Abstract
Background
Evidence shows both a tendency for research participants to conflate research and clinical care and limited public understanding of research. Conflation of research and care by participants is often referred to as the therapeutic misconception. Despite a lack of evidence, few studies have explicitly asked participants, and especially minors, to explain what they think research is and how they think it differs from regular medical care.
Methods
As part of a longer semi-structured interview evaluating assent and parental permission for research, adolescent research participants, including adolescents with illnesses and healthy volunteers (N=177), and their parents (N=177) were asked to describe medical research in their own words and say whether and how they thought being in medical research was different from seeing a regular doctor. Qualitative responses were coded and themes identified through an iterative process.
Results
When asked to describe medical research, the majority described research in terms of its goals of helping to advance science, develop treatments and medicines, and help others; fewer described research as having the goal of helping particular research participants, and fewer still in terms of the methods used in research. The majority of teen and parent respondents said being in research is different than seeing a regular doctor and explained this by describing different goals, different or more procedures, differences in the engagement of the doctors/researchers, and in logistics.
Conclusions
Adolescents participating in clinical research and their parents generally describe medical research in terms of its goals of advancing science and finding new medicines and treatments, sometimes in combination with helping the enrolled individuals. The majority perceives a difference between research and regular medical care and described these differences in various ways. Further exploration is warranted about how such perceived differences matter to participants and how this understanding could be used to enhance informed consent and the overall research experience.
Keywords: clinical research, ethics, assent and parental consent, therapeutic misconception
Ethically, it is important for research participants to understand that they are enrolled in research. Some assert that it is essential for research participants to appreciate the difference between clinical research and clinical care in order to give valid informed consent (Miller 2006). Yet, it is not always clear how well research participants understand what research is and the purpose and nature of the studies they join.
Despite a strong belief in the value of scientific research, evidence also shows that the American public has limited scientific literacy and health literacy (Institute of Medicine 2004; Miller 2007; National Science Foundation 2014). Understanding of research may be hampered both by limited scientific literacy in general as well as by the expectations that research participants often have when considering research enrollment (Pickersgill 2011; Miller 2000; Sankar P 2004).
Commentators have worried that research participants do not understand the difference between clinical research and clinical care. (Appelbaum, Lidz, and Grisso 2004; Henderson et al 2007; Snowdon, Elbourne, and Garcia 2007; Pentz et al. 2012). This misunderstanding is referred to as the “therapeutic misconception.” Although commentators do not always use the same definition of the therapeutic misconception, the general concern is that participants confuse research with medical care based on a misunderstanding or lack of appreciation that the goal of research is to produce generalizable knowledge and that research is not designed for their individualized benefit (Appelbaum, Lidz, and Grisso 2004; Chou and O’Rourke 2012; Henderson et al. 2007).
Mounting evidence from empirical studies suggests that participants often do have a therapeutic misconception (Appelbaum, Lidz, and Grisso 2004). Some, however, are skeptical about the pervasiveness or importance of the therapeutic misconception. Wendler (2012) argues, for example, that what participants should understand depends on the specific context and study and that focusing on the therapeutic misconception is a distraction. Kim and colleagues conclude from their data that motivation for benefit does not preclude understanding the purpose of research and the prevalence of a true “misconception” may be overstated because of how it is measured (Kim et al. 2009; Kim et al. 2013). Further, few studies report how participants, and especially minors, actually describe clinical research or describe the differences between clinical research and regular medical care not limited to their experiences in any particular study.
In the present study, we sought to learn how adolescent research participants and their parents describe medical research in general and how they explain the differences that they perceive between medical research and regular medical care.
METHODS
Study Design
This analysis is part of a larger, cross-sectional, descriptive, semi-structured interview study of adolescents enrolled in clinical research along with one of their parents. A purposeful sample of adolescents between 13 and 17 years of age with a variety of disorders as well as healthy volunteers who were currently enrolled in clinical research at the National Institutes of Health (NIH) Clinical Center or at Seattle Children’s Hospital and had enrolled in their respective research studies within the previous 6 months were invited to participate.
The clinical research team referred eligible adolescents to the interview study. Teens or parents also self-referred in response to posted fliers. Data were obtained through concurrent but separate face-to-face interviews in English with the adolescent and one parent.
