Abstract
Peripartum cardiomyopathy (PPCM) is characterized by the development of systolic heart failure in the last month of pregnancy or within the first 5 months postpartum. The disease affects between 1:300 and 1:3000 births worldwide. Heart failure can resolve spontaneously but often does not. Mortality rates, like incidence, vary widely based on location, ranging from 0% to 25%. The consequences of PPCM are thus often devastating for an otherwise healthy young woman and her newborn. The cause of PPCM remains elusive. Numerous hypotheses have been proposed, with mixed supporting evidence. Recent work has suggested that PPCM is a vascular disease, triggered by the profound hormonal changes of late gestation. We focus here on these new mechanistic findings, and their potential implication for understanding and treating PPCM.
Vignette
A 40-year-old woman delivered her second child by C-section uneventfully at 39 weeks of gestation. Three days later she developed shortness of breath and swelling in her legs. Two days later she experienced chest pain with exertion and went to the emergency room. On presentation, her blood pressure and heart rate were mildly elevated, and blood tests, EKG, and chest x-ray were unremarkable. Two hours later, while still in the emergency room, she developed anxiety, much worsened shortness of breath, hypoxemia, and hypotension. She required intubation and intravenous vasopressor support and was transferred to the intensive care unit. An echocardiogram revealed a dilated heart and an ejection fraction of 10% (normal >55%). The patient received aggressive medical management and remained in the intensive care unit for a week. Over 2 weeks, she slowly improved and was discharged home. An echocardiogram performed 6 months later revealed some improvement in her ejection fraction but she continued to have moderate cardiac dysfunction and stage C congestive heart failure [1].
Introduction
Peripartum cardiomyopathy (PPCM) can be, as in this case, a dramatic complication of pregnancy, usually occurring in otherwise healthy women of childbearing age. These women, with a young infant to care for, are often left with persistent cardiac dysfunction and have an elevated risk of death. Although recognized since the 18th century, the condition was not described in the medical literature until 1849 [2]. PPCM is currently defined based on the presence of four criteria: (1) development of symptomatic heart failure in the last month of pregnancy or within 5 months of delivery, (2) the absence of an identifiable cause of heart failure, (3) absence of preexisting heart disease prior to the last month of pregnancy, and (4) left ventricular systolic dysfunction (LV ejection fraction <45% and/or fractional shortening <30% on echocardiogram) [3]. In order to capture cases outside that relatively small window of time, recent European guidelines have suggested loosening the definition of PPCM to include cases of systolic heart failure occurring towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found [4]. Systematic population estimates of the incidence of PPCM are lacking for a variety of reasons, including under-diagnosis, misdiagnosis, and lack of a systematic reporting mechanism. Estimated incidence in the United States ranges from 1 in 1000 to 1 in 4000 pregnancies [5,6], but there are several well-described global hotspots, including Nigeria and Haiti, where incidence rates range as high as 1 in 100 to 1 in 300 [7,8]. Although a mechanism for this variance and clarification of incidence rates in other parts of the world remains to be elucidated, PPCM appears to be more common and carry a worse prognosis in women of African heritage [5,9–11]. The incidence rate in South Africa is 1 in 1000 live births [12]. In addition to racial differences in the risk of developing PPCM, classical risk factors include multiparity, multi-fetal pregnancy, advanced maternal age, preeclampsia, and gestational hypertension [3,13–18].
Clinical presentation and treatment
At the time of presentation, women usually present with wet/warm heart failure, although fulminant cardiovascular collapse can be seen when presentation for care is delayed. The symptoms of heart failure include shortness of breath (dyspnea) on exertion or at rest, difficulty breathing while supine (orthopnea), awakening abruptly due to shortness of breath (paroxysmal nocturnal dyspnea), and cough. Swelling of the feet, ankles, and abdomen, fatigue, weakness, and decreased exercise tolerance are also common presenting symptoms, which may be mistaken for sequelae of pregnancy rather than heart failure [19]. Objective signs of heart failure on examination include the presence of edema (swelling), crackles on pulmonary examination, elevated jugular venous pressure, ascites, hepatomegaly, a chest x-ray exhibiting cardiomegaly or pulmonary edema, and elevations in cardiac biomarkers including BNP and NT-proBNP.
