that were differentially expressed between the stocks were governed by additive gene interaction (33 to 42 %). Maternal dominance and over-dominance were also prevalent modes of inheritance, with no convincing evidence for a stock effect.
Conclusions
Our data indicate that even at this relatively early developmental stage, transcriptional differences exist between the two stocks and affect pathways that are relevant to wild versus domesticated environments. Many of the identified differentially perturbed pathways are involved in organogenesis, which is expected to be an active process at the eyed egg stage. The dominant effects are more largely due to the maternal line than to the origin of the stock. This finding is particularly relevant in the context of potential introgression between farmed and wild fish, since female escapees tend to have a higher spawning success rate compared to males.
Electronic supplementary material
The online version of this article (doi:10.1186/s12711-016-0200-6) contains supplementary material, which is available to authorized users.
Background
Atlantic salmon (Salmo salar L.) has been subject to domestication, including directional selection for economically important traits, since the aquaculture industry was first established in the early 1970s [1, 2]. These breeding programs, which now extend beyond 10 to 12 generations, have been very successful. For example, selection for growth rate, which was the primary target of all Atlantic salmon breeding programs, resulted in farmed fish that reach a body size that is 2 to 3 times larger than that of wild fish when reared under identical farming conditions [3–6]. However, economically important traits may not be beneficial under wild conditions, for example offspring survival is reduced in farmed salmon compared to the wild parental lines under natural conditions [7–10]. Given the magnitude of the phenotypic and genotypic differences between wild and farmed salmon, it is feasible to investigate how domestication in general, and selection for specific traits, have altered both the structure and expression of the Atlantic salmon genome.
The early stages of the life cycle of Atlantic salmon involve (1) hatching of eggs that are deposited in the gravel of rivers, (2) nesting of the sac fry in the gravel and feeding on their yolk-sac, (3) emergence of the sac fry from the gravel, which is a process known as swim-up, and finally (4) transition from endogenous to exogenous feeding. These critical developmental stages that are associated with high mortality play a major role in shaping the evolutionary trajectory of the individual and the population in general [9, 11, 12]. While numerous studies have investigated genetic differences between farmed and wild salmon, to date, relatively few studies have specifically focused on the critical early-life stages. Exceptions include studies on fertilization success rate [13], speed of embryonic development and growth prior to exogenous feeding [14–16], mortality in the wild [17], and gene transcription e.g. [18–20].
During the first phase of development, before the maternal-to-zygotic transition activates zygotic transcription, the embryo almost exclusively relies on maternal mRNA and proteins [21], and until the initiation of exogenous feeding, pre- and post-hatching embryos depend largely on maternally deposited yolk for energy provision [22]. Generally, the success rate of eggs from farmed fish is lower than that of eggs from wild fish, due to nominally suboptimal maternal resources [23], but these differences vary across species and time and can be reduced by improving fish husbandry, feed formulation and rearing conditions [24]. For example, the Atlantic cod aquaculture industry has yet to achieve optimal farming practices since the success rates of fertilization and hatching of eggs from farmed broodstock are significantly lower than those from wild broodstock [25]. In contrast, recent common garden studies have reported largely comparable fertilization (in vitro [13] and in-vivo [26]) and hatching success rates [16] between wild and domesticated Atlantic salmon stocks. The few detected differences were in egg size (which is indirectly affected by maternal body size) and hatching rate [16, 26]; these two factors are considered to be interlinked and to differ between any two given populations [27]. Although variability of these traits may affect success rate under natural conditions [9, 12], these parameters are not used, per se, to discriminate “high” from “low” quality eggs and embryos [23].
Salmonid maternal effects have been thoroughly investigated for easy-to-measure phenotypic traits, such as egg and fry size, which have a significant impact on early survival [9, 11, 12]. However, studies at the transcriptional level are scarce. Debes et al. [15] emphasized the fact that multi-generational genetic studies on salmonids rarely use reciprocal hybrids due to logistical constraints. Even when reciprocal hybrids are used, data are often averaged across hybrids, which tends to hide maternal effects. A previous study that explored transcriptional differences in the early stages of development between farmed and wild Atlantic salmon strains included only non-reciprocal hybrids that were generated by fertilizing domesticated eggs with wild milt [20]. Although this study documented dominant inheritance patterns in the F1 hybrids, the lack of fully reciprocal pedigrees precluded a further analysis of its primary source, i.e. domestication and/or maternal effects.
With the decreasing cost of broad-scale gene expression studies, transcriptomic profiling of fish embryos is starting to receive increased attention. Recently, researchers have started to investigate how gene expression varies during embryonic development [28–31] and have attempted to identify transcripts and markers associated with embryo quality [31, 32]. Renaut et al. [33] showed that gene expression in hybrid embryos is affected when divergent populations are crossed. In our study, we used a custom oligo-microarray as a tool to identify genes and gene pathways that display differential expression between embryos from wild and domesticated Atlantic salmon stocks that were reared under identical conditions. By including reciprocal hybrids in the experimental design, heritability patterns were assessed to specifically explore the relative importance of maternal versus domestication effects on embryonic gene expression.
Methods
Biological samples
This study used experimental crosses between (1) the domesticated Norwegian Mowi strain, which has been under directional selection for at least ten generations and for a range of economically important traits, and (2) wild brood fish that were collected from the River Figgjo in the south west of Norway. Numerous studies have investigated the characteristics of the Mowi strain [4–6, 9, 20, 34], and both strains are described in detail in [20].
The experiment was initiated on November 23, 2011 when gametes were stripped from four domesticated (Mowi) and four wild (Figgjo) salmon. Two independent sets of reciprocal crosses were established, each set using gametes from a pair of domesticated (D) and wild (W) parents to create four family combinations (i.e. pure wild, W × W; pure domesticated, D × D; and reciprocal hybrids W♀ × D♂ and D♀ × W♂). Fertilized eggs from each of the eight families were placed into individual family hatching trays under identical conditions. On February 2 2012, (approximately 410 days post-fertilisation), eyed ova from each family (n = 30) were sampled, transferred to RNA stabilisation buffer i.e. RNAlater (3.6 M ammonium sulfate, 18 mM sodium citrate, 15 mM EDTA, pH 5.2) and immediately pierced with a 25G syringe needle for rapid penetration of the preservative. After overnight incubation at 8 °C, the RNAlater solution was drained and the eggs were stored at −70 °C until RNA extraction.
The experiment was conducted in accordance with Norwegian regulations for the use of animals in research. No specific permit was required for this experiment because embryos were sampled prior to hatching.
RNA extraction and purification
Individual eyed eggs were homogenised in 1 mL Tri Reagent (Sigma-Aldrich®) using a Mini-Beadbeater-24 (BioSpec Products Inc.) and RNA was extracted following the manufacturer’s standard Tri Reagent protocol. RNA quantity and quality of individual embryos were assessed by spectrophotometry (NanoDrop ND-1000) and agarose gel electrophoresis, respectively. Each extraction yielded about 40 to 50 µg RNA. For each hybridisation sample (biological replicate), equal amounts of total RNA from eight individuals (four per family × two families) were pooled per reciprocal cross type (WW, DD, DW or WD) and then re-quantified and quality-assessed as described above (Fig. 1).
Fig. 1.
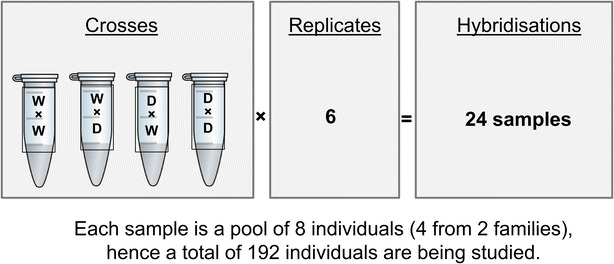
Schematic representation of the experimental design
Microarray experimental design
Microarray analysis was performed using a custom oligonucleotide microarray platform (Agilent) that included 4 × 44 K probes per slide (Salar3; ArrayExpress Accession number A-MEXP-2400). The general design of the microarray is described in [35] and was validated in subsequent studies e.g. [20, 35–38].
Dual-label hybridisations were undertaken, i.e. each experimental sample (Cy3-labelled) was competitively hybridised against a pooled reference control (Cy5-labelled) that included equimolar amounts from each experimental RNA sample. Thus, each experimental sample was assessed relative to a single common sample, which allowed a full range of comparisons between different states. The interrogations involved 24 separate hybridisations i.e. four reciprocal cross types (W♀ × W♂, D♀ × W♂; W♀ × D♂, D♀ × D♂) × six biological replicates (each replicate containing RNA from eight different individuals; four each from two families) (Fig. 1).
