Abstract
Objectives:
To assess the epidemiological profile of asthma in school going children in Srinagar, Kashmir.
Study design:
Cross-sectional study.
Setting:
Thirty-one schools with proportionate representation from both government and private schools as well as from primary, middle, and high schools.
Participants:
School children aged 10–16 years with equal representation of sex and all ages.
Main Outcome Measure:
Prevalence of current and past asthma.
Methods and Results:
After administering a modified pretested questionnaire, peak expiratory flow measurement was carried. Children who had asthma-like symptoms or positive family history of asthma or physician-labeled asthma were subjected to spirometry and bronchodilator reversibility. Out of 806 children, bronchial asthma was seen in 60 (prevalence of 7.4%) which included 34 boys and 26 girls. Majority of asthmatic children (78.3% [n = 47]) had probable asthma; 6.7% (n = 4) had definite asthma; and 15% (n = 9) had physician-diagnosed asthma. Majority of children had intermittent asthma (78.3% [n = 47]). Mild persistent asthma was seen in 12.7% (n = 7) and 10% (n = 6) had moderate persistent asthma. None of the children had severe persistent asthma. The prevalence of current asthma was 3.2% (n = 26). On univariate analysis, the factors found to be statistically significant were family history of asthma (odds ratio [OR] =8.174; confidence interval [CI] =4.403–15.178), seasonal cough (OR = 4.266; CI = 2.336–7.791), allergic rhinitis (OR = 2.877; CI = 1.414–5.852), atopic dermatitis (OR = 6.597; CI = 2.72–16.004), and obesity (OR = 6.074; CI = 2.308–18.034). On multivariate analysis, family history, seasonal cough, allergic rhinitis, atopic dermatitis, and obesity were found to be significant independent risk factors.
Conclusions:
Srinagar qualifies as a low prevalence area for bronchial asthma in the age group of 10–16 years. Majority of children had mild intermittent asthma resulting in under diagnosis and wrong treatment.
KEY WORDS: Asthma, Global Initiative for Asthma, International Study on Allergy and Asthma in Childhood, questionnaire
INTRODUCTION
Global Strategy for Asthma Management and Prevention Guidelines define asthma as “a chronic inflammatory disorder of airways associated with increased airway hyperresponsiveness, recurrent episodes of wheezing, breathlessness, chest tightness, and coughing.”[1]
Diagnosis of asthma is suspected in children with recurrent episodes of airflow obstruction characterized by recurrent wheeze/recurrent isolated cough/recurrent breathlessness/nocturnal cough/tightness of chest. Asthma being characteristically episodic, there may be no signs at the time of evaluation. Spirometry and peak flow meter can be used as an aid to diagnosis, provided the child can perform the test. Problems in case definition for community survey of childhood bronchial asthma have been circumvented by standard questionnaires.[2]
The proportion of Indian school children suffering from bronchial asthma has increased to more than double in the last 10 years.[3] The increase in prevalence of asthma in children may have serious implications in their adult life, as 40% of children with trivial wheeze and 70–90% of those with troublesome asthma continue to have symptoms in midadult life.[4] Children with asthma also have an increased risk of school absenteeism and hospitalizations when compared with unaffected children.[5]
Despite research work that has been directed at explaining why some individuals and not others develop asthma, etiology remains unknown. Investigation for possible reasons for variation in the epidemiological profile may be a more elusive source for clarification of some etiological aspects.
The aim of this study was to assess the epidemiological profile of asthma in school going children (10–16 years) in Srinagar, Kashmir.
METHODS
It was a community-based cross-sectional study carried out in the district Srinagar from 15th March 2012 to 15th May 2013. School children aged 10–16 years were eligible for the study.
A nonresponse rate of 10% was taken into consideration using the findings of a pilot study. Then, based on an anticipated prevalence of 12%, 95% confidence level and an absolute error of 2.4%, sample size of the study was calculated to be 806.[6] Sample was selected using multistage random sampling technique [Figure 1].
Figure 1.