Treatment for PPCM has historically been divided into acute and chronic heart failure management, similar to what is used for other forms of systolic heart failure. Mainstays of care in the acute phase include supportive therapies such as aggressive diuresis and the use of vasopressors and inotropes [19–22]. In extreme cases, the use of mechanical support devices such as LVADs and BiVADS may be necessary, either as a bridge to recovery or a bridge to cardiac transplantation [23]. Once stable and euvolemic, chronic management of PPCM is similar to that of systolic heart failure of other etiologies. Ace-inhibitors/angiotensin receptor blockers, beta-blockers, diuretics, and aldosterone antagonists are the mainstay of care with special caveats for lactating women to avoid the use of ace-inhibitors/angiotensin receptor blockers. No treatments specific to PPCM exist.
The prognosis of PPCM is quite variable, depending on ethnicity and locale. Early studies of PPCM revealed mortality rates in the 40–50% range. As the management of heart failure has improved, so has its prognosis. In the US, the contemporary mortality rate for women with PPCM ranges from 5% to 15% [24] and is higher in women of African descent [9,11,25], though one cohort reported no mortality in a group of 55 women followed up for over 3 years [26]. Globally, mortality rates range widely from 8% in Singapore [27] to ~20% of women who were followed up in a South African cohort [21] and up to 25% of women with PPCM in Haiti dying within 5 years [8]. Based on OPTN data as of November 28, 2014, 4% of transplants performed in the USA in women are for PPCM. Recovery of ejection fraction to the normal range is reported to occur in ~50% of cases, though there is a high recurrence rate with subsequent pregnancies. Duration of medical management and whether weaning of heart failure medications is appropriate have not been tested in randomized clinical trials.
PPCM as a vascular and hormonal disease
The cause of PPCM has remained unclear. Numerous hypotheses have been proposed, including myocarditis, autoimmunity against fetal antigens, fetal microchimerism, and dietary deficiencies of selenium or excessive salt intake. Recent research, however, suggests that PPCM is a vascular disease, triggered by late-gestational secretion of potent anti-angiogenic agents from the placenta and pituitary. Although this idea has been proposed in the past [28], experimental support for it has been lacking until recently. This new research is discussed in depth here.
Prolactin
Clinical observations and plasma analyses in women with PPCM have suggested an important role for apoptosis in PPCM [29,30], but what events cause apoptosis had not been clear. Based on these clinical observations, Denise Hilfiker-Kleiner and Helmut Drexler first presented experimental support for the notion that PPCM is a vascular disease that is triggered by the hormonal changes of late pregnancy, in a landmark article in 2007 [31]. The authors were studying a mouse model, in which the transcription factor STAT3 was deleted in cardiomyocytes, and pregnant female mice developed peripartum cardiomyopathy. Male controls, and nulliparous females, were unaffected. The authors proceeded to show that STAT3 controls a program that protects the heart against reactive oxygen species (ROS), largely by regulating the gene expression of superoxide dismutase 2 (SOD2), which neutralizes superoxides generated by the robust mitochondrial activity in beating cardiomyocytes. The absence of STAT3 thus led to increased ROS, leading in turn (via still unclear mechanisms) to the inappropriate secretion from the myocytes of peptidases, most notably cathepsin D (Fig.). Extracellular cathepsin D then proceeds to cleave the circulating pregnancy-specific hormone prolactin into a 16-kD fragment. This prolactin fragment was already previously well studied as a potent vasculotoxic protein, causing apoptosis in endothelial cells. And indeed, STAT3 cardiac knockout mice showed significant vascular dropout during late pregnancy. The most compelling aspect of this study, however, came from inhibiting prolactin secretion. Bromocriptine, an FDA-approved drug, is a dopamine D2-receptor agonist that inhibits secretion of prolactin from the pituitary. Treatment of STAT3 cardiac knockout mice with bromocriptine thus blocked prolactin production in these mice, and, strikingly, completely reversed the observed peripartum cardiomyopathy. These data thus convincingly showed that the hormone prolactin plays a key role in PPCM, at least in this murine model.
