Summary
Objective
Testing for antibodies against hepatitis B core antigen (anti-HBc) was introduced to detect blood donors suffering from occult hepatitis B infection. Confirmation of specification of reactive results in the anti-HBc screening assay is still a challenge for blood donation services.
Methods
Two different test strategies for confirmation of specification of reactive anti-HBc tests, one performed in our institute and one suggested by the German authority (Paul-Ehrlich-Institut (PEI)), were compared. The first strategy is based on one supplemental anti-HBc test, the other requires two supplemental anti-HBc tests.
Results
389 samples from 242 donors were considered. Both test strategies yielded concordant results in 117 reactive samples termed ‘true-positive’ or ‘specificity confirmed’, in 156 reactive samples termed ‘false-positive’ or ‘specificity not confirmed’, and in 99 negative samples. In 17 samples obtained from 11 donors, both test strategies gave discrepant results (‘false-positive’ but ‘specificity confirmed’). In 10 of 11 donors, a real HBV infection was very unlikely, one remained unclear. 30 donors considered ‘false-positive’ became negative in all anti-HBc tests after follow-up testing and thus eligible for donor re-entry.
Conclusions
The test strategy suggested by the PEI yielded no additional information but induced an overestimation of HBV infections and unnecessary look-back procedures. Many anti-HBc-reactive donors can be regained after follow-up testing.
Keywords: Hepatitis B virus, Anti-HBc, HBV DNA, Blood donor, Donor re-entry
Introduction
Occult HBV infection (OBI) is defined as the presence of HBV DNA without detectable hepatitis B surface antigene (HBsAg), except for window period HBV infections. HBV infection can be transmitted by transfusion not only by donations obtained from donors in the HBV window period infection but also by those from donors with OBI [1,2]; however, OBI can be detected by testing for antibodies against hepatitis B core antigen (anti-HBc) [3,4,5,6]. Therefore, anti-HBc testing has become an integral part in blood donor screening in many countries [7,8,9]. Compared to HBV DNA testing of minipools or even individual-donation nucleic acid testing (ID-NAT), it has the advantage of comparatively lesser costs. Moreover, it ensures a reliable detection of those HBV-infected donors who present only a low-level viremia, possibly not detectable by NAT due to low DNA concentration close to or below the detection limit of the NAT assay used. Even though the viral load is very low, these donors are able to transmit the hepatitis B by their blood components [10].
One drawback of anti-HBc testing, compared to NAT, is the missing ability to detect blood donors in the very early, pre-seroconversion HBV infection [11,12]. Another drawback is the lack of a confirmatory assay, which hinders further clarification if a sample tested repeatedly reactive in the screening assay. The main reasons for anti-HBc reactivity are resolved HBV infection and false-positive anti-HBc reactivity.
This impedes a clear counselling of otherwise eligible, but deferred donors and may result in an unnecessary donor loss. To avoid donor loss, the Food and Drug Administration (FDA) advised already in 1991 the repetition of anti-HBc testing at least at a later occasion [7]. If donors tested then repeatedly reactive again, they must ultimately be excluded from further donations.
Another test strategy was applied in our institution [13]: besides HBsAg testing and ID-NAT, it is based on the performance of a second anti-HBc test for clarification of the screening test result. A sample is considered true-positive for anti-HBc if at least the second anti-HBc test is reactive. In contrast to the FDA recommendation, it offers the possibility of a donor re-entry at any later date, even if one or even more false-positive screening results were obtained anytime in the past.
The German national authority (Paul-Ehrlich-Institut, PEI) recently suggested a further test strategy, based on two supplemental anti-HBc assays [9]: A sample tested repeatedly reactive in the screening assay can be considered as false-positive (so-called: ‘specificity not confirmed’) if it tested negative for HBsAg, HBV DNA by ID-NAT (<12 IU/ml 95% detection limit), and negative in the two supplemental anti-HBc tests. The donation can then be released. In the case of at least one supplemental reactive anti- HBc test (‘2:1 decision’), the anti-HBs titer must be ≥100 IU/l for release of the donation, provided that HBV DNA in ID-NAT and HBsAg are not detectable.
Performance of anti-HBc testing using a third test system might be cumbersome for some blood donations services but might lead to the receipt of additional donations. The aims of our study were i) to assess whether the performance of a third anti-HBc test yields additional information in the assessment of donors who tested repeatedly reactive for anti-HBc and ii) to assess how many of the donors, who tested false-positive for anti-HBc, can be regained by our re-entry strategy.
