Abstract
The Ras pathway transduces divergent signals determining normal cell fate and is frequently activated in hematopoietic malignancies, but the manner in which activation contributes to human leukemia is poorly understood. We report that a high level of activated H-Ras signaling in transduced primary human hematopoietic progenitors reduced their proliferation and enhanced monocyte/macrophage differentiation. However, the exposure of these cells to a farnesyltransferase inhibitor and establishment of a moderate level of Ras activity showed increased proliferation, an elevated frequency of primitive blast-like cells, and progenitors with enhanced self-renewal capacity. These results suggest that the amplitude of Ras pathway signaling is a determinant of myeloid cell fate and that moderate Ras activation in primitive hematopoietic cells can be an early event in leukemogenesis.
The Ras GTPases are critical components of signal transduction pathways that transmit information from the cell surface in order to control cellular proliferation, differentiation, and survival. These proteins convey specific signals from diverse sources through several effector pathways, but the mechanism by which a specific biological outcome is achieved is poorly understood (52). In hematopoietic cells, Ras proteins transmit signals from key fate-determining cytokine receptors, such as c-kit, granulocyte-macrophage colony-stimulating factor (CSF) receptor, macrophage colony-stimulating factor (M-CSF) receptor, and erythropoietin receptor (40). Direct activation of Ras by point mutation is among the most common genetic aberrations detected in hematological malignancies, occurring in more than 30% of cases of myelodysplastic syndrome (MDS), acute myeloblastic leukemia (AML), and juvenile myelomonocytic leukemia (JMML) (41). Ras is also indirectly activated by mutations in upstream genes such as Nf1 (in JMML) and Flt3 (in AML) or by the bcr-abl translocation (in chronic myeloid leukemia) (41). It is not clear, however, whether Ras activation represents an initiating event or a later step in leukemic transformation. The detection of different N-Ras mutations in distinct subclones derived from AML patients suggests that Ras mutations are late events that arise independently after the establishment of a preleukemia (3). Conversely, the high frequency of Ras mutations in preleukemic conditions such as MDS is indicative of an early event (20). However, experimental evidence for the role of Ras activation in the initiation of leukemia is lacking.
When activated Ras is expressed in primary fibroblasts, the typical response is p16/Rb- and p19ARF/p53-dependent cell cycle arrest followed by senescence (36, 47). In contrast, many immortalized cell lines become transformed upon the addition of constitutive Ras signaling. Thus, it appears that the cellular context—namely, the presence or absence of additional mutations—can determine the consequences of Ras activation. The introduction of Ras genes to leukemic cell lines has been the preferred method of investigating the role Ras plays in regulating hematopoiesis, but collectively, the results have been difficult to reconcile. Primitive murine FDCP-Mix cells transduced with mutant H-Ras exhibited either late-stage monocytic differentiation arrest (24) or normal monocytic differentiation with extended survival of neutrophil progenitors (13). When activated H-Ras was expressed in the human monoblastic cell line U937, monocytic differentiation was observed (31). Erythroleukemic TF1 cells responded to H-Ras expression either with factor-independent growth and proliferative hypersensitivity to some cytokines (31) or with inhibited proliferation and accelerated erythroid differentiation (21). Conflicting observations with different cell lines may be a result of different perturbations of the Ras pathway generated during establishment of the line and their restricted or altered developmental potential relative to primary cells. For these reasons, cell lines have limited utility for modeling the initiation of leukemia.
The impact of activated Ras signaling on normal hematopoiesis has also been studied by transplantation of primary murine hematopoietic cells transduced with an H-Ras-expressing vector. Lymphoid leukemia and lymphoma resulted (23), despite the association of mutant Ras with myeloid leukemia in humans. However, the transduced murine cells generated large monocytic colonies in vitro, and primitive myeloid cell lines were derived. Perturbed myelopoiesis was also observed in similar experiments employing murine cells transduced with N-Ras (30). In these studies, the frequency of myeloid progenitors was substantially reduced and disorders resembling human myeloproliferative disorder, chronic myeloid leukemia, and MDS eventually developed in some recipients. Although several different vectors have been used to express activated Ras genes in transgenic mice, these models have not recapitulated myeloid leukemia (1).
The varied and cell type-dependent consequences of activated Ras expression illustrate the importance of determining the effects of Ras activation in the most appropriate cellular context. To this end, the introduction of oncogenes to primitive primary human blood cells has emerged as a useful tool for the investigation of initiating events in leukemia (37). In one study, the effects of mutant Ras gene expression have been examined in primary human hematopoietic cells. The introduction of G12R N-Ras to umbilical cord blood (CB) cells had no specific effect on myelopoiesis but partially blocked erythroid-cell differentiation, causing a twofold decrease in the number of erythroid colonies (14). Although this does not reproduce a preleukemic phenotype, these results show that constitutively activated Ras can influence human hematopoiesis.
Most cell line, ex vivo, and transgenic models have not included an examination of the level or duration of Ras signaling. In standard vectors, activated Ras is driven by a strong promoter and is therefore likely to be more active than in a naturally transformed cell (49). However, differential duration or intensity of signaling has been proposed as an important mechanism by which Ras can transmit diverse and contradictory signals (52). Therefore, in addition to studying Ras in an appropriate cellular environment, it is important to examine the influence of signaling intensity.
Ras is a docking site for downstream effectors, and its activity is dependent upon membrane localization. In contrast to other Ras family members, H-Ras cannot employ geranylgeranylation as an alternate prenylation pathway for membrane binding and is therefore differentially sensitive to a farnesyltransferase inhibitor (FTI) such as SCH66177 (Schering-Plough, Kenilworth, N.J.) (2, 26, 40, 54, 57). Thus, it should be possible to use an FTI to modulate the signaling activity of an H-Ras transgene in target cells without affecting other Ras family members, although the precise specificity of SCH66177 within human hematopoietic stem or progenitor cells is not known. In human leukemia, mutations in H-Ras are observed much less frequently than in N-Ras (6). Functionally, however, the two proteins appear to be virtually interchangeable (18). This conclusion contrasts with what has been learned about K-Ras, which has a distinct protein trafficking mechanism (10) and, unlike H- or N-Ras, is essential for embryonic development (27). N-Ras may have a somewhat greater affinity for Raf than H-Ras (22), but both proteins employ this effector.
Evidence suggests that most subtypes of myeloid leukemia are maintained by leukemic stem cells (LSCs), with impaired differentiation capabilities that cause the accumulation of immature myeloid cells and the disruption of multiple hematopoietic lineages (5, 28). Indeed, it appears that the LSC arises from within the normal stem or progenitor cell compartment following leukemogenic alterations. By the retroviral introduction of activated H-Ras to these primitive cells and subsequent inhibition of farnesylation, we have demonstrated differential regulation of lineage commitment, differentiation, proliferation, and self-renewal. The combination of activated Ras and farnesyltransferase inhibition led to an increase in proliferation and self-renewal and a reduction in differentiation, suggesting that a moderate increase in Ras activity may be an early step in the development of hematopoietic malignancies.
MATERIALS AND METHODS
Purification of lineage-depleted (Lin−) human umbilical cells.
CB samples were obtained from placental and umbilical tissues scheduled for discard according to procedures approved by the institutional review board of Mount Sinai Hospital and the Hospital for Sick Children (Toronto, Ontario, Canada). Samples were collected in heparin and centrifuged on Ficoll-Paque (Pharmacia, Uppsala, Sweden) to obtain mononuclear cells. Lineage-committed cell depletion was achieved by negative selection with the StemSep system according to the manufacturer's protocol (Stem Cell Technologies, Inc., Vancouver, British Columbia, Canada). Cells expressing glycophorin A (GlyA), CD2, CD3, CD14, CD16, CD19, CD24, CD41, CD56, or CD66b were removed in this procedure. The efficiency of primitive cell enrichment was determined by flow cytometric assessment of CD34 expression (see below).