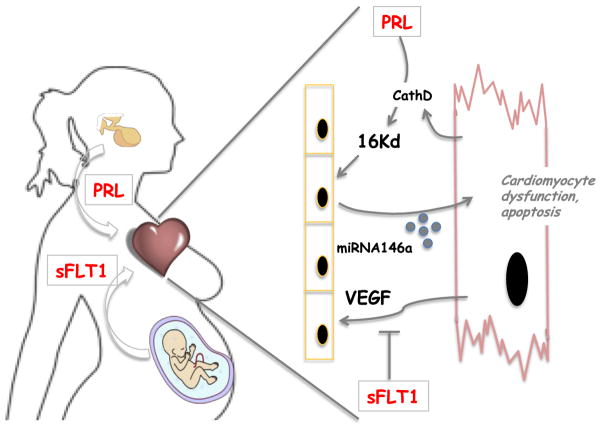
Fig.
PPCM as a hormonal and vascular disease: The peripartum period causes secretion of hormones from the pituitary (e.g., prolactin (PRL)) and placenta (e.g., sFlt1). PRL is converted to 16-kD Vasoinhibin by CathepsinD (CathD) secreted from cardiomyocytes. Vasoinhibin then both triggers endothelial cell (EC) apoptosis and the secretion from ECs of miRNA146a encapsulated in exosomes. These exosomes are internalized by cardiomyocytes, where miRNA146s targets the ErbB4 pathway and others, causing dysfunction and apoptosis. At the same time, sFlt1 binds to and inhibits VEGF signaling, leading to EC dysfunction and apoptosis. Ultimately, dwindling vascular support leads to metabolic insufficiency in cardiomyocytes and cardiomyopathy.
More recently, the same group probed more deeply into how 16-kD prolactin causes vascular damage [32,33]. They showed that endothelium exposed to 16-kD prolactin dramatically induces the expression of microRNA146a, which is then packaged and secreted in exosomes, small secreted lipid-encapsulated particles, which are in turn taken up by adjacent cardiomyocytes. The ultimate effect is microRNA-mediated suppression of target genes in the cardiomyocytes, most notably of the neuregulin/ErbB pathway, which was already known as an important suppressor of cardiomyocyte apoptosis. These elegant studies thus both unveiled a novel crosstalk between endothelial cells and cardiomyocytes in vivo and identified a mechanism by which prolactin causes cardiomyocyte toxicity in PPCM. Perhaps most strikingly, the authors show that circulating levels of microRNA146a are dramatically elevated in clinical cases of PPCM, and that these levels drop significantly with bromocriptine treatment. MicroRNA146a may thus be useful both as a biomarker of PPCM and a potential therapeutic target.
These compelling data have rapidly led to enthusiasm for testing bromocriptine in the clinic. Bromocriptine is widely used to suppress prolactin in patients with prolactinomas, but it also has been used, when indicated, to suppress lactation in the postpartum period. The latter indication is no longer recommended in the US, due to possible associations with hypertension, myocardial infarctions, and strokes, but it is still used elsewhere. A small open-label proof-of-concept pilot study was thus carried out in South Africa, in which bromocriptine was added to standard heart failure therapy in 10 of 20 women with confirmed newly diagnosed PPCM [34]. The outcomes were fairly dramatic: four women in the control arm died, while none in the bromocriptine arm did so, and recovery of ejection fraction was equally dramatically improved. However, there are several major limitations of this study that should be noted: enrolled numbers were small and highly selected from 93 suspected cases of PPCM, the trial was not blinded, and the number of events in the control arm was unusually large. A larger registry report from Germany that included 96 women, of whom 64 received bromocriptine, found that bromocriptine was significantly associated with improvement, though not full recovery of LV systolic function [22]. However, this study was not randomized, and selection bias is likely significant. It is also important to note that the use of bromocriptine is not without risks to the newborn, especially in the developing world, where the poor quality of water and poor access to infant formula render newborns heavily reliant on breast milk. There has been significant debate at international meetings over the role of bromocriptine in current management of patients with PPCM. The results of a larger, prospective randomized clinical trial that is ongoing in Germany (NCT00998556 clinicaltrials.gov) are eagerly awaited.
