Abstract
Background:
Previous studies suggested that zinc level was related to a certain diabetic microvascular complication. However, the relationship between zinc level and all the microvascular complications in type 2 diabetic patients remains unknown. The purpose of this study was to analyze the relationship between zinc level and each diabetic microvascular complication and identify the features related to low serum zinc level.
Methods:
We included the hospitalized patients with type 2 diabetes (T2D) at our department from May 30, 2013 to March 31, 2014. We initially compared the serum zinc levels between patients with specific microvascular complications and those without. We then analyzed the association between zinc level and each microvascular complication. Furthermore, we identified the unique features of patients with high and low serum zinc levels and analyzed the risk factors related to low zinc level.
Results:
The 412 patients included 271 with microvascular complications and 141 without any microvascular complications. Serum zinc level was significantly lower in patients with diabetic retinopathy (P < 0.001), diabetic nephropathy (DN, P < 0.001), or diabetic peripheral neuropathy (P = 0.002) compared with patients without that specific complication. Lower zinc level was an independent risk factor for DN (odds ratio = 0.869, 95% confidence interval = 0.765–0.987, P < 0.05). The subjects with lower serum zinc level had manifested a longer duration of diabetes, higher level of hemoglobin A1c, higher prevalence of hypertension and microvascular complications, and lower fasting and 2-h C-peptide levels.
Conclusions:
Lower serum zinc level in T2D patients was related to higher prevalence of diabetic microvascular complications, and represented as an independent risk factor for DN. Patients with lower zinc level were more likely to have a longer duration of diabetes, poorer glucose control, and worse β-cell function.
Keywords: Diabetic Nephropathy, Diabetic Peripheral Neuropathy, Diabetic Retinopathy, Type 2 Diabetes, Zinc
INTRODUCTION
Diabetes is a life-threatening chronic disease, with microvascular complications including diabetic retinopathy (DR), diabetic nephropathy (DN), and diabetic peripheral neuropathy (DPN), often bring about a huge burden to patients, their families, and the society.
Zinc plays a major role in the development of both type 1 and type 2 diabetes (T2D). Previous studies suggested that serum zinc level is associated with T2D, and loss-of-function mutations in zinc transporter-8 gene protect against T2D.[1,2] In type 1 diabetic patients, zinc transporter 8 autoantibody represents a key immunodiagnostic marker.[3]
Oxidative stress plays an important role in the development of diabetic microvascular complications. Zinc not only has an antioxidative effect, but also constitutes a key component of many antioxidases. It inhibits the damage associated with lipid peroxidation and induces the clearance of free radicals.[4] These findings suggest that zinc deficiency may be associated with the development of diabetic microvascular complications.
Moustafa[5] showed that zinc protects diabetic rats against oxidative changes in the retina and controls hyperglycemia. They also demonstrated that zinc is an antioxidant and a biological membrane stabilizer mediating the protection against DN. Khan et al.[6] showed that zinc supplementation for 12 weeks reduced albumin excretion in microalbuminuria of T2D patients probably via antioxidative mechanisms. Similarly, Hayee et al.[7] conducted a double-blind study and showed that serum zinc levels at baseline are significantly lower in patients with diabetic neuropathy when compared with healthy controls. After 6 weeks of therapy, values of fasting plasma glucose, 2-hour (2-h) plasma glucose after breakfast and motor nerve conduction velocity (median and common peroneal nerve) were altered significantly in patients who received zinc supplement compared with insignificant changes in those receiving placebo. Therefore, zinc therapy may lead to better glycemic control and improvement in DPN.
In recent years, many studies reported lower zinc levels in diabetic patients compared with healthy people.[8,9] Patients with microvascular complications had marked zinc deficiency compared with diabetic patients but without any microvascular complications. However, most of these studies were small in size and only investigated a single microvascular complication. However, some complication itself maybe the independent risk factor for another complication that might not be studied at the same time. Few studies evaluated the association between zinc level and all the microvascular complications, especially in the Asian population. Our study has included all the microvascular complications with a sample size larger than most of the previous studies.
