Abstract
X-linked inhibitor of apoptosis (XIAP) is an endogenous inhibitor of cell death that functions by suppressing caspases 3, 7, and 9. Here we describe the establishment of Jurkat-derived cell lines stably overexpressing either full-length XIAP or a truncation mutant of XIAP that can only inhibit caspase 9. Characterization of these cell lines revealed that following CD95 activation full-length XIAP supported both short- and long-term survival as well as proliferative capacity, in contrast to the truncation mutant but similar to Bcl-xL. Full-length XIAP was also able to inhibit CD95-mediated caspase 3 processing and activation, the mitochondrial release of cytochrome c and Smac/DIABLO, and the loss of mitochondrial membrane potential, whereas the XIAP truncation mutant failed to prevent any of these cell death events. Finally, suppression of XIAP levels by RNA interference sensitized Bcl-xL-overexpressing cells to death receptor-induced apoptosis. These data demonstrate for the first time that full-length XIAP inhibits caspase activation required for mitochondrial amplification of death receptor signals and that, by acting upstream of mitochondrial activation, XIAP supports the long-term proliferative capacity of cells following CD95 stimulation.
Apoptosis is a genetically programmed, biochemically ordered process by which cells die in response to defined stimuli and is a critical event in multicellular organisms for such processes as developmental progression, tissue homeostasis, and the immune response (33, 47, 68). Deregulation of apoptosis has been implicated in a range of human diseases, including cancer, neurodegenerative disorders, and autoimmunity (65). The principal mediators of apoptosis are members of the caspase family of cysteine proteases (13, 66), which are divided into two groups. Initiator caspases respond to and are activated by apoptotic stimuli, while effector caspases are cleaved and activated by the initiator caspases and are responsible for the majority of substrate proteolysis during cell death (7, 48, 60).
Among the molecules that regulate apoptosis are several members of the inhibitor of apoptosis (IAP) family (16, 27), which prevent cell death by acting as endogenous suppressors of caspase activity (18). The IAPs contain one to three copies of a domain known as the baculoviral IAP repeat (BIR), the defining motif of the IAP family. In addition, several IAP members contain a RING domain at the carboxyl terminus that in many cases has been shown to possess E3 ubiquitin-ligase activity (28, 29, 40, 74). The most thoroughly characterized member of the IAP family is X-linked inhibitor of apoptosis (XIAP) (21, 41, 67). XIAP contains three amino-terminal BIR domains and a carboxy-terminal RING domain, and it is the most potent endogenous inhibitor of caspases known, specifically targeting the initiator caspase 9 and the effector caspases 3 and 7, binding each with nanomolar affinity (18). Recent structural studies have revealed that determinants in BIR3 of XIAP are responsible for inhibition of caspase 9 (52, 61) and that the region immediately amino terminal to BIR2 is the major determinant for inhibition of caspases 3 and 7 (11, 30, 50).
Despite overlap of both function and substrate specificity among the many known caspases, a hierarchical cascade of activation exists (55), and the exact subset of caspases activated during apoptosis appears to be stimulus specific. By examining the patterns of caspase activation following many different apoptotic stimuli, at least two distinct pathways have emerged. Death signals originating from cellular stress, growth factor deprivation, radiation, and chemotherapeutic drugs activate an intrinsic apoptotic program that is mediated largely by the mitochondria. Mitochondrial release of cytochrome c into the cytoplasm induces the formation of an oligomeric complex containing cytochrome c and Apaf-1 (38). This complex, or “apoptosome” (1, 8, 9), supports the catalytic activation of caspase 9, which further cleaves and activates the effector caspase 3, resulting in the subsequent degradation of cellular death substrates. In addition to cytochrome c, the nuclear-encoded, mitochondrially localized proteins Smac/DIABLO (19, 69) and Omi/HtrA2 (26, 44, 70) are also released into the cytoplasm. Once released, these two proteins bind to XIAP in a manner similar to caspases (10, 42, 59) and thereby promote apoptosis by neutralizing the caspase inhibitory function of XIAP. Additionally, Omi/HtrA2 may further inactivate XIAP through proteolytic processing (58, 73).
In contrast to the intrinsic pathway, death receptor stimulation activates an extrinsic apoptotic program that often requires no mitochondrial involvement. Instead, death receptor ligation induces the formation of a death-inducing signaling complex (DISC) which contains the initiator caspase 8 (45). DISC formation activates caspase 8 through a proximityinduced dimerization mechanism requiring no autoproteolysis (5), which then cleaves and activates caspase 3, resulting in further cleavage of cellular targets. In some cell types, however, the linear progression from DISC formation to caspase 3 activation is insufficient to complete the cell death program, and amplification of the death receptor stimulus by engagement of the mitochondrially mediated cell death pathway is required (35, 51). One reported mechanism by which the mitochondrial pathway is engaged following death receptor activation is through caspase 8-mediated cleavage of Bid, a prodeath member of the Bcl-2 family. Once cleaved, truncated Bid translocates to the mitochondria (25, 37, 43), where it can induce the release of cytochrome c, Smac/DIABLO, and Omi/HtrA2. In this manner, death receptor signals may be amplified through formation and activation of the apoptosome, which can increase the activation of caspase 3, or through the neutralization of XIAP by Smac/DIABLO and Omi/HtrA2.
Much that is known about the caspase inhibitory properties of XIAP has been derived either from in vitro caspase activity studies employing recombinant proteins (17, 18) or from transient-expression studies of XIAP in intact cells (15, 20). While critical to advancing our understanding of XIAP function, due to their short-term nature these studies have been limited in that no examination of the long-term effects of XIAP expression has been possible. Here we describe a detailed characterization of the protective properties of XIAP stably overexpressed in Jurkat cells. Experiments evaluating both full-length XIAP and a truncation mutant of XIAP lacking the first two BIR domains (BIR3-sp-RING) show that the caspase 3 inhibitory property of XIAP, though dispensable for protection from etoposide treatment, is absolutely required for protection from activation of the death receptor CD95 (Apo-1/Fas). In contrast, full-length XIAP supports both long-term survival and proliferative capacity of cells following CD95 stimulation, an effect that stems from the ability of full-length XIAP to prevent caspase 3 and 9 activation, to inhibit the release of the mitochondrial proteins cytochrome c and Smac/DIABLO, and to suppress the loss of mitochondrial membrane potential. Finally, reduction of XIAP protein levels by RNA interference (RNAi) in cells overexpressing Bcl-xL results in sensitization to CD95-mediated death. These data are consistent with a model in which XIAP, through inhibition of caspase activity, functions upstream of mitochondrial activation and confers both apoptotic resistance and long-term proliferative capacity to cells by preventing mitochondrial amplification following death receptor engagement.
MATERIALS AND METHODS
Materials.
Reagents were obtained from the following sources: puromycin (Calbiochem, San Diego, Calif.); phosphate-buffered saline (PBS), G418, and protein G-coupled agarose beads (Invitrogen, Carlsbad, Calif.); fetal bovine serum (HyClone, Logan, Utah); cell culture media (Mediatech, Herndon, Va.); tetramethylrhodamine methyl ester (TMRM; Molecular Probes, Eugene, Oreg.); 20 μM N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin) (DEVD-AFC; BioMol, Plymouth Meeting, Pa.); PhiPhiLux-G1D2 (OncoImmunin, Gaithersburg, Md.); protease inhibitor cocktail tablets (Roche, Indianapolis, Ind.); etoposide (Bristol-Meyers Squibb, New York, N.Y.); soluble CD95 ligand (Alexis Biochemicals, San Diego, Calif.); bovine serum albumin, propidium iodide (PI), digitonin, and all other chemicals were from Sigma (St. Louis, Mo.). Antibodies were obtained from the following sources: anti-CD95 (CH.11; Upstate Biotechnology, Charlottesville, Va.); anti-XIAP, anti-FADD, and anti-Bcl-xL (Transduction Labs, Lexington, Ky.); anti-CD95 (sc8009; Santa Cruz Biotechnology, Santa Cruz, Calif.); anti-CD95 ligand, anti-cytochrome c, and anti-caspase 3 (Pharmingen, San Diego, Calif.); anti-caspase 9 (Stressgen, San Diego, Calif.); anti-FLIP-L/S (Alexis Biochemicals); anti-Smac/DIABLO (Calbiochem); anti-β-actin and anti-FLAG (M2; Sigma); anti-COX IV (Molecular Probes); antihemagglutinin (anti-HA; Covance, Princeton, N.J.); peroxidase-conjugated anti-mouse and anti-rabbit (Amersham, Piscataway, N.J.). Anti-caspase 8 was the kind gift of M. Peter (University of Chicago, Chicago, Ill.).
