Summary
Background
Polyvinyl chloride (PVC) plasticized with di(2-ethylhexyl) phthalate (DEHP) is commonly used for blood collection and storage. DEHP has protective effects on RBC membranes, but is also a toxin.
Methods
A paired study was conducted to investigate the influence of DEHP and two alternative plasticizers, 1,2-cyclohexane-dicarboxylic acid diisononyl ester (DINCH) and n-butyryl-tri-n-hexyl citrate (BTHC), on the preservation of RBCs stored for 42 days in PVC pediatric bags. The RBC membrane was evaluated for supernatant hemoglobin (Hb), release of extracellular microvesicles (EVs), osmotic fragility, deformability, and lipid composition.
Results
In BTHC-plasticized bags, the supernatant Hb increase during storage was 2 times greater than in DINCH- and DEHP-plasticized bags. By day 21, EV concentrations had doubled from day-5 levels in DINCH- and DEHP-, and trebled in BTHC-plasticized bags. RBC mean cell volumes were greater in BTHC- than in DINCH- or DEHP-plasticized bags (p < 0.001). Osmotic fragility differed significantly among plasticizers (p < 0.01). After day 21, RBC deformability decreased in all, but to a greater extent in the bags with BTHC. Phospholipid composition of RBCs and EVs was not different among plasticizers.
Conclusion
Membrane stabilization capacity differed among the plasticizers. RBC in BTHC bags stored more poorly, while DEHP and DINCH bags offered better protection against vesiculation, osmotic stress, and Hb loss.
Keywords: Red blood cell, Membrane, DINCH, DEHP, BTHC
Introduction
Plastic bag systems for whole blood collection, component separation, and storage are required for current blood banking practice and transfusion therapies. Bags consisting of different plastic materials are commercially available but polyvinyl chloride (PVC) plasticized with di(2-ethylhexyl) phthalate (DEHP) is the most popular material in use [1]. However, the toxicity of DEHP and congener phthalates has been and remains a topic of public concern [2,3]. The exposure of transfusion patients to the DEHP that leaches out of PVC blood bags during component storage has been widely discussed [4,5], with particular concern for pediatric recipients [6,7]. For red blood cell (RBC) components, replacing DEHP by a non-phthalate PVC plasticizer is challenged by the membrane protective effect that DEHP exerts on RBCs ex vivo. DEHP has been shown to improve stored RBC morphology [8], deformability [9], osmotic fragility [9,10] and microvesicle release without affecting 2,3-DPG and ATP levels [8]. The enhanced RBC in vivo recovery observed with DEHP [11] underlines the plasticizer's role in the plasma membrane preservation during storage.
The existing literature identifies n-butyryl-tri-n-hexyl citrate (BTHC), trioctyl-trimellitate (TOTM), and 1,2-cyclohexane-dicarboxylic acid diisononyl ester (DINCH) as the most studied alternatives for a PVC DEHP-free blood collection/storage system [7]. None of these plasticizers are devoid of potential health hazards but DINCH shows the lowest reproductive and developmental toxicity [6], which is also a DEHP-associated risk of concern for pediatric recipients. It has been previously shown that RBCs can be stored satisfactorily in BTHC-plasticized bags [12], despite inferior protection against hemolysis and vesiculation (assessed as the cell-free, membrane-bound protein concentration during storage) compared to DEHP [13]. DINCH has recently been reported to approach DEHP in terms of protection against hemolysis [14] with the advantage that it leaches less readily from the PVC matrix [15,16]. Interestingly, little is known about the RBC vesiculation under the influence of DINCH. This study was conducted to examine RBC vesiculation using a paired study including BTHC and DEHP.
Material and Methods
Red Cell Concentrates: Preparation and Shipping
Whole blood (WB) was donated by consenting volunteer research donors at the Canadian Blood Services NetCAD Laboratory, Vancouver, BC, as approved by the Canadian Blood Services Research Ethics Board (Protocol Reference # 2012.008). WB was collected into WB Sang Total Citrate-Phosphate-Dextrose (CPD) 500 ml (± 10%) blood bag sets (Macopharma, Tourcoing, France). WB units were cooled and stored at 20-24 °C on butane-1,4-diol cooling trays. 30 leukoreduced saline-adenine-glucose-mannitol (SAGM) red cell concentrate (RCC) units were produced from the WB units according to the buffy coat (top/bottom) method using PVC bags plasticized with DEHP. RCC pairs were pooled (n = 15 pools) and split into three types of pediatric bags: PVC/DEHP (Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany; P1459, lot 43FB17CA00), PVC/DINCH (Fresenius Kabi; T2440, lot 43FA10QA00), and PVC/BTHC (Fresenius Kabi; T2440, lot 43FA11QA00) in duplicate. Each bag was filled with 70-75 ml of RCC. One set of RCCs was shipped for testing to Edmonton, AB, in validated shipping containers packed with cold packs and monitored to ensure that the temperature was maintained between 1-10 °C. The other set remained in Vancouver, BC, for testing. All RCCs were stored at 2-6 °C and sampled on days 5, 21, 35, and 42 of storage. At the end of storage, units were assessed for sterility using the BacT/ALERT automated culture system (bioMérieux Canada Inc., St. Laurent, QC, Canada) to ensure that no contamination was introduced as a result of handling.