sFlt1 and preeclampsia
In parallel work, our lab has also implicated vascular toxicity and the hormonal milieu of late gestation in the etiology of PPCM. PGC-1alpha is a powerful transcriptional regulator of metabolic and angiogenic pathways in numerous cell types. We found that mice lacking PGC-1alpha in cardiomyocytes developed peripartum cardiomyopathy, much like the STAT3 cardiac KO mice [35]. Again, males and nulliparous females were unaffected. Like STAT3, PGC-1alpha controls the expression of SOD2, thus leading to elevated ROS in PGC-1alpha cardiac KO mice. Bromocriptine alone was insufficient to rescue the PPCM in these animals, however. This is because PCG-1alpha also directly regulates vascular health through the transcriptional induction of vascular endothelial growth factor (VEGF), the most widely studied angiogenic factor [36]. Absence of PGC-1alpha in the heart thus causes vasculotoxicity by two pathways: the activation of an anti-vascular 16-kD prolactin-mediated pathway, plus the loss of a pro-vascular VEGF-mediated pathway (Fig.). Accordingly, rescue of the PPCM phenotype in these animals was achieved only with the combination of bromocriptine and VEGF therapies. These findings, along with the STAT3 model, thus again support the notion that PPCM is largely a vascular disease, at least in these murine models.
The observations in the PGC-1alpha KO animals raised the following question: if the presence of prolactin in late gestation triggers the 16-kD pathway, what hormone might be inhibiting the VEGF pathway? There are two predominant sources of circulating hormones during pregnancy: the pituitary and the placenta. During late gestation in placental mammals, the placenta secretes various factors into the maternal circulation, including most notably a soluble version of the VEGF receptor 1 or soluble Fms-like tyrosine kinase 1 (sFlt1). sFlt1 binds to and neutralizes circulating VEGF family members, such that most free VEGF is in fact neutralized by sFlt1 during late gestation [37]. We therefore hypothesized that excess sFlt1 secreted by the placenta in late gestation constitutes a second hormonal insult to the vasculature of the heart. The heart defends itself from this insult, in part with robust local secretion of VEGF. Absence of PGC-1alpha decreases this local production of VEGF, which, when combined with high circulating sFlt1 of gestation, leads to vascular toxicity. Consistent with this notion, administering sFlt1 to nulliparous animals was sufficient to cause cardiomyopathy in PGC-1alpha cardiac KO animals, demonstrating that sFlt1 is a key component of the gravid state that triggers PPCM in these animals.
The placental secretion of sFlt1 is markedly elevated in preeclampsia, a common disease of pregnancy marked by hypertension and proteinuria [37]. Untreated, preeclampsia can progress to seizures, strokes, and death. Induction of delivery and removal of the placenta is the only treatment currently available. The maternal heart is thus exposed to significantly higher levels of sFlt1 during preeclampsia. Consistent with this, and with the notion that sFtl1 is toxic to the heart, women with preeclampsia have demonstrable cardiac dysfunction, as seen by echocardiography, though not always with clinical manifestations [35,38–40]. Women with gestational hypertension lack similar cardiac dysfunction, exonerating elevated blood pressure as the sole cause of the dysfunction. On the other hand, the extent of dysfunction correlates well with circulating levels of sFlt1, again supporting the idea that sFlt1 is cardiotoxic [35]. Consistent with these observations, preeclampsia is the strongest known risk factor for the development of PPCM [41]. Where it has been studied, approximately 25% of women with PPCM are co-diagnosed with preeclampsia, compared with an underlying prevalence of at most 3–5%. Similarly, 10–16% of PPCM cases occur in the context of twin gestations [42–44], which also have higher levels of sFlt1, likely from higher placental mass [45]. Together, these observations strongly support the notion that sFlt1, secreted from the placenta in late gestation, provides a toxic challenge to the heart, and that, in the absence of appropriate defenses, PPCM can ensue.
Many questions remain. Circulating levels of sFlt1 are elevated in a subset of women with PPCM [35]. This observation requires confirmation in a larger cohort. Moreover, correlation with clinical outcomes would be of great interest, e.g., does the persistence of excess sFlt1 correlate with worse outcome? The source of persistent sFlt1 is also unclear, as the placenta is no longer present after delivery. Is sFlt1 now being secreted from, for example, monocytes? [46]. Knowing this answer may provide therapeutic opportunity. Interestingly, mild elevations in sFlt1 have been noted in general cases of heart failure, suggesting possible mechanistic overlap between PPCM in more common forms of systolic failure. Understanding these processes may thus also inform other forms of heart failure.
