Abstract
Gene-environment interactions are known to play a key role in the development of rheumatoid arthritis (RA). Exposure to cigarette smoke (CS) is one of the strongest environmental risk factors associated with RA and has been shown to mediate a range of complex immunomodulatory effects from decreased T and B cell activation to depressed phagocytic function. The effects of CS on the function of TH17 cells, one of the key TH effector subsets implicated in RA pathogenesis, are not fully understood. IRF4 is one of the crucial transcription factors involved in TH-17 differentiation and is absolutely required for the production of IL-17 and IL-21 but, interestingly, inhibits the synthesis of IL-22. The production of IL-17 and IL-21 by IRF4 can be augmented by its phosphorylation by the serine-threonine kinase ROCK2. Given that CS has been reported to increase ROCK activity in endothelial cells, here we investigated the effects of CS on the ROCK2-IRF4 axis in T cells. Surprisingly, we found that CS leads to decreased ROCK2 activation and IRF4 phosphorylation in T cells. This effect was associated with increased IL-22 production. Using a GEF pull-down assay we furthermore identify ARHGEF1 as a key upstream regulator of ROCK2 whose activity in T cells is inhibited by CS. Thus CS can inhibit the ROCK2-IRF4 axis and modulate T cell production of IL-22.
1. Introduction
Rheumatoid arthritis (RA) is characterized by the infiltration of immune cells into the synovium eventually resulting in cartilage destruction and bone erosions (McInnes and Schett 2011). The development of RA is mediated through a complex interaction between environmental and genetic factors (Costenbader, Gay et al. 2012, Gerlag, Norris et al. 2015). Amongst environmental risk factors, cigarette smoke (CS) exposure has been strongly associated with the development of RA (Arnson, Shoenfeld et al. 2010, Hoovestol and Mikuls 2011, Klareskog, Malmstrom et al. 2011). CS has been shown to exert a number of complex immunomodulatory effects from decreased T and B cell activation to depressed phagocytic function to increased oxidative stress (Baka, Buzas et al. 2009). In line with the broad and multifaceted effects of CS on immune responses, exposure of mice to cigarette smoke has been reported to either augment or delay collagen-induced arthritis (CIA), with the latter effect being associated with lower autoantibody responses (Lindblad, Mydel et al. 2009, Chujo, Okamoto et al. 2010, Okamoto, Adachi et al. 2011).
CD4+ T helper cells play a key role in the pathogenesis of many autoimmune diseases, including RA. In particular, one of the TH effector subsets, the TH-17 subset has been implicated in the development of RA via its ability to produce key cytokines such as IL-17, IL-21, and IL-22 (Koenders and van den Berg 2015, Lubberts 2015). Aberrant production of IL-17 and IL-21 has been observed in murine models of RA and in patients affected by this disorder and blockade of IL-17- and IL-21-mediated responses has been found to be efficacious in ameliorating disease in murine models of RA (Pernis 2009). Higher expression levels of IL-22, a member of the IL-10 cytokine family, have also been observed in synovium from RA patients as well as in mice with CIA (Rutz, Eidenschenk et al. 2013, Yang and Zheng 2014, Xie, Huang et al. 2015).
Critical to TH-17 differentiation is a transcription factor, Interferon Regulatory Factor 4 (IRF4), which is absolutely required for IL-17 and IL-21 production (Brustle, Heink et al. 2007, Chen, Yang et al. 2008, Huber, Brustle et al. 2008). Interestingly, while IRF4 promotes the production of IL-17 and IL-21, it inhibits the synthesis of IL-22 (Valdez, Vithayathil et al. 2012). During a search for proteins interacting with IRF4, our laboratory isolated a novel protein termed Def6 (also known as IBP or SLAT) (Hotfilder, Baxendale et al. 1999, Gupta, Lee et al. 2003, Tanaka, Bi et al. 2003). DEF6 serves a crucial immunoregulatory role in vivo as shown by the fact that Def6-deficient mice crossed to a TCR transgenic mouse (DO11.10) spontaneously develop RA-like disease due to enhanced IRF4 activation and dysregulated IL-17 and IL-21 production (Chen, Yang et al. 2008). One of the key mechanisms by which DEF6 regulates IRF4 function is by inhibiting its ability to be phosphorylated by ROCK2 (Biswas, Gupta et al. 2010). The ROCK2-mediated phosphorylation of IRF4, indeed, increases its binding to the IL-17 and IL-21 promoters and leads to higher levels of IL-17 and IL-21 production (Biswas, Gupta et al. 2010). ROCK2 and its other isoform, ROCK1, are serine-threonine kinases, which normally become activated upon binding of active GTP-bound RhoA (Amano, Nakayama et al. 2010, Schofield and Bernard 2013, Thumkeo, Watanabe et al. 2013, Julian and Olson 2014). Aberrant ROCK activation has been implicated in the pathogenesis of cardiovascular, renal, and neurological disorders (Mueller, Mack et al. 2005, Zhou, Gensch et al. 2011, Komers 2013). Interestingly, smoking has been shown to activate RhoA and the ROCKs in non-hematopoietic cells (Chiba, Murata et al. 2005, Noma, Goto et al. 2005, Noma, Goto et al. 2007) and smokers exhibit increased leukocyte ROCK activity (Hidaka, Hata et al. 2010).
In view of the potential link between CS and the ROCKs here we explored whether CS could alter the ROCK2-IRF4 axis in T cells. To accomplish this goal we took advantage of the Def6−/− DO11.10 cell culture system because of the higher levels of activation of the ROCK2-IRF4 pathway in these T cells, which could facilitate the detection of any changes upon CSE exposure. Interestingly, exposure of T cells from Def6−/− DO11.10 mice to cigarette smoke extract (CSE) resulted in decreased activation of ROCK2 and lower levels of IRF4 phosphorylation. These effects were associated with increased synthesis of IL-22. Using a GEF pull-down assay we furthermore identify ARHGEF1 as a key upstream regulator of ROCK2 whose activity is inhibited by CSE. Thus CS can directly alter TH-17 function via effects on the ROCK2-IRF4 axis.
