Abstract
Background:
Pulmonary tuberculosis is not listed as a cause of pulmonary hypertension (PH). Scanty information is available in the literature regarding this issue.
Methods:
A group of patients with a history of pulmonary tuberculosis were diagnosed to have PH on the basis of a novel clinico-radio-echocardiographic criteria. Subdivided into two groups on the basis of the history of smoking, we looked for their demographic, spirometric, radiological characteristics along with the quality of life assessment.
Results:
A total of 40 patients (21 smokers and 19 nonsmokers) were found to have PH with history of pulmonary tuberculosis. The two groups were similar radiologically including the extent of fibrosis. The nonsmoker group had lower age range (52.16 ± 14.81 vs. 63.1 ± 10.05, P = 0.01), worse chronic obstructive pulmonary disease (COPD) assessment test score (16.11 ± 6.24 vs. 13.9 ± 5.6, P = 0.25) and higher pulmonary artery (PA) pressure (46.39 ± 7.44 vs. 44.55 ± 8.04, P = 0.46) compared to the smokers. Overall and for the smoker group, in particular, the spirometric pictures were favoring obstruction without reversibility as in COPD (forced expiratory volume in 1 second [FEV1] % as 64.26 ± 18.38 and 58.85 ± 14.61 with % of predicted FEV1 being 43.74 ± 17.26 and 42.38 ± 16.64 respectively). However, those with no history of smoking had restrictive changes as in diffuse parenchymal lung disease (DPLD) in their spirometry (FEV1/forced vital capacity [FVC] of 79.33 ± 19.93 and FVC as 49.67 ± 11.54% of predicted). The smoker group had far more obvious involvement of the small airways in terms of change in FEF25-75 compared to nonsmokers (FEF25-75 = 22.85 ± 19.68 vs. 63.83 ± 48.61).
Conclusion:
PH appears associated with the history of pulmonary tuberculosis. With or without a history of smoking, they pose two distinct phenotypes in spirometry as COPD and DPLD. The DPLD phenotype had worse quality of life.
KEY WORDS: Chronic obstructive pulmonary disease, chronic obstructive pulmonary disease assessment test score, diffuse parenchymal lung disease, interstitial lung disease, pulmonary hypertension, quality of life, spirometry, tuberculosis
INTRODUCTION
Tuberculosis, a WHO declared global epidemic in 1993 can affect virtually all organ system and cause a myriad of presentations. There is scanty report available regarding pulmonary hypertension (PH) been seen in the patients with tuberculosis or history of tuberculosis.[1,2] Here, we are presenting a cohort of patients in whom the history of pulmonary tuberculosis was forthcoming with no other apparent cause been present to explain the presence of PH. We are also forwarding their several characteristics as demographic, radiological, spirometric, and health status.
METHODS
The study was conducted at the Institute of Pulmocare and Research, Kolkata, a tertiary pulmonary out-patient services cum research institute following proper ethical clearance (Institutional Ethics Committee, Drugs Controller General of India registration No. ECR/159/Inst/WB/2013; approved on 21.04.2013). The diagnosis of PH was accomplished on the basis of a composite clinic-radio-echocardiographic criteria been practiced at the institute without doing right heart catheterization (RHC).[3] Such a diagnostic algorithm is based on the fact that whenever very highly sensitive and specific radiological signs are present favoring PH,[4,5] the co-presence of the appropriate clinical setting and echocardiographically measured raised PA pressure (systolic >40 mm of Hg.) confirms a diagnosis of PH.
Thus, the criteria been followed for the diagnosis of PH has been the presence of at least one point being positive from each of all the categories as
Clinical symptoms of pulmonary hypertension
Unexplained shortness of breath (SOB) and disproportionate SOB
Demonstration of desaturation in the consultation chamber with mild exercise
Other symptoms as unexplained fatigue, syncope, etc. associated with exercise-induced desaturation.
Chest X-ray
Dilated right, left or both main pulmonary arteries (PAs) in PA View
Fullness of the retro-sternal space from right ventricular enlargement
Gross PA dilatation/fullness of pulmonary bay.
High-resolution computed tomography chest
PA root diameter ≥aortic root diameter
PA branch diameter ≥accompany bronchial diameter in 3 or 4 lobes.
Doppler echocardiography
The measured PA systolic pressure ≥40 mm of Hg.
