Abstract
Introduction:
Superiority of topical instillation of drug into the cul-de-sac for the treatment of various ophthalmic complications can be validated with commercial availability of a large number of conventional formulations even though this mode of instillation still elicits limitations owing to poor ocular bioavailability. To overcome the drawbacks of conventional formulations, a large number of novel carriers have been investigated. In this perspective, a new novel nanocarrier, chondroitin sulfate (ChS)-chitosan (CS)-nanoparticles (NPs) are being evaluated for improved delivery of bromfenac sodium.
Materials and Methods:
Formulation was developed and optimized for CS, chondroitin, and initial drug concentration. Optimized formulation was evaluated for various in vitro aspects i.e., particles’ size, size distribution, zeta potential, shape and morphology, in vitro release profile, corneal permeation, corneal retention, corneal uptake, and ocular tolerance test.
Results:
The mean particle size, polydispersity index, zeta potential, and entrapment efficiency of optimized formulation were found to be 245.6 ± 14.22 nm, 0.187 ± 0.016, +37.59 ± 4.05 mV, and 71.72 ± 4.43%, respectively. Transmission electron microscopic analysis revealed a spherical shape of developed formulation. Further, formulation exhibited biphasic release profile and Korsmeyer–Peppas model was found to be the best fit model. Significantly high transcorneal permeation (1.62-fold) and corneal retention (1.92-fold) of bromfenac was observed through ChS-CS-NPs when compared with marketed eyedrops (P < 0.01). Furthermore, high corneal uptake of CHS-CS-NPs was confirmed by confocal laser scanning microscopy (CLSM). Safety profile of the developed formulation was established by hen's egg test-chorioallantoic membrane test.
Conclusion:
Encouraging outcomes of in vitro and ex vivo studies indicated that CHS-CS-NPs could be a potential substitute for improved ocular delivery.
Keywords: Bromfenac sodium, chitosan (CS), chondroitin sulfate (ChS), nanoparticles (NPs), ocular delivery, ocular drug delivery, transcorneal permeation
INTRODUCTION
Superiority of topical instillation of drug into the cul-de-sac for the treatment of various ophthalmic complications such as allergic conjunctivitis, postoperative pain, and glaucomatous conditions can be validated with the availability of a large number of conventional commercial formulations as it can offer high local concentration and consequently better efficacy and reduced systemic side effect. Despite of being a most preferred and convenient mode of administration, this route still elicits limitations owing to poor ocular bioavailability of drugs (<5%), which can be attributed to the following reasons: low corneal permeation, high turnover rate of tears and rapid nasolacrimal drainage, thus short residence time and consequently fast decay in drug concentration in ocular milieu.[1] Though, many drugs demonstrated good permeation across the cornea even then they necessitate frequent instillation to accomplish intended therapeutic effect due to short residence time, which may turn into undesirable side effects. Therefore, a number of formulations such as polymeric vehicles,[2] preformed gels or hydrogel,[3] in situ gelling systems,[4] and ocuserts[5] have been investigated to improve mean residence time of drugs into the cul-de-sac, thus improving the efficacy of drugs via sustained effect and improved absorption.[6] Preformed hydrogels or hydrogels limit their utility due to inaccurate and nonreproducible administration; they often cause blurred vision, and lachrymation while in situ gelling systems are free of these drawbacks and showed prolonged effect when compared with free solution and suspension.[4,7,8] However, there is scope for further improvement. Ocuserts demonstrated a sustained effect; unfortunately they cause crusting of eyelids due to their solidity, feeling of extraneous body into the eye, unintended removal during sleep, and interference with vision.[9,10]
Colloidal or nanoparticles (NPs) emerged as an elegant approach to enhance the ocular bioavailability of topically instilled drugs into the eye cavities; preserving the ease of delivery, these systems offer inimitable features such as nonirritancy, prolonged residence time, sustained effect, high corneal permeation, and reduced dosing frequency, and thus patient compliance and reduced systemic side effects.[11,12] Among them, polymeric NPs are the most investigated system to enhance the corneal and conjunctival penetration,[12,13,14,15] and most widely explored polymers for ocular delivery are poly (alkylcyanoacrylate), poly (ε-caprolactone), and poly(lactic acid) (PLA), poly(lactide-co-glycolide) (PLGA) Eudragits, polystyrene, and chitosan (CS).[16] PLA/PLGA is a Food and Drug Administration (FDA)-approved biodegradable polymer and thus, ideal for ocular delivery due to its biocompatibility, safety, regulatory approval as well as documented tolerance in animal models and potential in ophthalmology;[11,12,15] however, the short residence time limits its utility.[17] Designing of a nanocarrier system with mucoadhesive property would be a sensible approach toward the management of ocular implication.
Chondroitin sulfate (ChS) is a component of the human body, which is abundantly distributed into the extracellular matrix (ECM) of cartilage. It is a naturally occurring anionic polyelectrolyte comprising repeated disaccharide units of b-1, 4-linked D-glucuronic acid and b-1, 3-linked N-acetyl-galactosamine. It has been reported to use in the symptomatic treatment of osteoarthritis and possess anti-inflammatory property.[18] Being a component of body, it has got the FDA approval to be used as a skin substitute for inducing reepithelialization of wounds.[19,20] Moreover, it is being investigated for colon-targeted delivery.[21,22] In addition, it has shown promising potential in ocular gene therapy.[23,24] ChS is a water soluble matrix and proven unable to provide sustained release profile to drugs. CS, a naturally existing cationic polymer avidly forms a complexes with wide number of anionic poly-electrolytes including ChS.[25,26] The formed complex demonstrates a high affinity to water and able to soak a large amount of water and able to form hydrophilic swollen matrix, which is highly desirable to provide a sustained profile to any drug. Thus, it can be used for the effective delivery of wide variety of molecules.[27,28] Furthermore, it owns some obvious merits over cross-linking induced by tripolyphosphate such as higher yield, and more mechanical and chemical stability owing to highly cross-linked and dense network. In addition, CS possesses mucoadhesive property, which can add to it more value for improved delivery. Moreover, CS is considered to be the most potent polymer in ophthalmology as it is natural, nontoxic, nonirritant, biodegradable, and mucoadhesive in nature; it is also reported to have antibacterial property and the ability to open a tight junction and thus, enhance the paracellular transport of drugs.[29,30,31]
Hence, a new type of ChS-CS-NPs was conceptualized for improved ocular delivery. In our belief, ChS-CS has not been investigated so far for the ocular delivery of drugs. Therefore, the present work was aimed at the development and optimization of bromfenac-loaded ChS-CS NPs and evaluates them for various in vitro attributes such as particles’ size, size distribution, surface morphology, release profile, transcorneal permeation, corneal retention, corneal uptake by CLSM, and ocular irritancy test. Bromfenac sodium was used as model drug, which is basically a nonsteroidal anti-inflammatory drug (NSAID), indicated for postoperative inflammation and pain after cataract surgery.