METHODS
Participants
We included all the hospitalized T2D patients in our department from May 30, 2013 to March 31, 2014. All the patients were hospitalized for uncontrolled hyperglycemia or evaluation of the disease control and complications. All the participants were from the endocrine department. Diabetes was defined using the World Health Organization 1999 criteria: (1) typical symptoms with random plasma glucose ≥11.1 mmol/L; or (2) fasting plasma glucose ≥7.0 mmol/L; or (3) 2-h glucose level in oral glucose tolerance test ≥11.1 mmol/L. People without typical symptoms should repeat the plasma glucose test on the other day. Individuals fulfilling either of the above criteria were diagnosed with diabetes. The key diagnostic feature of T2D included a middle-age or older onset of diabetes, with a progressive disease. Patients were more likely to be overweight or obese, with only mild symptoms or without the typical symptoms. Patients were not prone to ketoacidosis. Patients retained residual β-cell function. The disease could be managed without insulin at the beginning stage. Islet cell and glutamic acid decarboxylase antibodies were both negative.
Diagnostic criteria of microvascular complications
All the participants were diagnosed at the time of hospital discharge. DR was diagnosed by fundus photography and further confirmed ophthalmologically. DN was diagnosed by urinary albumin/creatinine ratio (ACR) and blood creatinine levels. All the patients were asked to provide urine samples for 3 times. DN was diagnosed if the urinary ACR was higher than 30 mg/g twice, or if an abnormally elevated blood creatinine level was detected. Urinary infection and other types of nephropathy were excluded during the diagnosis of DN. DPN was diagnosed based on the results of physical examination and somatosensory evoked potentials.
Study design
Medical history was obtained including details of height, weight, and waist circumference in all the patients. We also obtained the results of hemoglobin A1c (HbA1c), fasting C-peptide, 2-h C-peptide, lipid profile (total cholesterol [TC], triglyceride [TG], high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C]), and serum zinc levels. Urinary ACR was calculated using the average of three sets of ACR results during hospitalization. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula: eGFR (ml∙min-1·1.73 m-2) = 186 × serum creatinine (SCr, mg/dl)−1.154 × age (years)−0.203 × (0.742, for female). Zinc level was assayed by nephelometry using the HITACHI LABOSPECT 008 (Hitachi, Japan); HbA1c was assayed by high-performance liquid chromatography method using Premier Hb9210 (PRIMUS, USA); C-peptide was assayed by electrochemiluminescence method using Cobas e411 (Roche, Switzerland); SCr was assayed by enzymic method using Beckman Coulter AU5800 (Beckman Coulter, USA); TC and TG were assayed by enzymic method using Beckman Coulter AU5800 (Beckman Coulter, USA); HDL-C and LDL-C were all assayed by direct method using Beckman Coulter AU5800 (Beckman Coulter); urine ACR was assayed by picric acid method using Cobas c311 (Roche).
We initially compared the serum zinc levels among patients with various numbers of microvascular complications. We also compared the features of patients with and without specific microvascular complication. A logistic regression analysis was further conducted to analyze the role of serum zinc as an independent risk factor for each microvascular complication. Finally, we compared the characteristics among patients with different serum zinc levels and performed logistic regression to determine the risk factors contributing to lower serum zinc levels.
Statistical methods
SPSS 19.0 (IBM, USA) was used to analyze the data. Normally distributed data were expressed as mean ± standard deviation (SD). Student's t-test was used to compare the parameters between two groups. Single-factor analysis of variance (ANOVA) was used to compare the parameters among multiple groups. Data with abnormal distribution were expressed as median (range). Mann-Whitney U nonparameter test was used to compare the parameters between two groups and the Kruskal-Wallis nonparametric test was used to compare the parameters among multiple groups. The chi-square test was used to compare the ratio among groups. A bivariate regression model was used to analyze the correlation between serum zinc level and each microvascular complication. Multiple regression analysis was used to determine the risk factors for low serum zinc level.
RESULTS
The 412 T2D patients included 233 (56.6%) males and 179 (43.4%) females. The mean age of the participants was 56 ± 14 years, and the medium duration of diabetes was 10 years. Seventy-eight patients (18.9%) had DR; 91 (22.1%) had DN and 234 (56.8%) had DPN. Sixty patients (14.6%) manifested two different microvascular complications and 36 patients (8.7%) exhibited all the three complications.
Association between zinc level and diabetic microvascular complications
We divided all the participants into four groups according to the number of diabetic complications. Among the 412 patients, 141 had none, 175 had one, 60 had two, and 36 had three microvascular complications. The results showed significantly lower zinc level in patients with microvascular complications [Figure 1].
Figure 1.
