Abstract
Both phospholipase D1 (PLD1) and PLD2 regulate degranulation when RBL-2H3 cells are stimulated via the immunoglobulin E receptor, FcɛRI. However, the activation mechanism for PLD2 is unclear. As reported here, PLD2 but not PLD1 is phosphorylated through the Src kinases, Fyn and Fgr, and this phosphorylation appears to regulate PLD2 activation and degranulation. For example, only hemagglutinin-tagged PLD2 was tyrosine phosphorylated in antigen-stimulated cells that had been made to express HA-PLD1 and HA-PLD2. This phosphorylation was blocked by a Src kinase inhibitor or by small interfering RNAs directed against Fyn and Fgr and was enhanced by overexpression of Fyn and Fgr but not by other Src kinases. The phosphorylation and activity of PLD2 were further enhanced by the tyrosine phosphatase inhibitor, Na3VO4. Mutation of PLD2 at tyrosines 11, 14, 165, or 470 partially impaired, and mutation of all tyrosines blocked, PLD2 phosphorylation and activation, although two of these mutations were detrimental to PLD2 function. PLD2 phosphorylation preceded degranulation, both events were equally sensitive to inhibition of Src kinase activity, and both were enhanced by coexpression of PLD2 and the Src kinases. The findings provide the first description of a mechanism for activation of PLD2 in a physiological setting and of a role for Fgr in FcɛRI-mediated signaling.
Phospholipase D (PLD) is activated via receptors in a wide variety of cells where it is thought to regulate intracellular signaling processes and functions such as membrane trafficking, cytoskeletal organization, and degranulation of mast cells (reviewed in references 15, 25, and 31). PLD catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid, which is rapidly converted to other biologically active molecules, namely, lysophosphatidic acid and diacylglycerol. In the presence of relatively low concentrations of primary alcohols, the production of phosphatidic acid is diverted to more metabolically inert phosphatidylalcohols by transphosphatidylation, a reaction that is unique to PLD and one that is utilized in the assay of PLD in vivo (39) and to unmask the physiologic roles of phosphatidic acid (62).
Two isoforms of PLD have been cloned, PLD1 and PLD2, with PLD1 existing as two variants, PLD1a and PLD1b (11, 21). PLD1 is activated in vitro by small GTPases such as ARF and Rho and protein kinase C (PKC) α in the presence of phosphatidylinositol 1,4-bisphosphate (PIP2) (4, 21, 37, 43, 55). There is also evidence that PLD1 can be regulated in vivo by Rho kinase (48), Ca2+/calmodulin-dependent kinase II (35), and PKC in a catalytically dependent or independent manner (21, 26, 63). PLD2, in contrast, is activated in vitro by PIP2 alone, and this activity is minimally affected by the small GTPases or PKCα (11, 32, 54). However, the mechanisms regulating PLD2 activity in vivo are unclear. There are reports of tyrosine phosphorylation of PLD1 (33, 36) and PLD2 (1, 44, 51) and indications from pharmacological studies that tyrosine phosphorylation may regulate PLD activity (6, 27, 36, 44). In addition, PLD2 was shown to associate with, and be phosphorylated by, the tyrosine kinase receptor for epidermal growth factor (EGF) (51) and by Src kinase (1, 42). Nevertheless, the role of such phosphorylation is uncertain. Although tyrosine-11 was identified as the specific residue phosphorylated in PLD2, mutation of this site enhanced basal PLD2 activity but had no effect on the magnitude of the PLD2 response to EGF (51).
Mast cells and blood basophils are responsible for a variety of allergic disorders (5, 59). These cells respond to immunoglobulin E (IgE)-directed antigens via the high-affinity receptor for IgE, namely, FcɛRI, by release of granules that contain preformed inflammatory mediators and the generation of inflammatory lipids and cytokines. PLD is thought to play an essential role in mast cell degranulation (7, 10, 58). PLD is activated in isolated mast cells (12) and cultured mast cell lines (10, 28, 30) by a variety of stimulants, including antigen. Cross-linking of the IgE/FcɛRI complex with antigen results in the recruitment and activation of Src kinases and subsequently other tyrosine kinases. The function of the individual PLD isoforms in mast cells has been studied in the RBL-2H3 cell line, which is now known to be an analog of rat mucosal mast cells (49). Studies with transiently expressed forms of both PLDs in RBL-2H3 cells indicate that PLD1b and PLD2 associate with granule membranes and the plasma membrane, respectively (7, 9), and that both isoforms are activated upon antigen stimulation (8, 40). The mechanisms of activation of these PLDs by antigen are unknown. However, the location of PLD2 at the plasma membrane makes this isoform particularly accessible to FcɛRI-associated tyrosine kinases.
As reported here, activation of PLD and degranulation in antigen-stimulated RBL-2H3 cells is inhibited by low concentrations of the Src kinase inhibitor PP2. We investigated whether Src kinases regulate PLD directly by tyrosine phosphorylation and, if so, whether this phosphorylation is essential for degranulation. We show by coexpression studies, site-directed mutagenesis, and the use of small interfering RNAs (siRNAs) directed against Src kinases that Fyn and Fgr phosphorylate PLD2 but not PLD1b in vitro and in vivo and that this phosphorylation is required for the activation of PLD2 in vivo. Furthermore, suppression of this phosphorylation or the activation of PLD2 itself by various strategies also results in suppression of degranulation in stimulated RBL-2H3 cells.
MATERIALS AND METHODS
Materials.
Materials were purchased from the following sources. Piceatannol, wortmannin, and Ro31-7549 were obtained from Alexis (San Diego, Calif.), and PP2 was from Calbiochem (La Jolla, Calif.). Antibodies to phosphotyrosine (PY) (4G10) and Src were from Upstate Biotechnology (Lake Placid, N.Y.). Antibodies to Lyn, Fyn, Fgr, and hemagglutinin (HA) tag were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). [3H]myristic acid was from DuPont-NEN (Boston, Mass.), and [γ-32P]ATP was from ICN Biomedicals, Inc. (Irvine, Calif.). Cell culture reagents were from Gibco/Invitrogen (Carlsbad, Calif.). Tris-glycine polyacrylamide gels were from Novex (San Diego, Calif.), trinitrophenyl (DNP)-specific monoclonal IgE and DNP-bovine serum albumin (BSA) were from Sigma (St. Louis, Mo.), and phosphatidylethanol (PEtOH) standard for the PLD assay was from Avanti-Polar Lipid (Alabaster, Ala.).
Extraction of RNA and reverse transcription-PCR (RT-PCR).
Total RNA was isolated from RBL-2H3 cells by using TRIzol reagent (Invitrogen) and was reverse transcribed with the Superscript first-strand synthesis system (Invitrogen) according to the manufacturer's protocol. PCR was performed at 94°C for 45 s at 55°C for 45 s and at 72°C for 60 s for 30 cycles. The following primer pairs were used: rat PLD1 forward (5′-GTGGGCAGTGTCAAGCGGGTCACC-3′) and reverse (5′-GCCAAAACCTAGTCTCCCCATGGA-3′), rat PLD2 forward (5′-ATGACTGTAACCCAGACGGCACTC-3′) and reverse (5′-CAGCTCCTGAAAGTGTCGGAATTT-3′), and rat GAPDH forward (5′-GTGGAGTCTACTGGCGTCTTC-3′) and reverse (5′-CCAAGGCTGTGGGCAAGGTCA-3′).
Cloning of Lyn, Fyn, Fgr, Src, and Yes from RBL-2H3 cells and mutation of HA-PLD2.
The Src kinases were cloned into pCMV Vector (Stratagene, La Jolla, Calif.) by PCR amplification with the following primers: 5′-TCCCCGCGGCACCGCGAGCGAGAAATATG-3′ and 5′-CCGCTCGAGTGGCTGCTGCTGATACTGC-3′ for LynB, 5′-GGAATTCGAGCTTGGATAATGGGCTGTG-3′ and 5′-GCGTCGACTCACAGGTTTTCACCGGGCTG-3′ for Fyn, 5′-GGAATTCGGAATGGGCTGTGTGTTCTGC-3′ and 5′-CCGCTCGAGGTCAGGCTATGTCTGGTCTCC-3′ for Fgr, 5′-GGAATTCATGGGCAGCAACAAGAGCAAG-3′ and 5′-CCGCTCGAGCACACAGTTCCTATAGGTTCT-3′ for c-Src, and 5′-TTCCCGCGGATGGGCTGCATTAAAAGTAAAG-3′ and 5′-GCGTCGACTTATAAATTTTCTCCTGGTTGG-3′ for Yes. Sequence and expression was confirmed by sequencing and Western blot analysis. Plasmids for human HA-PLD1b and murine HA-PLD2 (13) were kindly supplied by Michael A. Frohman (Institute for Cell and Developmental Biology, State University of New York, Stony Brook). Mutations of HA-PLD2 were performed by using a QuikChange site-directed mutagenesis kit (Stratagene) with the following primers: PLD2 Y11F, 5′-GAAGAACCTCTTTCCCTTTGGGGACTATCTGAAC-3′; PLD2 Y14F, 5′-CTGGCTGGAGTTCAGAAAGTCCCCATAGGGAAAG-3′; PLD2 Y165F, 5′-GCCAGCAAACAGAAATTCTTGGAAAATTACCTC-3′; and PLD2 Y470F, 5′-CAGGTCAGTCAGTCGGAATTGCACGTCATCCCAG-3′.
