Abstract
Background:
The optimal surgical management of nonfunctional pancreatic neuroendocrine tumors (NF-PNETs) is still controversial. Here, we evaluated the impact of lymph node status on postoperative recurrence in patients with NF-PNET and the potential of preoperative variables for predicting lymph node metastasis (LNM).
Methods:
In this mono-institutional retrospective cohort study conducted in 100 consecutive patients who underwent NF-PNET resection between January 2004 and December 2014, we evaluated risk factors for survival using the Kaplan–Meier method and the Cox regression model. Predictors of LNM were evaluated using the logistic regression model, and the power of predictive models was evaluated using receiver operating characteristic curve analysis.
Results:
Five-year disease-free survival of resected NF-PNET was 64.1%. LNM was independently associated with postoperative recurrence (hazard ratio = 3.995, P = 0.003). Multivariate analysis revealed tumor grade as an independent factor associated with LNM (G2 vs. G1: odds ratio [OR] =6.287, P = 0.008; G3 vs. G1: OR = 12.407, P = 0.001). When tumor grade was excluded, radiological tumor diameter >2.5 cm (OR = 5.430, P = 0.013) and presence of symptoms (OR = 3.366, P = 0.039) were significantly associated with LNM. Compared to neoplasms with radiological diameter >2.5 cm (32.1%), tumors ≤2.5 cm had an obviously lower risk of LNM (7.7%), indicating the reliability of this parameter in predicting LNM (area under the curve, 0.693). Incidentally discovered NF-PNETs ≤2.5 cm were associated with a low-risk of LNM and excellent survival.
Conclusions:
LNM is significantly associated with postoperative recurrence. Radiological tumor diameter is a reliable predictor of LNM in NF-PNETs. Our results indicate that lymphadenectomy in small (≤2.5 cm) NF-PNETs is not routinely necessary.
Keywords: Lymph Node Metastasis, Neuroendocrine Tumor, Nonfunctional Pancreatic Postoperative Recurrence, Tumor Diameter
INTRODUCTION
Pancreatic neuroendocrine tumors (PNETs) are a relatively rare and heterogeneous group of neoplasms comprising 1–2% of all pancreatic neoplasms and 2.6% of pancreatic cancers.[1,2,3,4] Due to the widespread use of high-quality imaging techniques, the incidence of PNETs has remarkably increased from 0.17 to 0.43/100,000 over the past three decades in the United States.[2,5] Furthermore, autopsy studies indicate that the prevalence of PNETs may be even higher.[6,7] Depending on whether clinical symptoms are related to excessive secretion of endocrine hormones, PNETs are divided into functional and nonfunctional PNETs (NF-PNETs). NF-PNETs are defined by the absence of a hormone hypersecretion syndrome and account for approximately 90% of all PNETs.[8] NF tumors are asymptomatic or present with nonspecific symptoms related to local mass effect or metastatic disease[7] and are associated with a poorer prognosis than functional tumors, probably due to delayed diagnosis and higher malignant potential.[6,9]
Compared to pancreatic ductal adenocarcinoma, NF-PNETs often have an indolent outcome but postoperative recurrence is not rare. Lymph node metastasis (LNM) is strictly correlated with survival in pancreatic ductal adenocarcinoma. Regarding NF-PNETs, lymph node status is regarded as an important prognostic factor in both the European Neuroendocrine Tumor Society (ENETS) TNM staging system and the American Joint Committee on Cancer (AJCC) cancer staging system.[10,11] In current guidelines, indications for regional lymphadenectomy in NF-PNETs are not consistent. The National Comprehensive Cancer Network (NCCN) guidelines do not advocate routine lymphadenectomy in tumors <2 cm, while this procedure is recommended for tumors that are 1–2 cm because of the risk of LNM.[12] Moreover, previous reports have demonstrated that only 30–40% of patients with NF-PNETs present with LNM at diagnosis,[13,14,15,16,17] which suggests the importance of preoperative recognition of patients at high-risk of LNM, who may benefit from regional lymphadenectomy. Therefore, the identification of reliable predictors of LNM is important in guiding clinical management decisions and avoiding an unnecessary lymphadenectomy in low-risk patients.
The aims of this study were: (1) To evaluate the impact of LNM on postoperative recurrence of patients with surgically treated NF-PNET; (2) to evaluate the feasibility of preoperative prediction of LNM in NF-PNETs using preoperatively available variables.
