Abstract
Among the large family of intermediate filament proteins, the keratin 8 and 18 (K8/K18) pair constitutes a hallmark for all simple epithelial cells, such as hepatocytes and mammary cells. Functional studies with different cell models have suggested that K8/K18 are involved in simple epithelial cell resistance to several forms of stress that may lead to cell death. We have reported recently that K8/K18-deprived hepatocytes from K8-null mice are more sensitive to Fas-mediated apoptosis. Here we show that upon Fas, tumor necrosis factor alpha receptor, or tumor necrosis factor alpha-related apoptosis-inducing ligand receptor stimulation, an inhibition of extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation sensitizes wild-type but not K8-null mouse hepatocytes to apoptosis and that a much weaker ERK1/2 activation occurs in K8-null hepatocytes. In turn, this impaired ERK1/2 activation in K8-null hepatocytes is associated with a drastic reduction in c-Flip protein, an event that also holds in a K8-null mouse mammary cell line. c-Flip, along with Raf-1, is part of a K8/K18-immunoisolated complex from wild-type hepatocytes, and Fas stimulation leads to further c-Flip and Raf-1 recruitment in the complex. This points to a new regulatory role of simple epithelium keratins in the c-Flip/ERK1/2 antiapoptotic signaling pathway.
Keratins form the intermediate filaments (IFs) of epithelial cells (10, 40). They constitute a family of over 20 proteins subdivided into type I (keratin 9 [K9] to K20) and type II (K1 to K8) subclasses, which are coordinately expressed and assembled as specific type I/type II pairs in differentiated epithelial cells (40). In simple epithelia, all cells contain the K8/K18 pair, and most of them express two or three other keratins in addition (37, 40). Genes encoding for K8 and K18 constitute the first keratin genes expressed in the embryo (22, 33). During the entire period of liver development, differentiating hepatocytes maintain solely K8 and K18, suggesting that this pair is involved in processes other than typical differentiation events in these simple epithelial cells (38). In line with data on epidermal keratins, we have shown that K8/K18 IFs contribute to maintain hepatocyte surface membrane integrity in response to mechanical stress (35). However, K8/K18 also exhibit resistance features in response to other forms of stress and to apoptosis (10, 38, 43).
Apoptosis may be initiated by different stimuli, particularly through involvement of death receptors (DRs), which in hepatocytes include Fas receptor (Fas), tumor necrosis factor receptor 1 (TNF-R1) and TNF-related apoptosis-inducing ligand (TRAIL) receptor 1 and 2 (TRAIL-R1 and -R2). For instance, stimulation of Fas by the Fas ligand (FasL) allows the recruitment of the adapter Fas-associated death domain (FADD) protein, which, with the initiator procaspase-8, forms the death-inducing signaling complex (27). This triggers procaspase-8 proteolytic activation, which in turn initiates several downstream apoptotic events (25). TNF-α activates two receptors, TNF-R1 and TNF-R2 (2), which normally, promote cell proliferation following binding of appropriate TRAF family members. In some cases, however, TNF-α stimulation of TNF-R1, but not of TNF-R2, which lacks the death domain, can lead to apoptosis through the consecutive recruitment of TNF-R1-associated death domain (TRADD) protein, FADD and procaspase-8 (36, 39). Still, some cell types are resistant to apoptosis after Fas or TNF-R1 stimulation due to an increased level of cellular FLICE inhibitory protein (c-Flip), a labile protein homologous to caspase 8 but exhibiting an inactive catalytic site (53). c-Flip level can be modulated by degradation via the ubiquitin/proteasome or other ill-defined proteolytic pathways (12, 26) or by upregulation through a process that responds to the activation of the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase 1 and 2 (ERK1/2) cascade (1). Still, c-Flip binds to Raf-1 and is responsible for the ERK1/2 activation in response to a DR stimulation (24, 44, 45) and, notably, the inhibition of this cascade can sensitize cells to apoptosis (19, 52).
Hepatocytes are particularly sensitive to Fas stimulation (41) and can, in fact, physiologically promote their own death via an autocrine mechanism (49), except that in toxin-mediated liver diseases the end result is pathological death (14, 28). The importance of K8 and K18 in liver diseases has recently been highlighted by the discovery of mutations associated with cryptogenic cirrhosis in humans (30, 31) and by the finding that hepatocyte damage is much more pronounced in toxin-exposed K8-null mouse liver (54). Similarly, we have shown that K8/K18 provide resistance to Fas-mediated apoptosis of mouse hepatocytes (15) and found that K8/K18 act as a modulator of Fas trafficking and caspase signaling. However, DR-mediated apoptosis can be downregulated upon activation of MAPK pathways in different cell lines (19, 52), and the same signaling pathways regulate keratin phosphorylation in epithelial cell lines (16). Using mouse hepatocytes and mammary cells as model systems, the study reported here shows that K8/K18 provide a resistance to DR-mediated apoptosis in part through a modulation of c-Flip level, a protein that regulates the antiapoptotic ERK1/2 signaling pathway in simple epithelial cells.
MATERIALS AND METHODS
Reagents, plasmids, and antibodies.
Isoflurane was purchased from Abbott Laboratories, Ltd. (Montreal, Quebec, Canada). Jo2 (no. 15400D) and EHS Matrigel Brand (no. 354234) were from BD Pharmingen (Mississauga, Ontario, Canada). Soluble mouse recombinant TRAIL (Apo2L; SE-722) was bought from BIOMOL Research Laboratories (Plymouth, Mass.). TNF-α, PD 98059, SB 203580, wortmanin, MG132, MG115, lactacystin, l-methionine, epidermal growth factor (EGF), and all other reagents were from Sigma Chemical Co. (Mississauga, Ontario, Canada). The plasmid encoding hemagglutinin (HA)-tagged c-Flip was a gift from Jürg Tschopp (University of Lausanne, Epalinges, Switzerland), the plasmid encoding a Raf-1 dominant-negative (Raf-C4 (7) was a gift from Ulf R. Rapp (University of Innsbruck, Innsbruck, Austria), and the plasmid encoding a MEK1 dominant-negative (MEK1AA) was kindly provided by Jean Charron (Laval University, Quebec, Canada). The methionine, l-[35S]-(NEG-009A), was from Perkin-Elmer life sciences (Boston, Mass.). The BETACOUNT, LSC Cocktail was bought from J. T. Baker (Phillipsburg, N.J.). The methionine-free DME medium (no. 21013-024) was from Invitrogen (Burlington, Ontario, Canada). The following antibodies were used: mouse anti-phospo-p44/42 MAPK (Thr202/Tyr204) E10 and anti-phospho-SAPK/JNK (Thr183/Tyr185) monoclonal antibody, rabbit anti-p44/42 MAPK, anti-phospho-p38 MAPK (Thr180/tyr182), anti-p38 MAPK, anti-SAPK/JNK, anti-phospho-Akt (Ser473), and anti-Akt polyclonal antibody (Cell Signaling Technology, Beverly, Mass.); rat anti-mouse K8 (TROMA-1), K18 (TROMA-2), and K19 (TROMA-3) monoclonal antibodies (from R. Kemler, Freiburg, Germany); rabbit anti-Fas (M20) and anti-p130Cas (C20) polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.); rabbit anti-caspase 8 polyclonal antibody (Calbiochem, San Diego, Calif.); rabbit anti-active caspase 3 polyclonal antibody (BD Pharmingen); rabbit anti-Flip CT polyclonal antibody (Upstate, Waltham, Mass.); mouse anti-Raf-1 monoclonal antibody (BD Transduction Laboratory, Franklin Lakes, N.J.); mouse anti-HA.11 monoclonal antibody (16B12; Babco, Richmond, Va.); mouse anti-keratin 7 monoclonal antibody (RCK105; ICN, Aurora, Ohio); mouse antivimentin monoclonal antibody (47); mouse antiactin monoclonal antibody (from J. L. Lessard, Cincinnati, Ohio); mouse anti-p21waf1/Cip1monoclonal antibody (clone CP74; Sigma Chemical Co., Mississauga, Ontario, Canada); mouse antikeratin (ab-1, C-11) and anti-human keratin 8 monoclonal antibody (ab-4, TS1) (NeoMarkers, Fremont, N.J.); horseradish peroxidase-goat anti-rabbit immunoglobulin G (IgG) and anti-mouse IgG (BIO/CAN, Mississauga, Ontario, Canada); Alexa 488-rabbit anti-green fluorescent protein (GFP) polyclonal antibody (A-21311), Alexa 488-goat anti-mouse (A-11001), and Alexa 594-goat anti-mouse (A-11005) IgG antibodies (Molecular Probes, Eugene, Oreg.).