Material and Methods
Test Strategies
All blood donations that were taken in the Institute of Transfusion Medicine of the University Hospital of Schleswig-Holstein in Lübeck and Kiel in the years 2013 and 2014 were considered. Blood donations were screened for anti-HBc and HBsAg using the chemiluminescence microparticle immunoassay (CMIA; Abbott ARCHITECT, Abbott GmbH, Wiesbaden, Germany). Results were indicated as sample to cut-off ratios (S/CO). A S/CO ratio of at least 1.0 was considered reactive.
Screening for HBV DNA by NAT (Cobas AmpliPrep/Cobas TaqMan 96, Roche Diagnostics, Mannheim, Germany; 95% detection limit of plasma of the single blood donation 864 IU/ml) was done in minipools that had been prepared within 18 h after donation comprising up to 96 samples. All tests were performed according to the manufacturer's instructions.
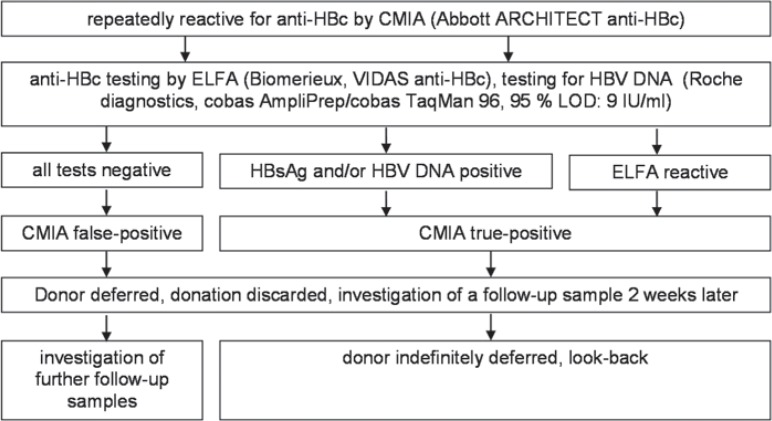
Reflecting our inhouse test strategy [13] (fig. 1), samples that tested repeatedly reactive for anti-HBc in the CMIA were supplementary tested for anti-HBc by a second assay. As the Abbott AxSYM assay is not available anymore, we used an enzyme-linked fluorescence immunoassay (ELFA; BioMerieux, Marcy-l'Etoile, France) as second assay as described below. HBV DNA was determined by ID-NAT (Cobas Ampliprep/Cobas TaqMan 96, 95% detection limit 9 IU/ml). HBsAg testing was also performed by CMIA but not considered for the final interpretation of anti-HBc test results; some donors did not remember whether they had been vaccinated or not, and their vaccination records have not been available.
Fig. 1.
Test strategy for the clarification of samples tested repeatedly reactive for anti-HBc according to the inhouse test strategy [13]. LOD = Limit of detection.
If a sample tested reactive in the second anti-HBc assay and/or was positive for HBV DNA, the donor was considered ‘true-positive’ for anti-HBc; otherwise, the donor was considered ‘false-positive’. In either case, the donor was deferred and the donation discarded, but a follow-up sample was requested for 2 weeks later and investigated likewise. If the follow-up sample tested again ‘false-positive’ (fig. 1), additional follow-up samples 6 months and 2 years later [13] were requested for. If any follow-up sample became negative in the CMIA and the supplemental anti-HBc test, the donor was permitted to donate again (re-entry), provided that neither HBsAg nor HBV DNA had become detectable meanwhile. If a donor tested still repeatedly reactive for anti-HBc after 2 years, the donor was indefinitely deferred.