Trained interviewers, independent of the clinical research team, interviewed adolescents and parents separately. The interview questions were multiple-choice or open-ended and assessed the following domains: (1) assent/parental permission, (2) motivations, (3) decision making, (4) knowledge and attitudes about research, (5) willingness to accept research risks, and (6) demographics and clinical history.
Parental and adolescent interviews were similar so that responses could be compared. Health status (classified as one of the following: healthy volunteer; minor condition; significant condition – chronic well-controlled; significant condition – chronic not well-controlled; or significant condition – life-threatening) was assessed at the time of the interview by medical providers within each participant’s primary research team. Further details about the methods are reported elsewhere (Wendler et al. 2012).
In this analysis, we report the results of two open-ended questions from the longer survey. These questions asked adolescents and parents to describe what medical research is and how it differs from regular clinical care. Each adolescent and parent were specifically asked the following questions:
Please describe in your own words what you think medical research is.
Would you say that being in a medical research study is: Just like seeing a regular doctor or different than seeing a regular doctor?
Those who replied that it was different were asked to explain how they thought it was different..
Data Analysis and Statistical Methods
Although the larger survey study was not designed as a qualitative study, we followed standard qualitative methods in analyzing the responses to these two questions. Interviewers asked the questions and recorded the responses verbatim, probing to clarify responses when necessary.
Teen responses and parental responses to each question were aggregated separately. The aggregate responses were read through independently by two investigators (IN, CG) to derive an overall impression and develop preliminary codes and concepts. Each coder developed separate codes based on patterns in the open-ended responses. Codes were then compared, combined, differences were reconciled, and codes were revised. Each coder applied the final codes to the responses. Discrepancies were reconciled by a third investigator.
Themes that described research and differences between medical research and regular care emerged from the coded responses. These themes were consistent with descriptions of research found in the literature. Major concepts, themes, and patterns were identified and compared. Direct quotations were selected for clarity and representativeness of the theme. We evaluated the frequency of themes to note any differences between responses from adolescents and parents, healthy and ill adolescents, and teens with previous research experience and those who had no previous experience.
Human Subjects Protection
The institutional review boards (IRBs) of the National Institute of Child Health and Human Development, Seattle Children’s Hospital, and Research Triangle International approved the study. Written parental permission and adolescent assent were obtained for each teen interview, along with informed consent for each parent’s interview. Teens were offered a $20 gift card for their participation in the interview.
RESULTS
Responses from a total of 354 respondents, 177 adolescents and 177 parents, are reported here. Characteristics of the sample are shown in Table 1 and have been reported elsewhere (Wendler et al. 2012). Slightly fewer than half (44%) of the teens had previous research experience, ranging from participation in one previous study to more than six previous studies. At the time of the interview, 39 teens (22%) were enrolled in research as healthy volunteers, 14 (7.9%) were in studies for a mental health condition, and 124 (70%) for a medical condition. The majority (62.7%) of the clinical studies that the teens were enrolled in at the time of the interview were classified as studies without the prospect of direct benefit.
TABLE 1.
Sample Characteristics N (%)
| Adolescents N=177 |
Parents N=177 |
|
|---|---|---|
| Gender | ||
| Female | 91 (51.4) | 135 (76.3) |
| Male | 86 (48.6) | 38 (21.5) |
| Mean Age (SD) | 15.1 (1.4) | 45.3 (6.8) |
| Hispanic/Latino* | 22 (12.4) | 17 (9.6) |
| Race* | ||
| White/Caucasian | 123 (69.5) | 128 (72.3) |
| Black/African American | 26 (14.7) | 20 (11.3) |
| Native American | 6 (3.4) | 3 (1.7) |
| Asian | 8 (4.5) | 5 (2.8) |
| Native Hawaiian/Pacific Islander | 2 (1.1) | 1 (0.5) |
| Other | 20 (15.8) | 20 (13.6) |
| Don’t know/Did not answer | 8 (4.4) | 4 (2.2) |
| Prior Research | ||
| 0 | 98 (55.9) | |
| 1–2 | 51 (28.8) | |
| 3–6 | 17 (9.6) | |
| >6 | 9 (5.1) | |
| Adolescent’s Illness Status§ | ||
| Healthy | 37 (20.9) | |
| Minor | 12 (6.8) | |
| Significant, Controlled | 76 (42.9) | |
| Significant, Not Controlled | 31 (17.5) | |
| Significant, Life Threatening | 21 (11.9) | |
Note. SD = standard deviation
Self defined: respondents could choose more than one race.