Mice.
Details on the establishment, maintenance, and genotyping of the K8-deficient FVB/N mouse colony were reported previously (3, 35). The mice were housed in the specific-pathogen-free animal facility at our research center, which included the absence of Helicobacter hepaticus (15). The experiments were performed according to the requirements of the Laval University Animal Care Committee.
Hepatocyte isolation and culture.
Hepatocytes were isolated from mice according to a modified version of the two-step collagenase method as described previously (15). The cells were plated at a density of 1.2 × 105 cells cm−2 on fibronectin-coated dishes in DME/F12 modified medium supplemented with sodium selenite (5 μg liter−1), insulin (5 mg liter−1), transferrin (5 mg liter−1), streptomycin (100 μg ml−1), and penicillin (100 U ml−1). After a 3-h attachment period, the culture medium was replaced by the same medium supplemented with dexamethasone (10−7 M) and EGF (20 ng ml−1).
MGT and MGT-K8 cell lines.
MGT 499 (MGT) is a cell line established from a primary culture of mammary gland adenocarcinoma cells originating from K8-null mice mated with transgenic mice (MT#634) carrying the middle T driven by the Moloney murine leukemia virus promoter (4). This cell line, generously provided by Hélène Baribault (Deltagen, Inc., Menlo Park, Calif.), is one of the 10 clones isolated by her laboratory (H. Baribault, unpublished data). The MGT-K8 is a clonal cell line generated in our laboratory by inserting a complete human K8 cDNA into MGT cells with a retrovirus vector as described previously (15). Both MGT and MGT-K8 cell lines exhibit the typical cobblestone-like morphology of simple epithelial cells in monolayer culture. These, plus additional phenotypic features of the two cell lines, will be described elsewhere. The cells were maintained in Dulbecco modified Eagle medium high glucose (Invitrogen, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum, 0.5 μg of insulin ml−1, 40 ng of dexamethasone ml−1, 0.1 mM β-mercaptoethanol, 3.5 μg of gentamicin ml−1, and 2 mM l-glutamine. Upon reaching confluency, cells were expanded by trypsin treatment (0.25% trypsin, 0.4% EDTA, 0.8% NaCl, and 0.2 g of glucose liter−1 in phosphate-buffered saline [PBS]-[pH 7.6]).
Assessment of apoptosis in culture.
The details on the acridine-orange based procedure were described previously (15). The apoptosis inducer (Jo2 [0.5 μg ml−1], TNF-α [0.1 μg ml−1], or TRAIL [1.0 μg ml−1]) was added for an 8-h period. Inhibition of the ERK1/2, p38, and Akt signaling pathways was obtained by the addition of PD 98059 (50 μM), SB 203580 (10 μM), and wortmanin (100 nM), respectively, 1 h prior to the addition of apoptosis inducer.
Transfection and infection.
Hepatocytes and MGT cells were seeded at 1.0 × 105 cells cm−2, and transfections with c-Flip-HA, a Raf-1 dominant-negative (Raf-C4), or a MEK1 dominant-negative cDNA (MEK1AA) were performed 52 h after seeding by using Effecten (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's instructions. The cultures were washed twice at 18 h posttransfection, and the cells were assayed 48 or 72 h later. The transfection efficiency in hepatocytes is 25 to 30%.
Using a protocol described previously (15), K8-null hepatocytes were infected with a retrovirus vector containing a full-length K8 cDNA, an internal ribosome entry site (IRES), and a GFP cDNA at 52 h postseeding at a ratio of 5 infectious virus per cell, with a medium change at 16 h after infection. The infection-transfection combination was obtained via a two-step protocol: at 52 h after seeding, cells were infected with the K8 retrovirus, and 24 h later they were washed and transfected according to the Effecten protocol. The relative intensity of the c-Flip-HA cell content was quantified by using the MetaMorph Software (Universal Imaging Corp., Downingtown, Pa.)
Immunofluorescence microscopy.
Cultured hepatocytes or MGT cells were rinsed twice in PBS, fixed with paraformaldehyde-PBS (2%) for 10 min at room temperature, and extracted with methanol (100%) 5 min at −20°C. After two consecutive washes with PBS, the labeling was performed with anti-HA (1/100) and/or anti-GFP-Alexa 488 (1/100) overnight at 4°C. Secondary antibody was incubated 60 min at room temperature (with Alexa 488- or 594-tagged goat anti-mouse immunoglobulin antibody [1/100]). Images were collected with the laser-scanning confocal microscopy by using the laser line 488 nm (Alexa 488; fluorescein isothiocyanate [FITC]) or 568 nm (phycoerythrin and Texas red) for fluorochrome excitation and a high-numerical-aperture (NA) (1.4-NA, 60×) oil immersion objective lens.
Western blotting.
For cultured cells, total proteins were extracted with 300 μl per 35-mm plastic petri dish of preheated (90°C) 2× sample buffer (15). For tissues, liver, pancreas, thymus, brain, colon, lung, and heart samples were weighed, snap-frozen in liquid nitrogen, and stored at −80°C until use. The frozen tissue was placed in 10 times its volume of homogenizing buffer with protease inhibitors and homogenized as described before (18). Proteins (20 μg) were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and electrotransferred onto a polyvinylidene difluoride membrane. The blots were incubated with the primary antibody and then with the horseradish peroxidase-conjugated secondary antibody. The staining was revealed with the SuperSignal West Pico kit (Pierce).
Immunoisolation.
For protein extraction, cells were washed twice with PBS and lysed with 300 μl per 35-mm plastic petri dish of ice-cold radioimmunoprecipitation assay buffer (1 mM EDTA, 1 mM EGTA, protease inhibitor cocktail [Complete; Roche, Mannheim, Germany]), 1% NP-40, 0.1% SDS, and 0.5% sodium deoxycholate in PBS [pH 7.4]) and incubated at 4°C for at least 20 min. Cells were scraped and ground with a syringe. The extract was centrifuged for 10 min at 10,000 × g at 4°C, and the supernatant was stored until immunoisolation with protein G-coupled magnetic beads (New England Biolabs). At least 80% of the keratin and essentially all of the c-Flip were solubilized by this extraction protocol. An antibody to mouse K8 (TROMA-1), K19 (TROMA-3), full-length c-Flip, or p130Cas was added to 500 μg of extracted proteins, previously precleared with the protein G-magnetic beads, followed by incubation overnight at 4°C with agitation. Protein G-magnetic beads (25 μl) were added, followed by a 90-min incubation period at 4°C with agitation. A magnetic field was then applied, and the beads were recovered and washed four times with radioimmunoprecipitation assay buffer. The beads were resuspended in 2× sample buffer and submitted to SDS-polyacrylamide gel electrophoresis and Western blotting.
c-Flip turnover and proteasome inhibition assays.
Wild-type and K8-null hepatocytes cultured in monolayers for 48 h were washed twice and then incubated for 1 h in methionine-free DMEM containing 0.2% bovine serum albumin. The medium was replaced with a methionine-free DMEM containing fresh 35S-labeled l-methionine (0.2 mCi/ml) and 0.2% bovine serum albumin. Incubation was continued for exactly 30 min, after which the medium from each dish was quickly withdrawn, and the monolayer was washed once and reincubated for a chase period of 0, 15, 30, or 45 min in the standard medium containing an excess of cold l-methionine (10 mM). Immunoisolation of [35S]methionine-labeled c-Flip was carried out as describe above, and the radioactivity was counted in a liquid scintillation counter (Beckman-Coulter).
Proteasome inhibition was obtained by adding MG132 (20 μM), MG115 (50 μM), or lactacystin (20 μM) to the medium, and the c-Flip protein level was monitored by Western blotting during a 48-h period.
