Abstract
Cystic echinococcosis (CE) is a zoonotic parasitic disease caused by the larval stages of the cestode Echinococcus granulosus. Worldwide, pulmonary hydatid cyst is a significant problem medically, socially, and economically. Surgery is the definitive therapy of pulmonary hydatidosis. Benzimidazoles may be considered in patients with a surgical contraindication. This review will focus on pathogenesis, lifecycle, clinical features, and management of pulmonary hydatid disease.
KEY WORDS: Benzimidazoles, cystic echinococcosis, Echinococcus granulosus, water-lily sign
INTRODUCTION
Human echinococcosis, also known as hydatid disease, is a zoonotic disease of worldwide distribution caused by the larval stage (metacestode) of the parasite belonging to the family Taeniidae and genus Echinococcus. Four species have been recognized to cause public health concerns. Echinococcus granulosus (E. granulosus) causes cystic echinococcosis (CE) and is the most common species to cause the human disease. Although Echinococcus multilocularis (E. multilocularis) is rare, it is the most virulent species and causes alveolar echinococcosis (AE). Echinococcus vogeli (E. vogeli) and Echinococcus oligarthrus (E. oligarthrus) cause polycystic echinococcosis.[1] Hydatid disease is a major zoonotic disease of significant public health and economic impact. CE is the most common presentation in human, contributing to more than 95% of the estimated 2–3 million global cases.[2] CE is endemic in many parts of the world, particularly the Mediterranean countries, Central Asia including the Tibetan Plateau, Northern and Eastern Africa, Australia, and South America.[3] Global burden of the human AE is approximately 18,235 new cases per annum with the majority (91%) occurring in China.[4] The impact of the disease on a human has been assessed by the disability-adjusted life years (DALYs) and the economic impact.[5] The socioeconomic impact of CE is enormous, and the calculated global burden in terms of DALYs is 1009,662 when under-reporting was taken into account. In terms of monetary loss, it stands at a staggering figure of USA $763,980,979.[6] The impact of AE is also enormous with a median of 666,434 DALYs per annum.[4]
LIFE CYCLE
Certain occupations pose an increased risk of CE such as slaughterers, tanners, stockbreeders, shepherds, butchers, and veterinarians or any job which makes a person to work closely with animals.[7] Adult E. granulosus is a tiny cestode of 3–6 mm long. The lifecycle of cestodes involves 2 or more hosts-intermediate host where immature parasite lives and definitive host which harbors the mature parasite. Definitive hosts in E. granulosus are carnivores such as dogs and wolves. Adult worm resides in the small intestine of the definitive hosts. Released eggs are ingested by intermediate hosts, such as sheep, goat, swine, cattle, horses, and camel. Eggs can survive for at least 1 year in the environment.[8] Ingested eggs hatch in the small intestine and release oncosphere, which penetrates the intestinal mucosa and enters the liver, lungs, or other organs via the blood or lymph, where they mature into hydatid cyst (metacestode larvae). The life cycle of the tapeworm is completed after the definitive host ingests the cyst containing organs of the infected intermediate host. Protoscolices evaginate in the intestinal mucosa and develop into adult worms in 32–80 days.[9] Human exposure occurs via the fecal-oral route, with ingestion of food or water contaminated by the feces of an infected definitive host, usually dogs. Humans act as the dead end for the parasite as they are not able to transmit the disease further. Echinococcus lifecycle occurs in two settings: Domestic and sylvatic form. Sheep is the most common intermediate host in the domestic cycle. The sylvatic cycle occurs in the wilderness of Alaska, Canada, North Scandinavia, and the former Soviet Union[10] where either wild dogs or wolves act as definitive hosts and moose and caribou act as intermediate hosts. It may involve the domestic cycle also,[1] and if it infects human, it may show a giant pulmonary cyst.[10] Definitive hosts of E. multilocularis are foxes and intermediate hosts include mice, rats, hamsters, gerbils, and squirrels. It is endemic in the Northern Hemisphere and China.
PATHOGENESIS
Hydatid disease involves the lungs by various mechanisms.[11] Human acquires the infection by the ingestion of eggs mixed with uncooked vegetables, fruits, and drinking water, or by handling the soil and dirt or by direct contact with animal hair containing eggs. Gastric and enteric digestion of eggs facilitates the release of embryos. Primary infestation is the result of the direct evolution of the hexacanth embryo. Embryos subsequently attach to the duodenal or jejunal wall by their hooklets and penetrate the intestinal wall. They reach the liver via the portal circulation. Most of the embryos are stuck in the liver sinusoids but embryos with diameters <0.3 mm may pass through the hepatic sinusoids and, through the hepatic vein and the inferior vena cava (IVC) enter the right heart and, finally, settle in the lungs.[12] Embryos can also reach the lungs via another route. They enter the thoracic duct via lymphatics of the small intestine and then through an internal jugular vein, and right side of the heart, and they enter the lungs.[13] This is a major pathway by which the hydatid larva bypasses the liver and involves the lungs. Another possible lymphatic pathway is the lymphatics of the dome of the liver and the diaphragm, which ascend to the parasternal and intercostal lymph nodes.[14] The third possible route is a venal-venous anastomosis in the liver and the space of Retzius. Another possibility is direct pulmonary exposure through the inhalation of air contaminated with Echinococcus eggs. Borrie et al. has shown that eggs administered to sheep via a tracheostomy resulted in the development of lung cysts.[15] Transdiaphragmatic dissemination via a bronchobiliary fistula due to transdiaphragmatic rupture of liver hydatid, and this mechanism may explain the simultaneous hepatopulmonary involvement. If embryos bypass the lungs, they can disseminate to the systemic circulation. The embryo gradually transforms into a hydatid cyst. Lungs may also become the site of secondary hydatidosis due to the rupture of a primary cyst resulting in dissemination of multiple daughter cysts and scolices. The secondary cyst may develop within the lung parenchyma, an adventitial layer of the primary cyst, intrabronchial, or metastatic.[11] Metastatic lung lesions develop due to rupture of the cyst into right heart chamber,[16] IVC,[17] or rupture of a bone cyst.[11]
HYDATID CYST STRUCTURE
Hydatid cyst is the cystic space-occupying lesion caused by the larval stage of the parasite Echinococcus. It can occur in any organ but is most commonly seen in the liver and lungs with respective frequencies of 60% and 20–30% of all cases.[18] Strictly speaking, hydatid cysts are only seen with E. granulosus, E. vogeli, and E. Oligarthrus. The lesion from E. multilocularis is called a “larval mass.” These are multiple irregular chambers intermixed with the host fibrous reaction and liver parenchyma.[19]
A hydatid cyst consists of three layers with hydatid fluid inside. The outermost layer is called the pericyst, ectocyst, or adventitial layer. It is formed by the host tissue's reaction to parasites. The next layer is the laminated membrane, or exocyst, and the innermost layer is known as the germinative layer or endocyst. The laminated layer is impermeable to bacteria, therefore, its rupture can cause secondary bacterial infections. The germinal layer is the most active layer of the cyst. The germinal layer produces laminated membranes outward and brood capsules and protoscoleces inward. A brood capsule is formed by inward budding of the germinal layer of the hydatid cyst.[19] It is a fluid-filled vesicle-like structure containing protoscoleces. Protoscoleces are the infective form and will grow to the adult stage after ingestion by a definitive host. The carbohydrate-rich laminated layer is formed from the germinal layer and functions to maintain the physical integrity of the hydatid cyst.[20] It also shields the germinal layer from host immune attack.[20] The hydatid fluid is colorless, odorless, and sterile.[13] Its electrolyte level and pH are similar to that of human serum with a density of 1.007–1.015[11] and an intracystic pressure of 36.6 cm H2O (range: 21–61 cm H2O).[21] Hydatid fluid is antigenic; it can cause an anaphylactic reaction when the cyst ruptures.
GROWTH OF THE CYST
The growth rate of the hydatid cyst depends on the softness of the organ and surrounding tissue elasticity.[13] Lung cysts grow faster than the liver cysts, as lungs are softer in consistency than liver. Negative pleural pressure may further accelerate the growth rate of the cysts.[22] Due to their more elastic lung tissues; cysts in children grow at a faster rate and become larger than in adults.[23] The diameters of the cysts grow at a variable rate. Bloomfield et al. reported a doubling time of 16–20 weeks.[24] Borrie reported that a hydatid cyst usually achieves 1–2 cm in diameter at the end of 6 months and up to 6 cm. in diameter within a year.[25] Various cysts in the same patient can show different growth rates. Romig et al.[26] reported that the majority (86%) of the cysts had an increase of <40% in 1 year. Cysts remained static, collapsed, or disappeared in 34% of cases.
GENETICS IN HYDATIDOSIS
E. granulosus is a complex parasite that exhibits genetic diversity. Molecular genetic analysis, using mitochondrial cytochrome C oxidase subunit 1 and NADH dehydrogenase 1 genes,[27] has identified 10 genotypes of E. granulosus to date. Recently, the 10 genotypes have been clustered into four different species: E. granulosus sensu stricto (G1–G3 complex), Echinococcus equinus (G4), Echinococcus ortleppi (G5), and Echinococcus Canadensis (G6–G10 complex).[28,29] Globally, the G1 genotype of E. granulosus has been found to infect humans more frequently than other species or strains.[24] Sharma et al.[30] demonstrated that the G1 (sheep strain) and G3 (buffalo strain) genotypes of E. granulosus are the predominant strains infecting humans in India. They also reported the first human CE case with the G5 genotype (cattle strain) in any Asian country. Genotyping of human CE is helpful, as it provides information on the transmission patterns of the parasite and data on the predominant prevalence of particular genotypes in humans. This data can provide valuable information in planning control strategies for human hydatidosis. Genotype diversity also explains the variability in development, antigenicity, pathologic patterns, and sensitivity to chemotherapeutic agents.[31,32,33]
CLINICAL FEATURES
The majority of patients had a longstanding history of contact with domestic animals (dogs) and/or farming animals (sheep). The clinical features of hydatid cysts depend on the site and size of the cyst and the presence of complications. Uncomplicated small, peripherally located cysts often remain asymptomatic and are discovered incidentally on chest radiography. Shehatha et al. reported asymptomatic cysts in 37% of 763 cases.[34] Clinical symptoms occur when the cysts grow large enough to exert mechanical effects on the adjacent structures or to develop complications.[18] Usually, cysts larger than 5 cm. in diameter may cause bronchial compression. Complications of pulmonary hydatid cysts include rupture, secondary infection, pneumothorax, and suppuration. Patients may develop sudden onset of chest pain, cough, fever, and hemoptysis after a cyst ruptures. Perforation of the cysts into a bronchiole and the resulting expectoration of the germinative membrane or the hooklets of the parasite are called hydatoptysis. Sometimes patients may also complain of a salty taste in the mouth, indicative of a ruptured pulmonary hydatid cyst. Another important feature of rupture is the development of hypersensitivity reactions ranging from urticaria and wheezing to anaphylaxis.[35,36] The presence of the parasites themselves can also produce a generalized toxic reaction.[37] A cough is the predominant symptoms in the majority of series. Darwish et al.[38] reported a cough in 54% of 206 patients with pulmonary hydatidosis. In another large series of 445 patients (383 adults and 62 children), cough was the predominant symptom detected in 79% of adults and in 93% of children.[39] The cough develops in pulmonary hydatidosis either due to bronchial irritation by the growing cyst or by direct rupture of the cyst into the bronchus. Other symptoms of pulmonary hydatid cysts include chest pain, breathlessness, expectoration, fever, hemoptysis, and anaphylactic phenomena.[40] Hemoptysis is more common in adults than in the pediatric population. Singh et al. reported hemoptysis as a presenting symptom in up to 70% of adult patients, but it is rare in pediatric patients.[41] The mechanism of hemoptysis in pulmonary hydatid disease includes pressure erosion of a bronchus, obstruction with secondary bronchial infection, or rupture of the cyst into the bronchus. Hydatid cysts can also erode major vessels, such as the aorta, and can cause massive hemoptysis.[42] In endemic areas, we should have a high degree of clinical suspicion and anybody with a chronic cough should have a screening chest X-ray and possibly an abdominal ultrasound. Liver cysts should be searched in all cases of pulmonary cysts for two reasons. First, the co-existence of pulmonary and hepatic hydatidosis is high, varying from 18.7% to 23.1% [Table 1]. Second, hepatic cysts can be asymptomatic. Kavukcu et al.[43] have shown that patients with only pulmonary cysts had a significantly higher mean age (33.1 ± 14.9 years) than patients with hepatopulmonary involvement (29.6 ± 11.7 years). Complicated cysts included perforation, infection, and lung cysts with pleural complications and calcification of cysts.[44] The most serious complication of perforated cysts is a secondary bacterial infection. Infection increases the density above 20 Hounsfield units, resulting in difficulty in differentiation from an abscess or neoplasm.[45]
Table 1.