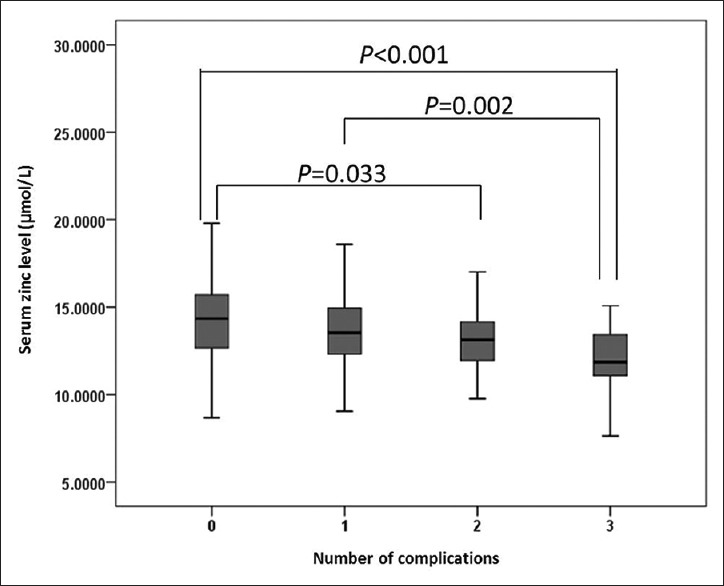
Zinc levels of patients with various number of diabetic microvascular complications.
Comparison of patients with and without specific diabetic microvascular complications
Among the 412 patients, 78 had DR. Sex, age, duration of diabetes, HbA1c, fasting C-peptide, 2-h C-peptide, LDL-C, eGFR, and zinc levels were significantly different between the two groups with and without DR. Zinc level in DR patients was significantly lower than in patients without retinopathy (P < 0.001). Patients with DR had a significantly longer duration of diabetes, higher HbA1c, lower C-peptide level, higher LDL-C level, and lower eGFR [Table 1].
Table 1.
Clinical characteristics of patients with and without diabetic retinopathy
| Items | Without DR | With DR | Statistic values | P |
|---|---|---|---|---|
| N | 334 | 78 | – | – |
| Male, n (%) | 198 (59) | 35 (45) | 5.344* | 0.023§ |
| Age (years), mean ± SD | 55 ± 14 | 60 ± 11 | −3.479† | 0.001§ |
| Waist (cm), mean ± SD | 96 ± 11 | 95 ± 12 | 0.731† | 0.465 |
| BMI (kg/m2), median (range) | 26.0 (17.7–43.2) | 25.2 (17.9–38.9) | −1.349‡ | 0.177 |
| Duration (years), median (range) | 9 (0–42) | 16 (2–37) | −6.691‡ | <0.001§ |
| Prevalence of hypertension, n (%) | 183 (54.8) | 52 (66.7) | 3.640* | 0.058 |
| HbA1c (%), median (range) | 8.5 (5.6–14.7) | 9.1 (5.8–13.9) | −2.822‡ | 0.005§ |
| C-P (ng/ml), median (range) | 1.97 (0.22–17.78) | 1.40 (0.06–4.65) | −3.731‡ | <0.001§ |
| 2-h C-P (ng/ml), median (range) | 4.74 (0.26–19.98) | 2.83 (0.11–11.86) | −5.149‡ | <0.001§ |
| TC (mmol/L), median (range) | 4.4 (2.0–12.0) | 4.7 (2.2–10.0) | −1.413‡ | 0.158 |
| TG (mmol/L), median (range) | 1.6 (0.5–18.3) | 1.6 (0.5–10.6) | −1.100‡ | 0.271 |
| HDL-C (mmol/L), median (range) | 0.9 (0.4–1.9) | 1.0 (0.4–1.7) | −1.284‡ | 0.199 |
| LDL-C (mmol/L), mean ± SD | 2.6 ± 0.8 | 2.8 ± 0.9 | −2.007† | 0.045§ |
| eGFR (ml·min-1·1.73 m-2), mean ± SD | 117 ± 34 | 103 ± 33 | 3.337† | 0.001§ |
| Zn (μmol/L), median (range) | 13.69 (8.68–27.90) | 12.80 (7.63–21.40) | −3.947‡ | <0.001§ |
*χ2 values; †t values, ‡Z values, §Statistical significance. DR: Diabetic retinopathy; SD: Standard deviation; BMI: Body mass index; HbA1c: Hemoglobin A1c; C-P: C-peptide; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate.
Among the 412 patients, 234 had DPN while 178 did not. Age, duration of diabetes, the prevalence of hypertension, eGFR and zinc level were significantly different between the two groups. Zinc level in patients with DPN was significantly lower than in those without (P < 0.01). Patients with DPN had a significantly longer duration of diabetes, higher prevalence of hypertension and lower eGFR [Table 2].
