Abstract
Bronchopleural fistula (BPF) is a well known complication of several pulmonary conditions posing challenging management problem and is often associated with high morbidity and mortality. Though no consensus exists on a definite closure management algorithm, strategies for closure widely include various methods like tube thoracostomy with suction, open surgical closure, bronchoscopy directed glue, coiling and sealants which now also includes use of occlusion devices. We report a case in which a novel method of delivery and closure of recurrent post-operative broncho-pleuro-cutaneous fistula by a duct occluder device was done transcutaneously which has not been previously described in literature.
KEY WORDS: Broncho-pleuro-cutaneous fistula, duct occluder device, transcutaneous closure
INTRODUCTION
Bronchopleural fistula (BPF) is a well-known complication among the several pulmonary conditions. Although BPF is a common postoperative complication of pulmonary resection, in developing countries it commonly occurs due to tuberculosis. Postoperative fistulas need open surgical closure and central BPF will usually require occlusion devices if they persist even after surgery. We present a case that offers a novel method of closure of a chronic postoperative broncho-pleuro-cutaneous fistula by a patent ductus arteriosus (PDA) occluder device.
CASE REPORT
A 25-year-old lady presented to our hospital with complaints of productive cough and streaky hemoptysis for the last 4 weeks. She had been treated in the past for smear positive pulmonary tuberculosis in 2000 for 3 months and again in 2005 for 9 months with first-line antituberculous (ATT) drugs with poor drug compliance. She was diagnosed to have relapse in November 2007, and was being treated with second-line ATT drugs. Her detailed workup showed radiographic evidence of large cavitary lesion in the right middle zone and fibrocystic opacities in the right upper zone. Reports of sputum smear for acid-fast bacilli (AFB) and culture for mycobacterium tuberculosis were negative. Second-line ATT drugs were continued and, the patient was reviewed on a monthly basis in our outpatient department (OPD). She had a persistent large cavity in the superior segment of the right lower lobe after 18 months of treatment and bronchiectasis in right upper lobe. The patient underwent right upper lobectomy and excision of cavity in right lower lobe but developed a discharging sinus with air leak from the thoracotomy wound after 1 month. The patient was bronchoscopically evaluated and was found to have a fistula at the stump of right upper lobe bronchus. The fistulous tract was delineated by contrast injection under fluoroscopy.
In view of poor general condition, the patient was not a candidate for surgical closure and was offered device occlusion of the fistula with duct occluder device. It was decided that the approach to the site of BPF would be transcutaneous route and monitoring of the release of a PDA duct occluder device will be done under bronchoscopy and fluoroscopy.
The BPF was measured to be 8 mm in diameter and it was decided that the size of the occluder device will be increased to achieve good wall approximation and to avoid postdeployment migration. A Extra- stiff wire 0.035” (Cook medical, United States of America) wire was passed through the working channel of the bronchoscope under conscious sedation to gain access into the BPF opening and onwards to the pleural space and then the sinus track. The bronchoscope was withdrawn from the wire to be repositioned by the side of the wire for better visual control of the procedure. The wire was torqued to guide it along the sinus track till the skin wound and was made to exit by pull-and-push technique under local anesthesia. An 8F PDA delivery sheath was passed transcutaneously over the wire and its tip was positioned in the BPF under fluoroscopic and bronchoscopic control. After removing the wire, nitinol PDA device (Lifetech™, Shenzhen, PR China) of size 10 mm (proximal waist), 8 mm (distal waist), 7 mm (length), and retention skirt of 2 mm on either side was selected and loaded into the delivery sheath. The device was pushed up to the proximal level of fistula and released under fluoroscopic and bronchoscopic control to assume the predetermined sizes and to occlude the fistula snugly in the shape of a mushroom [Figure 1].
Figure 1.