METHODS
Patients
This was a mono-institutional retrospective cohort study of the clinical records of 100 patients who underwent pancreatic surgery with curative intent for NF-PNET between January 2004 and December 2014. The diagnosis of PNET was confirmed by histopathology. All patients who were diagnosed with NF-PNET were included (n = 111), whereas syndromic patients (n = 1), patients lost to the postoperative follow-up (n = 6) and patients with distal metastasis (n = 4), were excluded from this study. Nonfunctioning neoplasms were defined by the lack of any clinical syndrome caused by excess hormonal secretion. In total, 100 patients with nonsyndromic, NF localized PNET were finally selected for this study. Information about clinical presentation, demographics, data regarding surgical procedures, postoperative course and complications, pathologic findings, and follow-up was collected. All patients underwent presurgical computed tomography (CT) evaluation of the dimensions, local invasiveness, and the presence of lymph node or distant metastasis. The radiological presurgical diameter was defined as the largest diameter on the CT scans.[18] The pathological diameter of neoplasms was defined as the largest diameter of the surgical specimens.
Surgical treatment of nonfunctional pancreatic neuroendocrine tumors
All patients underwent surgical resection with curative intent. Standard or parenchyma-preserving resection was selected for according to tumor size and anatomical location. Standard resections included pancreaticoduodenectomy or distal pancreatectomy with or without splenectomy. Parenchyma-preserving resections included middle pancreatectomy or enucleation.[19,20,21] All patients in this study had at least one lymph node sampled on resected specimens. Standard resections were always performed in association with regional lymphadenectomy. Lymphadenectomy during parenchyma-preserving resection was usually limited to the peripancreatic nodes. If lymph node involvement was suspected, regional lymphadenectomy was performed. The extent of regional lymphadenectomy was the same as that performed in cases of pancreatic ductal adenocarcinoma. For tumors located in the pancreatic head, regional nodes consisted of those located along the common bile duct, common hepatic artery, portal vein, superior mesenteric vein, posterior and anterior pancreatic head, and the right lateral wall of the superior mesenteric artery. For tumors located in the pancreatic body or tail, regional nodes included those along the common hepatic artery, celiac axis, splenic artery, and splenic hilum.
Pathological examination and staging system
The pathological diagnosis of PNETs depends on classic histological and immunohistochemical features. The surgical specimens of all cases were classified according to the World Health Organization (WHO) classification criteria (2010).[22] All PNETs were divided according to a grading scheme based on mitotic count or Ki67 index into G1 (mitotic count <2/10 high-power fields (HPF) and/or ≤2% Ki67 index), G2 (mitotic count 2–20/10 HPF and/or 3–20% Ki67 index), and G3 (mitotic count >20/10 HPF and/or >20% Ki67 index). The ENETS recommended TNM staging system was used for tumor staging.[10] Primary tumors (T stage) were classified into four categories: T1, tumor limited to the pancreas and size <2 cm; T2, tumor limited to the pancreas and size 2–4 cm; T3, tumor limited to the pancreas and size >4 cm or invading the duodenum or bile duct; and T4, tumor invading adjacent organs (stomach, spleen, colon, and adrenal gland) or the wall of large vessels (celiac axis or superior mesenteric artery). Primary tumor angioinvasion and local infiltration were also evaluated in pathological samples. Malignant behavior was defined as LNM or local invasion on histology.
Follow-up
All patients enrolled in this study underwent a postoperative clinical and radiological follow-up. All patients underwent a radiological examination by CT scans every 6–12 months after surgery, and magnetic resonance imaging was performed if necessary. If patients had any symptoms suspected to be associated with tumor progression during follow-up, a radiological examination was performed immediately to rule out recurrence or distant metastasis. The radiological examination was performed more frequently in patients with progressive disease or carcinoma. Disease-free survival (DFS) was calculated as the months between surgery and 30 Jun 2015 or the first documented disease recurrence. An acute postoperative mortality was defined as death which occurred within 30 days after surgery. The data on the operation and postoperative morbidity was collected from the electronic patient records of our institution. Phone interviews were conducted for all patients with a response rate of 95% (105 patients) and data on survival status, date of death, and tumor recurrence were collected. For all living patients, the last follow-up was updated by 30 Jun 2015.