2. Materials and Methods
2.1. Mice
C57BL/6 were obtained from Jackson Laboratory. Mice with DEF6 deficiency were generated by Lexicon Pharmaceuticals (Omnibank) using gene-trapping strategy and hence were originally termed Def6trap/trap mice (Fanzo, Yang et al. 2006). The original mice on a mixed 129XC57BL/6 background were backcrossed to Balb/c mice for >10 generations. Def6trap/trap mice on a Balb/c background were then crossed to DO11.10 TCR transgenic mice (Jackson Laboratory) to generate Def6trap/trap DO11.10 mice. To simplify the terminology, these mice will be referred to as Def6+/+ DO11.10 and Def6−/− DO11.10 mice in this manuscript. All mice used in the experiments were kept under specific pathogen–free conditions. Female mice (age 5 to 7 weeks old) were used in all experiments. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Hospital for Special Surgery.
2.2. CD4+ T cell isolation and cytokine production
CD4+ T cells were purified from the spleens using CD4+ T cell isolation kit from Miltenyi Biotech. For cytokine analysis, 1×106 cells/ml were stimulated with plate-bound anti-CD3ε (2 µg/ml) and soluble anti-CD28 (1 µg/ml) for 3 days and then rested in IL-2 (20 µg/ml) for 4 days. At day 7, cells were restimulated with plate-bound anti-CD3ε (2 µg/ml) and soluble anti-CD28 (1 µg/ml) for 24–48 hrs as previously described (Chen, Yang et al. 2008). Supernatants were analyzed for IL-17A (Biolegend), IL-21 (eBioscience), and IL-22 (Biolegend) production by ELISA.
2.3. Cell extracts and Western blotting
Nuclear and cytoplasmic extracts were prepared using the NE-PER Nuclear and Cytoplasmic extraction reagent kit as previously described (Chen, Yang et al. 2008, Biswas, Gupta et al. 2010). Anti-mouse ROCK1, ROCK2, IRF4 and ARHGEF1 Abs (Santa Cruz Biotechnology) were used to probe the Western blots according to the manufacturer’s instructions. Rabbit polyclonal Ab specific for phospho-IRF4 was generated by 21st Century Biochemicals Inc. using a synthetic phosphopeptide (YHRSIRH[pS][pS]IQE) corresponding to amino acids 439–450 of human IRF4 as an immunogen (the amino acid numbering in IRF4 sequence is based on GenBank accession no.U52682) and was previously described (Biswas, Gupta et al. 2010).
2.4. Real-time RT-PCR
Total RNA was isolated from cells using RNeasy Mini Kit (Qiagen GmbH). cDNAs were prepared and analyzed for expression of the gene of interest by quantitative real-time PCR (7500 Fast Real-Time PCR System, Applied Biosystems) using a Sybr-Green PCR master mix kit. PCR primers used for Il17, Il21, Il22, Irf4, and Rorγt have been previously described (Chen, Yang et al. 2008, Valdez, Vithayathil et al. 2012). Primers for mouse Arhgef1 were purchased from QIAGEN (Cat. QT02260727). The relative mRNA expression level of each gene was based on the comparison of the δCt value of restimulated DO11.10 Th0 sample normalized to β-actin.
2.5. ROCK1/ 2 kinase activity assays
ELISA
ROCK kinase activity in cell extracts was measured using the 96-well ROCK Activity Assay Kit (Cell Biolabs, Inc.) as previously described (Isgro, Gupta et al. 2013). Briefly, whole cell extracts (WCE) were prepared using 1% NP-40 lysis buffer. 10µg of the WCE were then incubated in a 96-well plate pre-coated with MYPT1, a ROCK substrate, in the presence of kinase reaction buffer containing ATP. After extensive washing, the wells were incubated with anti-phospho-MYPT1 (Thr696) antibody. After 1-hour, the wells were extensively washed and an HRP-conjugated secondary antibody was added for another hour. Substrate solution was then added to the wells and quantification performed on a spectrophotometer using 450nm as the primary wavelength. Active ROCK2 (1–4 ng) served as a positive control as per the manufacturer’s instructions.
ROCK1 and ROCK2 kinase activity assays
For these assays, which utilize exogenous recombinant MYPT1 as the ROCK substrate (Mong and Wang 2009), ROCK1 or ROCK2 were first immunoprecipitated from whole cell extracts of purified CD4+ T cells using an anti-ROCK1 or anti-ROCK2 antibody (Santa Cruz Biotechnology). The immunoprecipitated ROCK1 or ROCK2 was then subjected to an in vitro kinase reaction by incubating with purified recombinant MYPT1 substrate added exogenously in kinase buffer containing ATP according to the manufacturer’s instructions (Cell Biolabs). Phosphorylation of recombinant MYPT1 substrate by immunoprecipitated ROCK1 or ROCK2 was detected by Western blotting using an antiphospho-MYPT1 (T696) antibody.
2.6. Active ARHGEF1 pull-down
RhoA-G17A-conjugated agarose beads (Cell Biolabs; Cat. STA-431) were used to pull-down active ARHGEF1 from the whole cell extracts prepared from each condition, following the manufacturer’s instructions. Precipitated active ARHGEF1 was detected by Western blotting using an anti-ARHGEF1 antibody.
2.7. Cigarette Smoke Extract
Cigarette Smoke Extract (CSE) was prepared by bubbling the smoke of one cigarette (3R4F, University of Kentucky, Lexington, KY) through 25 ml of PBS for ten minutes. The cigarette smoke extract was pH balanced to 7.4 and sterile filtered prior to use (Mehra, Geraghty et al. 2012). We primarily used 0.625% CSE, which equates to ≈5 cigarettes per day (Cawood, Moriarty et al. 2007).
2.8 Statistical Analysis
Two-tailed Student’s t test was applied to all our in vitro studies. The statistical differences were considered significant when p-value < 0.05. Data were presented as mean ± SEM.