Supernatant Hemoglobin Concentration
RCCs were sampled (1,000 µl) and centrifuged (2,200 × g, 10 min, 4 °C). 40 µl of the supernatant was transferred to 1,000 µl Drabkin's reagent, mixed, kept in the dark for 20 min, and then read for light absorption at 540 nm (Spectramax Plus 384, Molecular Devices, Sunnyvale, CA, USA). As described elsewhere [17], the absorption intensity was converted to hemoglobin (Hb) concentration based on a reference concentration curve obtained using commercial Hb standards (Stanbio Laboratory, Boerne, TX, USA).
RBC Mean Corpuscular Volume and Mean Corpuscular Hb Concentration
RBC mean corpuscular volume (MCV) and mean corpuscular Hb concentration (MCHC) were determined using an automated cell counter (Coulter AcT, Beckman Coulter, New York, NY, USA).
RBC Deformability
RBC deformability was assessed using a laser-assisted optical rotational cell analyzer (Mechatronics, Zwaage, The Netherlands) and expressed as the maximum elongation index predicted at infinite shear stress (EImax). Briefly, RBCs were diluted 1:100 in a polyvinyl pyrrolidone solution and subjected to increasing shear stress (0.95, 1.69, 3.00, 5.34, 9.49, 16.87, 30.00 Pa) at 37.0 °C. The diffraction pattern produced by the scatter of the laser beam at each stress was plotted as a deformability curve. EImax was obtained using the Eadie-Hofstee transformation, as previously described [18].
Flow Cytometry: Monitoring CD235a, CD47 and Phospharidylserine (PS) Exposure
Flow cytometry and the use of Trucount tubes (BD Biosciences, San Jose, CA, USA) for glycophorin A-positive (CD235a+) extracellular vesicle (EV) quantification was performed as elsewhere described [19,20]. Briefly, RBCs were sampled from the units, and 5 µl were diluted with HEPES Ca2+ (2.5 mmol/l) buffered saline, pH 7.4, mixed with 5 µl each anti-CD235a fluorescein isothiocyanate (FITC), anti-CD47 phosphatidylethanolamine (PE) and Annexin V-APC, and transferred to Trucount tubes (BD Biosciences). Tubes were incubated for 15 min in the dark at room temperature and then vortexed before analysis using a FACSCalibur flow cytometer (BD Biosciences). Forward scatter and side scatter channels were set to log scale to accommodate the visualization of cells and vesicles in a single panel as shown elsewhere [20]. Acquisition was set up on the basis of time (3 min) without gating. For data analysis, the supernatant of a centrifuged RCC sample was used to set an adequate EV gate. RBC EVs were counted as CD235a+ EV-gated events.
Sizing of RCC-Associated EVs Using Dynamic Light Scattering
A Zetasizer Nano S (Malvern, Worcestershire, UK) was used for EV sizing. Uniform polystyrene microspheres of 100, 200 and 400 nm diameter (Bangs Laboratories, Fishers, IN, USA) as 0.01% in phosphate-buffered saline (PBS) were used to verify instrument operation. RCCs were sampled (1.5 ml) and centrifuged (2,200 × g, 10 min, 4 °C). Supernatants were collected and re-centrifuged (2,200 × g, 10 min, 4 °C). These supernatants were diluted with two parts of PBS and analyzed using sample refractive index = 1.43 (phospholipid liposomes), dispersant refractive index = 1.33 (water), system temperature = 25 °C, and sample equilibration time = 2 min. The size results were expressed in terms of Z-average.
Osmotic Fragility Assessment
Osmotic fragility was determined for RBCs stored in DEHP-plasticized bags on day 5 and for RBCs in all three bag types on day 43 of storage. Osmotic fragility was assessed at salt (NaCl) concentrations ranging from 0-0.9% as previously described [21]. Hb release was measured by spectrophotometry at 450 nm, with adjustment for albumin and bilirubin interference by absorbance at 380 nm and 415 nm [22].