Retrovirus preparation.
The construction of PG13/murine stem cell virus (MSCV)-Neo and PG13/MSCV-H-Ras-Neo producer cells has been previously described (23). These cells produced gibbon ape leukemia virus-pseudotyped virus at a titer of 4 × 105 to 8 × 105 G418-resistant CFU/ml when assayed on HeLa cells. Retroviral supernatants were collected from subconfluent cultures 12 h after the medium was changed from alpha-minimal essential medium (Gibco BRL, Burlington, Ontario, Canada) supplemented with 4% fetal bovine serum (FBS; Cansera, Rexdale, Ontario, Canada) to Iscove's modified Dulbecco's medium (IMDM; Gibco BRL) plus 4% FBS.
Retroviral gene transfer.
Infections of Lin− CB cells were carried out in flat-bottom 35-mm-diameter dishes (Nunc, Burlington, Ontario, Canada) that were coated with CH-296 fibronectin fragment (Retronectin; Takara Shuzo, Ltd., Otsu, Japan) at 4 μg/cm2. Cells were deposited at a density of 0.2× 106 to 0.6 × 106 in 2 ml per dish. Culture medium consisted of IMDM plus 4% FBS and cytokines at 37°C and 5% CO2. The cytokine mixture included 30-ng/ml thrombopoietin (Kirin Brewery, Tokyo, Japan), 10-ng/ml interleukin-6 (IL-6), 10-ng/ml granulocyte colony-stimulating factor (G-CSF), 100-ng/ml stem cell factor (SCF) (each from Amgen, Thousand Oaks, Calif.), and 100-ng/ml Flt-3 ligand (Immunex, Seattle, Wash.). After 18 to 24 h of prestimulation, the medium was replaced with supernatant from PG13/MSCV-H-Ras-Neo or PG13/MSCV-Neo producer cells plus cytokines. At this point, 5 nM SCH66177 FTI (Schering-Plough) was added to counteract the rapid differentiation induced by H-Ras expression in the earliest transduced cells. The duration of culture in the presence of virus was 48 to 72 h, including replacement by fresh retroviral supernatant, cytokines, and FTI every 12 h.
Liquid culture assays.
Retrovirally transduced Lin− CB cells were transferred to 24-well suspension plates (10 × 103 to 30 × 103 cells) or upright 25-cm2 suspension flasks (0.1 × 106 to 2.0 × 106 cells). For myeloid differentiation assays, cells were cultured in IMDM plus 4% FBS with 1.5-mg/ml G418 (Sigma, St. Louis, Mo.); a 50-ng/ml concentration (each) of G-CSF, M-CSF (Chemicon, Temecula, Calif.), and granulocyte-macrophage CSF (Amgen); 25-ng/ml IL-3 (Amgen); and 75-ng/ml SCF. In erythroid differentiation assays, StemPro-34 serum-free medium (Gibco/BRL) with 1.5-mg/ml G418, 10-U/ml erythropoietin, 1-ng/ml G-CSF, 2-ng/ml IL-3, and 20-ng/ml SCF was employed (37). When experiments included cultures containing an inhibitor(s), complete medium replacement was performed every 2 days in order to maintain a constant level of inhibition. In other assays, the medium was replaced at 4-day intervals. Drug selection was found to be >90% complete after 6 days and >98% complete after 10 days, as judged by comparison of the G418-selected and -unselected colony-forming cell (CFC) content following liquid culture selection (data not shown). When cells were collected for periodic analysis, flasks were briefly washed with Ca2+-Mg2+-free phosphate-buffered saline (Wisent, St. Bruno, Quebec, Canada), incubated for 5 min in cell dissociation buffer (Gibco/BRL), and then shaken to dislodge cells. This procedure often did not release a small fraction of tenaciously adherent cells, however, so these are likely underrepresented in the analyses.
Progenitor assays.
CFC assays were performed as previously described (17) and scored on days 10 to 13. Morphological designation of colony type by inverted light microscopy was confirmed by May-Grünwald-Giemsa (MGG) staining of cytospin preparations.
Flow cytometry.
Flow cytometric analysis was performed using a FACScalibur instrument and CellQuest software (Becton Dickinson, San Jose, Calif.). Isotype controls were mouse immunoglobulin G conjugated to fluorescein isothiocyanate (FITC; Becton Dickinson), phycoerythrin (PE; Becton Dickinson), or cyanin-5-PE (Coulter-Immunotech). Anti-CD34-FITC (Becton Dickenson) was used to evaluate the purity of Lin− CB samples, and phenotype assessment in culture was made using anti-CD15-FITC (Becton Dickinson), anti-CD14-FITC (Coulter-Immunotech) and anti-CD14-PE (Becton Dickinson), anti-CD33-cyanin-5-PE (Coulter-Immunotech), and anti-HLA-DR-PE (Becton Dickinson). The frequency of apoptotic cells was determined flow cytometrically using the Annexin V-FITC apoptosis detection kit (BD Biosciences, Mississauga, Ontario, Canada).
Statistical analysis.
The Student t test was used to evaluate significance in each set of values, assuming equal levels of variance. Mean values ± standard deviations (SD) are presented in this study, with one exception (Fig. 4A), where mean values ± standard errors are shown.
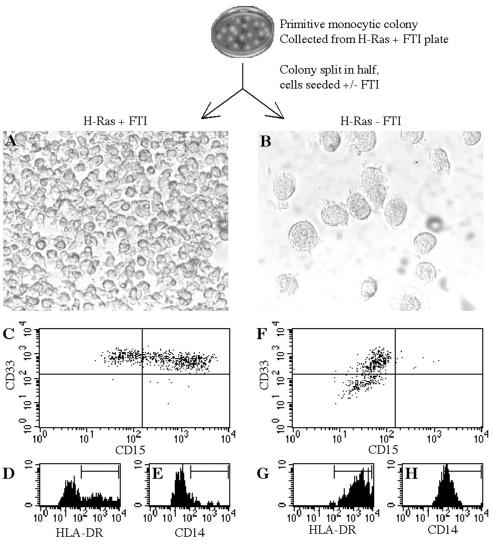
FIG. 4.
Withdrawal of FTI from inhibited H-Ras-expressing cells causes a cessation of proliferation and rapid differentiation. H-Ras-expressing cells were collected from primitive monocytic colonies formed in the presence of 50 nM FTI. The appearance of these cells after 4 days of suspension culture in the presence (A) or absence (B) of 10 nM FTI is indicated (magnification, ×100). The expression of CD33, CD15, HLA-DR, and CD14 on cells after 4 days with (C to E) or without (F to H) FTI was measured by flow cytometry.
RESULTS
Distinct levels of Ras signaling achieved by retroviral delivery of activated H-Ras coupled with partial inhibition by SCH66177 FTI.