RNA amplification and labelling
RNA from each biological replicate (pool of eight individuals) was amplified (TargetAmp™ 1-Round Aminoallyl-aRNA Amplification Kit, Epicentre Technologies Corporation) according to the manufacturer’s instructions. Following quality control (Nanodrop quantification and agarose gel electrophoresis), amplified RNA fragments (aRNA) were indirectly fluorescently labelled and purified. Briefly, dye suspensions (Cy3 and Cy5) in sufficient quantity for all labelling reactions were prepared by adding 42 µL of high-purity dimethyl sulfoxide (Stratagene) per tube of Cy dye (PA23001 or PA25001; GE HealthCare). Individual amplified samples (2.5 µL aRNA in 10.5 µL H2O) were denatured at 75 °C for 5 min, and then 3 µL 0.5 M NaHCO3 at pH 8.5 and 1.5 µL Cy3 dye were added. The common reference pool was labelled in the same way, but it was prepared in a single large-scale reaction i.e. 50 µg of pooled aRNA in 210 µL H2O were heat-denatured and then 60 µL 0.5 M NaHCO3 at pH8.5 and 20 µL Cy5 dye were added. All samples were incubated for 1 h at 25 °C in the dark, and purified through Illustra AutoSeq G-50 Dye Terminator columns (Qiagen). Dye incorporation and purity of all reactions were assessed spectrophotometrically (NanoDrop) and the products were also visualised on a fluorescent scanner (Typhoon Trio, GE Healthcare).
Microarray hybridisation and quality filtering
All hybridisations were performed at the same time using the Agilent Gene Expression Hybridisation Reagent Kit (Agilent Technologies) according to the manufacturer’s instructions. For each reaction, 825 ng of Cy5-labelled RNA reference pool and 825 ng of a Cy3-labelled RNA test sample were combined in 35 µL of H2O to which 20 µL fragmentation master mix were added (11 µL of 10 × blocking agent, 2 µL 25× fragmentation buffer and 7 µL H2O). The reactions were then incubated at 60 °C in the dark for 30 min, chilled on ice, and mixed with 55 µL of 2× GEx hybridisation buffer (pre heated at 37 °C). Following centrifugation (18,000g for 1 min), the samples were kept on ice until they were loaded (103 µL) onto the microarray slides (four arrays per slide). Samples from the six biological replicates were distributed across different slides. Hybridisation was carried out in a rotating rack oven (Agilent Technologies) at 65 °C, 10 rpm for 17 h.
Following hybridisation, the microarray slides were washed in Easy-DipTM slide staining containers (Canemco Inc.). First, a 1-min incubation at room temperature (approximately 20 °C) in Wash Buffer 1 was performed, with gentle shaking at 150 rpm (Stuart Orbital Incubator). Slides were then briefly dipped into Wash Buffer 1 pre-heated at 31 °C and placed into Wash Buffer 2 (31 °C) for 1 min with gentle shaking at 150 rpm. Finally, the slides were transferred to acetonitrile for 10 s and finally incubated in the stabilization and drying solution (Agilent) during 30 s. The slides were then air dried and scanned within 3 h.
Slides were scanned at 5 μm resolution on an Axon GenePix Pro scanner at 70 % laser power. The “auto PMT” function was set to adjust PMT for each channel such that <0.05 % of the features were saturated and the mean intensity ratio of the Cy3:Cy5 signals was close to 1. We used the Agilent Feature Extraction Software (v 9.5) to identify features and extract background subtracted raw intensity values that were then transferred to the GeneSpring GX (version 13) software [39] to perform quality filtering and normalisation steps. Intensity values less than 1 were adjusted to 1 and a Lowess normalisation was carried out. Stringent quality filtering ensured that features that represented technical controls, saturated probes, probe population outliers or probes which were not significantly different from the background (based on a two-sided t test implemented in the Feature Extraction software) were removed. Finally, probes were retained if they were positive and significant in at least 75 % of the arrays in any two of the experimental groups. As a result, 31,491 probes passed quality control and were analysed further.
Details of the microarray experiment were submitted to ArrayExpress under accession number E-MTAB-3677. The recording of the microarray experimental metadata complies with the MIAME (minimum information about a microarray experiment guidelines).
Microarray data analysis
Statistical analysis (T test and ANOVA) was performed by using the GeneSpring software (version 13), whereas the R software [40] was used for functional analysis (GAGE) and preparing graphs. Details of each analysis are provided below. To minimize repeat counting of the same gene, only transcripts that had BLAST [41] and/or KEGG annotations [42] were considered in the downstream analysis, and when multiple probes were present for the same gene, the probe with the lowest p value was chosen.
Functional analysis of the genetic differences between the offspring of wild and domesticated pure stocks was performed via the gage function of the GAGE (generally applicable gene-set/pathway analysis) package [43]. Gene-set tests establish correlations between functional groups and phenotype by detecting small but coordinated changes in gene expression [43]. Pairwise comparisons between replicates from domesticated fish embryos versus the average values for wild fish (‘1ongroup’ comparison) were performed and, as generally applied, results were considered significant if the corrected p value was >0.1. For ease of visualization and a more focused interpretation, pathways that were perturbed in both directions (2d) i.e. transcripts that were not restricted in terms of their direction of change, were further filtered by applying a p value cut off of 0.02. For a default (p ≤ 0.1) 2d pathway list, see Table S1 (see Additional file 1: Table S1). Since pathways that belong to the human disease functional group are particularly difficult to interpret in fish, this group was excluded from the gene-enrichment analysis. Significant pathways were further explored using the essGene function [43] to identify key genes. We used the package ggplot2 [44] to graphically represent the transcripts that were included in significantly perturbed pathways i.e. that varied by more than one standard deviation (SD) from the mean of all transcripts and differed significantly between domesticated and wild strains (t test unpaired unequal variance, p ≤ 0.05). When transcripts were represented in multiple KEGG groups, they were assigned the function for which the largest number of gene associations was found in the complete list.
To identify differentially-expressed transcripts between embryos of domesticated and wild salmon stocks, we performed a T test (unpaired unequal variance, Benjamini–Hochberg multiple-testing correction, corrected p ≤ 0.05) and applied a fold change filter ≥1.25 (see Additional file 1: Table S2) for details. Following hierarchical clustering (Pearson correlation), expression profiles of unique differentially-expressed transcripts between the two stocks were visualized as heatmaps (gplots package [45]).
To explore the heritability of differentially-expressed genes between stocks, one-way ANOVA (unequal variance) was performed with an FDR of 10 % (Benjamini–Hochberg) and Student Newman–Keuls (SNK) post hoc analysis. Differentially-expressed transcripts were assigned to the following categories of heritability:
Maternal effect: differentially-expressed transcripts that were identified between W♀ × W♂ versus D♀ × W♂ or D♀ × D♂ or W♀ × D♂;
Paternal effect: differentially-expressed transcripts that were identified between W♀ × W♂ versus W♀ × D♂ or D♀ × D♂ or D♀ × W♂;
Parental effect: differentially-expressed transcripts that were influenced by both maternal and paternal effects;
Maternal effect only: differentially-expressed transcripts that were influenced by maternal effects only;
Paternal effect only: differentially-expressed transcripts that were influenced by paternal effects only.
For normalised intensity values (ni) of the unique differentially-expressed genes: α = additivity = (Wni − Dni)/2 and δ = dominance = ((Wni + Dni)/2) − hybridni were calculated. The values for α and δ/α were plotted using the ggplot2 package [44]. A transcript with a level of gene expression in the hybrid that was midway between that for the parents had an additive effect (perfect additivity: δ/α = 0). A transcript with a level of gene expression in the hybrid that was close to that of one of the two parents had rather a dominant effect (domesticated dominance, δ/α = 1; wild dominance, δ/α = −1). Group memberships were assigned as follows by dividing the intervals into two parts:
additivity, if −0.5 < δ/α < 0.5;
wild dominance, if −1.5 < δ/α < −0.5;
domesticated dominance, if 0.5 < δ/α < 1.5;
over-dominance, if δ/α fell outside the interval between −1.5 and 1.5.
For ease of interpretation of the plots, genes with a δ/α above 5 were excluded from the scatter graph but were considered in the table on heritabilities.
Results
Functional analysis
For the functional analysis, KEGG annotation (KO) was used. Approximately 62 % of the probes that passed quality filtering were assigned KO numbers and about 31 % of these returned unique annotations. Hence, 6037 genes were included in the gene-set enrichment analysis, which revealed a range of pathways with significant differential gene expression between embryos of wild and domesticated salmon (Table 1). The ECM-receptor interactions pathway was down-regulated in the domesticated fish embryos compared to the wild fish embryos, whereas pathways that are involved in genetic information processing and metabolism functions were up-regulated. Genetic information processing-related pathways play a role in mRNA translation, whereas metabolism-related pathways are associated with carbohydrate, lipid and energy metabolism. In addition, the most significant two-way perturbed pathways were related to environmental information processing; cell signalling, in particular, and organismal systems; including digestive, immune and nervous systems. Most differentially-expressed transcripts and major contributors to these significant pathways were members of signal transduction pathways (Fig. 2). Other KEGG functional groups that displayed more than ten differentially-expressed genes included the immune system, cell communication and signalling molecules and interaction. There was considerable gene overlap between these groups, for details (see Additional file 1: Table S3).
