Abstract
Pulmonary toxicity related to the use of pegylated interferon alpha-2a during treatment of hepatitis C infections is rare; nonetheless, some cases with fatal outcomes have been reported. Evaluating patients’ pulmonary function is a key to diagnosis, follow-up and prognosis of several respiratory diseases, but case reports of respiratory manifestations related to the use of pegylated interferon alpha-2a have limited their findings to only baseline measurements. This paper examines the case of a 65-year-old woman with chronic hepatitis C virus infection who developed interstitial pneumonitis associated with pegylated interferon alpha-2a. Initial lung function evaluation revealed a marked reduction compared to an earlier assessment; the results were consistent with a moderate restricted pattern. Fortunately, over the ensuing 8 weeks of follow-up after discontinuing the drug, the patient recovered her lung function and experienced an overall improvement in her respiratory symptoms.
KEY WORDS: Interstitial pneumonia, lung function, pegylated interferon alpha-2a, restrictive pattern
INTRODUCTION
Persistent injurious stimuli derived from hepatitis C virus (HCV) infection can lead to cirrhosis and hepatocellular carcinoma.[1] Therapeutic strategies for HCV infections include a combination of pegylated interferon (IFN) and ribavirin.[2] However, several adverse effects associated with the use of pegylated IFN alpha-2a and -2b are known. Most reports describe flu-like symptoms, myalgia, fatigue, gastrointestinal, psychiatric and hematologic abnormalities. Pulmonary manifestations are rare, though findings included interstitial pneumonia, pleuritis, organized cryptogenic pneumonia and exacerbations of asthma.[3,4,5] The majority of the descriptions have been reported as a consequence of pegylated IFN α-2b;[5,6,7] while few events with pegylated IFN α-2a.[6,8,9,10]
Lung function evaluation is important for diagnosis, follow-up and prognosis in several respiratory diseases.[11,12,13] Reports of pulmonary complications due to the aforementioned treatment that have included lung function measurement are limited to baseline descriptions;[7,14] however, current standards recommend functional assessments as part of the follow-up procedure for all respiratory conditions that have received a therapeutic intervention.[15] Instead, cases that have had respiratory complications due to the treatment of HCV infection are considered to be discharged based in resolution of imaging abnormalities; notwithstanding, we must keep in mind that any change in X-ray scan following lung injury can occur much later and may not correspond to the evolution of symptoms.[16,17]
Therefore, the main purpose of this paper is to analyze the case of a woman in her mid-60s who developed interstitial pneumonia associated with pegylated IFN α-2a with special focus in lung function. Although is an infrequent adverse effect, the number of reports that developed any respiratory symptom is increasing; thus, it is important to remain alert in order to identify complications opportunely since in some scenarios has resulted in life-threatening conditions and death.[18,19]
Clinical case
A 64-year-old woman whose relevant clinical history included: (1) A well-controlled asthma based on symptoms (22-Point Asthma Control Test or ACT) and lung function measured by post-bronchodilator spirometry [FEV1/FVC 80%, FVC 2.26 L (76%p), and FEV1 1.81 L (80%p)]; and, (2) a history of mild, intermittent allergic rhinitis.
The patient was diagnosed with chronic HCV infection in 2007. Initially was treated with IFN α-2b + ribavirin. However, the medication was stopped in the next 3 weeks due to diarrhea and vomiting; thereafter, the treatment was modified to lamivudine + ribavirin for the subsequent 2 years and 6 months. Once again, the drug therapy was suspended because the patient complaint with gastrointestinal symptoms. Recently, in March 2014, she started a treatment with only IFN α-2a (180 μg per week).
After 8 weeks of treatment, the patient developed dyspnea when climbing stairs and progressed in the next 4 weeks being evident when she performed routine activities such as personal grooming and housework. In addition, the patient started to use short-acting bronchodilators due to excessive nighttime coughing, together with a perception of uncontrolled asthma (ACT 14 points). Moreover, the patient suffered isolated episodes of epistaxis that required cryotherapy, and recurrent episodes of flu-like symptoms.