Statistical analysis
Data were expressed as a mean ± standard deviation for continuous variables and as number and percentage for categorical variables. The comparison between subgroups was performed by the analysis of variance for quantitative variables and by the chi-square test or Fisher exact test for categorical variables, when necessary. Survival probability was estimated according to the Kaplan–Meier method. The Cox regression model was used in univariate and multivariate analyses to evaluate the independent predictive factors of postoperative recurrence. Hazard ratios (HR) and 95% confidence intervals (CI) were also calculated. Variables with P ≤ 0.05 in univariate analysis were included in the multivariate model. Logistic regression analysis performed in a stepwise fashion with backward selection was used to evaluate the value of clinical factors for predicting LNM and to establish the preoperative predictive model. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive power of the models through calculating the area under the curve (AUC).
A two-sided P < 0.05 was considered to indicate statistical significance. SPSS version 20.0 (IBM, USA) was used to perform all the data analysis.
RESULTS
Demographics, operative details, pathological findings
The clinical and pathological data of the 100 NF-PNETs are shown in Table 1. Among the 100 patients, more than 50% (n = 53) were symptomatic at the time of diagnosis. The most common presenting symptom was abdominal pain (n = 34). Overall, 81 patients (81%) underwent standard pancreatic resection, while 19 patients (19%) underwent parenchyma-preserving pancreatic resection. Postsurgical complications occurred in 55 patients (55%), among which, postoperative pancreatic fistula (POPF) (50.9%) was the most common. Approximately, 40% of POPFs were classified as grade A according to the International Study Group of Pancreatic Fistula criteria. 2 patients underwent reoperation before discharge due to postoperative hemorrhage. No operation-related mortality occurred.
Table 1.
Clinical and pathological data of patients with nonfunctioning PNETs
| Characteristics | All patients (n = 100) n (%) |
|---|---|
| Sex | |
| Male | 46 (46) |
| Female | 54 (54) |
| Age (years) | |
| ≤60 | 74 (74) |
| ˃60 | 26 (26) |
| Presence of symptoms | |
| No | 47 (47) |
| Yes | 53 (53) |
| Primary site | |
| Head | 55 (55) |
| Body/tail | 45 (45) |
| Tumor size (cm) | |
| <2 | 29 (29) |
| 2–4 | 42 (42) |
| ˃4 | 29 (29) |
| Surgery | |
| Enucleation | 9 (9) |
| Middle pancreatectomy | 10 (10) |
| Whipple | 43 (43) |
| Distal pancreatectomy | 38 (38) |
| Surgery approach | |
| Open | 69 (69) |
| Laparoscopic | 7 (7) |
| Robotic | 24 (24) |
| Morbidity | 55 (55) |
| Grade* | |
| Grade 1 | 61 (61) |
| Grade 2 | 24 (24) |
| Grade 3 | 15 (15) |
| Tb | |
| T1 | 29 (29) |
| T2 | 35 (35) |
| T3 | 31 (31) |
| T4 | 5 (5) |
| Node status | |
| Positive | 20 (20) |
| Negative | 80 (80) |
| ENETS stage† | |
| I | 27 (27) |
| IIa | 32 (32) |
| IIb | 21 (21) |
| IIIa | 0 |
| IIIb | 19 (19) |
| IV | 0 |
According to the 2010 WHO classification,[23] among the 100 NF-PNETs, 61 patients (61%) were diagnosed as G1 tumors, 24 (24%) patients as G2 tumors, and 15 (15%) as G3 tumors. Among the 85 patients with G1 or G2 tumors, 44 (51.8%) were asymptomatic. In contrast, among the patients with G3 tumors, 12 (80%) had symptoms before surgery. Compared to G1 neoplasms, G2 or G3 neoplasms had larger diameter (G2 vs. G1: 4.4 cm ± 2.6 cm vs. 2.8 cm ± 2.1 cm, P = 0.001; G3 vs. G1: 4.6 cm ± 2.0 cm vs. 2.8 cm ± 2.1 cm, P = 0.004). Overall, 20 patients presented with LNM (LN+). Tumor size in LN+ patients was larger than that in LN-patients (4.7 cm ± 2.6 cm vs. 3.2 cm ± 2.2 cm, P = 0.01).
According to the ENETS recommended TNM staging system,[10] 29 (29%) patients had T1 tumors, 35 (35%) had T2, 31 (31%) had T3, and 5 (5%) had T4. Among the 100 patients, 27 patients (27%) had Stage I neoplasms, 32 (32%) had Stage IIa, 22 (22%) had Stage IIb, and 19 (19%) had Stage IIIb. Malignant behavior was defined as LNM or local invasion on histology. Pathological examination revealed the presence of angioinvasion and perineural invasion in 15 patients (15%) and 7 patients (7%), respectively. Malignant behavior was found in 31 (31%) of all NF-PNETs and four (8.5%) of 47 patients with radiological tumor size <2.5 cm with LNM identified in 3 (75%) of these patients.