Various epidemiological studies of cystic hydatid disease
ANATOMICAL LOCATION
The most common organ involved in an adult is the liver followed by the lungs with respective frequencies of 60% and 20–30%.[18] The right lung is more commonly involved than the left.[46,47] Cysts in the lungs are usually solitary and mostly unilateral. Arinc et al.[48] reported unilateral cysts in 82.9% of cases. Similarly, Ghoshal et al.[49] reported unilateral single cysts in 81.13% cases. Lower lobe of the lungs is the most common site of pulmonary involvement, and there is a predilection for the posterior segments and the right lung, although Sadrizadeh et al. reported left lower lobe predominance.[50] About 60% of cases occur in the lower lobes. Bilateral involvement occurs in 20% of cases, and multiple cysts in 30% of cases.[51] There are certain unique characteristics of the pediatric hydatid cyst. Unlike an adult, lung involvement is more common than liver in the children, with frequencies of 64% and 28%, respectively.[52,53] Concomitant hepatic involvement is more common in adults than in children. Kanat et al. in a retrospective study reviewed the medical records of 145 patients with hydatid disease hospitalized over the last 10 years. They found a concomitant hepatic cyst in 79% of adults as compared to 33% of children.[54] Therefore, isolated pulmonary cysts are more common in children.
DIAGNOSIS
Diagnosis of hydatid cysts in endemic areas is suspected based on the presence of pulmonary cysts with a history of exposure to sheep and dogs. Radiology and serology are the principal diagnostic modality used to confirm the diagnosis. Peripheral blood eosinophilia is present in <25% of infected persons.[9] Leukocytosis and increased erythrocyte sedimentation rate are also observed. Peripheral blood eosinophilia and leukocytosis are more common in cases of ruptured cysts.[55] However, all these routine investigations are nonspecific and can be elevated in various conditions.
IMAGING TECHNIQUES
Chest imaging is the principal investigational modality for pulmonary hydatid cyst. Conventional X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) of the lungs are the various modalities useful in the diagnosis of thoracic hydatid cyst. Radiological presentation depends on whether the cysts are complicated or not.
The classical chest radiography of an intact cyst is a sharply
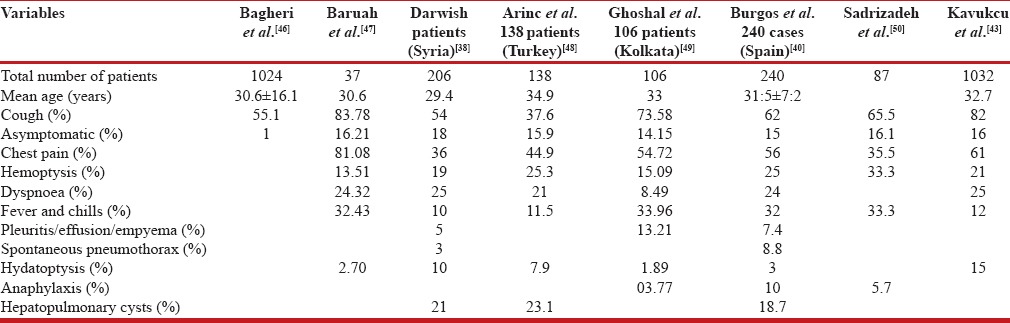
defined, round-to-oval homogenous opacity of variable size. The diameter of the thoracic hydatid cyst varies from 1 to 20 cm.[56] Centrally located cyst is smaller compared to peripherally located cyst as the major broncho-vascular structures in the central part of the lung prevents expansion of the centrally located cyst.[57] The cyst may become bizarre shaped by the pressures from adjacent broncho-vascular structures, mediastinum, pleura, and adjacent cysts. Balikian et al. reported bizarre polycyclic form as the most common shape seen in 40% of the cysts.[56] Adjacent relatively rigid anatomical structures may also produce a depression or indentation at the site of contact, radiologically known as the notch sign shown in Figure 1. The shape of the cyst may change radiologically during maximal inspiration and expiration known as the Escudero-Nimerov sign.[56] Pulmonary hydatid cysts may be single or multiple [Figure 2]. The single cyst is more common than multiple cysts. Ghosal et al.[50] reported single cyst and multiple cysts in 81.13% and 17.92% cases, respectively. Multiplicity depends on the number of the ova ingested and the number of embryos filtered via the liver and the lungs.[56] Multiple cysts when present are variable in diameter and can mimic malignancy.
Figure 1.
The notch sign. Chest radiology (posterior-anterior view) showing notch on the outer border of the left lung mass
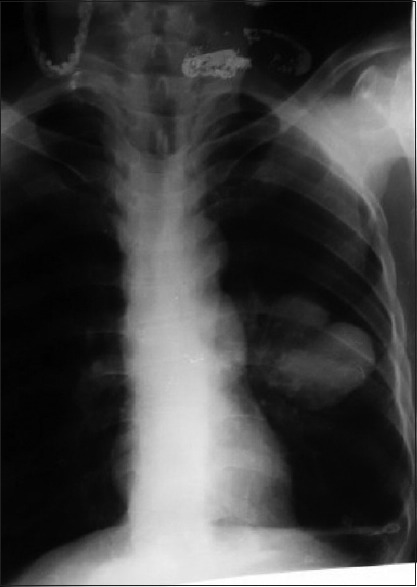
Figure 2.
Multiple hydatidosis lungs. Computed tomography thorax (mediastinal window) showing multiple fluid-filled cystic lesion along the mediastinal and costal pleura and lung parenchyma
Unlike extrapulmonary cysts, pulmonary hydatid cysts do not undergo calcification, and daughter cyst formation is also rare.[58,59] However, pericardial, pleural, and mediastinal cysts may calcify.[60] Low carbon dioxide content within the lung parenchyma reduces the serum calcium level despite the presence of sufficient phosphorus released by tissue necrosis for calcium precipitation. Pulmonary hydatid cyst can develop various local complications including rupture, secondary infection, host tissue reactions, and other complications.
Rupture
It is the most frequent complication of pulmonary hydatid disease occurring in 49% patients.[59] Rupture of the cyst may lead to significant clinical and radiological consequences. The cyst may rupture intrabronchially or into the pleural cavity. Spontaneous rupture may ensue due to the continuous production of hydatid fluid resulting in a rise in the intracystic pressure.[61] There are three types of rupture: Contained, communicating, and direct.[62]
Contained rupture
Endocyst layer is torn with intact pericyst and no intrabronchial communication. Imaging study may show floating membranes within the cyst. There is no risk of secondary bacterial infection as the outer layer is intact. Contained rupture can occur due to trauma, degeneration, or after chemotherapy.
Communicating rupture
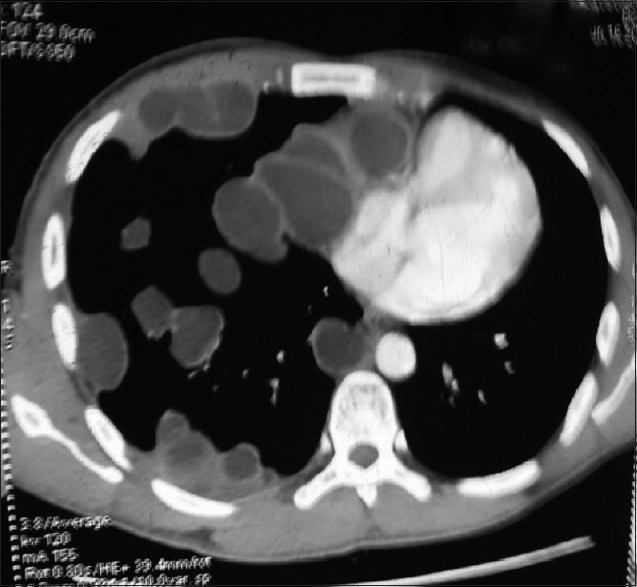
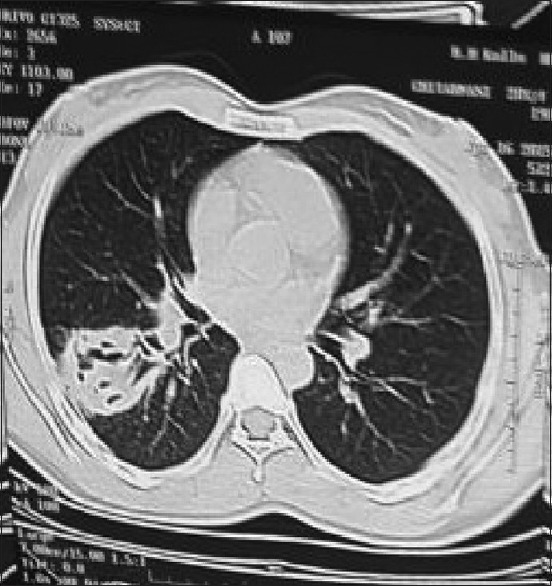
It consists of a tear of the endocyst along with perforation of pericyst by bronchi resulting in the escape of cyst contents via bronchioles. Patients complain of salty fluid in the mouth. Water-lily sign may be seen in partially evacuated cyst Figure 3a and b. Communicating rupture may lead to the transbronchial spread of hydatid disease.