Cell lines.
Jurkat cells were maintained in either Iscove's medium or RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 100 U of penicillin-streptomycin/ml at 37°C in an atmosphere of 95% air-5% CO2.
Transfections.
Parental Jurkat cells were electroporated with the following constructs: pBABE-puro, pEBB-HA, pEBB-HA-XIAP, pEBB-HA-BIR3-sp-RING, pcDNA3, and pcDNA3-Bcl-xL, which have been described elsewhere (4, 6, 49). Briefly, 107 cells per transfection were electroporated with 30 μg of pEBB-HA, pEBB-HA-XIAP, or pEBB-HA-BIR3-sp-RING-XIAP along with 3 μg of pBABE-puro, or with 30 μg of either pcDNA3 or pcDNA3-Bcl-xL. pBABE-puro-based transfectants were selected in the presence of 1 μg of puromycin/ml, and pcDNA3-based transfectants were selected in the presence of 1 mg of G418/ml. Single-cell clones were obtained by limited dilution of bulk cultures arising from selection. High-expressing single-cell clones were maintained in either 1 μg of puromycin/ml or 1 mg of G418/ml.
Cell lysate preparation.
Cells were harvested by centrifugation, washed in PBS, and resuspended in radioimmunoprecipitation assay (RIPA) lysis buffer (PBS containing 1% NP-40, 0.5% Na-deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) supplemented with 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 1 protease inhibitor cocktail tablet per 10 ml. Cells were incubated on ice for 20 min, and lysates were then cleared by centrifugation prior to protein concentration determination using the Bio-Rad protein assay kit according to the manufacturer's instructions.
Viability experiments.
For titrations, cells (3 × 105 cells/ml; 1.25 ml/sample) were left untreated or treated with various concentrations of either anti-CD95 (1 to 500 ng/ml) or etoposide (0.1 to 50 μg/ml) and incubated at 37°C for 20 h. For all subsequent experiments, anti-CD95 and etoposide were used at concentrations of 100 ng/ml and 10 μg/ml, respectively. For soluble CD95 ligand experiments, cells were left untreated or treated with 5 ng of CD95 ligand/ml in the presence of 1 μg of anti-FLAG M2/ml at 37°C for 20 h. Following treatment, cells were harvested by centrifugation, washed with PBS, resuspended in PBS plus 1% bovine serum albumin and 2 μg of PI/ml, and then analyzed by flow cytometry using a Coulter EPICS model XL-MCL flow cytometer.
Long-term proliferation.
Cells were left untreated or treated with either anti-CD95 or etoposide. Immediately following treatment, and once each day for 6 days thereafter, 50 μl from each culture was removed and cell density was determined by using a Beckman-Coulter model Z1 particle counter. Following 6 days of treatment, lysates were prepared from surviving cells as described above.
Extract preparation and immunoblot analysis.
Jurkat and Jurkat-derived cells (5 × 107 to 10 × 107 cells/treatment) were treated with anti-CD95 or etoposide for 0, 2, 4, or 6 h. Following treatment, extracts were prepared as described previously (2), with minor modifications. Briefly, cells were harvested and washed once with PBS. Cell pellets were resuspended at 3 × 107 cells/ml in digitonin extraction buffer (PBS containing 250 mM sucrose, 70 mM KCl, 1 mM phenylmethylsulfonyl fluoride, 200 μg of digitonin/ml, and 1 protease inhibitor cocktail tablet per 10 ml) and incubated on ice for 5 min. Samples were then centrifuged at 1,000 × g for 5 min; the pellets were lysed in RIPA lysis buffer as described above, and the supernatants (cytoplasmic extracts) were collected and centrifuged again at 1,000 × g for 5 min to clear any remaining debris. The protein concentrations of all extracts were then determined using the Bio-Rad protein assay kit.
Western blot analysis.
Cytoplasmic extracts (25 μg) or whole-cell lysates (60 μg) were prepared in LDS sample buffer (Invitrogen) and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using 4-to-12% gradient SDS-PAGE gels (Invitrogen), followed by transfer to nitrocellulose membranes (Invitrogen). Membranes were blocked with a 5% milk solution in Tris-buffered saline containing 0.02 to 0.2% Tween and then incubated with the indicated antibodies for 1 h at room temperature. Following washing, membranes were incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G secondary antibodies for 45 min at room temperature. Blots were visualized by enhanced chemiluminescence using Kodak XAR film.
Immunoprecipitations.
Whole-cell lysates were prepared in RIPA lysis buffer as described above, normalized for protein content, and immediately incubated with anti-HA antibodies for 2 h at 4°C. Protein G-coupled agarose beads were then added, and the incubation was continued for 1 h. Agarose beads, along with any bound immune complexes, were recovered by centrifugation and washed in RIPA buffer, and precipitated proteins were eluted from the agarose beads by adding LDS sample buffer and heating to 95°C for 5 min. Recovered proteins were then separated by electrophoresis, and immunoblot analysis was carried out as described above.
In vitro caspase assays.
Cytoplasmic extracts (12 μg; prepared as described above) were diluted with activation buffer [50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 6.9), 0.1 mM EDTA, 10% glycerol, and 2 mM dithiothreitol] in the presence of 20 μM DEVD-AFC to a final volume of 200 μl. AFC release over time (a total of 40 measurements at 75-s intervals) was then measured at 37°C using a Cytofluor 4000 multiwell plate reader (Applied Biosystems) with an excitation wavelength of 400 nm and an emission wavelength of 508 nm.
Intact cell caspase assays.
Cells (5 × 105 cells/ml; 1.25 ml/sample) were left untreated or treated with anti-CD95 and incubated at 37°C for 4 h. Following incubation, cells were harvested by centrifugation, resuspended in 50 μl of 10 μM PhiPhiLux-G1D2 substrate (OncoImmunin), and incubated for 1 h at 37°C. Samples were then washed once, resuspended in PBS, and analyzed by flow cytometry.
Mitochondrial membrane potential assays.
Cells were left untreated or treated with anti-CD95 or etoposide. At 20 h after treatment, cells were harvested and washed with PBS. Cells were then resuspended in PBS plus 200 nM TMRM and incubated at 37°C for 15 min. Samples were then placed on ice and analyzed by flow cytometry.
RNAi.
Cells (107 cells/transfection) were transfected with 16 μg of double-stranded RNA oligonucleotides by electroporation. Gene-specific targeting of XIAP was performed using an oligonucleotide corresponding to nucleotides 111 to 131 (AAGTGGTAGTCCTGTTTCAGC) of the coding sequence of XIAP. As a negative control, an oligonucleotide targeting nucleotides 322 to 342 (AAGACCCGCGCCGAGGTGAAG) of the coding sequence of green fluorescent protein (GFP) was used. At 24 h following transfection, viable cells from each sample were recovered by Ficoll-Paque gradient centrifugation (Roche) according to the manufacturer's instructions. At 48 h after transfection, a portion of cells from each transfection was used for viability experiments, as described above. The remaining cells were used for immunoblot analysis, as described above.