Based on the view of the adolescents’ clinical research team.
What is Medical Research?
When participants were asked to describe medical research in their own words, six distinct themes emerged: helping science (H1), helping others (H2), helping specific participants (P1), investigating specific participants (P2), description of methods, and experimentation. These themes are described in Table 2, accompanied by illustrative quotes. “Helping science” emerged from responses related to the goals of research as a scientific activity designed to generate knowledge that would advance science or medicine by developing treatments and cures for diseases. The closely related theme of “helping others” included responses that described research as an activity with the goal of learning something about treatment or prevention that would help other patients or future patients. Many respondents gave a description of research that expressed both themes, such as “Research consists of trying to figure out how to cure an illness or find out more about it in order to help other people.”
TABLE 2.
Themes and selected responses to the question “What is research?”
| Theme | Explanation | Illustrative Quotes |
|---|---|---|
| Helping science (H1) | Helping science or medicine, fighting diseases | “…trying to explore different ways to fight off certain diseases or find out more about them.” “…research into health, medicine, treatments, diagnoses, all aspects of medical care and how to make it better.” “…doing studies on different people so [that] doctors/scientists get information on different diseases so that they can find reasons or cures.” |
| Helping others (H2) | Helping other patients or future patients | “Research helps other people with the same illness or problems.” “…research in a particular disease or illness that can benefit anyone that has the disease in the future.” “Research consists of a group of people getting together for the benefit of mankind. It allows all of us to get together to look at diseases and make life better for those who are suffering.” |
| Both H1 and H2 | “Research consists of trying to figure out how to cure an illness or find out more about it in order to help other people.” “…investigations to determine what treatments will be effective for disease, the extent to which they will be effective, and the designated population of diseases.” |
|
| Helping specific Participants (P1) | Finding a treatment, cure, or diagnosis for a particular patient, helping that patient get better | “…medical researching where doctors study your illness.” “…taking a group of people, giving them some tests, applying some treatments, seeing the results for a specific illness or condition.” |
| Investigating specific participants (P2) | Figuring out a participant problem or doing a detailed study to find something that other doctors could not figure out | “…taking a look at the issue at hand and dissecting into it to determine the cause and other variables.” “…figuring out how different things work on different people who have the same disease.” |
| Both P1 and P2 | “Researchers are trying to find out what the person has and to find a cure for his/her condition.” “Research consists of getting to know all the people’s problems and what they are going through to help them get better.” |
|
| Methods | Methods: procedures, tests, data collection | “…conducting procedures to find out which will affect the patient, determining whether it will have positive or negative effects.” “…collecting information to find solutions to problems or review medical conditions.” |
| Experimentation | Guinea pigs, experiments, feeling used | “You’re a guinea pig. They throw stuff they make at you and say ‘whoops’ if it doesn’t work.” |
Two themes emerged from descriptions of the goals of research as more directed to the actual study participants. “Helping specific participants” included responses that described research as an activity designed to assist the participant to get better or to directly help the particular patient-participant by finding a treatment, cure, or diagnosis for him or her. A related theme of “investigating specific participants” encompassed responses that described the goal of research as helping the particular participant by intensely studying, as opposed to treating, a patient’s condition in order to understand it more clearly and discover new things about it, which might eventually allow appropriate treatment. In the latter cases, respondents described research as helping particular participants by figuring out a problem, studying the individual from head to toe, or attempting to learn something that other doctors could not figure out. Some responses, such as “Researchers are trying to find out what the person has and to find a cure for his/her condition” or “Research consists of getting to know all the people’s problems and what they are going through to help them get better” were coded as falling into both of these participant-specific categories.