Figure 3.
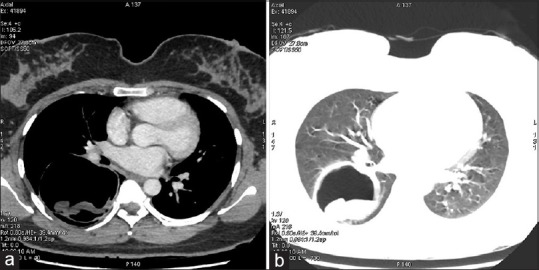
(a) A contrast-enhanced computed tomography scan of the chest (mediastinal window) showing a cyst in the right lower lobe of the lung containing a freely floating endocyst (the “Waterlily sign”). (b) A contrast-enhanced computed tomography scan of the chest (lung window) showing a cyst in the right lower lobe of the lung containing a freely floating endocyst (the “water lily sign”)
Direct rupture
It consists of tear of both the pericyst and endocyst allowing spillage of cyst contents into the pleural spaces. Direct rupture may have serious consequences such as anaphylaxis, secondary infection, secondary hydatid cyst formation, and recurrences after surgery. Peripherally located cyst due to a deficient pericyst and little host tissue support is more prone to direct rupture.[63]
CT thorax has an important role in patients with complicated pulmonary hydatid cyst. It helps in establishing the diagnosis and in differentiating from malignant and pyogenic infections of the lungs. Unruptured cyst is differentiated from other simple cysts by the presence of high attenuation wall at unenhanced CT.[59]
Radiological signs of ruptured pulmonary hydatid cyst:
Air-crescent or meniscus sign
The growth of the cyst may produce ischemic necrosis and erosions by the adjacent bronchioles. It results in air entry between the pericyst and exocyst layer producing a radiolucent crescent in the upper part of the cyst.[64] It signifies detachment, imminent rupture, and death of the parasite.[56,64] Other causes of meniscus sign include cavities with fungus balls and blood clots, carcinoma, pulmonary gangrene, tuberculosis, and sarcomas. Clinically, patient may have cough and hemoptysis.[56]
The “inverse crescent sign” is opposite to classic crescent sign. Here, the crescent shaped rim of air is present at the lower end of the cyst. It signifies separation of the membranes from the posterior aspect of the cyst without any anterior extension.
Water-lily sign or Camelotte sign
Following ruptures partial evacuation of the cyst fluid occurs, and the collapsed endocyst can be seen floating freely in the cyst fluid. It results in unevenness of the air-fluid interface, the so-called water-lily sign or Camelotte sign. Water-lily sign is seen in only 10–15% of lungs hydatid cysts.[65]
Cumbo sign
This is also known as the onion peel sign or double arch sign. As more air enters between pericyst and endocyst, endocyst rupture. It leads air entry inside endocyst and creation of air-fluid level surrounded by a crescent of air between the pericyst and the endocyst.
Air bubble sign
It consists of single or multiple small, rounded radiolucent areas with very sharp margins within solid media or pericystic areas and indicates ruptured infected hydatid cyst. It is best seen in the mediastinal window. Figure 4 is showing the air bubble sign. The air bubble sign has a high sensitivity and specificity. Yuncu et al.[66] has reported 85.7% sensitivity and 96.6% specificity of the air bubble sign. Compared to classical CT signs, the “air bubble” sign is more accurate in the correct diagnosis of ruptured, infected, solid hydatid cysts differentiating it from abscess or tumor. It avoids unnecessary invasive investigations and further delay in the diagnosis. Air enters the hydatid cyst by erosion by a bronchiole. Infection with a gas producing bacteria may also produce air bubbles sign.[67]
Figure 4.
Computed tomography chest (lung window) small air bubbles within the perforated pulmonary cysts named as “air bubble sign”
Signet ring sign
Bleb of air dissects into the wall of the cyst, giving it the shape of a ring. It indicates impending cyst rupture.[68]
Rising sun sign: Daughter cysts are rarely present in the lungs. When present, daughter cysts may appear in the lowest part as circular shadows amidst disintegrated endocyst membrane giving rise to the rising sun sign.[56]
THICK WALL CYST
Thick wall cysts usually about the pleura and are more than 10 mm in diameter. Koul et al. had shown evidence of infection in patients with thick wall cyst.[68]
All hydatid cysts begin as simple cyst and if daughter cysts or matrix (or both) develop they are termed type II cysts. Matrix is a gelatinous structure formed due to degeneration of the endocyst.[16]
The role of ultrasound in thoracic hydatid cyst is limited except when lesions are close to the thoracic wall. They are also useful in detecting concomitant liver involvement.
Figure 5 is showing cystic lesion in the RUL posterior segment with undulating membrane.
Figure 5.
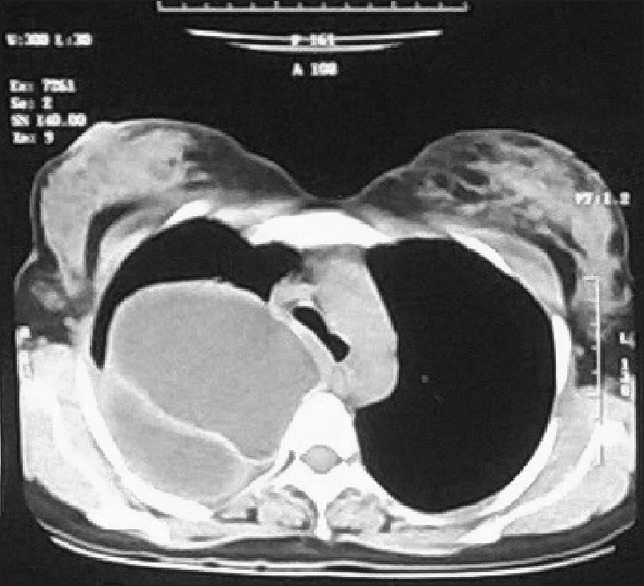
Computed tomography thorax (mediastinal window) showing cystic lesion in the RUL posterior segment with undulating membrane
Positron emission tomography (PET)
Positron emission tomography (PET) scan may show the “Doughnut sign” in a patient with ruptured hydatid cyst. It is characterized by uptake of the tracer along the wall of the cyst with the central photopenic area.[69] Yang et al. had shown differences in FDG uptake patterns depending on the presence or absence of cyst rupture. They demonstrated perilesional uptake on PET only in ruptured cysts.[70]
MRI is not routinely done as it is not superior to thoracic CT scan. MRI scan shows low signal intensity on T1-weighted images and high signal intensity on T2-weighted images of the cyst. MRI can demonstrate different developmental stages of the cyst except small calcifications. MRI is also useful in showing pericystic parenchymal, pleural, and soft tissue reactions. MRI can detect residual parasitic material remaining after surgery and can also show a response to chemotherapy.
Serology testing
Serological testing is useful in the primary diagnosis of hydatid cysts.[71] The majority of the serological tests is based on detecting antibody as it is more sensitive than antigen detection.[72] The enzyme-linked immunosorbent assay (ELISA), the indirect hemagglutination antibody assay (IHT), latex agglutination test, and immunoblot test (IB) are commonly used serological methods. Other serological based methods include the immunofluorescence antibody test and arc-5 immunoelectrophoresis (IEP). Most commonly used antigens are antigen 5 and antigen B.[73] Serological testing; however, have certain disadvantages. They may cause a false-positive reaction in people with other helminthic infections for example E. multilocularis and Taenia solium due to cross-reactions. Other causes include patients with cancer, chronic immune disorders, cirrhosis of liver, and presence of anti-P1 antibodies.[74] Seropositivity depends on the location, number, integrity, viability of the larval cyst, and the assay format.[75,76] The seropositivity rate is higher in hepatic cysts than in pulmonary cysts. The sensitivity of serological testing varies from 85% to 98% in hepatic cysts, and 50–60% in the pulmonary cyst. The sensitivity is higher in patients with multiorgan involvement, 90–100%.[77] False-negative serological tests are more common in intact pulmonary hydatid cysts as the antigens are sequestered within the cysts that may minimize the stimulation of host immunological machinery.[77] Likelihood of seropositivity is more in the case of ruptured cysts. Another limitation of the serological tests is in monitoring patients after surgery or pharmacotherapy as the antibody titer rises at 1–3 months after surgery and starts receding after 3 months. It takes another 12–24 months to become negative.[10]
In patients with initial negative serological testing, serotesting using a battery of different modalities such as IHT, latex agglutination, IEP, or radioallergosorbent testing should be done as a combination of several tests can improve the diagnostic accuracy.[78] Repeat the same serological test at different laboratories can also be helpful as a change in sensitivities can occur due to the batch-to-batch variation in the prepared antigens.[79]
The immunodiagnosis has higher sensitivity in patients with AE as it uses purified or recombinant E. multilocularis antigens. The sensitivity ranges between 91% and 100%, and specificity between 98% and 100%.[80] The problem with native antigens can be overcome by using recombinant antigens and synthetic peptides. Recombinant antigens show higher sensitivity than the native antigens. Moreover, recombinant technology can produce well-defined, standardized antigens in large quantity.[81] EpC1is a novel 32 kDa calcium-binding E. granulosus recombinant antigen, located in the germinal layer of the hydatid cyst, as well as the early protoscolex.[2] Li et al.[82] reported a sensitivity of 92.2% with EpC1 compared with 84.5% with native antigen B. in presurgical CE cases. The rate of cross-reactivity is also low as only 4.5% of AE and 9.3% of cysticercosis cases cross-reacted with EpC1, compared with more than 14% with antigen B. Among various sero-testing, specific IgG ELISA is the most sensitive method and the least sensitive are IEP and specific IgE ELISA. Specific IgE ELISA takes almost 5 years postsurgery to become negative.[83] IB tests using specific echinococcal antigen may be useful to confirm the seroreactivity, but this test is not available widely.[75]
MOLECULAR METHODS
Echinococcus-specific polymerase chain reaction (PCR) are useful in the diagnosis by identifying the parasite materials in the biological specimens resected or biopsied from patients. They are also useful in assessing the viability of parasite after chemotherapy.[84] Reverse transcription PCR is used to assess the viability of parasite following chemotherapy.
Diagnosis of hydatid cysts can be confirmed in seronegative individuals by demonstrating protoscolices/hydatid membranes or specific antigen also. Patients with ruptured pulmonary cysts may have protoscolices detected in the sputum or bronchial washings.