MATERIALS AND METHODS
Materials
Medium molecular weight CS with 75-85% degree of deacetylation (viscosity 200-800 cp, 1 wt % in 1% acetic acid solution) and ChS were procured from Sigma Aldrich (St. Louis, MO, USA). Bromfenac sodium was provided ex gratia by Jubilant Organosys Ltd., India. Methanol [A standard grade of analytical reagents (AR) grade], sodium chloride (AR grade), sodium carbonate (AR grade), and calcium chloride (AR grade) were purchased from SD Fine Chemicals Limited (Mumbai, India). Ultrapure water was procured with Milli-Q® Integral Water Purification System (Merck Millipore, Darmstadt, Germany). Goat eye was obtained from freshly slaughtered animals at a local abattoir.
Preparation of buffer solutions
Simulated tear fluids were prepared according to procedure as reported by Van Ooteghem.[32] In brief, sodium chloride (670 mg), sodium bicarbonate (200 mg), and calcium chloride dehydrated (8 mg) were dissolved in purified water and made the volume up to 100 mL and pH was adjusted to 7.4.
Preparation of chondroitin sulfate-chitosan-nanoparticles (ChS-CS-NPs)
In brief, a stock solution of CS [1% weight/volume (w/v)] was prepared by dispersing it into 2% v/v of acetic acid solution and kept on under mild stirring for 24 h. Similarly, 1% stock solution of ChS was prepared by dissolving it into double distilled water. Both solutions were serially diluted; subsequently pH of CS solution was adjusted to 5 followed by filtration before formulation development. Formulations were developed by the ionic gelation method with slight modification as discussed in a previous report.[33] Equal volume of ChS containing bromfenac sodium was added gradually into an equal volume of CS solution under the continuous magnetic stirring (600 rpm) at room temperature and was kept on stirring for 15 min to stabilize the NPs.
Characterization of developed formulation
Particle size, size distribution, and zeta potential
Freeze-dried formulation was dispersed in Milli-Q water (1 mg/2 mL); mean particles’ size, size distribution, and zeta potential were determined by dynamic light scattering method using Malvern Zetasizer (Zetasizer, HAS 3000; Malvern Instruments, Malvern, Worcestershire, UK) and data were processed by DTS software. All the measurements were performed in triplicate at a scattering angle of 90°C and 25°C temperature.
Particle morphology
Morphological analysis was performed by transmission electron microscopy (TEM; TOPCON002B, Tokyo, Japan, accelerating voltage 200 kV). Formvar-coated copper grid (Plano GmbH, Wetzlar, Germany) was soaked with NPs suspension and dried in open air and then stained with phosphotungstic acid solution (2% w/v). Image capture and analysis were performed by Digital Micrograph and Soft Imaging Viewer software (Olympus, Singapore).
Encapsulation efficiency
The entrapment efficiency of ChS-CS-NPs was estimated by previous reported method[34] using ultracentrifuge filtration tubes equipped with semi-permeable membrane (MW cutoff of 12 KD, Amicon® Merck Millipore, Darmstadt, Germany). Diluted sample was placed in the upper chamber followed by centrifugation for 15 min at 3,000 rpm. The filtrate was analyzed at absorption maxima (λmax) 270 nm by using ultraviolet–visible (UV-VIS) spectrophotometer (double beam, Shimadzu, Tokyo, Japan), and entrapment efficiency calculated by the following method;

In vitro release analysis
In vitro release profile of developed formulation was evaluated by dialysis bag method as described in a previous report with slight medication.[14] Formulation (equivalent to 1 mg of bromfenac) was suspended in 2 mL of simulated tear fluid (STF) placed into a dialysis bag (MW cutoff of 12 kDa); both the ends of the bag were tied and suspended into 20 mL of STF. Release study was performed at 32 ± 0.5°C and stirring speed (25 rpm). Samples (3 mL) were regularly withdrawn and analyzed by using UV-VIS spectrophotometer at absorption maxima (λmax) 270 nm (double beam, Shimadzu, Japan). Withdrawn volume was replaced with fresh media to maintain sink condition. A similar procedure was followed for marketed eyedrops. All measurements were conducted in triplicate.
In vitro transcorneal permeation study
Comparative in vitro transcorneal permeation of bromfenac through marketed eyedrops and ChS-CS-NPs was performed in goat cornea according to a previous report.[14,35] The goat cornea was mounted between donor and receiver compartments of Franz diffusion cell keeping epithelial surface toward donor side. 100 μL of marketed eyedrops (0.09%) or an equivalent amount of NPs suspended in 100 μL STF was poured into the donor compartment while receiver compartment was filled with STF (5 mL). All of the study was conducted at 32 ± 0.5°C; samples were withdrawn periodically (1 mL) up to 3 h and quantitatively analyzed at absorption maxima (λmax) 270 nm by using UV-VIS spectrophotometer (double beam, Shimadzu, Japan).
In vitro corneal retention study
Comparative in vitro corneal retention of bromfenac through marketed eyedrops and ChS-CS-NPs was performed in goat cornea according to previous reports with slight modifications.[14,36] Initially, cornea was fixed between donor and receiver compartment of Franz diffusion cell and allowed to stabilize for 15 min into prewarmed simulated tear fluid (32°C) for 15 min; subsequently, STF of donor chamber was replaced with 100 μL of marketed eyedrops/equivalent amount of NPs suspension in STF. After 2 h, cornea was taken out and softly rinsed with STF. Rinsed cornea was homogenized in an equivalent amount of STF followed by extraction in methanol. Resultant homogenate was centrifuged at 10,000 rpm for 10 min. The supernatant was passed through membrane filter (0.45 μM) and quantitatively analyzed at absorption maxima (λmax) 270 nm by UV-VIS spectrophotometer (double beam, Shimadzu, Japan).