Transient transfection of cells with HA-PLDs, PLD2(K758R) mutant, and Src kinases.
RBL-2H3 cells were grown as monolayers in minimal essential medium with Earle's salts, supplemented with glutamine, antibiotics, and 15% fetal bovine serum (3). Cells were transiently transfected with each DNA preparation (25 μg/2 × 107 cells unless stated otherwise) by electroporation (Bio-Rad Gene-Pulser; 960 μF, 250 V). Successful transfection was confirmed by Western blotting and by assay of PLD activity. Cells were used within 48 h of transfection.
Synthesis and transfection of siRNA against Fyn and Fgr.
Short hairpin siRNA constructs were designed around 21 nucleotide sequences that matched rat fyn (open reading frame nucleotides 984 to 1004) and fgr (open reading frame nucleotides 871 to 891). Sense and antisense RNA oligonucleotides that contained the loop sequence, CCACC, were synthesized by Lofstrand (Rockville, Md.) and cloned into the psiRNA-hH1zeo vector (Invivogen, San Diego, Calif.). The siRNA constructs (25 μg of DNA) were transfected into 2 × 107 cells, and cells were incubated in 500 μg of zeocin/ml for selection. Two weeks later cells were harvested for the studies described.
Cell stimulation, immunoprecipitation of HA-PLDs, and immunoblotting.
Transfected cells (∼1.0 × 106 cells/10-cm petri dishes) were washed with fresh growth medium 4 h after transfection and incubated with 50 ng of IgE/ml for 3 h. The cells were washed, and medium was replaced with a PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)]-buffered medium (25 mM PIPES [pH 7.2], 159 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 1 mM CaCl2, 5.6 mM glucose, and 0.1% fatty acid-free fraction V from bovine serum). Cells were stimulated with 25 ng of DNP-BSA/ml for 3 min or as indicated, chilled with ice to terminate stimulation, and then washed twice with ice-cold phosphate-buffered saline (PBS). Cells were lysed in 0.5 ml with ice-cold lysis buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 10% glycerol, 60 mM octyl-β-glucoside, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM nitrophenylphosphate, 0.7 μg of pepstatin/ml, and a protease inhibitor cocktail tablet). Lysates were kept on ice for 30 min and then centrifuged 15,000 × g for 15 min at 4°C. The supernatant fraction was “precleared” by addition of 50 μl of protein G-agarose. After gentle rocking for 1 h, the mixture was centrifuged. Samples of the supernatant fraction of equal protein content were used for immunoprecipitation. HA-PLDs were immunoprecipitated by overnight incubation (at 4°C with gentle rocking) with agarose-conjugated anti-HA antibody. The agarose was washed five times with a washing buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 0.1% Nonidet P-40, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM nitrophenylphosphate, 0.7 μg of pepstatin/ml, and a protease inhibitor cocktail tablet) and dissolved in 2× Laemmli buffer (29). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (BA85; Schleicher & Schuell). The immunoreactive proteins were detected by use of horseradish peroxidase-coupled secondary antibodies and enhanced chemiluminescence according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Assay of immunoprecipitated HA-PLD mutants in vitro.
Immunoprecipitated wild-type and mutated PLDs were assayed by measurement of the release of [3H]choline from the PLD substrate, (choline-[3H]methyl)dipalmitoylphosphatidylcholine. For this assay, 80 μl of each immunoprecipitated sample was added to 25 μl of a vesicle preparation that consisted of a mixture of phospholipids and 140,000 dpm of labeled substrate diluted with buffer to make a final volume of 125 μl exactly as described by Massenburg et al. (34). The mixture was incubated at 37°C for 1 h. The reaction was terminated by the addition of 1 ml of a mixture of chloroform-methanol-concentrated HCl (50:50:0.3 [vol/vol/vol]), followed by the addition of 0.35 ml of 1 M HCl-5 mM EGTA. The aqueous phase was assayed for [3H]choline by liquid scintillation counting.
Measurement of PLD activity in intact cells by the transphosphatidylation assay.
PLD-transfected RBL-2H3 cells were incubated for 3 h with 50 ng of IgE/ml in complete growth medium in 12-well plates (∼3.5 × 105 cells/well). [3H]myristic acid at 2 μCi/ml was added for the final 90 min of incubation. Cells were then incubated in PIPES-buffered medium (9) in the presence of 1% ethanol for 10 min before stimulation with 25 ng of DNP-BSA/ml for 3 min. [3H]PEtOH was assayed by minor modifications of a previously described procedure (2). The reaction was terminated by the addition of 1.5 ml of chloroform-methanol-4 N HCl (100:200:2 [vol/vol/vol]) to form a single phase. The mixture was separated into two phases by addition of 0.5 ml of chloroform that contained unlabeled phosphatidic acid and PEtOH (60 μg of each), as well as 0.5 ml of 0.1 N HCl. The phospholipids in the lower chloroform phase were separated by thin-layer chromatography. The [3H]PEtOH “spot” was excised and assayed by liquid scintillation counting (2).
Phosphorylation of HA-PLD2 by Src kinases in vitro.
The reversible immunoprecipitation system, Catch and Release (Upstate), was utilized according to the manufacturer's protocol to measure phosphorylation of HA-PLD2 in vitro. In this procedure, the cell lysate was diluted to 1 mg of protein/ml with the lysis-wash buffer, and 500 μl of the diluted lysates was transferred to spin columns. Then, 4 μg of antibody to HA-PLD2 or the Src kinases and 10 μl of the antibody capture affinity ligand were added to the diluted lysate. The spin columns were then gently rocked for 15 min at room temperature before centrifugation at 4,200 rpm for 10 min. The columns were washed twice with the lysis-wash buffer. HA-PLD2 and the Src kinases were eluted from the columns and analyzed by Western blotting. The phosphorylation of HA-PLD2 by Src kinases was assessed as follows. The eluates were added to a solution of 20 mM Tris (pH 7.4), 10 mM MgCl2, 1 mM dithiothreitol, 10 μg/ml of 4-(2-aminoethyl)benzensulfonyl fluoride, 0.1 mM EDTA, 0.1 mM sodium orthovanadate, 1 μg of aprotinin/ml, 0.1 μg of pepstatin A/ml, 0.05 μg of leupeptin/ml, 8 mM β-glycerophosphate, 2.5 mM NaF, 50 μM ATP, and 10 μCi of [γ32P]ATP. The mixture was incubated for 30 min at 30°C. Proteins were separated by SDS-PAGE and tyrosine phosphorylated, and 32P-labeled HA-PLD2 were detected by immunoblotting with antiphosphotyrosine antibody and by autoradiography, respectively.
Measurement of Src kinase activity.
Src kinases (Lyn, Fyn, Fgr, and Src) were immunoprecipitated from cell lysates with the kinase-specific antibodies noted above. The immunoprecipitates were assayed for kinase activity by use of an in vitro kinase assay kit (Tyrosine Kinase Assay Kit for Chemiluminescence Detection; Upstate) according to the manufacturer's instructions.
Measurement of degranulation.
Secretion of granules was determined by measuring the release of the granule marker, β-hexosaminidase with a colorimetric assay in which the release of p-nitrophenol from p-nitrophenyl-N-acetyl-β-d-glucosaminide is measured (41). Values were expressed as the percentage of intracellular β-hexosaminidase that was released into the medium.
Confocal microscopy.