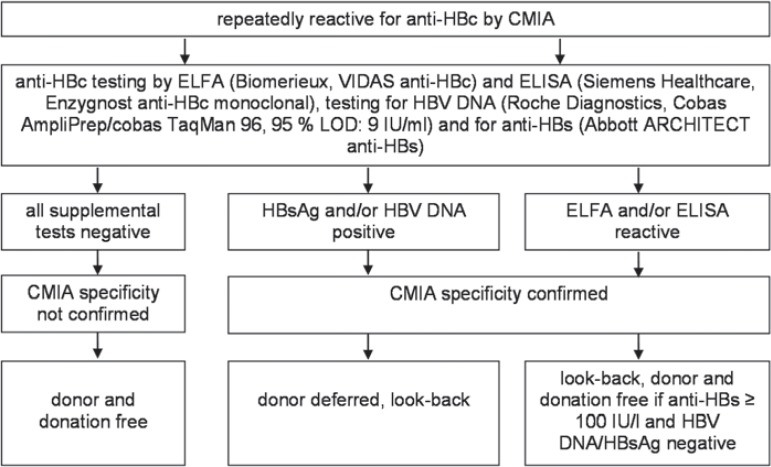
The other test strategy is based on the enactment of the PEI [9] and displayed in figure 2. It requires the performance of the anti-HBc test in a third test system and anti-HBs testing. Thus, an enzyme-linked immunoassay (ELISA, Enzygnost Anti-HBc monoclonal, Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) was performed additionally as described below. If at least one supplemental anti-HBc test became reactive, a look-back procedure was started. The donation could be used under the requirements displayed in figure 2[9].
Fig. 2.
Test strategy for the clarification of samples tested repeatedly reactive for anti-HBc according to the test strategy suggested by the PEI [9]. LOD = Limit of detection.
Follow-up samples of donors who tested reactive by CMIA anytime in the past but became negative during the observation period were also investigated by all supplemental tests.
Performance and Evaluation of Supplementary Anti-HBc Tests
ELFA
The VIDAS anti-HBc assay is a two-step enzyme immunoassay with fluorescent detection. The test was performed automatically by the device Mini Vidas (BioMerieux), results were calculated by the computer software and specified in ‘relative fluorescence values (RFV)’. A RFV ≥ 1.4 was considered negative. The manufacturer recommends to term samples presenting a RFV between 1.0 and 1.4 as equivocal and to investigate another sample, but for immediate decision if a look-back is necessary, a RFV < 1.4 was considered positive.
ELISA
The Enzygnost Anti-HBc monoclonal is a competitive one-step enzyme immunoassay. Test performance as well as evaluation of the result were carried out automatically by the BEPIII system (Siemens Healthcare Diagnostics Products GmbH). Results were indicated as S/CO ratios. A sample was considered positive if the S/CO ratio was ≤1.0 and negative if the ratio was ≥1.10. Samples with a S/CO ratio between 1.01 and 1.09 are equivocal according to the manufacturer's recommendations but were again considered positive in our analysis for the reason mentioned above.
Calculation of Mean Values and Confidence Intervals
Mean values and confidence intervals were calculated using MS Excel 2003 (Microsoft Corporation, Redmond, WA, USA).
Results
Results of Supplemental Anti-HBc Testing
During the observation period, 9,766 first-time donors and 20,168 repeat blood donors provided 109,603 donations (whole blood, platelet- and plasmapheresis).
389 samples (corresponding to 0.35% of all donations), taken either by blood donation or by follow-up, were considered during the study period.
The results of the CMIA as well as of the different supplemental anti-HBc tests are displayed in table 1. The inhouse and the PEI test strategy showed a good accordance in 370 samples: 117 out of 370 samples could be considered ‘true-positive’ or ‘specificity confirmed’. In 9 out of these 117 samples (all anti-HBc tests reactive) from 5 different donors, additional HBV DNA and HBsAg were detectable.
Table 1.
Results of supplemental anti-HBc testing and anti-HBs testing
| CMIA | ELFA | ELISA | Number (%) | Of those anti-HBs ≥ 100 IU/l (%) |
|---|---|---|---|---|
| + | + | + | 103a (26.5) | 55 (53.4) |
| + | + | − | 14b (3.6) | 5 (35.7) |
| + | − | + | 17c (4.4) | 4 (23.5) |
| − | + | + | 0 | 0 |
| + | − | − | 150 (38.6) | 51 (34.0) |
| − | + | − | 4d (1.0) | 2 (50.0) |
| − | − | + | 2e (0.5) | 1 (50.0) |
| − | − | − | 99 (25.4) | 43 (43.4) |
7 ELFA equivocal, 1 ELFA and ELISA equivocal.
11 ELFA equivocal.
2 ELISA equivocal.
2 ELFA equivocal.
1 ELISA equivocal.
The results also agreed in 154/370 samples which could be considered ‘false-positive’ or ‘specificity not confirmed’ (CMIA- or ELFA-reactive), and in 99 follow-up samples testing negative in all anti-HBc tests.
Two samples tested negative by CMIA and ELFA, but reactive in the ELISA.
