Abstract
Introduction:
The potent antifungal agent amphotericin B (AmB) is not freely soluble in water. The clinical use of AmB is limited by nephrotoxicity and poor water solubility. Polyamidoamine (PAMAM) dendrimer offers an identical carrier for drug binding that has the capacity to attach and discharge drugs in numerous ways.
Materials and methods:
In this research work, we explored the potential of PAMAM dendrimers to improve the solubility of AmB.
Results and discussion:
The experimental results indicated that the solubility of AmB was greatly enhanced in the presence of PAMAM dendrimer solutions. Results indicated that the solubility of AmB enhanced with increase in dendrimer generations as well as concentration. In vitro release studies of AmB in the presence of the third generation of PAMAM dendrimers was performed by the dialysis method. Our research work revealed that binding of drug into dendrimers led to sustained release of AmB in vitro.
Conclusion:
Based on the stability studies, it was concluded that the drug dendrimer complex should be stored in a dark place at a cool temperature.
Keywords: Amphotericin B (AmB), drug dendrimer conjugates, in vitro release studies, polyamidoamine (PAMAM) dendrimer, solubilization
INTRODUCTION
About $65 billion worth of drug income is accounted by active pharmaceutical ingredients (APIs) with suboptimal bioavailability. Just about 40% of the recently launched drugs are thrown out by the pharmaceutical companies and will never profit a patient due to decreased bioavailability owing to poor cell membrane permeability and water solubility. Novel drug delivery systems could assist in conquering this challenge. Among new drug delivery systems, nanotechnology has appeared as a novel research ground that mainly involves the design, synthesis, and formulation of compounds at the molecular level. As polymer technology has advanced over the past two centuries, the peculiar characteristics of macromolecules that are synthetically available have also developed.[1] The highly branched three-dimensional manmade polymeric macromolecules are known as dendrimers and are the mainly known element of the polymer science. Dendrimers have often been referred to as the “polymers of the 21st century.” Dendrimers have developed a young set of polymers with a versatile architecture and unique chemical structures.[2] The better encapsulating ability of dendrimers makes them excellent carriers for the delivery of drugs. It has been proved that dendrimers may have the potential to improve the solubility of low aqueous soluble drugs and as a carrier for bioactive materials.[3] Among them, the polyamidoamine (PAMAM) dendrimer is one of the well-known dendrimers and has been proven as a carrier to improve water solubility and the rate of dissolution of drugs such as ketoprofen,[4] ibuprofen,[5] aceclofenac,[6] and riboflavin.[7]
The potent antifungal agent amphotericin B (AmB) is often used intravenously for systemic fungal infections. The therapeutic application of AmB is restricted by nephrotoxicity and low water solubility, which guides in some events, renal impairment, especially with coadministered nephrotoxic drugs. Over the past several years, various efforts have been made to improve the solubility and in vitro dissolution property of AmB. The versatile architecture of dendrites such as their size, branching length, shape, and their surface functional groups permit the transformation of the dendrimers as per the requisites, constructs these macromolecules perfect carriers in drug delivery applications, and enhances the solubility of low aqueous soluble drugs.[8] Here, we made an attempt to incorporate AmB in PAMAM dendrimers of different generations (G1-G3) to explore the potential of PAMAM dendrimers to enhance the solubility of AmB. The solubilization mechanism and in vitro release studies of AmB-PAMAM complex were investigated. To enhance the efficacy and get a better acceptability profile, extensive efforts were made to reformulate AmB into a suitable drug delivery system. Thus, the search for an optimal formulation is still of great importance.
MATERIALS AND METHODS
Materials
AmB was purchased from Cipla Ltd. (Mumbai, Maharashtra, India). PAMAM dendrimer of different generations were received from Sigma Aldrich (Carlsbad, CA, USA). All other chemicals used were procured from SD Fine Chemicals (Mumbai, Maharashtra, India).