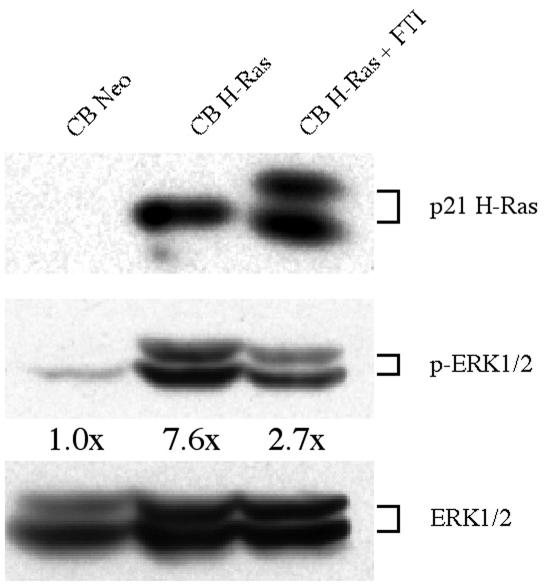
To determine the effects of Ras signaling on the fates of primitive hematopoietic cells, we transduced Lin− human CB cells with either an MSCV retroviral vector encoding G12R-activated H-Ras and a selectable neomycin resistance (Neor) marker (MSCV-H-Ras-Neo) or a control vector with Neor alone (MSCV-Neo). H-Ras was almost undetectable in control cells (Fig. 1), as expected (44), but MSCV-H-Ras-Neo-transduced cells showed substantial H-Ras expression. A portion of these was cultured in the presence of 10 nM SCH66177 in order to reduce Ras activity. The effects of moderate FTI inhibition are evident in Fig. 1, lane 3, as a second band corresponding to inactive nonfarnesylated H-Ras can be distinguished. Figure 1 also shows the levels of activated and total extracellular signal-regulated kinase 1 and 2 (ERK1/2), and the ratios of activated to total ERK1/2 relative to the levels in control cells. As indicated, the proportion of activated ERK1/2 was greatest in H-Ras-expressing cells, reduced in H-Ras-expressing cells in the presence of FTI, and lowest in control cells. These results show that the introduction of activated H-Ras expression and its partial inhibition by FTI permit modulation of the level of Ras signaling. Intracellular flow cytometric analysis of the levels of ERK activation in a group of myeloid leukemia samples showed that the range of observed ERK activation in these patients is comparable to that achieved with control and H-Ras-transduced CB cells in the presence or absence of FTI treatment (data not shown).
FIG. 1.
Farnesyltransferase inhibition permits an intermediate level of ERK activation in MSCV-H-Ras-Neo-transduced human Lin− CB cells. After 10 days of culture under selection, the levels of H-Ras, ERK1/2, and phosphorylated ERK1/2 in transduced cells were determined by Western blotting of cell lysates. The ratios of phosphorylated ERK1/2 to total ERK1/2, normalized to levels in the control, are indicated.
The proliferation and differentiation of hematopoietic cells in suspension culture are determined by the level of Ras signaling
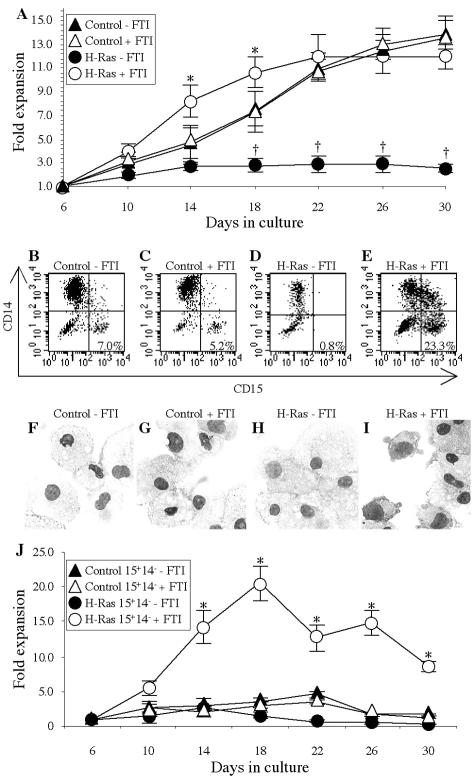
In myeloid leukemia, the differentiation of one or more hematopoietic lineages is blocked, allowing primitive cells to proliferate. To examine the role of Ras signaling in differentiation, transduced Lin− CB cells were seeded in suspension culture under conditions that support myelomonocytic lineages. Experimental measurement began 6 days after the completion of transduction, to permit selection of transduced cells by G418 resistance. As shown in Fig. 2A, control cell numbers expanded approximately 14-fold from days 6 to 30. H-Ras-expressing cells exhibited significantly impaired proliferation (P < 0.01 after day 14), achieving a maximum of 2.9- ± 0.6-fold expansion at day 22. In FTI, H-Ras-expressing cells had a significant proliferative advantage relative to controls (with or without FTI) at days 14 (P = 0.02) and 18 (P = 0.03). The advantage was transient, however: by day 30, inhibited H-Ras-expressing cells had expanded 11.9- ± 1.0-fold and were not significantly different in number relative to controls. Variation in cell number between these conditions could not be attributed to differential susceptibility to apoptosis; the percentages of annexin V-positive cells at days 10 and 22 ranged from 3 to 7% under each condition (data not shown) and were not significantly different.
FIG. 2.
Cells expressing activated H-Ras experience an early enhancement of proliferation and reduction in differentiation in the presence of FTI. (A) Transduced cells were cultured under continuous selection, and their proliferation was monitored beginning on day 6, when selection was >90% complete. The mean (from five experiments) levels of expansion (n-fold) ± SD are shown for control (triangles) and H-Ras-expressing (circles) cells either with (open symbols) or without (filled symbols) 10 nM FTI. An asterisk indicates significant difference (P ≤ 0.03) between results with H-Ras plus FTI and the control plus FTI, and a dagger indicates significant difference (P < 0.01) between results for H-Ras without FTI and the control without FTI. (B to E) The expression in a representative experiment of CD14 and CD15 on control cells (B), control cells plus FTI (C), H-Ras-expressing cells (D), and H-Ras-expressing cells plus FTI (E) was examined at day 14. The percentages of CD15+ CD14− cells are indicated. (F to I) MGG-stained cytospins of control cells (F), control cells plus FTI (G), H-Ras-expressing cells (H), and H-Ras-expressing cells plus FTI (I) are shown (magnification, ×400). (J) Mean expansion (n-fold) (five experiments) of the CD15+ CD14− fraction ± the SD in control (triangles) and H-Ras-expressing (circles) cells either with (open symbols) or without (filled symbols) 10 nM FTI. An asterisk indicates significant difference (P < 0.01) between results with H-Ras plus FTI and the control plus FTI.
The phenotypes of cells in these cultures at day 14 were evaluated by flow cytometry and morphological examination. Control (Fig. 2B) and control plus FTI (Fig. 2C) cultures were dominated by CD15− CD14+ macrophages (Fig. 2F and G), and most H-Ras-expressing cells were large CD15− CD14+/− macrophages (Fig. 2D and H). However, FTI-treated H-Ras-expressing cells had a substantially higher percentage of primitive monocytic CD15+ CD14− cells than the control cultures (23.3% versus 5.2 to 7.0%) (Fig. 2E) and a correspondingly higher number of cells with monoblastic or primitive monocytic morphology (Fig. 2I). When CD15+ CD14− FTI-treated H-Ras-expressing cells were isolated by flow sorting and examined, most had this primitive appearance (data not shown). A subset analysis of the number of cells with a CD15+ CD14− phenotype was then performed. Figure 2J shows that in FTI-treated H-Ras-expressing cell cultures, CD15+ CD14− cells expanded 20.5- ± 2.6-fold by day 18 before declining. In contrast, control or noninhibited H-Ras-expressing cells with this phenotype never expanded beyond 4.5-fold. Therefore, high levels of Ras signaling can induce rapid differentiation of primitive hematopoietic cells to large macrophages, whereas the combination of activated Ras and partial inhibition by FTI causes a transient block in differentiation and an accumulation of primitive monocytic cells.
FTI influences the fate of activated H-Ras-expressing cells in a dose-dependent manner.