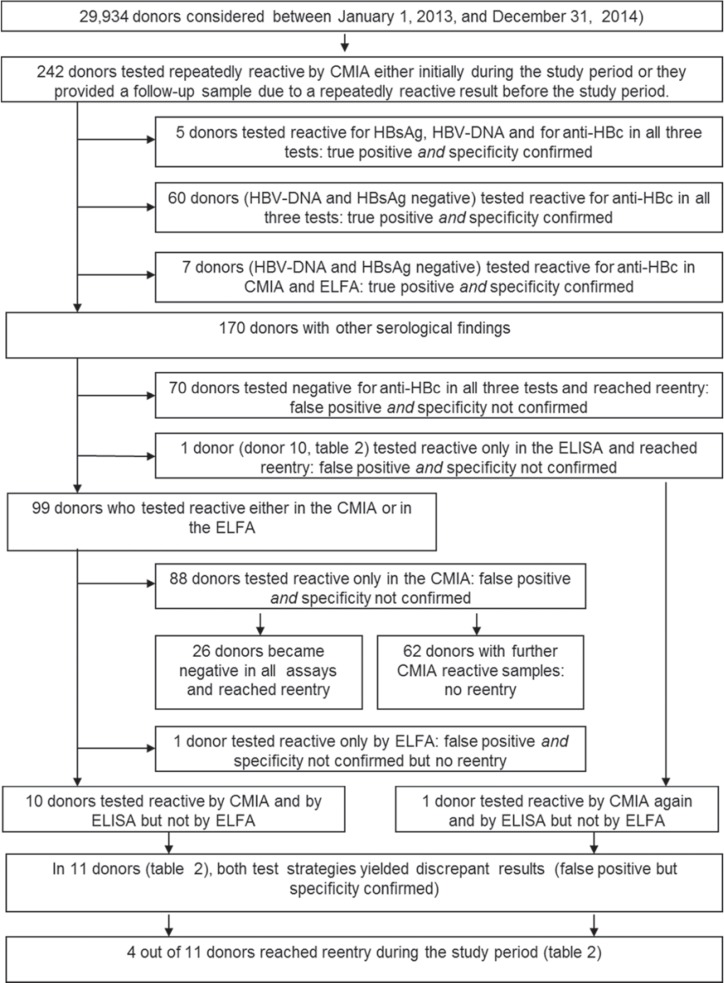
The 389 samples have been obtained from 242 donors (111 women and 131 men). In 72 of these donors, both test strategies yielded concordant results: they were considered ‘true-positive’ and ‘specificity confirmed’ (fig. 3). 159 donors were considered ‘false-positive’ and ‘specificity not confirmed’. They would have been eligible for blood donation according to the PEI test strategy. 96 of those reached re-entry due to our inhouse test strategy.
Fig. 3.
Final interpretation of supplemental HBV testing, related to the 242 donors who has been included in the study.
In 11 donors, who provided overall 22 samples (17 CMIA-reactive, 5 CMIA-negative), both test strategies yielded discrepant results leading to a different interpretation of the HBV status. The results of all assays are displayed in detail in table 2. These donors have been termed ‘false-positive’ by the inhouse test strategy but ‘specificity confirmed’ by the PEI test strategy due to the 2:1 decision.
Table 2.