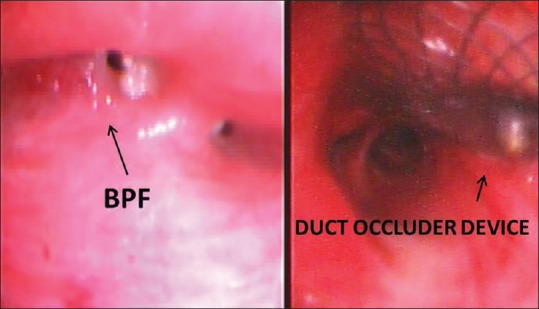
The BPF before and after the closure with duct occluder device in the right upper lobe bronchus
The device position was confirmed and there was complete cessation of air leak and cough experienced by the patient earlier. Patient was managed with broad spectrum antibiotics and anti-inflammatory drugs. On the fourth postprocedure day, the device was bronchoscopically checked and found in situ with good approximation of the device—well-aerated right lower lobe with no clinical or radiological evidence of air leak or device migration [Figure 2]. The patient showed remarkable improvement in general outlook and nutrition with good effort tolerance. She was discharged on the 20th postprocedure day. Patient is asymptomatic with increasing opacification of residual cavity and closure device in situ. The cutaneous opening has completely healed, and the nutritional status and effort tolerance level of the patient have improved.
Figure 2.
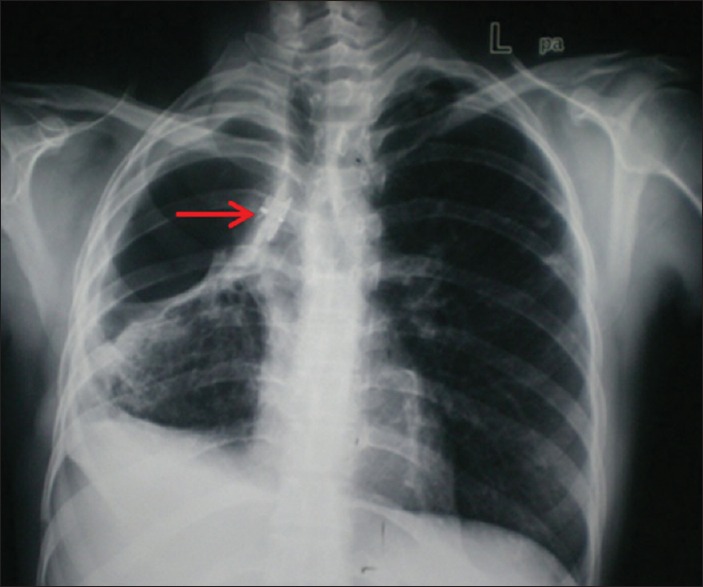
Chest radiograph showing duct occluder device in situ
DISCUSSION
BPF is an abnormal communication between the pleural space and the bronchial tree. It poses a management challenge as it is associated with high morbidity and mortality.[1] Post-pneumonectomy empyema (PNE) occurs in approximately 5% of pneumonectomy patients, and BPF appears in more than 80% of cases. The mortality of PNE with a BPF has been reported to be 11–13%. Persistent BPFs are often associated with multiple operations and prolonged hospitalization. Occasionally, small, uncomplicated BPFs may heal spontaneously, and in 20% of the patients, BPFs will close with drainage only. However, the remaining 80% of patients whose BPFs persist will require additional operative procedures. Various surgical procedures such as direct repair, thoracoplasty, myoplasty, omental transposition, and completion pneumonectomy have been described.[2] Surgical option appears to provide the definite closure but not always, as in the case we encountered. Endobronchial occlusion methods are now performed routinely with different bronchoscopes and a long list of embolic agents such as blood clots, gelatin, silicon rubber plugs, lead fishing sinkers, various sponge materials, tetracyclines, fibrin glue, cyanoacrylate, detachable balloons, metallic coils, stents, and septal occluder device with successful outcomes.[3,4,5]
In our case, we resorted to use an occluder device as BPF did not close in spite of conservative measures. The BPF was central and approximately 7–8 mm in size. The PDA duct occluder has traditionally been used by cardiologists for occluding PDA. We selected the nitinol duct occluder device and the transcutaneous route for its delivery as it was easier and more maneuverable. Fruchter et al. have published their experience of bronchoscopic closure of BPFs using Amplatzer devices with 5 F–7 F delivery sheath under fluoroscopic visualization.[6] The delivery sheath used by us was 8 F in size and was not compatible with the working channel of the bronchoscope. The occluder is shaped into a mushroom plug with a collar to secure the duct occluder in the stump of the right upper lobe bronchus. The transcutaneous delivery of device ensured that the larger proximal part is placed into the bronchus, thus preventing distal migration due to gravity into the pleura. To the best of our knowledge, this is the first report where PDA device has been used transcutaneously to close the BPF. Fruchter et al.[6] deployed Amplatzer devices bronchoscopically for the closure of BPF in patients without cutaneous fistula.