3. Results
3.1. Exposure of autoimmune CD4+ T cells to CSE induces the production of interleukin-22 (IL-22) under neutral conditions
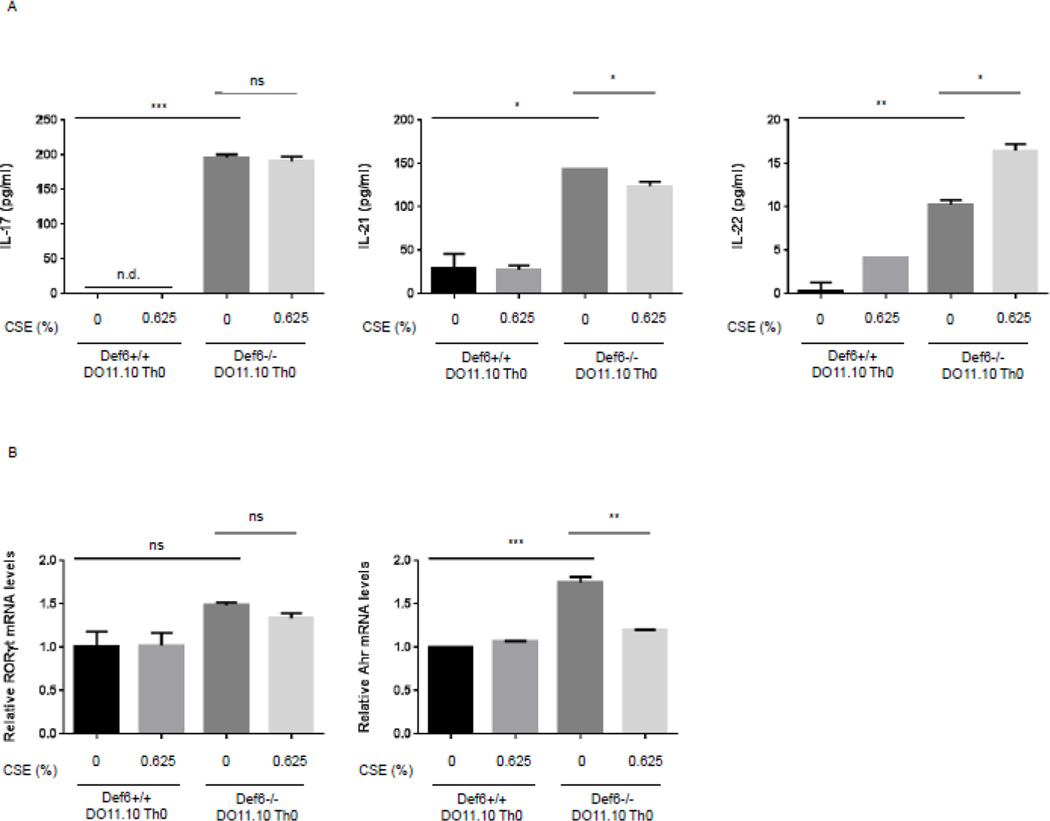
CS has previously been shown to increase ROCK activation in non-hematopoietic cells (Chiba, Murata et al. 2005, Noma, Goto et al. 2005, Noma, Goto et al. 2007). In view of the ability of the RhoA-ROCK pathway to promote the production of IL-17 and IL-21 by autoimmune T cells we proceeded to investigate whether CSE could further modulate the production of these cytokines. To gain insights into this question, we employed Def6−/− DO11.10 CD4+ T cells, which exhibit dysregulated ROCK2 activity and aberrant production of IL-17 and IL-21 under neutral conditions (Biswas, Gupta et al. 2010). CD4+ T cells were purified and expanded in vitro under neutral conditions and then restimulated for 24 hrs, culture conditions that have previously been shown to result in aberrant IL-17 and IL-21 production by Def6−/− DO11.10 CD4+ T cells as compared to control Def6+/+ DO11.10 CD4+ T cells (Chen, Yang et al. 2008). Pilot experiments demonstrated that addition of CSE at a concentration of >1.25% resulted in significant toxicity leading us to employ a CSE concentration of 0.625%, which was accompanied by no or minimal toxicity (data not shown). CSE was added upon restimulation of the cells and cytokine production in the supernatants assessed by ELISA. As compared to control CD4+ T cells, Def6−/− DO11.10 CD4+ T cells exhibited increased IL-17 and IL-21 production, which was not affected by exposure to CSE in a consistent manner (Fig. 1A). In line with these results, CSE did not alter the increased expression of RORγt, a key transcription factor controlling the production of IL-17 (Fig. 1B). Interestingly, however, we reliably observed that CSE upregulated IL-22 production (Fig. 1A). This effect was not due to increased expression of AHR, a transcription factor known to regulate IL-22 production (Fig. 1B). Exposure to CSE can thus lead to the upregulation of IL-22 production by autoimmune T cells.
Fig. 1. Effect of cigarette smoke extract (CSE) on cytokine production by Def6−/− DO11.10 CD4+ T cells.
CD4+ T cells from either Def6+/+ DO11.10 or Def6−/− DO11.10 mice were purified and cultured under neutral (Th0) (αCD3 2µg/ml, αCD28 1µg/ml) conditions for 3 days and then rested for 4 days in IL-2 (20ng/ml). After resting, CD4+ T cells were restimulated under neutral (Th0) conditions in the presence or absence of sterile CSE (0.625%) for 24 hours. (A) Cytokine levels of IL-17, IL-21, and IL-22 in culture supernatants were quantified by ELISA; n.d., cytokine levels non-detectable. (B) mRNA expression levels of RORγt and AHR were measured by quantitative real-time RT-PCR. Data are representatives of three independent experiments. Statistical analyses were performed by unpaired Student’s t test; ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
3.2. CSE exposure results in reduced IRF4 phosphorylation and ROCK2 kinase activity in Def6−/− DO11.10 CD4+ T cells
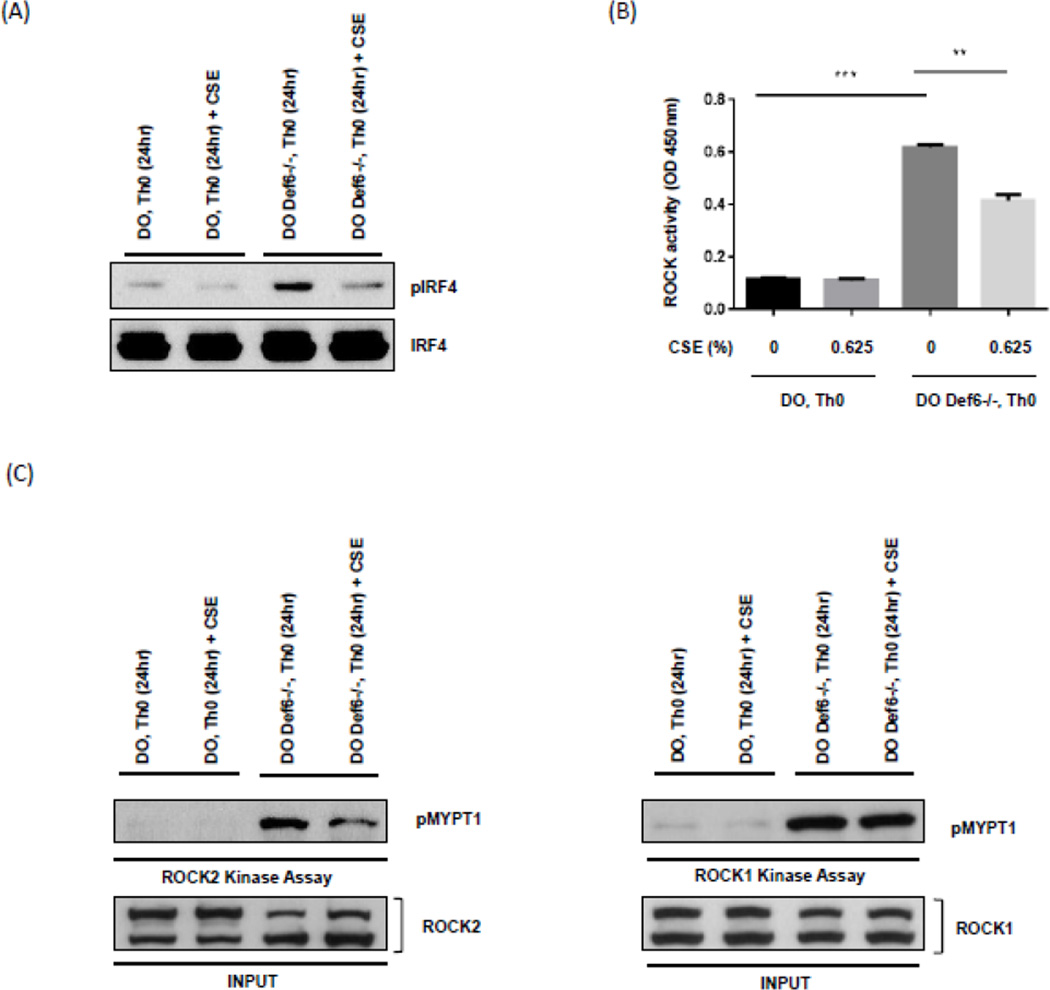