RESULTS
In order to more thoroughly characterize the function of XIAP in intact cells, the Jurkat T-cell leukemia line, which requires mitochondrial amplification during death receptor-mediated apoptosis (51), was used to establish stable, clonal cell lines overexpressing full-length wild-type (WT) XIAP. Previous reports (53, 54) suggested that the caspase 3 binding domain is not required for the protective effects of XIAP. Given the very high affinity of XIAP for caspase 3 (18) and in order to test the possibility that XIAP may exert a cytoprotective effect in a context- and stimulus-specific manner, we also established Jurkat-derived cells stably expressing the XIAP truncation mutant BIR3-sp-RING, which lacks the first two BIR domains of the WT protein and is therefore incapable of inhibiting caspase 3. As a positive control for resistance to apoptosis, Jurkat-derived lines overexpressing Bcl-xL were also established. Cells stably overexpressing each XIAP protein demonstrated XIAP levels that were three- to fivefold greater than those of endogenous XIAP in either parental or vector control cells (Fig. 1A). Similarly, Bcl-xL protein levels were approximately fourfold greater in the stably transfected cells than in parental or control transfected cells. Protein expression of a number of apoptosis regulators, including CD95, FADD, FLIPL, FLIPS, and caspases 8, 9, and 3, was also assessed by immunoblot analysis (Fig. 1A), and no changes in any of these molecules were observed among the four cell lines. CD95 ligand expression was undetectable in all cell lines tested by immunoblot analysis (data not shown).
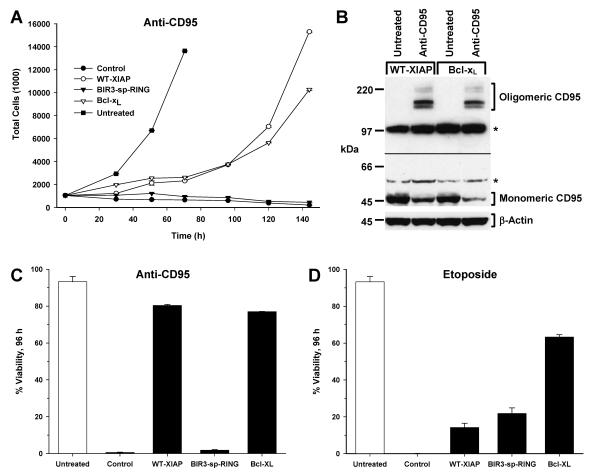
FIG. 1.
Protective properties of XIAP following anti-CD95 and etoposide. (A) Lysates were prepared from control transfected Jurkat cells or Jurkat-derived cells stably overexpressing either WT-XIAP, BIR3-sp-RING, or Bcl-xL and immunoblotted for the presence of XIAP, Bcl-xL, CD95, FADD, FLIPL, FLIPS, caspase 9, caspase 8, caspase 3, and β-actin. (B) Cells were treated overnight with various concentrations of anti-CD95 followed by PI staining and analysis by flow cytometry. (C) Cells were treated overnight with various concentrations of etoposide and analyzed as for panel B. (D) Cells were treated overnight with soluble CD95 ligand at 5 ng/ml in the presence of 1 μg of anti-FLAG/ml and analyzed as for panel B. For panels B, C, and D, the average ±1 standard deviation of multiple independent measurements is shown for each sample, and the data are representative of at least three independent experiments.
Sensitivities to apoptosis of cell lines stably overexpressing WT-XIAP, BIR3-sp-RING, or Bcl-xL were tested by titration of either the agonistic CD95 antibody CH.11 (Fig. 1B) or etoposide (Fig. 1C), or treatment with soluble CD95 ligand (Fig. 1D). Following overnight incubation, cell viability was assessed by staining with PI and flow cytometric analysis. After treatment with CH.11, WT-XIAP- and Bcl-xL-, but not BIR3-sp-RING-overexpressing cells, were significantly protected from death at all concentrations tested compared to control cells, and similar results were observed following treatment with CD95 ligand. The differences in anti-CD95 and CD95 ligand sensitivity observed were not due to differences in CD95 expression among the lines tested, as all lines possessed identical levels of CD95 protein (Fig. 1A). Since treatment with CH.11 and CD95 ligand yielded similar results, all subsequent experiments were preformed with CH.11. Following etoposide treatment, both WT-XIAP and BIR3-sp-RING, as well as Bcl-xL, effectively prevented cell death over the wide range of drug concentrations tested. These data suggest that the caspase 3 inhibitory properties of XIAP, while dispensable for protection from etoposide, are necessary for protection following anti-CD95.
The abilities of WT-XIAP, BIR3-sp-RING, and Bcl-xL cell lines to survive long-term administration of either anti-CD95 or etoposide were next assessed. Cells were treated with anti-CD95 or etoposide, and the cell density of each culture was determined every day for 6 days after the initial treatment. In this manner, the proliferative capacity of each cell line, as indicated by an increase in cell number over time, was determined. Following anti-CD95 administration, both WT-XIAP and Bcl-xL cells were capable not only of surviving long-term treatment (Fig. 2C) but also of resuming proliferation after a short lag phase, which was not the case for either the BIR3-sp-RING cells or control cells (Fig. 2A). To confirm that the proliferation observed for WT-XIAP and Bcl-xL cells was not due to selection of cells that express lower levels of CD95, immunoblot analysis for CD95 protein was performed following treatment (Fig. 2B). While there appeared to be a reduction in the levels of monomeric CD95 in both cell lines following treatment, oligomerized CD95 was readily detectable in long-term-treated cultures from both cell lines, in contrast to untreated samples that displayed no oligomerized CD95. Thus, the total levels of CD95 remained unchanged during treatment, suggesting that the long-term survival and proliferation observed for WT-XIAP and Bcl-xL cells was not due to a reduction in CD95 protein levels. Removal of anti-CD95 after 24 h had no effect on the survival or growth of WT-XIAP and Bcl-xL cells and was unable to restore either viability or proliferative capacity to the BIR3-sp-RING cells (data not shown). None of the cell lines examined were capable of proliferating following etoposide administration (data not shown), and only cells overexpressing Bcl-xL remained viable following long-term etoposide treatment (Fig. 2D).
FIG. 2.
Protective properties of XIAP following long-term anti-CD95 treatment. (A) Jurkat-derived cells were treated with anti-CD95 for 6 days. Proliferation was assessed by cell density determination every 24 h using a Coulter Counter. Representative data from untreated control cells are shown (filled squares). (B) Lysates were prepared from WT-XIAP- and Bcl-xL-expressing Jurkat cells prior to and following treatment with anti-CD95 for 6 days and then immunoblotted for the presence of CD95. Shown are two separate exposures of the same immunoblot, separated by the black line: the lower exposure (1 min) shows monomeric CD95, and the upper exposure (5 min) shows oligomeric CD95. Note that while levels of monomeric CD95 decreased following treatment, the presence of oligomeric CD95 following treatment accounted for this loss. As a loading control, immunoblot analysis for β-actin was also performed (lower panel). (C and D) Viability of cell lines shown in panel A (C) or of cells treated with etoposide (D) was assessed 96 h following treatment by PI staining followed by flow cytometry. The average ± standard deviation of multiple independent measurements is shown. Data are representative of at least three independent experiments.