Table 1.
Differentially-expressed pathways in domesticated versus wild embryos
| KEGG functional group | KEGG sub-group | KEGG pathway | p value | Direction of perturbation | Sac fry [20] | Feeding fry [20] |
|---|---|---|---|---|---|---|
| Cellular processes | Cell communication | Focal adhesion | 0.00051 | Two-way perturbed | ||
| Gap junction | 0.00036 | Two-way perturbed | ||||
| Environmental information processing | Signal transduction | Hippo signaling pathway | 0.00040 | Two-way perturbed | Up-regulated | |
| MAPK signaling pathway | 0.00101 | Two-way perturbed | ||||
| NF-kappa B signaling pathway | 0.00021 | Two-way perturbed | Down-regulated | Down-regulated | ||
| Wnt signaling pathway | 0.00213 | Two-way perturbed | Up-regulated | |||
| Signaling molecules and interaction | Cell adhesion molecules (CAM) | 0.00069/0.00144 | Up-regulated/two-way perturbed | |||
| Cytokine-cytokine receptor interaction | <0.00001 | Two-way perturbed | Down-regulated | |||
| Neuroactive ligand-receptor interaction | 0.00001 | Two-way perturbed | Two way perturbed | Down-regulated | ||
| ECM-receptor interaction | 0.00016 | Down-regulated | Up-regulated | |||
| Organismal systems | Circulatory system | Vascular smooth muscle contraction | 0.00032 | Two-way perturbed | ||
| Development | Osteoclast differentiation | 0.00019 | Two-way perturbed | Two way perturbed | ||
| Digestive system | Mineral absorption | 0.00011 | Up-regulated | |||
| Pancreatic secretion | 0.00164 | Two-way perturbed | Up-regulated | |||
| Salivary secretion | 0.00117 | Two-way perturbed | ||||
| Endocrine system | GnRH signaling pathway | 0.00014 | Two-way perturbed | |||
| Immune system | Chemokine signaling pathway | 0.00017 | Two-way perturbed | Down-regulated | Down-regulated | |
| Fc epsilon RI signaling | 0.00026 | Two-way perturbed | Down-regulated | Down-regulated | ||
| Natural killer cell mediated cytotoxicity | 0.00004 | Two-way perturbed | ||||
| T cell receptor signaling pathway | 0.00002 | Two-way perturbed | ||||
| Nervous system | Glutamatergic synapse | 0.00154 | Two-way perturbed | Down-regulated | ||
| Long-term potentiation | 0.00001 | Two-way perturbed | ||||
| Genetic information processing | Translation | Ribosome | 0.00383 | Up-regulated | Up-regulated | |
| RNA transport | 0.00174 | Up-regulated | Up-regulated | |||
| Metabolism | Carbohydrate metabolism | Fructose and mannose metabolism | 0.00183 | Up-regulated | ||
| Galactose metabolism | 0.00168 | Up-regulated | ||||
| Energy metabolism | Carbon fixation in photosynthetic organisms | 0.00494 | Up-regulated | Up-regulated | ||
| Glycan biosynthesis and metabolism | Glycosphingolipid biosynthesis—lacto and neolacto series | 0.00316 | Up-regulated | |||
| Lipid metabolism | Sphingolipid metabolism | 0.00229 | Up-regulated |
KEGG based functional representation of the pathways differentially perturbed between wild and domesticated embryos and their significance in a previous study conducted on sac and feeding fry
Fig. 2.
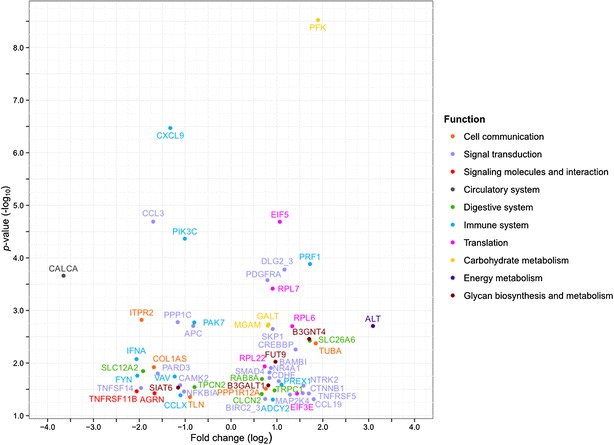
Key genes of the perturbed pathways. Differentially-expressed genes (T test p ≤ 0.05) between wild and domesticated embryos and identified as essential for the pathways perturbed between pure stocks (Table 1). Genes are plotted according to log2 fold change (domesticated vs. wild) and −log10 p value (T test), and color-coded by biological function. The list of plotted genes and values are included in Table S4 (see Additional file 1: Table S4)
Expression profiling
T tests identified 165 transcripts that showed significantly different gene expression levels between embryos of domesticated and wild salmon stocks and corresponded to 123 unique annotated transcripts. Hierarchical clustering of the differences in gene expression revealed both additive and dominant behaviours (Figs. 3, 4). The clusters with the most pronounced behaviour were related to maternal influence, such as the bottom cluster in Fig. 3 and the top cluster in Fig. 4, which both contain several cytochrome-related genes.
Fig. 3.
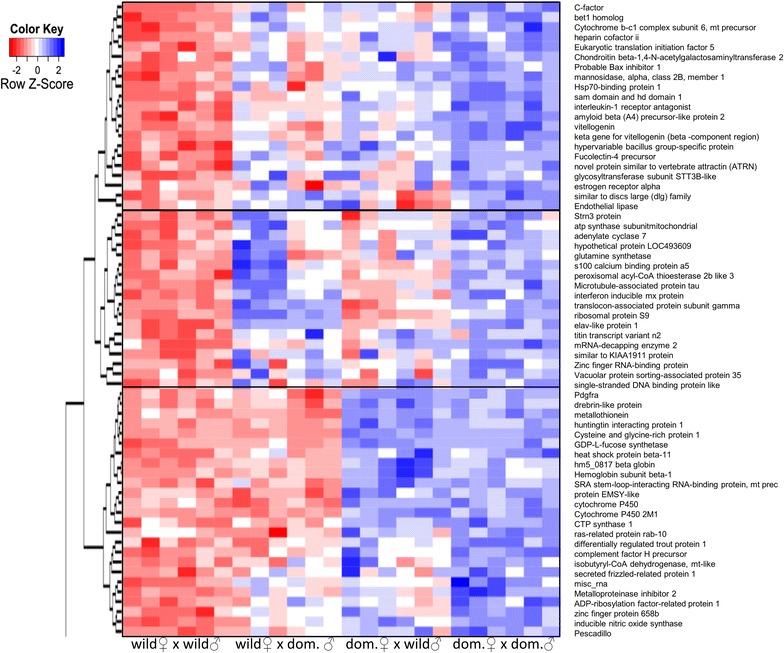
Up-regulated differentially expressed transcripts. Hierarchical clustering of the expression profiles of unique transcripts up-regulated in domesticated embryos compared to wild embryos
Fig. 4.
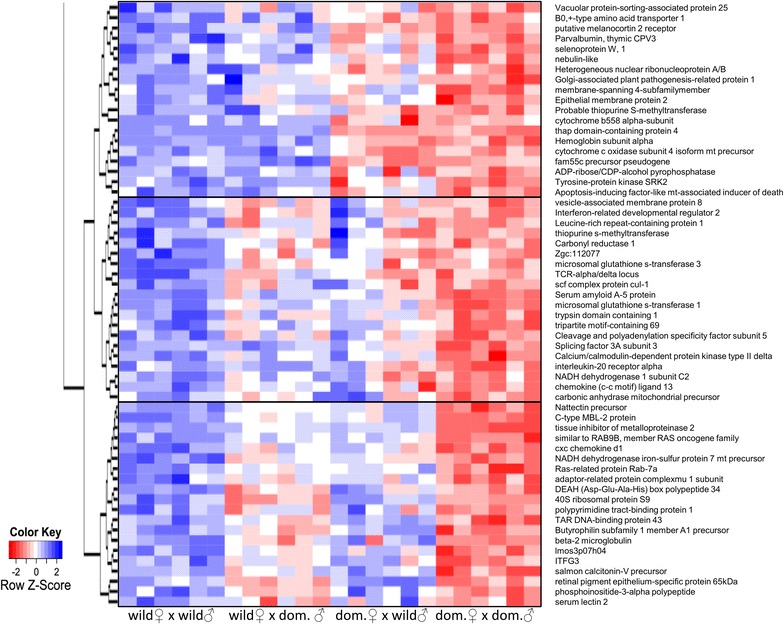
Down-regulated differentially expressed transcripts. Hierarchical clustering of the expression profiles of unique transcripts down-regulated in domesticated embryos compared to wild embryos
Heritability analyses
To further investigate the significance of parental effects that were revealed by expression profiling, additive and dominance parameters were calculated and plotted (Table 2; Fig. 5). Among the 208 transcripts that showed differential expression between the four experimental groups by one-way ANOVA, only two were significantly different between the pure crosses and were not considered further. There were no observed differences between hybrid × hybrid crosses that were not also seen between hybrid × pure crosses (Fig. 6).