Fiber-optic bronchoscopy
Bronchoscopy is not required in patients with pulmonary hydatid cysts with a typical clinico-radiological feature. However, fiber-optic bronchoscopy (FOB) is indicated in patients with atypical clinical and radiological features, when there is a suspicion of another diagnosis.[85,86] The most specific finding in FOB is the presence of a whitish yellow or white gelatinous membrane. FOB can help in taking a biopsy from a section of the membrane or bronchial aspirate can be analyzed for the presence of hooklets.[86] Komurcuoglu et al. performed the bronchoscopy in 34 patients with pulmonary hydatid disease and they demonstrated endobronchial cyst membrane in 7 (20%) patients. The diagnostic efficacy was 20.5%.[87] However, caution should be advised while doing bronchoscopy as it may provoke cyst rupture.
Treatment of pulmonary hydatid cyst
Pulmonary hydatid cysts is treated by pharmacotherapy and/or surgery. Surgical intervention is the treatment of choice though pharmacotherapy may also be useful in selected patients. Medical therapy of pulmonary hydatid cyst includes benzimidazoles group of drugs, for example, mebendazole (MBZ) or albendazole (ABZ). Indications of chemotherapy include smaller cysts, patients with contraindication for surgery: Poor surgical risk, refusal for surgery and multiorgan disease, multiple cysts, recurrent cysts, and patients with intraoperative spillage of hydatid fluid.[36,88] ABZ is preferred, as it has better bioavailability, can be used with lower doses and is more effective than MBZ.[89] ABZ also achieves a higher plasma and intracystic drug concentration. Its plasma concentration is 10–40 times higher than that of MBZ.[90] ABZ requires a minimum contact period of 11 days to have a significant response.[91] The usual recommended a dosage of ABZ is 10–15 mg/kg/day, taken twice daily, and that of MBZ is 40–50 mg/kg/day, thrice daily. Fat rich meals increase the bioavailability of the drugs.[92] The optimal duration of pharmacotherapy in pulmonary hydatidosis is not known, but it should be given for a minimum period of 3–6 months.
In the past, the drug was given in several 1-month courses interrupted by 14-day intervals. The interval was given to avoid hepatotoxicity.[93] However, studies have shown that continuous therapy is more efficacious than cyclic therapy with no increase in adverse effects.[92,94] Continuous therapy is more efficacious, as it achieves higher drug levels on a sustained basis and higher penetration into the parasitic mass. Moreover, with interrupted therapy, metacestodes may have a chance to recover gradually during the 14-day drug-free interval.[95] Limited long-term toxicity data in the early days of use may be the reason for cyclic therapy in the past.[96] The active metabolite of ABZ with larvicidal action is ABZ sulphoxide.[97] ABZ has poor water solubility, and fatty meals increase the bioavailability of the drug by 4–8-fold.[98] Benzimidazoles act by inhibiting microtubular assembly within the parasite, leading to impaired glucose absorption through the wall of the germinative cell layer of the larva. It results in glycogen depletion and degeneration of the endoplasmatic reticulum and mitochondria of the parasite, along with an increase in lysosomes and subsequent autolysis of cells.[99]
Randomized controlled trials
Keshmiri et al.,[100,101] in a placebo-controlled trial, demonstrated a better response to chemotherapy in cases of pulmonary and abdominal cysts than in cases of liver cysts. Various adverse effects reported are hepatotoxicity, bone marrow suppression, and alopecia. Both drugs are teratogenic in animal studies, so should be avoided during pregnancy (especially during the first trimester).[102] Bone marrow suppression is probably the class effect of the benzimidazole compounds. Alopecia is reported during long-term treatment with ABZ and is reversible after stoppage of the drug.[94] The response to ABZ is variable, depending on the size, number, age, structure, and localization of the cyst(s). Small cysts, multiple cysts, younger cysts with thin walls and/or high metabolic activities, and cysts without daughter cysts inside the mother cyst, show the most favorable responses.[103] The response is also better in young patients.[104] Variable response to chemotherapy is due to different strains or different intrinsic sensitivities of the cyst.[105,106] Keshmiri et al. reported good therapeutic response to ABZ in 91% of patients with pulmonary hydatidosis.[101] Messaritakis et al.[107] found a correlation between the efficacy of benzimidazoles and the size of the cyst with pulmonary cysts having a diameter of 4–5 cm responding better to MBZ treatment than larger cysts. Larger and older cysts show poor response as these cysts tend to have thicker adventitia, resulting in poor drug penetration.[108]
The role of praziquantel (PZQ) is less clearly defined in the treatment of hydatid cyst. PZQ is an isoquinoline that is highly effective against a variety of cestodes and trematodes. The standard dose of PZQ is 40 mg/kg once a week concomitantly with benzimidazoles.[92,109]
PZQ is a highly effective protoscolecidal agent, but it penetrates poorly into the mature hydatid cyst, and, therefore, does not inhibit cyst growth. At present, there is insufficient data for the routine use of combination therapy using ABZ and PZQ, but they can be used as an alternative to surgery in disseminated and inoperable cases.[110]
Contraindication of pharmacotherapy
Large cysts that are at risk of rupture
Inactive or calcified cysts
Bone marrow depression
Pregnancy, specifically the first trimester of pregnancy.
Anthelmintic therapy may promote rupture by causing degenerative changes in the cyst wall.[111] The risk is particularly high with large cysts of the lung (>6 cm diameter). Pharmacotherapy induced rupture has been reported as early as day 10 after the initiation of treatment but is mainly seen between the 2nd and 4th week of treatment. It can also be seen 1–2 months after treatment initiation. Therefore, patients on medical therapy should be closely followed up for at least 2 months. At 2 months, the cyst becomes smaller and fibrous and within 3–6 months, all of the empty cysts become fibrotic. Most of the lung cysts disappear by 5–14 months after treatment.[111]
Surgery
The indications for surgery include large cysts that are superficial and likely to rupture, infected cysts, cysts in vital anatomical locations, and cysts exerting substantial mass effect.[72]
The goal of surgical intervention includes removal of the entire cyst while preserving the lung parenchyma as much as possible and without allowing intraoperative spillage. Various surgical techniques available include enucleation, pericystectomy, cystostomy with capitonnage, open aspiration, and lung resection.[112] Prevention of intraoperative spillage can be achieved by placing gauze soaked with a hypertonic saline solution (20%) or povidone-iodine solution.[113]
Enucleation consists of removal of the cyst with its intact germinative membrane. It is suitable for the small pulmonary hydatid cysts <5 cm in diameter with little risk of rupture [Figure 6]. Removal of the cyst is aided by the intrabronchial positive pressure ventilation. Larger cysts should not be treated with this method as there is a risk of rupture.[114] With enucleation, pericyst remains as such; therefore, there is a risk of postoperative air leak and infection. Pericystectomy involves removal of hydatid cyst along with the pericyst. The proponents of this method include: Pericystic cavity may undergo amyloid degeneration and calcification.[115] Second, It can achieve complete removal of the parasite.[116] Opponents include: Pericyst is not a part of the parasite, and its removal may lead to increased risk of airway leak.
Figure 6.
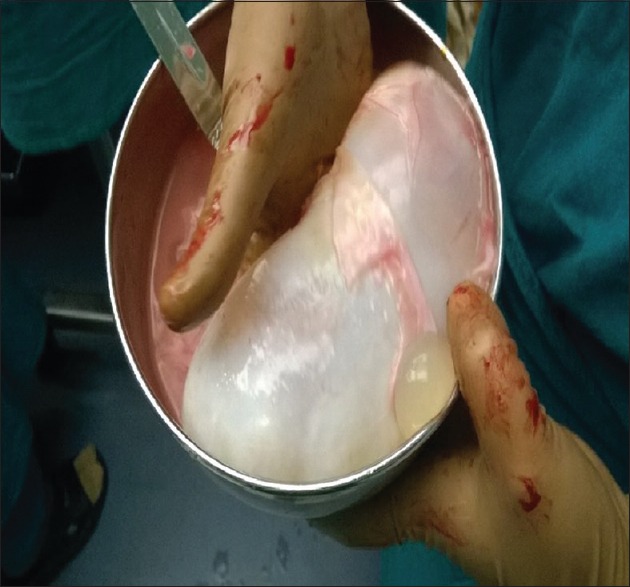
The completely enucleated cyst
Cystostomy with capitonnage is the preferred method of treatment of hydatid cyst. Cystotomy involves aspirating the cystic fluid and removing the germinative membrane (Barret technique). Capitonnage reduces the risk of the infection of the residual cavity, airway leak, and empyema formation, but there is a risk of disfigurement of the lung parenchyma.[117]
Other surgical methods include cystostomy with the closure of the bronchial openings alone. Capitonnage technique is skipped resulting in less disfigurement of the lung parenchyma. Rates of air leak and infection is increased.[118] The main indication of lobectomy include cysts involving more than 50% of the lobe, cysts with severe pulmonary suppuration not responding to treatment, multiple unilobar cysts, and sequelae of hydatid disease such as bronchiectasis, pulmonary fibrosis, or severe hemorrhage.[2]
In the case of multiple cysts, the priority of surgery should be given to cyst based on their susceptibility to rupture, size, and the risk of dissemination. The intact cyst should be treated first in patients with both an intact and a ruptured cyst. Large cysts require definite management of the residual space to avoid postoperative air leak and empyema formation.[114,119] There are various techniques available for the management of residual space. Cystotomy means closing off the bronchial openings only if any. Capitonnage obliterates the cyst cavity by folding the pericystic walls with sutures. It provides additional strength to the lung parenchyma and prevents postoperative air leak and empyema formation. However; studies have shown variable results regarding the role of capitonnage.[114,119,120,121]
Standard posterolateral thoracotomy is the preferred surgical approach. In patients with bilateral cysts, median sternotomy or two-stage thoracotomy can be used. In patients with cysts in different hemithoraces with one of the cysts ruptures, operate the unruptured cyst first owing to the risk of rupture. However, there is a risk of mediastinitis in the case of infected hydatid cysts. Surgery for pulmonary hydatid cysts is a safe procedure with a good outcome, low morbidity, and negligible mortality. Athanassiadi et al. in a meta-analysis involving 4255 patients with pulmonary hydatid disease undergoing surgical intervention reported a mortality rate of 1.45% and a morbidity rate of 0–17% with excellent cure rate.[122] Video-assisted thoracoscopic surgery is useful technique for the removal of superficial and small to moderate hydatid cysts and is associated with less morbidity compared to conventional surgery.[123]
Presurgical use
The role of the presurgical use of benzimidazoles is controversial. The proponent view is that preoperative use of benzimidazoles softens the cysts and reduces the intracystic pressure. It simplifies cysts removal and reduces the risk of secondary echinococcosis and recurrences. Benzimidazoles also reduce the viability of the protoscolex and cyst significantly.[124] However, a recent expert consensus for the diagnosis and treatment of cystic and AE advocates that benzimidazoles should be avoided preoperatively in larger lung cysts due to the risk of rupture.[74] Antihelminthic agents weaken the cyst wall, resulting in cyst rupture. This ruptured cyst later on becomes the source of recurrent infections. Wen et al. have reported the incidence of a cyst rupturing because of ABZ treatment to be 77.3% in 21 patients on ABZ treatment.[125]
Postsurgical follow-up
Postoperative all patients should receive ABZ (10 mg/kg per day) for 6 months to prevent recurrence of the disease.[112] The recurrence rate of pulmonary hydatid cyst without postoperative antihelminthic therapy is as high as 11%.[126] Liver function tests and chest radiography should be done monthly for the first 3 months. The follow-up then continued every 3 months by chest radiography until the end of postoperative year 1.[127]
THERAPEUTIC ASPIRATION
Percutaneous aspiration of pulmonary hydatid cyst has been reported in literature.[128,129] However, percutaneous aspiration is not a routine practice in pulmonary hydatid cyst due to the risk of rupture, dissemination, and anaphylaxis reaction. It is more commonly done in hepatic hydatid cysts.