Given the striking difference in the ability of WT-XIAP to protect cells from CD95-mediated death compared to BIR3-sp-RING, as well as the inability of both proteins to protect from long-term etoposide administration, a thorough characterization of the molecular events preceding apoptosis was undertaken. XIAP is a potent inhibitor of caspase 9 and caspase 3 but has no inhibitory effect on caspase 8 (17, 18). Since caspase activation is an early event in the apoptotic program, the activation of caspases 9 and 3 was examined in WT-XIAP and BIR3-sp-RING cells. Cytoplasmic extracts were prepared from control, WT-XIAP-, and BIR3-sp-RING-expressing cells following treatment with either anti-CD95 or etoposide for various times from 0 to 6 h, and caspase processing was examined by immunoblot analysis. As expected, no differences in caspase 8 processing were observed among these cell lines following treatment with anti-CD95 (Fig. 3A), and no processing of caspase 10 was observed (data not shown). Following anti-CD95 (Fig. 3A), processing of both caspase 9 and caspase 3 was clearly observed in control cells (Fig. 3A), and cleavage products were apparent as early as 2 h after treatment. In these cells, the processing of caspase 3 preceded that of caspase 9, an observation consistent with the direct cleavage of caspase 3 by caspase 8 during CD95-mediated apoptosis in Jurkat cells. Interestingly, when compared to control cells, BIR3-sp-RING cells showed nearly identical cleavage patterns for both caspase 9 and caspase 3. Since BIR3-sp-RING is capable of inhibiting caspase 9 processing following etoposide treatment (Fig. 3B), the observed cleavage of caspase 9 in BIR3-sp-RING cells suggests that this is a caspase 3-mediated event, consistent with previous reports (24, 55, 57). In contrast, in the WT-XIAP line there was little or no observable caspase 9 processing, and the pattern of caspase 3 processing was strikingly different: while the upper cleavage fragment of caspase 3 accumulated, albeit with slower kinetics and to a lesser extent than in control or BIR3-sp-RING cells, the smaller caspase 3 cleavage fragment was not detected, even at the latest time point examined (Fig. 3A). This pattern of caspase 3 processing is consistent with a recent report suggesting that XIAP binds to a partially processed, intermediate form of caspase 3 and that this binding prevents subsequent maturation of caspase 3 (62).
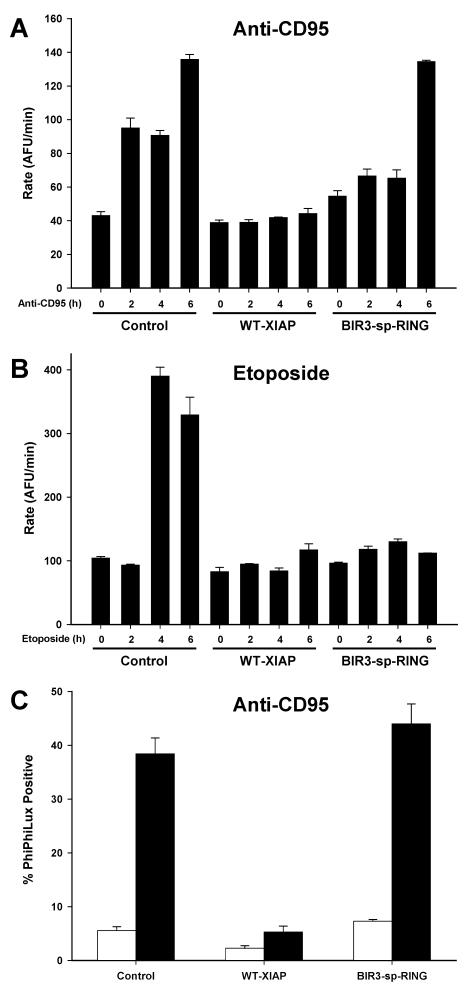
FIG. 3.
Caspase processing in Jurkat-derived cells. Jurkat-derived cells were treated with anti-CD95 (A) or etoposide (B) for 0, 2, 4, or 6 h. Cytoplasmic extracts were then prepared, and immunoblot analysis was performed to detect the presence of caspase 8 (A), caspase 9 (A and B), and caspase 3 (A and B). Arrows indicate the positions of the processed forms of each caspase. For caspase 8, three exposures of the same immunoblot are shown, separated by black lines: the upper exposure (1 min) shows unprocessed caspase 8, and the middle (10 min) and lower (180 min) exposures show processed caspase 8. Molecular mass markers are shown in kilodaltons. As a loading control, immunoblot analysis for β-actin was performed and appears below in Fig. 5.
Following etoposide treatment (Fig. 3B), the appearance of caspase 9 and caspase 3 cleavage products in control cells was similar to that following anti-CD95 treatment, with cleavage of both caspases apparent by 4 h. In contrast, the amount of cleaved caspase 9 and 3 observed in both WT-XIAP and BIR3-sp-RING cells was significantly reduced, even at the latest time point (Fig. 3B). These data indicate that while the caspase 3 inhibitory properties of XIAP are dispensable for preventing caspase processing following etoposide treatment, they are required to prevent caspase cleavage following anti-CD95 administration.
Since the immunoblot analysis detects caspase cleavage, but does not directly address caspase activity levels, the activity of caspase 3 was assessed both in vitro and in intact cells using fluorescent substrate cleavage assays. Using the same extracts prepared for immunoblot analysis (Fig. 3), caspase activity was tested by incubating these extracts with the caspase 3 substrate DEVD-AFC. Consistent with the immunoblot analysis, extracts from anti-CD95-treated control and BIR3-sp-RING cells contained significant caspase activity, whereas extracts from anti-CD95-treated WT-XIAP cells showed little to no caspase 3 activation (Fig. 4A). Furthermore, while control extracts contained caspase activity at the later time points following etoposide treatment, neither BIR3-sp-RING nor WT-XIAP extracts exhibited DEVD cleavage (Fig. 4B). To evaluate caspase 3 activity in the WT-XIAP line in vivo following anti-CD95 treatment, an additional caspase 3 activity assay was performed in intact cells by treating with anti-CD95 and then incubating cells with the cell-permeable caspase 3 substrate PhiPhiLux. When cleaved by activated caspase 3, this substrate becomes fluorescent, allowing the amount of caspase 3 activity to be quantified in intact cells by observing changes in fluorescence intensity (34). Cells were treated with anti-CD95, and caspase 3 activation was clearly observed in both control cells and BIR3-sp-RING cells, but no observable PhiPhiLux cleavage was present in WT-XIAP cells (Fig. 4C). These intact-cell results were consistent with both the immunoblot analysis and DEVD cleavage experiments and confirm that in WT-XIAP cells treated with anti-CD95, caspase 3 activity is inhibited.
FIG. 4.
Caspase activity in Jurkat-derived cells. (A and B) Jurkat-derived cells were treated with anti-CD95 (A) or etoposide (B) for 0, 2, 4, or 6 h. Cytoplasmic extracts were then prepared and tested for the presence of caspase 3 activity by incubation with the fluorogenic substrate DEVD-AFC. The average ± standard deviation of multiple independent measurements is shown, and data are representative of three independent experiments. (C) Cells were treated with anti-CD95 followed by incubation with the cell-permeable caspase 3 substrate PhiPhiLux and analysis by flow cytometry. The average ± standard deviation of positively fluorescent cells is shown.
Given the differences between WT-XIAP and BIR3-sp-RING in suppressing caspase activity and protecting from death following anti-CD95 treatment, mitochondrial events were next examined. Using the same cytoplasmic extracts prepared for the caspase immunoblot assays shown in Fig. 3 above, the release of both cytochrome c and Smac/DIABLO from the mitochondria to the cytoplasm was assessed by immunoblot analysis. Interestingly, while both cytochrome c and Smac/DIABLO were released from control and BIR3-sp-RING cells following anti-CD95 treatment, WT-XIAP cells failed to release either cytochrome c or Smac/DIABLO (Fig. 5A). This contrasted with results obtained from etoposide-treated extracts, where control, WT-XIAP, and BIR3-sp-RING lines all exhibited release of cytochrome c and Smac/DIABLO with similar kinetics, as release of both proteins was observed by 4 h (Fig. 5B). As a control for both stimuli, Bcl-xL cells were also tested and displayed no release of either mitochondrial protein following up to 6 h of treatment with either stimulus. Equivalent protein loading for all samples was confirmed by immunoblotting for β-actin (Fig. 5), and extract quality was determined by immunoblotting for cytochrome oxidase subunit IV (data not shown).
FIG. 5.