CONCLUSION
In conclusion, this endobronchial technique seems to be safe and effective to manage large BPFs. Some authors have reported the successful use of fibrin sealant in video-assisted thoracoscopic technique[7] as well as in computer tomography (CT)-guided percutaneous transthoracic application[8] to seal pulmonary air leaks but the drawback of these techniques is the need of an additional invasive procedure. We applied a simple endobronchial technique without general anesthesia, rigid bronchoscopy, thoracoscopy, or bronchography. If similar results are seen with ongoing experience, it may represent a major step forward in the management of a debilitating and difficult-to-treat complication after pulmonary resections in the resource-constrained settings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Khan JH, Rahman SB, McElhinney DB, Harmon AL, Anthony JP, Hall TS, et al. Management strategies for complex bronchopleural fistula. Asian Cardiovasc Thorac Ann. 2000;8:78–84. [Google Scholar]
- 2.Jain R, Baijal SS, Phadke RV, Pandey CK, Saraswat VA. Endobronchial closure of a bronchopleural cutaneous fistula using angiography catheters. AJR Am J Roentgenol. 2000;175:1646–8. doi: 10.2214/ajr.175.6.1751646. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe S, Shimokawa S, Yotsumoto G, Sakasegawa K. The use of a Dumon stent for the treatment of a bronchopleural fistula. Ann Thorac Surg. 2001;72:276–8. doi: 10.1016/s0003-4975(00)02533-9. [DOI] [PubMed] [Google Scholar]
- 4.Marwah V, Rajput AK, Madan H, Garg Y. Closure of chronic bronchopleural fistula using atrial septal occluder device. J Bronchology Interv Pulmonol. 2014;21:82–4. doi: 10.1097/LBR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 5.Lois M, Noppen M. Bronchopleural fistulas: An overview of the problem with special focus on endoscopic management. Chest. 2005;128:3955–65. doi: 10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]
- 6.Fruchter O, Kramer MR, Dagan T, Raviv Y, Abdel-Rahman N, Saute M, et al. Endobronchial closure of bronchopleural fistulae using Amplatzer devices: Our experience and literature review. Chest. 2011;139:682–7. doi: 10.1378/chest.10-1528. [DOI] [PubMed] [Google Scholar]
- 7.Mukaida T, Andou A, Date H, Aoe M, Shimizu N. Thoracoscopic operation for secondary pneumothorax under local and epidural anesthesia in high-risk patients. Ann Thorac Surg. 1998;65:924–6. doi: 10.1016/s0003-4975(98)00108-8. [DOI] [PubMed] [Google Scholar]
- 8.Samuels LE, Shaw PM, Blaum LC. Percutaneous technique for management of persistent airspace with prolonged air leak using fibrin glue. Chest. 1996;109:1653–5. doi: 10.1378/chest.109.6.1653. [DOI] [PubMed] [Google Scholar]