Giant hydatid cyst
Hydatid cysts may occasionally become very large. There is no universally accepted definition of giant hydatid cyst. However, it is usually defined as a cyst with the largest diameter more than 10 cm.[23,128] Giant hydatid cysts is a distinct clinical entity. It is more common in adolescents and children above 10 years[130] as the immature immune system and higher elasticity of the lung tissues allow more rapid growth of the cyst.[131] Celik et al.[132] reported giant cyst in 15% of 122 childhood cases of lung hydatid cyst, whereas Kosar et al.[119] reported it in 26.7% of 60 cases of childhood pulmonary hydatid cysts. The rate of growth in children ranges from a few millimeters to 5 cm/year.[12,132] Giant hydatid cysts develop more serious symptoms, frequent operative complications, and prolonged medical care.[130] Surgical intervention should be sought as early as possible, particularly in patients with giant hydatid cysts. Keramidas et al. reported complications of medical therapy in children with hydatid cysts with a diameter more than 6 cm. Surgery of the cysts complicated due to therapy was also problematic.[104]
Complications
The cyst that has ruptured into the bronchus or pleural cavity is called a complicated hydatid cyst. They may or may not be infected.[133] Rupture of pulmonary cysts may occur into the bronchus or pleura. Rupture into the bronchus is the most frequent complication of pulmonary hydatid disease.[134] Rupture of the cyst produces an allergic pneumonitis into the surrounding areas. This is reversible up to 10 days and after 10–14 days secondary bacterial pneumonia develops.[135]
Pleuro-pericardial complications
The cyst may also rupture into the pleural or pericardial cavity. Aribas et al. reported pleural and pericardial complications in 43 (29.7%) of 145 patients with hydatid disease. Pleural and pericardial complications were present in 40 (27.6%) and 3 (2.1%) patients, respectively.[136] Pleural involvement can occur in various ways. Primary involvement of the pleura occurs directly via hematological or lymphatic larval seeding. Other mechanisms include late complication of surgery or chest tube insertion during therapy for thoracic hydatid disease. However, the most important mechanism involves the rupture of a pulmonary or hepatic cyst inside the pleural space.[137] Development of secondary pleural hydatidosis is an uncommon event as it occurs in < 10% cases as the cyst becomes infertile due to the secondary bacterial infection.[137] Cysts located in the subpleural area may cause pressure necrosis of the pleura and subsequent risk of pleural complications.[138] Pleural rupture is more common in young age, male sex, and basal locations of the cyst.[139] Pleural and/or pericardial complications are associated with higher morbidity and the need for more radical resection. Therefore, prompt diagnosis and surgical intervention should be carried out wherever indicated to prevent the development of complications.[136]
Anaphylactic reactions
Anaphylactic reactions occur after rupture of cyst either spontaneously or during surgery or by blunt trauma.[140] Anaphylactic shock occurs as the high content of antigens within cyst fluid enters the circulation and activates IgE secretion and histamine release. Patients may develop following symptoms of anaphylaxis, such as rash, edema, bronchospasm, hemodynamic, and respiratory compromise.
Pulmonary embolism
Pulmonary artery involvement by hydatid cysts is very rare. It can occur by several mechanisms. The source of cystic emboli is liver, right cardiac chambers. Liver cyst ruptures into the IVC and through the right heart chambers enter into the pulmonary artery.[141] Another possible source can be the pulmonary cysts which can enter pulmonary circulation by producing breaks in the wall of the pulmonary vessels. The vesicles or the daughter cysts mechanically obstruct the blood flow, and there are no thrombi.[142] The treatment of choice of hydatid cyst embolism is surgery. The risk of surgery includes dissemination of the disease, anaphylactic shock, and pseudoaneurysm formation.
Other uncommon complications include aspergilloma within hydatid cavity,[143] co-existence tuberculosis and hydatid cyst,[144] lung hydatid abscess, persistent cavity, recurrence of daughter cysts, and fibrosis and bronchiectasis.
Prevention
Healthy livestock management practices
Dietary regulation of pet dogs
Education on proper hygiene. Hand washing with soap and warm water should be adopted religiously. Fruits and vegetables should be properly washed before consumption
Treatment of pet dogs in endemic areas with PZ (5 mg/kg) every 45 days
Effective livestock vaccine: Vaccination of livestock for prevention of pulmonary hydatid cyst should be an important goal in management. Recently EG95, a recombinant vaccine has been developed from the oncosphere for use in the parasite's intermediate host(s). It protects the sheep against E. granulosus infection, subsequently preventing human infection.[145] Similarly, Zhang et al.[146] reported significant suppression of worm growth and egg production by vaccinating the dog with the recombinant vaccine. Suppression of egg development would prevent transmission of infection to intermediate hosts.
CONCLUSION
CE is a zoonotic parasitic disease with global existence. Though it can involve any organ, liver and lungs are the most commonly involved organ. Patients remain asymptomatic for a longer period as the cyst grows slowly. Diagnosis is usually based on radiology supported by serological testing. Surgery is the treatment of choice of pulmonary hydatid cyst but in inoperable cases or in cases where surgery is contraindicated, medical therapy with benzimidazoles compounds may be tried. In view of its enormous human impact, prevention should be emphasized particularly in endemic areas. Vaccination has a great potential in containing the transmission of echinococcosis in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mandal S, Mandal MD. Human cystic echinococcosis: Epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac J Trop Med. 2012;5:253–60. doi: 10.1016/S1995-7645(12)60035-2. [DOI] [PubMed] [Google Scholar]
- 2.Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7:385–94. doi: 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- 3.Wahlers K, Menezes CN, Wong ML, Zeyhle E, Ahmed ME, Ocaido M, et al. Cystic echinococcosis in sub-Saharan Africa. Lancet Infect Dis. 2012;12:871–80. doi: 10.1016/S1473-3099(12)70155-X. [DOI] [PubMed] [Google Scholar]
- 4.Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. doi: 10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carabin H, Budke CM, Cowan LD, Willingham AL, rd, Torgerson PR. Methods for assessing the burden of parasitic zoonoses: Echinococcosis and cysticercosis. Trends Parasitol. 2005;21:327–33. doi: 10.1016/j.pt.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006;12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farahmand M, Yadollahi M. Echinococcosis: An occupational disease. Int J Occup Environ Med. 2010;1:88–91. [PubMed] [Google Scholar]
- 8.Santivanez S, Garcia HH. Pulmonary cystic echinococcosis. Curr Opin Pulm Med. 2010;16:257–61. doi: 10.1097/MCP.0b013e3283386282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moro P, Schantz PM. Echinococcosis: A review. Int J Infect Dis. 2009;13:125–33. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Khanfar N. Hydatid disease: A review and update. Curr Anesth Crit Care. 2004;15:173–83. [Google Scholar]
- 11.Ramos G, Orduña A, García-Yuste M. Hydatid cyst of the lung: Diagnosis and treatment. World J Surg. 2001;25:46–57. doi: 10.1007/s002680020007. [DOI] [PubMed] [Google Scholar]
- 12.Aletras H, Symbas PN. Hydatid disease of the lung. In: Shields TW, LoCicero J, Ponn RB, editors. General Thoracic Surgery. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 1113–22. [Google Scholar]
- 13.Halezeroglu S, Okur E, Tanyü MO. Surgical management for hydatid disease. Thorac Surg Clin. 2012;22:375–85. doi: 10.1016/j.thorsurg.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Isitmangil T, Toker A, Sebit S, Erdik O, Tunc H, Gorur R. A novel terminology and dissemination theory for a subgroup of intrathoracic extrapulmonary hydatid cysts. Med Hypotheses. 2003;61:68–71. doi: 10.1016/s0306-9877(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 15.Borrie J, Gemmell MA, Manktelow BW. An experimental approach to evaluate the potential risk of hydatid disease from inhalation of Echinococcus ova. Br J Surg. 1965;52:876–8. doi: 10.1002/bjs.1800521110. [DOI] [PubMed] [Google Scholar]
- 16.Odev K, Acikgözoglu S, Gormüs N, Aribas OK, Kiresi DA, Solak H. Pulmonary embolism due to cardiac hydatid disease: Imaging findings of unusual complication of hydatid cyst. Eur Radiol. 2002;12:627–33. doi: 10.1007/s003300100988. [DOI] [PubMed] [Google Scholar]
- 17.Röthlin MA. Fatal intraoperative pulmonary embolism from a hepatic hydatid cyst. Am J Gastroenterol. 1998;93:2606–7. doi: 10.1111/j.1572-0241.1998.00562.x. [DOI] [PubMed] [Google Scholar]