Mitochondrial protein release in Jurkat-derived cells. Jurkat-derived cells were treated with anti-CD95 (A) or etoposide (B) for 0, 2, 4, or 6 h. Cytoplasmic extracts were then prepared and immunoblotted for the presence of both cytochrome c and Smac. Equivalent protein loading was confirmed by immunoblotting for the presence of β-actin, and extract quality was determined by immunoblotting for the presence of cytochrome oxidase subunit IV (data not shown).
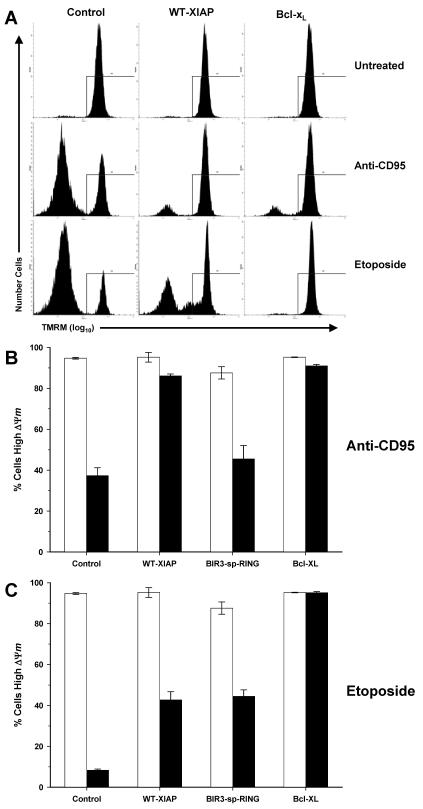
Caspase activation and mitochondrial protein release are two relatively early events in the progression of apoptosis. A subsequent event that is often caspase dependent is the loss of mitochondrial membrane potential, ΔΨm (31, 72) (see Fig. S1 in the supplemental material). To further characterize the differences in the protective properties of WT-XIAP versus BIR3-sp-RING, the loss of ΔΨm was examined. Cells were treated overnight with either anti-CD95 or etoposide, and ΔΨm was determined by flow cytometry (Fig. 6A) following staining with TMRM. Both Bcl-xL and WT-XIAP cells maintained ΔΨm following treatment with anti-CD95, whereas BIR3-sp-RING and control cells both lost ΔΨm (Fig. 6B). However, following etoposide treatment, only Bcl-xL cells were capable of maintaining normal ΔΨm, while control, WT-XIAP, and BIR3-sp-RING lines all showed a significant reduction of ΔΨm (Fig. 6C). Since only those cells that maintain ΔΨm survive long-term anti-CD95 treatment, these data correlate well with the results shown in Fig. 2 and suggest that XIAP supports the proliferative capacity of anti-CD95-treated cells, at least in part, by preventing the loss of ΔΨm.
FIG. 6.
Mitochondrial membrane potential in Jurkat-derived cells. (A) Representative raw flow cytometry data from untreated Jurkat-derived cells or cells treated with either anti-CD95 or etoposide and subsequently stained with TMRM. Lines indicate regions used to quantify TMRM-positive cells. (B) Quantification of ΔΨm in cells treated overnight with anti-CD95 followed by TMRM staining and analysis by flow cytometry. (C) Quantification of ΔΨm in cells treated overnight with etoposide followed by analysis as described for panel B. Bars represent the average ± standard deviation for multiple independent measurements from untreated (white bars) and treated (black bars) samples.
These data suggested that the caspase 3 inhibitory properties of XIAP are required to protect from anti-CD95 but are dispensable for suppression of cell death following etoposide treatment. Although caspase 9 and caspase 3 activation were clearly prevented in BIR3-sp-RING cells following etoposide treatment, the mechanism for this inhibition was unclear. BIR3-sp-RING may inhibit caspase 9 activity directly and thereby prevent caspase 9-dependent activation of caspase 3. Alternatively, since the third BIR domain of XIAP appears sufficient for binding to Smac/DIABLO in vitro (59), and since Smac/DIABLO is released from the mitochondria during etoposide-mediated death in BIR3-sp-RING cells (Fig. 5B), the possibility remained that BIR3-sp-RING might also bind Smac/DIABLO and therefore prevent the inhibition of endogenous XIAP. To assess if the BIR3-sp-RING used here (which lacks an additional 19 amino acids at the amino terminus compared to a BIR3-sp-RING truncation mutant detailed previously [59]) is indeed capable of functioning in this capacity, coimmunoprecipitation experiments were performed to determine whether BIR3-sp-RING is capable of binding to Smac/DIABLO. Under nonapoptotic conditions, XIAP and Smac/DIABLO are sequestered into cytoplasmic and mitochondrial compartments, respectively, and are therefore unable to interact. However, solubilization of both cytoplasmic and mitochondrial compartments prior to immunoprecipitation analysis permits any potential interactions between XIAP and Smac/DIABLO to occur postlysis. As shown in Fig. 7, immunoprecipitation of WT-XIAP followed by immunoblot analysis for the presence of Smac/DIABLO in precipitated complexes revealed a strong association between WT-XIAP and Smac/DIABLO. In contrast to WT-XIAP, Smac/DIABLO was undetectable in precipitated complexes from BIR3-sp-RING cells despite equivalent protein expression levels and immunoprecipitation efficiency. These data indicate that Smac/DIABLO and BIR3-sp-RING cannot interact and that the ability of BIR3-sp-RING to protect from etoposide-mediated death likely relies on direct inhibition of caspase 9.
FIG. 7.
XIAP-Smac coimmunoprecipitation. Whole-cell lysates from control, WT-XIAP, and BIR3-spRING cells were prepared and incubated with anti-HA antibodies followed by protein G-coupled agarose beads. Precipitated immune complexes were then immunoblotted for the presence of Smac/DIABLO (top panels) or the HA tag (bottom panels). Input samples contained 10% of the total protein used for immunoprecipitations. Specificity of immune complex precipitation was verified by control samples containing protein G-coupled agarose beads alone (data not shown).
Based on the above experiments evaluating caspase processing and activation, mitochondrial protein release, and loss of ΔΨm, XIAP appears capable of functioning upstream of mitochondrial activation in the prevention of cell death. Recent data suggest that in cells requiring mitochondrial amplification during death receptor-mediated apoptosis, the targeting of XIAP by the cytoplasmic release of Smac/DIABLO may play a more important role in apoptosis than the concurrent release of cytochrome c and subsequent apoptosome formation (14, 39, 62). Taken together, these data predict that in Bcl-xL cells, which are resistant to both etoposide- and anti-CD95-mediated death (Fig. 1 and 2), reducing XIAP protein levels would sensitize cells to anti-CD95 treatment. To test this hypothesis, XIAP protein levels were reduced in Bcl-xL cells by RNAi following transfection of short interfering RNA oligonucleotides (siRNA) specifically targeting XIAP. Forty-eight hours after transfection, the level of XIAP protein was greatly reduced (∼90%) by XIAP-specific siRNA compared to control siRNA-transfected cells (Fig. 8A). Treatment of these cells with anti-CD95 showed that the reduction of XIAP protein significantly reduced the protective effects of Bcl-xL (Fig. 8B). However, reduction of XIAP protein by RNAi had no effect upon the viability of Bcl-xL cells following etoposide treatment (Fig. 8C), suggesting that the points at which XIAP functions in regulating apoptosis are stimulus dependent. These data support the hypothesis that XIAP can function upstream of mitochondrial activation during death receptor-mediated apoptosis, and the data further implicate XIAP as a target of mitochondrial amplification during death receptor-induced cell death.
FIG. 8.