In some cases, respondents described research as a set of methods that might be used to conduct research (e.g., drug development or collecting information/data) rather than describing the goals [of research]. Very few respondents described research as experimentation similar to that with laboratory animals.
Overall, the most common themes emerging from both adolescent and parent responses described medical research as an activity with the goal of helping science or helping future patients through developing information and finding new treatments (H1 and H2). The second most common description of research was of an activity with the goal of treating or studying the individual patient; this theme was found in more parent responses than in responses from the teens. (See Figures 1 and 2.)
Figure 1.
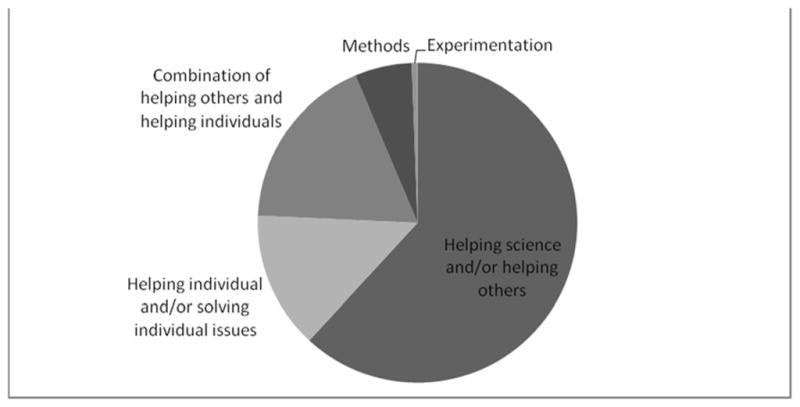
Teens’ responses to the question “What is medical research?”
Figure 2.
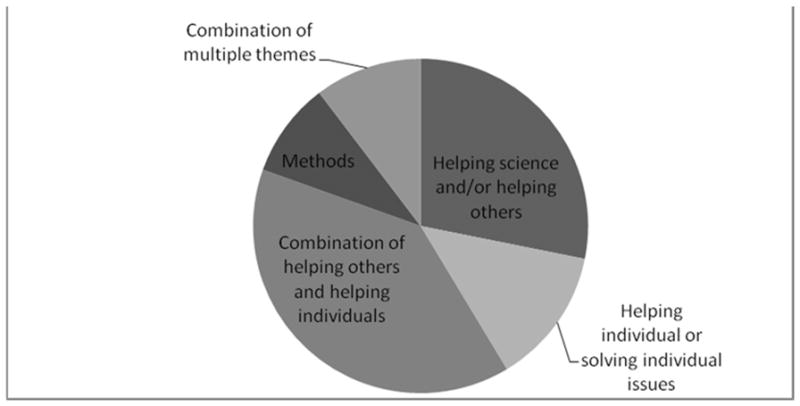
Parents’ responses to the question “What is medical research?”
Many responses captured more than one theme and were coded as such. For example, the following response was coded as conveying the first three themes (helping science, helping others, and helping the individual):
Research allows us to get new answers and to try things out and compare. It also allows us to get the latest information to help people who have problems similar to mine and patients like me..
Responses, especially from the parents, sometimes blended the themes of helping find new treatments for others with helping the particular participants. Examples from two teen participants illustrate the combination of helping others and helping particular individuals:
Studying stuff about the body and trying to find ways that can improve the treatment and diagnoses we have now.
Research is characterized by helping yourself while helping others in the future.
Still others described research as helping to advance science while solving a particular puzzle:
Medical research is studying about something that we don’t know a whole lot about to try to improve the lives of people who have medical problems.
Other responses combined themes expressing the goals of research with descriptions of methods:
I think it is where you are doing different things like the scans and helping find information for a study.
Adolescents usually expressed one theme in their responses, while parents often expressed a combination of themes. About half of the teens’ responses expressed a theme that was similar to their parents.
Descriptions of medical research as helping science or helping future patients through developing information and finding new treatments was the most common response from teens regardless of previous research experience or severity of illness. Teens who had no or only one previous research experience offered responses that described research as having the goal of treating or studying the individual patient more often than those with more research experience. Compared to the healthy volunteers, more of the teens with an illness described research as a scientific activity designed to help science, generate knowledge, develop treatments and cures, and help future patients. Teens with life-threatening illnesses gave responses related to treating or studying the individual patient (P1 and P2) more often than teens with other illness severities.