Multistage random sampling
Thirty-one schools were selected randomly for the study, with proportionate representation from both government and private schools, as well as from primary, middle, and high schools. Sample size was achieved when twenty-six students were selected from each school.
Informed consent was taken from parents of the subjects taken for study. Based on the International study on Allergy and Asthma in Childhood (ISSAC) and the European Community Respiratory Health Survey II Questionnaire, a modified questionnaire was formulated to fulfill the aims of the study.[7,8] The questionnaire was pretested. Subjects were interviewed as per the questionnaire (by postgraduate community medicine resident) after explaining the questions. Physical demonstration and audio recorded sound of wheezing was used whenever necessary. The cause of absenteeism, if any, in selected children was sought and such children were evaluated on next visit.
The questionnaire consisted of three proformas. Proforma I included biodata (age, sex, total family members, number and order of siblings, and literacy of parents) and questions regarding assessment of bronchial asthma and risk factors of exacerbation. Asthma exacerbation was defined as an asthma attack requiring medical attention or hospitalization. Direct questions regarding physician-diagnosed asthma were asked. These questions were filled by the interviewer. Proforma II included questions for assessing risk factors of bronchial asthma (family history of asthma, allergic rhinitis and atopic dermatitis, type of construction, shared bedrooms, mode of heating, pets, mold at home, and second-hand smoking). Questions dependent upon parent's knowledge in Proforma II were filled by parents in local language. Proforma III was for teachers for observation of any asthma-like symptoms (ALSs) or long absence from school because of ALSs.
After recording the responses, relevant general physical examination and systemic examination were done by a pediatrician. CDC Growth Charts 2000 were used for grading of body mass index (BMI). Obesity was defined as BMI >95th percentile matched for age and sex.
Peak expiratory flow rate (PEFR) was measured in standing position with Wrights mini peak flow meter after the demonstration. Highest of the three readings was taken as true value. Improvement in PEFR by >15% after bronchodilator was considered as reversible airway obstruction.
ALSs was defined as the presence of cough + whistling sounds in chest + breathlessness or cough + whistling sounds in chest/breathlessness or whistling sounds + breathlessness or presence of only asthma like cough.[9]
Children who had ALS or who had positive family history of asthma, but no ALS or who had physician-labeled asthma were subjected to spirometric examination.
Spirometry was performed by HELIOS 401 Spirometer (Recorders and Medicare Systems, Chandigarh). Spirometry was performed following the protocol by American Thoracic Society.[10] Three to eight readings were taken. The values were adjusted for gender and height. A prebronchodilator forced expiratory volume in 1s (FEV1)/forced vital capacity ratio of >0.80 was reported as normal and <0.80 suggested airway obstruction. Reversibility was demonstrated by a postbronchodilator increase in FEV1 by 12%.
Incompletely filled questionnaires and subjects not cooperating with the spirometry (n = 48) were excluded in the final analysis. In such cases, next consecutive subject was taken to achieve the desired sample size.
For the purpose of analysis, subjects with ALSs were further defined:[2,11]
Those children who had been labeled asthmatic by physicians and had a corroborative evidence of personal or family history of asthma/atopy or clinical response to bronchodilators at the time of ALS episodes, but with normal spirometry and chest examination were labeled as physician-diagnosed asthmatics.
Those children who had ALS and reversible airway disease or auscultatory wheeze (in the absence of other causes of wheeze) were labeled as definite asthmatics.
Those who had ALS with normal spirometry and chest examination, in the absence of physician-diagnosed asthma were labeled as probable asthma.
Children with no symptoms or supportive spirometry/peak flowmetry or relevant physical findings were labeled as nonasthmatic.
Further classification of asthma according to the severity of clinical features was done according to Global Initiative on Asthma into four Global Initiative for Asthma grades.[11]
Asthma was also classified according to duration of symptoms as:
Current asthma: Asthma symptoms within the last 12 months associated with airway hyperresponsiveness
Past asthma: Asthma ever in life.