Pelleting RCC-Associated EVs for Lipid Analysis
RCCs (180 ml) were centrifuged (2,200 × g, 20 min, 4 °C). Supernatants were collected and centrifuged (2,200 × g, 20 min, 4 °C) to remove cell debris. The cell debris-free supernatants were transferred to ultracentrifuge tubes and centrifuged (50,000 × g, 60 min, 4 °C) to precipitate the EVs. The bright red EV pellets obtained were suspended in PBS (500 µl) and stored at −20 °C for up to 16 weeks until required for lipid analysis.
Sample Preparation for Lipid Analysis
PBS-washed RBCs (100 μl) and PBS-stored EVs (100 μl) were spiked with 2 μg each internal standard dimyristoyl glycerophosphoethanolamine (PE 28:0) and dimyristoyl glycerophosphoserine (PS 28:0) and extracted with chloroform/isopropanol, as previously described [23]. The extraction solvents were evaporated using a stream of nitrogen and the extracts were reconstituted in 300 μl MeOH-CHCl3-20 mmol/l aqueous ammonium acetate (4:1:1, v/v/v) for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Reverse Phase LC-MS/MS
LC was performed using a Shimadzu (Kyoto, Japan) system consisting of controller (CBM20Alite), autosampler (SIL20A), two binary solvent pumps (LC20AD), and a column oven (CTO20AC) set at 60 °C. Lipid extracts were injected (10 µl) on an Acquity BEH Shield RP18 column (100 × 2.1 mm I.D., 1.7 µm, Waters, Wexford, Ireland) and gradient-eluted with H2O, 0.1% acetic acid, v/v (mobile phase A) and MeOH (mobile phase B) at a flow rate of 0.3 ml/min. The column outlet was coupled via a Turbo Spray ion source to an API 4000 triple quadrupole mass spectrometer (AB Sciex, Concord, ON, Canada). LC-MS/MS in the negative mode was applied to obtain precursor ions undergoing a neutral loss (NL) of 87 Da, and LC-MS/MS in the positive mode was applied to obtain precursor ions undergoing a NL of 141 Da. These were used for the selective monitoring of glycerophosphoserine (NL 87) and glycerophosphoethanolamine (NL 141) species. Instrument parameters applied to both ionization modes were: temperature = 450 °C, nebulizer gas (N) = 45 psi, heater gas (N) = 45 psi, curtain gas (N) = 10 psi, declustering potential = 110 V and collision cell exit potential = 15 V. In the negative mode, the ion spray voltage was 4,500 V and the collision energy 36 V. In the positive mode, the ion spray voltage was 5,500 V and the collision energy 30 V. Precursor ions were scanned within the range of 400-1,000 Da. Data acquisition and peak integration were performed using the Analyst v.1.5 software (AB Sciex). Compound-dependent instrument response differences were not accounted for and such assessment was out of the scope of present study.
Statistical Analysis
For analysis of osmotic fragility results, hemolysis data at 0.55% NaCl were square root-transformed to achieve normality. A one-way ANOVA for correlated samples using the transformed data was performed, and post-test analysis was performed with Tukey's HSD test (www.vassarstats.net). Comparison between means was performed using two-tailed t-test (unequal variance). For all analyses, a p value less than 0.05 was considered statistically significant.
Results
RBC EVs and Supernatant Hb Correlated Positively
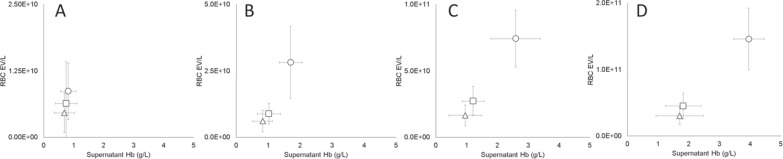
Supernatant Hb increased with the progression of the storage (fig. 1). Although supernatant Hb increased in all bag types, RCCs stored in BTHC-plasticized bags developed supernatant Hb levels two times higher than those stored in the bags plasticized with DINCH or DEHP (p < 0.001), which consistently had similar levels during storage. Increases in the concentration of Hb in the supernatant were accompanied by increases in the supernatant concentration of RBC EVs (CD235a+). On day 5, the concentration of RBC EVs ranged from 5.0 × 109 to 9.0 × 109 EV/l, with the DEHP and BTHC units at the minimum and maximum of this range, respectively (fig. 1A). On day 21, the concentration of RBC EVs had nearly doubled in the DINCH and DEHP groups, whereas the increase in the BTHC group was nearly threefold (fig. 1B), indicating that during the first 3 weeks of storage the rate of vesicle release was higher in the BTHC units (p < 0.001). Starting on day 21, the concentration of RBC EVs was higher in the BTHC units (p < 0.001). For the rest of the storage period, the vesiculation rate was less affected by differences in plasticizer. Between day 21 and day 35, the concentration of RBC EVs increased threefold in all units (fig. 1C); and between day 35 and day 42, a general twofold increase was observed (fig. 1D).