Suppression of XIAP sensitizes Bcl-xL cells to anti-CD95. (A) Control and Bcl-xL cells were transfected with siRNA oligonucleotides targeting XIAP or control siRNA oligonucleotides targeting GFP. Reduction of XIAP protein was assessed by immunoblot analysis performed on a portion of cells 48 h after transfection (top panel). Equivalent loading of each sample was confirmed by immunoblot analysis for the presence of β-actin (bottom panel). (B and C) The remaining cells were then treated overnight with either anti-CD95 (B) or etoposide (C) followed by PI staining and flow cytometry. The average ± standard deviation of multiple independent measurements is shown.
DISCUSSION
In this study, we established Jurkat-derived cell lines that stably overexpress either WT-XIAP or the truncation mutant BIR3-sp-RING, and we report here a detailed examination of the cytoprotective properties of these two XIAP variants. Short-term administration of agents that induce either intrinsic or extrinsic apoptotic programs revealed that while both variants of XIAP were equally capable of protecting cells from intrinsic apoptotic death, only the wild-type protein was further capable of protecting from the extrinsic stimulus, anti-CD95. Since the major functional difference between these two variants of XIAP is the ability to bind and inhibit caspase 3, these data strongly suggest that the caspase 3 inhibitory properties of XIAP, while dispensable for protection from intrinsic apoptotic stimuli, are required to prevent death receptor-mediated apoptosis. While previous reports have suggested that caspase 3 inhibition is not required for XIAP-mediated protection (53, 54), the fact that these studies only examined intrinsic cell death (following UV irradiation) highlights the stimulus-dependent nature of the protective properties of XIAP. When viability experiments were extended to examine long-term anti-CD95 administration, more striking differences in the protective properties of the two XIAP variants were observed: WT-XIAP conferred not only long-term survival, but also proliferative capacity to anti-CD95-treated cells, effects that were not observed with BIR3-sp-RING. These data are in agreement with the short-term protection data and further suggest that, since XIAP was as potent as Bcl-xL at protecting cells following anti-CD95, XIAP may function upstream of the mitochondria in protecting cells from CD95-mediated death.
Interestingly, both XIAP variants failed to protect cells and were incapable of supporting proliferative capacity following long-term administration of etoposide. Furthermore, Bcl-xL was also unable to facilitate eventual proliferation, though significantly higher levels of long-term viability by Bcl-xL were observed than with the XIAP variants. This observation contrasts with similar experiments examining Bcl-2-mediated protection of Jurkat cells, in which clonogenic survival was observed following a variety of intrinsic stimuli (3). However, this discrepancy may highlight differences among the various agents used to induce intrinsic cell death, since the previous report employed staurosporine, a protein kinase inhibitor, rather than etoposide, an inhibitor of topoisomerase II, used in the experiments shown here. By acting as a topoisomerase II inhibitor, etoposide induces DNA damage and subsequent cell cycle arrest at either the G1/S or G2/M transition (12). Given this mechanism of action, it is not surprising that despite remaining viable following long-term etoposide treatment, the Bcl-xL-overexpressing cells presented here were unable to reenter the cell cycle and continue proliferation.
Examination of caspase processing and activity following anti-CD95 revealed that in contrast to the BIR3-sp-RING mutant, WT-XIAP significantly inhibited both processing and activation of caspase 9 and caspase 3. Further, the partial processing of caspase 3 observed in WT-XIAP cells is consistent with the hypothesis that in the case of caspase 3, XIAP binds to a partially processed form of the zymogen and prevents subsequent cleavage to the fully active form (62). Following etoposide treatment, both XIAP variants equally inhibited caspase 9 and caspase 3, supporting the hypothesis that the caspase 3 inhibitory properties of XIAP are not required to protect cells from intrinsic proapoptotic stimuli. Moreover, since BIR3-sp-RING was incapable of binding Smac/DIABLO, it is likely that this protein prevents cell death through inhibition of caspase 9, rather than by preventing Smac/DIABLO from inhibiting endogenous XIAP.
By examining the events of mitochondrial amplification, a major insight into the mechanism by which XIAP supports long-term protection of cells from CD95-mediated death was obtained. Cytochrome c and Smac/DIABLO release, while not inhibited by either XIAP variant following etoposide treatment, was potently inhibited in WT-XIAP cells following anti-CD95 treatment. Furthermore, mitochondrial membrane potential was significantly reduced in both lines following etoposide treatment, yet remained normal in the WT-XIAP line. Several studies suggest that the preservation of mitochondrial integrity, in terms of preventing both the release of intermembrane proteins and the loss of membrane potential, is a major contributing factor in allowing cells not only to survive an apoptotic stimulus but also to proliferate once that stimulus is removed (3, 46). Our data agree with this view: only in cells that both failed to release mitochondrial proteins and continued to maintain mitochondrial membrane potential was cell death averted and proliferative capacity maintained. Since neither mitochondrial event occurs in WT-XIAP cells following anti-CD95 treatment, these observations support a model in which XIAP functions upstream of the mitochondria during death receptor-mediated death and that, by functioning prior to mitochondrial activation, XIAP can allow cells not only to survive, but to resume proliferation.
The functional consequences of cytochrome c and Smac/DIABLO release during death receptor-mediated apoptosis have recently been investigated, and it has been shown that Smac/DIABLO release plays a major role in allowing death to proceed (14, 62). Since XIAP is the predominant target of Smac/DIABLO (14, 39, 62), this suggests that preventing XIAP-mediated inhibition of caspase 3 may be more important than the activation of caspase 9 that occurs as a result of cytochrome c release. In support of this model, our data showed that reduction of XIAP protein levels by RNAi sensitized Bcl-xL cells to CD95-mediated death, in agreement with previous studies suggesting that the major consequence of mitochondrial amplification is the inhibition of XIAP by Smac/DIABLO.
Despite the data presented here, implicating an upstream regulatory role for XIAP in preventing death receptor-mediated apoptosis, the exact mechanism by which XIAP prevents mitochondrial amplification remains unclear. Neither full-length XIAP nor BIR3-sp-RING appears to affect the processing of caspase 8 following anti-CD95 treatment (Fig. 3A), and these results agree with previous reports describing the failure of XIAP to inhibit the activity of caspase 8 both in vitro (17, 18, 64) and in intact cells (22, 23, 32, 36, 71). It is therefore extremely unlikely that the protective effects of XIAP reported here are the result of direct caspase 8 inhibition. While it has been proposed that caspase 8-mediated Bid cleavage may play a role in mitochondrial amplification following death receptor activation (37, 43, 63), Bid cleavage has also been observed during intrinsic apoptosis induced by a variety of chemotherapeutic drugs (56), and caspase 8 activity has been shown to be dispensable for Bid cleavage following activation of intrinsic cell death in Jurkat cells (22). The implication of these studies is that caspase 3 plays a direct role in mediating Bid cleavage during intrinsic cell death and, indeed, at least one consensus caspase 3 cleavage site is present within the primary amino acid sequence of Bid (25). In light of these studies and along with the data presented here, one possible mechanism by which XIAP prevents CD95-mediated death in Jurkat cells is through preventing caspase 3-mediated Bid cleavage, an event that may be required for mitochondrial amplification during death receptor-mediated apoptosis. Alternatively, since Bid-deficient mice display no overt phenotype in CD95-mediated apoptosis in any cell type other than hepatocytes (75), it is possible that XIAP prevents death receptor amplification by inhibiting a caspase 3-dependent pathway that does not require Bid cleavage.
Taken together, the data presented here highlight a new position where XIAP can function in regulating apoptosis and support a model in which XIAP functions upstream of the mitochondria during CD95-mediated death through the inhibition of caspase 3. This inhibition prevents mitochondrial protein release and loss of membrane potential and further shows that the activity of caspase-3 is required for this mitochondrial amplification of death receptor signals. Finally, by preventing caspase 3-dependent mitochondrial amplification, XIAP not only protects cells from apoptosis but also supports the long-term proliferative capacity of cells following CD95 activation.
Supplementary Material
Acknowledgments
We are grateful to E. Burstein and J. Lewis for critical discussions and to A. Wilkinson for valuable technical assistance. We thank M. Peter for kindly providing anti-caspase-8.