Statistical analysis
The data were analyzed using appropriate statistical software; SPSS version 20 (Armonk, NY: IBM Corp). Frequencies were obtained using descriptive statistics. Odds ratio was calculated for various factors. The evaluation of independent risk factors was done by multiple logistic regression analysis.
RESULTS
The study included 411 boys and 395 girls, almost equally distributed in all ages. Majority of the subjects were Muslims (99%). Mean BMI of boys and girls was 17.79 and 18.68, respectively. The demographic profile and risk factors of asthma of baseline population of school children are given in Table 1.
Table 1.
Demography and risk factors
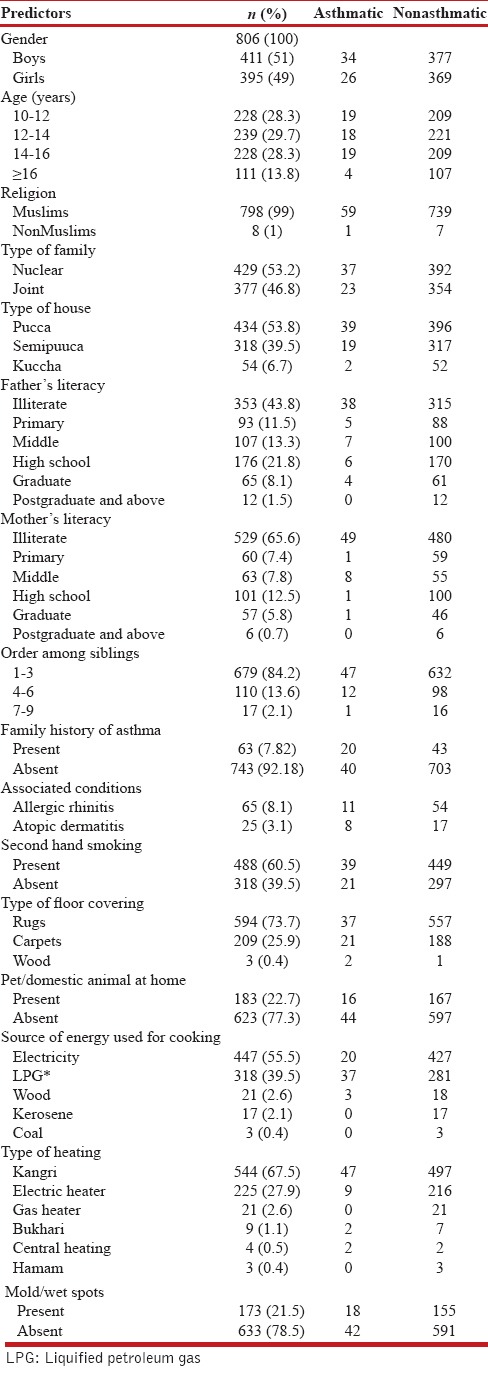
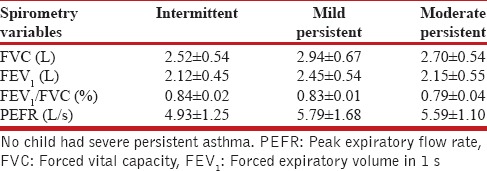
Prevalence of bronchial asthma was 7.4% (n = 60), which included 34 boys and 26 girls. The prevalence was more in boys (8.3%) than in girls (6.6%), with the prevalence in different age groups varying as per gender, the highest prevalence being in boys in the age group of 12–14 years (11.1%) and in girls in 10–12 years (9.2%). The prevalence of current asthma was 3.2% (n = 26) with prevalence in boys of 3.1% (n = 13) and in girls of 3.2% (n = 13). Of the 60 children with bronchial asthma; majority 78.3% (n = 47) had probable asthma, 6.7% (n = 4) were categorized as having definite asthma, and 15% (n = 9) had physician-diagnosed asthma. Table 2 depicts findings on spirometry and peak flowmetry in boys and girls with no asthma, probable asthma and physician-diagnosed asthma, and prebronchodilator and postbronchodilator results of boys with definite asthma. Of the 60 children with bronchial asthma, 78% (n = 47) had intermittent asthma, 12% (n = 7) had mild persistent asthma, 10% (n = 6) had moderate persistent asthma, and none had severe persistent asthma. Table 3 shows spirometry and peak flowmetry in children with different severities of asthma.