Corneal uptake of CSH-CS-NPs by confocal laser scanning microscopy (CLSM)
For the evaluation of cellular internalization (uptake), retention and penetration of NPs into the corneal epithelium, the excised goat cornea was treated with fluorescein sodium (FS) (fluorescent marker)-loaded NPs (suspended into STF) for 2 h. After scheduled time, cornea was rinsed gently with STF, mounted directly on the glass slide keeping epithelial side up and analyzed by using CLSM (Olympus FluoView FV 1000, Hamburg, Germany). Excitation was performed with green helium neon at 543-nm laser beam. The images were captured as stacks of serial optical sections in the Z axis under 20x oil objective to assess the penetrability of NPs.
Ocular tolerance test
Hen's egg test-chorioallantoic membrane (HET-CAM) test was used to assess the irritation potential of developed formulation.[35] In brief, fresh fertilized hen's eggs were procured from the local poultry farm. They were incubated at 37 ± 0.5°C and 40 ± 5% relative humidity for 9 days and rotated intermittently during the incubation period. Eggs with live embryo, intact yolk, and damaged CAM were rejected. On the 10th day of incubation, a 2 × 2 cm window was created at the equator of the egg and the inner membrane was removed to expose CAM. Separately, 0.5 mL each of the followings; normal saline (negative control) and sodium hydroxide (positive control) and drug loaded nanosuspension (test formulation) were softly placed on the surface of CAM and subjected to exposure of 5 min. After defined time interval, membrane were observed for possible vascular responses/injuries and scored according to the schemes.[12]
RESULTS AND DISCUSSION
Development and optimization of bromfenac-loaded ChS-CS-NPs
The ionic interaction between cationic amino group of CS and anionic sulfate group of ChS induces gelation and thus formation of NPs/aggregates. It may result in clear solution, opalescent suspensions, and aggregation depending on the concentration of CS, ChS, and drugs. The zone of opalescent is considered to be the most appropriate for development of NPs. Moreover, pH affects the particles size as well as yield of the formulation. In the present work, the pH of CS solution was kept at 5 as optimum particles’ size and maximum yield were reported at this pH.[33] In addition, bromfenac sodium showed poor solubility at lower pH, which resulted into the precipitation of drug/aggregation of formulation. As CS, ChS, and bromfenac concentration directly influence the particles’ size, entrapment efficiency, and zeta potential of formulations, thus controlling these parameters, the size, drug entrapment, and zeta potential can be manipulated. The effect of these factors discussed below;
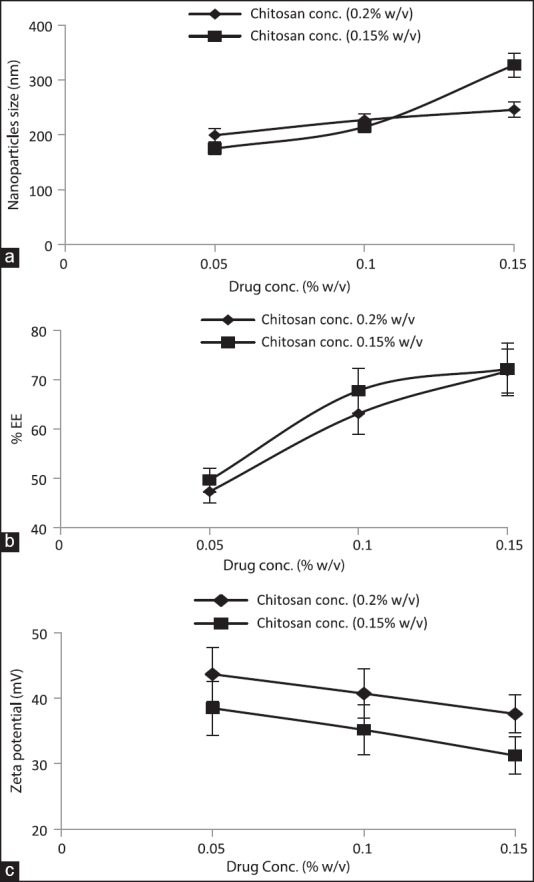
Effect of chitosan concentration
Table 1 and Figure 1 summarize the effect of the CS concentration on the size of NPs, their drug entrapment, and zeta potential keeping the drug concentration (0.2% w/v) and ChS concentration (0.1% w/v) constant. To study the effect of CS concentration, its concentration was taken in the range of 0.1-0.4%. At the lowest concentration of CS (0.1 w/v) aggregation was observed while NPs formation took place at the concentration range of 0.15-0.4% w/v [Table 1]. At this range, the size of NPs was found to increase on increasing the CS concentration [Figure 1a]. As discussed earlier, the formation of NPs occurs as a result of ionic interaction between cationic and anionic polyelectrolytes. Since the formation of NPs takes place at a specific range of cationic and anionic polyelectrolytes weight ratio, excess of any component results into aggregation.[33,37] Thus, aggregates at 0.1% w/v concentration of CS can be attributed to weight ratio (1/1). At this ratio, ChS molecules would be higher than required to stabilize the particles, which result in over-aggregation of ChS molecules on the ChS-CS-NPs owing to partial attraction between positive-negative charge of CS and ChS, respectively, thus aggregate formation. The formation of NPs at the concentration of range, 0.15-0.4% w/v, again can be explained by CS/ChS weight ratio. At these ratios, the amount of ChS was within the required range to stabilize the particles, which result in NPs formation while continuous increase in particles’ size upon increasing CS concentration can be attributed to the relatively lower amount of ChS (constant 0.1%) with respect to CS concentration, which results into the dominancy of repulsive force due to positive charge between CS-CS over the attractive force between CS and ChS. Higher attractive force is the prerequisite for the generation of smaller size NPs.[14,38] Thus, larger size NPs at higher CS concentration.
Table 1.
Effect of chitosan concentration on mean particles size, polydispersity index, entrapment efficiency and zeta potential
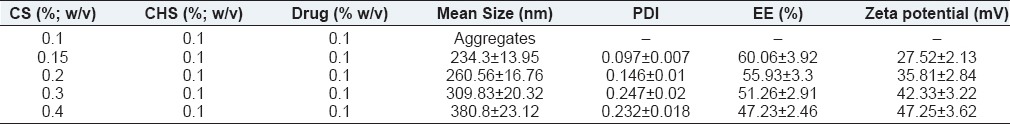
Figure 1.