The determination of the etiology of PH has been done through the evaluations based on the algorithm been used at the Institute. It included chest X-ray, high-resolution computed tomography chest, spirometry, and echocardiography as mandatory for all with pulmonary perfusion scan, pulmonary CT angiography, polysomnography, liver function tests, collagen profile, HIV serology, and others on a selective basis as a part of the real world practice protocol.
In the clinical evaluation, the history of tuberculosis was routinely recorded for all patients based on our previous experience;[6] the patients having such history were grouped separately and taken up for further analysis. We included simple anthropometric measurement as body mass index (BMI), pulse oximetry at rest, spirometry, chronic obstructive pulmonary disease (COPD) assessment test (CAT) as a measurement of health status, and abnormalities in chest X-rays to understand the peculiarities of these patients. Further, in relation to smoking history (past or present) we divided them into two groups and compared their lung function and other characteristics been included.
The existing radiological abnormalities of all the patients with PH and past history of pulmonary tuberculosis were scored observing an indigenous protocol of evaluating chest X-rays in which all the available abnormalities/types of lesions (as: Fibrosis, cavity, haze, nodule, etc.) on chest X-rays (PA view) of each patients were recorded by an independent radiologist on a scale of 0–5 as per the conventionally accepted zones on both sides of the film (upper, middle, and lower). Finally, the patients with PH with history of pulmonary tuberculosis with or without smoking history were statistically analyzed with unpaired Student's t-test and the correlation of the estimated PA pressure were done with the total radiological score and independently with fibrosis score using Pearson correlation coefficient.
RESULTS
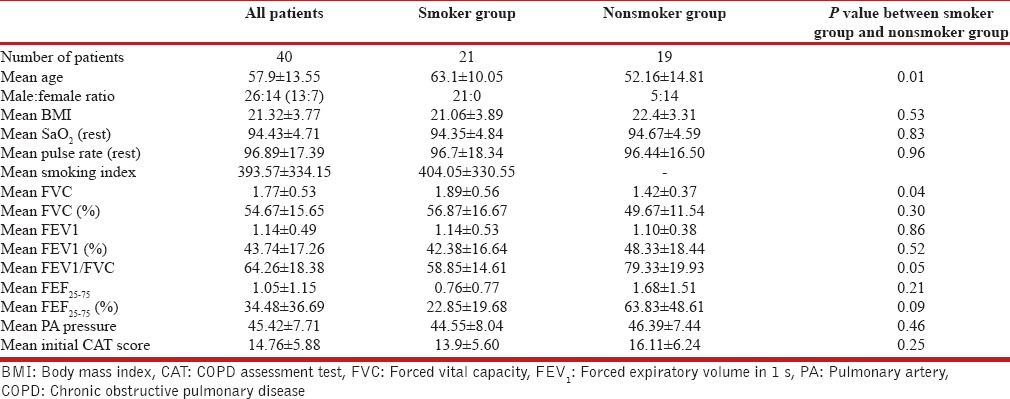
We had collected 40 patients having PH (26 males and 14 females; mean age 57.9 ± 13.55) with a history of treatment of tuberculosis. This forms 15% of the total number of PH patients been diagnosed through applying the mentioned criteria in a period from 2nd April, 2013 to 30th September 2013 (unpublished data). These patients showed mild PH (mean systolic PA pressure 45.42 ± 7.71 mm of Hg), overall spirometric characteristics as COPD (postbronchodilator forced expiratory volume in 1 second [FEV1]% as 64.26 ± 18.38 with % of FEV1 predicted being 43.74 ± 17.26) with a mean CAT score of 14.76 ± 5.88. When subdivided into two groups based on the presence of a history of smoking (past and/or continuing), we found that the two groups differ distinctly based on the available variables [Table 1]. The smokers (mean smoking index 404) show feature of poorly reversible airflow obstruction as COPD (postbronchodilator FEV1/forced vital capacity [FVC] =58.85 ± 14.61 with % FEV1 = 42.38 ± 16.64) with male preponderance (male: female as 21:0) and features of small airway obstruction (FEF25-75 = 22.85 ± 19.68). The nonsmokers, however, demonstrate female preponderance (male: female as 5:14) feature as diffuse parenchymal lung disease (DPLD) (postbronchodilator FEV1/FVC =79.33 ± 19.93 with % FVC =49.67 ± 11.54) without feature of small airway obstruction (FEF25-75 = 63.83 ± 48.61). The two groups were therefore named them as COPD and DPLD phenotypes of tuberculosis related PH; the former shows slightly lower BMI, higher mean age (63.1 ± 10.05 vs. 52.16 ± 14.81), lower SaO2 at rest (94.35 ± 4.84 vs. 94.67 ± 4.59 percent) with lower PA pressure (44.55 ± 8.04 vs. 46.39 ± 7.44 mm of Hg) when compared to the DPLD phenotype. Despite being younger and having a relatively better BMI (22.4 ± 3.31 compared to 21.06 ± 3.89 in the COPD phenotype), the later (the DPLD phenotype) has worse lung function parameters, higher estimated PA pressure (systolic 46.39 ± 7.44) and the CAT Score (16.11 ± 6.24 vs. 13.9 ± 5.60) [Table 1].