During the physical examination both nostrils with hematic clots and scabs; in the chest, fine bilateral inspiratory crackles were identified.
Based on these clinical findings, high resolution computed tomography (HRCT) of the chest was performed [Figure 1]. The most significant findings were the presence of diffuse ground glass opacities in the lung lower lobes.
Figure 1.
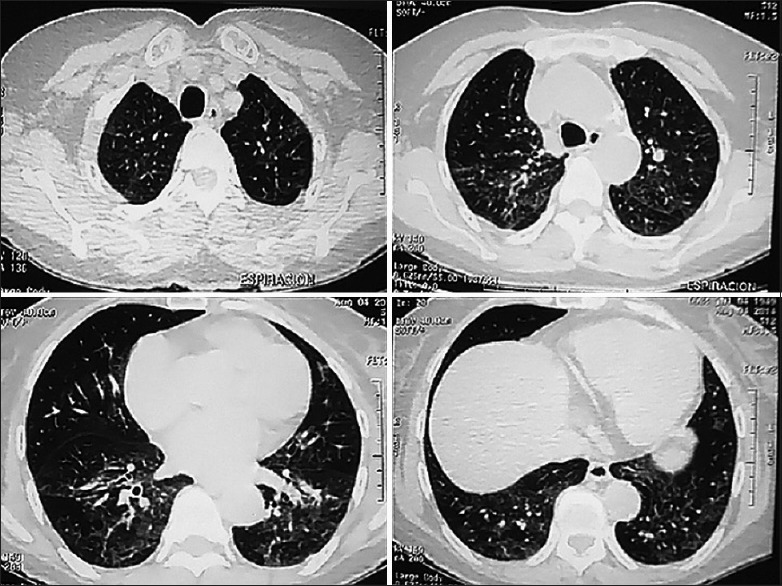
Initial high-Rresolution computed tomography (HRCT) of the chest demonstrates bilateral interstitial infiltrates. The main abnormalities were ground-glass opacities more prominent in the lower lung lobes
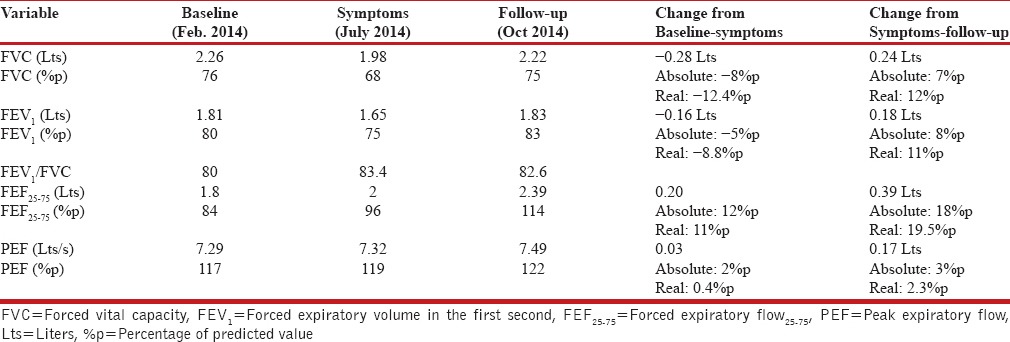
The lung function evaluation showed a moderate restrictive pattern [Table 1] with mild hypoxemia while breathing on room air (SpO2 of 93%) Based on these findings it was decided to stop the medication (IFN α-2a) and maintain only with asthma treatments (Salmeterol/Fluticasone 50/250 mcg BID).
Table 1.
Description of lung function evaluation (baseline, symptoms and follow-up)
In the follow-up evaluation (8 weeks after symptom onset), there was no evidence of epistaxis, flu-like symptoms, and a marked improvement in the perception of dyspnea during physical activity. A second HRCT was ordered, together with an analysis of lung function. The images from the scan are showed in Figure 2; while the functional results are reported in Table 1; in addition, SpO2 of 97% while breathing on room air.