Table 2.
Spirometry in boys and girls
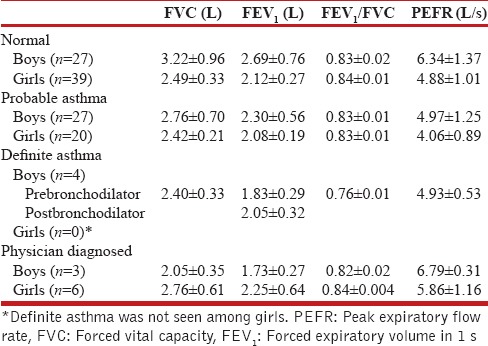
Table 3.
Spirometry and peak flowmetry in children with different grades of asthma
Wheeze was present in 42% (n = 28) and breathlessness was present in 71% (n = 43). For their respiratory symptoms, 22 patients of asthma had visited a physician and 38 had been given medicines by local pharmacists or medical shopkeepers.
The risk factors that were attributed to cause exacerbation among asthmatics are shown in Table 4.
Table 4.
Risk factors of exacerbation among asthmatics

Prevalence of asthma was significantly higher in children with seasonal cough (12.7%), obesity (42.8%), history of atopic dermatitis and allergic rhinitis (32% and 16.9%, respectively), and family history of asthma (31.7%). The prevalence was also significantly higher in children who had illiterate fathers (11.3%) or educated mothers (12.7%) or homes where wood as fuel (14.3%) or central heating was used (50%). Prevalence of asthma was higher, but not statistically significant in nuclear families (8.6%), sibling order 4–6 (10.9%), molds/wet spots at home (10.4%), pet at home (8.7%), pucca house (9%), partial breastfeeding (10.3%), or those exposed to second-hand smoking (8%).
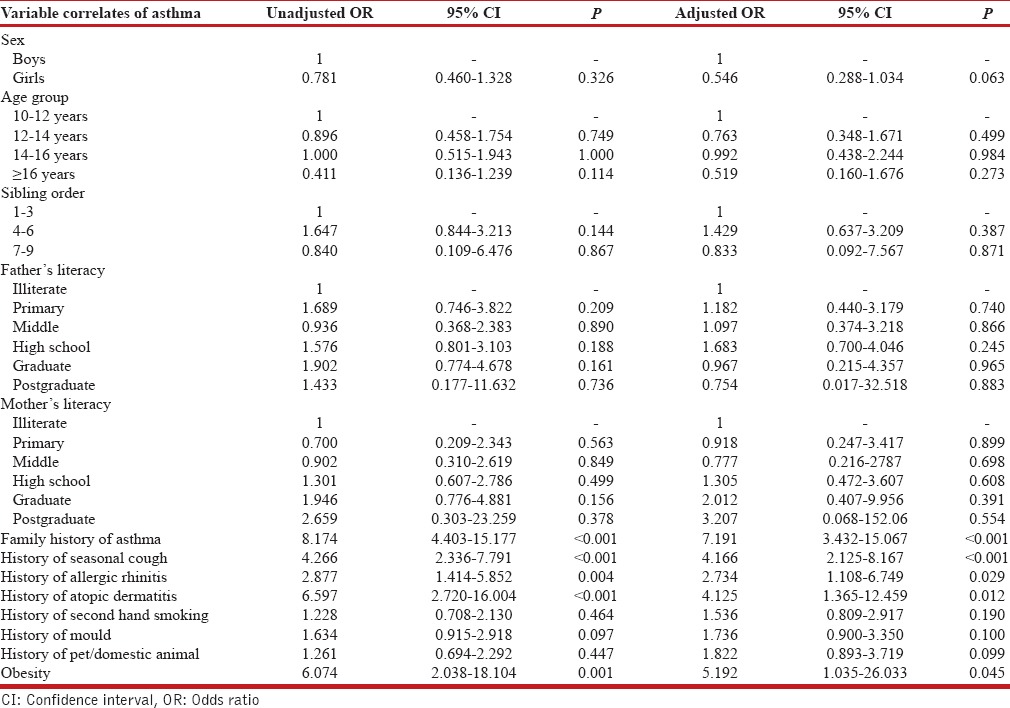
On univariate analysis, the risk factors found to be statistically significant with asthma were family history of asthma, seasonal cough, allergic rhinitis, atopic dermatitis, and obesity [Table 5]. On multivariate analysis, family history, seasonal cough, allergic rhinitis, atopic dermatitis, and obesity were found to be significant independent risk factors for asthma [Table 5].