The increased production of IL-22 upon CSE exposure was surprising given that IRF4 normally inhibits IL-22 production and that Def6−/− DO11.10 CD4+ T cells are known to exhibit enhanced IRF4 activity due to increased phosphorylation by ROCK2 (Biswas, Gupta et al. 2010, Valdez, Vithayathil et al. 2012). We thus proceeded to directly evaluate the effects of CSE on the ROCK2-pIRF4 axis. Interestingly, CSE-treated Def6−/−DO11.10 CD4+ T cells exhibited lower levels of phosphorylated IRF4 compared to unexposed samples, although CSE did not significantly affect total IRF4 levels (Fig. 2A). In line with these findings a ROCK ELISA assay, which measures total ROCK activity (encompassing both ROCK1 and ROCK2) demonstrated lower levels of ROCK kinase activity (Fig. 2B). To directly assess whether ROCK2 rather than ROCK1 was affected by CSE we immunoprecipitated ROCK1 or ROCK2 and directly tested their kinase activity by incubating the precipitates with MYPT1. Exposure to CSE inhibited ROCK2 kinase activity to a much greater extent than ROCK1 activity (Fig. 2C). Thus, CSE selectively reduces ROCK2 kinase activity resulting in decreased IRF4 phosphorylation.
Fig. 2.
Exposure of Def6−/− DO11.10 CD4+ T cells to cigarette smoke extract (CSE) inhibits IRF4 phosphorylation and ROCK2 kinase activity. CD4+ T cells from either Def6+/+ DO11.10 (DO Th0) or Def6−/− DO11.10 (DO Def6−/− Th0) mice were purified and cultured under neutral (Th0) (αCD3 2µg/ml, αCD28 1µg/ml) conditions for 3 days and then rested for 4 days in IL-2 (20ng/ml). After resting, CD4+ T cells were restimulated under neutral (Th0) conditions in the presence or absence of sterile CSE (0.625%) for 24 hours. (A) Nuclear extracts from each condition were assayed for IRF4 phosphorylation by Western blotting using an antibody directed against phosphorylated IRF4 (pIRF4) (upper panel). Total IRF4 levels were evaluated by reprobing with an antibody against total IRF4 (lower panel). (B) Total ROCK kinase activity in whole cell extracts from each condition was assessed by an ELISA-based assay. (C) ROCK2 (left) and ROCK1 (right) kinase activity was examined by incubating immunoprecipitated ROCK2 or ROCK1 with purified recombinant MYPT1 (rMYPT1) as substrate. Phosphorylated rMYPT1 (pMYPT1) (shown in upper panel) was then detected using anti-phospho-MYPT1 antibody. Total ROCK2 or ROCK1 levels for each sample are shown in the lower panel. Data are representatives of two independent experiments. Statistical analyses were performed by unpaired Student’s t test; ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
3.3. CSE reduces activation of ARHGEF1, an upstream regulator of the RhoAROCK pathway
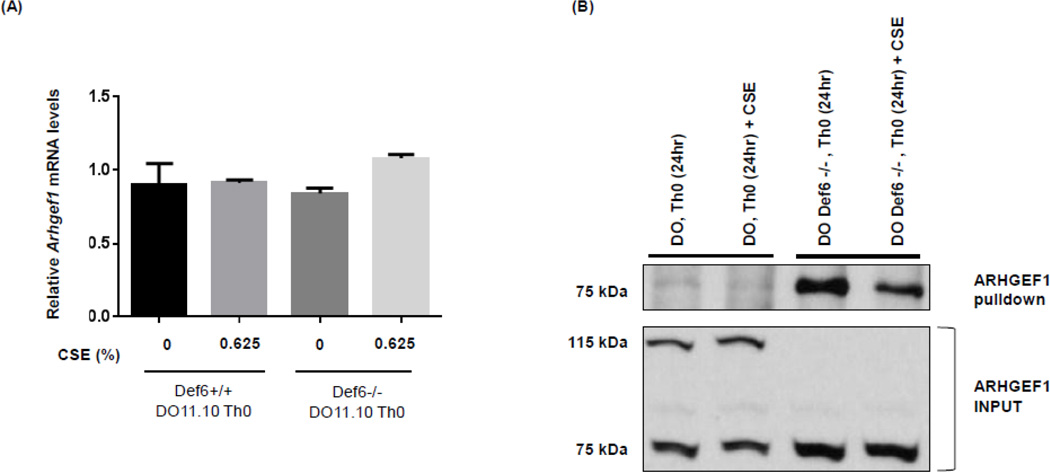
ROCK activation is primarily regulated upon binding of activated (or GTP-bound) RhoA to its RhoA Binding Domain (RBD). GTP loading of RhoA is, in turn, controlled by the activation of Rho guanine nucleotide exchange factors (GEFs) (Amano, Nakayama et al. 2010, Schofield and Bernard 2013, Thumkeo, Watanabe et al. 2013, Julian and Olson 2014). While a number of RhoGEFs exist within a cell, ARHGEF1 is known to be a major regulator of RhoA activation (Hart, Jiang et al. 1998, Kozasa, Jiang et al. 1998, Guan, Torres et al. 2013, Cook, Rossman et al. 2014). Real-time PCR analysis demonstrated that ARHGEF1 is expressed in Def6−/− DO11.10 CD4+ T cells at levels equivalent to Def6+/+ DO11.10 CD4+ T cells and that its mRNA expression is not affected by exposure to CSE (Fig. 3A). To investigate whether the decreased activity of ROCK2 could be accounted for by changes in the activity level of ARHGEF1, we next employed an active Rho-GEF pull-down assay (Arthur, Ellerbroek et al. 2002, Garcia-Mata, Wennerberg et al. 2006). In this assay, agarose beads coupled with a nucleotide free form of RhoA (G17A), which stabilizes the interaction between RhoA and the activated RhoGEF, are