Table 2.
Clinical characteristics of patients with and without diabetic peripheral neuropathy
| Items | Without DPN | With DPN | Statistic values | P |
|---|---|---|---|---|
| N | 178 | 234 | – | – |
| Male, n (%) | 107 (60) | 126 (54) | 1.616* | 0.229 |
| Age (years), mean ± SD | 53 ± 16 | 59 ± 11 | −4.815† | <0.001§ |
| Waist (cm), mean ± SD | 96 ± 12 | 96 ± 11 | −0.079† | 0.937 |
| BMI (kg/m2), median (range) | 25.6 (17.7–43.2) | 25.9 (17.9–38.9) | −0.203‡ | 0.839 |
| Duration (years), median (range) | 7 (0–31) | 11 (0–42) | −5.945‡ | <0.001§ |
| Prevalence of hypertension, n (%) | 90 (50.6) | 145 (62.0) | 5.365* | 0.021§ |
| HbA1c (%), median (range) | 8.7 (5.6–14.7) | 8.7 (5.8–13.9) | −0.018‡ | 0.986 |
| C-P (ng/ml), median (range) | 1.99 (0.22–7.11) | 1.79 (0.06–17.78) | −1.870‡ | 0.061 |
| 2-hour C-P (ng/ml), median (range) | 4.67 (0.26–19.98) | 4.26 (0.11–15) | −1.726‡ | 0.084 |
| TC (mmol/L), median (range) | 4.5 (2.0–12.0) | 4.4 (2.2–10.0) | −1.05‡ | 0.294 |
| TG (mmol/L), median (range) | 1.6 (0.6–18.3) | 1.6 (0.5–15.3) | −0.39‡ | 0.696 |
| HDL-C (mmol/L), median (range) | 1.0 (0.4–1.8) | 1.0 (0.4–1.9) | −0.57‡ | 0.569 |
| LDL-C (mmol/L), mean ± SD | 2.7 ± 0.9 | 2.6 ± 0.9 | 0.797† | 0.426 |
| eGFR (ml·min-1·1.73 m-2), mean ± SD | 119 ± 34 | 111 ± 34 | 2.442† | 0.015§ |
| Zn (μmol/L), median (range) | 13.99 (8.14–23.55) | 13.23 (7.63–27.90) | −3.159‡ | 0.002§ |
*χ2 values, †t values, ‡Z values, §Statistical significance. DPN: Diabetic peripheral neuropathy; SD: Standard deviation; BMI: Body mass index; HbA1c: Hemoglobin A1c; C-P: C-peptide; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate.
Among the 412 patients, 91 had DN while 321 had no DN. The results showed that age, duration of diabetes, prevalence of hypertension, HbA1c, HDL-C, eGFR, and zinc level were significantly different between the two groups. Zinc level in DN patients was significantly lower than in patients without retinopathy (P < 0.01). Patients with DN had a significantly longer duration of diabetes, higher prevalence of hypertension, higher HbA1c, lower HDL-C level, and lower eGFR [Table 3]. We further divided the DN patients according to their urinary ACR into three groups: (1) normal group with ACR <30 mg/g (n = 321), (2) microalbuminuria group with 30 ≤ACR <300 mg/g (n = 67), and (3) macroalbuminuria group with ACR ≥300 mg/g (n = 24). A significantly lower zinc level was observed in patients with higher ACR level [P < 0.01, Figure 2].
Table 3.
