Abstract
Introduction:
Psychiatric disorders, especially anxiety and depression have been reported to have an increased prevalence in chronic obstructive pulmonary disease (COPD) patients, but there is a paucity of data from India.
Aims and Objectives:
Aim of our study is to study the frequency of psychiatric comorbidities in COPD patients and their correlation with severity of COPD, as per global initiative for obstructive lung disease guidelines.
Materials and Methods:
This study was conducted in outpatient department of a tertiary care hospital (King George's Medical University). A total of 74 COPD patients were included in this study and compared with 74 controls. The diagnosis and severity of COPD were assessed by spirometry. Psychiatric comorbidities were assessed using the Mini International Neuropsychiatric Interview questionnaire.
Results:
The frequency of psychiatric comorbidities was significantly higher (P < 0.05) in COPD patients (28.4%) as compared to controls (2.7%). As regards to severity, the frequency was significantly increased in severe and very severe COPD. The frequency of psychiatric comorbidities in COPD patients increased significantly with the increase in duration of symptoms being present in 67% of patients with duration of symptoms more than 10 years and only 23% of patients with duration of symptoms ≤5 years.
Conclusion:
The frequency of psychiatric comorbidities is increased in COPD patients as compared to controls. We recommend that all patients with COPD should be screened for psychiatric comorbidity, if any.
KEY WORDS: Anxiety, chronic obstructive pulmonary disease, psychiatric comorbidities
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), a common preventable and treatable disease, is characterized by persistent airflow limitation that is usually progressive.[1] Exacerbations and comorbidities contribute to the overall severity in individual patients. Its prevalence is 5.0% among Indian males and approximately 3.2% among Indian females over 35 years of age.[2]
The projection for 2020 indicates that COPD will be the third leading cause of death worldwide and the fifth leading cause of years lost through early mortality or handicap in terms of disability-adjusted life years.[3] Anxiety and depressive symptoms are common in patients affected by COPD, even when their disease is mild in terms of forced expiratory volume (FEV1) and respiratory symptoms. The etiology between COPD and depression continues to be complex and likely bidirectional. As with schizophrenia, the higher rates of smoking seen in patients with depression could lead to the higher prevalence rate of COPD in depressed patients.[4] One hypothesis suggests that chronic hypoxemia may lead to disruptions of noradrenergic and dopaminergic synthesis, release, and replenishment that ultimately lead to depressive symptoms. Furthermore, chronic hypoxemia may also lead to poor oxygenation in the periventricular and subcortical regions of the brain, which are vulnerable regions to hypoperfusion and lead to similar brain MRI changes as seen in patients with depression.[5]
Similar to COPD and depression, the association between COPD and anxiety appears to be multifactorial and bidirectional. Mikkelsen et al. further outlined possible explanatory models for a common pathophysiology between COPD and anxiety, including the hyperventilation model, carbon dioxide hypersensitivity model, and cognitive-behavioral model.[6] However, psychiatric comorbidities in COPD patients are often under diagnosed and contribute to the morbidity in these patients. Increased awareness is, therefore, essential to diagnose and treat psychiatric comorbidities in such patients. This study aims to study the frequency of psychiatric comorbidities in COPD outpatients and to correlate the severity of COPD with psychiatric comorbidities.
MATERIALS AND METHODS
The present study was conducted in a tertiary care hospital of Uttar Pradesh, India. The first and second patients with COPD attending medicine or respiratory OPD were selected for this study, and normal healthy individuals without COPD were taken as control from the general population. The controls were randomly assigned, and age and gender matched. The present study was a hospital-based, cross-sectional, observational study conducted from August 2013 to August 2014.
Inclusion criteria
All patients diagnosed to have COPD by pulmonary function tests were included in this study. The severity of COPD in these patients was determined by pulmonary function tests, and global initiative for obstructive lung diseases (GOLD) criteria was used for staging. The patients were required to consent for this study, and our population consisted of individuals between 30 and 80 years.
Exclusion criteria
Those patients who were not consenting for the study or who had a primary psychiatric disorder except for substance abuse disorder (tobacco) diagnosed by Mini International Neuropsychiatric Interview (MINI) version 6.0.0 were excluded from this study. Individuals who had a comorbid medical condition (congestive heart failure, diabetes mellitus, hypertension, and cerebrovascular accident) or those taking oral corticosteroids were also excluded from the study. Patients who presented with an acute exacerbation were excluded from the study.