incubated with cell extracts and the presence of active ARHGEF1 visualized by western blotting with an ARHGEF1 antibody. Interestingly, while Def6+/+ DO11.10 CD4+ T cells expressed both a 100 kDa and a 75 kDa isoform of ARHGEF1, Def6−/− DO11.10 CD4+ T cells expressed primarily the shorter 75 kDa isoform (Fig. 3B). Consistent with the higher levels of ROCK2 activity in Def6−/− DO11.10 CD4+ T cells, ARHGEF1 activity was higher in Def6−/− DO11.10 CD4+ T cells than in Def6+/+ DO11.10 CD4+ T cells (Fig. 3B). Importantly, exposure to CSE substantially diminished the activity of ARHGEF1 in Def6−/− DO11.10 CD4+ T cells (Fig. 3B). Thus, Def6−/− DO11.10 CD4+ T cells exhibit increased levels of ARHGEF1 activity suggesting that this is the key regulator of ROCK2 activation in these cells. The inhibitory effects of CSE on ARHGEF1 activity, but not on its mRNA or protein expression, furthermore, suggest that the capacity of CSE to decrease ROCK2 activation in these cells relies on its ability to interfere with ARHGEF1 activation.
Fig. 3. CSE decreases the activity of ARHGEF1 in Def6−/− DO11.10 CD4+ T cells.
CD4+ T cells from either Def6+/+ DO11.10 (DO, Th0) or Def6−/− DO11.10 (DO Def6−/−, Th0) mice were purified and cultured under neutral (Th0) (αCD3 2µg/ml, αCD28 1µg/ml) conditions for 3 days and then rested for 4 days in IL-2 (20ng/ml). After resting, CD4+ T cells were restimulated under neutral (Th0) conditions in the presence or absence of sterile CSE (0.625%) for 24 hours. (A) mRNA expression levels of Arhgef1 were measured by quantitative real-time RT-PCR. Statistical analyses were performed by unpaired Student’s t test; ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) RhoA G17A-conjugated agarose beads were used to pull-down active ARHGEF1 from whole cell extracts of CD4+ T cells prepared from each condition. Precipitated active ARHGEF1 (upper panel) and total ARHGEF1 (input) levels in each sample (lower panel) were detected by Western blotting using an antibody against ARHGEF1. Data are representatives of two independent experiments.
3.4. Exposure to CSE upregulates IL-22 production in TH-17 cells from non-autoimmune mice
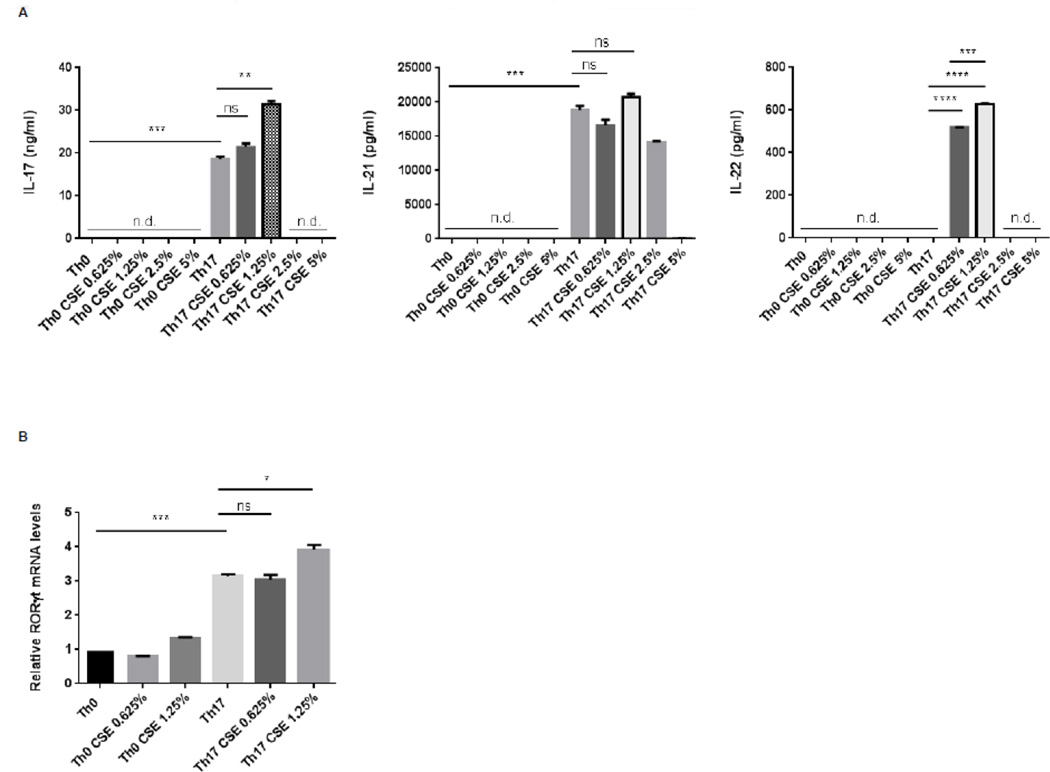
Unlike what is observed with Def6−/− DO11.10 CD4+ autoimmune T cells, production of IL-22 by nonautoimmune T cells requires exposure to specific cytokine milieus such as those encountered upon TH-17 differentiation (Rutz, Eidenschenk et al. 2013). To evaluate whether the effects of CSE on IL-22 production could also be observed in differentiating TH-17 cells, we sorted naïve T cells from C57BL/6 mice and differentiated them under neutral (TH0) or TH-17 conditions for 3 days in the presence/absence of increasing doses of CSE (Fig. 4A). Similar to the DO11.10 T cell system, CSE doses >1.25% exerted profound inhibitory effects on cytokine production due to its toxic effects on T cells. While the effects of lower doses (0.625% and 1.25%) of CSE on IL-17 and IL-21 production were again variable, addition of CSE at those doses consistently upregulated IL-22 production by TH-17 cells (Fig. 4A). No effects of CSE could instead be detected in TH0 cells (Fig. 4A). The upregulation of IL-22 by 0.625% CSE furthermore was not accompanied by changes in the expression of RORγt in cells cultured under TH-17-polarizing condition (Fig. 4B). Exposure to low-levels of CSE thus leads to the upregulation of IL-22 production by non-autoimmune T cells exposed to TH-17 differentiating condition.