To determine whether the degree of differentiation arrest in H-Ras-expressing cells could be controlled with different concentrations of FTI, cells were grown in liquid cultures and in erythromyeloid CFC progenitor assays. Levels of CD15 and CD14 expression on control cells were comparable in cultures at day 14 with 0, 5, or 20 nM FTI, but H-Ras-expressing cells exhibited an increased fraction of CD15− CD14+ cells and a reduction of CD15− CD14− cells in the presence of 5 nM FTI and the appearance of a substantial, primitive CD15+ CD14− population with 20 nM FTI (data not shown). As indicated in Table 1, neither the number of colonies produced by control cells nor their phenotypes were significantly altered by increasing the concentration of FTI. However, the number of colonies produced by H-Ras-expressing cells was increased by Ras inhibition, reaching a maximum of 2.49- ± 0.48-fold with 100 nM FTI. The percentage of colonies classified as primitive monocytic (described below) (Fig. 3E) was also FTI sensitive, reaching a maximum of 16.9% ± 7.4% at 200 nM FTI versus 2.8% ± 2.9% for uninhibited H-Ras-expressing cells or 1.7% ± 3.0% for uninhibited control cells. Higher concentrations of FTI were required in these semisolid assays, likely because the inhibitor could not be continually replaced during medium exchange as it was in the liquid cultures.
TABLE 1.
FTI dose influences the number and primitiveness of H-Ras-expressing CFCs
| Vector | FTI treatment (nM) | Relative colony counta | % Primitive CFU-Mb |
|---|---|---|---|
| MSCV-Neo | 0 | 1 | 1.7 ± 3.0 |
| 25 | 1.01 ± 0.09 | 1.6 ± 2.4 | |
| 50 | 0.98 ± 0.08 | 1.5 ± 4.1 | |
| 100 | 0.91 ± 0.09 | 1.0 ± 2.1 | |
| 200 | 0.9 ± 0.11 | 1.8 ± 4.5 | |
| 400 | 0.9 ± 0.08 | 1.9 ± 3.5 | |
| MSCV-H-Ras-Neo | 0 | 1 | 2.8 ± 2.9 |
| 25 | 1.64 ± 0.21 | 10.2 ± 6.1 | |
| 50 | 2.01 ± 0.29 | 15.0 ± 7.7 | |
| 100 | 2.49 ± 0.48 | 15.1 ± 8.7 | |
| 200 | 1.93 ± 0.51 | 16.9 ± 7.4 | |
| 400 | 1.03 ± 0.32 | 9.3 ± 6.2 |
Number of colonies formed relative to the number formed without treatment. Values are means±SD.
Percentage of primitive monocytic colonies (high level of colony cellularity and high number of phenotypically primitive cells). Values are means ± SD.
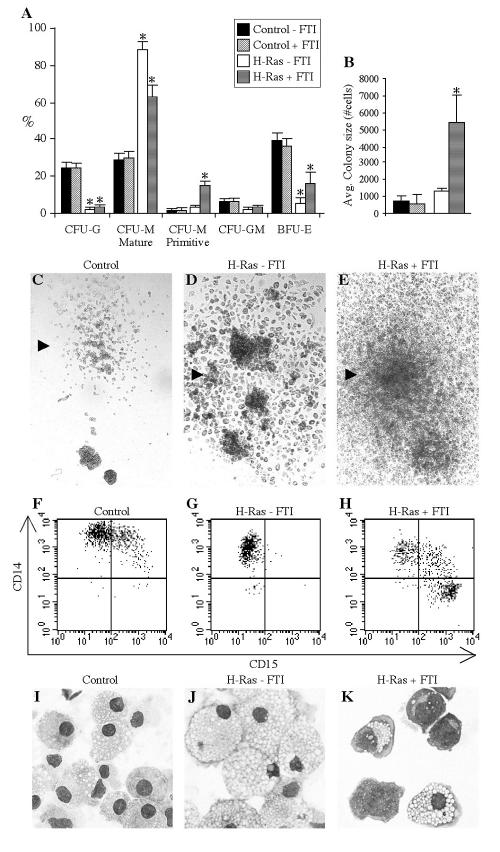
FIG. 3.
The amplitude of Ras signaling influences lineage selection and the proliferative capacity of hematopoietic progenitors. (A) Transduced cells were plated in CFC assays in the presence or absence of 50 nM FTI, as indicated. The mean percentages ± standard errors of CFU-G, primitive and mature CFU-M, and granulomonocytic and BFU-E progenitors scored on day 11 of culture (nine experiments) are shown. An asterisk indicates significant difference (P < 0.01) between results with H-Ras-expressing samples and their control counterparts. (B) The average size of colonies (±SD) generated by transduced progenitors in the presence or absence of FTI was determined by collecting all cells from day 12 methylcellulose plates, counting them, and dividing the cells by the number of colonies present. The results of three experiments, with two methylcellulose plates per condition per experiment, are presented. An asterisk indicates significant difference (P < 0.03) between results with H-Ras plus FTI and the control plus FTI. (C to E) Representative monocytic colonies on control, H-Ras without FTI, and H-Ras plus FTI plates (magnification, ×25). (F to H) Flow cytometric assessment of CD15 and CD14 expression on cells from representative single monocytic colonies on control, H-Ras without FTI, and H-Ras plus FTI plates. (I to K) MGG-stained cytospins of cells from representative monocytic colonies on control, H-Ras without FTI, and H-Ras plus FTI plates (magnification, ×400).
The developmental program of colony-forming progenitors is affected by Ras signaling.
To distinguish between the fate-altering effects of activated Ras signaling in mature cells or in their progenitors, control and H-Ras-transduced Lin− CB cells were tested in CFC assays in the presence or absence of FTI. Figure 3A shows the distribution of colony types obtained in 13 experiments. The most common colony type generated by control progenitors with or without FTI was made up of erythroid-burst-forming units (BFU-E) (36.7% ± 3.8% or 39.3% ± 3.9% of cells, respectively), whereas erythroid differentiation was significantly less common among H-Ras-expressing cells (5.4% ± 2.5% of cells without FTI [P = 5 × 10−7] and 16.2% ± 5.7% of cells with FTI [P = 0.007] were BFU-E). A significant deficit of granulocytic CFU progenitors (CFU-G) was also found in the H-Ras-expressing populations (2.9% ± 1.0% of cells with FTI [P = 4 × 10−8] and 2.1% ± 1.0% of cells without FTI [P = 5 × 10−7] were CFU-G) compared to the level in controls (24.1% ± 2.1% and 24.1% ± 2.5% of cells were CFU-G, respectively). Instead, H-Ras-expressing cells demonstrated a dramatic preference for the monocyte/macrophage lineage; 91.4% ± 3.9% of uninhibited H-Rasexpressing progenitors and 78.0% ± 7.1% of inhibited H-Ras-expressing progenitors were monocytic CFUs (CFU-M). However, in the case of FTI-treated cells, a significantly greater number of these monocytic colonies were classified as primitive by morphology, surface phenotype, and proliferative potential (see below). Such progenitors were very rare in the progenitor pools of normal samples. The primitive colonies produced by inhibited H-Ras-expressing cells contained significantly higher cell numbers than those formed by control or uninhibited H-Ras-expressing cells (P < 0.03) (Fig. 3B). Representative monocytic colonies formed by control, H-Ras-expressing, or inhibited H-Ras-expressing cells are shown in Fig. 3C to E. Uninhibited H-Ras-expressing cells formed monocytic colonies containing very large, adherent cells with unusually dark coloration attributable to phagocytosis of methylcellulose (Fig. 3D). Cells from control monocytic colonies often contained a less-mature CD15+ CD14+ subfraction (Fig. 3F), whereas those from uninhibited H-Ras-expressing colonies were uniformly CD15− (Fig. 3G). Primitive monocytic colonies formed by FTI-treated H-Ras-expressing cells invariably contained an immature CD15+ CD14− population (Fig. 3H). As was the case with suspension culture analyses, this phenotype was associated with a primitive, blast-like morphology in the corresponding cytospin (Fig. 3K). Cells from control monocytic (Fig. 3I) and uninhibited H-Ras-expressing monocytic (Fig. 3J) colonies were small and lightly vacuolated or large and highly vacuolated macrophages, respectively.