A two-hit hypothesis
Taken together, these recent developments suggest that PPCM stems from the coincidence of two “hits”: one hit is the late-gestational vasculotoxic hormonal environment, including sFlt1 and prolactin, and the second hit is an inability, in some women, to withstand this vasculotoxic insult.
The hormonal hypothesis of the first hit is consistent with the timing of PPCM. Pregnancy presents significant hemodynamic and metabolic challenges to the heart, including large increases in heart rate, stroke volume, total vascular volume, and cardiac output [47]. However, these hemodynamic changes occur predominantly near the end of the first trimester or beginning of the second trimester, long before PPCM typically presents. Indeed, an ongoing registry in Europe of pregnancy-associated heart disease, with more than 1000 cases thus far, showed that women with preexisting heart disease (e.g., valvular or myocardial disease) presented with clinical heart failure predominantly in the second trimester, consistent with the timing of hemodynamic changes [48]. On the other hand, PPCM presented largely in the postpartum period. It remains possible that the hemodynamic stress of delivery itself contributes to PPCM, but the use of cesarean section, which can limit this stress, does not seem to reduce the likelihood of developing PPCM.
The nature of the predisposition to PPCM (the second “hit”) unfortunately remains speculative at this point. One possibility is that an acute event, such as viral myocarditis, might weaken cardiac pro-vascular defenses. Such an explanation would unify the current hypothesis with previous theories of PPCM, but evidence remains weak. Another possible second hit is a genetic predisposition. A few studies potentially favor this idea. One genome-wide association study (GWAS) found an association, with genome-wide significance, between PPCM and one polymorphism located near the PTHLH gene [49]. The functional significance of this polymorphism is not clear, although PTHLH has been implicated as an angiogenesis inhibitor [50]. The study was also limited to less than 100 cases, underscoring the difficulty of carrying out GWAS with rare diseases like PPCM. Another interesting series of observations is the identification, in some families where both PPCM and dilated cardiomyopathies (DCM) occur, of mutations in genes known to cause familiar DCM, most notably titin [51,52]. Such families with coincident histories of DCM are only a small subset of PPCM, however. It may be that familial DCM predisposes to PPCM but does not explain most cases of PPCM, reflecting the likely heterogeneous nature of the disease. Genetic analyses of unselected cohorts of women with PPCM are needed.
Conclusions and future studies
The recent findings outlined above paint a compelling picture of PPCM as a disease caused by a vasculotoxic hormonal state of late gestation, superimposed on a permissive heart with insufficient pro-vascular defenses. Evidence for the model rests on both significant experimental mouse data and clinical epidemiological observations. Nevertheless, the proposition that PPCM is a vascular and hormonal disease is still in its early stages and remains poorly discussed, if at all, in most current reviews.
The new conceptualization of the pathophysiology of PPCM raises a number of exciting questions that will need to be addressed in the coming years, including the following: Can the novel findings inform the design of new biomarkers to diagnose the disease and/or to predict recovery? miRNA146a appears particularly promising. What is the “2nd hit”, i.e., what causes the predisposition to PPCM? The rapid development of modern genomic tools should greatly facilitate addressing at least the genetic component of this question. Does sFlt1 primarily affect VEGFA or does neutralization of other family members like VEGFB, recently demonstrated to play a key role in endothelial metabolism [53], also contribute to cardiac dysfunction? Are there other hormones involved, in addition to prolactin and sFlt1? Preeclampsia, for example, is also triggered by the placental secretion of soluble endoglin, a potent inhibitor or the TGFbeta pathway [54]. Do STAT3 and PGC-1alpha play a role in human PPCM or have they simply provided convenient and informative mouse models of the disease? Is bromocriptine a viable treatment option, and which subset of women with PPCM would benefit the most from this therapy? Organizing multinational collaborations that include access to clinical samples will be imperative for these and many other questions. Despite these many remaining unanswered questions, the resurgence of both clinical and basic research in PPCM, and the emerging model of how the disease unfolds, offers new hope for women with this often tragic ailment.
Footnotes
The authors have indicated there are no conflicts of interest.