Fig. 4. Effects of cigarette smoke extract (CSE) on cytokine production by nonautoimmune CD4+ T cells cultured under either Th0 or Th17 conditions.
FACS-sorted naïve CD4+ T cells (CD4+, CD62L+, CD44−, CD25−) from wild-type C57BL/6 female mice were cultured under either neutral conditions (Th0) (αCD3 2µg/ml, αCD28 1µg/ml) or Th17 polarizing conditions (αCD3 2µg/ml, αCD28 1µg/ml, IL-6 100ng/ml, TGFβ 2ng/ml) for 4 days in the presence or absence of the indicated dose of sterile cigarette smoke extract (CSE). (A) Cytokine levels of IL-17A (left panel), IL-21 (middle panel), and IL-22 (right panel) in the culture supernatants were quantified by ELISA; n.d., cytokine level non-detectable. (B) mRNA expression levels of RORγt was measured by quantitative real-time RT-PCR. Statistical analyses were performed by unpaired Student’s t test; ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Data are representatives of three independent experiments.
4. Discussion
Given the pathogenic role of TH-17 cells in RA and the strong association between CS and the development of RA here we evaluated whether CS exerts direct effects on the ability of CD4+ T cells from either autoimmune or nonautoimmune mice to produce TH-17 cytokines. We found that exposure to CS consistently resulted in upregulation of IL-22 production. This was associated with the ability of CSE to downregulate ROCK2 activation and the phosphorylation of IRF4, a known negative regulator of IL-22 production (Valdez, Vithayathil et al. 2012). Interestingly, the inhibitory effects of CSE on ROCK2 activity were accompanied by a decrease in the activation of ARHGEF1, an upstream regulator of RhoA. Thus CS can alter T cell function by modulating the activity of the RhoA-ROCK2 pathway.
The ability of CSE to increase IL-22 production could provide a potential new mechanism by which CS can promote the development of RA. Indeed increased IL-22 production has been observed in peripheral blood mononuclear cells and joints of RA patients and serum levels of IL-22 have been associated with disease severity (Rutz, Eidenschenk et al. 2013, Yang and Zheng 2014, Xie, Huang et al. 2015). Furthermore, IL-22 can mediate a number of effects that have been linked to RA pathogenesis including driving the production of pro-inflammatory cytokines like IL-1β, IL-6, and TNFα, increasing expression of matrix metalloproteinase (MMP)-9, and promoting osteoclastogenesis. In line with these findings mice deficient in IL-22 exhibit delayed onset of CIA and decreased disease severity (Geboes, Dumoutier et al. 2009). Intriguingly, the capacity of CS to drive IL-22 production may also underlie the known beneficial effects of smoking in inflammatory bowel disease (Motley, Rhodes et al. 1987), where the antimicrobial and tissue regenerative functions of IL-22 may exert protective rather than pathogenic functions.
Unlike the consistent effects that CSE exerted on IL-22 production, its ability to modulate the synthesis of IL-17 and IL-21 was more variable possibly due to the complexities underlying the regulation of these different cytokines in CD4+ T cells. Indeed, despite the employment of some shared transcriptional modules, important differences exist in the control of IL-17 and IL-22 production (Rutz, Eidenschenk et al. 2013). Indeed activation of STAT3 is critical for the production of IL-22 but does promote IL-17 production by itself. In contrast, although the TH-17 master regulator-RORγt- is a positive regulator of both IL-17 and IL-22 expression, RORγt alone can drive the expression of IL-17 but may regulate IL-22 indirectly. The requirement for the aryl hydrocarbon receptor (AHR) in the control of IL-22 production also differs depending on the presence or absence of TH-17 promoting cytokines like TGFβ whereby ligand-dependent activation of AHR is required to overcome the effects of c-Maf, which is induced downstream of TGF-β signaling and inhibits the production of IL-22 but promotes IL-17 synthesis.
Similarly to c-Maf, IRF4 is an activator of IL-17 and IL-21 but a repressor of IL-22 production. The simultaneous ability of CSE to downregulate IRF4 phosphorylation and upregulate IL-22 production thus suggests that the inhibitory effects of IRF4 may be more sensitive to its phosphorylation levels than its activating functions. Alternatively, distinct kinetics may be at play whereby the effects of CSE on IRF4 phosphorylation, which require a series of inhibitory events starting with a decrease in the activation of ARHGEF1, may not take place in sufficient time to interfere with the ability of IRF4 to drive the production of IL-17 and IL-21. Given the known ability of CSE to mediate the activation of AHR (Esakky, Hansen et al. 2012) it is also possible that CSE induces IL-22 production via a dual mechanism whereby it concomitantly promotes the activity of a transcriptional activator (AHR) while interfering with that of an inhibitor (IRF4). This dual mechanism may furthermore explain the different effects of CSE on IL-17 production in TH-17 cells versus the Def6−/− DO11.10 CD4+ T cells since AHR would be expressed at high levels in the TH-17 cultures but not in the Def6−/− DO11.10 cultures due to the presence of TGF-β in the former but not the latter setting.