RT-PCR assay.
Total RNA was prepared by using TRIzol reagent (Invitrogen), and reverse transcription-PCR (RT-PCR) was performed by using SuperScript One-Step RT-PCR with Platinum Taq (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized and amplified with the following primers: c-Flip, 5′-AATGTGGACTCTAAGCCCCTGCAACC-3′ and 5′-CGTAGGAGCCAGGATGAGTTTCTTCC-3′; β-actin, 5′-GTGGGCCGCCCTAGGCACCAG-3′ and 5′-CTCTTTGATGTCACGCACGATTTC-3′; and 14-3-3γ, 5′-GGCCATGAAGAACGTGAC-3′ and 5′-GTAGGCCTTCTCAGACGACTC-3′. The conditions were as follows: for cDNA synthesis, 1 cycle of 50°C for 30 min, followed by incubation at 94°C for 2 min, and for PCR amplification, 25 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 40 s. The PCR products were analyzed on 2% agarose gels and stained with ethidium bromide.
RESULTS
ERK1/2 inhibition increases the sensitivity of WT but not K8-null hepatocytes to Fas-induced apoptosis.
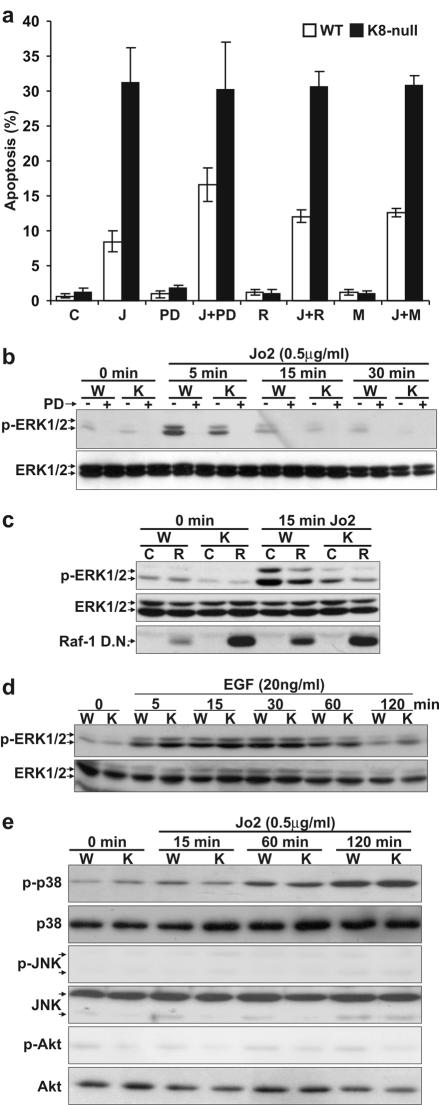
As we reported previously (15), at day 2 postseeding, cultured K8-null hepatocytes were three-fold more sensitive than wild-type (WT) hepatocytes at 8 h after the addition of Jo2 (Fig. 1a). However, Fas stimulation activates ERK1/2 and the inhibition of the pathway sensitizes cells to apoptosis (19, 52). We thus assessed the Fas-induced apoptosis after treatment with the MEK1/2 inhibitor, PD 98059. WT hepatocytes were markedly (twofold) sensitized to Fas-induced apoptosis, whereas K8-null hepatocytes were not (Fig. 1a). In the same way, inhibition of the ERK1/2 pathway via the expression of a Raf-1 dominant-negative or a MEK1 dominant-negative also sensitized WT hepatocytes to Fas-mediated apoptosis (Fig. 1a).
FIG. 1.
ERK1/2 inhibition increases the sensitivity of WT but not K8-null hepatocytes to Fas-induced apoptosis. (a) Upon an 8-h exposure to 0.5 μg of Jo2 (J) ml−1, K8-null hepatocytes in primary culture are three- to fourfold more responsive to Jo2 than are WT hepatocytes. The addition of PD 98059 (PD) 1 h prior to Jo2 addition leads to a twofold increase in WT but not in K8-null hepatocyte apoptosis (P < 0.0002). The expression of a Raf-1 (R) or a MEK1 (M) dominant negative also increases apoptosis only in WT hepatocytes in response to Jo2 (P < 0.001). (b) The kinetics of ERK1/2 activation (p-ERK1/2) upon Jo2 treatment in presence or absence of PD 98059 (PD) shows a reduction in K8-null (K) versus WT (W) hepatocytes. No change is observed in ERK1/2 content upon Jo2 treatment. (c) The expression of a dominant-negative Raf-1 (R) reduces the activation of ERK1/2 after Jo2 treatment in both WT and K8-null hepatocytes. C refers to nontransfected (control) hepatocytes; no variation was observed in ERK1/2 content. The Raf-1 dominant-negative (Raf-1 D.N.) level was assessed with the Raf-1 monoclonal antibody. (d) Kinetics of ERK1/2 activation (p-ERK1/2) after treatment with EGF (20 ng ml−1) showing a comparable activation of ERK1/2 in K8-null (K) versus WT (W) hepatocytes. No change is observed in ERK1/2 content upon EGF treatment. (e) The activation of the p38 pathway (anti-phospho p38 [p-p38]) between WT and K8-null hepatocytes in response to Jo2 is not different, whereas the JNK (anti-phospho JNK [p-JNK]) and Akt (anti-phosphoAkt [p-Akt]) pathways are not activated. No changes are seen in total p38, JNK, or Akt upon Jo2 treatment.
We then compared the ERK1/2 activation in response to Jo2-Fas stimulation. As shown in Fig. 1b, the ERK1/2 activity increased within 15 min, much more prominently in WT than in K8-null hepatocytes. A pretreatment with PD 98059 confirmed the potency of the inhibitor for the ERK1/2 pathway. We next sought to determine whether the activity of Raf-1 was required for Fas-mediated activation of the ERK1/2 pathway. As shown in Fig. 1c, the expression of a Raf-1 dominant-negative cDNA reduced the Fas-mediated activation of ERK1/2 in both WT and K8-null hepatocytes, showing indeed that Raf-1 is involved in Fas-mediated ERK1/2 activation.
EGF can protect against apoptosis in part via a Ras-dependent activation of Raf-1, which leads to ERK1/2 activation (17). The 120-min kinetics of ERK1/2 activation after EGF treatment (Fig. 1d) established that the deficit of ERK1/2 activation after Fas stimulation in K8-null hepatocytes did not merely reflect a general defect in ERK1/2 activation.
In some cell models, Fas can activate p38 (51) and cells can be protected from Fas-mediated apoptosis via the PI 3′-kinase/Akt signaling pathway (46). Our assessment of the Fas-induced apoptosis after a pretreatment with inhibitors of p38 (SB 203580) or Akt (wortmannin) activation revealed that the inhibitors were unable to sensitize WT and K8-null hepatocytes (data not shown). We also monitored p38, c-Jun N-terminal kinase (JNK) and Akt activation in response to Fas stimulation. As shown in Fig. 1e, the addition of Jo2 led to a maximum activation of p38 at 120 min but not in a differential manner between WT and K8-null hepatocytes, whereas essentially no activation occurred for JNK or Akt within the same period, confirming that the loss of K8/K18 selectively affects the Fas/ERK1/2 signaling interplay.
ERK1/2 inhibition also affects hepatocyte sensitivity to TNF-α- and TRAIL-induced apoptosis.