Figure 2.
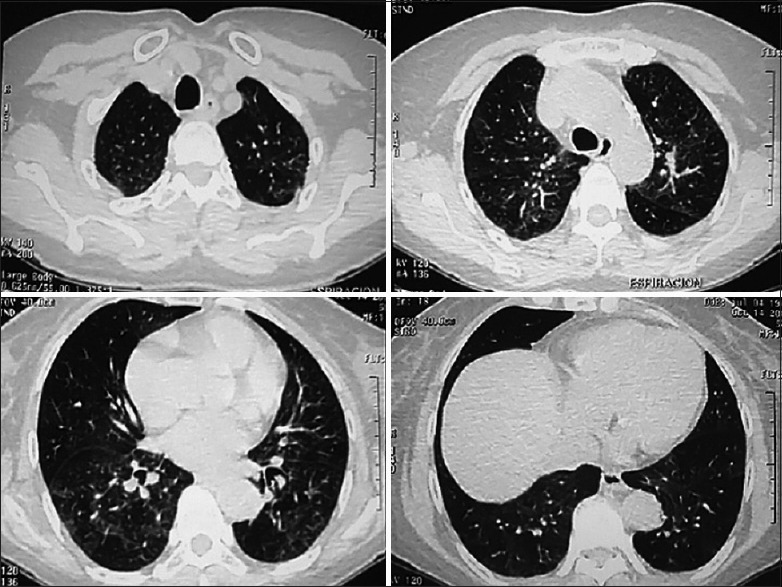
Follow-up (10 weeks) high-resolution computed tomography (HRCT) of the chest. There is still evidence of ground-glass opacities; however, less attenuation regarding the initial HRCT scan
At present, the patient remains in stable condition with both her asthma (ACT Questionnaire = 20 points) and pulmonary function under control [Figure 3]. Possible options for a new treatment scheme to control chronic HCV infection are being analyzed.
Figure 3.
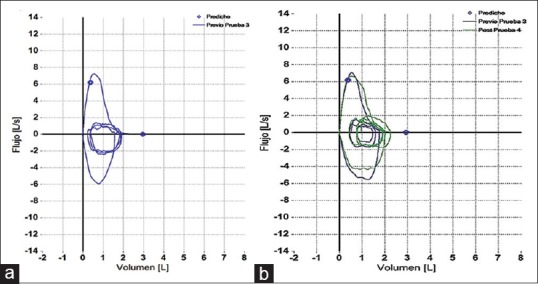
(a) Baseline and (b) Follow-up flow-volume loops
DISCUSSION
The current case was considered relevant because it illustrates the evolution of the pulmonary function in a patient with chronic HCV infection who developed an interstitial pneumonia related to the use of IFN α-2a. Also, this case adds evidence about pulmonary toxicity related to this specific drug; the awareness of the side effects will lead us to recognize such eventualities since some of them may result in permanent lung damage or even fatal outcomes.[7,18,19]
It is important to emphasize that current treatments for chronic HCV infections are not exempted from adverse events; the frequency of pulmonary toxicity is less than 1%.[4] Moreover, the prevalence of interstitial pneumonia is uncertain, some estimates place it between 0.01 and 0.3%;[14] the precise mechanism that causes such damage has not been clarified.