To determine whether continuous farnesyltransferase inhibition was necessary for the phenotype and increased proliferation of primitive monocytic colonies generated by H-Ras-expressing cells, 10-day-old colonies were individually collected, divided into two equal parts, and cultured in suspension with or without FTI. Figure 4A depicts cells growing in suspension after 4 days of continued inhibition; these were nearly all nonadherent and had expanded 2.2- ± 0.6-fold (10 experiments). As shown in Fig. 4C to E, such cells were CD33+ CD14low/−, generally CD15+, and HLA-DR−. In sharp contrast, 4 days after being released from inhibition, cells from the other half of the original colony were large and adherent, had a macrophage-like appearance (Fig. 4B), and had not changed significantly in number (0.8- ± 0.3-fold expansion; 10 experiments). Cytometric analysis confirmed the phenotypic maturity of these cells (Fig. 4F to H). Differentiation induced by the withdrawal of Ras inhibition was irreversible; the addition of FTI at day 4 and after additional time in culture did not induce proliferation or alter the cell phenotype (data not shown). These results indicate that activated H-Ras expression alters progenitor lineage distribution to favor the monocyte/macrophage lineage but that sustained farnesyltransferase inhibition can partially block differentiation and permit the accumulation of monocytic progenitors with increased proliferation and self-renewal potential.
H-Ras expression impairs erythroid differentiation.
Impairment of the erythroid lineage is common in most hematopoietic malignancies and is associated with preleukemia (33). The reduced frequency of erythroid progenitors in H-Ras-expressing populations suggested a block in the differentiation of this lineage. To determine the stage at which this block occurred, we evaluated the differentiation of transduced cells in suspension culture under conditions that support erythroid differentiation. Control cells progressively gained GlyA expression and lost CD36 expression while progressing from progenitors to early and then late erythroblasts, as has been previously reported (37). In contrast, we found that virtually no uninhibited H-Ras-expressing cells and fewer than 3% of inhibited H-Ras-expressing cells acquired GlyA (data not shown). This finding indicates that even a modest elevation of Ras activity can cause an early block of erythroid differentiation, preventing the appearance of erythroblasts.
Self-renewal capacity of H-Ras-expressing progenitors with FTI treatment.
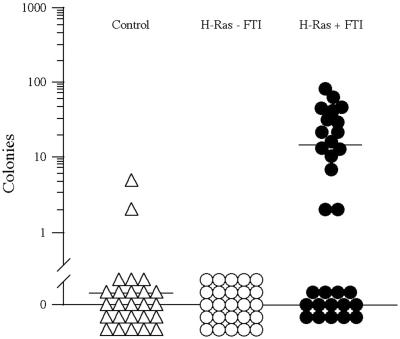
Self-renewal is the key property that distinguishes stem cells from other cells in the hematopoietic system, and increased self-renewal is strongly associated with leukemogenic transformation (16). The extent of this increase, as measured by the frequency of clonogenic progenitors capable of forming secondary colonies by replating, correlates with poor clinical outcome (35). Individual myeloid colonies from control, H-Ras-expressing, and FTI-treated H-Ras-expressing primary CFC cultures were collected at day 11 and tested for the capacity to generate new colonies. As shown in Fig. 5, only 2 of 25 control colonies were able to generate secondary colonies (mean, 0.24 secondary colonies per primary colony) and 0 of 25 H-Ras-expressing CFCs formed secondary colonies. In contrast, 16 of 30 FTI-treated H-Ras-expressing colonies formed large numbers of secondary colonies (mean, 15.1 secondary colonies per primary colony; range, 2 to 107). Thus, the combination of activated H-Ras expression and partial inhibition by FTI can dramatically enhance progenitor self-renewal.
FIG. 5.
Partially inhibited H-Ras permits secondary colony formation. Individual myeloid colonies produced by transduced progenitors were collected and reseeded in a second CFC assay under the same conditions as in the first. The number of secondary colonies formed per primary colony is indicated.
Inhibition of PI3K or p38 MAPK can partially substitute for farnesyltransferase inhibition.
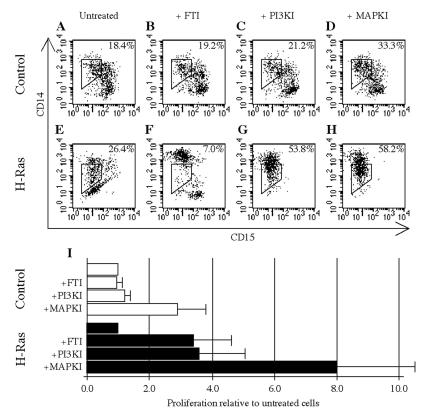
To investigate which Ras effectors are responsible for transmitting fate-determining signals, we tested whether inhibition of phosphatidylinositol 3-kinase (PI3K) by LY294002 (Calbiochem) or p38 mitogen-activated protein kinase (MAPK) by SB203580 (Calbiochem) (PI3K and MAPK inhibited with SB203580 are subsequently referred to as PI3KI and MAPKI, respectively) could substitute for FTI. The CD15 CD14 expression phenotypes of cultured control cells were not strongly influenced by these inhibitors (Fig. 6A to D), with the exception of a 1.8-fold increase in the percentage of CD15− CD14low/+ cells during MAPK treatment (Fig. 6D). The phenotypes of uninhibited and FTI-treated H-Ras-expressing cells (Fig. 6E and F) were as previously described, but the influence of PI3KI (Fig. 6G) or MAPKI (Fig. 6H) on cell phenotype was distinct from that of FTI. An increase in the percentage of CD15− CD14low/+ cells was observed (2.0- or 2.2-fold increase, relative to levels in uninhibited cells), but CD14− cells, which represent the majority of uninhibited H-Ras-expressing cells, were nearly absent from PI3KI- or MAPKI-containing cultures. MAPKI was also found to cause a 2.9- ± 0.9-fold increase in control cell number, whereas the other inhibitors exerted no meaningful effect on proliferation (Fig. 6I). In H-Ras-expressing cells, FTI (3.4- ± 1.2-fold) and PI3KI (3.6- ± 1.5-fold) enhanced proliferation at comparable levels, but MAPKI caused a significantly greater 8.0- ± 2.5-fold increase in cell number than FTI (P < 0.02). However, the enhancement of proliferation of control cells by MAPKI indicates that part of this effect is independent of H-Ras. CFC assays were also performed on H-Ras-expressing cells in the presence of MAPKI or PI3KI, but the number and gross phenotype of the resulting colonies were indistinguishable from those generated by untreated H-Ras-expressing cells (data not shown). Thus, although PI3K or p38 MAPK inhibition can enhance H-Ras-expressing cell proliferation in suspension culture at levels comparable to those produced by FTI, the distinct cellular differentiation phenotypes of the resulting cells suggest that neither of these effectors is fully responsible for the influence of Ras upon hematopoietic cell fate.
FIG. 6.
The consequences of PI3K or p38 MAPK inhibition are distinct from Ras inhibition. Following transduction, control and H-Ras-expressing cells were cultured for 10 days under selection and in the presence of the indicated inhibitor(s). Concentrations of 50 μm PI3KI or 100 μm MAPKI (which were found to be optimal for the proliferation of control and H-Ras-expressing cells) were used. (A to H) CD15 and CD14 expression of these cells as measured by flow cytometry, including the percentage of cells contained within a gate defining the CD15− CD14low fraction. (I) Expansion of cell number in the presence of an inhibitor(s) at day 10, with uninhibited control and H-Ras-expressing cultures both normalized to 1.0. Values are presented as mean normalized expansions ± SD from three experiments.