- 18.Gottstein B, Reichen J. Hydatid lung disease (echinococcosis/hydatidosis) Clin Chest Med. 2002;23:397–408, ix. doi: 10.1016/s0272-5231(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 19.Díaz A, Casaravilla C, Irigoín F, Lin G, Previato JO, Ferreira F. Understanding the laminated layer of larval Echinococcus I: Structure. Trends Parasitol. 2011;27:204–13. doi: 10.1016/j.pt.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Díaz A, Casaravilla C, Allen JE, Sim RB, Ferreira AM. Understanding the laminated layer of larval Echinococcus II: Immunology. Trends Parasitol. 2011;27:264–73. doi: 10.1016/j.pt.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Yuksel M, Kir A, Ercan S, Batirel HF, Baysungur V. Correlation between sizes and intracystic pressures of hydatid cysts. Eur J Cardiothorac Surg. 1997;12:903–6. doi: 10.1016/s1010-7940(97)00266-2. [DOI] [PubMed] [Google Scholar]
- 22.Dincer SI, Demir A, Sayar A, Gunluoglu MZ, Kara HV, Gurses A. Surgical treatment of pulmonary hydatid disease: A comparison of children and adults. J Pediatr Surg. 2006;41:1230–6. doi: 10.1016/j.jpedsurg.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 23.Halezeroglu S, Celik M, Uysal A, Senol C, Keles M, Arman B. Giant hydatid cysts of the lung. J Thorac Cardiovasc Surg. 1997;113:712–7. doi: 10.1016/S0022-5223(97)70228-9. [DOI] [PubMed] [Google Scholar]
- 24.Bloomfield JA. Protean radiological manifestations of hydatid infestation. Australas Radiol. 1966;10:330–43. doi: 10.1111/j.1440-1673.1966.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 25.Borrie J. Fifty thoracic hydatid cysts. Br J Surg. 1962;50:268–87. doi: 10.1002/bjs.18005022107. [DOI] [PubMed] [Google Scholar]
- 26.Romig T, Zeyhle E, Macpherson CN, Rees PH, Were JB. Cyst growth and spontaneous cure in hydatid disease. Lancet. 1986;1:861. doi: 10.1016/s0140-6736(86)90974-8. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez E, Cáceres O, Náquira C, Garcia D, Patiño G, Silvia H, et al. Molecular characterization of Echinococcus granulosus from Peru by sequencing of the mitochondrial cytochrome C oxidase subunit 1 gene. Mem Inst Oswaldo Cruz. 2010;105:806–10. doi: 10.1590/s0074-02762010000600013. [DOI] [PubMed] [Google Scholar]
- 28.Nakao M, McManus DP, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. 2007;134(Pt 5):713–22. doi: 10.1017/S0031182006001934. [DOI] [PubMed] [Google Scholar]
- 29.Thompson RC. The taxonomy, phylogeny and transmission of Echinococcus. Exp Parasitol. 2008;119:439–46. doi: 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Sehgal R, Fomda BA, Malhotra A, Malla N. Molecular characterization of Echinococcus granulosus cysts in north Indian patients: Identification of G1, G3, G5 and G6 genotypes. PLoS Negl Trop Dis. 2013;7:e2262. doi: 10.1371/journal.pntd.0002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson RC, Lymbery AJ. The nature, extent and significance of variation within the genus Echinococcus. Adv Parasitol. 1988;27:209–58. doi: 10.1016/s0065-308x(08)60356-5. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RC, McManus DP. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 2002;18:452–7. doi: 10.1016/s1471-4922(02)02358-9. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RC, McManus DP. Aetiology: Parasites and life-cycles. In: Eckert J, Gemmell M, Meslin FX, Pawlowski Z, editors. WHOI/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: World Organisation for Animal Health; 2001. pp. 1–19. [Google Scholar]
- 34.Shehatha J, Alizzi A, Alward M, Konstantinov I. Thoracic hydatid disease; a review of 763 cases. Heart Lung Circ. 2008;17:502–4. doi: 10.1016/j.hlc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Martínez S, Restrepo CS, Carrillo JA, Betancourt SL, Franquet T, Varón C, et al. Thoracic manifestations of tropical parasitic infections: A pictorial review. Radiographics. 2005;25:135–55. doi: 10.1148/rg.251045043. [DOI] [PubMed] [Google Scholar]
- 36.Morar R, Feldman C. Pulmonary echinococcosis. Eur Respir J. 2003;21:1069–77. doi: 10.1183/09031936.03.00108403. [DOI] [PubMed] [Google Scholar]
- 37.Petrov D, Athanassiadi K. Hydatid disease of lung. Eur Respir Monogr. 2013;61:107–21. [Google Scholar]
- 38.Darwish B. Clinical and radiological manifestations of 206 patients with pulmonary hydatidosis over a ten-year period. Prim Care Respir J. 2006;15:246–51. doi: 10.1016/j.pcrj.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montazeri V, Sokouti M, Rashidi RM. comparison of pulmonary hydatid disease between children and adults. Tanaffos. 2007;6:13–8. [Google Scholar]
- 40.Burgos R, Varela A, Castedo E, Roda J, Montero CG, Serrano S, et al. Pulmonary hydatidosis: Surgical treatment and follow-up of 240 cases. Eur J Cardiothorac Surg. 1999;16:628–34. doi: 10.1016/s1010-7940(99)00304-8. [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Vimesh P, Nadeem SA. Massive Hemoptysis in Children – Unusual Presentation in Pulmonary Hydatid Disease. [Last accessed on 2015 May 28]. Available from: http://www.ctsnet.org/sections/clinicalresources/clinicalcases/article-10.html .
- 42.Harris DG, Van Vuuren WM, Augustyn J, Rossouw GJ. Hydatid cyst fistula into the aorta presenting with massive hemoptysis. Case report and literature review. J Cardiovasc Surg (Torino) 2001;42:565–7. [PubMed] [Google Scholar]
- 43.Kavukcu S, Kilic D, Tokat AO, Kutlay H, Cangir AK, Enon S, et al. Parenchyma-preserving surgery in the management of pulmonary hydatid cysts. J Invest Surg. 2006;19:61–8. doi: 10.1080/08941930500444586. [DOI] [PubMed] [Google Scholar]
- 44.Crausaz PH. Surgical treatment of the hydatid cyst of the lung and hydatid disease of the liver with intrathoracic evolution. J Thorac Cardiovasc Surg. 1967;53:116–29. [PubMed] [Google Scholar]
- 45.Köktürk O, Oztürk C, Diren B, Unsal M, Ayla K. “Air bubble”: A new diagnostic CT sign of perforated pulmonary hydatid cyst. Eur Radiol. 1999;9:1321–3. doi: 10.1007/s003300050840. [DOI] [PubMed] [Google Scholar]
- 46.Bagheri R, Haghi SZ, Amini M, Fattahi AS, Noorshafiee S. Pulmonary hydatid cyst: Analysis of 1024 cases. Gen Thorac Cardiovasc Surg. 2011;59:105–9. doi: 10.1007/s11748-010-0690-z. [DOI] [PubMed] [Google Scholar]
- 47.Baruah N, Saikia PP, Baruah RA, Das RS. Management of pulmonary hydatid cysts in a tertiary care centre in Northeast India. Indian J Thorac Cardiovasc Surg. 2014;30:203–6. [Google Scholar]
- 48.Arinc S, Kosif A, Ertugrul M, Arpag H, Alpay L, Unal O, et al. Evaluation of pulmonary hydatid cyst cases. Int J Surg. 2009;7:192–5. doi: 10.1016/j.ijsu.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Ghoshal AG, Sarkar S, Saha K, Sarkar U, Kundu S, Chatterjee S, et al. Hydatid lung disease: An analysis of five years cumulative data from Kolkata. J Assoc Physicians India. 2012;60:12–6. [PubMed] [Google Scholar]
- 50.Sadrizadeh A, Haghi SZ, Masuom SH, Bagheri R, Dalouee MN. Evaluation of the effect of pulmonary hydatid cyst location on the surgical technique approaches. Lung India. 2014;31:361–5. doi: 10.4103/0970-2113.142118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–94. doi: 10.1148/rg.232025704. [DOI] [PubMed] [Google Scholar]
- 52.Haliloglu M, Saatci I, Akhan O, Ozmen MN, Besim A. Spectrum of imaging findings in pediatric hydatid disease. AJR Am J Roentgenol. 1997;169:1627–31. doi: 10.2214/ajr.169.6.9393178. [DOI] [PubMed] [Google Scholar]
- 53.Tsakayiannis E, Pappis C, Moussatos G. Late results of conservative surgical procedures in hydatid disease of the lung in children. Surgery. 1970;68:379–82. [PubMed] [Google Scholar]
- 54.Kanat F, Turk E, Aribas OK. Comparison of pulmonary hydatid cysts in children and adults. ANZ J Surg. 2004;74:885–9. doi: 10.1111/j.1445-1433.2004.03022.x. [DOI] [PubMed] [Google Scholar]
- 55.Ozyurtkan MO, Balci AE. Surgical treatment of intrathoracic hydatid disease: A 5-year experience in an endemic region. Surg Today. 2010;40:31–7. doi: 10.1007/s00595-009-4063-0. [DOI] [PubMed] [Google Scholar]
- 56.Balikian JP, Mudarris FF. Hydatid disease of the lungs. A roentgenologic study of 50 cases. Am J Roentgenol Radium Ther Nucl Med. 1974;122:692–707. doi: 10.2214/ajr.122.4.692. [DOI] [PubMed] [Google Scholar]
- 57.Kuzucu A, Ulutas H, Reha Celik M, Yekeler E. Hydatid cysts of the lung: Lesion size in relation to clinical presentation and therapeutic approach. Surg Today. 2014;44:131–6. doi: 10.1007/s00595-012-0484-2. [DOI] [PubMed] [Google Scholar]
- 58.Rai SP, Panda BN, Ganguly D, Bharadwaj R. Pulmonary hydatid: Diagnosis and response to hypertonic saline irrigation and albendazole. Med J Armed Forces India. 2005;61:9–12. doi: 10.1016/S0377-1237(05)80109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turgut AT, Altinok T, Topçu S, Koşar U. Local complications of hydatid disease involving thoracic cavity: Imaging findings. Eur J Radiol. 2009;70:49–56. doi: 10.1016/j.ejrad.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Taori K, Sanyal R, Rathod J, Mahajan S, Jajoo G, Saxena V, et al. CT appearances of hydatid disease at various locations. Australas Radiol. 2006;50:298–305. doi: 10.1111/j.1440-1673.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- 61.Aktan AO, Yalin R. Preoperative albendazole treatment for liver hydatid disease decreases the viability of the cyst. Eur J Gastroenterol Hepatol. 1996;8:877–9. [PubMed] [Google Scholar]