To the best of our knowledge, previous reports of any respiratory side effect due to the treatment with IFN α-2a or -2b describes improvements in clinical symptoms, pharmacological strategies implemented to treat this adverse effects, time to chest X-ray resolution and outcome;[14] however, very few are focused on lung function evolution.[20]
Patients who manifest interstitial pneumonia while using IFN tend to develop any complaint within an average time of 12 weeks, though the range varies from 2 to 48 weeks. Most reports are linked to IFN alpha-2b.[7]
The main intervention for the side effects includes suspending the drug immediately and, some reports recommend beginning treatment with a systemic corticosteroid (CS). However, the precise indication to use systemic CS is uncertain since there is no guideline that clarifies which patients are candidates for it, or the exact dosage and time required to treat the adverse effects.[14] It is important to note that among cases previously reported who were taking IFN α-2a, 66.7% were resolved simply by suspending the drug.[3,4,10]
Resolution of the clinical symptoms occurs between 8 and 12 weeks.[14,20] The case analyzed here allows us to add that evolution toward improved pulmonary function coincided with improvements in the symptomatology.
Our findings emphasize the importance of lung function evaluation during the follow-up, since it can identify early functional changes and the severity of such abnormalities. Usually, the functional pattern in this group of patients consists in pulmonary restriction.[14,21]
The only study that has evaluated pulmonary function in patients with chronic HCV infection undergoing treatment with IFN plus ribavirin was published in 2013.[22] Foster et al. described a group of patients who were subjected to treatment for 24 weeks, and then were re-evaluated in the subsequent 24 weeks. According to their functional findings, the average allowable decrease in the forced vital capacity (FVC) is 6% at the end of treatment. The maximum effect occurs in the first 12 weeks and, in general, after suspending the drug the abnormalities subside in the ensuing 24 weeks. Nonetheless, 13% of cases persisted with a decrease equal to or greater than 10%. Therefore, despite the fact that the patient in our case experienced a substantial decrease in her lung function (-12.4%p in FVC) after 12 weeks of treatment with IFN α-2a, she recovered it over the ensuing 3 months, once the drug was suspended.
Based on these findings, clinicians that evaluates and treats this group of patients should be encouraged to monitor their lung function in order to opportunely detect any alterations that prevent disease progression, since in isolated cases may lead to death due to events of acute respiratory failure.[14,19] Timely recognition will make it possible to develop therapeutic strategies designed to reduce complications.
CONCLUSIONS
Frequent monitoring of the pulmonary function of patients with chronic HCV infections who are prescribed a combined treatment with IFN and ribavirin may alert clinicians to the onset of respiratory complications. Also, functional follow-up during at least 8 weeks will help define the need-or lack there of-for other pharmacological strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author would like to thank Paul Kersey for correcting errors in language usage.
REFERENCES
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–51. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 3.Kumar KS, Russo MW, Borczuk AC, Brown M, Esposito SP, Lobritto SJ, et al. Significant pulmonary toxicity associated with interferon and ribavirin therapy for hepatitis C. Am J Gastroenterol. 2002;97:2432–40. doi: 10.1111/j.1572-0241.2002.05999.x. [DOI] [PubMed] [Google Scholar]
- 4.Midturi J, Sierra-Hoffman M, Hurley D, Winn R, Beissner R, Carpenter J. Spectrum of pulmonary toxicity associated with the use of interferon therapy for hepatitis C: Case report and review of the literature. Clin Infect Dis. 2004;39:1724–9. doi: 10.1086/425746. [DOI] [PubMed] [Google Scholar]