RBL-2H3 cells were transfected with wild-type or mutated HA-PLDs by electroporation as described above. The cells were then suspended in complete growth medium, transferred to Lab-Tek chambered coverslips (Nalge Nunc International, Naperville, Ill.) and then incubated overnight at 37°C. The cultures were washed three times with phosphate-buffered saline (PBS). Cultures were fixed in 4% formaldehyde in PBS for 10 min, washed, and permeabilized with 0.5% Triton X-100 for 15 min. The fixed cells were washed again before incubation for 60 min with a blocking reagent, 1% bovine serum albumin in PBS. The coverslips were incubated for 2 h with a solution of the primary antibody in 1% bovine serum albumin in PBS, washed, and then incubated with rhodamine-conjugated secondary antibody for 45 min. The coverslips were washed, and mounts were prepared by using a Prolonged Antifade Kit (Molecular Probes, Eugene, Oreg.). Confocal images were obtained with a Bio-Rad MRC 1024 confocal laser-scanning microscope with an Apochromat ×60 objective lens.
RESULTS
Tyrosine phosphorylation of PLD2 and its suppression by the Src kinase inhibitor, PP2.
Analysis by RT-PCR revealed that RBL-2H3 cells express message for PLD2 and less-abundant message for PLD1b (Fig. 1A). In the absence of reliable immunoprecipitating antibodies for these PLDs, studies were conducted with RBL-2H3 cells made to express human HA-PLD1b (hereafter referred to as HA-PLD1) or murine HA-PLD2. Immunoprecipitation of the HA-tagged PLDs and immunoblotting with antibodies against HA and phosphotyrosine showed that HA-PLD2 but not HA-PLD1 was tyrosine phosphorylated (Fig. 1B). The extent of PLD2 phosphorylation (Fig. 1B) and the PLD activity (Fig. 1C) were increased after antigen stimulation. The increased phosphorylation was evident within 1.5 min and reached a maximum by 15 min (Fig. 1D). The concentration of antigen used (25 ng/ml) for this and subsequent experiments elicited maximal degranulation of RBL-2H3 cells (40 to 50% [data not shown]).
FIG. 1.
RBL-2H3 cells express message for PLD1b and PLD2, but only PLD2 is tyrosine phosphorylated after antigen stimulation. (A) The presence of mRNA for the PLD isoforms in RBL-2H3 cells was determined by RT-PCR. RBL-2H3 cells were then transiently transfected with HA-PLD1b or HA-PLD2 DNA-constructs and primed with DNP-specific IgE before stimulation with antigen (25 ng of DNP-BSA/ml) for 3 min (B and C) or the times indicated (D). The PLDs were immunoprecipitated with anti-HA antibody and separated by SDS-PAGE for detection of the HA-PLDs and tyrosine phosphorylated PLDs (pY-PLD) with anti-HA and antiphosphotyrosine antibodies. Representative immunoblots from three experiments, as well as the average values for relative densities of the phosphorylated PLDs, as determined by densitometry, are shown in panels B and D. Transfected cells were also labeled with [3H]myristic acid for measurement of PLD activity by the transphosphatidylation assay in intact cells to verify that transfection with HA-PLD2 also enhanced cellular PLD activity (shown in panel C). The results are expressed as the percentage of 3H-lipid fraction recovered as [3H]PEtOH as described in Materials and Methods. Values are means ± the standard error of the mean (SEM) of three experiments. Key: NS, nonstimulated; Ag, antigen stimulated; pY-PLD, tyrosine phosphorylated PLD1 and PLD2; **, significant difference at the P < 0.01 level.
Various kinase inhibitors were tested to identify the type of kinase involved in PLD2 phosphorylation. Of these, the Src kinase inhibitor PP2 alone suppressed antigen-induced tyrosine phosphorylation of PLD2 (Fig. 2A). Piceatannol, wortmannin, and Ro31-7549 (which inhibit Syk, phosphatidylinositol 3-kinase, and PKC, respectively) were inactive to indicate that only Src kinases regulated, either directly or indirectly, tyrosine phosphorylation of PLD2. The inhibition of PLD2 phosphorylation by PP2 was concentration dependent and was apparent with as little as 2 μM PP2 (Fig. 2B). Near-maximal inhibition was observed with 6.0 μM PP2, and the estimated concentration for 50% inhibition was 3.0 μM.
FIG. 2.
Tyrosine phosphorylation of PLD2 is suppressed by the Src kinase inhibitor, PP2. RBL-2H3 cells were transiently transfected with HA-PLD2 cDNA and primed with DNP-specific IgE. PP2 (20 μM), piceatannol (120 μM; Pi), wortmannin (100 nM; Wort), or Ro31-7549 (10 μM; Ro31) (A) or the indicated concentrations of PP2 (B) were added 10 min before stimulation with 25 ng of DNP-BSA/ml (Ag) for 3 min. Some cells were left unstimulated (NS). HA-PLD2 was immunoprecipitated with anti-HA antibody and separated by SDS-PAGE for detection of HA-PLD2 with anti-HA antibody and tyrosine phosphorylated PLD2 (pY-PLD2) with antiphosphotyrosine antibody. Representative immunoblots from three experiments, and average values for relative densities of the phosphorylated PLD2, as determined by densitometry, are shown.
Coexpression of Fyn or Fgr enhances tyrosine phosphorylation of expressed HA-PLD2.
Endogenous Lyn, Fyn, Fgr, and c-Src were detected in RBL-2H3 cells by immunoblotting (Fig. 3A) and by cloning their cDNA (see Materials and Methods). The cloned Src kinases were coexpressed with HA-PLD2 to identify which of them could phosphorylate PLD2 in vivo (Fig. 3B). Both basal and antigen-stimulated tyrosine phosphorylation of HA-PLD2 were enhanced in cells cotransfected with Fyn or Fgr compared to cells transfected with the vector alone. Cotransfection with Lyn produced minimal enhancement of phosphorylation and cotransfection with c-Src, if anything, appeared to suppress antigen-induced HA-PLD2 phosphorylation. The differences in phosphorylation were not attributable to differences in activities of the Src kinases in transfected cells (Fig. 3C). For example, immunoprecipitation and assay of Fgr indicated that this kinase exhibited the least activity while promoting the most abundant phosphorylation of PLD2 (compare Fig. 3C and B). Conversely, c-Src kinase exhibited the highest activity while promoting the least phosphorylation of PLD2. Also, Lyn and Fyn transfected cells possessed similar kinase activities but differed in their ability to phosphorylate PLD2 as noted above. However, we detected no increase in activity of any of these kinases upon antigen stimulation (see Fig. 3C). Others have noted such lack of activation of Fyn and Lyn in antigen-stimulated RBL-2H3 cells (Juan Rivera, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health [unpublished data]), possibly because the proportion of available intracellular Src kinase that is recruited by FcɛRI during stimulation is relatively small (17, 61).
FIG. 3.
Tyrosine phosphorylation of PLD2 is enhanced upon overexpression of Fyn or Fgr. Immunoblots of lysates of RBL-2H3 cells were prepared for detection of endogenous Src kinases (A). RBL-2H3 cells were cotransfected with cDNA constructs for HA-PLD2 and the indicated Src kinases or vector. Cells were then primed with DNP-specific IgE. Cells were not stimulated (NS) or stimulated with 25 ng of DNP-BSA/ml (Ag) for 3 min. HA-PLD was immunoprecipitated (IP) with anti-HA antibody, and immunoblots were probed with antiphosphotyrosine and anti-HA antibodies for detection of tyrosine phosphorylated PLD2 (pY-PLD2) and HA-PLD2 (PLD2). Average values for relative densities of phosphorylated PLD2, as determined by densitometry, are indicated also (B, upper panel). Immunoblots were also prepared from cell lysates to verify expression of the individual Src kinases; representative immunoblots from three experiments are shown in panel B. (C) The individual Src kinases were also immunoprecipitated from the cell lysates for assay of Src-kinase activity by the chemiluminescence procedure described in Materials and Methods. Values are means ± the SEM from three experiments, and chemiluminescence is expressed as counts per second (cps).
The enhancement of HA-PLD2 phosphorylation by Fyn and Fgr was dependent on the level of expression of either Src kinase and showed a progressive increase with increasing levels of the expressed Src kinase (upper panels of Fig. 4). No such increase was observed in cells cotransfected with increasing amounts of Lyn cDNA (data not shown) to suggest that PLD2 was not a substrate for Lyn kinase activity. The time course of antigen-induced HA-PLD2 phosphorylation in cells cotransfected with either Fyn or Fgr (lower panels, Fig. 4A and B) was comparable to that observed in cells not transfected with these kinases (i.e., Fig. 1D). That is, increased phosphorylation was apparent at 1 min and reached a maximum by 15 min. Of particular interest, both Fyn and Fgr coimmunoprecipitated with HA-PLD2 in nonstimulated cells, but the extent of this association appeared to decrease after stimulation with antigen (lower panels, Fig. 4A and B). This result strongly suggests that phosphorylation and/or activation of PLD2 promotes dissociation of the Src kinases from PLD2 early in the FcɛRI-mediated cascade of signaling events. The reason for this dissociation is unknown and requires further investigation.