Response themes were similar from teen participants whether they were seen in Seattle or at the NIH, whether they were male or female, and regardless of age. Four teens and three parents did not answer this question.
Is Being in a Medical Research Study Just Like Seeing a Regular Doctor or Different Than Seeing a Regular Doctor?
The majority of adolescents (74%) and parents (85%) responded that being in medical research is different from seeing a regular doctor. A greater proportion of the adolescents who said that there was no difference between research and seeing a regular doctor had no prior research experience than the full cohort. Otherwise, they were similar by gender, age, and health status.
Four general themes emerged from the explanations of those who had responded that medical research was different than seeing a regular doctor: differences in goals, differences in the doctors or teams, in the procedures, and in logistics. Sub-themes were also identified under the categories of goals and of doctors/teams. Explanations from five teens did not fit any of the codes. (See Table 3.)
TABLE 3.
Themes and descriptions of in responses to the question “How is being in medical research is different from seeing a regular doctor?”
| Theme | Sub-themes and Explanation | Illustrative quotes |
|---|---|---|
|
| ||
| Logistics | Differences in research facility’s distance from home, the length of stay, the frequency of visits, the amount of time involved, and the required paper work | “When you see a regular doctor you don’t have to stay there for so long.” “All appointments are in a short amount of time.” |
|
| ||
| Goals | G1: medical research has a more detailed, complex, and holistic approach | “You are not just being examined: research checkups are more detailed.” (G1) “More thorough testing and more information is gathered about the patient’s overall (condition)/health.” (G1) |
| G2: research is more uncertain and focused on learning | “They [researchers] know what to do but don’t know how results will come out. It’s something new.” (G2) “It [research] is investigational; it focuses more on investigation than treatment.” (G2) |
|
| G3: research serves to help others rather than the individual patient | “[Research is] beneficial to you and the people doing the research, while [a visit with a] regular doctor [is] just beneficial to you.” (G3) “In this research study, you participate when you are healthy, not when you are ill. Research can also help others besides the participants.” (G3) “Usually, at a checkup, a doctor gives you medicine if you are sick. Here, they (research doctors) are trying to improve your health and find more information about it.” (G3) |
|
| G4: medical research is specialized and has a specific target. | “They [research doctors)]are looking into one specific area; it (medical research) is much more specialized.” (G4) | |
|
| ||
| Procedures | Differences in the number or type of procedures and details. Research involves more procedures or different procedures than the regular doctor, as well as more questions and more probing | “They [researchers] do more things with you like all the imaging and sedation.” “They do a lot more studies, and they take more blood from you, check your body, and ask you a lot more questions.” “Regular doctors are less nosy.” “There are a lot more questionnaires.” |
|
| ||
| Doctors/ research team | Differences in the approach of the research team and relationships with patients | |
| D1: differences in professionalism, involvement, friendliness of research doctors/team | “There are different criteria for patients. They [research doctors] are more consistent with keeping me updated frequently. The doctors keep patients more informed.” (D1) “You’re seeing several different types of doctors, and they’re studying a very thorough evaluation of everything.” (D1) “[Research doctors have] more time for you; they are more interested in depth and details.” “The doctor doesn’t come in every day, but when he does, he tells me what’s changed and what hasn’t. [A regular doctor] comes in every day.” |
|
| D2: differences in the number of doctors and size of the team | “You have a lot more doctors, a lot of different ones that you have to see in research.” (D2) “They have more doctors that treat different things.” (D2) |
|
| D3: differences in how well you know the research team (strangers) | “They [Research doctors at the NIH] are less involved.” (D3) “There are no ongoing relationships. The researcher looks at a broader spectrum…”(D3) |
|
Many responses from both adolescents and parents explaining the differences combined concepts from more than one theme. For instance, respondents noted differences in both logistics and in the doctors:
I’m traveling from Virginia to here [the NIH], and they [research doctors] are specialists here.
I fly here to see the doctor (…) the doctors here are more knowledgeable.
Some responses combined differences in both procedures and goals:
They [research doctors] do more in order to learn more.