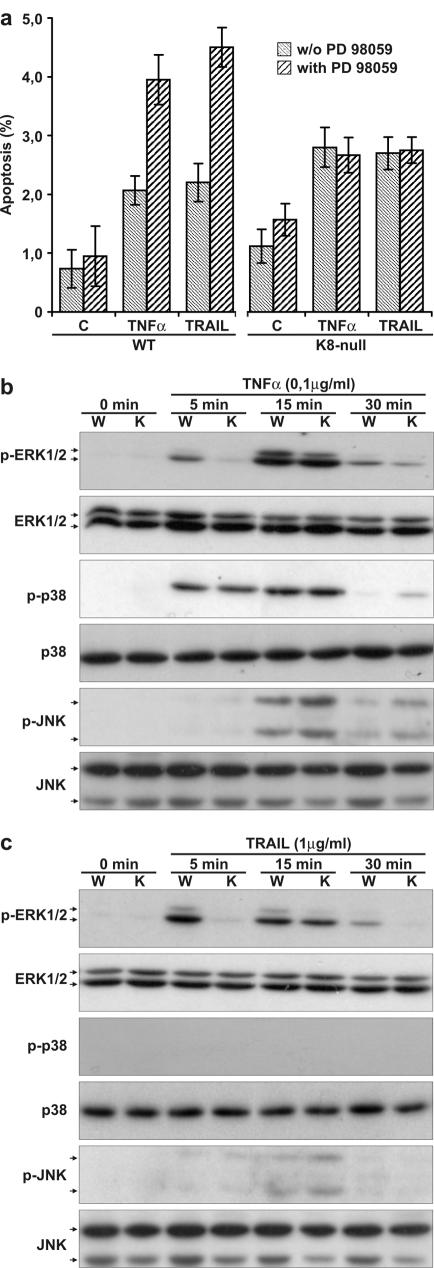
The addition of TNF-α or TRAIL to primary cultured hepatocytes induces a low level of apoptosis, with no significant difference between WT and K8-null hepatocytes (15). The results reported here confirmed those findings and further demonstrated that a pretreatment with PD 98059 led to a major sensitization of WT but not of K8-null hepatocytes upon TNF-α or TRAIL addition (Fig. 2a). Still, the addition of either TNF-α or TRAIL led to a differential activation of the ERK1/2 pathway in WT versus K8-null hepatocytes at 5 min (Fig. 2b and c), followed by a second activation at 15 min, with no apparent difference between WT and K8-null hepatocytes. In addition, TNF-α is a natural activator of the p38 signaling pathway (5) and can activate the JNK pathway (42). As shown in Fig. 2b, the activation did occur but without appreciable difference between WT and K8-null hepatocytes. Similarly, TRAIL induced a weak but nondifferential JNK activation between WT and K8-null hepatocytes and no detectable activation of p38 (Fig. 2c). Taken together, the data show that the K8/K18 loss perturbs specifically ERK1/2 activation in response to Fas, TNF-α or TRAIL.
FIG. 2.
ERK1/2 inhibition affects hepatocyte sensitivity to TNF-α- and TRAIL-induced apoptosis. (a) The addition of PD 98059 1 h prior to an 8 h addition of TNF-α (0.1 μg ml−1) or TRAIL (1.0 μg ml−1) leads to a twofold increase in WT but not in K8-null hepatocyte apoptosis. (b) The time-dependent MAPK activation upon TNF-α (0.1 μg ml−1) stimulation shows a reduced activation of ERK1/2 (p-ERK1/2) at 5 min in K8-null (K) versus WT (W) hepatocytes but no differential activation of p38 (p-p38) or JNK (p-JNK). (c) The data with TRAIL (1.0 μg ml−1) show a reduced activation of ERK1/2 (p-ERK1/2) at 5 min in K8-null (K) versus WT (W) hepatocytes but no differential activation of JNK (p-JNK) and p38 (p-p38). No differences are observed in total MAPK (ERK1/2, p38, and JNK) after treatment with TNF-α or TRAIL.
Correlation between K8 expression and c-Flip content in liver and other K8-containing tissues.
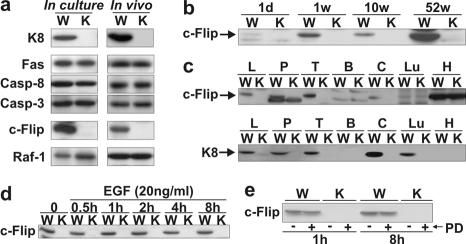
There is accumulating evidence showing that c-Flip mediates the activation of ERK1/2 in response to DR-mediated apoptosis via an interaction with Raf-1 (24, 44, 45). Hence, in light of the above data showing that the K8/K18 loss perturbed ERK1/2 activation in response to Fas, we assessed whether the c-Flip level was perturbed in primary WT versus K8-null hepatocyte cultures. As shown in Fig. 3a, a drastic reduction of c-Flip protein level occurred as a result of the K8/K18 loss. In comparison, the respective levels of Fas, caspase 8 or caspase 3 in WT versus K8-null hepatocytes remained unchanged, while the Raf-1 level slightly increased in K8-null hepatocytes.
FIG. 3.
c-Flip content correlates with K8 expression in liver and other K8-containing tissues. (a) Western blots show no difference in Fas, caspase 8 (Casp-8), caspase 3 (Casp-3), and Raf-1 protein contents of WT (W) and K8-null (K) hepatocytes but the absence of c-Flip in K8-null hepatocytes both in culture and in the liver (in vivo). (b) No c-Flip was detected in K8-null (K) mice of different ages (d = day; w = week). (c) c-Flip protein is lost in the K8-null (K) counterpart of K8-containing tissue (liver [L], pancreas [P], thymus [T], colon [C], and lung [Lu]), whereas it is present in heart (H) and absent in brain (B) tissues. (d) The time course evaluation of c-Flip content after EGF treatment shows no increase in WT (W) hepatocytes and no reappearance in K8-null (K) hepatocytes. (e) The addition of PD 98059 (PD) to WT (W) or K8-null (K) hepatocytes over an 8 h-period did not affect c-Flip expression.
In the same way, the loss of K8/K18 in whole liver led to a dramatic downregulation of c-Flip without affecting the levels of Fas, Raf-1, caspase 8, or caspase 3 (Fig. 3a), and this loss of c-Flip was age independent (Fig. 3b). In light of these correlative findings in liver, we also monitored c-Flip levels in other tissues containing or not containing K8/K18. Figure 3c shows that the same correlation was found in pancreas, colon, and thymus, which all contain K8/K18. The heart expressed c-Flip in both WT and K8-null mice, whereas c-Flip was not detected in the brain, even in the WT mice, which is in line with data reported previously (21).
In endothelial cells, the c-Flip level is in part regulated via ERK1/2 activation (1), and we thus used the observations given above on EGF-mediated ERK1/2 activation in cultured hepatocytes (Fig. 1e) to determine the relationship between ERK1/2 activation and c-Flip protein level. As shown in Fig. 3d, the EGF stimulation did not affect the c-Flip level in WT hepatocytes nor did it lead to a c-Flip reappearance in K8-null hepatocytes. Conversely, a PD 98059 treatment by using the above conditions (Fig. 1b) did not result in a decreased c-Flip level in WT hepatocytes (Fig. 3e). Thus, the regulation of c-Flip level is not ERK1/2 dependent in EGF-stimulated WT and K8-null hepatocytes, and the reduced c-Flip level in K8-null hepatocytes is not due to the weak activation of ERK1/2 by Fas.
c-Flip downregulation in K8-null hepatocytes occurs at the translation level.