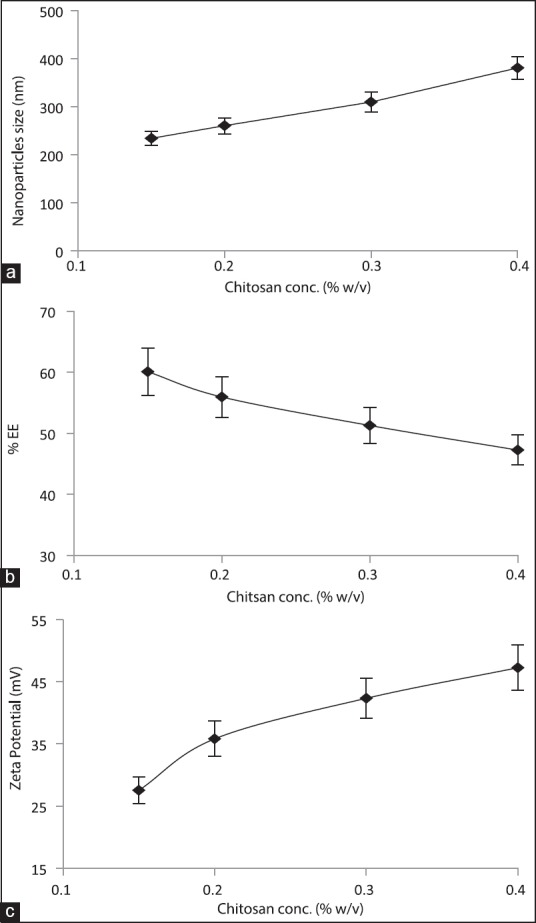
Effect of chitosan concentration on (a) mean particle size (b) entrapment efficiency (c) zeta potential
The effect of CS concentration on the drug entrapment was demonstrated in Figure 1b. Upon increasing CS concentration, the drug entrapment was found to reduce and vice versa [Table 1]. It can be explained by the relative lager size of particles owing to the relatively loose network within NPs due to more repulsion among CS-CS amino groups at a higher CS concentration, thus more leaching of drug and consequently poor entrapment and vice versa.
Figure 1c demonstrates the effect of CS concentration on the zeta potential. Zeta potential was found to increase upon increasing CS concentration. The observation can be attributed to the availability of more number of positively charged CS molecules.
Effect of chondroitin sulfate concentration
Table 2 and Figure 2 summarize the effect of ChS concentration on the size of NPs, their drug entrapment, and zeta potential while keeping drug concentration (0.2% w/v) and CS concentration (0.2% w/v) constant [Table 2]. To study the effect of chondroitin concentration, its concentration was taken in the range of 0.05-0.1% w/v. Upon increasing ChS concentration, initially the size of particles was found to decrease followed by increment. The initial reduction in particles’ size upon increasing chondroitin concentration at constant CS concentration (0.15% w/v) can be attributed to the optimum amount of ChS molecules, which could establish the optimum balance between attractive force (among CS-ChS molecules) and repulsive force (among CS-CS molecules), which result in more effective shrinkage of polymer and consequently smaller size particles. It was further validated by increasing the ChS concentration but at increased CS concentration (0.2% w/v). A similar pattern was observed as found at CS concentration 0.15% w/v [Table 2]; however, the size was found to be higher in the respective ChS concentration [Table 2]. It can be justified by the loose aggregation of excess ChS molecules on the NPs owing to the dominance of repulsive force over attractive force and consequently larger size particles.
Table 2.
Effect of chondroitin sulphate concentration on mean particles size, polydispersity index, entrapment efficiency and zeta potential
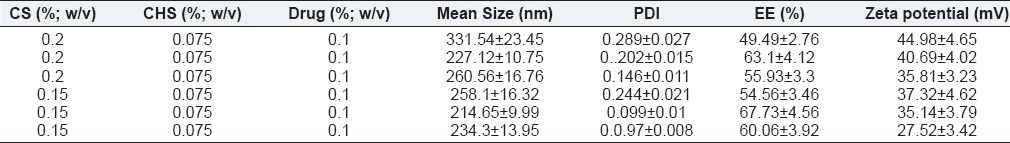
Figure 2.
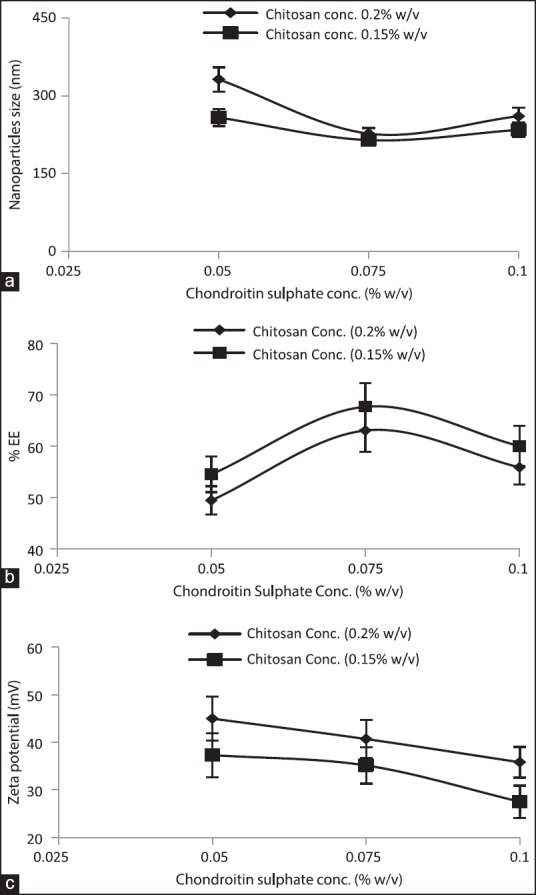
Effect of chondroitin sulfate concentration on (a) mean particle size (b) entrapment efficiency (c) zeta potential
The effect of chondroitin concentration on the drug entrapment was demonstrated in Figure 2b. Upon increasing ChS concentration, initially, drug entrapment was found to be increased and then reduced [Table 2]. Initial increment in entrapment efficiency can be explained by the denser network within particles while at a lower ChS concentration its molecules would be insufficient to induce a dense network; consequently, there is a loose network and thus, more leaching of drug. The reduction in entrapment efficiency upon further increasing ChS concentration can be attributed to more affinity of CS to anionic ChS molecules than anionic drug. Thus, at higher ChS concentration its molecules would have interacted more avidly with CS than the drug molecules, which in turns into lower entrapment.