One of the most interesting aspects of our studies was the finding that the inhibitory effects of CSE on the ROCK2-IRF4 axis were associated with its ability to decrease the activation of a known upstream regulator of the RhoA-ROCK pathway, ARHGEF1. CSE is a complex mixture of substances including acrolein, nicotine, methyl vinyl ketone and volatile reactive oxygen species (Takanami, Moriyama et al. 2009, Noya, Seki et al. 2013). The precise component that diminished ARHGEF1 activity in T cells remains to be determined. However, it is important to note that oxidants, which are present in CSE, can alter the ability of guanine nucleotide exchange factors to interact with their targets (Wey, Phan et al. 2014). Thus, oxidative protein modifications could potentially explain why ARHGEF1 bound RhoA less avidly in the CSE treated T cells. While no effects of CSE on the expression of ARHGEF1 were observed, we did detect different ARHGEF1 isoforms in wt versus Def6-deficient T cells. Since ARHGEF1 is known to be a 27-exon gene with multiple splice variants whose precise functions have not been defined (Muppidi, Schmitz et al. 2014), it will also be important in future studies to delineate whether these ARHGEF1 isoforms are differentially susceptible to the effects of CSE. Intriguingly, previous studies have shown that ARHGEF1-deficient mice spontaneously develop pulmonary pathology characterized by key features of emphysema with increased expression and activity of MMPs, airspace enlargement, and decreased lung elastance (Hartney, Brown et al. 2010). Of additional interest was the finding that macrophages lacking ARHGEF1 produce increased levels of MMP-2, -9 and -12 upon adhering to fibronectin (Hartney, Gustafson et al. 2011) suggesting that ARHGEF1 is critical for the ability of immune cells to properly sense cues derived from the extracellular matrix, a parameter that could be of great relevance to RA pathophysiology.
While the inhibitory effects of CSE on the ROCK2-IRF4 axis might seem surprising in view of previous work demonstrating high total ROCK activity levels in pulmonary arteries and in leukocytes of smokers (Hidaka, Hata et al. 2010), the ability of CSE to dampen this axis is consistent with the known capacity of CS to exert a wide-range of immunosuppressive effects. In line with these inhibitory effects, furthermore, nicotine has previously been shown to decrease the frequencies of IL-17 producing cells in a murine model of IBD although IL-22 production in those studies was not evaluated (Liu, Han et al. 2014). Furthermore, the short-term immunosuppressive effects of CS may eventually promote inflammatory sequelae because of associated effects like a decreased ability to fight infections. In this regard, it is important to note that CS exerted greater inhibitory effects on ROCK2 than on ROCK1 activation thus potentially altering the balance between these two isoforms. This finding raises the possibility that the elevated levels of ROCK activity in smokers may reflect primarily increases in ROCK1. The development of tools designed to specifically assess the activity of the two isoforms as well as the generation of genetic approaches aimed at dissecting the precise role of the two isoforms will be critical to shed insights into this possibility.
Highlights.
Direct exposure of CD4+ T cells to cigarette smoke extract (CSE) enhances IL-22 production.
Treatment with CSE inhibits the RhoA-ROCK2 signaling pathway and reduces phosphorylation of IRF4.
Decreased activation of the RhoA-ROCK2 pathway by CSE is associated with a reduction in the activation of ARHGEF1, an upstream regulator of the RhoA-ROCK axis.
Acknowledgments
The research was supported by the Rheumatology Research Foundation Innovative Research Grant, the Peter Jay Sharp Foundation, and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chien-Huan Weng, Email: chw2019@med.cornell.edu.
Sanjay Gupta, Email: guptasa@hss.edu.
Patrick Geraghty, Email: Patrick.Geraghty@downstate.edu.
Robert Foronjy, Email: robertforonjy@downstate.edu.
Alessandra B. Pernis, Email: pernisa@hss.edu.
References
- Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67(9):545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem. 2002;277(45):42964–42972. doi: 10.1074/jbc.M207401200. [DOI] [PubMed] [Google Scholar]
- Baka Z, Buzas E, Nagy G. Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res Ther. 2009;11(4):238. doi: 10.1186/ar2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, Bhagat G, Pernis AB. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120(9):3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8(9):958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- Cawood TJ, Moriarty P, O'Farrelly C, O'Shea D. Smoking and thyroid-associated ophthalmopathy: A novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92(1):59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29(6):899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Murata M, Ushikubo H, Yoshikawa Y, Saitoh A, Sakai H, Kamei J, Misawa M. Effect of cigarette smoke exposure in vivo on bronchial smooth muscle contractility in vitro in rats. Am J Respir Cell Mol Biol. 2005;33(6):574–581. doi: 10.1165/rcmb.2005-0177OC. [DOI] [PubMed] [Google Scholar]
- Chujo S, Okamoto S, Sunahara R, Adachi M, Yamada K, Hayashi H, Takii T, Hayakawa K, Onozaki K. Cigarette smoke condensate extracts augment collagen-induced arthritis in mice. Int Immunopharmacol. 2010;10(10):1194–1199. doi: 10.1016/j.intimp.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2014;33(31):4021–4035. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenbader KH, Gay S, Alarcon-Riquelme ME, Iaccarino L, Doria A. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev. 2012;11(8):604–609. doi: 10.1016/j.autrev.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Esakky P, Hansen DA, Drury AM, Moley KH. Cigarette smoke condensate induces aryl hydrocarbon receptor-dependent changes in gene expression in spermatocytes. Reprod Toxicol. 2012;34(4):665–676. doi: 10.1016/j.reprotox.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Fanzo JC, Yang W, Jang SY, Gupta S, Chen Q, Siddiq A, Greenberg S, Pernis AB. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J Clin Invest. 2006;116(3):703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Wennerberg K, Arthur WT, Noren NK, Ellerbroek SM, Burridge K. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, Matthys P. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60(2):390–395. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- Gerlag DM, Norris JM, Tak PP. RA: from risk factors and pathogenesis to prevention: Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxford) 2015 doi: 10.1093/rheumatology/kev347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Torres RM, Hartney JM. The influence of Arhgef1 on pulmonary leukocyte function. Immunol Res. 2013;55(1–3):162–166. doi: 10.1007/s12026-012-8360-0. [DOI] [PubMed] [Google Scholar]