Table 1.
The patients of PH with history of pulmonary tuberculosis and their status as a whole and with or without history of smoking as regards different variables been in concern, the difference between the latter two groups appear significant (marked in bold) as regards age, mean forced vital capacity, and mean forced expiratory volume in 1 s/forced vital capacity
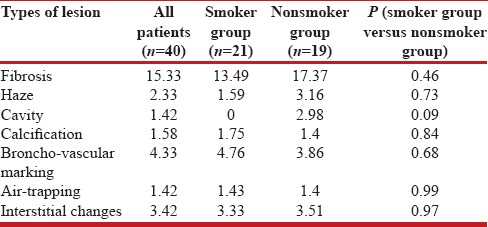
Several radiological abnormalities were also detected as fibrosis, haze, cavity, calcification, increases broncho-vascular markings, bullae, air-trapping, interstitial changes, mixed lesions, nodule, organization, pleural thickening and prominent hila. The major few are charted in Table 2 where the percentage of each abnormality has been noted. This percentage of abnormality has been derived applying the formula as the abnormality score × 100/maximum possible score for a particular abnormality.
Table 2.
The radiological abnormalities of the patients of PH with history of tuberculosis as a whole and with or without history of smoking in terms of percentage of abnormality as regards fibrosis, haze, cavity, calcification, bronchovascular markings, air-trapping and interstitial changes
When we tried to correlate the PA pressure with different radiological abnormalities, all the patients taken together as a whole show a poor positive co-relationship with the total radiological abnormality score [Figure 1a], and a poor negative co-relationship when the total fibrosis score [Figure 1b]. This association is maintained for total radiological abnormality score [Figure 1c and e] and the total fibrosis score [Figure 1d and f] when looked separately at the COPD and DPLD phenotypes.
Figure 1.
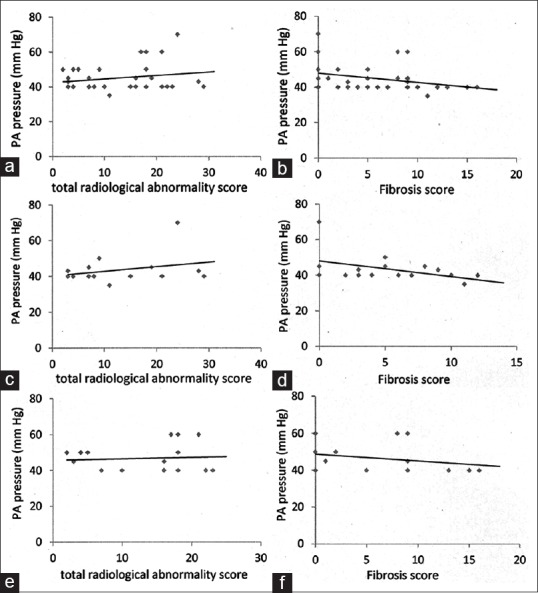
The correlation of tuberculosis associated pulmonary artery pressure to the total score and the fibrosis score (in chest X-ray) has been displayed. The upper panel (a and b) shows the overall co-relationship while the intermediate panel (c and d) and the lower panel (e and f) show the co-relationship of the “chronic obstructive pulmonary disease phenotype” and the “diffuse parenchymal lung disease phenotype” with the total abnormality score and the fibrosis score
DISCUSSION
PH, contributed by varied reasons, appears to be frequently encountered in our OPD practice and it was incidental to find approximately 15% of them having a preceding history of pulmonary tuberculosis without any other forthcoming cause for PH. This entity of “tuberculosis-related PH,” as we name it, is not well-recognized and not been given any space in all the classifications of PH.[7] Kapoor had described 66 patients of cor pulmonale with pulmonary tuberculosis mainly based on clinical and ECG findings.[2] Considering the data of early chemotherapeutic period, it appears that cor pulmonale happened to remain a common sequel of tuberculosis.[8,9,10] After the advent of effective anti-tubercular chemotherapy, the literature in this regard has been sparse. Recently, Patel et al. have described PH in six out of 50 (12%) cases of PH to develop from tuberculosis.[11] The diagnosis was based mainly on echocardiographic findings.[11] Again, in another recent cross-sectional study, 14 consecutive patients cured of tuberculosis were diagnosed to have PH (based on echocardiographic measurement of PA systolic pressure as ≥40 mm of Hg.[12] These patients are akin to those in our series where history of treatment of tuberculosis has been entertained in the clinical exercise for the diagnosis of PH. We have, however, established the presence of PH with a better objectivity using highly specific radiological and imaging parameters[4,5,13,14] in addition to Doppler echocardiography. This eliminates the scope of false positive interpretation in echocardiography.[15]