Table 5.
Univariate and multivariate analysis of risk factors of asthma
Prevalence of allergic rhinitis and atopic dermatitis was 8.1% (n = 65) and 3.1% (n = 25), respectively.
DISCUSSION
ISSAC study compared the prevalence rates of asthma at 155 centers in 56 countries in children aged between 6–7 and 13–14 years. The prevalence was greater in English speaking countries. Prevalence was 17–30% in the United Kingdom, New Zealand, and Australia whereas areas of low prevalence (1–7%) included Eastern Europe, China, and Indonesia.[12] There is a wide variation of asthma even in India, with prevalence ranging from 3.3% in Lucknow to 11.6% in New Delhi.[6,13] It has been seen that prevalence is more in urban than in rural areas.[14] We observed a prevalence of 7.4% in school children aged between 10 and 16 years. Wide differences in samples, different methodologies, lack of consistency in age groups, rural-urban variation, study instruments, and criteria for positive diagnosis are reasons for such a wide variation in estimated prevalence. Comparison of childhood asthma prevalence studies done in India in last decade is shown in Table 6.
Table 6.
Prevalence of childhood asthma in different studies in India
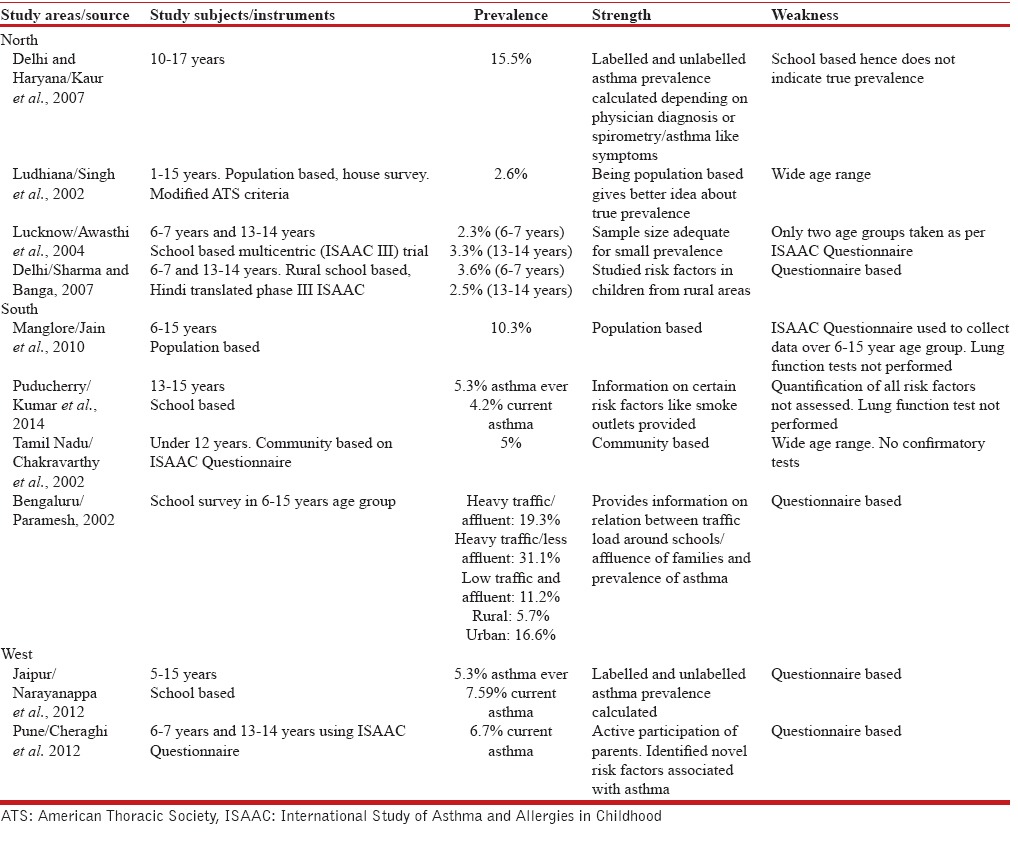
Asthma prevalence is increasing globally due to urbanization, air pollution, and environmental tobacco smoke. A steady rise in prevalence has also been documented in Bengaluru in India.[15] No reference study in the same age group is available in our state to look for change in trends.