Figure 2c summarizes the effect of chondroitin concentration on the zeta potential. Continuous reduction in zeta potential, i.e., shifting toward zero was observed upon increasing chondroitin concentration. The plausible reason for continuous reduction in zeta potential could be more neutralization of cationic groups with anionic ChS molecules and thus, reduction in positive zeta potential.
To further validate the influence of ChS concentration on the particles’ size, drug entrapment, and zeta potential, formulations were also developed keeping CS concentration 0.15% w/v. The effect of ChS concentration was demonstrated in Figure 2, which revealed a similar influence as observed for CS concentration, 0.2% w/v [Figure 2 and Table 2].
Effect of drug concentration
Table 3 and Figure 3 summarize effect of drug concentration on the size of NPs, their drug entrapment, and zeta potential while keeping ChS concentration (0.075% w/v) and CS concentration constant (0.2% w/v). To study the effect of bromfenac concentration, its concentration was taken in the range of 0.05% to 0.15% w/v.
Table 3.
Effect of initial drug concentration on mean particles size, polydispersity index, entrapment efficiency and zeta potential
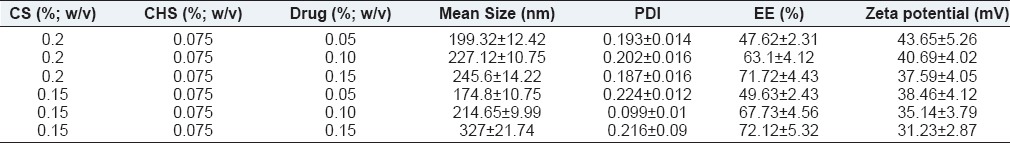
Figure 3.
Effect of drug concentration on (a) mean particle size (b) entrapment efficiency (c) zeta potential
Initial drug concentration directly influenced the particles’ size, i.e., increasing the drug concentration particles size was found increase [Figure 3a and Table 3]. The relationship between drug concentration and particle size can be attributed to enhance repulsive force owing to accumulation/entrapment of drug since bromfenac is an anionic drug, which might have synergized the repulsive force induced by ChS-ChS molecules. Moreover, drug molecules would have competitively prevented the ChS molecules to interact with CS molecules, which are able to shrink particles more effectively; thus, the above discussed influence would be more prominent at high drug concentration and consequently there would be lager particles. These observations were further validated by taking CS at lower concentration (0.15% w/v). The aforementioned effects were found to be more prominent than observed at 0.2% w/v of CS. It can be justified for the fact that at lower concentration of CS, drug molecules would have synergized the repulsive force more prominently and thus the larger size of the particles [Table 3, formulation 1 versus 4, 2 versus 5, 3 versus 6]. It is worth noting that further increment of drug concentration (0.2% w/v) led to aggregation of particles at both concentrations of CS, which further confirmed the effect of drug concentration on the attributes of the NPs.
The effect of initial drug concentration on the entrapment efficiency was demonstrated in Figure 3b, likely to particles size, initial drug concentration also affected the drug entrapment directly i.e. entrapment efficiency was found increase upon increasing drug concentration. The high drug entrapment at high drug concentration can be explained by effective collision between drug molecules and CS molecules, since number of effective collision are directly proportional to number of reacting molecules. Similar findings were observed when CS was taken at a lower concentration, 0.15% w/v and was found to be more prominent than at 0.2% w/v CS concentration [Table 3, formulation 3 versus 6].
Figure 3c demonstrated the influence initial drug concentration on the zeta potential of NPs. It can be attributed to drug entrapment, as discussed above; the more the initial drug concentration, higher the drug entrapment and consequently lower the zeta potential owing to more neutralization of positive charge of CS through anionic drug molecules.
In vitro characterization of nanoparticles
The effect of different variables such as CS, ChS, and initial drug concentration were studies and their effect on the particles’ size, entrapment efficiency, and zeta potential that were studied and summarized [Tables 1–3 and Figures 1–3]. The particles’ size, zeta potential, and drug entrapment were found in the range of 174.8 ± 10.75 nm to 380.8 ± 23.12 nm, +27.52 ± 3.42 mV to +47.25 ± 3.62 mV, and 47.23 ± 2.46% to 72.12 ± 5.32%, respectively. Among the different formulations, optimized formulation was selected by keeping the particles’ size and drug entrapment in view and subjected to further evaluation. The optimized formulation showed the particles’ size, 245.6 ± 14.22 nm [Figure 4a], polydispersity index to be 0.187 ± 0.016, entrapment efficiency to be 71.72 ± 4.43%, and zeta potential 37.59 ± 4.05 mV [Figure 4b]. The shape and surface morphology of NPs was determined by the transmittance electron microscope (TEM, TOPCON002B, Tokyo, Japan), which demonstrated a spherical shape with smooth surfaces [Figure 4c]. Moreover, TEM micrograph demonstrated the particles’ size in the range of 200-300 nm. The NPs revealed by TEM are in corroboration with size determined by dynamic light scattering method.
Figure 4.