Clinical characteristics of patients with and without diabetic nephropathy
| Items | Without DN | With DN | Statistic values | P |
|---|---|---|---|---|
| N | 321 | 91 | – | – |
| Male, n (%) | 186 (58) | 47 (52) | 1.144* | 0.338 |
| Age (years), mean ± SD | 55 ± 14 | 60 ± 14 | −2.727† | 0.007§ |
| Waist (cm), mean ± SD | 95 ± 11 | 97 ± 13 | −0.993† | 0.322 |
| BMI (kg/m2), median (range) | 25.8 (17.7–40.8) | 25.6 (17.9–43.2) | −0.086‡ | 0.932 |
| Duration (years), median (range) | 10 (0–42) | 13 (0–37) | −3.325‡ | 0.001§ |
| Prevalence of hypertension, n (%) | 163 (50.8) | 72 (79.1) | 23.242* | <0.001§ |
| HbA1c (%), median (range) | 8.5 (5.6–14.7) | 9.1 (5.8–13.2) | −2.279‡ | 0.023§ |
| C-P (ng/ml), median (range) | 1.89 (0.06–8.58) | 1.86 (0.11–17.78) | −0.296‡ | 0.767 |
| 2-h C-P (ng/ml), median (range) | 4.41 (0.11–18.09) | 3.90 (0.60–19.98) | −1.654‡ | 0.098 |
| TC (mmol/L), median (range) | 4.5 (2.0–10.8) | 4.5 (2.2–12.0) | −0.513‡ | 0.608 |
| TG (mmol/L), median (range) | 1.6 (0.5–15.3) | 1.6 (0.5–18.3) | −0.25‡ | 0.803 |
| HDL-C (mmol/L), median (range) | 1.0 (0.4–1.9) | 0.9 (0.4–1.7) | −2.063‡ | 0.039§ |
| LDL-C (mmol/L), mean ± SD | 2.7 ± 0.8 | 2.6 ± 0.9 | 0.115† | 0.908 |
| eGFR (ml·min-1·1.73 m-2), mean ± SD | 118 ± 33 | 101 ± 34 | 4.354† | <0.001§ |
| Zn (μmol/L), median (range) | 13.80 (8.68–27.9) | 12.65 (7.63–22.80) | −4.29‡ | <0.001§ |
*χ2 values, †t values, ‡Z values, §Statistical significance. DN: Diabetic nephropathy; SD: Standard deviation; BMI: Body mass index; HbA1c: Hemoglobin A1c; C-P: C-peptide; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate.
Figure 2.
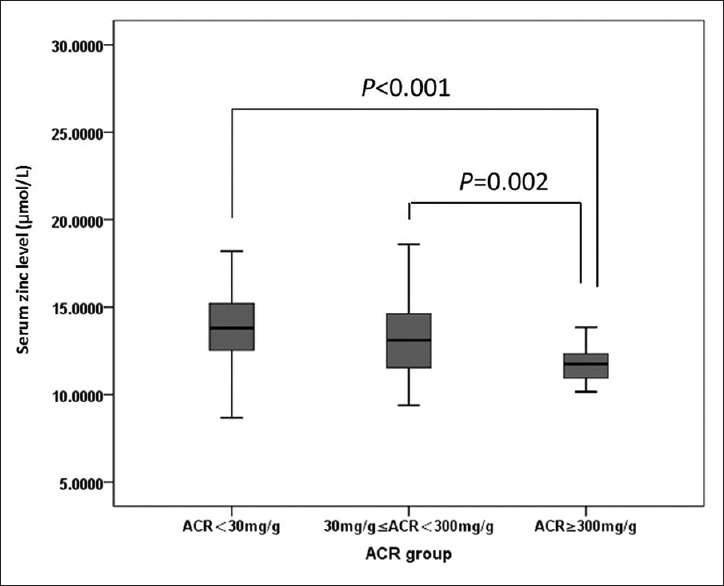
Comparison of zinc levels in patients with different urinary albumin/creatinine ratio. ACR: Albumin/creatinine ratio.
Logistic regression analysis of zinc levels and diabetic microvascular complications
Table 4 summarizes the association between serum zinc level and each diabetic microvascular complication. A negative correlation is shown between zinc level and DN. After adjustment for age, duration of diabetes, prevalence of hypertension, body mass index (BMI), HbA1c and eGFR, there was a significant negative correlation between zinc level and DN (odds ratio: 0.869; 95% confidence interval: 0.765–0.987; P < 0.05). Whereas no significant correlations were found between zinc level and either DR or DPN.
Table 4.
Logistic regression analysis of zinc level and diabetic microvascular complications in patients with type 2 diabetes
| Microvascular complications | OR (95% CI) | P |
|---|---|---|
| DR | 0.900 (0.782–1.035) | 0.138 |
| DN | 0.869 (0.765–0.987) | 0.031* |
| DPN | 0.967 (0.882–1.060) | 0.468 |
*Statistical significance. OR: Odds ratio; 95% CI: Confidence interval; DR: Diabetic retinopathy; DN: Diabetic nephropathy; DPN: Diabetic peripheral neuropathy.
Patient profile under different levels of serum zinc and risk factors for lower serum zinc level
We divided all the patients into three groups according to their zinc levels. Zinc levels in upper tertile group ranged from 14.53 to 27.90 μmol/L. The middle tertile range of zinc level was 12.74–14.53 μmol/L and the lower tertile was 7.63–12.74 μmol/L. Patients with low zinc level showed a higher prevalence of all the three diabetic microvascular complications. Compared with the patients with zinc levels within the middle and upper tertiles, those with zinc level within the lower tertile were older and had a longer duration of diabetes, higher HbA1c, lower C-peptide, TC, TG and eGFR levels [Table 5].