Results of supplemental HBV tests in donors with discrepant results in the both test strategies
| Donor number / sample drawn at day | CMIA mean S/CO | ELFA | ELISA | HBV-NAT, 95 % LOD: 9 IU/ml | HBsAg | Anti-HBs | Vaccinated | Look-back / days between last negative and first reactive donation | Re-entry |
|---|---|---|---|---|---|---|---|---|---|
| 1 / 0 | 1.01 | 2.59 | 0.92 | neg | neg | 0 | n.a. | neg / 168 | yes |
| 1 / 7 | 0.91 | 2.29 | 1.11 | neg | neg | 0 | n.a. | n.a. | |
| 2 / 0 | 3.86 | 1.62 | 1.05 | neg | neg | 7 | n.s.* | n.d. | no |
| 3 / 0 | 1.27 | 2.45 | 1.07 | neg | neg | 344 | yes | neg / 182 | yes |
| 3 / 94 | 0.64 | 2.39 | 1.13 | neg | neg | 278 | yes | n. a. | |
| 4 / 0 | 1.24 | 2.6 | 0.82 | neg | neg | 0 | n.a. | n.d. | no |
| 5 / 0 | 1.1 | 2.0 | 0.24 | neg | neg | 0 | n.a. | neg / 207 | no |
| 5 / 21 | 0.46 | 2.35 | 0.36 | neg | neg | 0 | n.a. | yes | |
| 5 / 181 | 1.46 | 2.13 | 0.28 | neg | neg | 0 | n.a. | no | |
| 6 / 0 | 1.19 | 2.12 | 0.14 | neg | neg | 0 | n.a. | neg / 82 | no |
| 6 / 14 | 1.29 | 2.13 | pos** | neg | neg | 0 | n.a. | no | |
| 6 / 84 | 1.14 | 2.06 | 0.09 | neg | neg | 0 | n.a. | no | |
| 7 / 0 | 1.27 | 1.52 | 0.11 | neg | N eg | 85 | yes | n.d. | no |
| 7 / 21 | 1.36 | 2.29 | 0.15 | neg | neg | 72 | yes | no | |
| 8 / 0 | 2.2 | 2.23 | 0.20 | neg | neg | 0 | n.a. | neg / 107 | no |
| 8 / 11 | 1.36 | 2.17 | 0.52 | neg | neg | 0 | n.a. | no | |
| 9 / 0 | 3.0 | 2.31 | 0.61 | neg | neg | 286 | yes | neg / 140 | no |
| 9 / 12 | 2.3 | 2.03 | 0.71 | neg | neg | 245 | yes | no | |
| 10 / 0 | 0.86 | 2.43 | 1.04 | neg | neg | 149 | yes | n.d. | yes |
| 3 donations without pathological findings meanwhile provided by donor 10 | |||||||||
| 10 / 519 | 1.24 | 2.28 | 0.23 | neg | neg | 123 | yes | neg / 75 | no |
| 10 / 630 | 0.62 | 2.08 | 2.15 | neg | neg | 122 | yes | yes | |
| 11 / 0 | 2.07 | 2.22 | 0.81 | neg | neg | 11 | yes | neg / 201 | no |
n.a. = Not applicable; neg = negative; n.d. = not done; pos = positive.
Not specified.
No S/CO ratio was reported in one sample.
Results of Donor Look-Back
In overall 19 donors, a look-back procedure was mandatory. Of those, 8 tested reactive in all the anti-HBc tests, and 3 tested reactive only in the CMIA and the ELFA. The samples of these 11 donors were considered ‘true-positive’ as well as ‘specificity confirmed’. In the samples of 8 donors, performance of the both test strategies yielded discrepant results (‘false-positive’ but ‘specificity confirmed’, table 2). In the archive samples of the last donation tested negative for anti-HBc, no HBV DNA was detectable, not only in those of the 8 donors but also in those of the other 11 donors with concordant results in both test strategies. Overall, the 19, HBV DNA-negative archives samples have been obtained at a mean time of 177 days (range 69-756 days, 95% confidence interval 108-247 days) before the first anti-HBc-reactive test result was measured.
Donor Re-Entry during the Observation Period
28 donors (26 ‘false-positive’ and ‘specificity not confirmed’, 2 ‘false-positive’ but ‘specificity confirmed’) reached a definitive donor re-entry during the study period. 20 blood donors became eligible for re-entry already at the next follow-up investigation, in 7 donors, two consecutive follow-up investigations were required before re-entry was reached, and 1 donor became eligible for re-entry by the third follow-up investigation.
However, 2 donors presented a more complex course. Both provided a sample tested reactive in the CMIA and the ELISA, but not in the ELFA, and are thus displayed in table 2: Donor 5 tested reactive in the CMIA by the first donation during the observation period, achieved the re-entry already by the second follow-up investigation but became reactive anew by the consecutive donation. Donor 10 tested reactive by CMIA before the observation period, became eligible for re-entry by the first follow-up investigation during the observation period and provided 3 donations without any pathological findings. Afterwards, the donor tested reactive by CMIA again but just reached the re-entry during the observation period anew.
Discussion
At the present time, besides anti-HBc testing, HBsAg testing is mandatory in Germany. Although many blood donation services voluntarily perform additional minipool HBV NAT testing, maintaining of anti-HBc testing in combination with HBsAg testing is essential to reliably detect most of the HBV infections [14]. As only few virions can be enough for transmission of the HBV infection [15], even the performance of high-sensitive ID HBV NAT may not prevent transfusion-transmitted HBV infection at any case [10].