FIG. 4.
Tyrosine phosphorylation of PLD2 is dependent on level of expression of Fyn and Fgr and on duration of stimulation with antigen. RBL-2H3 cells were cotransfected with cDNA constructs for HA-PLD2, along with vector, Fyn, or Fgr. For the upper panels, the amount of cDNA was varied as indicated and for the lower panels, 6.25 μg of DNA was used. Cells were primed with DNP-specific IgE and cells were either not stimulated (NS) or stimulated with 25 ng of antigen DNP-BSA/ml (Ag) for 3 min or for the times indicated in the lower panels. HA-PLD2 was immunoprecipitated (IP) for detection of tyrosine phosphorylated PLD2 (pY-PLD2) and HA-PLD2 (all panels) and for coimmunoprecipitated Fyn or Fgr (lower panels). In addition, immunoblots prepared from cell lysates were probed for Fyn or Fgr as indicated. Representative immunoblots from three experiments are shown.
Other experiments showed that coexpression of Fyn and Fgr enhanced phosphorylation of HA-PLD2 to a greater extent than expression of the individual kinases in both nonstimulated cells and antigen-stimulated cells. A typical immunoblot is shown in Fig. 5A. Densitometric analysis of the immunoblots suggested that the enhanced phosphorylation was either additive or possibly synergistic (Fig. 5B).
FIG. 5.
Fyn and Fgr together enhance phosphorylation of PLD2 in vivo. RBL-2H3 cells were cotransfected with cDNA constructs for HA-PLD2 along with vector, Fyn, Fgr, or the combination of the two Src kinases. Cells were stimulated with antigen (Ag) or not stimulated (NS) for 3 min, and immunoprecipitates (IP) of HA-PLD2 and cell lysates were prepared for the detection of tyrosine-phosphorylated PLD2 (pY-PLD2), HA-PLD2, Fyn and Fgr as described for previous figures. Representative blots (A) and densitometric data (B) from two experiments are shown.
Fyn and Fgr, but not Lyn, phosphorylate HA-PLD2 in vitro.
Studies were conducted in vitro to determine whether or not immunoprecipitated Fyn and Fgr phosphorylate PLD2 directly. HA-PLD2, Lyn, Fyn, and Fgr were recovered by immunoprecipitation from cells made to overexpress these proteins. The immunoprecipitated Src kinases were then incubated with immunoprecipitated HA-PLD2 in the presence of [32P]ATP as described in Materials and Methods. Both Fyn and Fgr caused phosphorylation of PLD2, as indicated by formation of 32P-labeled PLD2 and an increase in levels of tyrosine phosphorylated PLD2, whereas Lyn was inactive in this regard (Fig. 6A). Measurement of Src kinase activity revealed that the immunoprecipitates of Lyn and Fyn contained comparable levels of activity, whereas immunoprecipitates of Fgr contained ∼30% less activity (Fig. 6B). Therefore, the failure of Lyn to phosphorylate HA-PLD2 was not the result of inherently low Lyn kinase activity in the immunoprecipitates.
FIG. 6.
Fyn and Fgr phosphorylate PLD2 in vitro. Separate batches of cells were made to overexpress HA-PLD2, Lyn, Fyn, or Fgr, and each of these proteins was immunoprecipitated. (A) The indicated mixtures of these proteins were incubated with [γ32P]ATP, and the proteins were separated by SDS-PAGE for the detection of tyrosine phosphorylated PLD2 (pY-PLD2) with antiphosphotyrosine antibody, [32P]PLD2 by autoradiography, and the indicated Src kinases as described in Materials and Methods. The results shown are representative of three experiments. (B) In addition, the immunoprecipitated Src kinases were assayed for kinase activity. Values are means ± the SEM from three experiments and are expressed as a percentage of the activity of immunoprecipitated Lyn. BL, assay blank.
Evidence that Fyn and Fgr regulate phosphorylation and activation of PLD2, as well as degranulation.
To assess the physiological relevance of tyrosine phosphorylation of PLD2 by Fyn and Fgr, cells were transfected with kinase-specific siRNAs to suppress the expression of these kinases. Transfection with siRNA targeted against Fyn or Fgr resulted in suppression of expression of the targeted kinase, tyrosine phosphorylation of PLD2 (Fig. 7A), and degranulation (Fig. 7B). Both siRNAs were equally effective in suppressing degranulation.
FIG. 7.
Suppression of expression Fyn and Fgr reduces tyrosine phosphorylation of PLD2 and degranulation. RBL-2H3 cells were made to express siRNAs (RNAi) directed against Fyn or Fgr or as a control green fluorescent protein (GFP). (A) Expression of Fyn and Fgr, as well as tyrosine-phosphorylated PLD2 (pY-PLD2) and PLD2 protein, was determined by immunoblotting. (B) Cells were also stimulated with antigen for 15 min to assess the effects on degranulation as determined by the release of the granule marker, β-hexosaminidase. Values are expressed as percentages of cellular β-hexosaminidase that was released into the medium and are the means ± the SEM of values from three experiments. The asterisks indicate a significant decrease in release (P < 0.01).
Other experiments were designed to determine whether PLD2 itself was regulating degranulation. Two approaches were used. The first took advantage of the PLD-catalyzed transphosphatidylation reaction in which cells were incubated with 50 mM 1-butanol which diverts production of the PLD product, phosphatidic acid, to phosphatidylbutanol (62). This strategy was used to unmask the physiological role of phosphatidic acid because there are no cell permeable inhibitors of PLD. As shown in Fig. 8A, antigen-stimulated degranulation was suppressed by 1-butanol but not by tertiary-butanol, which is not a substrate for transphosphatidylation and serves as a control for nonspecific effects of the alcohol. The second approach utilized a catalytically inactive mutant of HA-PLD2 (K758R) (55). Expression of this mutant blocked the antigen-induced activation of PLD (Fig. 8B and inset) and degranulation (Fig. 8C). Expression of wild-type HA-PLD2, as in Fig. 1, enhanced the activation of PLD and degranulation (Fig. 8B and C).
FIG. 8.
Impairment of PLD function is associated with reduction of degranulation. (A) PLD function was altered by stimulating cells with antigen in the absence or presence of 50 mM 1-butanol (Bu) or, as a control, tertiary butanol (tBu). (B and C) Alternatively, cells were transfected with the catalytically inactive mutant PLD2 (K758R) or, for comparison, with the empty vector (V) or wild-type PLD2 (W). The levels of expression of the HA-PLDs are shown in the inset in panel B. As noted in the text, 1-butanol but not tertiary butanol subverts the production of phosphatidic acid to phosphatidylbutanol. Cells were stimulated with antigen for 15 min for measurement of the release of the granule marker, β-hexosaminidase (A and C), and for PLD activity in [3H]myristate-labeled cells (B) as described in previous legends. Degranulation is expressed as a percentage of cellular β-hexosaminidase that is released into the medium (A) or as a percentage of release observed in vector-expressing cells (C). PLD activity is expressed as a percentage of 3H-lipid fraction recovered as [3H]PEtOH. Values are means ± the SEM from three experiments, and asterisks indicate a significant difference from antigen-stimulated cells in absence of butanol (A) or cells expressing wild-type PLD2 (*, P < 0.05; **, P < 0.01).
For further verification of the pathway Fyn/Fgr → PLD2 → degranulation, cells were made to overexpress HA-PLD2, Fyn, and Fgr in various combinations. Coexpression of Fyn and Fgr with HA-PLD2 enhanced the activation of PLD by antigen compared to cells that expressed HA-PLD2 alone (Fig. 9A). In addition, Fyn and Fgr individually and in combination augmented degranulation in antigen-stimulated cells (Fig. 9B).
FIG. 9.
Coexpression of Fyn or Fgr with HA-PLD2 enhances PLD activation and degranulation. RBL-2H3 cells were cotransfected with cDNA constructs for HA-PLD2, along with Fyn, Fgr, or both Src kinases. The activation of PLD in [3H]myristate-labeled cells (A) and degranulation (B) after stimulation of cells with antigen for 3 min were determined as described for previous figures. The data are expressed as the percentages of 3H-phospholipids recovered as [3H]PEtOH (A) or the enhancement of degranulation relative to that observed in cells made to express HA-PLD2 alone (B). The stimulated release of β-hexosaminidase in the latter cells was 17%. Values are the mean value from three experiments. Bars indicate ± the SEM, and the asterisk indicates a significant increase in response at P < 0.05.