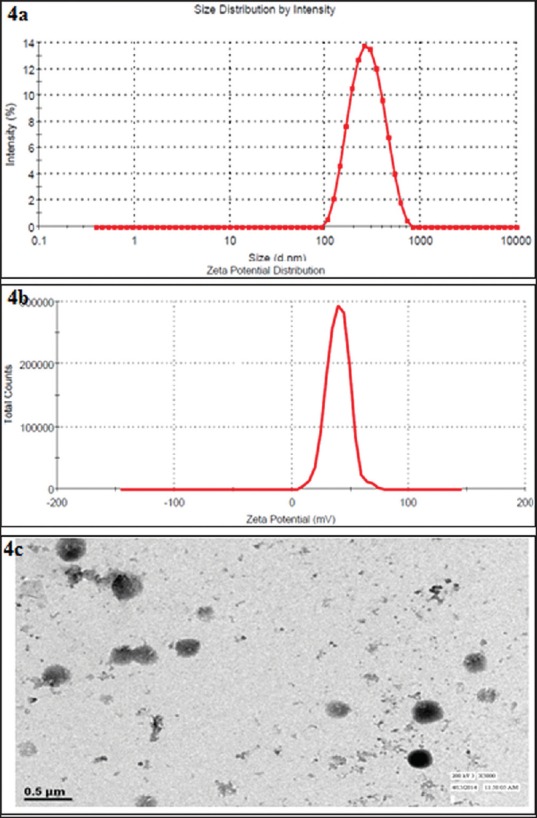
Particle size analysis (a) size distribution curve (b) zeta potential curve (c) transmission electron micrograph
In vitro release profile
Developed formulation demonstrated biphasic release pattern, initial burst release, 24.23 ± 1.82% in 1 h followed by sustained release profile, 94.36 ± 6.36% in 24 h [Figure 5]. As anticipated, conventional eyedrops showed immediate release profile ∼97% within 30 min. The burst profile can be explained by rapid hydration of NPs, which resulted in leaching of loosely bound/surface adhered drug molecules. The sustained effect can be attributed to the swelling property of CS; thus, entrapped drug within the swelled matrix diffuses out gradually. In vitro release was fitted into a different kinetic model, which revealed Korsmeyer-Peppas as the best fit model (r2, 0.98) with an exponent value <0.5 that suggested Fickian diffusion process. The release profile of NPs can be seen as an advantage toward improved delivery; initial burst effect would be able to achieve minimum effective concentration (MEC) while sustained release would be able to maintain MEC for over a longer period.
Figure 5.
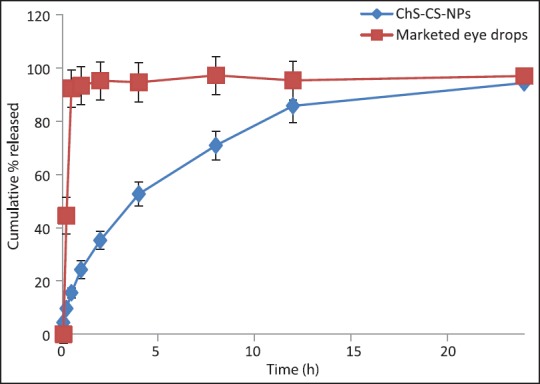
In vitro release profile of bromfenac sodium through marketed eyedrops and ChS-CS-NPs
In vitro transcorneal permeation
ChS-CS-NPs demonstrated a cumulative permeation of bromfenac 44.79 ± 2.54 μg/cm2/h while it was found to be 25.63 ± 1.82 μg/cm2/h for marketed eyedrops. Bromfenac permeation was found to be 1.62-fold higher through ChS-CS-NPs, P < 0.01 [Figure 6]. The findings are in corroboration with a previous report.[38] Enhanced permeation can be justified with enhanced retention at the corneal surface due to mucoadhesive nature of CS-NPs owing to ionic interaction between protonated amino group of CS and negative charge of corneal mucin.[14,39] In addition, endocytic uptake of NPs as well as improved transport of released drug via paracellular route due to widening of tight junction in the presence of CS.[30]
Figure 6.

In vitro transcorneal permeation across goat cornea
In vitro corneal retention study
Quantitative estimation of bromfenac sodium into the cornea treated with marketed eyedrop and ChS-CS-NPs was performed. It is worth noting that the cornea treated with ChS-CS-NPs (0.308 μg/mg) demonstrated significantly high bromfenac content in the cornea when compared with marketed eyedrop (0.16 μg/mg), P < 0.01 [Figure 7]. Significantly high drug content into the cornea treated with ChS-CS-NPs can be justified by mucoadhesive propriety of ChS-CS-NPs and thus, prolonged retention over the cornea. Moreover, it can be explained by endocytosis of NPs.[40,41]
Figure 7.
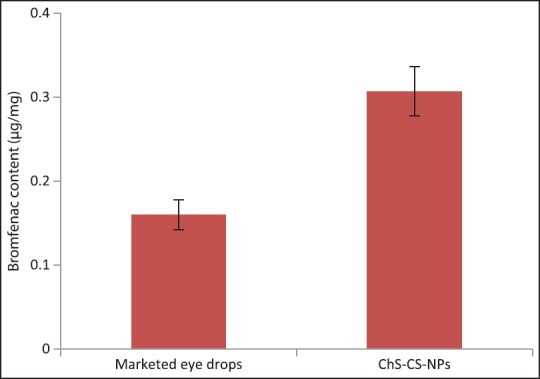
In vitro corneal retention of bromfenac sodium into cornea treated with marketed eye drops and ChS-CS-NPs after 2 h of treatment
Corneal uptake of ChS-CS-NPs by confocal laser scanning microscopy
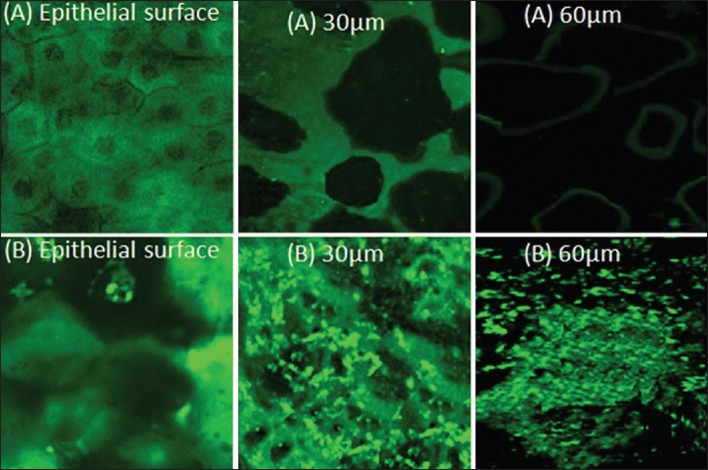
Corneal uptake of ChS-CS-NPs was evaluated by using CLSM. Cornea treated with fluorescein sodium (FS) demonstrated a homogenous distribution throughout the corneal surface; however, it got diminished within different depths, 30 μm and 60 μm (inner layers) of the cornea and limited to intracellular spaces. However, a number of intense and green spots can be seen at the surface, in different depths as well as intracellular spaces between corneocytes [Figure 8]. It can be attributed to endocytic uptake of NPs. Moreover, improved transport through the paracellular route can be attributed to the ability of CS to open tight junctions.[40,41]
Figure 8.