Table 5.
Clinical characteristics of people with different serum zinc levels
| Items | Upper tertile | Middle tertile | Lower tertile | Statistic values | P |
|---|---|---|---|---|---|
| N | 137 | 137 | 138 | – | – |
| Male, n (%) | 90 (66) | 77 (56) | 66 (48) | 8.943* | 0.011‡ |
| Age (years), mean ± SD | 51 ± 14 | 57 ± 13 | 61 ± 12 | 20.634† | <0.001‡ |
| Waist (cm), mean ± SD | 97 ± 11 | 95 ± 11 | 95 ± 12 | 1.631† | 0.197 |
| BMI (kg/m2), median (range) | 26.1 (18.6–43.2) | 25.4 (18.1–35.7) | 25.4 (17.7–40.0) | 5.239* | 0.073 |
| Duration (years), median (range) | 7 (0–26) | 10 (0–31) | 13 (0–42) | 26.665* | <0.001‡ |
| Prevalence of hypertention, n (%) | 67 (48.9) | 83 (60.6) | 85 (61.6) | 5.57* | 0.062 |
| HbA1c (%), median (range) | 8.3 (5.6–14.7) | 8.4 (5.8–13.5) | 9.1 (5.8–14.5) | 6.784* | 0.034‡ |
| C-P (ng/ml), median (range) | 2.14 (0.45–4.79) | 1.87 (0.06–17.78) | 1.69 (0.11–7.11) | 13.984* | 0.001‡ |
| 2-h C-P (ng/ml), median (range) | 5.10 (0.75–19.98) | 4.29 (0.11–18.09) | 3.84 (0.60–11.11) | 17.565* | <0.001‡ |
| TC (mmol/L), median (range) | 4.7 (2.0–12.0) | 4.4 (2.5–10.8) | 4.3 (2.2–10.0) | 6.434* | 0.040‡ |
| TG (mmol/L), median (range) | 1.9 (0.6–18.3) | 1.6 (0.6–14.9) | 1.4 (0.5–10.6) | 24.546* | <0.001‡ |
| HDL-C (mmol/L), median (range) | 0.9 (0.4–1.9) | 1.0 (0.6–1.8) | 1.0 (0.4–1.8) | 5.424* | 0.066 |
| LDL-C (mmol/L), mean ± SD | 2.6 ± 0.9 | 2.6 ± 0.8 | 2.7 ± 0.9 | 0.511† | 0.601 |
| eGFR (ml·min-1·1.73 m-2), mean ± SD | 1216 ± 35 | 114 ± 32 | 109 ± 34 | 4.792† | 0.009‡ |
| Prevalence of DR, n (%) | 14 (10.2) | 26 (19.0) | 38 (27.5) | 13.433* | 0.001‡ |
| Prevalence of DN, n (%) | 18 (13.1) | 25 (18.2) | 48 (34.8) | 20.473* | <0.001‡ |
| Prevalence of DPN, n (%) | 63 (46.0) | 85 (62.0) | 86 (62.3) | 9.778* | 0.008‡ |
*χ2 values, †F values, ‡Statistical significance. SD: Standard deviation; BMI: Body mass index; HbA1c: Hemoglobin A1c; C-P: C-peptide; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate; DR: Diabetic retinopathy; DN: Diabetic nephropathy; DPN: Diabetic peripheral neuropathy.
The logistic regression analysis revealed that serum zinc level was negatively correlated with age, diabetes duration, HbA1c level, and the prevalence of DN, and was positively correlated with TG level [Table 6].
Table 6.
Logistic regression analysis of risk factors related to low serum zinc level
| Factors | OR (95% CI) | P |
|---|---|---|
| Sex | 1.200 (0.674–2.136) | 0.535 |
| Age | 0.955 (0.928–0.983) | 0.002* |
| Diabetes duration | 0.950 (0.905–0.996) | 0.034* |
| HbA1c | 0.744 (0.632–0.877) | 0.000* |
| C-P | 0.959 (0.707–1.300) | 0.787 |
| 2-h C-P | 1.069 (0.930–1.228) | 0.349 |
| TC | 0.916 (0.695–1.207) | 0.534 |
| TG | 1.503 (1.196–1.888) | 0.000* |
| eGFR | 1.200 (0.674–2.136) | 0.535 |
| DR | 0.855 (0.367–1.995) | 0.718 |
| DN | 0.326 (0.150–0.711) | 0.005* |
| DPN | 0.848 (0.479–1.501) | 0.571 |
*Statistical significance. OR: Odds ratio; CI: Confidence interval; HbA1c: Hemoglobin A1c; C-P: C-peptide; TC: Total cholesterol; TG: Triglyceride; eGFR: Estimated glomerular filtration rate; DR: Diabetic retinopathy; DN: Diabetic nephropathy; DPN: Diabetic peripheral neuropathy.