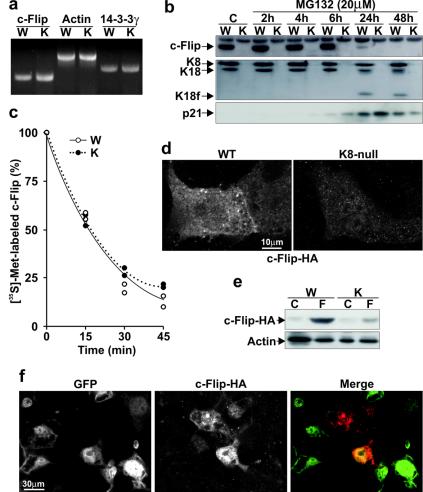
The c-Flip deficit observed in K8-null liver and hepatocytes led us to assess whether it was due to a defect in expression or in stability. As shown in Fig. 4a, the mRNA content was not affected by the lack of K8/K18, implying a regulation at the posttranscriptional level. The next step was then to determine whether K8/K18 modulate c-Flip degradation or translation. With regard to degradation, other data have indicated that in some cases c-Flip can be degraded by proteasomes (12, 26). However, as shown in Fig. 4b, the addition of the inhibitor MG132 did not lead to the reappearance of c-Flip in K8-null hepatocytes. To demonstrate that MG132 inhibited proteasome-mediated protein degradation, we verified its effect on p21, a typical target of proteasomes (32). The results showed an increase in p21 content in both WT and K8-null hepatocytes, thus confirming the efficiency of the inhibitor (Fig. 4b). In contrast, there was no increase in the WT hepatocyte c-Flip content after MG132 addition. Moreover, the inhibition at 24 h led to apoptosis, as shown by the cleavage of K18 and c-Flip (Fig. 4b). Furthermore, the use of two other proteasome inhibitors, MG115 and lactacystin confirmed that the proteasome machinery is not involved in c-Flip degradation (data not shown). We thus sought to determine whether the c-Flip turnover was increased in K8-null hepatocytes. As shown in Fig. 4c, the results from the pulse-chase assay revealed that the disappearance kinetics of [35S]methionine-labeled endogenous c-Flip was comparable in both WT and K8-null hepatocytes, with a half-life of 15 to 20 min, implying therefore that the c-Flip deficit was due to a reduced translation. Moreover, by transfecting a c-Flip-HA cDNA into WT and K8-null hepatocytes, we showed that the c-Flip-HA protein, as revealed by both immunofluorescence staining and Western blotting, was maintained at a much lower level in K8-null than WT hepatocytes (Fig. 4d and e). Together, these results indicate that K8/K18 regulate c-Flip expression at the translation level.
FIG. 4.
c-Flip downregulation in K8-null hepatocytes occurs at the posttranscriptional level. (a) RT-PCR showing no difference in c-Flip mRNA content between WT (W) and K8-null (K) hepatocytes. As controls, no variations are detected in actin and in 14-3-3γ mRNA levels. (b) Proteasome inhibition with 20 μM MG132 does not rescue the c-Flip in K8-null hepatocytes (K) (K18f, fragment of K18). Used as a control, the level of p21 protein increases after MG132 treatment in both WT and K8-null hepatocytes. (c) The disappearance kinetics of [35S]methionine-labeled c-Flip after a pulse-chase point to a comparable turnover in WT and K8-null hepatocytes. (d) HA-tagged c-Flip cDNA transfection shows an increased c-Flip-HA protein content in transfected WT versus K8-null hepatocytes. (e) A Western blot reveals a higher c-Flip-HA protein content in WT (W) than in K8-null (K) hepatocytes after transfection (F) of a c-Flip-HA cDNA; the “C” refers to nontransfected (control) hepatocytes. (f) K8-null hepatocytes infected with a recombinant K8 cDNA-IRES-EGFP cDNA retrovirus, followed by transfection with a c-Flip-HA vector, show that the expression of c-Flip-HA (red in merge image) is stronger in K8 expressing cells (GFP, green in merge image).
We also determined whether the c-Flip deficit observed in K8-null hepatocytes was reversed after the reinsertion of the WT K8 protein. The transfer of a complete K8 cDNA into 52-h K8-null hepatocyte cultures with a retroviral vector did not lead to the reappearance of endogenous c-Flip, in spite of the fact that K8 was well expressed (data not shown). However, when the retroviral transfer of K8 cDNA was combined with transfection of the c-Flip-HA cDNA into K8-null hepatocytes, a two- to threefold increase in c-Flip-HA level occurred in all of the K8-null hepatocytes that reexpressed full-length K8 protein (Fig. 4f).
c-Flip interacts with a K8/K18-containing complex.
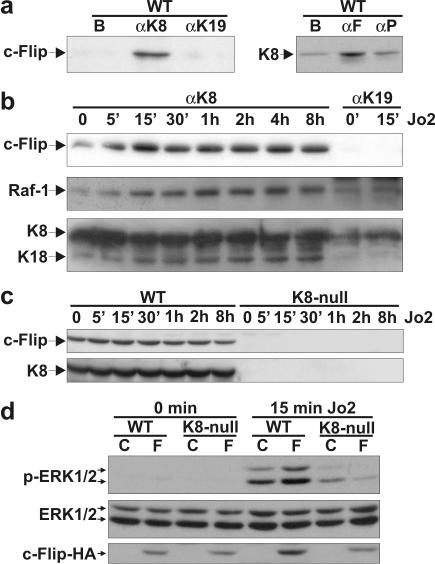
Previous reports have indicated that K8 and K18 can directly interact with TNF-R2 and TRADD, respectively (9, 20). Here, we used the magnetic bead immunoisolation approach to examine the interaction between K8/K18 and c-Flip. For instance, the anti-K8 antibody (TROMA-1) immunoisolated c-Flip (Fig. 5a) from a solubilized WT-hepatocyte protein extract and, conversely, the anti-c-Flip was able to immunoisolate K8 (Fig. 5a), thus showing that c-Flip and K8/18 were parts of the same complex, even in absence of Jo2 stimulation. As a control for the observed specificity of interaction of c-Flip with the K8-containing complex, we selected TROMA-3, an antibody directed against K19 (not present in hepatocytes) generated under conditions similar to those used for TROMA-1 (8), and obtained no immunoisolation of c-Flip (Fig. 5a and b). Similarly, we used an anti-p130Cas antibody as a negative control for the anti-c-Flip antibody and obtained no particular immunoisolation of K8, i.e., a low K8 immunodetection equivalent to that obtained with the magnetic beads alone (Fig. 5a).
FIG. 5.
c-Flip interacts with a K8/K18-containing complex. (a) Immunoisolation with an anti-K8 (αK8) antibody and subsequent Western blotting with an anti-c-Flip antibody show that c-Flip is present in the complex containing K8 in WT hepatocytes. As controls, magnetic beads (B) alone and an anti-K19 (αK19) does not immunoisolate c-Flip. Conversely, the use of an anti-c-Flip (αF) antibody confirms the presence of K8 in the immunoisolate. As a control, an anti-p130Cas (αP) does not immunoisolate K8, the K8 blotting being equivalent to that obtained with the magnetic beads (lane B) alone. (b) Immunoisolation with anti-K8 (αK8) starting from WT hepatocytes treated with Jo2 (0.5 μg ml−1) reveals an increase binding of c-Flip to a K8-containing complex in a time-dependent manner. Moreover, a progressive Raf-1 recruitment to the complex is observed during the same stimulation period. The Western blot with the PAN (ab-1) antibody confirms that K8 and K18 are indeed present in the immunoisolate complex and no increase is seen during the Jo2 time course. As a control, the anti-K19 (αK19) does not immunoisolate c-Flip, Raf-1, K8, or K18. (c) Western blots of c-Flip and K8 showing no increase in either protein when WT (W) or K8-null (K) hepatocytes are stimulated with Jo2 (0.5 μg ml−1). (d) The expression of c-Flip-HA (F) in WT and K8-null hepatocytes, followed by a Jo2 treatment increases ERK1/2 activation only in WT hepatocytes. The “C” refers to nontransfected (control) hepatocytes; no variation was observed in ERK1/2 content. The c-Flip-HA level was assessed with the antibody to the HA-epitope.
To assess the importance of the complex within the context of Fas-mediated apoptosis, we first monitored the c-Flip content in K8-immunoisolates obtained in WT hepatocytes at different time periods after Jo2 stimulation. As shown in Fig. 5b, Fas stimulation led to a further c-Flip recruitment in the K8/K18-containing complex, reaching a maximum at 15 min poststimulation. Moreover, in line with other reports revealing a c-Flip/Raf-1 interaction (24, 44), Raf-1 was also detected in the immunoisolated complex of nonstimulated cells, and the Jo2 stimulation led to a subsequent rapid Raf-1 recruitment. The recruitment of Raf-1 was slightly delayed compared to that of c-Flip. No c-Flip or Raf-1 immunoisolation was obtained at 15 min poststimulation, by using the negative control anti-K19 antibody (Fig. 5b). A complementary control confirmed that both K8 and K18 were present in the immunoisolates, and while their level remained constant during the time course of Jo2 stimulation, c-Flip and Raf-1 were progressively recruited in the complex (Fig. 5b). In the same way, parallel control immunoblots with total proteins confirmed that the progressive c-Flip recruitment in the immunoisolates was not due to an increase in total c-Flip and K8 content in response to the Jo2 stimulation (Fig. 5c).