Asthma is more prevalent in boys than in girls. Jain et al. attributed it to increased bronchial lability in males.[16] In our study, gender distribution of asthma ever was in favor of boys, although current asthma prevalence was similar in both sex groups. Sex affects the development of asthma in a time-dependent manner. Male sex is a risk factor for asthma in prepubertal children whereas female sex is a risk factor for persistence of asthma into adulthood. When segregated by gender and age, asthma was seen more in boys in the age group of 12–14 years and more in girls in the age group of 14–16 years. This was similar to findings observed by Gupta et al.[17]
Majority of the subjects in our study had illiterate parents and there was a significant association between father's illiteracy and asthma. Golshan et al. also noted father's illiteracy to be a significant risk factor.[18] Mother's illiteracy was not associated with asthma in their children. Jain et al., found no significant association between literacy level of parents and prevalence of asthma.[16]
In Kashmir, during the winter season, different appliances utilizing coal, wood, liquefied petroleum gas, and electricity as fuel are used to keep warm. Kangri is a traditional mode of heating, being kept very close to the body. Our study showed that prevalence of asthma was highest in subjects using “Bukhari” as a mode of heating in which either wood or sawdust is used. A study conducted by Singh et al. showed that asthma was more prevalent in families using smoke producing fuels as compared to other families.[19]
In our study, a significant relationship was observed between family history and asthma. First comprehensive study for inheritance in asthma was undertaken by cook and varider veer in 1916. They came to the conclusion that familial association was due to genetic component. Since then, a number of studies have shown an association between family history and asthma.[16] Paramesh observed that the incidence of asthma in children, if one parent is having asthma, is 18%, in case of one sibling having asthma, the incidence is 1.65% and where grandparents have asthma, the incidence is 4%.[15] Despite running in families, identification of asthma gene has been elusive with over 100 genes found to be associated with asthma. There is a report of no familial association as well.[20] The clinical relationship among asthma, allergic rhinitis, and atopic dermatitis, the so-called “allergic triad,” is well known. Several longitudinal studies provide evidence for a characteristic sequential development of “atopic march” during childhood: Atopic dermatitis and food allergy typically develop in infancy followed by asthma and/or allergic rhinitis in childhood. The vast majority (~80%) of patients with asthma have allergic rhinitis, whereas 19% to 38% of patients with allergic rhinitis have coexisting asthma.[21] We found a similar relationship.