Table 2.
Proportions of differentially-expressed genes that display various inheritance patterns
| Life stage | Hybrid type | Unique genes | Wild overdominant (%) | Wild dominant (%) | Additive (%) | Domesticated dominant (%) | Domesticated overdominant (%) | Experiment |
|---|---|---|---|---|---|---|---|---|
| Embryo | wild♀ × domesticated♂ | 162 | 18.5 | 27.8 | 33.3 | 14.2 | 6.2 | Current study |
| Embryo | domesticated♀ × wild♂ | 156 | 4.5 | 9.0 | 42.3 | 23.1 | 21.2 | Current study |
| Fry | domesticated♀ × wild♂ | 25 | 0.0 | 0.0 | 48.0 | 52 | 0.0 | [20] |
| Feeding fry | domesticated♀ × wild♂ | 313 | 1.6 | 6.1 | 45.0 | 42.2 | 5.1 | [20] |
Based on a heritability analysis of the differentially expressed genes and a comparison of the inheritance patterns to a previous study conducted in sac and feeding fry. For explanation of the various categories see the “Methods” section
Fig. 5.
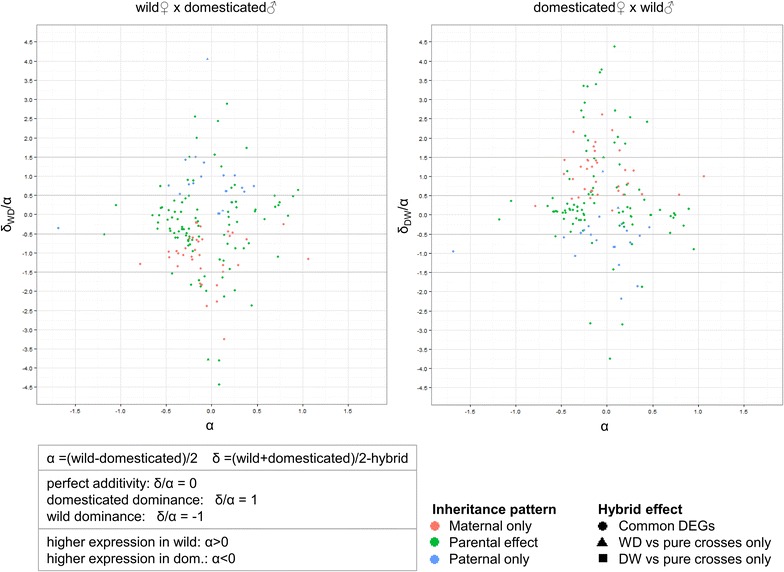
Heritability predictions of the differentially-expressed genes between the two hybrid stocks. DEG differentially-expressed gene, WD wild♀ × domesticated♂, DW domesticated♀ × wild♂
Fig. 6.
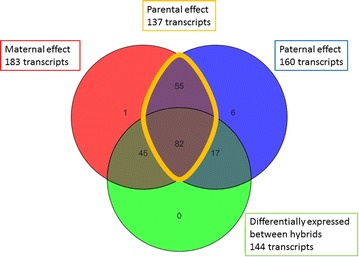
Number of transcripts differentially expressed between stocks and their inheritance pattern. Differences observed between hybrid and pure crosses are categorized as influenced by maternal, paternal or parental effects (see “Methods” for details). The number of differentially-expressed transcripts identified between hybrid crosses is also shown
The remaining 206 differentially-expressed transcripts corresponded to 165 unique genes that were further analysed. The vast majority of the differences (153 genes) were shared by both hybrid crosses, whereas nine and three additional genes were unique to either the W♀ × D♂ or D♀ × W♂ hybrids, respectively. For the reciprocal hybrids, most transcripts displayed either an intermediate level of expression (33.3 and 42.3 %) or dominance versus over-dominance (27.8 vs. 18.5 % and 23.1 vs. 21.2 %, for the reciprocal hybrids, respectively), which was in favour of a maternal effect (Table 2). However, W♀ × D♂ hybrids showed a stronger combined (wild or domesticated dominance) dominance effect (42 vs. 32.1 %) and a weaker additive effect (33.3 vs. 42.3 %) compared to D♀ × W♂ hybrids.
Since most of the transcriptomic differences detected between the two stocks were shared by both reciprocal hybrids, expression levels of the transcripts for the hybrids and the pure crosses were compared to determine whether these were primarily influenced by domestication or parental line factors. Visualisation of the dominance behaviour (Fig. 5) showed that most transcripts that were differentially expressed between the two stocks were either additive or maternally-dominant. For ease of visualization, 15 over-dominant genes were excluded from the scatterplot (Fig. 5), due to large δ/α.
Discussion
In the literature, the first comparisons of genome-wide gene transcription of Atlantic salmon fry based on microarray data reported that five to seven generations of domestication and selection had induced heritable changes of gene expression in cultured relative to wild fish [18, 19]. Differences were observed in common pathways but did not necessarily involve the same genes within a given pathway, which is referred to as differences in ‘genetic architecture’ between stocks. A more recent study [20] demonstrated that whereas common differences could be observed between different life stages, some of the affected key pathways were stage-dependent. Since the experimental designs of these earlier studies included the analysis of D♀ × W♂ hybrids only, it was not possible to distinguish dominant parental effects from domestication effects.
Our study aimed at expanding existing knowledge on transcriptomic differences between wild and domesticated Atlantic salmon by investigating embryos for the first time, and using reciprocal hybrids to help dissect parental effects from the effects of domestication. Focusing on early-life stages has also the benefit of minimizing environmental effects and the impact of early-growth divergence on the transcriptome due to the fact that farmed salmon outgrow wild salmon by up to threefold by 4 months into feeding [5]. However, this approach has several inherent limitations. The microarray analysis is restricted to the set of preselected probes on the platform. Analysis of transcriptomes for whole embryos is likely to be relatively insensitive to differences in tissue-specific transcript expression, especially from smaller organs and low abundance cell types. For example, cellular signalling occurs in all cells regardless of the tissue of origin and, thus, the members of these pathways are expressed across all organs [46]. Organism-wide expression of cell signalling genes may provide support for the detection of this function when gene expression of whole individuals is studied. It is also important to bear in mind that since our study used only one wild and one domesticated salmon stock, some of the observed differences may be specific to these stocks and not necessarily reflect a domestication effect per se.
Overall, we identified pathways that are related with metabolic, immune and nervous system, genetic and environmental information processing functions for which altered gene expression was observed between the wild and domesticated Atlantic salmon embryos that were studied here. Our findings build on earlier studies of whole-animal transcriptomic responses to domestication in a number of fish species and for different life stages [18–20, 31, 32]. In the next section, we examine in more detail some of the key pathways that we identified, in order to set the observed differences in a biological context.
Domestication is a form of adaptation
Domestication is possible because “organic beings” have the ability to adapt to the changing environment, which is imposed on them [47]. As such, one would expect that the biological pathways that are relevant to adaptation to a farm environment would be differentially expressed between wild and domesticated fish. Cell signalling mediates responses to internal and external environmental cues and therefore may be affected by domestication. Cell signalling is involved in the control of the basal level of cell replication, differentiation and apoptosis and the regulation of metabolic events, including the ability to receive signals and to respond to constantly altered physiological requirements. Such control is achieved through the action of three broad classes of signalling molecules: neurotransmitter substances, hormones and cytokines or growth factors [46]. In lower vertebrates, such as fish, cytokines and neuropeptides play roles both in the neuroendocrine and immune systems, including responses to stress [48, 49]. It has been suggested that the fish domestication process involves increased selection pressure on genes and pathways which contribute to improved tolerance to acute and chronic stress, since individuals that perform better under farm conditions are more likely to be selected for broodstock [5, 50]. As a key mediator of the stress response, modulation of cellular signalling is expected to play a role in the process of domestication as clearly shown in our study by the detection of differential expression of stress-associated nervous and endocrine pathways between the two fish stocks. In particular, we found that the glutamatergic synapse pathway differed between the two fish stocks, as previously reported between wild and domesticated Atlantic salmon fry [20]. Changes in this pathway are known to be associated with domestication in pigs and the expression of glutamate receptors that affect the neural control of eating behaviour is linked to tameness [51]. In addition, two other pathways that are linked with domestication in birds [52] were highlighted in our study, i.e. long-term potentiation, which has a role in memory consolidation [53] and GnRH signaling, which is a master regulator of vertebrate reproduction [54].