We then explored the biological significance of the complex in the context of c-Flip/ERK1/2 anti-apoptotic signaling. Since Raf-1 is required for Fas activation of the ERK1/2 pathway (Fig. 1c) and since c-Flip can interact with Raf-1 (24, 44), we determined whether the addition of c-Flip-HA in WT and K8-null hepatocytes modulated the Fas-mediated activation of ERK1/2. As shown in Fig. 5d, transfection with c-Flip-HA cDNA led to increased ERK1/2 activation in WT hepatocytes. In addition, the ERK1/2 activation remained low in c-Flip-HA transfected K8-null hepatocytes, suggesting that the presence of K8/K18 was necessary for the Fas-mediated activation of the c-Flip/ERK1/2 signaling pathway.
c-Flip downregulation in a K8-null simple epithelium cell line.
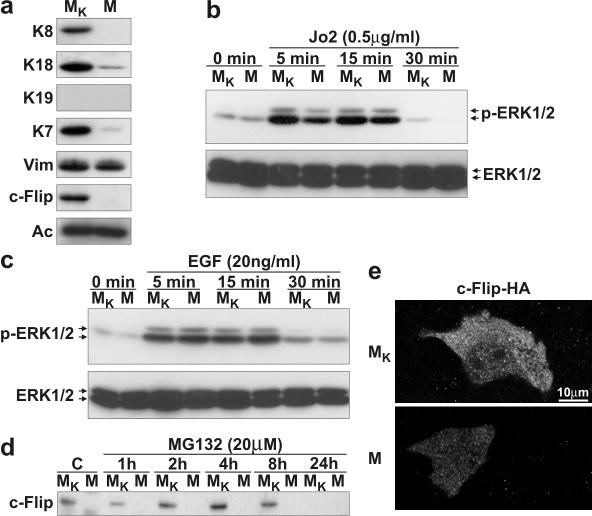
To further document the significance of the above findings in WT versus K8-null hepatocytes, we sought to determine whether the observed functional link between K8/K18 and c-Flip expressions could be observed in another cell model. To address this question, we used the MGT (M) mammary gland cell line derived from K8-null mice and MGT cells which stably express a complete K8 cDNA (MGT-K8; MK). IF-based typing revealed that MGT cells lack K8 and K19 but express a low level of K7 and K18, along with vimentin (Fig. 6a). After the K8 insertion in these cells (MGT-K8) a major increase occurred in K18 and K7 contents but not in vimentin (in Fig. 6a). Interestingly, just as in the case of K8-null hepatocytes, MGT cells exhibited a c-Flip deficit, but it was recovered upon reexpression of K8 (Fig. 6a). As reported for other cell lines (16), Jo2 stimulation of MGT and MGTK8 did not lead to apoptosis (data not shown), but in the light of the above c-Flip data, we used them to assess whether the K8/K18 modulation of c-Flip/ERK1/2 signaling observed in hepatocytes could be extended to another type of simple epithelial cells.
FIG. 6.
Lack of c-Flip in a K8-null simple epithelium cell line. (a) Western blots of a K8-null (MGT [M]) and a K8-reexpressing (MGT-K8 [MK]) epithelial cells line reveal the presence of K18, K7, and vimentin (Vim) in MGT cells, with increased K18 and K7 contents after the stable expression of a K8 cDNA (MGT-K8). c-Flip was not detected in MGT cells, and the reappearance of K8 (MGT-K8) led to a c-Flip reexpression. (b) The kinetics of ERK1/2 activation (p-ERK1/2) upon Jo2 treatment shows a reduction in K8-null MGT (M) versus K8 reexpressing MGT-K8 (MK) cells. No change was observed in ERK1/2 content upon Jo2 treatment. (c) Time course of ERK1/2 activation (p-ERK1/2) after treatment with EGF (20 ng ml−1) showing a comparable activation of ERK1/2 in MGT (M) versus MGT-K8 (MK) cells. (d) Proteasome inhibition by 20 μg of MG132 does not rescue the loss of c-Flip in K8-null MGT epithelial cells (M). (e) The transfection of a HA-tagged c-Flip cDNA in MGT (M) versus MGT-K8 (MK) cells shows an increased c-Flip-HA protein content in K8-reexpressing MGT-K8 versus K8-null MGT epithelial cells.
We thus compared the kinetics of ERK1/2 activation in response to Jo2-Fas stimulation. As shown in Fig. 6b, ERK1/2 activity was increased in a time-dependent manner upon Fas stimulation with the maximum occurring within 15 min and, notably, this rapid upregulation was higher in MGT-K8 (MK) than in MGT (M) cells. Moreover, as for hepatocytes (Fig. 1d), the deficit of ERK1/2 activation after Fas stimulation in MGT cells was not due to a general defect, since no differential ERK1/2 activation was observed between MGT cells and MGT-K8 cells upon EGF stimulation (Fig. 6c). Furthermore, the addition of MG132 did not lead to the reappearance of c-Flip in MGT cells (Fig. 6d). Still, the transfection of a c-Flip-HA cDNA into MGT and MGT-K8 cells revealed that c-Flip was maintained at a much lower level in MGT cells (Fig. 6e). All together, the data indicate that not only the c-Flip deficit observed in K8-null tissues is maintained in K8-null cells in culture, but the K8/K18 modulation of c-Flip/ERK1/2 signaling is not dependent on the simple epithelial cell types.
DISCUSSION
Here we provide the first direct evidence for a mechanistic association between K8/K18 expression, c-Flip content, and ERK1/2 activation upon DR stimulation in simple epithelial cells. The key observation is that a loss of keratins in hepatocytes and mammary gland cells results in a drastic c-Flip deficit which is associated with a decreased activation of ERK1/2 after Fas stimulation, thus showing that a maximal activation of the antiapoptotic ERK1/2 signaling depends on K8/18- and c-Flip-mediated events. We have reported recently that K8-null hepatocytes are three- to fourfold more sensitive than WT hepatocytes to Fas-mediated apoptosis, which is associated with a more prominent targeting of Fas to the surface of K8-null hepatocytes and an increased caspase activation (15), an apoptotic signaling event that may in part be due to the c-Flip deficit reported here. Nevertheless, a PD 98059 pretreatment of Fas-stimulated WT hepatocytes leads to a twofold death enhancement. We then conclude that the K8/K18-dependent resistance to Fas-mediated apoptosis of hepatocytes occurs through a regulation of Fas density at the surface and a concerted c-Flip-dependent modulation of caspase activation and ERK1/2 activation.
Fas activation of ERK1/2 in WT versus K8-null hepatocytes and mammary gland K8-null MGT versus MGT-K8 cells occurs within 15 min, which is in line with the early ERK1/2 activation reported recently using Fas-stimulated HeLa cells (52). Since an inhibition of ERK1/2 signaling increases WT hepatocyte apoptosis, it appears that this Fas-mediated activation of the ERK1/2 signaling pathway constitutes a significant antiapoptotic event such as, for instance, when cells have to rapidly switch off the apoptotic signaling machinery. In addition, there is good evidence that another mitogen-activated protein kinase signaling pathway, i.e., JNK, is activated in Fas-stimulated HT-29 cells at 9 to 12 h poststimulation (16). Although we observed a slight activation of JNK in Fas-stimulated WT and K8-null hepatocytes at 8 h poststimulation, it occurs without any differential activation (unpublished results). Since at 8 h after Fas stimulation the apoptosis is well under way in hepatocytes, with caspases being already activated 2 h after stimulation (15), we think that the JNK activation might be related to alternative pathways activated in cells undergoing apoptosis (11, 48).