Procedures
The study was approved by the ethical and research committee of King George's Medical University, and the selected patients were briefed about the study. The study was designed as a cross-sectional case–control study and the enrolled patients were subject to a detailed clinical history and physical examination. In the clinical history, duration of COPD with history of complications and treatment were elicited. History of the presence of risk factors such as smoking, hypertension, and diabetes mellitus was enquired. After that, detailed physical examination was carried out which included general examination and systemic examination. The patients were subjected to routine investigations such as complete blood count, serum electrolytes, renal function tests, liver function tests, viral markers, and chest X-ray. The pulmonary function test was done using RMS Helios 702 Spirometer (Recorders and Medicare Systems Pvt. Ltd., MEDSPIROR, India). The following parameters were obtained from the test: FEV1, forced vital capacity (FVC), FEV1/FVC ratio. FEV1 and FVC and the FEV1/FVC ratio were the main parameters used to stage the COPD patients, according to GOLD guidelines. Postbronchodilator spirometry (salbutamol 2.5 mg by nebulization) was performed in all the patients to exclude the diagnosis of bronchial asthma.
Psychiatric comorbidities were assessed by the following questionnaire
The MINI is a short structured diagnostic interview, developed jointly by psychiatrists and clinicians in the United States and Europe, for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision and International Classification of Diseases, Tenth Revision psychiatric disorders. It was designed to meet the need for a short but accurate-structured psychiatric interview for multicenter clinical trials and epidemiology studies and to be used as a first step in outcome tracking in nonresearch clinical settings. The MINI is divided into modules identified by letters each corresponding to a diagnostic category. All questions must be rated. Rating is done to the right of each question by circling either yes or no. When necessary, the clinician should ask for examples, and the patient should be encouraged to ask for clarification when needed. We have used MINI version 6.0.0 in our study.
Statistical analysis
This is a cross-sectional, case–control study in which prevalence of psychiatric comorbidities in COPD was evaluated.
RESULTS
Baseline characteristics
A total of 200 subjects were enrolled in this study. Of these, 74 were normal healthy volunteers, and 126 were COPD patients diagnosed clinically. Of these, 52 were excluded by PFT analysis. Age of the patients (n = 74) ranged from 38 years to 76 years. The mean age of cases was 56.97 ± 10.11 years when compared with 54.92 ± 9.09 years for controls. The percentage of males in cases and controls was equal at 77%. A majority of cases (62%), as well as controls (55%), were from a rural background.
Risk factors for chronic obstructive pulmonary disease
A significant difference was found with regards to cigarette smoke exposure [Table 1]. Seventy-one percentage of cases were smokers as compared to 8% in controls. The mean pack years of smoking were significantly higher in cases (24.32 ± 12.15) as compared to controls (6.5 ± 2.74). Among smokers, a significant proportion of cases (77%) had quit smoking. The exposure to biomass-based smoke (Chulha) was significant as a risk factor in the causation of COPD as 13.5% of the cases chiefly females had an exposure history as compared to none in the controls.
Table 1.
Smoking history of study population
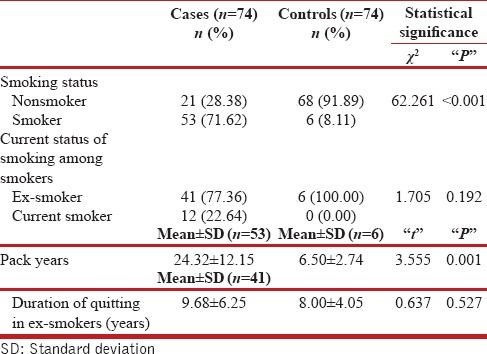
Characteristics of cases
The duration of symptoms was <5 years in most of our cases (77%) with the mean duration of 4.94 ± 3.78 years. The mean FEV1 in our cases was 38.18% ±13.67% compared to 89.89% ±1.32% in controls. The mean FEV1/FVC ratio in our cases was 56.74% ±9.20% as compared to 96.85% ±2.57% in controls. A majority of our patients (60.8%) had severe COPD as per GOLD criteria.