Mutational analysis reveals several sites of tyrosine phosphorylation that participate in PLD2 activation.
Examination of the amino acid sequences of both PLDs indicated several tyrosines that are unique to PLD2, four (Y11, Y14, Y165, and Y470) in rat and mouse PLD2 and two (Y165 and Y470) in human PLD2. We investigated the effects of mutation of murine PLD2 at each of these sites (Y to F) and of all four sites together (Y11/14/165/470F) in cells made to express these mutants (Fig. 10). Antigen-stimulated phosphorylation of PLD2 was partially impaired by all single point mutations and was virtually abolished by mutation of all four sites (Fig. 10A). Indeed, the completely mutated PLD2 reduced the levels of PLD2 phosphorylation to below those observed in nonstimulated cells. A similar pattern was observed in the stimulation of PLD activity by antigen. The individual mutations partially impaired, and all four mutations abolished, PLD activation (Fig. 10B).
FIG. 10.
Mutation of tyrosine sites that are unique to PLD2 impair phosphorylation and activation of PLD2. Cells were made to express HA-PLD2 with point mutations (Y to F) at tyrosine residues 11, 14, 165, or 470 or all four residues (all), as well as HA-PLD2 (Wild or W). (A) Cells were stimulated for 3 min with antigen (Ag) or left unstimulated (NS) for immunoprecipitation and immunoblotting for detection of tyrosine-phosphorylated HA-PLD2 (upper bands) and HA-PLD (lower bands). The blots are representative of three such experiments. (B) The PLD activity was also determined after cells were labeled with [3H]myristic acid for the determination of cellular PLD activity by the transphosphatidylation assay. The data are expressed as the fold increase in activity in antigen-stimulated cells versus the basal activity in nonstimulated cells. (C) Immunoprecipitates of wild-type and mutated HA-tagged PLDs from lysates of nonstimulated cells were assayed for intrinsic PLD activity in vitro in the presence of PIP2. The data are expressed as the percentage of activity observed with immunoprecipitated wild-type PLD2. Values in panels B and C are the means ± the SEM of three experiments, and asterisks indicate significant difference from values obtained with wild-type PLD2 (*, P < 0.05; **, P < 0.01). (D) In addition, cells were made to express the HA-PLD2 mutants and were examined by confocal microscopy to determine the intracellular distribution of the mutated PLDs.
The effect of the mutations described above on the catalytic activity of PLD was investigated by immunoprecipitation of expressed HA-PLD2 from nonstimulated cells, followed by measurement of PLD activity in the presence of the PLD activator, PIP2. Mutations at Y11, Y14, and Y165 had no effect on this activation and, therefore, did not influence the intrinsic catalytic activity of HA-PLD2 (Fig. 10C). However, mutation of Y470 resulted in partial impairment (∼50%) in activation to indicate some possible loss of catalytic activity. The effects of the mutations on the cellular location of HA-PLD2 was also investigated by confocal microscopy (Fig. 10D). As in previous studies (9), expressed HA-PLD2 was located primarily in the plasma membrane. Mutation of Y14 clearly caused aberrant localization of HA-PLD2, whereas the other individual mutations did not do so. Although the role of Y14 and Y470 phosphorylation is uncertain because of the caveats noted above, the studies do indicate that the mutations Y11F and Y165F impeded phosphorylation and activation of PLD2 without impairment of catalytic activity and localization of PLD2.
Further examination of the role of Src-mediated phosphorylation on PLD activation and degranulation.
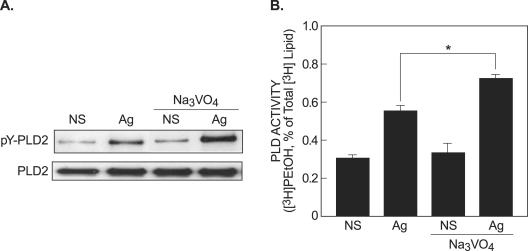
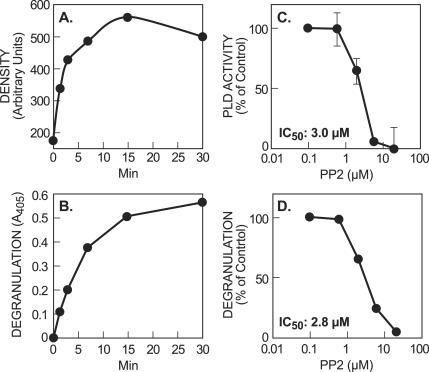
To examine further the possible consequences of enhanced tyrosine phosphorylation of PLD2, cells were stimulated in the absence or presence of the phosphatase inhibitor, sodium orthovanadate. Sodium orthovanadate substantially enhanced antigen-stimulated tyrosine phosphorylation of expressed HA-PLD2 (Fig. 11A). This effect was associated with enhanced activation of PLD in intact cells (Fig. 11B). Therefore, phosphorylation of PLD2 appeared to be linked to the activation of PLD. Measurement of the kinetics of tyrosine phosphorylation of expressed HA-PLD2 and degranulation showed that after antigen stimulation PLD2 phosphorylation preceded degranulation and reached a maximum by 15 min or at a time when the rate of degranulation was declining (Fig. 12A and B). Suppression of endogenous PLD activity by the Src kinase inhibitor, PP2, was closely correlated with suppression of degranulation (Fig. 12C and D). The estimated 50% inhibitory concentration for PP2 was ∼3.0 μM or a value identical to that observed for the suppression of phosphorylation of expressed HA-PLD2 (Fig. 2). These data were thus entirely consistent with the notion that PLD regulated degranulation and that Src kinase-mediated phosphorylation of PLD2 might be essential for this regulation.
FIG. 11.
Sodium orthovanadate enhances antigen-induced tyrosine phosphorylation and activation of PLD2. Cells made to express HA-PLD2 were stimulated with antigen (Ag) or not stimulated (NS) for 3 min in the absence or presence of 100 μM sodium orthovanadate. (A) HA-PLD2 was immunoprecipitated from whole-cell lysates, and immunoblots were prepared for detection of tyrosine phosphorylated PLD2 (pY-PLD2) and HA-PLD2 (PLD2). (B) Cells were also labeled with [3H]myristic acid for the assay of PLD activity in intact cells by the transphosphatidylation assay. The values are means ± the SEM from three experiments, and the asterisk indicates a significant difference (P < 0.01).
FIG. 12.
Tyrosine phosphorylation and PLD activation correlate with degranulation. RBL-2H3 cells made to express HA-PLD2 were primed with DNP-specific IgE and stimulated with DNP-BSA for the indicated times. (A) The levels of tyrosine phosphorylated PLD was assessed after immunoprecipitation of HA-PLD2 by immunoblotting and densitometric analysis. (B) The concentration of the granule marker, β-hexosaminidase, that was released into the medium was also determined by colorimetric assay (A405, absorbance at 405 nm). (C and D) The indicated concentrations of Src kinase inhibitor, PP2, were added to [3H]myristate-labeled RBL-2H3 cells 10 min before stimulation with DNP-BSA for 3 min, and the PLD activity in intact cells (C) and degranulation (D) were determined as described in previous figure legends. The data are expressed as the percentage of PLD activity or degranulation that was observed in the absence of PP2. Values are means from three experiments. Bars indicate ± the SEM where these fall outside the datum points.
DISCUSSION
Antigen-induced release of preformed inflammatory mediators from mast cell granules is the primary cause of immediate symptoms of IgE-mediated allergic diseases. Recent studies suggest that activation of PLD is an absolute requirement for the stimulated release of granules (7, 10, 58). As reported here, PLD2, which is probably the major PLD isoform in RBL-2H3 cells, is phosphorylated in vitro and in vivo through the actions of the Src kinases Fyn and Fgr. Furthermore, this phosphorylation is associated with activation of PLD and degranulation in antigen-stimulated mast cells. These findings point to a mechanism for the activation of PLD2 by the IgE receptor, FcɛRI, and provide additional evidence for an essential role for PLD in mast cell degranulation. However, an additional signal or signals such as the production of PIP2 by type I phosphatidylinositol 4-phosphate 5-kinase (23) are probably required for full activation of PLD2 in vivo for reasons to be discussed later.