DISCUSSION
Here, we have found that the combination of H-Ras signaling and farnesyltransferase inhibition can alter hematopoietic lineage commitment, differentiation, proliferation, and self-renewal. This study provides the first demonstration of an enhancement of proliferation and self-renewal in primary human hematopoietic cells mediated by activated Ras and suggests that the level of Ras activity is critical to its role in leukemogenesis and normal hematopoiesis.
An important consideration in evaluating these results is the potentially complex effects of farnesyltransferase inhibition. Although the modulation of Ras activity by FTI treatment was demonstrated by a reduction of ERK activity, it is possible that inhibition of farnesylation on other proteins may have contributed to the observed effects. The roles of non-Ras FTI targets have been suggested by results in cell lines and in clinical trials, where drug sensitivity does not correlate with Ras mutational status (12, 46). If the enhancement of proliferation and self-renewal of the activated H-Ras-expressing cells by FTI treatment in the present study occurred in a Ras-independent manner, it would imply the existence of a proliferation-promoting agent that is negatively regulated by farnesylation. However, this is unlikely, for several reasons. (i) Control cells exhibited no enhancement of proliferation or self-renewal at any FTI concentration. (ii) The membrane binding and function of H-Ras is extremely sensitive to FTI, permitting the use of drug concentrations 10- to 100-fold lower than have been shown to affect cells with wild-type Ras or mutations in K-Ras (2, 12). (iii) The reported consequences of FTI inhibition of non-Ras targets are apoptosis or cell cycle arrest by inhibition of spindle formation (46), whereas we have observed proliferation and self-renewal. (iv) Neither of the non-Ras farnesylated proteins proposed as key FTI targets is relevant here; the activity of RhoB is independent of its farnesylation status (10, 29), and Rheb, which is functionally regulated by FTI (9), is not expressed in human hematopoietic cells (K. Takenaka, unpublished data). Thus, although some caution should remain with respect to the exclusivity of the FTI effects on H-Ras, it is very likely to have been the key target of the drug.
The expression of activated H-Ras within primary human hematopoietic cells, with or without its partial inhibition by FTI, promotes monocytic lineage cell fate. This result is concordant with the observation that large monocytic colonies arose from mice transplanted with H-Ras-transduced bone marrow (23), the induction by Ras of monocyte-like differentiation in some hematopoietic cell lines (24, 31), and the impairment of monocytic differentiation when antisense Ras oligonucleotides were added to human CD34+ cells (50). Furthermore, clinical evidence suggests that Ras mutations may be associated with monocytic subtypes of AML and MDS (19, 38, 56). Additionally, Ras has been implicated as a mediator of signaling from M-CSF, which stimulates survival, proliferation, and differentiation of cells in the monocytic lineage. Our results support the idea that the molecular regulation required for progenitors to commit to the monocytic/macrophage lineage involves Ras and that the magnitude of differentiation is regulated by the level of Ras activity. The observation of an erythrocyte deficit associated with Ras activation may indicate that differentiation in this lineage is inhibited by even a moderate level of continuous Ras activity. This is consistent with existing experimental data regarding the erythropoiesis-inhibiting properties of activated Ras in human cells (14) and suggests that Ras mutations might contribute to the impairment of erythropoiesis associated with myelodysplasia and preleukemia (7, 43).
Our results suggest that the amplitude of Ras signaling is a critical factor determining the balance between proliferation and differentiation. When activated H-Ras was introduced to primary primitive human hematopoietic cells, a proliferative deficit was observed. This is consistent with the antiproliferative phenotype observed in many hematopoietic cell lines and primary fibroblasts upon the addition of activated Ras (31, 47). However, the intermediate level of Ras signaling generated by partial inhibition had the opposite effect, causing an enhancement of proliferation and an increase in the number of phenotypically and functionally primitive monoblastic cells. This was particularly apparent in primitive colony-forming progenitors, which generated significantly larger colonies and had substantially greater self-renewal capacity. Enhancement of proliferation was contingent upon maintenance of intermediate Ras activity; when Ras-expressing cells were released from inhibition, rapid differentiation occurred and proliferation ceased. These results suggest that sustained, moderate Ras activity can induce proliferation but that even brief exposure to a high level of Ras signaling causes differentiation. Since most studies of Ras in hematopoiesis have employed strong promoters without modulation of expression or activity, it is possible that Ras activity was too high to induce proliferation. Recently, the effects of different levels of Ras signaling were tested in primary human fibroblasts (15). A vector with inducible H-Ras expression was used to show that a high level of Ras promoted senescence but that intermediate activity enhanced proliferation and was tumorigenic when combined with hTERT. This system is analogous to the mechanism by which the PC12 rat pheochromocytoma cell line uses ERK activation to transmit both differentiation signals from nerve growth factor and proliferation signals from epidermal growth factor (25, 32). In the hematopoietic system, the level of B-cell receptor signaling (which employs the Ras pathway) has been shown to determine whether B-1 or follicular and/or marginal zone differentiation occurs (8). Furthermore, the level of Raf signaling in NIH 3T3 cells (48, 55) and the extent of ERK activation in the hepatocellular carcinoma cell line HepG2 (53) have been shown to control the balance between proliferation and senescence. Thus, the level of Ras activity is an important fate-determining signal in multiple cell types. In fibroblasts, the mechanism behind this effect has been described as differential sensitivity of Ras effector proteins such as mitogen-activated protein kinase kinases 3 and 6 (15). The model reported here should permit investigation of this issue within primary hematopoietic cells.
The observation that inhibition of PI3K in activated H-Ras-expressing cells enhanced cell proliferation at a level comparable to that of Ras inhibition suggests that this effector pathway is important for exerting such control. PI3K/Akt signaling is necessary for the proliferation of normal and leukemic human CD34+ cells (4, 34), but our results indicate that the level of such signaling may also be important. Interestingly, p38 MAPK inhibition enhanced proliferation in both control and activated H-Ras-expressing cells. Although p38 activation has been associated with cytokine-mediated proliferation in human and murine leukemic cell lines (39, 51), p38 signaling was moderately antiproliferative in our experiments. This finding is consistent with the correlation between Ras-induced p38 activation and senescence in human fibroblasts (15). However, the phenotypic and functional differences between FTI-treated and PI3K- or MAPK-treated H-Ras-expressing cells indicate that additional pathways are involved in transmitting fate-determining signals within these primary human hematopoietic cells.
In addition to the effects of constitutive Ras signaling on differentiation and proliferation, we found a marked effect on self-renewal. The moderate Ras activity that delayed the differentiation and increased the proliferation of a primitive monocytic-blast-like population also caused enhanced self-renewal, which is a critical aspect of the leukemogenic process (16, 42, 58). Normal committed progenitors of all myeloerythroid lineages almost never have self-renewal capacity, but this property is inappropriately retained by leukemic progenitors. The increased proliferation and self-renewal, accumulation of primitive blast-like cells, and impairment of erythropoiesis observed in this study is reminiscent of a preleukemic state. Thus, this in vitro model may provide insight into the early stages of MDS, AML, or perhaps JMML. Since moderate Ras activation in the context of a normal primitive cell appears to promote a preleukemic state, we propose that mutations that produce low or moderate levels of Ras activation can be initiating events in myeloid leukemia. This does not preclude a role for Ras in the progression or maintenance of the disease but increases the importance of this pathway as a therapeutic target. One potential limitation of our approach is the low proportion of human AML cases with mutations in H-Ras relative to the proportions of other Ras family members (N-Ras in particular). Although the two genes are functionally very similar, it will be useful to test whether modulation of the level of N-Ras activity produces cell fate alterations different than those generated by H-Ras.