Fig. 1.
Changes in the concentration of RBC EVs (CD235a+) and supernatant Hb as a function of storage time (A day 5, B day 21, C day 35, D day 42) and blood bag plasticizer (Δ = DEHP, □ = DINCH, ○ = BTHC; n = 15/group; graphs display means ± SD).
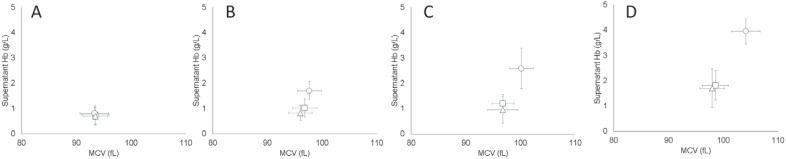
RBC MCV and Supernatant Hb Correlated Positively
In all bag types, the MCV of RBCs increased with time (p < 0.001), indicating cell swelling during storage (fig. 2). However, RBCs stored in BTHC-plasticized bags had larger MCVs than those stored in the bags plasticized with DINCH or DEHP (p < 0.001), which consistently displayed equivalent MCVs during the storage. Increases in MCV were accompanied by increases in supernatant Hb, which was already twofold greater in the BTHC units on day 21 compared to DINCH and DEHP units (p < 0.001).
Fig. 2.
Changes to RBC MCV and supernatant Hb during storage (A day 5, B day 21, C day 35, D day 42) and as a function of the bag plasticizer (Δ = DEHP, □ = DINCH, ○ = BTHC; n = 15/group; graphs display means ± SD).
The Average Size of RCC EVs Changed as a Function of Storage Time
The average size of the RCC EVs increased towards 200 nm from day 5 to day 42 of storage in the 3 bag types (p < 0.001; fig. 3), suggesting that in the RCCs there was a mixture of exosomes (50-100 nm) and plasma membrane microvesicles (ectosomes, 200-250 nm) changing in proportion during storage [20], which is consistent with the accumulation of RBC EVs (ectosomes) during storage (fig. 1).
Fig. 3.
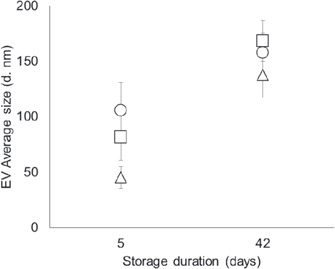
The average size of EVs (unlabeled) on day 5 and on day 42 of storage, as influenced by the bag plasticizer (Δ = DEHP, □ = DINCH, ○ = BTHC; n = 15/group; graphs display means ± SD).
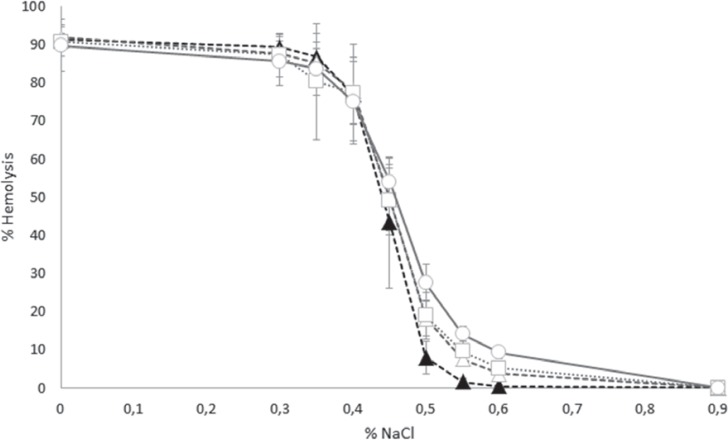
Osmotic Fragility as a Function of Plasticizer
Osmotic fragility curves displayed the characteristic sigmoidal distribution for all samples tested (fig. 4). The curve appeared to shift to the right with increasing storage time suggesting an increase in osmotic fragility of the stored RBCs. For statistical purposes, the amount of hemolysis at a fixed concentration of salt (0.55% NaCl) was selected to interrogate potential differences in osmotic fragility of RBCs stored in the 3 different plastics. At day 43 and at 0.55% NaCl, RBCs stored in BTHC bags displayed the highest hemolysis (mean ± SD 14.1 ± 2.0; range 10.5-17.0; median 14.3), followed by RBCs stored in DINCH bags (mean ± SD 9.6 ± 2.6; range 6.4-14.7; median 9.4), and finally RBCs stored in DEHP bags (mean ± SD 7.6 ± 1.7; range 5.5-11.2; median 7.0; p < 0.01). Regardless of the container, the 43-day stored RBCs all displayed higher hemolysis at 0.55% NaCl than the RBCs stored in DEHP for only 5 days (mean ± SD 1.6 ± 1.0; range 0.5-3.8; median 1.3; p < 0.01).