There are certain interesting features in the observation as we tried to analyze the data depending on the available variables and more so on making subclasses on the presence of a history of smoking. Overall, these patients behave as COPD as far as the spirometric interpretation of their lung function is concerned and they have a poor quality of life (the mean CAT score being 14.76 ± 5.88). Further, the smokers who belong to a relatively higher age group with lower PA pressure, behave as patients of COPD on spirometry while the nonsmokers patients with PH having a history of tuberculosis demonstrate restriction in spirometry like that of the DPLD patients. When the comparison is drawn between these two groups as far as the small airway affection is concerned (FEF25-75), the small airway obstruction appear obvious in smokers (the mean FEF25-75 being 0.76 ± 0.77; 22.85 ± 19.68 of predicted) but not so in nonsmoker population (the mean FEF25-75 being 1.68 ± 1.51; 63.83 ± 48.61of predicted). The two groups do not have much difference in their cumulative score on fibrosis and interstitial changes [Table 2].
Radiologically, the patients of PH with a history of tuberculosis have shown an overall direct but weak co-relationship to the total radiological abnormalities, and a poor but negative co-relationship with the degree of fibrosis [Figure 1]. This suggests that the likely mechanism of development of PH in these patients with a history of tuberculosis is unlikely correlated to the fibrotic pathology/process of healing in tuberculosis which has been conjectured previously.[13]
It appears difficult to explain why the health status, PA pressure, and the mean FVC (1.89 ± 0.56 L vs. 1.42 ± 0.37 L; P = 0.04) is relatively better in the so called COPD phenotype compared to the so called DPLD phenotype group when it is expected that two suspected etiologies (COPD and tuberculosis) together could likely make the PH worse. It would have been better if we could bring the COPD population with PH without history of pulmonary tuberculosis for comparison.
Since these patients, to our experience, show a COPD-like a lung function, we included CAT (COPD assessment test) to evaluate the quality of life. CAT has been a validated test for COPD quality of life assessment.[16,17] Incidentally, with the line of thought as COPD, we divided the patients into two groups to see the effect of smoking, the predominant cause of COPD. To our surprise, we found that the nonsmoker group (the DPLD phenotype) does not show features of airflow obstruction. Their status measured through CAT score appears relatively worse and tallies with the significantly lower FVC as mentioned above. Although CAT was developed to assess the health status in COPD, it has been used in several other lung disease including DPLD.[18] Interestingly, tuberculosis has been proposed to be etiologically related to the development of COPD.[19,20] It is interesting to postulate that possibly some subset of pulmonary tuberculosis patients develops PH and some develop both PH and COPD and possibly again, those developing PH and COPD together pose a lower degree of stimuli to develop PH; only future research can prove the validity of such proposition.
The results further lead to a Conjecture that possibly the observed lung function change is not because of smoking-induced COPD per se, but it is likely secondary to a COPD-like state been developed as a result of tuberculosis. Indeed, thus, the question of association versus co-incidence to explain the presence of PH in patients with history of tuberculosis remains an enigma at the end of our observation. For the sake of argument, if we exclude the patients with a history of smoking, still we are left with 50% of these patients with PH where the etiological explanation of PH remains difficult with the available investigations and the existing knowledge. Our experience supports the previous observation that tuberculosis can possibly cause PH. Hence, to our opinion, history of pulmonary tuberculosis should be sought in all the cases of PH been detected in our country. It may be interesting to ponder the reason for the relatively lower PA pressure and better health status in the so-called “COPD phenotype” (where there could be likely COPD as well with history of tuberculosis) compared to the “DPLD phenotype” (where the history of tuberculosis is present alone).