The specificity of anti-HBc tests is not always satisfactory [13,16,17,18], and. although confirmation assays are under way [19], they are not yet broadly available, and experiences in the daily routine are limited to few laboratories. Thus, the question remains how to deal with donors who tested reactive in the anti-HBc test, how to clarify a reactive result, and how to enable donors tested ‘false-positive’ a re-entry. Although the donor loss in an anti-HBc ‘low-endemic’ country like Germany would be comparatively low, if all anti-HBc reactive donors would be deferred, this donor loss must be added to the donor loss caused by other reasons. The purpose of both test strategies compared is to clarify as much reactive screening test results as possible and thereby to sustain as much donors as possible without compromising the recipients of blood components.
Basis for further clarification whether a repeatedly reactive screening tests result is ‘false-positive’ or ‘specificity not confirmed’ is, beside ID-NAT, the performance of additional anti-HBc tests both in our inhouse as well as in the PEI strategy. While only one additional anti-HBc test is performed when using our inhouse strategy, thus making it more convenient for blood donation services, two additional anti-HBc tests are applied by the PEI strategy. Both test strategies yielded accordance in 370 (95.1%) samples: 117 (30.1%) out of those were considered ‘true-positive’ or ‘specificity confirmed’. The same applies in 154 (39.6%) samples tested reactive in only one anti-HBc test (‘false-positive’ or ‘specificity not confirmed’) as well as in 99 (25.4%) previously reactive and currently completely negative samples (table 1). While in 2 samples (only ELISA-reactive), the final interpretation was discrepant but not really different (negative and ‘specificity not confirmed’), in 17 (4.4%, table 2) samples the final interpretation was inconsistent. These 17 samples have been considered ‘false-positive’ by the inhouse strategy but ‘specificity confirmed’ using the strategy suggested by the PEI. Thus, in the majority of samples, the performance of a third anti-HBc test did not contribute to an improved clarification of the screening test results. Detailed consideration of the 11 donors who provided these 17 samples suggested that rather the inhouse strategy was right than the PEI strategy: In 10 out of the 11 donors, a real HBV infection was rather unlikely because no anti-HBs was detectable (donors 1, 4, 5, 6 and 8, table 2) or, if so, the donors had a history of vaccination (donors 3, 7, 9, 10, 11). None of the donors had detectable HBV DNA in its plasma, and, if an archive sample was investigated by NAT, it tested also negative (donors 1, 3, 5, 6, 8, 9, 10, 11). The interjacent periods were rather short, making a meanwhile occurrence and disappearance of HBV DNA in terms of a new HBV infection and its resolution very unlikely. Four donors provided a CMIA-negative sample during the study period (donors 1, 3, 5, 10). Only in 1 donor (donor 2), a decision whether or not a real HBV infection had been occurred was not possible. Thus, the value of a third anti-HBc test for clarification of reactive screening test results can be reconsidered: the yield of additional information seems to be low. Its performance, contrariwise, induced an overestimation of ‘real’ HBV infection, unnecessary look-back procedures, and unnecessarily unsettles the donors, but requires a cumbersome and costly third anti-HBc test system.
The inhouse and the PEI strategy deal with donors who tested reactive for anti-HBc in a different manner: each donor with reactive anti-HBc test result, irrespective whether ‘true-positive’ or ‘false-positive’ is initially deferred by the inhouse strategy. The approach suggested by the PEI is more complex: the donor is further permitted to donate if the anti-HBc specificity is ‘not confirmed’, or, if specificity is ‘confirmed’, only if HBV DNA in ID-NAT is negative and the anti-HBs titer is more than 100 IU/l. In the latter case, future anti-HBc testing can be omitted, but anti-HBs testing is mandatory every 2 years.
That way, also donors with a subsided HBV infection are permitted to donate. However, anti-HBs titer and HBV DNA are determined by a random sample, and further donations with low anti-HBs titer and detectable HBV DNA might be missed in single cases of OBI. Such a scenario is excluded, if the ‘inhouse’ strategy is applied.