Fig. 4.
Osmotic fragility of red cell concentrate as influenced by the bag plasticizer. Osmotic fragility was assessed by measuring hemolysis at salt concentrations ranging from 0–0.9%. (▲ = DEHP, day 5; Δ = DEHP, day 43; □ = DINCH, day 43; and ○ = BTHC, day 43; n = 15; graphs display means ± SD).
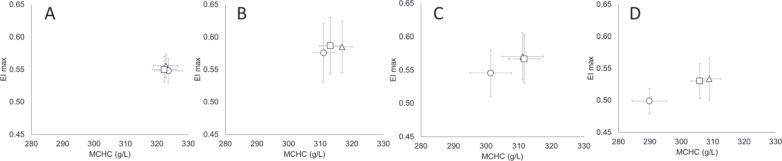
RBC Deformability as a Function of the RCC Storage
RBCs stored in blood bags plasticized with DEHP, DINCH or BTHC displayed a similar elongation index (EImax) and MCHC on day 5 (fig. 5). On day 21, the ability of RBCs to elongate increased by about 5% (higher EImax) in all bag groups (p < 0.05) while the respective MCHC was decreased by about 5% (p < 0.001), suggesting that cells were more deformable due to a drop in cytoplasmic viscosity. On day 35, although the MCHC continued to decrease, particularly in the BTHC group (3%, p < 0.001), deformability impairments (lower EImax values, p < 0.05) in the order of 2% (DEHP) to 5% (BTHC) were noted. On day 42, all RBCs were less deformable (6-9%) than on day 35 (p < 0.001) despite continued decreases in MCHC, particularly in the BTHC group (4%, p < 0.001).
Fig. 5.
Changes to RBC deformability and MCHC as a function of the storage time (A day 5, B day 21, C day 35, D day 42) and bag plasticizers (Δ = DEHP, □ = DINCH, ○ = BTHC; n = 15/group; graphs display means ± SD). EImax: maximum elongation index predicted at an infinite shear stress.
Phospholipid Analysis
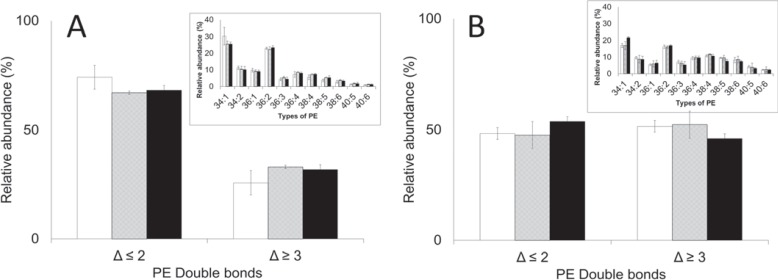
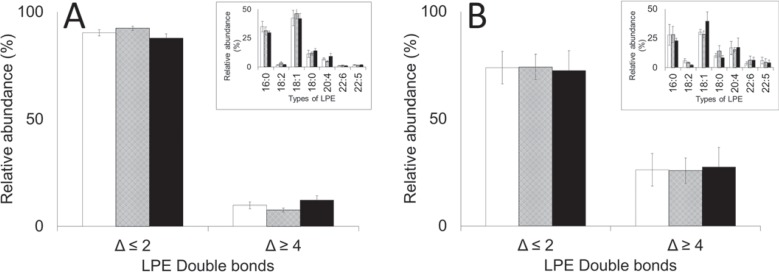
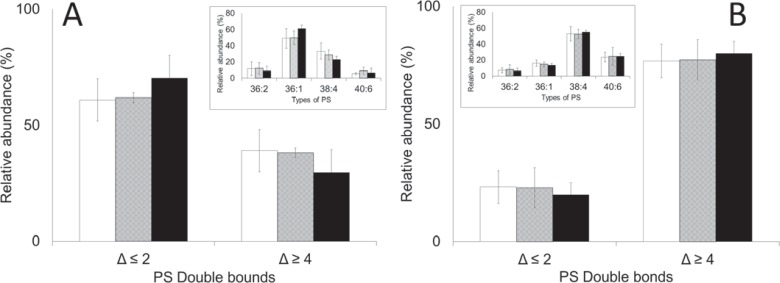
To explore plasticizer-induced changes to the RBC membrane, the distribution of PEs and PSs in RBCs and EVs was examined. RBCs stored in bags plasticized with DINCH, BTHC or DEHP were not different in regards to the composition of PEs, lysophosphatidylethanolamines (LPEs) and PSs (fig. 6A, 7A, 8A). Similarly, the EVs accumulated in the respective bags were no different in regards to the composition of the above mentioned lipids (fig. 6B, 7B, 8B). Irrespective of plasticizer, however, the more unsaturated lipids were enriched in the extract of EVs (p < 0.05). These included PEs containing 3 or more carbon-carbon (C-C) double bonds (Δ ≥ 3; fig. 6), LPEs and PSs containing 4 or more C-C double bonds (Δ ≥ 4; fig. 7, 8) particularly.