Long-term outcomes
The 1, 5, and 8-year DFS was 90.2, 64.1–47.1%, respectively [Figure 1]. During the follow-up period, 21 patients (21%) presented postoperative recurrence. Of these patients, 15 had liver metastasis and six had a local recurrence. Three patients underwent reoperation due to local recurrence. Variables associated with DFS in the univariate analysis are shown in Table 2. In the multivariate analysis, lymph node positive (HR = 3.995, 95% CI: 1.585–10.06, P = 0.003), angioinvasion (HR = 4.049, 95% CI: 1.472 - 11.135, P = 0.007), and high tumor grading (G3 vs. G1 + G2: HR = 7.286, 95% CI: 2.7797–18.980, P = 0.000048) were significantly associated with decreased DFS in patients with resected NF-PNET [Table 2]. The 5- and 8-year DFS was 79.4% and 71.4%, respectively, for LN-patients compared to 24.9% and 8.3%, respectively, for patients with LN+ disease (P = 0.000001) [Figure 2].
Figure 1.
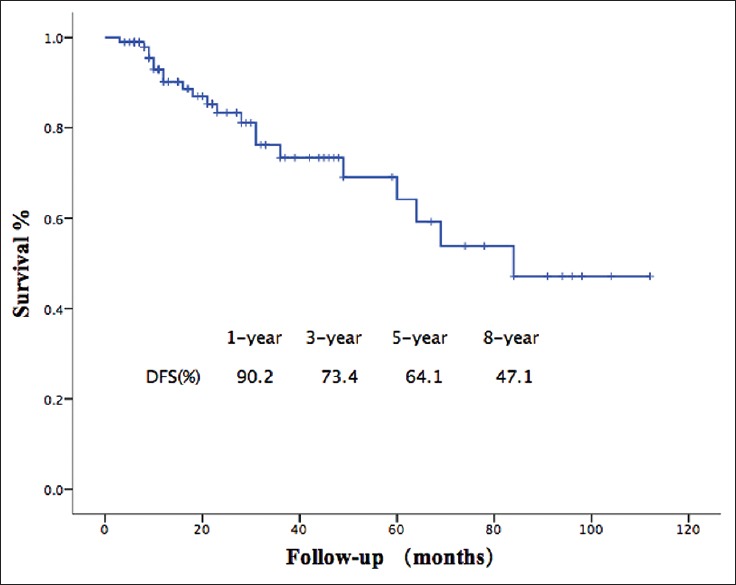
Disease-free survival of patients with nonfunctional pancreatic neuroendocrine tumors. The 1, 5, and 8-year disease-free survival was 90.2%, 64.1% and 47.1%, respectively.
Table 2.
Univariate and multivariate analysis of risk factors of DFS
| Items | n | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| 5 years DFS (%) ± SD | P | HR (95% CI) | P | ||
| Gender | |||||
| Male | 46 | 53.8 ± 14.3 | 0.207 | ||
| Female | 54 | 71.2 ± 9.8 | |||
| Age (years) | |||||
| ≤60 | 74 | 73.6 ± 7.4 | 0.267 | ||
| >60 | 26 | 57.7 ± 15.6 | |||
| Presence of symptom | |||||
| No | 47 | 68.8 ± 16.9 | 0.053 | ||
| Yes | 53 | 60.1 ± 9.3 | |||
| CA199 (U/ml) | |||||
| Normal | 88 | 69.6 ± 8.6 | 0.036 | 1 | |
| Elevated | 12 | 32.7 ± 18.4 | 1.622 (0.535–4.916) | 0.393 | |
| Tumor size (cm)* | |||||
| ≤2 | 35 | 100 | 0.014 | 1 | |
| >2 | 65 | 53.9 ± 9.2 | 1.635 (0.158–16.895) | 0.680 | |
| Tumor location | |||||
| Head | 55 | 63 ± 11.5 | 0.806 | ||
| Body/tail | 45 | 64.9 ± 11.5 | |||
| Grade† | |||||
| Grade 1 + Grade 2 | 85 | 78.8 ± 7.9 | 0.000000 | 1 | |
| Grade 3 | 15 | 10.5 ± 9.6 | 7.286 (2.797–18.980) | 0.000048 | |
| T‡ | |||||
| T1 + T2 | 64 | 92.4 ± 5.2 | 0.000019 | 1 | |
| T3 + T4 | 36 | 33.2 ± 11.8 | 2.798 (0.765–10.235) | 0.120 | |
| Nodal status | |||||
| Negative | 80 | 79.4 ± 8.3 | 0.000001 | 1 | |
| Positive | 20 | 24.9 ± 12.0 | 3.995 (1.585–10.06) | 0.003 | |
| Angioinvasion | |||||
| No | 85 | 73.3 ± 7.1 | 0.000011 | 1 | |
| Yes | 15 | 23.3 ± 13.16 | 4.049 (1.472–11.135) | 0.007 | |
| Perineural invasion | |||||
| No | 93 | 69.7 ± 8.5 | 0.000000 | 1 | |
| Yes | 7 | 0 | 2.215 (0.440–11.048) | 0.332 | |
| LN examined | |||||
| ≤6 | 60 | 60 ± 11.4 | 0.618 | ||
| >6 | 40 | 68.8 ± 11.7 | |||
| Focality | |||||