A regular doctor doesn’t take as much blood. They just check once. Here, they [research doctors] have to figure everything out.
[There are] more questions and less treatment.
They [research doctors] are just asking questions; it [research] is not necessarily beneficial to the individual.
Still other responses noted differences in both procedures and research doctors:
You get studied more and they [research doctors] know more than [doctors] at a regular hospital.
There’s a lot more to do; there are a lot of different doctors.
Some responses about the differences combined multiple themes:
You are enrolled in more tests. You see several different doctors. You see the doctor more often than you see a regular doctor and you probably get more benefits because of the additional visits.
Parents most frequently explained the difference between research and regular medical care in terms of goals and emphasized the value of research in finding treatments, its focus on detail, and on one specific concept or disease. In contrast, teens more often explained the difference as more procedures and more uncertainty about outcomes in research.
Parents tended to explain the difference by describing the roles and attitudes of research doctors compared to regular doctors more often than teens explained it this way. In these cases, both parents and teens usually described the research doctor and team as more knowledgeable, more involved, and more invested in learning and finding answers compared to regular doctors. A small number of parents explained that the researcher was not a doctor or that the researcher only saw the teen infrequently. Teens often pointed out a difference in the number or type of procedures in research (e.g., more blood tests, MRIs, CAT scans) and more questions in research.
Some respondents’ explanations of differences focused on specific experiences rather than goals or engagement. For example, one teen offered:
You usually don’t get stuck seeing a regular doctor, it isn’t an all day experience, you don’t get paid seeing a regular doctor, but here they do more tests and ask more questions.
And another:
They [research doctors] don’t do a checkup, they do MRIs, stick you with needles, and ask you 2 hours of questions in front of a computer.
Parents explained for example, that [research is] “more attention, less rushed” or “when you are in a study they look more closely for adverse effects to the vaccines/medications…”
DISCUSSION
Studies have observed that a large percentage of research participants confuse or conflate research with medical care and suffer from a potentially problematic therapeutic misconception (Appelbaum, Grisso, and Lidz 2004; Appelbaum et al. 2012, Chou and O’Rourke 2012). In our study, the majority of teen research participants and their parents described research by describing the goals of finding new treatments and advancing science in an effort to help others. The majority also said that medical research is different from seeing a regular medical doctor and offered a variety of explanations regarding how the two activities differ.
Our findings suggest that adolescent research participants and their parents generally understand that the goal of research is advancing science and finding treatments and cures to help people with diseases, sometimes including the participant herself. They also describe research as a process of solving a puzzle, investigating something that others cannot figure out or studying someone’s illness through tests and probing to understand it better. Some describe research as more directly designed to help a particular patient either through interventions or searching for explanations.
Overall. their responses suggest that they have a realistic and accurate general description of medical research. We did not, however, evaluate their understanding of, and motivations for, the particular clinical study in which the adolescent was participating.
The findings from this study demonstrate that understanding about research is often complex and multifactorial, as respondents described research as having the goal of helping others while sometimes also helping themselves. While responses to our questions often contained more than one theme, especially from parents, the majority of responses reflected an understanding that the goal of research is producing scientific or medical knowledge and helping others. Our data can be helpful in further investigations and dialogue about the normative and practical distinctions between research and medical care (Kass et al. 2013) and how important understanding these distinctions might be for valid consent to research.
Most previous studies assessing research participants’ understanding of research and the therapeutic misconception ask the respondent questions about the study he or she is participating in, and responses may reflect a combination of understanding, appreciation, and motivations. Few studies have explicitly asked people to explain what they think medical research is in general, or whether and how they think participating in research differs from receiving regular medical care.
In this study, we used open-ended questions to query adolescent research participants and their parents about how they would describe research in their own words and explain whether and how being in medical research was different from receiving regular medical care. Open-ended questions provide insights into research participants’ views that may differ from other types of inquiry, as others have shown that results and conclusions depend on how questions are posed (Kim et al. 2013; Weinfurt et al. 2012).