Fig. 6.
PE composition of A RBCs and B EVs on day 42. Inserts: the types of PE analyzed, coded by the number of esterified carbons double bonds (Δ). Main: the types of PE grouped according to the number of double bonds (Δ). Average values ± SD (n = 3/group). Bar color: white = DINCH, grey = BTHC, black = DEHP.
Fig. 7.
LPE composition of A RBCs and B EVs on day 42. Inserts: the types of LPE analyzed, coded by the number of esterified carbons double bonds (Δ). Main: the types of LPE grouped according to the number of double bonds (Δ). Average values ± SD (n = 3/group). Bar color: white = DINCH, grey = BTHC, black = DEHP.
Fig. 8.
PS composition of A RBCs and B EVs on day 42. Inserts: the types of PS analyzed, coded by the number of esterified carbons double bonds (Δ). Main: the types of LPE grouped according to the number of double bonds (Δ). Average values ± SD (n = 3/group). Bar color: white = DINCH, grey = BTHC, black = DEHP.
Discussion
The aim of this study was to compare the RBC membrane preservation capacity of DEHP with the membrane preservation capacity of the alternative plasticizers DINCH and BTHC (citrate). In this paired study (pool-and-split) conducted with leukoreduced RCCs stored in pediatric bags, we found that the membrane stabilization capacity differed among bags manufactured with different plasticizers. Many parameters indicated that, overall, RBCs stored in BTHC-plasticized bags showed poorer membrane preservation capacity than those in DINCH- or DEHP-plasticized bags. A previous study from 1991, found that overall RBC quality in DEHP- and BTHC-plasticized PVC was generally comparable [24]. Our results, focused on events at the RBC membrane, would suggest differently. Irrespective of plasticizer, the data showed a positive correlation between increasing levels of RBC EVs (CD235a+) and supernatant Hb during storage. For these parameters, the plasticizer influenced the rate with which the concentration of EVs and supernatant Hb increased with time. RBC vesiculation levels and rates were similar in DEHP- and DINCH-plasticized bags. In contrast, BTHC-plasticized bags provided less protection against vesiculation, osmotic stress, and Hb loss. By day 21, the levels of vesiculation and supernatant Hb in the BTHC bags were approximately twice as high as those seen in DINCH and DEHP bags. The RBCs stored in BTHC bags also became larger (higher MCV) than those stored in DINCH- or DEHP-plasticized bags, which suggests that the membrane stabilization with DEHP is associated with, but not necessarily limited to, membrane mechanisms in control of RBC volume. The progressive swelling of the RBCs during storage inversely correlated, as expected, with the changes of MCHC. These two parameters combined, MCV and MCHC, indicated that the RBCs became less viscous (decreased Hb concentration) with the progression of the storage. Should only viscosity be considered, the RBC deformability should proportionally increase. As shown, all units displayed this simple association up to the 3rd week of the storage. Then, despite lower viscosity, deformability decreased, clearly indicating a storage time point (between the 3rd and 5th week in our study) when changes to the mechanical properties of the membrane made RBCs more rigid. Fundamental changes to the cytoskeleton (e.g. oxidation) and surface area loss due to vesiculation might have at this point become critical. It is thus not surprising that the RCCs presenting more RBC EVs - those stored in PVC-BTHC bags - were also the RCCs containing the most rigid RBCs.
Research by others have demonstrated that exosomes (∼50 nm) and microvesicles (∼200 nm) from endothelial cells, leukocytes, and platelets exceed the amount of RBC EVs (i.e., microvesicles) in the plasma of healthy individuals (blood donors) [25] as well as in blood bank-produced RCCs as they enter storage [26]. While we did not look at non-RBC EVs in this study, this prior work led us to postulate that the average size increase of the RCC EVs during storage reflected the accumulation of RBC EVs (microvesicles) over exosomes (non-RBC origin) caused by the RBC vesiculation. The measurements of EV size did not capture any particular influence of the plasticizers, suggesting that the analyzed EVs had similar dimensions. Flow cytometry analysis also did not capture any particular influence of the plasticizers on the exposed amounts of PS and CD47 in the RBC EVs. LC-MS analysis also did not capture any particular influence of the plasticizers on the composition of EVs in terms of PEs and PSs, suggesting the presence of a similar membrane asymmetry pattern before vesicle release. The combination of these results suggested that none of the plasticizers exerted a direct interference on the mechanism of vesiculation.