| Unifocal | 97 | 64.6 ± 8.3 | 0.807 | ||
| Multifocal | 3 | 66.7 ± 27.2 | |||
*Size on resected specimens; †WHO 2010 classification;[22] ‡ENETS recommended TNM staging system.[10] DFS: Disease-free survival; SD: Standard deviation; HR: Hazard ratio; 95% CI: 95% confidence interval; LN: Lymph node; ENETS: European Neuroendocrine Tumor Society; TNM: Tumor-node-metastasis; WHO: World Health Organization.
Figure 2.
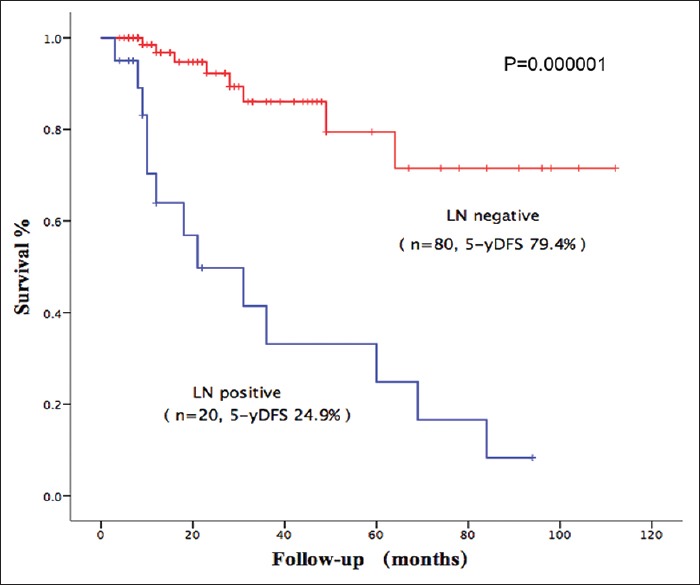
Impact of lymph node status on disease-free survival. The 5-year disease-free survival was 79.4% for lymph node-patients compared to 24.9% for patients with LN + disease (P = 0.000001), LN: Lymph node.
Predictors of lymph node metastasis
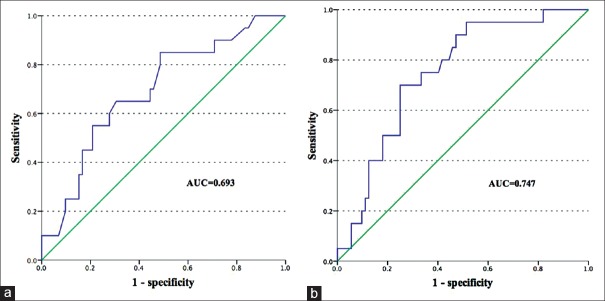
Among these 100 patients with NF-PNETs, 92 who underwent regional lymphadenectomy were selected for analysis of predictors of LNM. Of these patients, LNM was eventually confirmed in 20 (21.7%). Variables that could be measured preoperatively were selected for the univariate analysis of the feasibility of preoperative prediction of LN status. In the univariate analysis, factors associated with LN metastasis were radiological tumor diameter >2.5 cm (odds ratio [OR] =5.667, P = 0.010), Elevated CA199 (OR = 4.714, P = 0.017), high tumor grading (G2:G1, OR = 6.125, P = 0.007; G3:G1, OR = 14.000, P = 0.000322), and presence of symptoms (OR = 3.545, P = 0.026) [Table 3]. In the multivariate analysis, tumor grading (G2 vs. G1: OR = 6.287, P = 0.008; G3 vs. G1: OR = 12.407, P = 0.001) was an independent predictor of LNM [Table 3]. The rate of LNM progressively increased from G1 to G3 (G1, G2 vs. G3: 7.5%, 33.3% vs. 53.3%). Considering the difficulty of preoperative retrievability of tumor grade, we excluded tumor grade from the analysis, and as a result, radiological tumor size >2.5 cm (OR = 5.430, P = 0.013) and presence of symptoms (OR = 3.366, P = 0.039) were independently associated with LNM [Table 3]. Radiological diameter was consistent with pathological diameter (34.1 mm vs. 34.7 mm, P = 0.237). When tumor size was treated as a continuous variable, the correlation with LN metastasis also reached significance (OR = 1.313, P = 0.016). ROC analysis demonstrated radiological tumor diameter was a reliable and feasible predictor of LNM in patients with resectable NF-PNET with an AUC of 0.693 [Figure 3a]. Compared to neoplasms with radiological size >2.5 cm (32.1%), tumors ≤2.5 cm had an obviously lower risk of LNM (7.7%). The various clinicopathologic factors reviewed in this study stratified by a tumor size cut-off of 2.5 cm are summarized in Table 4. Compared to tumors ≤2.5 cm, tumors >2.5 cm had higher tumor grade and greater malignant potential. A cut-off of >2.5 