The majority, but not all, participants in this study explicitly said that being in medical research was different from seeing a regular medical doctor. Teens said that research was different less often than their parents. The explanations from those who said the two activities were different suggest that they perceive medical research and medical care as distinct activities with some overlap. These participants described various kinds of differences between medical research and care, including differences in goals, uncertainty, methods (especially the number and type of procedures), the ways research and non-research doctors relate to patients, and logistics.
Teens often described aspects of research that are underemphasized in discussions of informed consent and assent, but that might have relevance for participants’ decisions, such as differences in the number and type of procedures and questions posed by researchers to participants. These kinds of differences may be salient for teens and others deciding about research, especially as the differences reflect their own, sometimes extensive, research experiences. Differences in procedures and logistics may not be highlighted in study information but could be important to include in discussions with prospective teen participants.
The teens’ parents, in contrast, more often explained that the goals or the doctor’s engagement differed between research and regular medical care. Some respondents’ explanations of research as different from regular medical care highlighted details specific to their own experiences, like being rushed or poked. Others, however, offered descriptions more in tune with understanding that the goals of the two activities are distinct, as exemplified by the following quotes:
Because you are not here for your own health, but for bigger purposes.
[Research is] not for you but for others, when you go to the doctor you expect an outcome for yourself.
In sum, we found that: respondents generally understood the goals of research and differences from regular medical care, suggesting that misunderstanding is not the reason for a therapeutic misconception; since understanding and motivation for research participation is multifactorial, a critical component in evaluating either is the manner in which questions are asked; and teens, and to some extent their parents, find differences between research and regular medical care that are not always discussed or described but may be meaningful to teens.
Limitations
Our findings are limited as they come from a purposeful sample of adolescents who were already enrolled in research and their parents, and included only English-speaking participants at two sites. A large proportion of our sample also had previous research experience. Hence, our results are not generalizable to those who have never participated in research.
Further, we did not investigate how the adolescents or their parents understood or described the particular research study in which the teen was enrolled. Researchers who were involved in designing the questions also conducted some of the interviews and were involved in coding the responses. One independent coder, however, had not been involved in any interviews, and several other interviewers were not involved in coding. We did not use triangulation or similar methods to further validate our findings.
CONCLUSIONS
To our knowledge, this is the first study that included teens across the age spectrum with a wide variety of illnesses and types of studies as well as healthy volunteers. The open-ended questions led to rich descriptions of how these teens and their parents explain research. Further research using both qualitative and quantitative methods is warranted about how perceived differences between research and regular medical care matter to participants, how general understanding pertains to particular research decisions, and how research teams could use this understanding to enhance informed consent and the overall research experience.
Acknowledgments
The authors appreciate the generous willingness of the adolescents and parents who agreed to participate in this study, and the research teams that referred them to us. We also greatly appreciate the contributions of Research Triangle International, Social Systems International, and Stats Collaborative. We further acknowledge Tara Brennan, Megan Sabo, Libby Brockman, and Brie Kohrt who helped to coordinate and conduct interviews.
FUNDING: This research was supported by National Institutes of Health (NIH) Office of Evaluation, the NIH Clinical Center Department of Bioethics, the Intramural Research Program, National Cancer Institute, Center for Cancer Research, and the Institute for Translational Health Sciences (UL1TR000423).
Footnotes
AUTHOR CONTRIBUTIONS: The authors contributed to this project through conception and design (CG, DW, LW, BW), collection and assembly of data (CG, LW, BW, DW), analysis and interpretation (IN, CG, DW), drafting of the article (IN, CG), critical revision (all), and final approval (all).
DISCLAIMER: The views expressed are the authors’ own. They do not reflect any position or policy of the National Institutes of Health, U.S. Public Health Service, or the Department of Health and Human Services.
CONFLICTS OF INTEREST: The authors declare that they have no relevant financial relationships or other conflicts of interest to report.
ETHICAL APPROVAL: This study was approved by the institutional review boards at the National Institute of Child Health and Human Development, Seattle Children’s Hospital, and Research Triangle International.