The biphasic ERK1/2 activation in response to TNF-α or TRAIL stimulation suggests a two-step process: a first step that is K8/K18 and c-Flip dependent and a second step that is independent of K8/K18 and c-Flip. The occurrence of the first peak in response to the three DR ligands, which depends on the presence or absence of K8/18, suggests the existence of common K8-dependent signaling cascade that includes a c-Flip modulation of the ERK1/2 pathway. In contrast, the second activation peak of ERK1/2 in TNF-α- or TRAIL-stimulated hepatocytes is a K8/K18-independent event, suggesting that a second activation event occurs at a different level. One candidate for this activation in TNF-α-induced signaling is MAPK-activating death domain protein, a Rab3-GEP-like protein which binds to TNF-R1 and TRADD and the overexpression of which can activate ERK1/2 (6). Moreover, the present data showing JNK activation in response to TNF-α or TRAIL and p38 activation in response to TNF-α or Fas, all in a K8/K18-independent manner, demonstrate that K8/K18 constitute an intermediate element in the pathway that links DRs to ERK1/2 but not to p38 nor JNK activation.
The intriguing aspect of c-Flip being part of a K8-containing complex is that it takes place in the absence of Fas stimulation, suggesting a close interaction. Similarly, there is good evidence indicating that K8 is capable of binding to the cytoplasmic domain of TNF-R2 (9) and K18 to the C-terminal portion of TRADD (20). The data reported here show that c-Flip is part of a K8-containing complex, although our pulldown findings with GST-K8 and c-Flip proteins do suggest that c-Flip does not bind directly to K8 itself (unpublished data). Moreover, Raf-1 is also part of the complex and, upon Fas stimulation, the c-Flip and Raf-1 contents increase in the complex. Taken together, these findings suggest that a signaling complex containing K8/K18, c-Flip and Raf-1 play a central antiapoptotic function in Fas-stimulated simple epithelial cells.
The present results show that the c-Flip content is drastically reduced in all of the K8-null mouse tissues that normally express this keratin in WT mice, even though these tissues contain other simple epithelium keratins such as K7 and K18 and also vimentin, and this association is confirmed in the MGT cell line. Since c-Flip was recovered in the MGT-K8 cell line and since the connection between K8 and c-Flip expressions holds in all of the K8-containing cells, how can the nonrecovery of c-Flip in K8-null hepatocytes in primary culture after the 5-day complete K8 cDNA expression be explained? We thought that for primary hepatocytes the 5-day K8 expression period was not long enough to reestablish efficient c-Flip synthesis and, in line with this interpretation, we found that a 5-day K8-cDNA expression in MGT cells led to <40% of the recovery level of c-Flip obtained in the MGT-K8 cell line (unpublished results). In addition, given the present results indicating that c-Flip translation is perturbed in K8-null cells, it could be that the recovery of c-Flip upon K8 reexpression requires the reestablishment of a K8/K18 IF configuration that allows proper c-Flip mRNA processing for optimal translation. In fact, we believe that K8/K18 IFs might associate with c-Flip mRNA, a likely perspective that is based on substantial evidence indicating that a large fraction of mRNAs and components of the translation machinery are associated with the cytoskeleton, mainly actin microfilaments, with subsets of mRNAs being apparently associated with IFs (23, 34). In any case, the present data show that a K8/K18 loss is associated with a profound c-Flip reduction in mouse tissues, primary hepatocytes, and a mammary gland cell line and that this reduction provides a molecular mechanism by which K8/K18 modulate the ERK1/2 antiapoptotic signaling.
c-Flip is a labile protein and the addition of cycloheximide leads to its rapid disappearance and to cell sensitization to DR-mediated apoptosis (13, 29). In some cell lines, PPARα or p53 stimulation results in a proteasome-dependent degradation of c-Flip, whereas in others its degradation can be proteasome independent (12, 26). In addition, the present results show that the regulation of c-Flip content can also occur at the level of translation. On these grounds, c-Flip can be viewed as a sensor of the cell integrity, such that upon cell stress or damage, its cytoplasmic level can be downregulated in various ways to enable apoptosis to take place rapidly and efficiently. For instance, apoptosis is essential for an efficient clearance of irreversibly damaged hepatocytes, and this dramatic event creates a hyperphosphorylation and a reorganization of K8/K18 IFs into aggregates (50). According to this scenario, such an IF network perturbation can lead to a reduced c-Flip level concomitantly with an increased Fas density at the cell surface, resulting in an accelerated Fas-mediated apoptosis.
Acknowledgments
We thank H. Baribault for the gift of the MGT cell line; R. Kemler for the TROMA-1, -2, and -3 hybridomas; J. L. Lessard for the anti-actin monoclonal antibody; and J. Tschopp, U. R. Rapp, and J. Charron for the plasmids encoding HA-tagged c-Flip, Raf-1, and MEK1 (dominant negative), respectively. We are grateful of S. Champetier for producing the K8-containing retrovirus and D. Savoie for helping with the MGT cell line. We also thank J. Huot and A. Anderson for helpful discussions and critical reading of the manuscript.
This study was supported by a grant from Canadian Institutes of Health Research and a grant from The Cancer Research Society.
REFERENCES
- 1.Aoudjit, F., and K. Vuori. 2001. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-Flip and implications for anoikis. J. Cell Biol. 152:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., and V. M. Dixit. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11:255-260. [DOI] [PubMed] [Google Scholar]
- 3.Baribault, H., J. Penner, R. V. Iozzo, and M. Wilson-Heiner. 1994. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 8:2964-2973. [DOI] [PubMed] [Google Scholar]
- 4.Baribault, H., M. Wilson-Heiner, W. Muller, J. Penner, and N. Bakhiet. 1997. Functional analysis of mouse keratin 8 in polyoma middle T-induced mammary gland tumours. Transgenic Res. 6:359-367. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, J. R., and J. S. Pober. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482-6491. [DOI] [PubMed] [Google Scholar]
- 6.Brown, T. L., and P. H. Howe. 1998. MADD is highly homologous to a Rab3 guanine-nucleotide exchange protein (Rab3-GEP). Curr. Biol. 8:R191. [PubMed]
- 7.Bruder, J. T., G. Heidecker, and U. R. Rapp. 1992. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 6:545-556. [DOI] [PubMed] [Google Scholar]
- 8.Brulet, P., C. Babinet, R. Kemler, and F. Jacob. 1980. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc. Natl. Acad. Sci. USA 77:4113-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caulin, C., C. F. Ware, T. M. Magin, and R. G. Oshima. 2000. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J. Cell Biol. 149:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulombe, P. A., and M. B. Omary. 2002. “Hard” and “soft” principles defining the structure, function and regulation of keratin intermediate filaments. Curr. Opin. Cell Biol. 14:110-122. [DOI] [PubMed] [Google Scholar]
- 11.Deak, J. C., J. V. Cross, M. Lewis, Y. Qian, L. A. Parrott, C. W. Distelhorst, and D. J. Templeton. 1998. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc. Natl. Acad. Sci. USA 95:5595-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukazawa, T., T. Fujiwara, F. Uno, F. Teraishi, Y. Kadowaki, T. Itoshima, Y. Takata, S. Kagawa, J. A. Roth, J. Tschopp, and N. Tanaka. 2001. Accelerated degradation of cellular FLIP protein through the ubiquitin-proteasome pathway in p53-mediated apoptosis of human cancer cells. Oncogene 20:5225-5231. [DOI] [PubMed] [Google Scholar]
- 13.Fulda, S., E. Meyer, and K. M. Debatin. 2000. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 60:3947-3956. [PubMed] [Google Scholar]