This work was supported in part by the University of Michigan Biomedical Scholars Program (to C.S.D.) and grants R01 GM65813 (to L.H.B.), GM20435 (to E.C.), and T32 CA09676 (to J.C.W.) from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Acehan, D., X. Jiang, D. G. Morgan, J. E. Heuser, X. Wang, and C. W. Akey. 2002. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9:423-432. [DOI] [PubMed] [Google Scholar]
- 2.Adrain, C., E. M. Creagh, and S. J. Martin. 2001. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 20:6627-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarante-Mendes, G. P., D. M. Finucane, S. J. Martin, T. G. Cotter, G. S. Salvesen, and D. R. Green. 1998. Anti-apoptotic oncogenes prevent caspase-dependent and independent commitment for cell death. Cell Death Differ. 5:298-306. [DOI] [PubMed] [Google Scholar]
- 4.Birkey Reffey, S., J. U. Wurthner, W. T. Parks, A. B. Roberts, and C. S. Duckett. 2001. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-β signaling. J. Biol. Chem. 276:26542-26549. [DOI] [PubMed] [Google Scholar]
- 5.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. [DOI] [PubMed] [Google Scholar]
- 6.Boise, L. H., and C. B. Thompson. 1997. Bcl-xL can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc. Natl. Acad. Sci. USA 94:3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 8.Cain, K., S. B. Bratton, C. Langlais, G. Walker, D. G. Brown, X. M. Sun, and G. M. Cohen. 2000. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J. Biol. Chem. 275:6067-6070. [DOI] [PubMed] [Google Scholar]
- 9.Cain, K., D. G. Brown, C. Langlais, and G. M. Cohen. 1999. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem. 274:22686-22692. [DOI] [PubMed] [Google Scholar]
- 10.Chai, J., C. Du, J. W. Wu, S. Kyin, X. Wang, and Y. Shi. 2000. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406:855-862. [DOI] [PubMed] [Google Scholar]
- 11.Chai, J., E. Shiozaki, S. M. Srinivasula, Q. Wu, P. Dataa, E. S. Alnemri, and Y. Shi. 2001. Structural basis of caspase-7 inhibition by XIAP. Cell 104:769-780. [DOI] [PubMed] [Google Scholar]
- 12.Clifford, B., M. Beljin, G. R. Stark, and W. R. Taylor. 2003. G2 arrest in response to topoisomerase II inhibitors: the role of p53. Cancer Res. 63:4074-4081. [PubMed] [Google Scholar]
- 13.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. [DOI] [PubMed] [Google Scholar]
- 14.Deng, Y., Y. Lin, and X. Wu. 2002. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deveraux, Q. L., E. Leo, H. R. Stennicke, K. Welsh, G. S. Salvesen, and J. C. Reed. 1999. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 18:5242-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deveraux, Q. L., and J. C. Reed. 1999. IAP family proteins-suppressors of apoptosis. Genes Dev. 13:239-252. [DOI] [PubMed] [Google Scholar]
- 17.Deveraux, Q. L., N. Roy, H. R. Stennicke, T. Van Arsdale, Q. Zhou, S. M. Srinivasula, E. S. Alnemri, G. S. Salvesen, and J. C. Reed. 1998. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deveraux, Q. L., R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300-304. [DOI] [PubMed] [Google Scholar]
- 19.Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33-42. [DOI] [PubMed] [Google Scholar]
- 20.Duckett, C. S., F. Li, Y. Wang, K. J. Tomaselli, C. B. Thompson, and R. C. Armstrong. 1998. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol. Cell. Biol. 18:608-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duckett, C. S., V. E. Nava, R. W. Gedrich, R. J. Clem, J. L. Van Dongen, M. C. Gilfillan, H. Shiels, J. M. Hardwick, and C. B. Thompson. 1996. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 15:2685-2694. [PMC free article] [PubMed] [Google Scholar]
- 22.Engels, I. H., A. Stepczynska, C. Stroh, K. Lauber, C. Berg, R. Schwenzer, H. Wajant, R. U. Janicke, A. G. Porter, C. Belka, M. Gregor, K. Schulze-Osthoff, and S. Wesselborg. 2000. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene 19:4563-4573. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira, C. G., S. W. Span, G. J. Peters, F. A. Kruyt, and G. Giaccone. 2000. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 60:7133-7141. [PubMed] [Google Scholar]
- 24.Fujita, E., J. Egashira, K. Urase, K. Kuida, and T. Momoi. 2001. Caspase-9 processing by caspase-3 via a feedback amplification loop in vivo. Cell Death Differ. 8:335-344. [DOI] [PubMed] [Google Scholar]
- 25.Gross, A., X. M. Yin, K. Wang, M. C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S. J. Korsmeyer. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156-1163. [DOI] [PubMed] [Google Scholar]
- 26.Hegde, R., S. M. Srinivasula, Z. Zhang, R. Wassell, R. Mukattash, L. Cilenti, G. DuBois, Y. Lazebnik, A. S. Zervos, T. Fernandes-Alnemri, and E. S. Alnemri. 2001. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts IAP-caspase interaction. J. Biol. Chem. 277:432-438. [DOI] [PubMed] [Google Scholar]
- 27.Holcik, M., and R. G. Korneluk. 2001. XIAP, the guardian angel. Nat. Rev. Mol. Cell Biol. 2:550-556. [DOI] [PubMed] [Google Scholar]
- 28.Hu, S., and X. Yang. 2003. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J. Biol. Chem. 278:10055-10060. [DOI] [PubMed] [Google Scholar]
- 29.Huang, H.-K., C. A. P. Joazeiro, E. Bonfoco, S. Kamada, J. D. Leverson, and T. Hunter. 2000. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J. Biol. Chem. 275:26661-26664. [DOI] [PubMed] [Google Scholar]
- 30.Huang, Y., Y. C. Park, R. L. Rich, D. Segal, D. G. Myszka, and H. Wu. 2001. Structural basis of caspase inhibition by XIAP. Differential roles of the linker versus the BIR domain. Cell 104:781-790. [PubMed] [Google Scholar]
- 31.Johnson, B. W., E. Cepero, and L. H. Boise. 2000. Bcl-xL inhibits cytochrome c release but not mitochondrial depolarization during the activation of multiple death pathways by tumor necrosis factor-α. J. Biol. Chem. 275:31546-31553. [DOI] [PubMed] [Google Scholar]
- 32.Kahns, S., M. Kalai, L. D. Jakobsen, B. F. Clark, P. Vandenabeele, and P. H. Jensen. 2003. Caspase-1 and caspase-8 cleave and inactivate cellular parkin. J. Biol. Chem. 278:23376-23380. [DOI] [PubMed] [Google Scholar]
- 33.Kerr, J. F., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komoriya, A., B. Z. Packard, M. J. Brown, M. L. Wu, and P. A. Henkart. 2000. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J. Exp. Med. 191:1819-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwana, T., J. J. Smith, M. Muzio, V. Dixit, D. D. Newmeyer, and S. Kornbluth. 1998. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J. Biol. Chem. 273:16589-16594. [DOI] [PubMed] [Google Scholar]
- 36.Leverkus, M., M. R. Sprick, T. Wachter, T. Mengling, B. Baumann, E. Serfling, E. B. Brocker, M. Goebeler, M. Neumann, and H. Walczak. 2003. Proteasome inhibition results in TRAIL sensitization of primary keratinocytes by removing the resistance-mediating block of effector caspase maturation. Mol. Cell. Biol. 23:777-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 38.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 39.Li, S., Y. Zhao, X. He, T. H. Kim, D. K. Kuharsky, H. Rabinowich, J. Chen, C. Du, and X. M. Yin. 2002. Relief of extrinsic pathway inhibition by the bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J. Biol. Chem. 277:26912-26920. [DOI] [PubMed] [Google Scholar]