Synthesis of polyamidoamine-amphotericin B conjugates
Results of dendrimer-mediated solubility studies suggested that the solubility of AmB was enhanced to increase in generation as well as dendrimer concentration. The optimized G3 dendrimer-based formulations were selected for advanced studies. The AmB was dissolved in dendrimer solutions to a concentration of 2 mg/mL after dilution with distilled water. These were vortexed for 24 h at room temperature. The vortexed samples were spun at 14,000 rpm for 10 min. These were then filtered through 0.22 μm membrane filter. These conjugates were used for in vitro release studies.[9]
Ultraviolet-visible spectroscopy
AmB in phosphate buffer (pH 7.4) gives sharp absorbance at 407 nm. A standard curve of AmB was constructed by using various concentrations. Jasco V-630 spectrophotometer (Jasco, Easton, USA) was used to determine the drug content in the dendrimer and further, the mixture was diluted with distilled water. The sharp peaks obtained for AmB-dendrimer mixture at 407 nm would be solely from AmB since dendrimer in the solutions gives no sharp peaks at 407 nm. The amount of AmB was estimated by correlating the absorbance value with the standard curve.
Solubility studies
Determination of the effect of dendrimer generation and concentration on the solubility of amphotericin B
The solubility of AmB was determined using an equilibrium solubility technique.[10] Diluted solutions of PAMAM dendrimers (G1-G3) in a concentration of 0.05-0.2% weight/volume (w/v) was prepared. The excess amount of drug was then added to each of the test solutions. These were vortexed for 24 h at room temperature. The vortexed samples were spun at 10,000 rpm for 10 min. These were then filtered through a 0.22-μm membrane filter (Merck Life Science Private Limited, Mumbai) followed by spectrophotometric measurements of absorbance using ultraviolet (UV) spectroscopy.[11] Drug loaded dendrimer solutions were scanned between 200 nm and 450 nm in Jasco V-630 spectrophotometer to analyze the drug loading as well as the effect of solubilization at λmax. Three repeats were conducted. The generation of dendrimer with maximum solubilization/drug loading was selected for further studies.
Determination of pH-dependent solubility of amphotericin B
This work was extended to ascertain the influence of pH on dendrimer-mediated solubilization of AmB.[11] The study was conducted in different pH conditions (4, 7.4, and 10). A choice of these pH values was to provide typical acidc/basic states since we believed that the solubilization process of AmB was significantly affected by its ionization status. To ascertain the pH-dependent solubility of AmB, an excess amount of drug was added to glass vials containing concentrations of 0.05-0.2% w/v of G3 PAMAM dendrimers (Sigma Aldrich, Carlsbad, USA) in pH 4.0 acetate buffer. A comparative method was taken after for pH 7.4 and 10 too. The rest of the method was similar to the procedure followed to interpret the impact of concentration of denrimers on the solubility of AmB.
In vitro release studies
In vitro release studies of AmB in the vicinity of the third generation of PAMAM dendrimers was performed by the dialysis method.[12] AmB was dissolved in dendrimer solutions to a final concentration of 2 mg/mL after being diluted with distilled water. Pure AmB was dissolved in a small quantity of dimethyl sulfoxide and then diluted with distilled water and used as a control. Drug dendrimer solution of about 5 mL was filled in dialysis bags [molecular weight (MW) cutoff = 1000 Daltons (Himedia, Mumbai, India)], which was pretreated with pH 7.4 phosphate buffer. The dialysis bags were suspended in 100 mL of phosphate buffer of pH 7.4 manitained at 37± 0.5°C under constant stirring. A perfect sink condition was achieved by withdrawing 1 mL of the sample from the outer phase, and the outer phase was again replenished with the same volume of dissolution medium. The quantity of drug released at each time interval for 12 h was estimated at 407 nm using UV spectrophotometer (Jasco V-630, Japan).
Stability studies
Stability studies of AmB-G3 PAMAM were conducted under dark and light conditions. The dark and light conditions were achieved by keeping 10 mL of the sample in amber-colored and colorless glass vials, respectively. The samples were kept at 0°C, room temperature, and accelerated temperature (60 ± 2°C) in a temperature controlled over for about 6 weeks. The samples were taken out and examined at the start and periodically (every week) for up to 6 weeks for any turbidity, color change, consistency, precipitation, and drug leakage. The percent drug leakage was determined by estimating a rise in drug release from the drug-dendrimer complex throughout storage. Drug dendrimer solution of about 5 mL was filled in dialysis bags (MW cutoff = 1,000 Daltons, Himedia, India), which was pretreated with pH 7.4 phosphate buffer and dialyzed against 50 mL of dissolution medium. The samples were observed for the amount of drug spectrophotometrically at 407 nm. The method was repeated at a weekly interval for up to 6 weeks. The data were analyzed to understand the conditions suitable for the storage of the formulation.