Potential trade-off between immune function and growth
In addition to pathways that are involved in the adaptation to a farm environment, domestication may also affect pathways, which enhance farm traits that are important to broodstock selection, in particular increased growth.
Up-regulated mRNA translation
In addition to its role in the response to stress, the mitogen activated protein kinase (MAPK) signalling pathway also regulates mRNA translation and classical MAPK signalling promotes protein synthesis [55]. Thus, selection for improved growth traits in domesticated fish may explain why MAPK signalling pathways were enriched in our analysis. Previous studies have reported that MAPK signalling pathways are also involved in the domestication process of birds [52] and mammals [56–58]. Up-regulation of ribosome and RNA transport pathways in domesticated salmon embryos may also reflect processes which can enhance growth. Ribosomes are the site of protein synthesis, which is principally regulated at the initiation stage of translation, thus allowing plasticity of expression. The differential expression of translation initiation factors 3E and 5and large ribosomal subunits 6 and 7 that we identified in our study, are also involved in the regulation of protein synthesis [59]. Previous studies have reported that genes that affect protein synthesis, and hence growth, are over-represented in comparisons of expression levels of transcripts between wild and domesticated salmonid stocks [18, 20, 60–62]. By comparing fast- and slow-growing rainbow trout lines (Oncorhynchus mykiss), Xu et al. [29] concluded that up-regulation of genes that affect protein synthesis occurred at stages that corresponded to the earlier onsets of developmental processes in fast-growing families, i.e. as early as 15 days post-fertilisation.
Up-regulated metabolic pathways
During early development, the embryo relies on yolk sac-derived nutrients to sustain its growth and survival. These include yolk lipids, which are the source of essential fat-soluble vitamins and triacylglycerol, and cholesterol, which is a required component of cell signalling molecules, membrane components, and sources of fuel [63]. Many of the digestive functions that occur in hatched fry are known to be active in embryos [64], particularly after the eyeing stage as examined in the current study. Several pathways that impact lipid, carbohydrate and energy metabolism functions were up-regulated in the domesticated Atlantic salmon embryos compared to the wild embryos. These findings mirror those reported for feeding fry from the same two stocks [20], although the specific pathways differed. This possibly reflects differences in the processes for metabolizing yolk deposits and external food. For example, carbohydrate metabolism pathways that are differentially expressed between wild and domesticated embryos are involved in fructose, mannose and galactosemetabolism, whereas glycolysis/gluconeogenesis and propanoate metabolism pathways were identified in the feeding fry life-stage. Sphingolipid metabolism was detected as a differentially-expressed lipid metabolism pathway in the embryo stage, whereas fatty acid degradation and elongation and glycerolipid metabolism were differentially perturbed in the feeding fry stage. Indeed, activation of the glycolytic and fatty acid pathways is associated with gene expression changes that occur during the transition from endogenous to exogenous feeding of fish [65].
Down-regulation of immune pathways
Cell signalling is particularly important during embryonic development [66] and the reciprocal gene regulation in both directions is characteristic of these regulatory pathways [43]. Major overlaps between members of signalling and immune pathways may mask the direction of change of immune pathways. For this reason, the expression of some key genes was investigated, including representatives of different groups of cytokines i.e.: four chemokines (CCL and CXCL), three tumour necrosis factor (TNF) ligands/receptors, and an interferon α (IFN-α). Most of these genes had a lower level of expression in domesticated embryos than in their wild counterparts. Chemokines and TNF play a pivotal role in immune function, but some members are also involved in stress responses and developmental processes [67–69]. It was previously suggested that domestication in salmonids may have resulted in immunosuppression, due to a trade-off between growth and immune functions [3]. In addition, since domesticated fish generally display a higher tolerance to stress, immune genes may have been collaterally selected during domestication [5, 50].
Two cytokines, i.e. C–C motif chemokine 19 (CCL19) and TNFR superfamily member 5 (TNFRSF5) are not down-regulated in domesticated versus wild embryo salmon, contrary to the behaviour expected based on the above argument. CCL19 is referred to as a homeostatic or dual function chemokine [70] and has a role in the formation of the embryonic axis in zebrafish [71]. Hence, it may have a more important role in developmental functions than in immune functions. TNFRSF5 does not play a role in any of the significantly differentially-expressed immune pathways and was detected only in signalling pathways. It should be noted that, interferon regulatory factor 7 (IRF7), a transcription factor that is known to regulate IFN-α genes [72] and which, in our study, was down-regulated in the domesticated salmon embryos, has been proposed as a marker for assessing egg quality in Atlantic halibut (Hippoglossus hippoglossus) and is associated with hatching success [31].
Organogenesis
Two cell communication pathways and the cell adhesion molecules pathways were differentially expressed between wild and domesticated Atlantic salmon embryos but not in yolk-sac fry or feeding fry [20], which probably reflects life-stage specific differences between stocks. These and several other differentially-expressed signalling pathways identified in our study (but not necessarily unique to embryos), are all known to participate in organ development. For example, the Hippo signalling pathway, which was differentially expressed between wild and domesticated strains, is involved in determining organ size and mediates crosstalk between other pathways [73]. NF-KB/IKB proteins, in addition to their immune function, are vital for organogenesis, e.g. zebrafish notochord development [74]. The wnt signalling pathway which is responsible for tissue morphogenesis, is up-regulated in domesticated Atlantic salmon sac fry [20]. According to Steinberg’s differential adhesion hypothesis, the basis of organ self-assembly is the segregation of cells with similar adhesive properties to achieve the most thermodynamically-stable pattern [75]. Thus, WNT proteins and cellular communication/cell adhesion pathways are closely linked [76] and we found that they were differentially expressed between wild and domesticated Atlantic salmon embryos. Sphingolipids, and their more complex, glycosylated derivatives, glycosphingolipids, as well as being components of cell membranes are also involved in cell signalling and adhesion [77]. In line with this, glycan and lipid metabolism pathways were up-regulated in the domesticated salmon embryos. The epithelial–mesenchymal transition (EMT) is a process during which tightly adjoined basal polarity epithelial cells acquire migratory mesenchymal properties [78]. This process involves most of the differentially-expressed signalling and cellular communication pathways identified in our study, including MAPK, NF-kappa B, and wnt signalling, cytokine–cytokine receptor interactions, ECM-receptor interactions, cell and focal adhesion and gap junction. The role of the epithelial–mesenchymal transition during development has an effect on organ development and neural crest cell migration [79]. Although changes that occur during neural crest development through domestication may provide an explanation for some of the shared similarities between domesticated species [80], its role in organ development fits the sampling timeline better. Sampling took place after eyeing of embryos, which occurs in the last third of embryogenesis. This phase of development is characterized by organogenesis, with the appearance of fins and the formation of the internal organs and circulatory system. Eyeing occurs in stage 24 of the development of salmons, whereas the neural tube is considered to be formed by stage 14 [64, 81].
Parental effects on gene expression
For genes that are significantly differentially expressed between pure crosses, gene expression in the hybrids ranged from intermediate to fully polarized expression towards one or the other parent. Hierarchical clustering revealed that the behaviour of a number of genes in the hybrids reflected that of the maternal parent (wild or farmed). Within this group, there was a high abundance of cytochrome-related genes, which are involved in oxidative phosphorylation (mitochondrial subunit/precursors of the cytochrome b-c1 complex subunit 6 and cytochrome c oxidase subunit 4 isoform, NADH dehydrogenase 1 subunit C2 and NADH dehydrogenase iron-sulfur protein 7 and an ATP synthase) and the metabolism of xenobiotics (microsomal glutathione s-transferase 1 and 3 and cytochrome P450, family 2, subfamily D). Previous studies have reported that these processes have been affected by domestication in a number of fish species including brook charr (Salvelinus fontinalis), Atlantic salmon and Atlantic cod (Gadus morhua) [32, 61, 82]. Crockford [83–85] proposed that domestication is the product of heterochrony, i.e. changes in developmental rates and/or timing that are induced by thyroid hormone-induced oxidative reaction and metabolism rates, to which carbohydrates and lipids contribute. Two haemoglobin subunits were also differentially regulated between wild and domesticated Atlantic salmon embryos and clustered with genes that showed maternal influence (Figs. 2, 3). Previously, haemoglobin genes were also shown to be differentially regulated between multiple wild and domesticated brook charr reciprocal hybrids, which suggests consistent parental effects [86].