References
- 1.Arany ZP, Walker CM, Wang L. Case records of the Massachusetts General Hospital. Case 22–2014. A 40-year-old woman with postpartum dyspnea and hypoxemia. N Engl J Med. 2014;371:261–9. doi: 10.1056/NEJMcpc1304163. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie C. Clinical contribution to the pathology, diagnosis, and treatment of certain chronic diseases of the heart. Edinburgh Med Surg J. 1849;2:333–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson GD, Veille J, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, et al. Peripartum cardiomyopathy: National heart, lung, and blood institute and office of rare diseases (National Institutes of Health) workshop recommendations and review. J Am Med Assoc. 2000;283:1183–8. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 4.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–78. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 5.Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JWY, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100:302–4. doi: 10.1016/j.amjcard.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 6.Deneux-Tharaux C, Berg C, Bouvier-Colle MH, Gissler M, Harper M, Nannini A, et al. Underreporting of pregnancy-related mortality in the United States and Europe. Obstet Gynecol. 2005;106:684–92. doi: 10.1097/01.AOG.0000174580.24281.e6. [DOI] [PubMed] [Google Scholar]
- 7.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clinic Proc. 2005;80:1602–6. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 8.Isezuo SA, Abubakar SA. Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethn Dis. 2007;17:228–33. [PubMed] [Google Scholar]
- 9.Goland SMK, Hatamizadeh P, Elkayam U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail. 2013;19:214–8. doi: 10.1016/j.cardfail.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Fett JD, Murphy JG. Infant survival in Haiti after maternal death from peripartum cardiomyopathy. Int J Gynaecol Obstet. 2006;94:135–6. doi: 10.1016/j.ijgo.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Gentry MB, Dias JK, Luis A, Patel R, Thornton J, Reed GL. African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol. 2010;55:654–9. doi: 10.1016/j.jacc.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai D, Moodley J, Naidoo D. Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Trop Doct. 1995;25:118–23. doi: 10.1177/004947559502500310. [DOI] [PubMed] [Google Scholar]
- 13.Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli TM. Peripartum cardiomyopathy: a comprehensive review. Int J Cardiol. 2007;118:295–303. doi: 10.1016/j.ijcard.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Brown CS, Bertolet BD. Peripartum cardiomyopathy: a comprehensive review. Am J Obstet Gynecol. 1998;178:409–14. doi: 10.1016/s0002-9378(98)80034-3. [DOI] [PubMed] [Google Scholar]
- 15.Desai P. Peripartum Cardiomyopathy: a review. J Obstet Gynecol India. 2010;60:25–32. [Google Scholar]
- 16.Johnson-Coyle L, Jensen L, Sobey A. Peripartum cardiomyopathy: review and practice guidelines. Am J Crit Care. 2012;21:89–99. doi: 10.4037/ajcc2012163. [DOI] [PubMed] [Google Scholar]
- 17.Karaye KM, Henein MY. Peripartum cardiomyopathy: a review article. Int J Cardiol. 2011;164:333–8. doi: 10.1016/j.ijcard.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 18.Ntusi NBA, Mayosi BM. Aetiology and risk factors of peripartum cardiomyopathy: a systematic review. Int J Cardiol. 2009;131:168–79. doi: 10.1016/j.ijcard.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Hunt SA. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, Hilfiker-Kleiner D, et al. Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart. 2012;99:308–13. doi: 10.1136/heartjnl-2012-302760. [DOI] [PubMed] [Google Scholar]
- 22.Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman H, Bose R, Smith R, Copeland JG. Treatment of peripartum cardiomyopathy with mechanical assist devices and cardiac transplantation. Ann Thorac Surg. 2010;89:1211–7. doi: 10.1016/j.athoracsur.2009.12.064. [DOI] [PubMed] [Google Scholar]
- 24.Pillarisetti J, Kondur A, Alani A, Reddy M, Reddy M, Vacek J, et al. Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter-defibrillator use. J Am Coll Cardiol. 2014;63(25 Pt A):2831–9. doi: 10.1016/j.jacc.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Elkayam U. Risk of subsequent pregnancy in women with a history of peripartum cardiomyopathy. J Am Coll Cardiol. 2014;64:1629–36. doi: 10.1016/j.jacc.2014.07.961. [DOI] [PubMed] [Google Scholar]