RESULTS AND DISCUSSIONS
Solubility studies
A series of solubility studies were conducted to determine the influence of dendrimer generation and concentration on the solubility of AmB in the presence of PAMAM dendrimers. In this study, we explored the influence of G1-G3 PAMAM dendrimers in the process of solubilization. The solubility of AmB was determined at room temperature and the results are shown in Figures 1 and 2. It was found that the extremely poor aqueous solubility of AmB has been extensively enhanced by association with PAMAM dendrimers compared with distilled water. The solubility of AmB in the dendrimer solutions increased in an approximately linear manner with an increase of dendrimer concentration. The enhancement of solubility of AmB was due to the internal architecture that are offered to incorporate AmB molecules (host-guest interaction) and theses versatile features make them excellent carriers for drug delivery applications.[12] It was clear that the solubility of AmB was influenced by different generations of PAMAM dendrimers are illustrated in Figure 1. The solubility of AmB in higher generations of PAMAM solution was in fact higher than those in the lower ones.[13] The solubility of AmB in PAMAM solutions depends on surface amino groups of PAMAM dendrimers; thus, a molecule of higher generation of PAMAM particle has a higher ability to absorb and interact with the AmB molecule than that of lower one. Results suggested that the solubility of AmB increased with increase in dendrimer concentration as well as generation.
Figure 1.
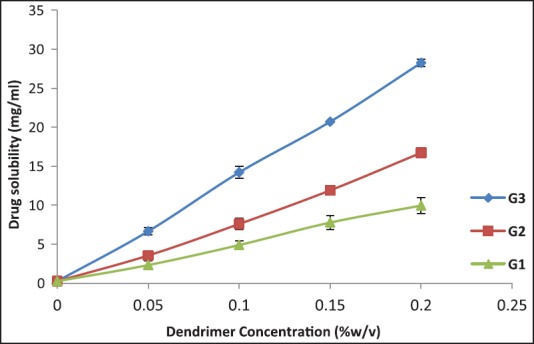
Solubility of amphotericin B (AmB) in the presence of increasing concentrations of PAMAM dendrimers
Figure 2.
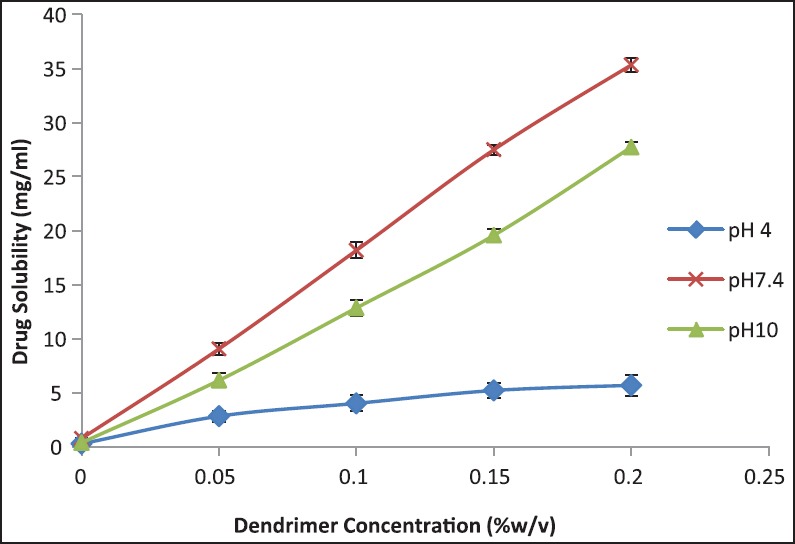
Solubility profile of AmB at different pH conditions with increasing concentration of PAMAM G3 dendrimers
It was found that the extremely low water solubility of AmB has been extensively improved by associating with PAMAM dendrimers compared with that in distilled water. The solubility of AmB in the dendrimer solutions increased in an approximately linear manner with an increase of dendrimer concentration. The enhancement of solubility of AmB was due to the internal cavities that are extended to incorporate AmB molecules and these versatile features make them excellent carriers for drug delivery applications.[11,14] From the Figure 1, it was clear that the solubility of AmB was influenced by different generations of PAMAM dendrimers. The solubility of AmB is usually increased with a higher generation of dendrimers because of the effect of size and surface amino groups on solubility.[13] The effect of dendrimer concentration on the solubility of AmB was shown in Figure 1. The solubility of AmB enhanced in a linear fashion with increasing concentration of the dendrimer. Results showed that the solubility of AmB enhances the increase in dendrimer generation as well as compactness.