Chronic obstructive pulmonary disease and psychiatric comorbidity
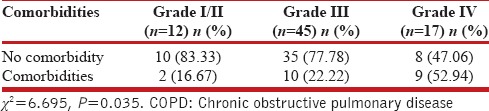
The overall frequency of psychiatric comorbidities was 28.4% as compared to 2.7% in controls [Table 2]. The frequency was the highest for substance abuse disorder with a prevalence of 16%. The gender-based difference in the frequency of psychiatric comorbidities in COPD patients was statistically significant for substance abuse disorder which was more prevalent in males as compared to females with a P = 0.039. The frequency of psychiatric comorbidities in COPD patients increased with the severity of COPD [Table 3]. The frequency of comorbidities in Grade IV COPD was 53% as compared to 16.67% in Grade I/II (P = 0.035). The frequency of psychiatric comorbidities was also affected by the duration of symptoms. In patients with duration of symptoms <5 years, the frequency was 22.8% compared to the frequency of 66.67% in patients with symptoms more than 10 years (P < 0.001). The relationship of the frequency of psychiatric comorbidities with respect to the number of exacerbations per year was not significant, although the frequency was higher in those with symptoms more than 10 years history compared to none in patients with ≤2 exacerbations per year.
Table 2.
Psychiatric co-morbidity as per MINI version 6.0.0
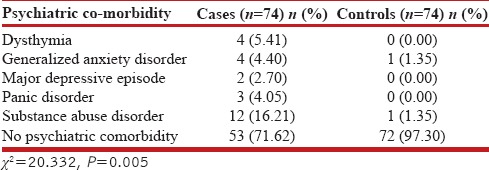
Table 3.
COPD severity and psychiatric comorbidities in cases (n=74)
DISCUSSION
Psychiatric comorbidities are more prevalent among COPD patients than among healthy subjects. In our study, 71.6% of cases were smokers compared to 8.1% in controls (P < 0.001). In a study in Northern Sweden by Lindberg et al., the prevalence of COPD among men who were either current or ex-smokers was 89%.[7] The prevalence of COPD among males who had a positive smoking history in our study was 91%, and these findings go hand in hand with the study by Lindberg et al. The mean pack years in cases was 24.32 ± 12.15 while in controls it was 6.5 ± 2.74 (P = 0.001). These findings of ours are consistent with smoking being a risk factor in pathophysiology of COPD. Among the smokers, a significant proportion of cases (77%) had quit smoking. The history of smoking was predominantly found in males with only a single female case having a positive history of exposure to tobacco smoke.
A statistically significant difference in Chulha (biomass based fuel) exposure was found (P = 0.001). In our study 13.5% of cases had a positive history of exposure to Chulhas utilizing biomass fuels. This finding corroborates with Chulha exposure utilizing biomass fuels being a risk factor in the causation of COPD. The study by Hu et al. observed that people exposed to biomass smoke have an odds ratio of 2.44 (95% confidence interval, 1.9–3.33) for developing COPD, relative to those not exposed to biomass smoke.[8]
In our study, cases had psychiatric comorbidity of 28.4% as compared to 2.7% in controls (P = 0.005). In an Indian study conducted by Sharma et al., the prevalence of psychiatric comorbidity was estimated to be around 44.8% as compared to 24.3% in controls. The lower frequency of psychiatric comorbidities in our study could be explained by the fact that we have used a different tool for the diagnosis of psychiatric comorbidities (MINI), compared to the use of Global Mental Health Assessment Tool-Primary Care version by the study conducted by Sharma et al[9] The lower frequency of psychiatric comorbidities in our study can be due to the fact that we excluded other medical comorbidities and inpatients. It is a well-known fact that the prevalence of psychiatric comorbidities especially anxiety and depression increase with other medical illnesses.
The most frequent psychiatric comorbidity in our patients was substance abuse disorder with a frequency of 16.21%. Among the substance abuse disorders, the highest frequency was for abuse of nontobacco products such as cannabis. This finding can be explained by the fact that most of our cases had a positive smoking history, and many of the patients were still dependent on tobacco substitutes. In the study by Sharma et al., the most prevalent psychiatric comorbidity was anxiety disorders. This difference is due to the reason that substance abuse disorders were not considered in the study by Sharma et al.[9]
In our study, the frequency of anxiety disorders was 9.5% as compared to 1.4% in controls. The study by Kahraman et al. found that the prevalence of anxiety in COPD patients was 30.7% as compared to 16.4% in controls.[10] The lower frequency of anxiety disorders in our study could be explained by the fact that Hospital Anxiety and Depression Scale-subscale of anxiety was employed in the above-mentioned study whereas we relied on a structured psychiatric tool. In the study by Sharma et al., the prevalence of anxiety disorders was 20.6%.[10] The prevalence rates of anxiety in patients with COPD ranges between 10% and 19% in patients with stable disease.[11] This compares with our findings and consolidates the fact that the prevalence of anxiety disorders is lower in stable COPD patients as compared to patients who have been assessed when in acute exacerbation.