Maternal effects are known to be particularly frequent during the embryonic stage of fish [21, 22] but there is also a growing body of evidence for paternal effects [87]. In our study, most of the transcripts that were differentially expressed between a hybrid and a pure cross were common to both reciprocals. These shared differences were more likely to show dominance with respect to the origin of the mother rather than the origin of the stock, which indicates maternal dominance. We observed that differential expression of wild♀ × domesticated♂ hybrids showed a slightly higher combined dominance (42 vs. 32.1 %) and lower additivity (33.3 vs. 42.3 %) than domesticated♀ × wild♂ hybrids. In line with these results, Bougas et al. [88] highlighted the relevance of additivity (54.3 %) and the importance of maternal effects (40 %) when comparing the inheritance of gene expression of wild-domesticated brook charr hybrids.
Implications for interactions between wild and farmed salmonids
Fish escaping from commercial farms and subsequent genetic interactions with wild conspecifics represent a major environmental challenge to a sustainable Atlantic salmon aquaculture industry [89]. Each year, hundreds of thousands of farmed Atlantic salmon escape into the wild. Although many of these remain unaccounted for, a significant number do enter rivers [90, 91] and interbreeding between wild and farmed salmon that lead to genetic changes of the wild populations has been reported in Ireland and Norway [92–96]. This has caused significant international concern over the long-term fitness of wild populations, given that wild salmon populations may display local adaptations to the rivers they inhabit [97], and that the offspring of farmed salmon show reduced survival in the wild compared to the offspring of wild salmon [7–9, 98]. The transcriptomic differences that were identified in our study may reflect the influence of different selection pressures acting on wild and domesticated Atlantic salmon populations. Adaptation to a farm environment is unlikely to be advantageous under natural conditions. The high prevalence of maternal effects is of particular concern, since domesticated females are more likely to contribute to gene flow from farm escapees than males [99].
Conclusions
Our findings document the differential expression of gene pathways between Atlantic salmon eyed embryos of wild and domesticated origin, which were fertilised and incubated under identical conditions. The data indicate that even at this early developmental stage, transcriptional differences between the stocks exist and affect pathways that are relevant to wild and domesticated environments. By analysing the data from reciprocal hybrids, the potential significance of maternal effects in wild × domesticated hybrids and the relatively high percentage of over-dominant gene expression, which may be typical of the embryo stage, were highlighted. In order to draw more general conclusions regarding the outcome of the genetic interactions between wild and domesticated fish, more evidence is required from future studies on multiple strains, rather than single strains as was the case here.
Authors’ contributions
BB performed the experimental sampling, carried out the lab work, data analysis and wrote the first draft of the manuscript. KAG organized the production and maintenance of the crosses. All authors contributed to the conception of the study, experimental design and revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was financed by the Norwegian Research Council project INTERACT. We would like to thank Lise Dyrhovden, Ivar Helge Matre and Dr. Monica F. Solberg for their assistance in producing the fish which used in this study. The authors also acknowledge the support of the MASTS pooling initiative (The Marine Alliance for Science and Technology for Scotland) in the completion of this study. MASTS is funded by the Scottish Funding Council (Grant Reference HR09011) and contributing institutions.
Competing interests
The authors declare that they have no competing interests.
Additional file
10.1186/s12711-016-0200-6 List of differentially-expressed 2d pathways. The complete list of differentially-expressed 2d pathways that were identified by an adjusted p ≤ 0.1. Table S2. List of differentially-expressed transcripts. Details of the transcripts identified as differentially expressed between wild and domesticated embryos. Table S3. Essential genes of the differentially-expressed pathways. Gene members of pathways that contribute most to their differential expression. Table S4. Key genes of the differentially-expressed pathways.
Contributor Information
Beatrix Bicskei, Email: bicskei.beatrix@hotmail.co.uk.
John B. Taggart, Email: j.b.taggart@stir.ac.uk
Kevin A. Glover, Email: kevin.glover@imr.no
James E. Bron, Email: j.e.bron@stir.ac.uk
References
- 1.Gjedrem T. Possibilities for genetic gain in salmonids. Aquaculture. 1975;6:23–29. doi: 10.1016/0044-8486(75)90087-3. [DOI] [Google Scholar]
- 2.Gjedrem T. The first family-based breeding program in aquaculture. Rev Aquac. 2010;2:2–15. doi: 10.1111/j.1753-5131.2010.01011.x. [DOI] [Google Scholar]
- 3.Glover KA, Bergh Ø, Rudra H, Skaala Ø. Juvenile growth and susceptibility to Aeromonas salmonicida subsp. salmonicida in Atlantic salmon (Salmo salar L.) of farmed, hybrid and wild parentage. Aquaculture. 2006;254:72–81. doi: 10.1016/j.aquaculture.2005.10.040. [DOI] [Google Scholar]
- 4.Glover KA, Ottera H, Olsen RE, Slinde E, Taranger GL, Skaala O. A comparison of farmed, wild and hybrid Atlantic salmon (Salmo salar L.) reared under farming conditions. Aquaculture. 2009;286:203–210. doi: 10.1016/j.aquaculture.2008.09.023. [DOI] [Google Scholar]
- 5.Solberg MF, Skaala Ø, Nilsen F, Glover KA. Does domestication cause changes in growth reaction norms? A study of farmed, wild and hybrid Atlantic salmon families exposed to environmental stress. PLoS One. 2013;8:e54469. doi: 10.1371/journal.pone.0054469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solberg MF, Zhang Z, Nilsen F, Glover KA. Growth reaction norms of domesticated, wild and hybrid Atlantic salmon families in response to differing social and physical environments. BMC Evol Biol. 2013;13:234. doi: 10.1186/1471-2148-13-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming IA, Hindar K, Mjølnerød IB, Jonsson B, Balstad T, Lamberg A. Lifetime success and interactions of farm salmon invading a native population. Proc Biol Sci. 2000;267:1517–1523. doi: 10.1098/rspb.2000.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGinnity P, Prodöhl P, Ferguson A, Hynes R, Maoiléidigh NO, Baker N, et al. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc Biol Sci. 2003;270:2443–2450. doi: 10.1098/rspb.2003.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skaala Ø, Glover KA, Barlaup BT, Svåsand T, Besnier F, Hansen MM, et al. Performance of farmed, hybrid, and wild Atlantic salmon (Salmo salar) families in a natural river environment. Can J Fish Aquat Sci. 2012;69:1994–2006. doi: 10.1139/f2012-118. [DOI] [Google Scholar]
- 10.Besnier F, Glover ZA, Lien S, Kent M, Hansen MM, Shen X, et al. Identification of quantitative genetic components of fitness variation in farmed, hybrid and native salmon in the wild. Heredity (Edinb). 2015;115:47–55. doi: 10.1038/hdy.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einum S, Fleming IA. Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proc R Soc Lond B. 1999;266:2095–2100. doi: 10.1098/rspb.1999.0893. [DOI] [Google Scholar]