Conclusion
This analysis of EV concentration, size and lipid composition allowed us to conclude that DINCH and DEHP provide equivalent protection to RBCs against vesiculation. In contrast, RCCs stored in BTHC-plasticized bags generated more EVs and had higher levels of supernatant hemoglobin. The RBCs stored in BTHC-plasticized bags were larger, more rigid, and more sensitive to osmotic challenge than RBCs stored in the other containers. Collectively, this data would suggest that DINCH would be a superior alternative to BTHC for replacing DEHP in red cell storage bags. The lack of differences in the composition of PEs and PSs of RBCs and EVs across the plasticizer groups suggested, however, that the protection against vesiculation does not come from a direct intervention on the mechanism of vesiculation. The membrane-protective effect appears to be related to the control of cell swelling.
Sources of Funding
This study was supported financially by the Canadian Blood Services which is funded by federal (Health Canada), provincial and territorial Ministries of Health. This study was conducted in collaboration with Fresenius Kabi Deutschland GmbH, which supported the study by providing the pediatric bags for testing.
Author Contributions
BB, KS, DVD, JA: research design; BB, KS, AdSP: data acquisition; BB, KS, DVD, JA: analysis and interpretation; BB: manuscript drafting; BB, KS, AdSP, DVD, JA: revisions and approval.
Disclosure Statement
This study was a collaboration with Fresenius Kabi Deutschland GmbH, who provided the pediatric bags that were used in this study and reviewed the manuscript before submission. Dr. Dana V. Devine serves on the Fresenius Kabi Deutschland GmbH Expert Panel. The other authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank the following at the Canadian Blood Services NetCAD facility in Vancouver, BC: Tamiko Stewart, research assistant for donor recruitment and scheduling; Joanne Ross, clinic assistant; Ann Frankish and Judy Fung, nurses at the blood collection clinics, Riki Roberts and Nobu Nakane, both research assistants, for the production of red cell components for the study. The authors thank Deborah Chen, Canadian Blood Services and the University of British Columbia, Vancouver, BC, for conducting osmotic fragility assay testing and Dr. Geraldine Walsh, Canadian Blood Services scientific writer, for assistance with manuscript preparation and editing. The authors also acknowledge the generous contribution of the blood donors who made this study possible.
References
- 1.Prowse CV, de Korte D, Hess JR, van der Meer PF, Biomedical Excellence for Safer Transfusion (BEST) Collaborative Commercially available blood storage containers. Vox Sang. 2014;106:1–13. doi: 10.1111/vox.12084. [DOI] [PubMed] [Google Scholar]
- 2.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Heal. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Naarala J, Korpi A. Cell death and production of reactive oxygen species by murine macrophages after short term exposure to phthalates. Toxicol Lett. 2009;188:157–160. doi: 10.1016/j.toxlet.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Rael LT, Bar-Or R, Ambruso DR, Mains CW, Slone DS, Craun ML, Bar-Or D. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxid Med Cell Longev. 2009;2:166–171. doi: 10.4161/oxim.2.3.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasakawa S, Mitomi Y. Di-2-ethylhexylphthalate (DEHP) content of blood or blood components stored in plastic bags. Vox Sang. 1978;34:81–86. doi: 10.1111/j.1423-0410.1978.tb03727.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Vliet ED, Reitano EM, Chhabra JS, Bergen GP, Whyatt RM. A review of alternatives to di (2-ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit. J Perinatol. 2011;31:551–560. doi: 10.1038/jp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children's health. Curr Opin Pediatr. 2013;25:247–254. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estep TN, Pedersen RA, Miller TJ, Stupar KR. Characterization of erythrocyte quality during the refrigerated storage of whole blood containing di-(2-ethylhexyl) phthalate. Blood. 1984;64:1270–1276. [PubMed] [Google Scholar]
- 9.Labow RS, Card RT, Rock G. The effect of the plasticizer di(2-ethylhexyl)phthalate on red cell deformability. Blood. 1987;70:319–323. [PubMed] [Google Scholar]