- Gupta S, Lee A, Hu C, Fanzo J, Goldberg I, Cattoretti G, Pernis AB. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Human Immunol. 2003;64:389–401. doi: 10.1016/s0198-8859(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280(5372):2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Hartney JM, Brown J, Chu HW, Chang LY, Pelanda R, Torres RM. Arhgef1 regulates alpha5beta1 integrin-mediated matrix metalloproteinase expression and is required for homeostatic lung immunity. Am J Pathol. 2010;176(3):1157–1168. doi: 10.2353/ajpath.2010.090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartney JM, Gustafson CE, Bowler RP, Pelanda R, Torres RM. Thromboxane receptor signaling is required for fibronectin-induced matrix metalloproteinase 9 production by human and murine macrophages and is attenuated by the Arhgef1 molecule. J Biol Chem. 2011;286(52):44521–44531. doi: 10.1074/jbc.M111.282772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T, Hata T, Soga J, Fujii Y, Idei N, Fujimura N, Kihara Y, Noma K, Liao JK, Higashi Y. Increased leukocyte rho kinase (ROCK) activity and endothelial dysfunction in cigarette smokers. Hypertens Res. 2010;33(4):354–359. doi: 10.1038/hr.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoovestol RA, Mikuls TR. Environmental exposures and rheumatoid arthritis risk. Curr Rheumatol Rep. 2011;13(5):431–439. doi: 10.1007/s11926-011-0203-9. [DOI] [PubMed] [Google Scholar]
- Hotfilder M, Baxendale S, Cross MA, Sablitzky F. Def-2,-3,-6,-8, novel mouse genes differentially expressed in the hematopoietic system. Br. J. Haematol. 1999;106:335–344. doi: 10.1046/j.1365-2141.1999.01551.x. [DOI] [PubMed] [Google Scholar]
- Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Low E, Lohoff M. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008;105(52):20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgro J, Gupta S, Jacek E, Pavri T, Duculan R, Kim M, Kirou KA, Salmon JE, Pernis AB. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65(6):1592–1602. doi: 10.1002/art.37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L, Malmstrom V, Lundberg K, Padyukov L, Alfredsson L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin Immunol. 2011;23(2):92–98. doi: 10.1016/j.smim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Koenders MI, van den Berg WB. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol Sci. 2015;36(4):189–195. doi: 10.1016/j.tips.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Komers R. Rho kinase inhibition in diabetic kidney disease. Br J Clin Pharmacol. 2013;76(4):551–559. doi: 10.1111/bcp.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280(5372):2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Lindblad SS, Mydel P, Jonsson IM, Senior RM, Tarkowski A, Bokarewa M. Smoking and nicotine exposure delay development of collagen-induced arthritis in mice. Arthritis Res Ther. 2009;11(3):R88. doi: 10.1186/ar2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Han B, Li P, Wang Z, Fan Q. Activation of alpha7nAChR by nicotine reduced the Th17 response in CD4(+)T lymphocytes. Immunol Invest. 2014;43(7):667–674. doi: 10.3109/08820139.2014.914532. [DOI] [PubMed] [Google Scholar]
- Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11(10):562. doi: 10.1038/nrrheum.2015.128. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- Mehra D, Geraghty PM, Hardigan AA, Foronjy R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS One. 2012;7(12):e52889. doi: 10.1371/journal.pone.0052889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009;182(4):2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- Motley RJ, Rhodes J, Ford GA, Wilkinson SP, Chesner IM, Asquith P, Hellier MD, Mayberry JF. Time relationships between cessation of smoking and onset of ulcerative colitis. Digestion. 1987;37(2):125–127. doi: 10.1159/000199478. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4(5):387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Schmitz R, Green JA, Xiao W, Larsen AB, Braun SE, An J, Xu Y, Rosenwald A, Ott G, Gascoyne RD, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Vaidehi N, Staudt LM, Cyster JG. Loss of signalling via Galpha13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516(7530):254–258. doi: 10.1038/nature13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Goto C, Nishioka K, Hara K, Kimura M, Umemura T, Jitsuiki D, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Higashi Y. Smoking, endothelial function, and Rho-kinase in humans. Arterioscler Thromb Vasc Biol. 2005;25(12):2630–2635. doi: 10.1161/01.ATV.0000189304.32725.bd. [DOI] [PubMed] [Google Scholar]
- Noma K, Goto C, Nishioka K, Jitsuiki D, Umemura T, Ueda K, Kimura M, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Liao JK, Higashi Y. Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49(6):698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noya Y, Seki K, Asano H, Mai Y, Horinouchi T, Higashi T, Terada K, Hatate C, Hoshi A, Nepal P, Horiguchi M, Kuge Y, Miwa S. Identification of stable cytotoxic factors in the gas phase extract of cigarette smoke and pharmacological characterization of their cytotoxicity. Toxicology. 2013;314(1):1–10. doi: 10.1016/j.tox.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Adachi M, Chujo S, Yamada K, Akita K, Itoh S, Takii T, Hayakawa K, Onozaki K. Etiological role of cigarette smoking in rheumatoid arthritis: Nasal exposure to cigarette smoke condensate extracts augments the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun. 2011;404(4):1088–1092. doi: 10.1016/j.bbrc.2010.12.118. [DOI] [PubMed] [Google Scholar]
- Pernis AB. Th17 cells in rheumatoid arthritis and systemic lupus erythematosus. J Intern Med. 2009;265(6):644–652. doi: 10.1111/j.1365-2796.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252(1):116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol. 2013;48(4):301–316. doi: 10.3109/10409238.2013.786671. [DOI] [PubMed] [Google Scholar]
- Takanami Y, Moriyama T, Kosaka Y, Nakayama T. Analysis of hydrogen peroxide in an aqueous extract of cigarette smoke and effect of pH on the yield. Biosci Biotechnol Biochem. 2009;73(10):2222–2225. doi: 10.1271/bbb.90324. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Bi K, Kitamura R, Hong S, Altman Y, Matsumoto A, Tabata H, Lebedeva S, Bushway PJ, Altman A. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity. 2003;18:403–414. doi: 10.1016/s1074-7613(03)00054-2. [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92(10–11):303–315. doi: 10.1016/j.ejcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Valdez PA, Vithayathil PJ, Janelsins BM, Shaffer AL, Williamson PR, Datta SK. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity. 2012;36(4):668–679. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey M, Phan V, Yepez G, Heo J. Superoxide inhibits guanine nucleotide exchange factor (GEF) action on Ras, but not on Rho, through desensitization of Ras to GEF. Biochemistry. 2014;53(3):518–532. doi: 10.1021/bi401528n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Huang C, Li J. Interleukin-22 and rheumatoid arthritis: emerging role in pathogenesis and therapy. Autoimmunity. 2015;48(2):69–72. doi: 10.3109/08916934.2014.959165. [DOI] [PubMed] [Google Scholar]
- Yang X, Zheng SG. Interleukin-22: a likely target for treatment of autoimmune diseases. Autoimmun Rev. 2014;13(6):615–620. doi: 10.1016/j.autrev.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32(3):167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]