- 26.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152:509–13. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Chee KH. Favourable outcome after peripartum cardiomyopathy: a ten-year study on peripartum cardiomyopathy in a university hospital. Singapore Med J. 2013;54:28–31. doi: 10.11622/smedj.2013007. [DOI] [PubMed] [Google Scholar]
- 28.Homans DC. Peripartum cardiomyopathy. N Engl J Med. 1985;312:1432–7. doi: 10.1056/NEJM198505303122206. [DOI] [PubMed] [Google Scholar]
- 29.Sliwa K, Forster O, Libhaber E, Fett JD, Sundstrom JB, Hilfiker-Kleiner D, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–6. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 30.Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol. 2000;35:701–5. doi: 10.1016/s0735-1097(99)00624-5. [DOI] [PubMed] [Google Scholar]
- 31.Hilfiker-Kleiner D, Sliwa K, Drexler H. Peripartum cardiomyopathy: recent insights in its pathophysiology. Trends Cardiovasc Med. 2008;18:173–9. doi: 10.1016/j.tcm.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123:2143–54. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajou K, Herkenne S, Thijssen VL, D’Amico S, Nguyen NQ, Bouche A, et al. PAI-1 mediates the antiangiogenic and profibrinolytic effects of 16k prolactin. Nat Med. 2014;20:741–7. doi: 10.1038/nm.3552. [DOI] [PubMed] [Google Scholar]
- 34.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465–73. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 35.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arany Z, Foo SY, Ma Y, Ruas J, Bommi-Reddy A, Girnun GD, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature. 2008;451:1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 37.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57:85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 39.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–15. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 40.Shahul S, Rhee J, Hacker MR, Gulati G, Mitchell JD, Hess P, et al. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2D speckle-tracking imaging study. Circ Cardiovasc Imaging. 2012;5:734–9. doi: 10.1161/CIRCIMAGING.112.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:1715–23. doi: 10.1016/j.jacc.2013.08.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamiya CA, Kitakaze M, Ishibashi-Ueda H, Nakatani S, Murohara T, Tomoike H, et al. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders. Results from the Japanese Nationwide survey of peripartum cardiomyopathy. Circ J. 2011;75:1975–81. doi: 10.1253/circj.cj-10-1214. [DOI] [PubMed] [Google Scholar]
- 43.Kao DP, Hsich E, Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail. 2013;1:409–16. doi: 10.1016/j.jchf.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–5. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 45.Bdolah Y, Lam C, Rajakumar A, Shivalingappa V, Mutter W, Sachs BP, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:428 e1–e6. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 46.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–73. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Liu LX, Arany Z. Maternal cardiac metabolism in pregnancy. Cardiovasc Res. 2014;101:545–54. doi: 10.1093/cvr/cvu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruys TP, Roos-Hesselink JW, Hall R, Subirana-Domenech MT, Grando-Ting J, Estensen M, et al. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart. 2014;100:231–8. doi: 10.1136/heartjnl-2013-304888. [DOI] [PubMed] [Google Scholar]
- 49.Horne BD, Rasmusson KD, Alharethi R, Budge D, Brunisholz KD, Metz T, et al. Genome-wide significance and replication of the chromosome 12p11. 22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ Cardiovasc Genet. 2011;4:359–66. doi: 10.1161/CIRCGENETICS.110.959205. [DOI] [PubMed] [Google Scholar]
- 50.Bakre MM, Zhu Y, Yin H, Burton DW, Terkeltaub R, Deftos LJ, et al. Parathyroid hormone-related peptide is a naturally occurring, protein kinase A-dependent angiogenesis inhibitor. Nat Med. 2002;8:995–1003. doi: 10.1038/nm753. [DOI] [PubMed] [Google Scholar]
- 51.van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JD, Paulus WJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121:2169–75. doi: 10.1161/CIRCULATIONAHA.109.929646. [DOI] [PubMed] [Google Scholar]
- 52.van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, Hilfiker-Kleiner D, Bollen IA, Sliwa K, et al. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J. 2014;35:2165–73. doi: 10.1093/eurheartj/ehu050. [DOI] [PubMed] [Google Scholar]
- 53.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–21. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 54.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]