- 40.Li, X., Y. Yang, and J. D. Ashwell. 2002. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 416:345-347. [DOI] [PubMed] [Google Scholar]
- 41.Liston, P., N. Roy, K. Tamai, C. Lefebvre, S. Baird, G. Cherton-Horvat, R. Farahani, M. McLean, J.-E. Ikeda, A. MacKenzie, and R. G. Korneluk. 1996. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349-353. [DOI] [PubMed] [Google Scholar]
- 42.Liu, Z., C. Sun, E. T. Olejniczak, R. P. Meadows, S. F. Betz, T. Oost, J. Herrmann, J. C. Wu, and S. W. Fesik. 2000. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature 408:1004-1008. [DOI] [PubMed] [Google Scholar]
- 43.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 44.Martins, L. M., I. Iaccarino, T. Tenev, S. Gschmeissner, N. F. Totty, N. R. Lemoine, J. Savopoulos, C. W. Gray, C. L. Creasy, C. Dingwall, and J. Downward. 2001. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a Reaper-like motif. J. Biol. Chem. 277:439-444. [DOI] [PubMed] [Google Scholar]
- 45.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mootha, V. K., M. C. Wei, K. F. Buttle, L. Scorrano, V. Panoutsakopoulou, C. A. Mannella, and S. J. Korsmeyer. 2001. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J. 20:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagata, S. 1996. Apoptosis: telling cells their time is up. Curr. Biol. 6:1241-1243. [DOI] [PubMed] [Google Scholar]
- 48.Nicholson, D. W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028-1042. [DOI] [PubMed] [Google Scholar]
- 49.Richter, B. W. M., S. S. Mir, L. J. Eiben, J. Lewis, S. B. Reffey, A. Frattini, L. Tian, S. Frank, R. J. Youle, D. L. Nelson, L. D. Notarangelo, P. Vezzoni, H. O. Fearnhead, and C. S. Duckett. 2001. Molecular cloning of ILP-2, a novel member of the inhibitor of apoptosis protein (IAP) family. Mol. Cell. Biol. 21:4292-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riedl, S. J., M. Renatus, R. Schwarzenbacher, Q. Zhou, C. Sun, S. W. Fesik, R. C. Liddington, and G. S. Salvesen. 2001. Structural basis for the inhibition of caspase-3 by XIAP. Cell 104:791-800. [DOI] [PubMed] [Google Scholar]
- 51.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K.-M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiozaki, E. N., J. Chai, D. J. Rigotti, S. J. Riedl, P. Li, S. M. Srinivasula, E. S. Alnemri, R. Fairman, and Y. Shi. 2003. Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell 11:519-527. [DOI] [PubMed] [Google Scholar]
- 53.Silke, J., P. G. Ekert, C. L. Day, C. J. Hawkins, M. Baca, J. Chew, M. Pakusch, A. M. Verhagen, and D. L. Vaux. 2001. Direct inhibition of caspase 3 is dispensable for the anti-apoptotic activity of XIAP. EMBO J. 20:3114-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silke, J., C. J. Hawkins, P. G. Ekert, J. Chew, C. L. Day, M. Pakusch, A. M. Verhagen, and D. L. Vaux. 2002. The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites. J. Cell Biol. 157:115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slee, E. A., S. A. Keogh, and S. J. Martin. 2000. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 7:556-565. [DOI] [PubMed] [Google Scholar]
- 57.Srinivasula, S. M., M. Ahmad, T. Fernandes-Alnemri, and E. S. Alnemri. 1998. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1:949-957. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasula, S. M., S. Gupta, P. Datta, Z. Zhang, R. Hegde, N. Cheong, T. Fernandes-Alnemri, and E. S. Alnemri. 2003. Inhibitor of apoptosis proteins are substrates for the mitochondrial serine protease Omi/HtrA2. J. Biol. Chem. 278:31469-31472. [DOI] [PubMed] [Google Scholar]
- 59.Srinivasula, S. M., R. Hegde, A. Saleh, P. Datta, E. Shiozaki, J. Chai, R. A. Lee, P. D. Robbins, T. Fernandes-Alnemri, Y. Shi, and E. S. Alnemri. 2001. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410:112-116. [DOI] [PubMed] [Google Scholar]
- 60.Stennicke, H. R., Q. L. Deveraux, E. W. Humke, J. C. Reed, V. M. Dixit, and G. S. Salvesen. 1999. Caspase-9 can be activated without proteolytic processing. J. Biol. Chem. 274:8359-8362. [DOI] [PubMed] [Google Scholar]
- 61.Sun, C., M. Cai, R. P. Meadows, N. Xu, A. H. Gunasekera, J. Herrmann, J. C. Wu, and S. W. Fesik. 2000. NMR structure and mutagenesis of the third BIR domain of the inhibitor of apoptosis protein XIAP. J. Biol. Chem. 275:33777-33781. [DOI] [PubMed] [Google Scholar]
- 62.Sun, X. M., S. B. Bratton, M. Butterworth, M. Macfarlane, and G. M. Cohen. 2002. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of XIAP. J. Biol. Chem. 277:11345-11351. [DOI] [PubMed] [Google Scholar]
- 63.Tafani, M., N. O. Karpinich, K. A. Hurster, J. G. Pastorino, T. Schneider, M. A. Russo, and J. L. Farber. 2002. Cytochrome c release upon Fas receptor activation depends on translocation of full-length Bid and the induction of the mitochondrial permeability transition. J. Biol. Chem. 277:10073-10082. [DOI] [PubMed] [Google Scholar]
- 64.Tamm, I., M. Trepel, M. Cardo-Vila, Y. Sun, K. Welsh, E. Cabezas, A. Swatterthwait, W. Arap, J. C. Reed, and R. Pasqualini. 2003. Peptides targeting caspase inhibitors. J. Biol. Chem. 278:14401-14405. [DOI] [PubMed] [Google Scholar]
- 65.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 66.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 67.Uren, A., M. Pakusch, C. Hawkins, K. L. Puls, and D. L. Vaux. 1996. Cloning and expression of apoptosis inhibitory proteins homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc. Natl. Acad. Sci. USA. 93:4974-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaux, D. L., G. Haecker, and A. Strasser. 1994. An evolutionary perspective on apoptosis. Cell 76:777-779. [DOI] [PubMed] [Google Scholar]
- 69.Verhagen, A. M., P. G. Ekert, M. Pakusch, J. Silke, L. M. Connolly, G. E. Reid, R. L. Moritz, R. J. Simpson, and D. L. Vaux. 2000. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43-53. [DOI] [PubMed] [Google Scholar]
- 70.Verhagen, A. M., J. Silke, P. G. Ekert, M. Pakusch, H. Kaufmann, L. M. Connolly, C. L. Day, A. Tikoo, R. Burke, C. Wrobel, R. L. Moritz, R. J. Simpson, and D. L. Vaux. 2001. HtrA2 promotes cell death through its serine protease activity and its ability to antagonise inhibitor of apoptosis proteins. J. Biol. Chem. 277:445-454. [DOI] [PubMed] [Google Scholar]
- 71.Wagenknecht, B., T. Glaser, U. Naumann, S. Kugler, S. Isenmann, M. Bahr, R. Korneluk, P. Liston, and M. Weller. 1999. Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ. 6:370-376. [DOI] [PubMed] [Google Scholar]
- 72.Waterhouse, N. J., J. C. Goldstein, O. von Ahsen, M. Schuler, D. D. Newmeyer, and D. R. Green. 2001. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J. Cell Biol. 153:319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang, Q. H., R. Church-Hajduk, J. Ren, M. L. Newton, and C. Du. 2003. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev. 17:1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang, Y., S. Fang, J. P. Jensen, A. M. Weissman, and J. D. Ashwell. 2000. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288:874-877. [DOI] [PubMed] [Google Scholar]
- 75.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.