DISCUSSION
Our study showed that the increased incidence of diabetic microvascular complications was accompanied by decreased serum zinc level in patients with T2D. However, evidence supporting the role of zinc insufficiency as an etiological factor of diabetic microvascular complications is scarce.
In our study, we found lower zinc levels in DR patients than in those without DR, consistent with previous studies, suggesting that zinc might play an important role in the development of DR.[5,9,10] Zinc is a retinal protective factor, by stabilizing the membrane structure, activating metallothionein, clearing free radicals and inhibiting lipid peroxidation, it may reduce the expression of vascular endothelial growth factor, and inhibit the neovascularization and exudation.[11] Our results suggest that in T2D patients with a relatively low zinc level, the protective effect of the anti-oxidative zinc may be reduced, and the risk of DR may be elevated. Our study failed to reveal any independent relationship between zinc level and DR, perhaps due to the limited sample size. Furthermore, we failed to adjust the zinc intake from the food, leading to possible impact on the serum zinc level. Finally, we did not collected the detailed information of DR, which may be helpful for us to further analyze the association between zinc level and different stage of DR.
Our study also showed that the zinc level was significantly lower in patients with elevated urinary ACR, patients with an ACR over 300 mg/g were associated with the lowest zinc levels in serum. Our study also suggested that serum zinc level was an independent risk factor for DN. A previous animal study had shown that zinc supplementation raises the zinc levels in renal tubular epithelial cells, up-regulates the expression of metallothionein in the renal tubular cells, and inhibits the renal oxidative damage, inflammation and expression of connective tissue growth factor via its antioxidative effect.[12] Since zinc may exhibit a protective effect in DN, patients with low zinc level were more likely to show an elevated ACR. Brun et al.[13] showed much higher urinary zinc excretion in diabetic rats with albuminuria, concluding that incipient nephropathy in terms of microalbuminuria was associated with a highly significant increase in zinc excretion.
Our study also showed that zinc levels were significantly lower in patients with DPN. Oxidative stress was a major cause of DPN.[14] Migdalis et al.[15] demonstrated a negative relationship between zinc level and lipid peroxidation. Therefore, a lower serum zinc level in patients with DN in our study might be attributed to the elevated lipid peroxidation. Another animal study showed that zinc is a protective factor in DN via regulation of metallothionein expression and inhibition of oxidative stress.[16] Therefore, in patients with a lower zinc level, this protective effect may be abrogated.
In our study, we found that a decrease in zinc level may led to an increase in the proportion of metabolic disorders in females, which indicates a higher risk of zinc metabolic disorders in women. Studies done by McNair et al.[17] had shown that urinary zinc excretion in women is higher than in men, which might lower the serum zinc level. Our study also showed that zinc levels in the serum were lower in patients with longer duration of diabetes, similar to the results reported previously.[18] We have also showed that patients with lower zinc level had higher HbA1c and lower C-peptide levels, suggesting that patients with lower zinc level manifested worse glucose control and severe β-cell dysfunction.
However, our study was a cross-sectional study. The results of our study only suggest an association between zinc level and diabetic microvascular complications. The other limitation relates to the failure to adjust the zinc intake and urine zinc excretion, which may be an important confounding factor. A randomized controlled trial is needed to establish the causal relationship between serum zinc level and the development of diabetic microvascular complications.
In conclusion, our study suggested that lower serum zinc level in T2D patients was related to higher prevalence of diabetic microvascular complications and represented as an independent risk factor for DN. Patients with lower serum zinc level were more likely to have a longer duration of diabetes, poorer glucose control, and worse β-cell function. Older age, longer diabetes duration, higher HbA1c level, and the prevalence of DN were risk factors related to the lower serum zinc level.