In our study, the frequency of clinically significant depression was 8.1% in cases as compared to 0% in controls. The prevalence of depression as observed by Sharma et al. was 13.2%.[10] However, studies from other countries reported the prevalence of depression in patients with COPD varying from 6% to 56%.[12,13,14] The study by Negi et al. found a prevalence of depression of 33%.[15] There is no standardized approach for the diagnosis of depression in COPD patients because of the differences in the methodology and variability of the screening questionnaires in cut-off points to determine a diagnosis of depression. Hence, the wide range that is observed among different studies. Maurer et al. also found a prevalence of depression in COPD patients to vary from 10% to 42%.[11] The differences were attributed to the differences in sampling and variability in diagnostic instruments and cut-off scores.[16]
The higher prevalence of depression in COPD could be attributed to biological factors like increased levels of 5-hydroxyindoleacetic acid as reported by Sekiduka-Kumano et al.[17] The other possible factors responsible for increased prevalence of psychiatric comorbidities in COPD patients could be psychological factors such as low self-esteem and low self-worth resulting from suffering a chronic medical illness. Social factors such as social isolation and low productivity may also contribute to this increased prevalence.
The gender-based differences in frequency of psychiatric comorbidity in cases were significant in our study for substance abuse disorder with a frequency of nearly 19.3% in males as compared to 0% in females. This finding of ours was statistically significant at a P = 0.047. The gender-based difference in frequency of anxiety disorders was present in our study. About 17.64% of females had anxiety disorders compared to 7.01% of males, although this observation was not statistically significant. This finding though goes in concordance with the study by Laurin et al. in which the prevalence was 1.5 times more common in women as compared to men in cases.[18] In our study, gender was also not associated with depression in COPD patients, which was in accordance with the past studies.[19] Our results showed a slight increase in the frequency of depression in males (7%) as compared to females (5.88%). In India, the male patients had higher likelihood of COPD compared to female; however, in the USA, the rate is reversed.[20,21] Hence, the factors associated with depression could be disease-related factors rather than gender-specific factors.
In our study, the frequency of psychiatric comorbidities increased with the severity of COPD. This finding of ours was statistically significant (P = 0.035).
In our study, there was a significant difference in the frequency of psychiatric comorbidities with durations of symptoms of COPD as evidenced by a P < 0.001. The frequency of psychiatric comorbidities in cases with duration of symptoms more than 10 years was nearly 67%.
Our study showed that the frequency of psychiatric comorbidities increased with the increase in number of exacerbations of COPD per year. This finding, however, lacked any statistical significance with a P = 0.254. The lack of any statistical significance could be explained by the variation in the distribution of cases with a majority having <3 exacerbations. Gudmundsson et al. commented that incompletely or untreated depression and anxiety have major implications on the increased frequency of admissions and prolonged length of stay.[22]
The multivariate analysis for the frequency of psychiatric comorbidities in relation to very severe COPD, duration of symptoms more than 5 years and more than 2 exacerbations in an year had a high specificity (94.3%) but a poor sensitivity of 23.8%.
The strengths of our study are as follows
Our study utilized a structured analytical tool for the estimation of frequency of psychiatric comorbidities in COPD patients
In our study, stable patients who were attending OPD were enrolled. In COPD patients, many of the symptoms are shared with those of anxiety disorders, particularly exaggerated during an exacerbation. Hence, the decision to enroll only stable outpatients for our study.
Limitations of our study are as follows
The study was conducted in a single center
The number of subjects enrolled in our study was small. Hence, this study might need validation on a much larger scale for the exact estimation of prevalence of psychiatric comorbidities
In this study, staging of COPD was made by an older version of GOLD guideline, by spirometry finding alone. Updated gold guideline (2013) mandate inclusion of severity of symptoms by modified British Medical Research Council questionnaire and COPD assessment test and risk of exacerbations.