We have previously shown that the leukemic clone is organized as a hierarchy produced by rare LSCs and have postulated that the target for transformation is the hematopoietic stem cell rather than a committed progenitor (5, 28, 37). Understanding the specific nature of the target cell and the earliest molecular events in leukemogenesis will permit targeting of the cell reservoir that maintains the disease, including cells that may not yet have the full repertoire of mutations present in active leukemic clones. A gene transfer protocol specifically designed for stem cell transduction and/or the selection of a more aggressively purified primitive cell fraction will permit testing of whether constitutive Ras signaling in the stem cell compartment might result in even more abnormal growth characteristics.
Acknowledgments
This work was supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society, the Canadian Institutes for Health Research, the Stem Cell Network of the National Centres of Excellence, the Canadian Genetic Diseases Network of the National Centres of Excellence, and a Canada Research Chair.
We thank Ljilijana Petrovic of Mount Sinai Hospital (Toronto, Ontario, Canada) for obtaining CB samples, Gisele Knowles and Sherry Zhao (Department of Immunology, Hospital for Sick Children, Toronto) for fluorescence-activated cell sorting of CB subfractions, and Teresa Hawley (Holland Laboratory, American Red Cross, Rockville, Md.) for creating the PG13/MSCV-H-Ras producer line. We are also grateful to Dwayne Barber (Princess Margaret Hospital, Toronto) for his gift of antibodies and Ras effector inhibitors, to Bruce Patterson (Princess Margaret Hospital) for his valuable assistance in interpreting cell morphology, to David Hedley for antibodies and advice regarding intracellular flow cytometry, and to Robert Bishop (Schering-Plough) for the generous gift of FTI compounds which made this project possible. Finally, we thank Sean Egan, Dwayne Barber, Robert Bishop, and Atsushi Miyajima for their critical reading of the manuscript.
REFERENCES
- 1.Adams, J. M., and S. Cory. 1991. Transgenic models of tumor development. Science 254:1161-1167. [DOI] [PubMed] [Google Scholar]
- 2.Ashar, H. R., L. James, K. Gray, D. Carr, M. McGuirk, E. Maxwell, S. Black, L. Armstrong, R. J. Doll, A. G. Taveras, W. R. Bishop, and P. Kirschmeier. 2001. The farnesyl transferase inhibitor SCH 66336 induces a G(2)→M or G(1) pause in sensitive human tumor cell lines. Exp. Cell Res. 262:17-27. [DOI] [PubMed] [Google Scholar]
- 3.Bashey, A., R. Gill, S. Levi, C. J. Farr, R. Clutterbuck, J. L. Millar, I. B. Pragnell, and C. J. Marshall. 1992. Mutational activation of the N-ras oncogene assessed in primary clonogenic culture of acute myeloid leukemia (AML): implications for the role of N-ras mutation in AML pathogenesis. Blood 79:981-989. [PubMed] [Google Scholar]
- 4.Birkenkamp, K. U., M. T. Esselink, W. Kruijer, and E. Vellenga. 2000. An inhibitor of PI3-K differentially affects proliferation and IL-6 protein secretion in normal and leukemic myeloid cells depending on the stage of differentiation. Exp. Hematol. 28:1239-1249. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet, D., and J. E. Dick. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3:730-737. [DOI] [PubMed] [Google Scholar]
- 6.Bos, J. L. 1989. ras oncogenes in human cancer: a review. Cancer Res. 49:4682-4689. [PubMed] [Google Scholar]
- 7.Bowen, D. 1995. What is ineffective erythropoiesis in myelodysplastic syndromes? Leuk. Lymphoma 18:243-247. [DOI] [PubMed] [Google Scholar]
- 8.Casola, S., K. L. Otipoby, M. Alimzhanov, S. Humme, N. Uyttersprot, J. L. Kutok, M. C. Carroll, and K. Rajewsky. 2004. B cell receptor signal strength determines B cell fate. Nat. Immunol. 5:317-327. [DOI] [PubMed] [Google Scholar]
- 9.Castro, A. F., J. F. Rebhun, G. J. Clark, and L. A. Quilliam. 2003. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J. Biol. Chem. 278:32493-32496. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Z., J. Sun, A. Pradines, G. Favre, J. Adnane, and S. M. Sebti. 2000. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J. Biol. Chem. 275:17974-17978. [DOI] [PubMed] [Google Scholar]
- 11.Choy, E., V. K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I. E. Ivanov, and M. R. Philips. 1999. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98:69-80. [DOI] [PubMed] [Google Scholar]
- 12.Cox, A. D., and C. J. Der. 1997. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim. Biophys. Acta 1333:F51-F71. [DOI] [PubMed] [Google Scholar]
- 13.Darley, R. L., and A. K. Burnett. 1999. Mutant RAS inhibits neutrophil but not macrophage differentiation and allows continued growth of neutrophil precursors. Exp. Hematol. 27:1599-1608. [DOI] [PubMed] [Google Scholar]
- 14.Darley, R. L., T. G. Hoy, P. Baines, R. A. Padua, and A. K. Burnett. 1997. Mutant N-RAS induces erythroid lineage dysplasia in human CD34+ cells. J. Exp. Med. 185:1337-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng, Q., R. Liao, B. L. Wu, and P. Sun. 2004. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J. Biol. Chem. 279:1050-1059. [DOI] [PubMed] [Google Scholar]
- 16.Dick, J. E. 2003. Stem cells: self-renewal writ in blood. Nature 423:231-233. [DOI] [PubMed] [Google Scholar]
- 17.Dorrell, C., O. I. Gan, D. S. Pereira, R. G. Hawley, and J. E. Dick. 2000. Expansion of human cord blood CD34(+)CD38(−) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood 95:102-110. [PubMed] [Google Scholar]
- 18.Ehrhardt, A., G. R. Ehrhardt, X. Guo, and J. W. Schrader. 2002. Ras and relatives—job sharing and networking keep an old family together. Exp. Hematol. 30:1089-1106. [DOI] [PubMed] [Google Scholar]
- 19.Farr, C. J., R. K. Saiki, H. A. Erlich, F. McCormick, and C. J. Marshall. 1988. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc. Natl. Acad. Sci. USA 85:1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher, A., R. L. Darley, and R. Padua. 1997. The molecular basis of myelodysplastic syndromes. Haematologica 82:191-204. [PubMed] [Google Scholar]
- 21.Ge, Y., Z. H. Li, M. S. Marshall, H. E. Broxmeyer, and L. Lu. 1998. Involvement of H-ras in erythroid differentiation of TF1 and human umbilical cord blood CD34 cells. Blood Cells Mol. Dis. 24:124-137. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton, M., and A. Wolfman. 1998. Ha-ras and N-ras regulate MAPK activity by distinct mechanisms in vivo. Oncogene 16:1417-1428. [DOI] [PubMed] [Google Scholar]
- 23.Hawley, R. G., A. Z. Fong, B. Y. Ngan, and T. S. Hawley. 1995. Hematopoietic transforming potential of activated ras in chimeric mice. Oncogene 11:1113-1123. [PubMed] [Google Scholar]
- 24.Hibi, S., J. Lohler, J. Friel, C. Stocking, and W. Ostertag. 1993. Induction of monocytic differentiation and tumorigenicity by v-Ha-ras in differentiation arrested hematopoietic cells. Blood 81:1841-1848. [PubMed] [Google Scholar]