The study has several weaknesses. Despite the argument forwarded, the paucity of the hemodynamic data through (RHC) happened to be a limitation of the study since we have no information apart from the fact of raised PA pressure in our population. We have used CAT score for the assessment of the health status in these patients, but CAT has not been used or validated in patients with PH; functional assessment as 6 min’ walk test would have a better adjunct. A higher number of patients could have elaborated the impact of the radiological abnormalities in a better way.
CONCLUSION
From the observation, it appears that the “tuberculosis associated PH” as described by us is a distinct entity. Tuberculosis may have a causal association with PH and history of pulmonary tuberculosis, therefore, should be sought in all cases of PH, especially in the developing world. History of smoking apparently makes two distinct phenotypes in these patients as (a) COPD phenotype, and (b) interstitial lung disease phenotype; the later looks worse as per the PA pressure and the health status. This association of tuberculosis and PH needs further elaboration and investigation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Padmavati S, Arora R. Sex differences in chronic cor pulmonale in delhi. Br J Dis Chest. 1976;70:251–9. doi: 10.1016/0007-0971(76)90040-1. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor SC. Pulmonary hypertension in Pulmonary Tuberculosis. Indian J Tuberc. 1950;6:50–64. [Google Scholar]
- 3.Ganguly D. Editorial: Diagnosis and treating PH: Dilemma on evidence and ethics. Pulmo Face. 2014;XIV:1. [Google Scholar]
- 4.Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest. 1998;113:1250–6. doi: 10.1378/chest.113.5.1250. [DOI] [PubMed] [Google Scholar]
- 5.Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: The ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14:270–8. doi: 10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Saha D, Bhattacherjee PD, Das SK, Dey R, Ghosh M, Dutta l, et al. Group III pulmonary hypertension: Relative frequency of different etiologies in a referral pulmonary OPD. The Pulmo- Face. 2013;XIII:3–8. [Google Scholar]
- 7.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the european respiratory society (ERS), endorsed by the international society of heart and lung transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 8.Walzer I, Frost TT. Cor pulmonale; a consideration of clinical and autopsy findings. Dis Chest. 1954;26:192–8. [PubMed] [Google Scholar]
- 9.Kozlowski H, Maldyk E. Anatomopathological changes in the heart in patients dead from pulmonary tuberculosis. Gruzlica. 1955;23:693–9. [PubMed] [Google Scholar]
- 10.Corbetta V, Pozzi G, Scoccia S. Pulmonary heart disease in tuberculosis patients. G Ital Della Tuberc. 1955;9:283–90. [PubMed] [Google Scholar]
- 11.Patel V, Khaped K, Solanki B, Patel A, Rathod H, Patel J. Profile of pulmonary hypertension patients coming to Civil Hospital, Ahmedabad. Int J Res Med. 2013;2:94–7. [Google Scholar]
- 12.Ahmed AE, Ibrahim AS, Elshafie SM. Pulmonary hypertension in patients with treated pulmonary tuberculosis: Analysis of 14 consecutive cases. Circ Respir Pulm Med. 2011;5:1–5. doi: 10.4137/CCRPM.S6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanemoto N, Furuya H, Etoh T, Sasamoto H, Matsuyama S. Chest roentgenograms in primary pulmonary hypertension. Chest. 1979;76:45–9. doi: 10.1378/chest.76.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Bush A, Gray H, Denison DM. Diagnosis of pulmonary hypertension from radiographic estimates of pulmonary arterial size. Thorax. 1988;43:127–31. doi: 10.1136/thx.43.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerstingl C, Schueler R, Bors L, Momcilovic D, Pabst S, Nickenig G, et al. Diagnostic value of echocardiography in the diagnosis of pulmonary hypertension. PLoS One. 2012;7:e38519. doi: 10.1371/journal.pone.0038519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 17.Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38:29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 18.Someya F, Nakagawa T. Application of the COPD assessment test (CAT) to Patients with interstitial lung disease. Health. 2014;6:2562–9. [Google Scholar]
- 19.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–8. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos LM, Sulmonett N, Ferreira CS, Henriques JF, de Miranda SS. Functional profile of patients with tuberculosis sequelae in a university hospital. J Bras Pneumol. 2006;32:43–7. doi: 10.1590/s1806-37132006000100010. [DOI] [PubMed] [Google Scholar]