CONCLUSION
The frequency of psychiatric comorbidities is significantly increased in COPD patients as compared to controls and the frequency increases with the severity and duration of symptoms of COPD. The gender-based differences in the frequency are significant for substance abuse disorder and anxiety disorders. As a primary care physician, it is important to recognize the presence of symptoms suggestive of psychiatric illness and to institute proper treatment for the same.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Jindal SK. COPD: The unrecognized epidemic in India. J Assoc Physicians India. 2012;60(Suppl):14–6. [PubMed] [Google Scholar]
- 3.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18:213–21. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 4.Jain A, Lolak S. Psychiatric aspects of chronic lung disease. Curr Psychiatry Rep. 2009;11:219–25. doi: 10.1007/s11920-009-0034-9. [DOI] [PubMed] [Google Scholar]
- 5.Norwood R. Prevalence and impact of depression in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2006;12:113–7. doi: 10.1097/01.mcp.0000208450.50231.c6. [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58:65–70. doi: 10.1080/08039480310000824. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg A, Bjerg A, Rönmark E, Larsson LG, Lundbäck B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2006;100:264–72. doi: 10.1016/j.rmed.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Hu G, Zhou Y, Tian J, Yao W, Li J, Li B, et al. Risk of COPD from exposure to biomass smoke: A metaanalysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 9.Sharma BB, Singh S, Sharma VK, Choudhary M, Singh V, Lane S, et al. Psychiatric morbidity in chronic respiratory disorders in an Indian service using GMHAT/PC. Gen Hosp Psychiatry. 2013;35:39–44. doi: 10.1016/j.genhosppsych.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Kahraman H, Orhan FO, Sucakli MH, Ozer A, Koksal N, Sen B. Temperament and character profiles of male COPD patients. J Thorac Dis. 2013;5:406–13. doi: 10.3978/j.issn.2072-1439.2013.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, et al. Anxiety and depression in COPD: Current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S–56S. doi: 10.1378/chest.08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan VS, Ramsey SD, Giardino ND, Make BJ, Emery CF, Diaz PT, et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007;167:2345–53. doi: 10.1001/archinte.167.21.2345. [DOI] [PubMed] [Google Scholar]
- 13.Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27:788–94. doi: 10.1183/09031936.06.00130605. [DOI] [PubMed] [Google Scholar]
- 14.Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, et al. The effects of depressed mood on smoking cessation: Mediation by postcessation self-efficacy. J Consult Clin Psychol. 2003;71:292–301. doi: 10.1037/0022-006x.71.2.292. [DOI] [PubMed] [Google Scholar]
- 15.Negi H, Sarkar M, Raval AD, Pandey K, Das P. Presence of depression and its risk factors in patients with chronic obstructive pulmonary disease. Indian J Med Res. 2014;139:402–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Van Ede L, Yzermans CJ, Brouwer HJ. Prevalence of depression in patients with chronic obstructive pulmonary disease: A systematic review. Thorax. 1999;54:688–92. doi: 10.1136/thx.54.8.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekiduka-Kumano T, Kawayama T, Ito K, Shoji Y, Matsunaga K, Okamoto M, et al. Positive association between the plasma levels of 5-hydroxyindoleacetic acid and the severity of depression in patients with chronic obstructive pulmonary disease. BMC Psychiatry. 2013;13:159. doi: 10.1186/1471-244X-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurin C, Lavoie KL, Bacon SL, et al. Sex differences in the prevalence of psychiatric disorders and psychological distress in patients with COPD. Chest. 2007;132(1):148–155. doi: 10.1378/chest.07-0134. [DOI] [PubMed] [Google Scholar]
- 19.Cleland JA, Lee AJ, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract. 2007;24:217–23. doi: 10.1093/fampra/cmm009. [DOI] [PubMed] [Google Scholar]
- 20.Jindal SK. Emergence of chronic obstructive pulmonary disease as an epidemic in India. Indian J Med Res. 2006;124:619–30. [PubMed] [Google Scholar]
- 21.Helms SL, Ni FZ, Darbishire PL. COPD: Investigating the gender paradigm shift. [Last accessed on 2012 Jul 12];US Pharmacist. 2011 36:HS-30–HS-34. Available from: http://www.uspharmacist.com/content/d/health%20systems/c/29854/ [Google Scholar]
- 22.Gudmundsson G, Gislason T, Janson C, Lindberg E, Suppli Ulrik C, Br, BrC E, et al. Depression, anxiety and health status after hospitalisation for COPD: A multicentre study in the Nordic countries. Respir Med. 2006;100:87–93. doi: 10.1016/j.rmed.2005.04.003. [DOI] [PubMed] [Google Scholar]