- 25.Huff, K., D. End, and G. Guroff. 1981. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J. Cell Biol. 88:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James, G., J. L. Goldstein, and M. S. Brown. 1996. Resistance of K-RasBV12 proteins to farnesyltransferase inhibitors in Rat1 cells. Proc. Natl. Acad. Sci. USA 93:4454-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, L., D. Greenbaum, K. Cichowski, K. Mercer, E. Murphy, E. Schmitt, R. T. Bronson, H. Umanoff, W. Edelmann, R. Kucherlapati, and T. Jacks. 1997. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 11:2468-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapidot, T., C. Sirard, J. Vormoor, B. Murdoch, T. Hoang, J. Caceres-Cortes, M. Minden, B. Paterson, M. Caligiuri, and J. E. Dick. 1994. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367:645-648. [DOI] [PubMed] [Google Scholar]
- 29.Lebowitz, P. F., P. J. Casey, G. C. Prendergast, and J. A. Thissen. 1997. Farnesyltransferase inhibitors alter the prenylation and growth- stimulating function of RhoB. J. Biol. Chem. 272:15591-15594. [DOI] [PubMed] [Google Scholar]
- 30.MacKenzie, K. L., A. Dolnikov, M. Millington, Y. Shounan, and G. Symonds. 1999. Mutant N-ras induces myeloproliferative disorders and apoptosis in bone marrow repopulated mice. Blood 93:2043-2056. [PubMed] [Google Scholar]
- 31.Maher, J., D. Baker, N. Dibb, and I. Roberts. 1996. Mutant ras promotes haemopoietic cell proliferation or differentiation in a cell-specific manner. Leukemia 10:83-90. [PubMed] [Google Scholar]
- 32.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 33.May, S. J., S. A. Smith, A. Jacobs, A. Williams, and R. Bailey-Wood. 1985. The myelodysplastic syndrome: analysis of laboratory characteristics in relation to the FAB classification. Br. J. Haematol. 59:311-319. [DOI] [PubMed] [Google Scholar]
- 34.Myklebust, J. H., H. K. Blomhoff, L. S. Rusten, T. Stokke, and E. B. Smeland. 2002. Activation of phosphatidylinositol 3-kinase is important for erythropoietin-induced erythropoiesis from CD34(+) hematopoietic progenitor cells. Exp. Hematol. 30:990-1000. [DOI] [PubMed] [Google Scholar]
- 35.Nara, N., G. J. Chen, I. Murohashi, S. Tohda, Y. Imai, J. Tomiyama, K. Nagata, T. Suzuki, S. Tanikawa, and S. Shiina. 1992. The in vitro growth patterns and drug sensitivities of leukemic blast progenitors among the subtypes of acute myelocytic leukemia. Exp. Hematol. 20:904-908. [PubMed] [Google Scholar]
- 36.Palmero, I., C. Pantoja, and M. Serrano. 1998. p19ARF links the tumour suppressor p53 to Ras. Nature 395:125-126. [DOI] [PubMed] [Google Scholar]
- 37.Pereira, D. S., C. Dorrell, C. Y. Ito, O. I. Gan, B. Murdoch, V. N. Rao, J. P. Zou, E. S. P. Reddy, and J. E. Dick. 1998. Retroviral transduction of TLS-ERG initiates a leukemogenic program in normal human hematopoietic cells. Proc. Natl. Acad. Sci. USA 95:8239-8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radich, J. P., K. J. Kopecky, C. L. Willman, J. Weick, D. Head, F. Appelbaum, and S. J. Collins. 1990. N-ras mutations in adult de novo acute myelogenous leukemia: prevalence and clinical significance. Blood 76:801-807. [PubMed] [Google Scholar]
- 39.Rausch, O., and C. J. Marshall. 1999. Cooperation of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways during granulocyte colony-stimulating factor-induced hemopoietic cell proliferation. J. Biol. Chem. 274:4096-4105. [DOI] [PubMed] [Google Scholar]
- 40.Rebollo, A., and A. C. Martinez. 1999. Ras proteins: recent advances and new functions. Blood 94:2971-2980. [PubMed] [Google Scholar]
- 41.Reuter, C. W., M. A. Morgan, and L. Bergmann. 2000. Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies? Blood 96:1655-1669. [PubMed] [Google Scholar]
- 42.Reya, T., S. J. Morrison, M. F. Clarke, and I. L. Weissman. 2001. Stem cells, cancer, and cancer stem cells. Nature 414:105-111. [DOI] [PubMed] [Google Scholar]
- 43.Sawada, K., N. Sato, A. Notoya, T. Tarumi, S. Hirayama, H. Takano, K. Koizumi, T. Yasukouchi, M. Yamaguchi, and T. Koike. 1995. Proliferation and differentiation of myelodysplastic CD34+ cells: phenotypic subpopulations of marrow CD34+ cells. Blood 85:194-202. [PubMed] [Google Scholar]
- 44.Schaefer, C., L. Grouse, K. Buetow, and R. L. Strausberg. 2001. A new cancer genome anatomy project web resource for the community. Cancer J. 7:52-60. [PubMed] [Google Scholar]
- 45.Sebti, S. M. 2003. Blocked pathways: FTIs shut down oncogene signals. Oncologist 8(Suppl. 3):30-38. [DOI] [PubMed] [Google Scholar]
- 46.Sepp-Lorenzino, L., Z. Ma, E. Rands, N. E. Kohl, J. B. Gibbs, A. Oliff, and N. Rosen. 1995. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 55:5302-5309. [PubMed] [Google Scholar]
- 47.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 48.Sewing, A., B. Wiseman, A. C. Lloyd, and H. Land. 1997. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol. Cell. Biol. 17:5588-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shannon, K. 1995. The Ras signaling pathway and the molecular basis of myeloid leukemogenesis. Curr. Opin. Hematol. 2:305-308. [DOI] [PubMed] [Google Scholar]
- 50.Skorski, T., C. Szczylik, M. Z. Ratajczak, L. Malaguarnera, A. M. Gewirtz, and B. Calabretta. 1992. Growth factor-dependent inhibition of normal hematopoiesis by N-ras antisense oligodeoxynucleotides. J. Exp. Med. 175:743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasa, S. P., and P. D. Doshi. 2002. Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways cooperate in mediating cytokine-induced proliferation of a leukemic cell line. Leukemia 16:244-253. [DOI] [PubMed] [Google Scholar]
- 52.Tan, P. B., and S. K. Kim. 1999. Signaling specificity: the RTK/RAS/MAP kinase pathway in metazoans. Trends Genet. 15:145-149. [DOI] [PubMed] [Google Scholar]
- 53.Tsukada, Y., K. Miyazawa, and N. Kitamura. 2001. High intensity ERK signal mediates hepatocyte growth factor-induced proliferation inhibition of the human hepatocellular carcinoma cell line HepG2. J. Biol. Chem. 276:40968-40976. [DOI] [PubMed] [Google Scholar]
- 54.Whyte, D. B., P. Kirschmeier, T. N. Hockenberry, I. Nunez-Oliva, L. James, J. J. Catino, W. R. Bishop, and J. K. Pai. 1997. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 272:14459-14464. [DOI] [PubMed] [Google Scholar]
- 55.Woods, D., D. Parry, H. Cherwinski, E. Bosch, E. Lees, and M. McMahon. 1997. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol. Cell. Biol. 17:5598-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yunis, J. J., A. J. Boot, M. G. Mayer, and J. L. Bos. 1989. Mechanisms of ras mutation in myelodysplastic syndrome. Oncogene 4:609-614. [PubMed] [Google Scholar]
- 57.Zhang, F. L., P. Kirschmeier, D. Carr, L. James, R. W. Bond, L. Wang, R. Patton, W. T. Windsor, R. Syto, R. Zhang, and W. R. Bishop. 1997. Characterization of Ha-ras, N-ras, Ki-Ras4A, and Ki-Ras4B as in vitro substrates for farnesyl protein transferase and geranylgeranyl protein transferase type I. J. Biol. Chem. 272:10232-10239. [DOI] [PubMed] [Google Scholar]
- 58.Zon, L. I. 2001. Hematopoiesis: a developmental approach. Oxford University Press, New York, N.Y.