- 12.Einum S, Fleming IA. Selection against late emergence and small offspring in Atlantic salmon (Salmo salar) Evolution. 2000;54:628–639. doi: 10.1111/j.0014-3820.2000.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 13.Yeates SE, Einum S, Fleming IA, Holt WV, Gage MJ. Assessing risks of invasion through gamete performance: farm Atlantic salmon sperm and eggs show equivalence in function, fertility, compatibility and competitiveness to wild Atlantic salmon. Evol Appl. 2014;7:493–505. doi: 10.1111/eva.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser DJ, Minto C, Calvert AM, Eddington JD, Hutchings JA. Potential for domesticated-wild interbreeding to induce maladaptive phenology across multiple populations of wild Atlantic salmon (Salmo salar) Can J Fish Aquat Sci. 2010;67:1768–1775. doi: 10.1139/F10-084. [DOI] [Google Scholar]
- 15.Debes PV, Fraser DJ, McBride MC, Hutchings JA. Multigenerational hybridisation and its consequences for maternal effects in Atlantic salmon. Heredity (Edinb). 2013;111:238–247. doi: 10.1038/hdy.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solberg MF, Fjelldal PG, Nilsen F, Glover KA. Hatching time and alevin growth prior to the onset of exogenous feeding in farmed, wild and hybrid norwegian atlantic salmon. PLoS One. 2014;9:e113697. doi: 10.1371/journal.pone.0113697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skaala Ø, Glover KA, Barlaup BT, Borgstrøm R. Microsatellite DNA used for parentage identification of partly digested Atlantic salmon (Salmo salar) juveniles through non-destructive diet sampling in salmonids. Mar Biol Res. 2013;10:323–328. doi: 10.1080/17451000.2013.810757. [DOI] [Google Scholar]
- 18.Roberge C, Einum S, Guderley H, Bernatchez L. Rapid parallel evolutionary changes of gene transcription profiles in farmed Atlantic salmon. Mol Ecol. 2006;15:9–20. doi: 10.1111/j.1365-294X.2005.02807.x. [DOI] [PubMed] [Google Scholar]
- 19.Roberge C, Normandeau E, Einum S, Guderley H, Bernatchez L. Genetic consequences of interbreeding between farmed and wild Atlantic salmon: insights from the transcriptome. Mol Ecol. 2008;7:314–324. doi: 10.1111/j.1365-294X.2007.03438.x. [DOI] [PubMed] [Google Scholar]
- 20.Bicskei B, Bron JE, Glover KA, Taggart JB. A comparison of gene transcription profiles of domesticated and wild Atlantic salmon (Salmo salar L.) at early life stages, reared under controlled conditions. BMC Genomics. 2014;15:884. [DOI] [PMC free article] [PubMed]
- 21.Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development. 2009;136:3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- 22.Kamler E. Resource allocation in yolk-feeding fish. Rev Fish Biol Fish. 2007;18:143–200. doi: 10.1007/s11160-007-9070-x. [DOI] [Google Scholar]
- 23.Brooks S, Tyler CR, Sumpter JP. Egg quality in fish: what makes a good egg? Rev Fish Biol Fish. 1997;7:387–416. doi: 10.1023/A:1018400130692. [DOI] [Google Scholar]
- 24.Bobe J, Labbé C. Egg and sperm quality in fish. Gen Comp Endocrinol. 2010;165:535–548. doi: 10.1016/j.ygcen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Lanes CFC, Bizuayehu TT, Bolla S, Martins C, de Oliveira Fernandes JM, Bianchini A, et al. Biochemical composition and performance of Atlantic cod (Gadus morhua L.) eggs and larvae obtained from farmed and wild broodstocks. Aquaculture. 2012;324–5:267–275. doi: 10.1016/j.aquaculture.2011.10.036. [DOI] [Google Scholar]
- 26.Lush L, Burt K, Hamoutene D, Camarillo-Sepulveda N, Perez-Casanova JC, Kenny S, et al. Size and ATP content of unfertilized eggs from farmed and wild Atlantic salmon in Newfoundland. North Am J Aquac. 2014;76:138–142. doi: 10.1080/15222055.2014.886648. [DOI] [Google Scholar]
- 27.Mills D. Ecology and management of atlantic salmon. 1. London: Chapman & Hall; 1989. [Google Scholar]
- 28.Jantzen SG, Sanderson DS, von Schalburg KR, Yasuike M, Marass F, Koop BF. A, 44 K microarray dataset of the changing transcriptome in developing Atlantic salmon (Salmo salar L.) BMC Res Notes. 2011;4:88. doi: 10.1186/1756-0500-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu P, McIntyre LM, Scardina J, Wheeler PA, Thorgaard GH, Nichols KM. Transcriptome profiling of embryonic development rate in rainbow trout advanced backcross introgression lines. Mar Biotechnol (NY). 2011;13:215–231. doi: 10.1007/s10126-010-9283-1. [DOI] [PubMed] [Google Scholar]
- 30.Škugor A, Krasnov A, Andersen Ø. Genome-wide microarray analysis of Atlantic cod (Gadus morhua) oocyte and embryo. BMC Genomics. 2014;15:594. doi: 10.1186/1471-2164-15-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mommens M, Fernandes JMO, Tollefsen KE, Johnston IA, Babiak I. Profiling of the embryonic Atlantic halibut (Hippoglossus hippoglossus L.) transcriptome reveals maternal transcripts as potential markers of embryo quality. BMC Genomics. 2014;15:829. doi: 10.1186/1471-2164-15-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanes CFC, Bizuayehu TT, de OliveiraFernandes JM, Kiron V, Babiak I. Transcriptome of Atlantic cod (Gadus morhua L.) early embryos from farmed and wild broodstocks. Mar Biotechnol (NY) 2013;15:677–694. doi: 10.1007/s10126-013-9527-y. [DOI] [PubMed] [Google Scholar]
- 33.Renaut S, Nolte AW, Bernatchez L. Gene expression divergence and hybrid misexpression between lake whitefish species pairs (Coregonus spp. Salmonidae) Mol Biol Evol. 2009;26:925–936. doi: 10.1093/molbev/msp017. [DOI] [PubMed] [Google Scholar]
- 34.Solberg MF, Zhang Z, Glover KA, Favnebøe M, Zhang Z, Alan K. Are farmed salmon more prone to risk than wild salmon? Susceptibility of juvenile farm, hybrid and wild Atlantic salmon Salmo salar L. to an artificial predator. Appl Anim Behav Sci. 2015;162:67–80. doi: 10.1016/j.applanim.2014.11.012. [DOI] [Google Scholar]
- 35.Tacchi L, Bickerdike R, Douglas A, Secombes CJ, Martin SAM. Transcriptomic responses to functional feeds in Atlantic salmon (Salmo salar) Fish Shellfish Immunol. 2011;31:704–715. doi: 10.1016/j.fsi.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Morais S, Taggart JB, Guy DR, Bell JG, Tocher DR. Hepatic transcriptome analysis of inter-family variability in flesh n-3 long-chain polyunsaturated fatty acid content in Atlantic salmon. BMC Genomics. 2012;13:410. doi: 10.1186/1471-2164-13-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Rubio L, Morais S, Evensen Ø, Wadsworth S, Ruohonen K, Vecino JLG, et al. Functional feeds reduce heart inflammation and pathology in Atlantic salmon (Salmo salar L.) following experimental challenge with Atlantic salmon reovirus (ASRV) PLoS One. 2012;7:e40266. doi: 10.1371/journal.pone.0040266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Santis C, Bartie KL, Olsen RE, Taggart JB, Tocher DR. Nutrigenomic profiling of transcriptional processes affected in liver and distal intestine in response to a soybean meal-induced nutritional stress in Atlantic salmon (Salmo salar) Comp Biochem Physiol Part D Genomics Proteomics. 2015;15:1–11. doi: 10.1016/j.cbd.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Chu L, Scharf E, Kondo T. GeneSpring: tools for analyzing microarray expression data. Genome Inf. 2001;12:227–229. [Google Scholar]
- 40.Development Core Team R. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 41.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 42.Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformics. 2009;10:161. doi: 10.1186/1471-2105-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wickham H. Ggplot2: Elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- 45.Warnes AGR, Bolker B, Bonebakker L, Huber W, Liaw A, Lumley T, et al. gplots: various R programming tools for plotting data. R package version 2.12. 1. 2013. http://CRAN.R-project.org/package=gplots.