- 5.Bini EJ, Weinshel EH. Severe exacerbation of asthma: A new side effect of interferon-alpha in patients with asthma and chronic hepatitis C. Mayo Clin Proc. 1999;74:367–70. doi: 10.4065/74.4.367. [DOI] [PubMed] [Google Scholar]
- 6.Hegade VS, Sood R, Saralaya D, Moreea S. Pulmonary complications of treatment with pegylated interferon for hepatitis C infection-two case reports. Ann Hepatol. 2013;12:629–33. [PubMed] [Google Scholar]
- 7.Slavenburg S, Heijdra YF, Drenth JP. Pneumonitis as a consequence of (peg) interferon-ribavirin combination therapy for hepatitis C: A review of the literature. Dig Dis Sci. 2010;55:579–85. doi: 10.1007/s10620-009-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins RS, Machado JA, Teixeira R. Secondary bronchiolitis obliterans organizing pneumonia during treatment of chronic hepatitis C: Role of pegylated interferon alfa-2a. Rev Soc Bras Med Trop. 2012;45:655–6. doi: 10.1590/s0037-86822012000500023. [DOI] [PubMed] [Google Scholar]
- 9.Atug O, Akin H, Alahdab YO, Ceyhan B, Tozun N, Ozdogan O. Interstitial pneumonitis associated with pegylated interferon-alpha 2a and ribavirin for chronic hepatitis C infection: A case report. Cases J. 2009;2:6464. doi: 10.1186/1757-1626-2-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renou C, Germain S, Harafa A, Martin S, Larroque O, Muller P, et al. Interstitial pneumonia recurrence during chronic hepatitis C treatment. Am J Gastroenterol. 2005;100:1625–6. doi: 10.1111/j.1572-0241.2005.50006_9.x. [DOI] [PubMed] [Google Scholar]
- 11.Jegal Y, Kim DS, Shim TS, Lim CM, Do Lee S, Koh Y, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171:639–44. doi: 10.1164/rccm.200403-331OC. [DOI] [PubMed] [Google Scholar]
- 12.Cortes-Telles A, Forkert L, O’Donnell DE, Moran-Mendoza O. Idiopathic pulmonary fibrosis: New insights on functional characteristics at diagnosis. Can Respir J. 2014;21:e55–60. doi: 10.1155/2014/825606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index.An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–85. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji FP, Li ZX, Deng H, Xue HA, Liu Y, Li M. Diagnosis and management of interstitial pneumonitis associated with interferon therapy for chronic hepatitis C. World J Gastroenterol. 2010;16:4394–9. doi: 10.3748/wjg.v16.i35.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puente Maestú L, García de Pedro J. Lung function tests in clinical decision-making. Arch Bronconeumol. 2012;48:161–9. doi: 10.1016/j.arbres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Bruns AH, Oosterheert JJ, El Moussaoui R, Opmeer BC, Hoepelman AI, Prins JM. Pneumonia recovery: Discrepancies in perspectives of the radiologist, physician and patient. J Gen Intern Med. 2010;25:203–6. doi: 10.1007/s11606-009-1182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittl RL Jr, Schwab RJ, Duchin JS, Goin JE, Albeida SM, Miller WT. Radiographic resolution of community-acquired pneumonia. Am J Respir Crit Care Med. 1994;149:630–5. doi: 10.1164/ajrccm.149.3.8118630. [DOI] [PubMed] [Google Scholar]
- 18.Carrillo-Esper R, González-Avila D, Uribe-Rios M, Méndez-Sánchez N. Interstitial pneumonitis associated with pegylated interferon alpha-2b therapy for chronic hepatitis C: Case report. Ann Hepatol. 2008;7:87–90. [PubMed] [Google Scholar]
- 19.Abi-Nassif S, Mark EJ, Fogel RB, Hallisey RK., Jr Pegylated interferon and ribavirin-induced interstitial pneumonitis with ARDS. Chest. 2003;124:406–10. doi: 10.1378/chest.124.1.406. [DOI] [PubMed] [Google Scholar]
- 20.Kang EJ, Kim DK, Jeon SR, Choi HS, Jeong SW, Jang JY, et al. Interstitial pneumonitis in a patient with chronic hepatitis C and chronic renal failure on interferon therapy. Korean J Gastroenterol. 2011;58:47–52. doi: 10.4166/kjg.2011.58.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 22.Foster GR, Zeuzem S, Pianko S, Sarin SK, Piratvisuth T, Shah S, et al. Decline in pulmonary function during chronic hepatitis C virus therapy with modified interferon alpha and ribavirin. J Viral Hepat. 2013;20:e115–23. doi: 10.1111/jvh.12020. [DOI] [PubMed] [Google Scholar]