Financial support and sponsorship
This study was supported by grants from the National High Technology Research and Development Program of China (863 Program, No. 2012AA02A50) and the Beijing Municipal Science and Technology Commission Funding (No. D131100005313008).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Flannick J, Thorleifsson G, Beer NL, Jacobs SB, Grarup N, Burtt NP, et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014;46:357–63. doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shan Z, Bao W, Zhang Y, Rong Y, Wang X, Jin Y, et al. Interactions between zinc transporter-8 gene (SLC30A8) and plasma zinc concentrations for impaired glucose regulation and type 2 diabetes. Diabetes. 2014;63:1796–803. doi: 10.2337/db13-0606. [DOI] [PubMed] [Google Scholar]
- 3.Wenzlau JM, Frisch LM, Hutton JC, Fain PR, Davidson HW. Changes in zinc transporter 8 autoantibodies following type 1 diabetes onset: The type 1 diabetes genetics consortium autoantibody workshop. Diabetes Care. 2015;38(Suppl 2):S14–20. doi: 10.2337/dcs15-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas JP, Bachowski GJ, Girotti AW. Inhibition of cell membrane lipid peroxidation by cadmium- and zinc-metallothioneins. Biochim Biophys Acta. 1986;884:448–61. doi: 10.1016/0304-4165(86)90195-9. [DOI] [PubMed] [Google Scholar]
- 5.Moustafa SA. Zinc might protect oxidative changes in the retina and pancreas at the early stage of diabetic rats. Toxicol Appl Pharmacol. 2004;201:149–55. doi: 10.1016/j.taap.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Khan MI, Siddique KU, Ashfaq F, Ali W, Reddy HD, Mishra A. Effect of high-dose zinc supplementation with oral hypoglycemic agents on glycemic control and inflammation in type-2 diabetic nephropathy patients. J Nat Sci Biol Med. 2013;4:336–40. doi: 10.4103/0976-9668.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayee MA, Mohammad QD, Haque A. Diabetic neuropathy and zinc therapy. Bangladesh Med Res Counc Bull. 2005;31:62–7. [PubMed] [Google Scholar]
- 8.Farvid MS, Jalali M, Siassi F, Hosseini M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care. 2005;28:2458–64. doi: 10.2337/diacare.28.10.2458. [DOI] [PubMed] [Google Scholar]
- 9.Hasan NA. Effects of trace elements on albumin and lipoprotein glycation in diabetic retinopathy. Saudi Med J. 2009;30:1263–71. [PubMed] [Google Scholar]
- 10.Duzguner V, Kaya S. Effect of zinc on the lipid peroxidation and the antioxidant defense systems of the alloxan-induced diabetic rabbits. Free Radic Biol Med. 2007;42:1481–6. doi: 10.1016/j.freeradbiomed.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Miao X, Sun W, Miao L, Fu Y, Wang Y, Su G, et al. Zinc and diabetic retinopathy. J Diabetes Res 2013. 2013 doi: 10.1155/2013/425854. 425854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem. 2010;21:237–46. doi: 10.1016/j.jnutbio.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Brun JF, Fons C, Fussellier M, Bardet L, Orsetti A. Urinary zinc and its relationships with microalbuminuria in type I diabetics. Biol Trace Elem Res. 1992;32:317–23. doi: 10.1007/BF02784617. [DOI] [PubMed] [Google Scholar]
- 14.Farvid MS, Homayouni F, Amiri Z, Adelmanesh F. Improving neuropathy scores in type 2 diabetic patients using micronutrients supplementation. Diabetes Res Clin Pract. 2011;93:86–94. doi: 10.1016/j.diabres.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Migdalis IN, Triantafilou P, Petridou E, Varvarigos N, Totolos V, Rigopoulos A. Lipid peroxides in type 2 diabetic patients with neuropathy. Res Commun Mol Pathol Pharmacol. 2005;117-118:5–12. [PubMed] [Google Scholar]
- 16.Liu F, Ma F, Kong G, Wu K, Deng Z, Wang H. Zinc supplementation alleviates diabetic peripheral neuropathy by inhibiting oxidative stress and upregulating metallothionein in peripheral nerves of diabetic rats. Biol Trace Elem Res. 2014;158:211–8. doi: 10.1007/s12011-014-9923-9. [DOI] [PubMed] [Google Scholar]
- 17.McNair P, Kiilerich S, Christiansen C, Christensen MS, Madsbad S, Transbol I. Hyperzincuria in insulin treated diabetes mellitus - Its relation to glucose homeostasis and insulin administration. Clin Chim Acta. 1981;112:343–8. doi: 10.1016/0009-8981(81)90457-5. [DOI] [PubMed] [Google Scholar]
- 18.Al-Maroof RA, Al-Sharbatti SS. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J. 2006;27:344–50. [PubMed] [Google Scholar]