One must bear in mind the possibility of missing anti-HBc true-positive donations in single cases, as the sensitivity of anti-HBc tests is not always sufficient [20]. A blood donor, presenting an OBI with an intermittent viremia close to or below the cut-off of the NAT assay used, might be missed by two anti-HBc tests and thus permitted to donate by the PEI strategy. Albeit this event may very rarely occur, it should be at least considered in terms of transfusion medicine and especially safety of blood products, an area of expertise dealing with likelihoods of one to several millions. Otherwise, 154 samples tested reactive by CMIA or by ELFA only. These donations are directly regained by the PEI test strategy (while accepting the mentioned risk) but initially lost by the inhouse test strategy. However, such donors might though at least partly be regained: a donor re-entry in donors who tested repeatedly reactive at two occasions in the past but become non-reactive in a current investigation has been proposed already in 2008 as a safe and feasible approach [21]. Another, similar approach has been proposed in the year 2011 [13]. 100 different donors (fig. 3, one donor (donor 10, table 2) reached re-entry twice) became eligible for re-entry due to the results in 101 samples provided by them (non-reactive in CMIA and ELFA, HBsAg and HBV DNA not detectable), of these 30 became anti-HBc-reactive during our observation period. Missing of anti-HBc ‘true-positive’ samples is also possible if serial follow-up samples are tested. Reasons could be a disappearance of anti-HBc (which is unlikely after such a short period) or a failure of the anti-HBc assays used. But in any case, serial testing for HBV DNA by ID-NAT, at the initial reactive donation as well as at every follow-up investigation, is a requirement for the re-entry. Thereby, a higher safety level is reached as the probability of missing a low-level viremic donation is reduced due to investigation of serial samples by ID-NAT.
In conclusion, the PEI as well as our inhouse test strategy showed a good accordance in samples considered ‘specificity confirmed’ or ‘true-positive’, and in samples termed ‘false-positive’ and ‘specificity not confirmed’.
Therefore, performance of a third anti-HBc test did not improve the final interpretation of repeatedly reactive anti-HBc screening test results, but led to an overestimation of HBV infections in 10 donors. If anti-HBc-reactive donors are initially deferred, donor loss can be minimized by performing follow-up investigations and defining criteria for a donor re-entry.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Acknowledgement
The authors are indebted to the staff of the blood donor's laboratory for the excellent technical assistance.
References
- 1.Satake M, Taira R, Yugi H, Hino S, Kanemitsu K, Ikeda H, Tadokoro K. Infectivity of blood components with low hepatitis B virus DNA levels identified in a lookback program. Transfusion. 2007;47:1197–1205. doi: 10.1111/j.1537-2995.2007.01276.x. [DOI] [PubMed] [Google Scholar]
- 2.Levicnik-Stezinar S, Rahne-Potokar U, Candotti D, Lelie N, Allain JP. Anti-HBs positive occult hepatitis B virus carrier blood infectious in two transfusion recipients. J Hepatol. 2008;48:1022–1025. doi: 10.1016/j.jhep.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Hennig H, Puchta I, Luhm J, Schlenke P, Goerg S, Kirchner H. Frequency and load of hepatitis B virus DNA in first-time blood donors with antibodies to hepatitis B core antigen. Blood. 2002;100:2637–2641. doi: 10.1182/blood-2002-03-0798. [DOI] [PubMed] [Google Scholar]
- 4.Kleinman SH, Kuhns MC, Todd DS, Glynn SA, McNamara A, DiMarco A, Busch MP, Retrovirus Epidemiology Donor Study Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion. 2003;43:696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 5.Roth WK, Weber M, Petersen D, Drosten C, Buhr S, Sireis W, Weichert W, Hedges D, Seifried E. NAT for HBV and anti-HBc testing increase blood safety. Transfusion. 2002;42:869–75. doi: 10.1046/j.1537-2995.2002.00128.x. [DOI] [PubMed] [Google Scholar]
- 6.Dreier J, Kröger M, Diekmann J, Götting C, Kleesiek K. Low-level viraemia of hepatitis B virus in an anti-HBc- and anti-HBs-positive blood donor. Transfus Med. 2004;14:97–103. doi: 10.1111/j.0958-7578.2004.0486.x. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. Center for Biologics Evaluation and Research FDA recommendations concerning testing for antibody to hepatitis B core antigen (Anti-HBc) www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/OtherRecommendationsforManufacturers/Memorandumto BloodEstablishments/UCM062847.pdf (last accessed October 16, 2015)
- 8.O'Brien SF, Fearon MA, Yi QL, Fan W, Scalia V, Muntz IR, Vamvakas EC. Hepatitis B virus DNA-positive, hepatitis B surface antigen-negative blood donations intercepted by anti-hepatitis B core antigen testing: the Canadian Blood Services experience. Transfusion. 2007;47:1809–1815. doi: 10.1111/j.1537-2995.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 9.Paul-Ehrlich-Institut Bekanntmachung über die Zulassung von Arzneimitteln - Abwehr von Arzneimittelrisiken Stufe II - (Neufassung: Testung auf Antikörper gegen Hepatitis-B-Core-Antigen (anti-HBc) im Blutspendewesen) vom: 07.02.2014. Bundesanzeiger, BAnz AT 18.03.2014: B6.