- 46.Elliott WH, Elliott DC. Biochemistry and molecular bBiology. 4. Oxford: Oxford University Press; 2009. [Google Scholar]
- 47.Darwin C. The variation of animals and plants under domestication. 2. London: John Murray; 1875. [Google Scholar]
- 48.Tort L. Stress and immune modulation in fish. Dev Comp Immunol. 2011;35:1366–1375. doi: 10.1016/j.dci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Nardocci G, Navarro C, Cort PP, Montoya M, Valenzuela B, Jara P, et al. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol. 2014;40:531–538. doi: 10.1016/j.fsi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Øverli Ø, Winberg S, Pottinger TG. Behavioral and neuroendocrine correlates of selection for stress responsiveness in rainbow trout: a review. Integr Comp Biol. 2005;45:463–474. doi: 10.1093/icb/45.3.463. [DOI] [PubMed] [Google Scholar]
- 51.Moon S, Kim H, Lee KT, Kwak W, Lee T, Lee SW, et al. A genome-wide scan for signatures of directional selection in domesticated pigs. BMC Genomics. 2015;16:130. doi: 10.1186/s12864-015-1330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nätt D, Rubin CJ, Wright D, Johnsson M, Beltéky J, Andersson L, Jensen P. Heritable genome-wide variation of gene expression and promoter methylation between wild and domesticated chickens. BMC Genomics. 2012;13:59. doi: 10.1186/1471-2164-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei HP, Yao YY, Zhang RW, Zhao XF, Du JL. Activity-induced long-term potentiation of excitatory synapses in developing zebrafish retina in vivo. Neuron. 2012;75:479–489. doi: 10.1016/j.neuron.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 54.Onuma TA, Ding Y, Abraham E, Zohar Y, Ando H, Duan C. Regulation of Ttemporal and spatial organization of newborn GnRH neurons by IGF signaling in zebrafish. J Neurosci. 2011;31:11814–11824. doi: 10.1523/JNEUROSCI.6804-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, et al. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amaral AJ, Ferretti L, Megens HJ, Crooijmans RPM, Nie H, Ramos-Onsins SE, et al. Genome-wide footprints of pig domestication and selection revealed through massive parallel sequencing of pooled DNA. PLoS One. 2011;6:e14782. doi: 10.1371/journal.pone.0014782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang S, Li X, Li K, Fan B, Tang Z. A genome-wide scan for signatures of selection in Chinese indigenous and commercial pig breeds. BMC Genet. 2014;15:7. doi: 10.1186/1471-2156-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park W, Kim J, Kim HJ, Choi J, Park JW, Cho HW, et al. Investigation of de novo unique differentially expressed genes related to evolution in exercise response during domestication in thoroughbred race horses. PLoS One. 2014;9:e91418. doi: 10.1371/journal.pone.0091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson RJ, Hellen CUT, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devlin RH, Sakhrani D, Tymchuk WE, Rise ML, Goh B. Domestication and growth hormone transgenesis cause similar changes in gene expression in coho salmon (Oncorhynchus kisutch) Proc Nat Acad Sci USA. 2009;106:3047–3052. doi: 10.1073/pnas.0809798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sauvage C, Derôme N, Normandeau E, St-Cyr J, Audet C, Bernatchez L. Fast transcriptional responses to domestication in the brook charr Salvelinus fontinalis. Genetics. 2010;85:105–112. doi: 10.1534/genetics.110.115071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White SL, Sakhrani D, Danzmann RG, Devlin RH. Influence of developmental stage and genotype on liver mRNA levels among wild, domesticated, and hybrid rainbow trout (Oncorhynchus mykiss) BMC Genomics. 2013;14:673. doi: 10.1186/1471-2164-14-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson JL, Carten JD, Farber SA. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011;101:111–141. doi: 10.1016/B978-0-12-387036-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vernier JM. Chronological table of the embryonic development of rainbow trout, Salmo gairdneri Rich. Ann Embryol Morphog. 1969;2:495–520. [Google Scholar]
- 65.Mennigen JA, Skiba-Cassy S, Panserat S. Ontogenetic expression of metabolic genes and microRNAs in rainbow trout alevins during the transition from the endogenous to the exogenous feeding period. J Exp Biol. 2013;216:1597–1608. doi: 10.1242/jeb.082248. [DOI] [PubMed] [Google Scholar]
- 66.Yang H, Zhou Y, Gu J, Xie S, Xu Y, Zhu G, et al. Deep mRNA sequencing analysis to capture the transcriptome landscape of zebrafish embryos and larvae. PLoS One. 2013;8:e64058. doi: 10.1371/journal.pone.0064058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ottaviani E, Franceschi C. The neuroimmunology of stress from invertebrates to man. Prog Neurobiol. 1996;48:421–440. doi: 10.1016/0301-0082(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 68.Alejo A, Tafalla C. Chemokines in teleost fish species. Dev Comp Immunol. 2011;35:1215–1222. doi: 10.1016/j.dci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Wiens GD, Glenney GW. Origin and evolution of TNF and TNF receptor superfamilies. Dev Comp Immunol. 2011;35:1324–1335. doi: 10.1016/j.dci.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 70.Peatman E, Liu Z. Evolution of CC chemokines in teleost fish: a case study in gene duplication and implications for immune diversity. Immunogenetics. 2007;59:613–623. doi: 10.1007/s00251-007-0228-4. [DOI] [PubMed] [Google Scholar]
- 71.Wu SY, Shin J, Sepich DS, Solnica-Krezel L. Chemokine GPCR signaling inhibits? Catenin during zebrafish axis formation. PLoS Biol. 2012;10:e1001403. doi: 10.1371/journal.pbio.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marié I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Correa RG, Tergaonkar V, Ng JK, Izpisua-belmonte JC, Verma IM, Dubova I. Characterization of NF-kB/IkB proteins in zebra fish and their involvement in notochord development. Mol Cell Biol. 2004;24:5257–5268. doi: 10.1128/MCB.24.12.5257-5268.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 76.Rao TP, Kühl M. An updated overview on wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 77.Lahiri S, Futerman AH. The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci. 2007;64:2270–2284. doi: 10.1007/s00018-007-7076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;29:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilkins AS, Wrangham RW, Fitch WT. The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics. 2014;197:795–808. doi: 10.1534/genetics.114.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velsen FPJ. Embryonic development in eggs of sockeye salmon, Oncorhynchus nerka. Can Spec Publ Fish Aquat Sci. 1980.
- 82.Debes PV, Normandeau E, Fraser DJ, Bernatchez L, Hutchings J. Differences in transcription levels among wild, domesticated, and hybrid Atlantic salmon (Salmo salar) from two environments. Mol Ecol. 2012;21:2574–2587. doi: 10.1111/j.1365-294X.2012.05567.x. [DOI] [PubMed] [Google Scholar]
- 83.Crockford SJ. Thyroid rhythm phenotypes and hominid evolution: a new paradigm implicates pulsatile hormone secretion in speciation and adaptation changes. Comp Biochem Physiol A: Mol Integr Physiol. 2003;135:105–129. doi: 10.1016/S1095-6433(02)00259-3. [DOI] [PubMed] [Google Scholar]
- 84.Crockford SJ. Animal domestication and vertebrate speciation : a paradigm for the origin of species. Greater Victoria: University of Victoria; 2004. [Google Scholar]
- 85.Crockford SJ. Rhythms of life: thyroid hormone and the origin of species. Victoria: Trafford Publishing; 2006. [Google Scholar]
- 86.Bougas B, Audet C, Bernatchez L. The influence of parental effects on transcriptomic landscape during early development in brook charr (Salvelinus fontinalis, Mitchill) Heredity (Edinb). 2013;110:484–491. doi: 10.1038/hdy.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang L, Zhang J, Wang JJ, Wang L, Zhang L, Li G, et al. Sperm, but not oocyte, DNA methylome is inherited by zebrafish early embryos. Cell. 2013;153:773–784. doi: 10.1016/j.cell.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bougas B, Granier S, Audet C, Bernatchez L. The transcriptional landscape of cross-specific hybrids and its possible link with growth in brook charr (Salvelinus fontinalis Mitchill) Genetics. 2010;186:97–107. doi: 10.1534/genetics.110.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taranger GL, Karlsen Ø, Bannister RJ, Glover KA, Husa V, Bjørn A, et al. Risk assessment of the environmental impact of Norwegian Atlantic salmon farming. ICES J Mar Sci. 2015;72:997–1021. doi: 10.1093/icesjms/fsu132. [DOI] [Google Scholar]
- 90.Youngson AF, Webb JH, Maclean JC, Whyte BM. Frequency of occurrence of reared Atlantic salmon in Scottish salmon fisheries. ICES J Mar Sci. 1997;54:1216–1220. doi: 10.1016/S1054-3139(97)80028-8. [DOI] [Google Scholar]
- 91.Fiske P, Lund R, Hansen L. Relationships between the frequency of farmed Atlantic salmon, Salmo salar L., in wild salmon populations and fish farming activity in Norway, 1989–2004. ICES J Mar Sci. 2006;63:1182–1189. doi: 10.1016/j.icesjms.2006.04.006. [DOI] [Google Scholar]
- 92.Crozier WW. Evidence of genetic interaction between escaped farmed salmon and wild Atlantic salmon (Salmo salar L.) in a Northern Irish river. Aquaculture. 1993;113:19–29. doi: 10.1016/0044-8486(93)90337-X. [DOI] [Google Scholar]
- 93.Clifford SL, McGinnity P, Ferguson A. Genetic changes in Atlantic salmon (Salmo salar) populations of Northwest Irish rivers resulting from escapes of adult farm salmon. Can J Fish Aquat Sci. 1998;55:358–363. doi: 10.1139/f97-229. [DOI] [Google Scholar]
- 94.Skaala O, Wennevik V, Glover K. Evidence of temporal genetic change in wild Atlantic salmon, Salmo salar L., populations affected by farm escapees. ICES J Mar Sci. 2006;63:1224–1233. doi: 10.1016/j.icesjms.2006.04.005. [DOI] [Google Scholar]
- 95.Glover KA, Quintela M, Wennevik V, Besnier F, Sørvik AGE, Skaala Ø. Three decades of farmed escapees in the wild: a spatio-temporal analysis of Atlantic salmon population genetic structure throughout Norway. PLoS One. 2012;7:e43129. doi: 10.1371/journal.pone.0043129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glover KA, Pertoldi C, Besnier F, Wennevik V, Kent M, Skaala Ø. Atlantic salmon populations invaded by farmed escapees: quantifying genetic introgression with a Bayesian approach and SNPs. BMC Genet. 2013;14:74. doi: 10.1186/1471-2156-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biol Rev Camb Philos Soc. 2007;82:173–211. doi: 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 98.McGinnity P, Stone C, Taggart JB, Cooke D, Cotter D, Hynes R, et al. Genetic impact of escaped farmed Atlantic salmon (Salmo salar L) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. ICES J Mar Sci. 1997;54:998–1008. [Google Scholar]
- 99.Fleming IA, Jonsson B, Gross MR, Lamberg A, Fleming IA. An experimental study of the reproductive behaviour and success of farmed and wild Atlantic salmon (Salmo salar) J Appl Ecol. 1996;33:893–905. doi: 10.2307/2404960. [DOI] [Google Scholar]