The activation of signaling pathways in antigen-stimulated mast cells is dependent initially on the interaction of FcɛRI with the Src kinases Lyn and Fyn and subsequently on the downstream activation of Syk and other tyrosine kinases (45). However, propagation of the full array of activating signals is thought to require the assembly of two distinct clusters of signaling molecules at the plasma membrane (46). One is assembled around the linker for activation of T cells (LAT), and the other is assembled around the Grb2-associated binder-2 (Gab2). The LAT cluster of proteins enables propagation of Syk-mediated signals. These signals include the phosphatidylinositol 3-kinase-dependent phosphorylation of Tec kinases, Btk and Itk, which in turn phosphorylate and activate PLCγ1 and PLCγ2 to promote a calcium signal through the generation of inositol 1,4,5-trisphosphate (47). The Gab2 cluster consists of Fyn and the Src homology 2 domain-containing protein tyrosine phosphatase, SHP-2, in addition to phosphatidylinositol 3-kinase. Less is known about the function of this cluster, but it appears to facilitate phosphatidylinositol 3-kinase-dependent phosphorylation of the survival factor Akt by the phosphoinositide-dependent kinase and the activation of PKC (20, 45). These clusters appear to localize in distinct but different regions of the plasma membrane (60).
Current models for mast cell activation, such as the one described above, do not accommodate PLD because its linkages to FcɛRI are unknown. Nevertheless, our results suggest that PLD2 is linked to FcɛRI through Fyn and possibly Fgr. In studies to be reported elsewhere, PLD2 becomes localized within discrete micropatches on the plasma membrane after antigen stimulation. Also, tyrosine phosphorylation of PLD2 is prevented by prior treatment of RBL-2H3 cells with methyl-β-cylodextrin, a lipid raft dispersing agent. It is likely, therefore, that the interaction of PLD2 with Fyn occurs within specialized domains of the plasma membrane. The aforementioned association of Fyn with Gab2 suggest that PLD2 possibly associates with the Gab2/Fyn complex. If this scenario is correct, the interaction of PLD2 with the Gab2/Fyn complex might provide one signal for degranulation and complement other essential signals that are mediated via LAT, namely, the activation of PLCγ for the generation of a calcium signal.
With respect to Fgr, this Src kinase has not been previously implicated in FcɛRI-mediated signaling in mast cells. Fgr is most highly expressed by mature blood granulocytes and monocytes, as well as tissue macrophages. Although Fgr negatively regulates Fcγ receptor-mediated phagocytosis in macrophages (19), it appears to play a positive role in integrin- or chemokine-mediated responses in macrophages (52), eosinophils (14, 57), and neutrophils (38). Of particular interest, Fgr has been identified as a downstream target of PLD-derived phosphatidic acid in neutrophils stimulated with chemotactic peptide (50). In this situation, Fgr tyrosine phosphorylating activity was found to be dependent on phosphatidic acid (50). If this finding is applicable to antigen-stimulated mast cells, the possibility exists that PLD2 might promote its own phosphorylation by Fgr through the production of phosphatidic acid. A possible analogous situation is the ability of phosphatidic acid to activate phosphatidylinositol 4-phosphate 5-kinase (23) which, as noted earlier, catalyzes the production of PIP2, the only known stimulant of PLD2 activity. Both Fgr and phosphatidylinositol 4-phosphate 5-kinase may thus provide the means of sustaining or amplifying PLD2 activation.
There are indications from previous work that Src kinases interact with PLD2. Src kinase(s) is thought to be responsible for enhanced PLD activity in v-Ras-transformed cells (24), whereas other studies indicate that c-Src phosphorylates PLD2 and to a much lesser extent PLD1 in EGF-stimulated cells through direct interaction of the N-terminal phox (PX) domain of PLD2 with the catalytic domain of c-Src (1). This interaction did not alter PLD activity but rather enhanced Src catalytic activity possibly as a consequence of the production of phosphatidic acid by PLD.
It would appear from our studies in mast cells that tyrosine phosphorylation of PLD2 leads to activation of PLD2, but it is not clear whether this phosphorylation directly activates PLD2 or promotes association of PLD2 with other regulatory molecules or signaling complexes. Increased tyrosine phosphorylation of PLD2 is associated with increased PLD2 activity (Fig. 1 and 11) and mutation of the tyrosine residues that are phosphorylated reduces the activation of PLD2 (Fig. 10B). However, the effects of mutation of Tyr-14 and Tyr-470 are complex. Mutation of Tyr-14 leads to aberrant intracellular distribution of PLD2, dissociation from Fyn and Fgr, and decreased phosphorylation (Fig. 10). The reason for the mislocalization of the Y14 mutant is still under investigation but current studies indicate that it colocalizes with PLD1 on secretory granules (unpublished data). If so, Y14 may allow proper localization of PLD2 on the plasma membrane at least in rodent mast cells. The effect of mutation at Tyr-470 is ambiguous because of the impaired activation of the Tyr-470 mutant by PIP2 (Fig. 10C). Nevertheless, this does not exclude the possibility that basal phosphorylation of Tyr-470 is critical for basal PLD2 activity. Of the four tyrosines that are phosphorylated, only Tyr-165 and Tyr-470 are conserved in rat, mouse, and human PLD2. It is notable that these two residues reside in strategic locations of PLD2. Tyr-470 is close to one of two HKD motifs, whereas Tyr-165 lies within the PX domain of PLD2. The two PLD HKD motifs (16, 18) provide the essential core for PLD catalytic activity (55). The precise role of the PX domain is unknown (18) but deletion of the NH2-terminal region, which includes the PX domain and a pleckstrin homology domain, disrupts the regulation of PLD activity and the association of PLD1 with membranes (22, 53, 54, 56). One proposal is that the NH2-terminal region normally represses PLD1 catalytic activity, and this repression is alleviated by stimulatory molecules such as ARF, Rho, and PKCα (56). A similar scenario is envisaged for PLD2 because truncation of the NH2-terminal domain renders PLD2 sensitive to ARF (54). If so, phosphorylation Tyr-165 in the PLD2 PX domain could conceivably alter the tertiary structure of PLD2 and allow interaction of PLD2 with other regulatory molecules.
The present study also indicates a close correlation between PLD2 tyrosine phosphorylation and degranulation. PLD2 phosphorylation preceded degranulation (Fig. 12A and B), both events were equally sensitive to inhibition of Src kinase activity (Fig. 7 and 12C and D), and both were enhanced by coexpression of PLD2 and the Src kinases (Fig. 9). Other correlations were noted between the activation of PLD and degranulation (Fig. 8) in support of previous findings (9). These findings, therefore, point to the possibility that PLD2 tyrosine phosphorylation is a regulatory step in degranulation.
In summary, our findings indicate that PLD2, which is associated primarily with the plasma membrane in RBL-2H3 cells, is phosphorylated at multiple sites by FcɛRI-activated Fyn and Fgr. This is the first indication of a direct link between FcɛRI and PLD2. Furthermore, this phosphorylation is essential for PLD activation and possibly degranulation in vivo. Although it is possible that the phosphorylation in itself is sufficient for PLD2 activation, our view based on the findings described above is that the phosphorylation of tyrosines, particularly those located within or near the PX domain and HKD motif, induces conformational changes of PLD2 which allow interaction with other regulatory molecules. The production of phosphatidic acid by PLD2 could conceivably promote these interactions in a manner analogous to that conceived for the interaction of c-Src with PLD2 in response to EGF (1) as noted above. In addition to these considerations, the rapidity of PLD2 phosphorylation (Fig. 12A) and of the dissociation of the Src kinase/PLD2 complex (Fig. 4) suggest that PLD2 activation is an early rather than a late event in mast cell activation. Given the key role of mast cells in allergic diseases and of PLD in mast cell degranulation, these findings have relevance to the pathology and treatment of mast cell-related allergic disease.
Acknowledgments
The studies conducted in the Department of Immunology, College of Medicine, Konkuk University, Chungju, Korea, were supported by the Ministry of Science and Technology through the Bio-Food and Drug Research Center at Konkuk University.