- 10.Horowitz B, Stryker MH, Waldman AA, Woods KR, Gass JD, Drago J. Stabilization of red blood cells by the plasticizer, diethylhexylphthalate. Vox Sang. 1985;48:150–155. doi: 10.1111/j.1423-0410.1985.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 11.AuBuchon JP, Estep TN, Davey RJ. The effect of the plasticizer di-2-ethylhexyl phthalate on the survival of stored RBCs. Blood. 1988;71:448–452. [PubMed] [Google Scholar]
- 12.Seidl S, Gosda W, Reppucci AJ. The in vitro and in vivo evaluation of whole blood and red cell concentrates drawn on CPDA-1 and stored in a non-DEHP plasticized PVC container. Vox Sang. 1991;61:8–13. doi: 10.1111/j.1423-0410.1991.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 13.Draper CJ, Greenwalt TJ, Dumaswala UJ. Biochemical and structural changes in RBCs stored with different plasticizers: the role of hexanol. Transfusion. 2002;42:830–835. doi: 10.1046/j.1537-2995.2002.00138.x. [DOI] [PubMed] [Google Scholar]
- 14.Dumont LJ, Baker S, Dumont DF, Herschel L, Waters S, Calcagni K, Sandford C, Radwanski K, Min K, David RM, Otter R. Exploratory in vitro study of red blood cell storage containers formulated with an alternative plasticizer. Transfusion. 2012;52:1439–1445. doi: 10.1111/j.1537-2995.2011.03506.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhong R, Wang H, Wu X, Cao Y, He Z, He Y, Liu J. In vitro investigation of the effect of plasticizers on the blood compatibility of medical grade plasticized poly (vinyl chloride) J Mater Sci Mater Med. 2013;24:1985–1992. doi: 10.1007/s10856-013-4950-1. [DOI] [PubMed] [Google Scholar]
- 16.Haishima Y, Kawakami T, Hasegawa C, Tanoue A, Yuba T, Isama K, Matsuoka A, Niimi S. Screening study on hemolysis suppression effect of an alternative plasticizer for the development of a novel blood container made of polyvinyl chloride. J Biomed Mater Res B Appl Biomater. 2014;102:721–728. doi: 10.1002/jbm.b.33052. [DOI] [PubMed] [Google Scholar]
- 17.Acker JP, Hansen AL, Kurach JD, Turner TR, Croteau I, Jenkins C. A quality monitoring program for red blood cell components: in vitro quality indicators before and after implementation of semiautomated processing. Transfusion. 2014;54:2534–2543. doi: 10.1111/trf.12679. [DOI] [PubMed] [Google Scholar]
- 18.Stadnick H, Onell R, Acker JP, Holovati JL. Eadie-Hofstee analysis of red blood cell deformability. Clin Hemorheol Microcirc. 2011;47:229–239. doi: 10.3233/CH-2010-1384. [DOI] [PubMed] [Google Scholar]
- 19.Almizraq R, Tchir JD, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013;53:2258–2267. doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- 20.Bicalho B, Pereira AS, Acker JP. Buffy coat (top/bottom)- and whole-blood filtration (top/top)-produced red cell concentrates differ in size of extracellular vesicles. Vox Sang. 2015;109:214–220. doi: 10.1111/vox.12272. [DOI] [PubMed] [Google Scholar]
- 21.Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc Natl Acad Sci U S A. 1997;94:7566–7571. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han V, Serrano K, Devine DV. A comparative study of common techniques used to measure haemolysis in stored red cell concentrates. Vox Sang. 2010;98:116–123. doi: 10.1111/j.1423-0410.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 23.Bicalho B, Holovati JL, Acker JP. Phospholipidomics reveals differences in glycerophosphoserine profiles of hypothermically stored red blood cells and microvesicles. Biochim Biophys Acta. 2013;1828:317–326. doi: 10.1016/j.bbamem.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Hogman CF, Eriksson L, Ericson A, Reppucci AJ. Storage of saline-adenine-glucose-mannitol-suspended red cells in a new plastic container: polyvinylchloride plasticized with butyryl-n-trihexyl-citrate. Transfusion. 1991;31:26–29. doi: 10.1046/j.1537-2995.1991.31191096180.x. [DOI] [PubMed] [Google Scholar]
- 25.de Vooght KM, Lau C, de Laat PP, van Wijk R, van Solinge WW, Schiffelers RM. Extracellular vesicles in the circulation: are erythrocyte microvesicles a confounder in the plasma haemoglobin assay? Bio Soc Trans. 2013;41:288–292. doi: 10.1042/BST20120254. [DOI] [PubMed] [Google Scholar]
- 26.Danesh A, Inglis HC, Jackman RP, Wu S, Deng X, Muench MO, Heitman JW, Norris PJ. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123:687–696. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]