Confocal image of the corneal epithelium incubated with (a) FS-SOL and (b) FS-ChS-CS-NPs
Ocular tolerance test (hen's egg test-chorioallantoic membrane)
Ocular tissues are very sensitive to foreign substances or chemicals. Considering safety issues, optimized formulation ChS-CS-NPs was assessed by hen's egg test-chorioallantoic membrane to establish its ocular irritancy profile into the chorioallantoic membrane of the hen's egg. Being rapid, sensitive, and cost-effective, it is considered to be a good technique for the assessment of ocular sensitivity to any chemical. In addition, it does not associate with any ethical issue. Furthermore, the chorioallantoic membrane chick embryo is considered equivalent to rabbit eye tissue with respect to veins, arteries, and capillaries, and its responses to any foreign materials. The ChS-CS-NPs were evaluated for its irritancy profile and compared with negative control (normal saline) and positive control (0.1 N NaOH) [Figure 9]. Positive control demonstrated mean score 5.33 while negative control showed zero score. ChS-CS-NPs also showed zero score, which is identical to negative control (normal saline). Thus, ChS-CS-NPs can be considered as nonirritant and safe to use as ocular delivery carrier.
Figure 9.
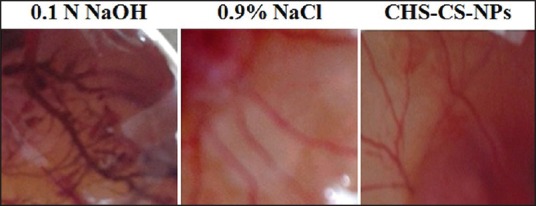
Vascular responses to (a) 0.1 N NaOH solution (b) saline solution, and (c) ChS-CS-NPs at 5 min post application
CONCLUSION
Bromfenac-loaded ChS-CS-NPs were successfully developed and optimized in terms of CS concentration, ChS concentration, and initial drug concentration. Optimized formulation demonstrated particles size in nano dimension with very good polydispersity index. Moreover, formulation demonstrated reasonable entrapment of bromfenac, the small hydrophilic molecules. In addition, significantly high transcorneal permeation and retention of bromfenac could be achieved through ChS-CS-NPs. Moreover, CLSM study demonstrated high uptake of developed formulation. ChS-CS-NPs showed safety profile comparable to normal saline. Thus, the said formulation could be an excellent alternative to conventional eyedrops; however, there is a need of stringent clinical studies to make it clinically viable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ameeduzzafar, Ali J, Bhatnagar A, Kumar N, Ali A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int J Biol Macromol. 2014;65:479–91. doi: 10.1016/j.ijbiomac.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Anitha A, Deepa N, Chennazhi K P, Nair SV, Tamura H, Jayakumar R. Development of mucoadhesive thiolated chitosan nanoparticles for biomedical applications. Carbohydr Polym. 2011;83:66–73. [Google Scholar]
- 3.Araújo J, Vega E, Lopes C, Egea MA, Garcia ML, Souto EB. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf B Biointerfaces. 2009;72:48–56. doi: 10.1016/j.colsurfb.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Badawi AA, El-Laithy HM, El Qidra RK, El Mofty H, El dally M. Chitosan based nanocarriers for indomethacin ocular delivery. Arch Pharm Res. 2008;31:1040–9. doi: 10.1007/s12272-001-1266-6. [DOI] [PubMed] [Google Scholar]
- 5.Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems — Recent advances. Prog Retin Eye Res. 1998;17:33–58. doi: 10.1016/s1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 6.Calvo P, Vila-Jato JL, Alonso MJ. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int J Pharm. 1997;153:41–50. [Google Scholar]
- 7.Chien Y. Ocular drug delivery and delivery systems. In: Chien Y, editor. Novel Drug Delivery Systems. 2nd ed. New York: Taylor & Francis; 1991. pp. 269–70. [Google Scholar]
- 8.Chopra S, Patil GV, Motwani SK. Release modulating hydrophilic matrix systems of losartan potassium: Optimization of formulation using statistical experimental design. Eur J Pharm Biopharm. 2007;66:73–82. doi: 10.1016/j.ejpb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Davies NM, Farr SJ, Hadgraft J, Kellaway IW. Evaluation of mucoadhesive polymers in ocular drug delivery. II. Polymer-coated vesicles. Pharm Res. 1992;9:1137–44. doi: 10.1023/a:1015891419676. [DOI] [PubMed] [Google Scholar]
- 10.de Campos AM, Diebold Y, Carvalho EL, Sánchez A, Alonso MJ. Chitosan nanoparticles as new ocular drug delivery systems: In vitro stability, in vivo fate, and cellular toxicity. Pharm Res. 2004;21:803–10. doi: 10.1023/b:pham.0000026432.75781.cb. [DOI] [PubMed] [Google Scholar]
- 11.Denuziere A, Ferrier D, Domard A. Chitosan-chondroitin sulphate and chitosan-hyaluronate polyelectrolyte complexes. Physico-chemical aspects. Carbohydr Polym. 1996;29:317–23. [Google Scholar]
- 12.Denuziere A, Ferrier D, Damour O, Domard A. Chitosan-chondroitin sulphate and chitosan-hyaluronate polyelectrolyte complexes: Biological properties. Biomaterials. 1998;19:1275–85. doi: 10.1016/s0142-9612(98)00036-2. [DOI] [PubMed] [Google Scholar]
- 13.Dodane V, Amin Khan M, Merwin JR. Effect of chitosan on epithelial permeability and structure. Int J Pharm. 1999;182:21–32. doi: 10.1016/s0378-5173(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 14.Felt O, Baeyens V, Zignani M, Buri P, Gurny R. Mucosal drug delivery ocular Encyclopedia of controlled drug delivery. Geneva: University of Geneva; 1999. pp. 605–22. [Google Scholar]