REFERENCES
- 1.Ahn, B. H., S. Y. Kim, E. H. Kim, K. S. Choi, T. K. Kwon, Y. H. Lee, J. S. Chang, M. S. Kim, Y. H. Jo, and D. S. Min. 2003. Transmodulation between phospholipase D and c-Src enhances cell proliferation. Mol. Cell. Biol. 23:3103-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, H., O. H. Choi, P. F. Fraundorfer, K. Yamada, H. M. S. Gonzaga, and M. A. Beaven. 1996. Sustained activation of phospholipase D via adenosine A3 receptors is associated with enhancement of antigen- and Ca2+-ionophore-induced secretion in a rat mast cell line. J. Pharm. Exp. Ther. 276:837-845. [PubMed] [Google Scholar]
- 3.Ali, H., J. R. Cunha-Melo, W. F. Saul, and M. A. Beaven. 1990. The activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen stimulated RBL-2H3 cells: evidence for a novel adenosine receptor. J. Biol. Chem. 265:745-753. [PubMed] [Google Scholar]
- 4.Bae, C. D., D. S. Min, I. N. Fleming, and J. H. Exton. 1998. Determination of interaction sites on the small G protein RhoA for phospholipase D. J. Biol. Chem. 273:11596-11604. [DOI] [PubMed] [Google Scholar]
- 5.Beaven, M. A., and T. R. Hundley. 2003. Mast cell related diseases: genetics, signaling pathways, and novel therapies, p. 307-355. In T. H. Finkel and J. S. Gutkind (ed.), Signal transduction and human disease. John Wiley & Sons, Inc., Hoboken, N.J.
- 6.Bourgoin, S., and S. Grinstein. 1992. Peroxides of vanadate induce activation of phospholipase D in HL-60 cells: role of tyrosine phosphorylation. J. Biol. Chem. 267:11908-11916. [PubMed] [Google Scholar]
- 7.Brown, F. D., N. Thompson, K. M. Saqid, J. M. Clark, D. Powner, N. T. Thompson, R. Solari, and M. J. O. Wakelam. 1998. Phospholipase D1 localizes to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol. 8:835-838. [DOI] [PubMed] [Google Scholar]
- 8.Chahdi, A., W. S. Choi, Y. M. Kim, P. F. Fraundorfer, and M. A. Beaven. 2002. Serine/threonine kinases synergistically regulate phospholipase D1 and 2 and secretion in RBL-2H3 mast cells. Mol. Immunol. 38:1269-1276. [DOI] [PubMed] [Google Scholar]
- 9.Choi, W. S., Y. M. Kim, C. Combs, M. A. Frohman, and M. A. Beaven. 2002. Phospholipase D1 and 2 regulate different phases of exocytosis in mast cells. J. Immunol. 168:5682-5689. [DOI] [PubMed] [Google Scholar]
- 10.Cissel, D. S., P. F. Fraundorfer, and M. A. Beaven. 1998. Thapsigargin-induced secretion is dependent on activation of a cholera toxin-sensitive and a phosphatidylinositol-3-kinase-regulated phospholipase D in a mast cell line. J. Pharmacol. Exp. Ther. 285:110-118. [PubMed] [Google Scholar]
- 11.Colley, W. C., T. C. Sung, R. Roll, J. Jenco, S. M. Hammond, Y. Altshuller, D. Bar-Sagi, A. J. Morris, and M. A. Frohman. 1997. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7:191-201. [DOI] [PubMed] [Google Scholar]
- 12.Dinh, T. T., and D. A. Kennerly. 1991. Assessment of receptor-dependent activation of phosphatidylcholine hydrolysis by both phospholipase D and phospholipase C. Cell Regul. 2:299-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du, G., P. Huang, B. T. Liang, and M. A. Frohman. 2004. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol. Biol. Cell 15:1024-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Shazly, A., N. Yamaguchi, K. Masuyama, T. Suda, and T. Ishikawa. 1999. Novel association of the src family kinases, hck and c-fgr, with CCR3 receptor stimulation: a possible mechanism for eotaxin-induced human eosinophil chemotaxis. Biochem. Biophys. Res. Commun. 264:163-170. [DOI] [PubMed] [Google Scholar]
- 15.Exton, J. H. 1997. Phospholipase D: enzymology, mechanisms of regulation, and function. Physiol. Rev. 77:303-320. [DOI] [PubMed] [Google Scholar]
- 16.Exton, J. H. 2002. Regulation of phospholipase D. FEBS Lett. 531:58-61. [DOI] [PubMed] [Google Scholar]
- 17.Faeder, J. R., W. S. Hlavacek, I. Reischl, M. L. Blinov, H. Metzger, A. Redondo, C. Wofsy, and B. Goldstein. 2003. Investigation of early events in FcɛRI-mediated signaling using a detailed mathematical model. J. Immunol. 170:3769-3781. [DOI] [PubMed] [Google Scholar]
- 18.Frohman, M. A., T. C. Sung, and A. J. Morris. 1999. Mammalian phospholipase D structure and regulation. Biochim. Biophys. Acta 1439:175-186. [DOI] [PubMed] [Google Scholar]
- 19.Gresham, H. D., B. M. Dale, J. W. Potter, P. W. Chang, C. M. Vines, C. A. Lowell, C. F. Lagenaur, and C. L. Willman. 2000. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J. Exp. Med. 191:515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu, H., K. Saito, L. D. Klaman, J. Shen, T. Fleming, Y. Wang, J. C. Pratt, G. Lin, B. Lim, J. P. Kinet, and B. G. Neel. 2001. Essential role for Gab2 in the allergic response. Nature 412:186-190. [DOI] [PubMed] [Google Scholar]
- 21.Hammond, S. M., J. M. Jenco, S. Nakashima, K. Cadwallader, Q. M. Gu, S. Cook, Y. Nozawa, G. D. Prestwich, M. A. Frohman, and A. J. Morris. 1997. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-α. J. Biol. Chem. 272:3860-3868. [DOI] [PubMed] [Google Scholar]
- 22.Hodgkin, M. N., M. R. Masson, D. Powner, K. M. Saqib, C. P. Ponting, and M. J. O. Wakelam. 2000. Phospholipase D regulation and localization is dependent upon a phosphatidylinositol 4,5-bisphosphate-specific PH domain. Curr. Biol. 10:43-46. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins, G. H., P. L. Fisette, and R. A. Anderson. 1994. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem. 269:11547-11554. [PubMed] [Google Scholar]
- 24.Jiang, H., Z. Lu, J. Q. Luo, A. Wolfman, and D. A. Foster. 1995. Ras mediates the activation of phospholipase D by v-Src. J. Biol. Chem. 270:6006-6009. [DOI] [PubMed] [Google Scholar]
- 25.Jones, D., C. Morgan, and S. Cockcroft. 1999. Phospholipase D and membrane traffic: potential roles in regulated exocytosis, membrane delivery and vesicle budding. Biochim. Biophys. Acta 1439:229-244. [DOI] [PubMed] [Google Scholar]
- 26.Kim, Y., J. M. Han, J. B. Park, S. D. Lee, Y. S. Oh, C. Chung, T. G. Lee, J. H. Kim, S. K. Park, J. S. Yoo, P. G. Suh, and S. H. Ryu. 1999. Phosphorylation and activation of phospholipase D1 by protein kinase C in vivo: determination of multiple phosphorylation sites. Biochemistry 38:10344-10351. [DOI] [PubMed] [Google Scholar]
- 27.Kumada, T., H. Miyata, and Y. Nozawa. 1993. Involvement of tyrosine phosphorylation in IgE receptor-mediated phospholipase D activation in rat basophilic leukemia (RBL-2H3) cells. Biochem. Biophys. Res. Commun. 191:1363-1368. [DOI] [PubMed] [Google Scholar]
- 28.Kumada, T., S. Nakashima, H. Miyata, and Y. Nozawa. 1994. Potent activation of phospholipase D by phenylarsine oxide in rat basophilic leukemia (RBL-2H3) cells. Biochem. Biophys. Res. Commun. 199:792-798. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 30.Lin, P., and A. M. Gilfillan. 1992. The role of calcium and protein kinase C in the IgE-dependent activation of phosphatidylcholine-specific phospholipase D in a rat mast (RBL-2H3) cell line. Eur. J. Biochem. 207:163-168. [DOI] [PubMed] [Google Scholar]
- 31.Liscovitch, M., M. Czarny, G. Fiucci, and X. Tang. 2000. Phospholipase D: molecular and cell biology of a novel gene family. Biochem. J. 345:401-415. [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez, I., R. S. Arnold, and J. D. Lambeth. 1998. Cloning and initial characterization of a human phospholipase D (hPLD2): ADP-ribosylation factor regulates hPLD2. J. Biol. Chem. 273:12846-12852. [DOI] [PubMed] [Google Scholar]