Solubility profiles of AmB conducted in the vicinity of G3 PAMAM dendrimers at pH 4, 7.4, and 10 are shown in Figure 2. Under optimized concentration of PAMAM G3 (0.2% w/v) at pH 7.4 and 10.0, solubility of AmB was extremely higher than at pH 4.0. The solubility of AmB enhanced in the order of pH 7.4 > 10.0 > 4.0 in the AmB-G3 PAMAM dendrimer formulations. Dendrimers show micellar structure and their structure remains stable at even higher concentrations of solvents. The solubility enhancement of drugs by the use of dendrimers is perhaps because of hydrogen bonding, hydrophobic interactions, and electrostatic attraction between the drug and surface functional groups of the dendrimers.[15,16,17]
In vitro release studies
The potency of the drug-dendrimer complex was investigated by release studies. In vitro release of AmB-PAMAM G3 complex was carried out in pH 7.4 phosphate buffer. Pure AmB was released (66.26%) in 6 h, whereas AmB-dendrimer complex exhibited the delayed release of the drug [Figure 3]. After 10 h, 75.76% release was accomplished for the pure AmB while 42.72% release was shown by AmB-G3 PAMAM complex. The release profile of AmB from the drug dendrimer complexes was in a sustained manner compared to pure AmB. This was probably due to hrophobicity of AmB, which permits it to reside little longer in the hydrophobic pockets of the dendrimers. These results strongly suggested that electrostatic interaction might involve an important role in the release of drugs from dendritic mixtures.
Figure 3.
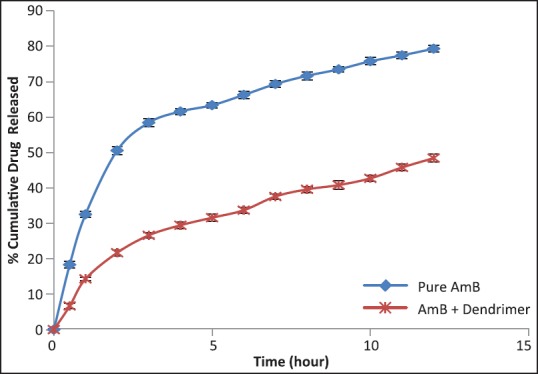
In vitro release of AmB in G3 PAMAM dendrimer solution compared with the pure AmB release behavior
Stability studies
The stability studies of AmB-G3 PAMAM was conducted under different storage conditions and results that are shown in Table 1. Results showed that AmB-G3 PAMAM complex was stable at an accelerated temperature up to 60 ± 2°C in the dark. There was no alteration in the color, consistency, and turbidity was observed in the drug dendrimer complex placed in 0°C and room temperature. Percent drug leakage was determined and the outcomes are illustrated in Figure 4. The percent drug leakage was found to be maximum under 60 ± 2°C in the dark. Based on the stability studies, it was concluded that the drug dendrimer complex should be stored in a dark place at a cool temperature.
Table 1.
Stability studies of AmB-G3 PAMAM dendrimer formulation
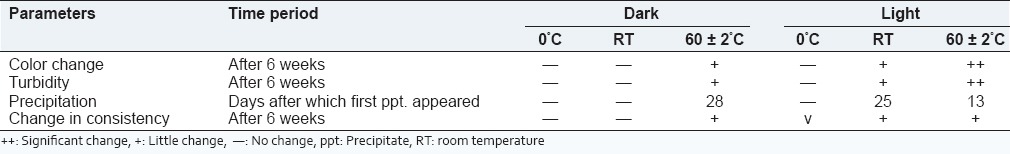
Figure 4.
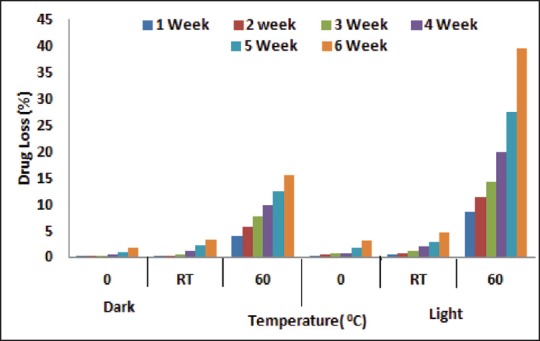
Drug leakage from AmB-G3 PAMAM complex under diverse storage conditions
CONCLUSION
PAMAM dendrimers with terminal amine functional groups have the ability to enhance the solubility of low aqueous soluble drugs, for instance, AmB. The solubility of AmB depends on the generation of the dendrimer, concentration of the dendrimer, and medium pH. Dendrimers can also control release profile of the drug from the drug-dendrimer conjugates. Our study presented that the conjugation of AmB with PAMAM dendrimers led to sustained release of drug in vitro. Even though dendrimer-based drug delivery is in its early phase, it tenders numerous features in the delivery of drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Support from Nitte University, Mangalore, Karnataka, India and National Institute of Immunology, Delhi, India were highly appreciated.