Asthma is more frequently observed in obese subjects. An increase in intraabdominal pressure on the diaphragm and fat mass on chest wall leads to mass loading of the thorax, resulting in a reduction of respiratory compliance and changes in airway resistance.[22] Further systemic and airway inflammation of obesity and asthma are interlinked by systemic spillover of “adipokines.”[23] Our study showed a higher prevalence of bronchial asthma in subjects with obesity both in boys and girls. These results are also supported by Figueroa-Muñoz, who found a clear association between obesity and asthma in 4- and 11-year-old children in the United Kingdom.[24] In India, studies done in adults have found a strong association between asthma and obesity.[25]
A number of modifiable risk factors were attributed as triggers for exacerbation. It was not confirmed whether these triggers were true or perceived, as skin allergen testing was not done. Pollen was also attributed. That could be accounted by an increase in a number of popular trees grown in Kashmir. Respiratory viral infections trigger an attack of asthma. Onset of new season, particularly winter was significantly associated with asthma such as cough.
Asthma is often under-diagnosed.[26] Physician label of asthma was given only in 15% of asthmatics partly because of visits to local pharmacists and medical shopkeepers. Only 5.3% had physician-diagnosed asthma ever in urban schools in Jaipur.[27]
Measurements of lung functions provide an assessment of the severity of airflow limitations and its reversibility. Only 15 subjects had ever undergone spirometry, which represents underutilization of basic tools for diagnosis and management. This is even true of the capital city of India.[28]
Majority of children had intermittent asthma with less severe symptoms. This caused the parents to seek the advice of pharmacists rather than doctors. Ng Man Kwong et al. in an earlier study found that increasing prevalence of asthma diagnosis is because of mild symptoms. They found that symptoms with frequency of wheeze ≤3 times in a year were more frequent.[29]
Our study derives strength from the fact that spirometry, peak flowmetry, and bronchodilator reversibility were performed in children with ALSs and children were further categorized. Data on lung function in different categories of asthma are also presented. Pure questionnaire-based surveys have limitations due to difference in comprehension by the respondents, misclassification of symptoms of asthma, and underreporting due to social stigma.
The limitation of our study is that it is a school-based cross-sectional study. Therefore, true prevalence and direct causal relationship with risk factors cannot be implied.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Gruchalla RS, Wolf RL, Yawn BP, Cartar L, Gan V, et al. Development and validation of school-based asthma and allergy screening questionnaires in a 4-city study. Ann Allergy Asthma Immunol. 2004;93:36–48. doi: 10.1016/S1081-1206(10)61445-7. [DOI] [PubMed] [Google Scholar]
- 3.Pal R, Barua A. Prevalence of childhood bronchial asthma in India. Ann Trop Med Public Health. 2008;1:73–5. [Google Scholar]
- 4.Horak E, Lanigan A, Roberts M, Welsh L, Wilson J, Carlin JB, et al. Longitudinal study of childhood wheezy bronchitis and asthma: Outcome at age 42. BMJ. 2003;326:422–3. doi: 10.1136/bmj.326.7386.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid J, Marciniuk DD, Cockcroft DW. Asthma management in the emergency department. Can Respir J. 2000;7:255–60. doi: 10.1155/2000/296273. [DOI] [PubMed] [Google Scholar]
- 6.Chhabra SK, Gupta CK, Chhabra P, Rajpal S. Risk factors for development of bronchial asthma in children in Delhi. Ann Allergy Asthma Immunol. 1999;83:385–90. doi: 10.1016/S1081-1206(10)62835-9. [DOI] [PubMed] [Google Scholar]
- 7.Faniran AO, Peat JK, Woolcock AJ. Prevalence of atopy, asthma symptoms and diagnosis, and the management of asthma: Comparison of an affluent and a non-affluent country. Thorax. 1999;54:606–10. doi: 10.1136/thx.54.7.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi M, Rezzani C, Biino G, Marinoni A. Asthma-like symptoms assessment through ECRHS screening questionnaire scoring. J Clin Epidemiol. 2003;56:238–47. doi: 10.1016/s0895-4356(02)00613-3. [DOI] [PubMed] [Google Scholar]
- 9.Global Strategy for Asthma Management and Prevention. [Last accessed on 2014 Jul 01]. Update From: NHLB/WHO; 2002. Available from: http://www.ginasthma.com .