References
- Appelbaum P, Anatchkova M, Albert K, Dunn L, Lidz C. Therapeutic misconception in research subjects: development and validation of a measure. Clinical Trials. 2012;9(6):748–761. doi: 10.1177/1740774512456455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P, Lidz C, Grisso T. Therapeutic misconception in clinical research: frequency and risk factors. IRB: Ethics and Human Research. 2004;26(2):1–8. [PubMed] [Google Scholar]
- Chou PH, O’Rourke N. Development and initial validation of the Therapeutic Misunderstanding Scale for use with clinical trials research participants. Aging and Mental Health. 2012;16(2):145–153. doi: 10.1080/13607863.2011.602962. [DOI] [PubMed] [Google Scholar]
- Grady C, Wiener L, Abdoler E, et al. Assent in research: the voices of adolescents. Journal of Adolescent Health. 2014;54(5):515–520. doi: 10.1016/j.jadohealth.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GE, Churchill LR, Davis AM, et al. Clinical trials and medical care: defining the therapeutic misconception. PLoS Medicine. 2007;4(11):e32. doi: 10.1371/journal.pmed.0040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Report brief. [accessed October 18, 2014];Health Literacy: A Prescription to End Confusion. 2004 Available at: http://www.iom.edu/~/media/Files/Report%20Files/2004/Health-Literacy-A-Prescription-to-End-Confusion/healthliteracyfinal.pdf.
- Kass N, Faden R, Goodman S, Pronovost P, Tunis S, Beauchamp T. The research-treatment distinction: a problematic approach for determining which activities should have ethical oversight. Hastings Center Report. 2013;43(1):S4–S15. doi: 10.1002/hast.133. [DOI] [PubMed] [Google Scholar]
- Kim SY, deVries R, Wilson R, et al. Research participants’ “irrational” expectations: common or commonly mismeasured? IRB: Ethics and Human Research. 2013;35(1):1–9. [PubMed] [Google Scholar]
- Kim SY, Schrock L, Wilson R, et al. An approach to evaluating the therapeutic misconception. IRB: Ethics and Human Research. 2009;31(5):7–14. [PMC free article] [PubMed] [Google Scholar]
- Miller FG. Revisiting the Belmont Report: the ethical significance of the distinction between clinical research and medical care. APA Newsletter on Philosophy and Medicine. 2006;5(20):10–14. [Google Scholar]
- Miller J. Public understanding of, and attitudes toward, scientific research: what we know and what we need to know. Public Understanding of Science. 2004;13(3):273–294. [Google Scholar]
- Miller M. Phase I cancer trials: a collusion of misunderstanding. Hastings Center Report. 2000;30(4):34–42. [PubMed] [Google Scholar]
- National Science Foundation. Science and Engineering Indicators. Arlington, VA: 2014. Chapter 7: science and technology: public attitudes and understanding. (NSB14-01). Available at: http://www.nsf.gov/statistics/seind14/index.cfm/chapter-7. [Google Scholar]
- Pentz RD, White M, Harvey RD, et al. Therapeutic misconception, misestimation, and optimism in participants enrolled in phase 1 trials. Cancer. 2012;118(18):4571–4578. doi: 10.1002/cncr.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickersgill M. Research, engagement and public bioethics: promoting socially robust science. Journal of Medical Ethics. 2011;37(11):698–701. doi: 10.1136/jme.2010.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar P. Communication and miscommunication in informed consent to research. Medical Anthropology Quarterly. 2004;18(4):429–446. doi: 10.1525/maq.2004.18.4.429. [DOI] [PubMed] [Google Scholar]
- Snowdon C, Elbourne D, Garcia J. Declining enrolment in a clinical trial and injurious misconceptions: is there a flipside to the therapeutic misconception? Clinical Ethics. 2007;2(4):193–200. [Google Scholar]
- Weinfurt K, Seils D, Lin L, et al. Research participants’ high expectations of benefit in early-phase oncology trials: are we asking the right question? Journal of Clinical Oncology. 2012;30(35):4396–4400. doi: 10.1200/JCO.2011.40.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D. Time to stop worrying about the therapeutic misconception. Journal of Clinical Ethics. 2012;23(3):272–289. [PubMed] [Google Scholar]
- Wendler D, Abdoler E, Wiener L, Grady C. Views of adolescents and parents on pediatric research without the potential for clinical benefit. Pediatrics. 2012;130(4):692–699. doi: 10.1542/peds.2012-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]