REFERENCES
- 1.Lee CC, MacKay JA, Fréchet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2003;23:1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 2.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S. A new class of polymers: Starburst-dendritic macromolecules. Polym J. 1985;17:117–32. [Google Scholar]
- 3.Gupta U, Agashe HB, Asthana A, Jain NK. Dendrimers: Novel polymeric nanoarchitectures for solubility enhancement. Biomacromolecules. 2006;7:649–58. doi: 10.1021/bm050802s. [DOI] [PubMed] [Google Scholar]
- 4.Yiyun C, Tongwen X, Rongqiang F. Polyamidoamine dendrimers used as solubility enhancers of ketoprofen. Eur J Med Chem. 2005;40:1390–3. doi: 10.1016/j.ejmech.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Milhem OM, Myles C, McKeown NB, Attwood D, D’Emanuele A. Polyamidoamine starburst dendrimers as solubility enhancers. Int J Pharm. 2000;197:239–41. doi: 10.1016/s0378-5173(99)00463-9. [DOI] [PubMed] [Google Scholar]
- 6.Patel J, Garala K, Basu B, Raval M, Dharamsi A. Solubility of aceclofenanc in polyamidoamine dendrimer solutions. Int J Pharm Invest. 2011;1:135–8. doi: 10.4103/2230-973X.85962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipowicz A, Wołowiec S. Solubility and In vitro transdermal diffusion of riboflavin assisted by PAMAM dendrimers. Int J Pharm. 2011;408:152–6. doi: 10.1016/j.ijpharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Fréchet JM. Designing dendrimers for drug delivery. Pharm Sci Technolo Today. 1999;2:393–401. doi: 10.1016/s1461-5347(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 9.Ma M, Cheng Y, Xu Z, Xu P, Qu H, Fang Y, et al. Evaluation of polyamidoamine (PAMAM) dendrimers as drug carriers of anti-bacterial drugs using sulfamethoxazole (SMZ) as a model drug. Eur J Med Chem. 2007;42:93–8. doi: 10.1016/j.ejmech.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi T, Connors A. Phase-solubility techniques. In: Reilly CN, editor. Advances in Analytical Chemistry and Instrument. New York: John Wiley; 1965. pp. 117–212. [Google Scholar]
- 11.Devarakonda B, Hill RA, Liebenberg W, Brits M, de Villiers MM. Comparison of the aqueous solubilization of practically insoluble niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int J Pharm. 2005;304:193–209. doi: 10.1016/j.ijpharm.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Na M, Yiyun C, Tongwen X, Yang D, Xiaomin W, Zhenwei L, et al. Dendrimers as potential drug carriers. Part II. Prolonged delivery of ketoprofen by in vitro and in vivo studies. Eur J Med Chem. 2006;41:670–4. doi: 10.1016/j.ejmech.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Morris JJ, Lopina ST. Polyethylene glycol-polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J Colloid Interface Sci. 2004;273:148–54. doi: 10.1016/j.jcis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Esfand R, Tomalia DA. Poly (amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–36. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 15.Hawker CJ, Wooley KL, Fréchet JM. Unimolecular micelles and globular amphiphiles: Dendritic macromolecules as novel recyclable solubilization agents. J Chem Soc Perkin Trans. 1993;1:1287–97. [Google Scholar]
- 16.Newkome GR, Moorefield CN, Baker GR, Saunders MJ, Grossman SH. Unimolecular micelles. Angew Chem Int Ed Engl. 1991;30:1178–80. [Google Scholar]
- 17.Stevelmens S, Hest JC, Jansen JF, van Boxtel DA, de Brabander-van den Berg EM, Miejer EW. Synthesis, characterization and guest-host properties of inverted unimolecular dendritic micelles. J Am Chem Soc. 1996;118:7398–9. [Google Scholar]