- 62.Lewall DB, McCorkell SJ. Rupture of echinococcal cysts: Diagnosis, classification, and clinical implications. AJR Am J Roentgenol. 1986;146:391–4. doi: 10.2214/ajr.146.2.391. [DOI] [PubMed] [Google Scholar]
- 63.Lewall DB. Hydatid disease: Biology, pathology, imaging and classification. Clin Radiol. 1998;53:863–74. doi: 10.1016/s0009-9260(98)80212-2. [DOI] [PubMed] [Google Scholar]
- 64.Beggs I. The radiology of hydatid disease. AJR Am J Roentgenol. 1985;145:639–48. doi: 10.2214/ajr.145.3.639. [DOI] [PubMed] [Google Scholar]
- 65.von Sinner W. Advanced medical imaging and treatment of human cystic echinococcosis. Semin Roentgenol. 1997;32:276–90. doi: 10.1016/s0037-198x(97)80022-3. [DOI] [PubMed] [Google Scholar]
- 66.Yuncu G, Ors KS, Sevinc S, Karabulut N, Alper H. The diagnostic value of the ‘Air Bubble Sign’ in complicated pulmonary hydatid cysts. J Thorac Cardiovasc Surg. 2007;133:1524–74. [Google Scholar]
- 67.von Sinner WN, Rifai A, te Strake L, Sieck J. Magnetic resonance imaging of thoracic hydatid disease. Correlation with clinical findings, radiography, ultrasonography, CT and pathology. Acta Radiol. 1990;31:59–62. [PubMed] [Google Scholar]
- 68.Koul PA, Koul AN, Wahid A, Mir FA. CT in pulmonary hydatid disease: Unusual appearances. Chest. 2000;118:1645–7. doi: 10.1378/chest.118.6.1645. [DOI] [PubMed] [Google Scholar]
- 69.Kadam SG, Basu S, Joshi JM. Doughnut sign on FDG-PET scan in a ruptured lung hydatid cyst. Indian J Chest Dis Allied Sci. 2012;54:185–7. [PubMed] [Google Scholar]
- 70.Yang L, Maldjian PD, Peters S. FDG PET/CT findings of a ruptured pulmonary hydatid cyst with histopathological correlation. Clin Imaging. 2013;37:798–801. doi: 10.1016/j.clinimag.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Ben Nouir N, Gianinazzi C, Gorcii M, Müller N, Nouri A, Babba H, et al. Isolation and molecular characterization of recombinant Echinococcus granulosus P29 protein (recP29) and its assessment for the post-surgical serological follow-up of human cystic echinococcosis in young patients. Trans R Soc Trop Med Hyg. 2009;103:355–64. doi: 10.1016/j.trstmh.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 72.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 73.Poretti D, Felleisen E, Grimm F, Pfister M, Teuscher F, Zuercher C, et al. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am J Trop Med Hyg. 1999;60:193–8. doi: 10.4269/ajtmh.1999.60.193. [DOI] [PubMed] [Google Scholar]
- 74.Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 75.Brunetti E, White AC., Jr Cestode infestations: Hydatid disease and cysticercosis. Infect Dis Clin North Am. 2012;26:421–35. doi: 10.1016/j.idc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Verastegui M, Moro P, Guevara A, Rodriguez T, Miranda E, Gilman RH. Enzyme-linked immunoelectrotransfer blot test for diagnosis of human hydatid disease. J Clin Microbiol. 1992;30:1557–61. doi: 10.1128/jcm.30.6.1557-1561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnes TS, Deplazes P, Gottstein B, Jenkins DJ, Mathis A, Siles-Lucas M, et al. Challenges for diagnosis and control of cystic hydatid disease. Acta Trop. 2012;123:1–7. doi: 10.1016/j.actatropica.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 78.Force L, Torres JM, Carrillo A, Buscà J. Evaluation of eight serological tests in the diagnosis of human echinococcosis and follow-up. Clin Infect Dis. 1992;15:473–80. doi: 10.1093/clind/15.3.473. [DOI] [PubMed] [Google Scholar]
- 79.List C, Qi W, Maag E, Gottstein B, Muller N, Felger I. Serodiagnosis of Echinococcus spp. infection: Explorative selection of diagnostic antigens by peptide microarray. PLoS Negl Trop Dis. 2010;4:e771. doi: 10.1371/journal.pntd.0000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao N, Mamuti W, Yamasaki H, Sako Y, Nakao M, Nakaya K, et al. Evaluation of use of recombinant Em18 and affinity-purified Em18 for serological differentiation of alveolar echinococcosis from cystic echinococcosis and other parasitic infections. J Clin Microbiol. 2003;41:3351–3. doi: 10.1128/JCM.41.7.3351-3353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carmena D, Benito A, Eraso E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: An update. Acta Trop. 2006;98:74–86. doi: 10.1016/j.actatropica.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Zhang WB, Wilson M, Ito A, McManus DP. A novel recombinant antigen for immunodiagnosis of human cystic echinococcosis. J Infect Dis. 2003;188:1951–60. doi: 10.1086/379976. [DOI] [PubMed] [Google Scholar]
- 83.Zarzosa MP, Orduña Domingo A, Gutiérrez P, Alonso P, Cuervo M, Prado A, et al. Evaluation of six serological tests in diagnosis and postoperative control of pulmonary hydatid disease patients. Diagn Microbiol Infect Dis. 1999;35:255–62. doi: 10.1016/s0732-8893(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 84.Siles-Lucas MM, Gottstein BB. Molecular tools for the diagnosis of cystic and alveolar echinococcosis. Trop Med Int Health. 2001;6:463–75. doi: 10.1046/j.1365-3156.2001.00732.x. [DOI] [PubMed] [Google Scholar]
- 85.Saygi A, Oztek I, Güder M, Süngün F, Arman B. Value of fibreoptic bronchoscopy in the diagnosis of complicated pulmonary unilocular cystic hydatidosis. Eur Respir J. 1997;10:811–4. [PubMed] [Google Scholar]
- 86.Yilmaz A, Tuncer LY, Damadoglu E, Sulu E, Takir HB, Selvi UB. Pulmonary hydatid disease diagnosed by bronchoscopy: A report of three cases. Respirology. 2009;14:141–3. doi: 10.1111/j.1440-1843.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- 87.Komurcuoglu B, Ozkaya S, Cirak AK, Yalniz E, Polat G. Pulmonary hydatid cyst: The characteristics of patients and diagnostic efficacy of bronchoscopy. Exp Lung Res. 2012;38:277–80. doi: 10.3109/01902148.2012.684757. [DOI] [PubMed] [Google Scholar]
- 88.Dakak M, Genç O, Gürkök S, Gözübüyük A, Balkanli K. Surgical treatment for pulmonary hydatidosis (a review of 422 cases) J R Coll Surg Edinb. 2002;47:689–92. [PubMed] [Google Scholar]
- 89.Anadol D, Ozçelik U, Kiper N, Göçmen A. Treatment of hydatid disease. Paediatr Drugs. 2001;3:123–35. doi: 10.2165/00128072-200103020-00005. [DOI] [PubMed] [Google Scholar]
- 90.Morris DL, Gould SE. Serum and cyst concentrations of mebendazole and flubendazole in hydatid disease. Br Med J (Clin Res Ed) 1982;285:175. doi: 10.1136/bmj.285.6336.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor DH, Morris DL, Richards KS. Echinococcus granulosus: In vitro maintenance of whole cysts and the assessment of the effects of albendazole sulphoxide and praziquantel on the germinal layer. Trans R Soc Trop Med Hyg. 1989;83:535–8. doi: 10.1016/0035-9203(89)90282-4. [DOI] [PubMed] [Google Scholar]
- 92.Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ. 1996;74:231–42. [PMC free article] [PubMed] [Google Scholar]
- 93.Halezeroglu S, Okur E, Tanyü OM. Surgical management for hydatid disease. Thorac Surg Clin. 2011;22:375–85. doi: 10.1016/j.thorsurg.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 94.De Rosa F, Lastilla MG, Franchi C, Teggi A. Advances of medical treatment of human hydatidosis. Recenti Prog Med. 1996;87:346–52. [PubMed] [Google Scholar]
- 95.Liu YH, Wang XG, Gao JS, Qingyao Y, Horton J. Continuous albendazole therapy in alveolar echinococcosis: Long-term follow-up observation of 20 cases. Trans R Soc Trop Med Hyg. 2009;103:768–78. doi: 10.1016/j.trstmh.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: State of the art, problems, and perspectives. Am J Trop Med Hyg. 2008;79:301–11. [PubMed] [Google Scholar]
- 97.Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79–93. doi: 10.1016/s0001-706x(96)00640-7. [DOI] [PubMed] [Google Scholar]
- 98.Lange H, Eggers R, Bircher J. Increased systemic availability of albendazole when taken with a fatty meal. Eur J Clin Pharmacol. 1988;34:315–7. doi: 10.1007/BF00540964. [DOI] [PubMed] [Google Scholar]
- 99.Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990;6:112–5. doi: 10.1016/0169-4758(90)90227-u. [DOI] [PubMed] [Google Scholar]
- 100.Keshmiri M, Baharvahdat H, Fattahi SH, Davachi B, Dabiri RH, Baradaran H, et al. Albendazole versus placebo in treatment of echinococcosis. Trans R Soc Trop Med Hyg. 2001;95:190–4. doi: 10.1016/s0035-9203(01)90162-2. [DOI] [PubMed] [Google Scholar]
- 101.Keshmiri M, Baharvahdat H, Fattahi SH, Davachi B, Dabiri RH, Baradaran H, et al. A placebo controlled study of albendazole in the treatment of pulmonary echinococcosis. Eur Respir J. 1999;14:503–7. doi: 10.1034/j.1399-3003.1999.14c05.x. [DOI] [PubMed] [Google Scholar]
- 102.Venkatesan P. Albendazole. J Antimicrob Chemother. 1998;41:145–7. doi: 10.1093/jac/41.2.145. [DOI] [PubMed] [Google Scholar]
- 103.Schantz PM, Van den Bossche H, Eckert J. Chemotherapy for larval echinococcosis in animals and humans: Report of a workshop. Z Parasitenkd. 1982;67:5–26. doi: 10.1007/BF00929509. [DOI] [PubMed] [Google Scholar]
- 104.Keramidas D, Mavridis G, Soutis M, Passalidis A. Medical treatment of pulmonary hydatidosis: Complications and surgical management. Pediatr Surg Int. 2004;19:774–6. doi: 10.1007/s00383-003-1031-4. [DOI] [PubMed] [Google Scholar]
- 105.Rostami Nejad M, Nazemalhosseini Mojarad E, Nochi Z, Fasihi Harandi M, Cheraghipour K, Mowlavi GR, et al. Echinococcus granulosus strain differentiation in Iran based on sequence heterogeneity in the mitochondrial 12S rRNA gene. J Helminthol. 2008;82:343–7. doi: 10.1017/S0022149X0804594X. [DOI] [PubMed] [Google Scholar]