- 10.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 11.Koshak EA. Classification of asthma according to revised 2006 GINA: Evolution from severity to control. Ann Thorac Med. 2007;2:45–6. doi: 10.4103/1817-1737.32228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal R, Dahal S, Pal S. Prevalence of bronchial asthma in Indian children. Indian J Community Med. 2009;34:310–6. doi: 10.4103/0970-0218.58389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awasthi S, Kalra E, Roy S, Awasthi S. Prevalence and risk factors of asthma and wheeze in school-going children in Lucknow, North India. Indian Pediatr. 2004;41:1205–10. [PubMed] [Google Scholar]
- 14.Chakravarthy S, Singh RB, Swaminathan S, Venkatesan P. Prevalence of asthma in urban and rural children in Tamil Nadu. Natl Med J India. 2002;15:260–3. [PubMed] [Google Scholar]
- 15.Paramesh H. Epidemiology of asthma in India. Indian J Pediatr. 2002;69:309–12. doi: 10.1007/BF02723216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain A, Vinod Bhat H, Acharya D. Prevalence of bronchial asthma in rural Indian children: A cross sectional study from South India. Indian J Pediatr. 2010;77:31–5. doi: 10.1007/s12098-009-0308-6. [DOI] [PubMed] [Google Scholar]
- 17.Gupta MK, Sharma BS, Chandel R. Prevalence of asthma in urban school children in Jaipur, India. Pediatr Res. 2011;70:517. doi: 10.1007/s13312-012-0188-0. [DOI] [PubMed] [Google Scholar]
- 18.Golshan M, Mohammad-Zadeh Z, Khanlar-Pour A, Iran-Pour R. Prevalence of asthma and related symptoms in junior high school children in Isfahan, Iran. Monaldi Arch Chest Dis. 2002;57:19–24. [PubMed] [Google Scholar]
- 19.Singh D, Sobti PC, Arora V, Soni RK. Epidemiological study of asthma in rural children. Indian J Community. 2002;27:167–70. [Google Scholar]
- 20.Pokharel PK, Kabra SK, Kapoor SK, Pandey RM. Risk factors associated with bronchial asthma in school going children of rural Haryana. Indian J Pediatr. 2001;68:103–6. doi: 10.1007/BF02722022. [DOI] [PubMed] [Google Scholar]
- 21.Ober C, Yao TC. The genetics of asthma and allergic disease: A 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–33. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 23.Shore SA. Obesity and asthma: Implications for treatment. Curr Opin Pulm Med. 2007;13:56–62. doi: 10.1097/MCP.0b013e3280110196. [DOI] [PubMed] [Google Scholar]
- 24.Figueroa-Muñoz JI, Chinn S, Rona RJ. Association between obesity and asthma in 4-11 year old children in the UK. Thorax. 2001;56:133–7. doi: 10.1136/thorax.56.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra V. Effect of obesity on asthma among adult Indian women. Int J Obes Relat Metab Disord. 2004;28:1048–58. doi: 10.1038/sj.ijo.0802700. [DOI] [PubMed] [Google Scholar]
- 26.Kaur J, Chugh K, Sachdeva A, Satyanarayana L. Under diagnosis of asthma in school children and its related factors. Indian Pediatr. 2007;44:425–8. [PubMed] [Google Scholar]
- 27.Narayanappa D, Rajani HS, Mahendrappa KB, Ravikumar VG. Prevalence of asthma in urban school children in Jaipur, Rajasthan. Indian Pediatr. 2012;49:835–6. doi: 10.1007/s13312-012-0188-0. [DOI] [PubMed] [Google Scholar]
- 28.Kotwani A, Chhabra SK, Tayal V, Vijayan VK. Quality of asthma management in an urban community in Delhi, India. Indian J Med Res. 2012;135:184–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Ng Man Kwong G, Proctor A, Billings C, Duggan R, Das C, Whyte MK, et al. Increasing prevalence of asthma diagnosis and symptoms in children is confined to mild symptoms. Thorax. 2001;56:312–4. doi: 10.1136/thorax.56.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]