- 33.Marcil, J., D. Harbour, P. H. Naccache, and S. Bourgoin. 1997. Human phospholipase D1 can be tyrosine phosphorylated in HL-60 granulocytes. J. Biol. Chem. 272:20660-20664. [DOI] [PubMed] [Google Scholar]
- 34.Massenburg, D., J. S. Han, M. Liyanage, W. A. Patton, S. G. Rhee, J. Moss, and M. Vaughan. 1994. Activation of rat brain phospholipase D by ADP-ribosylation factors 1, 5, and 6: separation of ADP-ribosylation factor-dependent and oleate-dependent enzymes. Proc. Natl. Acad. Sci. USA 91:11718-11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min, D. S., N. J. Cho, S. H. Yoon, Y. H. Lee, S. J. Hahn, K. H. Lee, M. S. Kim, and Y. H. Jo. 2000. Phospholipase C, protein kinase C, Ca2+/calmodulin-dependent protein kinase II, and tyrosine phosphorylation are involved in carbachol-induced phospholipase D activation in Chinese hamster ovary cells expressing muscarinic acetylcholine receptor of Caenorhabditis elegans. J. Neurochem. 75:274-281. [DOI] [PubMed] [Google Scholar]
- 36.Min, D. S., E. G. Kim, and J. H. Exton. 1998. Involvement of tyrosine phosphorylation and protein kinase C in the activation of phospholipase D by H2O2 in Swiss 3T3 fibroblasts. J. Biol. Chem. 273:29986-29994. [DOI] [PubMed] [Google Scholar]
- 37.Min, D. S., S. K. Park, and J. H. Exton. 1998. Characterization of a rat brain phospholipase D isozyme. J. Biol. Chem. 273:7044-7051. [DOI] [PubMed] [Google Scholar]
- 38.Mocsai, A., E. Ligeti, C. A. Lowell, and G. Berton. 1999. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J. Immunol. 162:1120-1126. [PubMed] [Google Scholar]
- 39.Morris, A. J., M. A. Frohman, and J. Engebrecht. 1997. Measurement of phospholipase D activity. Anal. Biochem. 252:1-9. [DOI] [PubMed] [Google Scholar]
- 40.O'Luanaigh, N., R. Pardo, A. Fensome, V. Allen-Baume, D. Jones, M. R. Holt, and S. Cockcroft. 2002. Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 13:3730-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozawa, K., Z. Szallasi, M. G. Kazanietz, P. M. Blumberg, H. Mischak, J. F. Mushinski, and M. A. Beaven. 1993. Ca2+-Dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells: reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 268:1749-1756. [PubMed] [Google Scholar]
- 42.Parinandi, N. L., S. Roy, S. Shi, R. J. Cummings, A. J. Morris, J. G. N. Garcia, and V. Natarajan. 2001. Role of Src kinase in diperoxovanadate-mediated activation of phospholipase D in endothelial cells. Arch. Biochem. Biophys. 396:231-242. [DOI] [PubMed] [Google Scholar]
- 43.Park, S. K., J. J. Provost, C. D. Bae, W. T. Ho, and J. H. Exton. 1997. Cloning and characterization of phospholipase D from rat brain. J. Biol. Chem. 272:29263-29271. [DOI] [PubMed] [Google Scholar]
- 44.Parmentier, J. H., M. M. Muthalif, A. E. Saeed, and K. U. Malik. 2001. Phospholipase D activation by norepinephrine is mediated by 12(S)-, 15(S)-, and 20-hydroxyeicosatetraenoic acids generated by stimulation of cytosolic phospholipase A2: tyrosine phosphorylation of phospholipase D2 in response to norepinephrine. J. Biol. Chem. 276:15704-15711. [DOI] [PubMed] [Google Scholar]
- 45.Parravinci, V., M. Gadina, M. Kovarova, S. Odom, C. Gonzalez-Espinosa, Y. Furumoto, S. Saitoh, L. E. Samelson, J. J. O'Shea, and J. Rivera. 2002. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 3:741-748. [DOI] [PubMed] [Google Scholar]
- 46.Rivera, J. 2002. Molecular adapters in FcɛRI signaling and the allergic response. Curr. Opin. Immunol. 14:688-693. [DOI] [PubMed] [Google Scholar]
- 47.Saitoh, S., R. Arudchandran, T. S. Manetz, W. Zhang, C. L. Sommers, P. E. Love, J. Rivera, and L. E. Samelson. 2000. LAT is essential for FcɛRI-mediated mast cell activation. Immunity 12:525-535. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt, M., M. Vob, P. A. Oude Weernink, J. Wetzel, M. Amano, K. Kaibuchi, and K. H. Jakobs. 1999. A role for Rho-kinase in Rho-controlled phospholipase D stimulation by the m3 muscarinic acetylcholine receptor. J. Biol. Chem. 274:14648-14654. [DOI] [PubMed] [Google Scholar]
- 49.Seldin, D. C., S. Adelman, K. F. Austen, R. L. Stevens, A. Hein, J. P. Caulfield, and R. G. Woodbury. 1985. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc. Natl. Acad. Sci. USA 82:3871-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sergeant, S., K. A. Waite, J. Heravi, and L. C. McPhail. 2001. Phosphatidic acid regulates tyrosine phosphorylating activity in human neutrophils: enhancement of Fgr activity. J. Biol. Chem. 276:4737-4746. [DOI] [PubMed] [Google Scholar]
- 51.Slaaby, R., T. Jensen, H. S. Hansen, M. A. Frohman, and K. Seedorf. 1998. PLD2 complexes with the EGF receptor and undergoes tyrosine phosphorylation at a single site upon agonist stimulation. J. Biol. Chem. 273:33722-33727. [DOI] [PubMed] [Google Scholar]
- 52.Suen, P. W., D. Ilic, E. Caveggion, G. Berton, C. H. Damsky, and C. A. Lowell. 1999. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J. Cell Sci. 112:4067-4078. [DOI] [PubMed] [Google Scholar]
- 53.Sugars, J. M., S. Cellek, M. Manifava, J. Coadwell, and N. T. Ktistakis. 2002. Hierarchy of membrane-targeting signals of phospholipase D1 involving lipid modification of a pleckstrin homology domain. J. Biol. Chem. 277:29152-29161. [DOI] [PubMed] [Google Scholar]
- 54.Sung, T. C., Y. M. Altshuller, A. J. Morris, and M. A. Frohman. 1999. Molecular analysis of mammalian phospholipase D2. J. Biol. Chem. 274:494-502. [DOI] [PubMed] [Google Scholar]
- 55.Sung, T. C., R. L. Roper, Y. Zhang, S. A. Rudge, R. Temel, S. M. Hammond, A. J. Morris, B. Moss, J. Engebrecht, and M. A. Frohman. 1997. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 16:4519-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung, T. C., Y. Zhang, A. J. Morris, and M. A. Frohman. 1999. Structural analysis of human phospholipase D1. J. Biol. Chem. 274:3659-3666. [DOI] [PubMed] [Google Scholar]
- 57.Vicentini, L., P. Mazzi, E. Caveggion, S. Continolo, L. Fumagalli, J. A. Lapinet-Vera, C. A. Lowell, and G. Berton. 2002. Fgr deficiency results in defective eosinophil recruitment to the lung during allergic airway inflammation. J. Immunol. 168:6446-6454. [DOI] [PubMed] [Google Scholar]
- 58.Way, G., N. O'Luanaigh, and S. Cockcroft. 2000. Activation of exocytosis by cross-linking of the IgE receptor is dependent on ADP-ribosylation factor 1-regulated phospholipase D in RBL-2H3 mast cells: evidence that the mechanism of activation is via regulation of phosphatidylinositol 4,5-bisphosphate synthesis. Biochem. J. 346:63-70. [PMC free article] [PubMed] [Google Scholar]
- 59.Wedemeyer, J., M. Tsai, and S. J. Galli. 2000. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 12:624-631. [DOI] [PubMed] [Google Scholar]
- 60.Wilson, B. S., J. R. Pfeiffer, Z. Surviladze, E. A. Gaudet, and J. M. Oliver. 2001. High resolution mapping of mast cell membranes reveals primary and secondary domains of FcɛRI and LAT. J. Cell Biol. 154:645-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashita, T., S. Y. Mao, and H. Metzger. 1994. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc. Natl. Acad. Sci. USA 91:11251-11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, C. H., S. Y. Liu, and V. Panagia. 1996. The transphosphatidylation activity of phospholipase D. Mol. Cell. Biochem. 157:101-105. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, Y., Y. M. Altshuller, S. M. Hammond, and M. A. Frohman. 1999. Loss of receptor regulation by a phospholipase D1 mutant unresponsive to protein kinase C. EMBO J. 18:6339-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]