- 15.Guinedi AS, Mortada ND, Mansour S, Hathout RM. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm. 2005;306:71–82. doi: 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007;14:507–15. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 17.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine. 2010;6:324–33. doi: 10.1016/j.nano.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Iouv M, Dumais G, du Souich P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 2008;16(Suppl 3):S14–8. doi: 10.1016/j.joca.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Jain GK, Pathan SA, Akhter S, Jayabalan N, Talegaonkar S, Khar RK, et al. Microscopic and spectroscopic evaluation of novel PLGA-chitosan Nanoplexes as an ocular delivery system. Colloids Surf B Biointerfaces. 2011;82:397–403. doi: 10.1016/j.colsurfb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Jayaprakash PS, James CC, Rajan NS, Saisivam S, Nagarajan M. Design and evaluation of ketorolac tromethamine ocuserts. Indian J Pharm Sci. 2000;62:334–8. [Google Scholar]
- 21.Kaskoos RA. Investigation of moxifloxacin loaded chitosan-dextran nanoparticles for topical instillation into eye: In-vitro and ex-vivo evaluation. Int J Pharm Investig. 2014;4:164–73. doi: 10.4103/2230-973X.143114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirker KR, Luo Y, Nielson JH, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials. 2002;23:3661–71. doi: 10.1016/s0142-9612(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 23.Kirker KR, Luo Y, Morris SE, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel as supplemental wound dressings for donor sites. J Burn Care Rehabil. 2004;25:276–86. doi: 10.1097/01.bcr.0000124790.69026.3d. [DOI] [PubMed] [Google Scholar]
- 24.Konat Zorzi G, Contreras-Ruiz L, Párraga JE, López-García A, Romero Bello R, Diebold Y, et al. Expression of MUC5AC in ocular surface epithelial cells using cationized gelatin nanoparticles. Mol Pharm. 2011;8:1783–8. doi: 10.1021/mp200155t. [DOI] [PubMed] [Google Scholar]
- 25.Malhotra M, Mujumdar DK. In vivo ocular availability of Ketorolac following ocular instillations of aqueous, oil, and ointment formulations to normal corneas of rabbits: A technical Note. AAPS PharmSciTech. 2005;6:E523–6. doi: 10.1208/pt060365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandal B, Halder KK, Dey SK, Bhoumik M, Debnath MC, Ghosh LK. Development and physical characterization of chloramphenicol loaded biodegradable nanoparticles for prolonged release. Pharmazie. 2009;64:445–9. [PubMed] [Google Scholar]
- 27.Mi FL, Shyu SS, Peng CK, Wu YB, Sung HW, Wang PS, et al. Fabrication of chondroitin sulphate-chitosan composite artificial extracellular matrix for stabilization of fibroblast growth factor. J Biomed Mater Res A. 2006;76:1–15. doi: 10.1002/jbm.a.30298. [DOI] [PubMed] [Google Scholar]
- 28.Paliwal SK, Chauhan R, Sharma V, Majumdar DK, Paliwal S. Entrapment of ketorolac tromethamine in polymeric vehicle for controlled drug delivery. Indian J Pharm Sci. 2009;71:687–91. doi: 10.4103/0250-474X.59555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paolicelli P, de la Fuente M, Sánchez A, Seijo B, Alonso MJ. Chitosan nanoparticles for drug delivery to the eye. Expert Opin Drug Deliv. 2009;6:239–53. doi: 10.1517/17425240902762818. [DOI] [PubMed] [Google Scholar]
- 30.Pardeike J, Weber S, Haber T, Wagner J, Zarfl HP, Plank H, et al. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int J Pharm. 2011;419:329–38. doi: 10.1016/j.ijpharm.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Park YJ, Lee YM, Lee JY, Seol YJ, Chung CP, Lee SJ. Controlled release of platelet-derived growth factor-BB from chondroitin sulphate-chitosan sponge for guided bone regeneration. J Control Release. 2000;67:385–94. doi: 10.1016/s0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 32.Porporatto C, Bianco ID, Correa SG. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J Leukoc Biol. 2005;78:62–9. doi: 10.1189/jlb.0904541. [DOI] [PubMed] [Google Scholar]
- 33.Saettone MF, Salminen L. Ocular inserts for topical delivery. Adv Drug Del Rev. 1995;16:95–106. [Google Scholar]
- 34.Sintov A, Di-Capua N, Rubinstein A. Cross-linked chondroitin sulphate: Characterization for drug delivery purposes. Biomaterials. 1995;16:473–8. doi: 10.1016/0142-9612(95)98820-5. [DOI] [PubMed] [Google Scholar]
- 35.Spielmann H. Ocular irritation. In: Castle JV, Gomez MJ, editors. In Vitro Methods in Pharmaceutical Research. San Diego, CA: Academic Press; 1997. pp. 265–87. [Google Scholar]
- 36.Van Ooteghem MM. Formulation of ophthalmic solutions and suspensions: Problems and advantages. In: Edman P, editor. Biopharmaceutics of Ocular Drug Delivery. Boca Raton, FL: CRC Press; 1993. pp. 27–42. [Google Scholar]
- 37.Wang LF, Wang JM, Chiang YL. Insolubilization of sodium chondroitin sulphate by forming a semi-interpenetrating polymer network with acrylic acid: A potential carrier for colon-specific drug delivery. J Appl Polym Sci. 2002;85:114–22. [Google Scholar]
- 38.Warsi MH, Anwar M, Garg V, Jain GK, Talegaonkar S, Ahmad FJ, et al. Dorzolamide-loaded PLGA/vitamin E TPGS nanoparticles for glaucoma therapy: Pharmacoscintigraphy study and evaluation of extended ocular hypotensive effect in rabbits. Colloids Surf B Biointerfaces. 2014;122:423–31. doi: 10.1016/j.colsurfb.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Woodley J. Bioadhesion: New possibilities for drug administration? Clin Pharmacokinet. 2001;40:77–84. doi: 10.2165/00003088-200140020-00001. [DOI] [PubMed] [Google Scholar]
- 40.Zaki R, Hosny KM, Khames A, Abd-elbary A. Ketorolac tromethamine in-situ ocular hydro gel; preparation, characterization, and in-vivo evaluation. Int J Drug Deliv. 2011;3:535–45. [Google Scholar]
- 41.Zorzi GK, Párraga JE, Seijo B, Sánchez A. Hybrid nanoparticle design based on cationized gelatin and the polyanions dextran sulfate and chondroitin sulfate for ocular gene therapy. Macromol Biosci. 2011;11:905–13. doi: 10.1002/mabi.201100005. [DOI] [PubMed] [Google Scholar]