- 10.Taira R, Satake M, Momose S, Hino S, Suzuki Y, Murokawa H, Uchida S, Tadokoro K. Residual risk of transfusion-transmitted hepatitis B virus (HBV) infection caused by blood components derived from donors with occult HBV infection in Japan. Transfusion. 2013;53:1393–1404. doi: 10.1111/j.1537-2995.2012.03909.x. [DOI] [PubMed] [Google Scholar]
- 11.Wendel S, Levi JE, Biagini S, Candotti D, Allain JP. A probable case of hepatitis B virus transfusion transmission revealed after a 13-month-long window period. Transfusion. 2008;48:1602–1608. doi: 10.1111/j.1537-2995.2008.01723.x. [DOI] [PubMed] [Google Scholar]
- 12.Servant-Delmas A, Chuteau C, Lefort C, Piquet Y, Chevaleyre S, Betbeze V, Delhoume M, Hantz S, Alain S, Laperche S. Two cases of transfusion-transmitted hepatitis low-endemic country before implementation of HBV nucleic acid testing. Transfusion. 2013;53:291–296. doi: 10.1111/j.1537-2995.2012.03736.x. [DOI] [PubMed] [Google Scholar]
- 13.Juhl D, Luhm J, Görg S, Ziemann M, Hennig H. Evaluation of algorithms for the diagnostic assessment and the reentry of blood donors who tested reactive for antibodies against hepatitis B core antigen. Transfusion. 2011;51:1477–1485. doi: 10.1111/j.1537-2995.2010.03031.x. [DOI] [PubMed] [Google Scholar]
- 14.Lieshout-Krikke RW, Molenaar-de Backer MW, van Swieten P, Zaaijer HL. Surface antigen-negative hepatitis B virus infection in Dutch blood donors. Eur J Clin Microbiol Infect Dis. 2014;33:69–77. doi: 10.1007/s10096-013-1930-9. [DOI] [PubMed] [Google Scholar]
- 15.Komiya Y, Katayama K, Yugi H, Mizui M, Matsukura H, Tomoguri T, Miyakawa Y, Tabuchi A, Tanaka J, Yoshizawa H. Minimum infectious dose of hepatitis B virus in chimpanzees and difference in the dynamics of viremia between genotype A and genotype C. Transfusion. 2008;48:286–294. doi: 10.1111/j.1537-2995.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 16.Ollier L, Laffont C, Kechkekian A, Doglio A, Giordanengo V. Detection of antibodies to hepatitis B core antigen using the Abbott ARCHITECT anti-HBc assay: analysis of borderline reactive sera. J Virol Methods. 2008;154:206–209. doi: 10.1016/j.jviromet.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Nübling CM, Scheiblauer H, Chudy M, Walch LA, Seifried E, Roth WK, Hourfar MK. Anti-HBc screening of blood donors: a comparison of nine anti-HBc tests. Vox Sang. 2006;91:237–243. doi: 10.1111/j.1423-0410.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 18.Niederhauser C, Mansouri Taleghani B, Graziani M, Stolz M, Tinguely C, Schneider P. Blood donor screening: how to decrease the risk of transfusion-transmitted hepatitis B virus? Swiss Med Wkly. 2008;138:134–141. doi: 10.4414/smw.2008.12001. [DOI] [PubMed] [Google Scholar]
- 19.Huzly D, Nassal M, Vorreiter J, Falcone V, Neumann-Haefelin D, Gerlich WH, Panning M. Simple confirmatory assay for anti-HBc reactivity. J Clin Virol. 2011;51:283–284. doi: 10.1016/j.jcv.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Gerlich WH, Glebe D, Schüttler CG. Deficiencies in the standardization and sensitivity of diagnostic tests for hepatitis B virus. J Viral Hepat. 2007;14(suppl 1):16–21. doi: 10.1111/j.1365-2893.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 21.Katz L, Strong DM, Tegtmeier G, Stramer S. Performance of an algorithm for the reentry of volunteer blood donors deferred due to false-positive test results for antibody to hepatitis B core antigen. Transfusion. 2008;48:2315–2322. doi: 10.1111/j.1537-2995.2008.01844.x. [DOI] [PubMed] [Google Scholar]