- 106.Rosenzvit MC, Camicia F, Kamenetzky L, Muzulin PM, Gutierrez AM. Identification and intra-specific variability analysis of secreted and membrane-bound proteins from Echinococcus granulosus. Parasitol Int. 2006;55(Suppl):S63–7. doi: 10.1016/j.parint.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Messaritakis J, Psychou P, Nicolaidou P, Karpathios T, Syriopoulou B, Fretzayas A, et al. High mebendazole doses in pulmonary and hepatic hydatid disease. Arch Dis Child. 1991;66:532–3. doi: 10.1136/adc.66.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anadol D, Göçmen A, Kiper N, Ozçelik U. Hydatid disease in childhood: A retrospective analysis of 376 cases. Pediatr Pulmonol. 1998;26:190–6. doi: 10.1002/(sici)1099-0496(199809)26:3<190::aid-ppul6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 109.Mandour ME, el Turabi H, Homeida MM, el Sadig T, Ali HM, Bennett JL, et al. Pharmacokinetics of praziquantel in healthy volunteers and patients with schistosomiasis. Trans R Soc Trop Med Hyg. 1990;84:389–93. doi: 10.1016/0035-9203(90)90333-a. [DOI] [PubMed] [Google Scholar]
- 110.Jamshidi M, Mohraz M, Zangeneh M, Jamshidi A. The effect of combination therapy with albendazole and praziquantel on hydatid cyst treatment. Parasitol Res. 2008;103:195–9. doi: 10.1007/s00436-008-0954-z. [DOI] [PubMed] [Google Scholar]
- 111.Todorov T, Vutova K, Donev S, Ivanov A, Katzarov K, Takov D. The types and timing of the degenerative changes seen in the cysts during and after benzimidazole treatment of cystic echinococcosis. Ann Trop Med Parasitol. 2005;99:649–59. doi: 10.1179/136485905X65125. [DOI] [PubMed] [Google Scholar]
- 112.Nabi MS, Waseem T. Pulmonary hydatid disease: What is the optimal surgical strategy? Int J Surg. 2010;8:612–6. doi: 10.1016/j.ijsu.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 113.Koul PA, Singh AA, Ahanger AG, Wahid A, Sofi BA. Optimal duration of preoperative anti-helminthic therapy for pulmonary hydatid surgery. ANZ J Surg. 2010;80:354–7. doi: 10.1111/j.1445-2197.2009.05089.x. [DOI] [PubMed] [Google Scholar]
- 114.Bilgin M, Oguzkaya F, Akçali Y. Is capitonnage unnecessary in the surgery of intact pulmonary hydatic cyst? ANZ J Surg. 2004;74:40–2. doi: 10.1046/j.1445-1433.2003.02684.x. [DOI] [PubMed] [Google Scholar]
- 115.Perez-Fontana V. Nuevo metodo de operar en el quiste hidatico del pulmon. Arch Pediatr Urug. 1948;9:5. [PubMed] [Google Scholar]
- 116.Kontaxis AN. Surgical treatment of pulmonary hydatidosis. J Thorac Cardiovasc Surg. 1983;85:799–800. [PubMed] [Google Scholar]
- 117.Lichter I. Surgery of pulmonary hydatid cyst – The Barrett technique. Thorax. 1972;27:529–34. doi: 10.1136/thx.27.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turna A, Yilmaz MA, Haciibrahimoglu G, Kutlu CA, Bedirhan MA. Surgical treatment of pulmonary hydatid cysts: Is capitonnage necessary? Ann Thorac Surg. 2002;74:191–5. doi: 10.1016/s0003-4975(02)03643-3. [DOI] [PubMed] [Google Scholar]
- 119.Kosar A, Orki A, Haciibrahimoglu G, Kiral H, Arman B. Effect of capitonnage and cystotomy on outcome of childhood pulmonary hydatid cysts. J Thorac Cardiovasc Surg. 2006;132:560–4. doi: 10.1016/j.jtcvs.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 120.Eren MN, Balci AE, Eren S. Non-capitonnage method for surgical treatment of lung hydatid cysts. Asian Cardiovasc Thorac Ann. 2005;13:20–3. doi: 10.1177/021849230501300105. [DOI] [PubMed] [Google Scholar]
- 121.Erdogan A, Ayten A, Demircan A. Methods of surgical therapy in pulmonary hydatid disease: Is capitonnage advantageous? ANZ J Surg. 2005;75:992–6. doi: 10.1111/j.1445-2197.2005.03594.x. [DOI] [PubMed] [Google Scholar]
- 122.Athanassiadi K, Kalavrouziotis G, Loutsidis A, Bellenis I, Exarchos N. Surgical treatment of echinococcosis by a transthoracic approach: A review of 85 cases. Eur J Cardiothorac Surg. 1998;14:134–40. doi: 10.1016/s1010-7940(98)00144-4. [DOI] [PubMed] [Google Scholar]
- 123.Alpay L, Lacin T, Atinkaya C, Kiral H, Demir M, Baysungur V, et al. Video-assisted thoracoscopic removal of pulmonary hydatid cysts. Eur J Cardiothorac Surg. 2012;42:971–5. doi: 10.1093/ejcts/ezs338. [DOI] [PubMed] [Google Scholar]
- 124.Morris DL. Pre-operative albendazole therapy for hydatid cyst. Br J Surg. 1987;74:805–6. doi: 10.1002/bjs.1800740918. [DOI] [PubMed] [Google Scholar]
- 125.Wen H, Yang WG. Public health importance of cystic echinococcosis in China. Acta Trop. 1997;67:133–45. doi: 10.1016/s0001-706x(97)00051-x. [DOI] [PubMed] [Google Scholar]
- 126.Tor M, Atasalihi A, Altuntas N, Sulu E, Senol T, Kir A, et al. Review of cases with cystic hydatid lung disease in a tertiary referral hospital located in an endemic region: A 10 years’ experience. Respiration. 2000;67:539–42. doi: 10.1159/000067470. [DOI] [PubMed] [Google Scholar]
- 127.Türk F, Yuncu G, Karabulut N, Türk T, Ozban M, Zümrütbas EA, et al. A single-center large-volume experience in the surgical management of hydatid disease of the lung with and without extrapulmonary involvement. World J Surg. 2013;37:2306–12. doi: 10.1007/s00268-013-2122-6. [DOI] [PubMed] [Google Scholar]
- 128.Mawhorter S, Temeck B, Chang R, Pass H, Nash T. Nonsurgical therapy for pulmonary hydatid cyst disease. Chest. 1997;112:1432–6. doi: 10.1378/chest.112.5.1432. [DOI] [PubMed] [Google Scholar]
- 129.Akhan O, Ozmen MN, Dinçer A, Göçmen A, Kalyoncu F. Percutaneous treatment of pulmonary hydatid cysts. Cardiovasc Intervent Radiol. 1994;17:271–5. doi: 10.1007/BF00192450. [DOI] [PubMed] [Google Scholar]
- 130.Karaoglanoglu N, Kurkcuoglu IC, Gorguner M, Eroglu A, Turkyilmaz A. Giant hydatid lung cysts. Eur J Cardiothorac Surg. 2001;19:914–7. doi: 10.1016/s1010-7940(01)00687-x. [DOI] [PubMed] [Google Scholar]
- 131.Lamy AL, Cameron BH, LeBlanc JG, Culham JA, Blair GK, Taylor GP. Giant hydatid lung cysts in the Canadian northwest: Outcome of conservative treatment in three children. J Pediatr Surg. 1993;28:1140–3. doi: 10.1016/0022-3468(93)90149-f. [DOI] [PubMed] [Google Scholar]
- 132.Celik M, Senol C, Keles M, Halezeroglu S, Urek S, Haciibrahimoglu G, et al. Surgical treatment of pulmonary hydatid disease in children: Report of 122 cases. J Pediatr Surg. 2000;35:1710–3. doi: 10.1053/jpsu.2000.19219. [DOI] [PubMed] [Google Scholar]
- 133.Kuzucu A, Soysal O, Ozgel M, Yologlu S. Complicated hydatid cysts of the lung: Clinical and therapeutic issues. Ann Thorac Surg. 2004;77:1200–4. doi: 10.1016/j.athoracsur.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 134.Dogan R, Yüksel M, Cetin G, Süzer K, Alp M, Kaya S, et al. Surgical treatment of hydatid cysts of the lung: Report on 1055 patients. Thorax. 1989;44:192–9. doi: 10.1136/thx.44.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sadrieh M, Dutz W, Navabpoor MS. Review of 150 cases of hydatid cyst of the lung. Dis Chest. 1967;52:662–6. doi: 10.1378/chest.52.5.662. [DOI] [PubMed] [Google Scholar]
- 136.Aribas OK, Kanat F, Gormus N, Turk E. Pleural complications of hydatid disease. J Thorac Cardiovasc Surg. 2002;123:492–7. doi: 10.1067/mtc.2002.119341. [DOI] [PubMed] [Google Scholar]
- 137.Skerrett SJ, Plorde JJ. Parasitic infections of the pleural space. Semin Respir Med. 1992;13:242–58. [Google Scholar]
- 138.Ozer Z, Cetin M, Kahraman C. Pleural involvement by hydatid cysts of the lung. Thorac Cardiovasc Surg. 1985;33:103–5. doi: 10.1055/s-2007-1014097. [DOI] [PubMed] [Google Scholar]
- 139.Bouchikh M, Achir A, Maidi M, Ouchen F, Fenane H, Benosman A. Intrapleural rupture of pulmonary hydatid cysts. Rev Pneumol Clin. 2014;70:203–7. doi: 10.1016/j.pneumo.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 140.Topuzlar M, Eken C, Ozkurt B, Khan F. Possible anaphylactic reaction due to pulmonary hydatid cyst rupture following blunt chest trauma: A case report and review of the literature. Wilderness Environ Med. 2008;19:119–23. doi: 10.1580/07-WEME-CR-1561.1. [DOI] [PubMed] [Google Scholar]
- 141.Oztürk C, Agildere AM, Cila A, Balkanci F. Pulmonary arterial embolism secondary to hydatid cyst of the liver. Can Assoc Radiol J. 1992;43:374–6. [PubMed] [Google Scholar]
- 142.Lioulias A, Kotoulas C, Kokotsakis J, Konstantinou M. Acute pulmonary embolism due to multiple hydatid cysts. Eur J Cardiothorac Surg. 2001;20:197–9. doi: 10.1016/s1010-7940(01)00734-5. [DOI] [PubMed] [Google Scholar]
- 143.Manzoor MU, Faruqui ZS, Ahmed Q, Uddin N, Khan A. Aspergilloma complicating newly diagnosed pulmonary echinococcal (hydatid) cyst: A rare occurrence. Br J Radiol. 2008;81:e279–81. doi: 10.1259/bjr/23110281. [DOI] [PubMed] [Google Scholar]
- 144.Abraham JV, Mathur MR, Sisodia A, Devgarha S, Yadav A. Coexistent pulmonary hydatid disease and tuberculosis in an adult male. Egypt J Chest Dis Tuberc. 2013;62:651–3. [Google Scholar]
- 145.Gauci C, Heath D, Chow C, Lightowlers MW. Hydatid disease: Vaccinology and development of the EG95 recombinant vaccine. Expert Rev Vaccines. 2005;4:103–12. doi: 10.1586/14760584.4.1.103. [DOI] [PubMed] [Google Scholar]
- 146.Zhang W, Zhang Z, Shi B, Li J, You H, Tulson G, et al. Vaccination of dogs against Echinococcus granulosus, the cause of cystic hydatid disease in humans. J Infect Dis. 2006;194:966–74. doi: 10.1086/506622. [DOI] [PubMed] [Google Scholar]