- 14.Galle, P. R., W. J. Hofmann, H. Walczak, H. Schaller, G. Otto, W. Stremmel, P. H. Krammer, and L. Runkell. 1995. Involvement of the CD95 (APO-1/fas) receptor and ligand in liver damage. J. Exp. Med. 182:1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert, S., A. Loranger, N. Daigle, and N. Marceau. 2001. Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J. Cell Biol. 154:763-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, T., A. Stepulak, T. H. Holmstrom, M. B. Omary, and J. E. Eriksson. 2002. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J. Biol. Chem. 277:10767-10774. [DOI] [PubMed] [Google Scholar]
- 17.Hingorani, S. R., and D. A. Tuveson. 2003. Ras redux: rethinking how and where Ras acts. Curr. Opin. Gen. Dev. 13:6-13. [DOI] [PubMed] [Google Scholar]
- 18.Ho-Kim, M. A., A. Bédard, M. Vincent, and P. A. Rogers. 1991. Dystrophin: a sensitive and reliable immunochemical assay in tissue and cell culture homogenates. Biochem. Biophys. Res. Commun. 181:1164-1172. [DOI] [PubMed] [Google Scholar]
- 19.Holmstrom, T. H., I. Schmitz, T. S. Soderstrom, M. Poukkula, V. L. Johnson, S. C. Chow, P. H. Krammer, and J. E. Eriksson. 2000. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 19:5418-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inada, H., I. Izawa, M. Nishizawa, E. Fujita, T. Kiyono, T. Takahashi, T. Momoi, and M. Inagaki. 2001. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J. Cell Biol. 155:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, B. W., C. Grund, E. Schmid, K. Burki, W. W. Franke, and K. Illmensee. 1980. Formation of cytoskeletal elements during mouse embryogenesis: intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation 17:161-179. [DOI] [PubMed] [Google Scholar]
- 23.Jansen, R. P. 1999. RNA-cytoskeletal associations. FASEB J. 13:455-466. [PubMed] [Google Scholar]
- 24.Kataoka, T., R. C. Budd, N. Holler, M. Thome, F. Martinon, M. Irmler, K. Burns, M. Hahne, N. Kennedy, M. Kovacsovics, and J. Tschopp. 2000. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr. Biol. 10:640-648. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann, S. H., and M. O. Hengartner. 2001. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 11:526-534. [DOI] [PubMed] [Google Scholar]
- 26.Kim, Y., N. Suh, M. Sporn, and J. C. Reed. 2002. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J. Biol. Chem. 277:22320-22329. [DOI] [PubMed] [Google Scholar]
- 27.Kischkel, F. C., S. Hellbardt, I. Behrmann, M. Germer, M. Pawlita, P. H. Krammer, and M. E. Peter. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo, T., T. Suda, H. Fukuyama, M. Adachi, and S. Nagata. 1997. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3:409-413. [DOI] [PubMed] [Google Scholar]
- 29.Kreuz, S., D. Siegmund, P. Scheurich, and H. Wajant. 2001. NF-κB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol. Cell. Biol. 21:3964-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku, N. O., R. Gish, T. L. Wright, and M. B. Omary. 2001. Keratin 8 mutations in patients with cryptogenic liver disease. N. Engl. J. Med. 344:1580-1587. [DOI] [PubMed] [Google Scholar]
- 31.Ku, N. O., T. L. Wright, N. A. Terrault, R. Gish, and M. B. Omary. 1997. Mutation of human keratin 18 in association with cryptogenic cirrhosis. J. Clin. Investig. 99:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon, Y. H., A. Jovanovic, M. S. Serfas, H. Kiyokawa, and A. L. Tyner. 2002. P21 functions to maintain quiescence of p27-deficient hepatocytes. J. Biol. Chem. 277:41417-41422. [DOI] [PubMed] [Google Scholar]
- 33.Lane, E. B., B. L. Hogan, M. Kurkinen, and J. I. Garrels. 1983. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature 303:701-704. [DOI] [PubMed] [Google Scholar]
- 34.Liu, G., W. M. Grant, D. Persky, V. M. Latham, Jr., R. H. Singer, and J. Condeelis. 2002. Interactions of elongation factor 1alpha with F-actin and beta-actin mRNA: implications for anchoring mRNA in cell protrusions. Mol. Biol. Cell 13:579-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loranger, A., S. Duclos, A. Grenier, J. Price, M. Wilson-Heiner, H. Baribault, and N. Marceau. 1997. Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am. J. Pathol. 151:1673-1683. [PMC free article] [PubMed] [Google Scholar]
- 36.Mak, T. W., and W. C. Yeh. 2002. Signaling for survival and apoptosis in the immune system. Arthritis Res. 4:S243-S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marceau, N. 1994. Epithelial cell lineages in developing, restoring, and transforming liver: evidence for the existence of a “differentiation window.” Gut 35:294-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marceau, N., A. Loranger, S. Gilbert, N. Daigle, and S. Champetier. 2001. Keratin-mediated resistance to stress and apoptosis in simple epithelial cells in relation to health and disease. Biochem. Cell Biol. 79:543-555. [PubMed] [Google Scholar]
- 39.Micheau, O., and J. Tschopp. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181-190. [DOI] [PubMed] [Google Scholar]
- 40.Moll, R., W. W. Franke, and D. L. Schiller. 1982. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11-24. [DOI] [PubMed] [Google Scholar]
- 41.Nagata, S. 1999. Fas ligand-induced apoptosis. Annu. Rev. Genet. 33:29-55. [DOI] [PubMed] [Google Scholar]
- 42.Natoli, G., A. Costanzo, A. Ianni, D. J. Templeton, J. R. Woodgett, C. Balsano, and M. Levrero. 1997. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science 275:200-203. [DOI] [PubMed] [Google Scholar]
- 43.Oshima, R. G. 2002. Apoptosis and keratin intermediate filaments. Cell Death Differ. 9:486-492. [DOI] [PubMed] [Google Scholar]
- 44.Park, S. J., Y. Y. Kim, J. W. Ju, B. G. Han, S. I. Park, and B. J. Park. 2001. Alternative splicing variants of c-FLIP transduce the differential signal through the Raf or TRAF2 in TNF-induced cell proliferation. Biochem. Biophys. Res. Commun. 289:1205-1210. [DOI] [PubMed] [Google Scholar]
- 45.Park, S. J., Y. Y. Kim, J. Y. Lim, G. J. Seo, J. Kim, S. I. Park, and B. J. Park. 2001. Opposite role of Ras in tumor necrosis factor-alpha-induced cell cycle regulation: competition for Raf kinase. Biochem. Biophys. Res. Commun. 287:1140-1147. [DOI] [PubMed] [Google Scholar]
- 46.Roberts, R. A., N. H. James, and S. C. Cosulich. 2000. The role of protein kinase B and mitogen-activated protein kinase in epidermal growth factor and tumor necrosis factor alpha-mediated rat hepatocyte survival and apoptosis. Hepatology 31:420-427. [DOI] [PubMed] [Google Scholar]
- 47.Royal, I., L. Raptis, B. J. Druker, and N. Marceau. 1996. Downregulation of cytokeratin 14 gene expression by the polyoma virus middle T antigen is dependent on c-Src association but independent of full transformation in rat liver nonparenchymal epithelial cells. Cell Growth Differ. 7:737-743. [PubMed] [Google Scholar]
- 48.Rudel, T., F. T. Zenke, T. H. Chuang, and G. M. Bokoch. 1998. p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J. Immunol. 160:7-11. [PubMed] [Google Scholar]
- 49.Rust, C., and G. J. Gores. 2000. Hepatocyte transplantation in acute liver failure: a new therapeutic option for the next millennium? Liver Transpl. 6:41-43. [DOI] [PubMed] [Google Scholar]
- 50.Stumptner, C., M. B. Omary, P. Fickert, H. Denk, and K. Zatloukal. 2000. Hepatocyte cytokeratins are hyperphosphorylated at multiple sites in human alcoholic hepatitis and in a mallory body mouse model. Am. J. Pathol. 156:77-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toyoshima, F., T. Moriguchi, and E. Nishida. 1997. Fas induces cytoplasmic apoptotic responses and activation of the MKK7-JNK/SAPK and MKK6-p38 pathways independent of CPP32-like proteases. J. Cell Biol. 139:1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran, S. E., T. H. Holmstrom, M. Ahonen, V. M. Kahari, and J. E. Eriksson. 2001. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J. Biol. Chem. 276:16484-16490. [DOI] [PubMed] [Google Scholar]
- 53.Tschopp, J., M. Irmler, and M. Thome. 1998. Inhibition of Fas death signals by FLIPs. Curr. Opin. Immunol. 10:552-558. [DOI] [PubMed] [Google Scholar]
- 54.Zatloukal, K., C. Stumptner, M. Lehner, H. Denk, H. Baribault, L. G. Eshkind, and W. W. Franke. 2000. Cytokeratin 8 protects from hepatotoxicity, and its ratio to cytokeratin 18 determines the ability of hepatocytes to form Mallory bodies. Am. J. Pathol. 156:1263-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]