cm was associated with a sensitivity of 85% for the presence of LN+ disease. The negative predictive value of tumor size ≤2.5 cm was 92.3%. Other cut-offs were also examined [Table 5]. With the purpose of promoting the predictive power, we constructed a preoperative predictive model of LN metastasis based on radiological tumor size combined with symptoms. ROC analysis was performed to evaluate the predictive power of this model. The AUC of this model was 0.747 [Figure 3b], and the probability of LN+ for every patient was calculated. For the patients with an LN+ risk of ≤20%, 89.6% of these patients were, actually, LN-negative. Of the 21 incidentally discovered patients with tumors size ≤2.5 cm on CT scans, only one (4.8%) had LNM and none presented a postoperative recurrence during follow-up.
Table 3.
Univariate and multivariate logistic regression analysis of LN metastasis
| Items | Univariate analysis | Multivariate analysis | Multivariate analysis† | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Gender | ||||||
| Male | 1 | |||||
| Female | 0.504 (0.184–1.382) | 0.183 | ||||
| Age (years) | ||||||
| ≤60 | 1 | |||||
| >60 | 0.607 (0.181–2.032) | 0.418 | ||||
| Tumor location | ||||||
| Head | 1 | |||||
| Body/tail | 0.907 (0.148–1.240) | 0.899 | ||||
| CA125 | ||||||
| Normal | 1 | |||||
| Elevated | 2.750 (0.693–10.909) | 0.150 | ||||
| CA199 | ||||||
| Normal | 1 | 1 | 1 | |||
| Elevated | 4.714 (1.324–16.788) | 0.017 | 3.731 (0.869–16.021) | 0.077 | 2.832 (0.717–11.189) | 0.137 |
| Radiological size | ||||||
| ≤2.5 cm | 1 | 1 | 1 | |||
| >2.5 cm | 5.667 (1.527–21.032) | 0.010 | 2.456 (0.561–10.755) | 0.233 | 5.430 (1.431–20.603) | 0.013 |
| Symptoms | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 3.345 (1.165–10.793) | 0.026 | 2.319 (0.670–8.032) | 0.184 | 3.366 (1.063–10.656) | 0.039 |
| Grading* | ||||||
| Grade 1 | 1 | 1 | / | / | ||
| Grade 2 | 6.125 (1.626–23.073) | 0.007 | 6.287 (1.615–24.478) | 0.008 | / | / |
| Grade 3 | 14.000 (3.324–58.969) | 0.000332 | 12.407 (2.827–54.450) | 0.001 | / | / |
*WHO 2010 classification;[22] †Multivariate analysis excluding tumor grade. OR: Odds ratio; 95% CI: 95% confidence interval; LN: Lymph node; WHO: World Health Organization.
Figure 3.
Receiver operating characteristic curve of predictors of lymph node metastasis. The area under the curve of radiological tumor size (a) is 0.693, while the area under the curve of the constructed predictive model (b) is 0.747.
Table 4.
Clinicopathologic factors stratified by radiological tumor size
| Items | ≤2.5 cm (n = 39) | >2.5 cm (n = 53) | P |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 15 (38.5) | 28 (52.8) | 0.172 |
| Female | 24 (61.5) | 25 (47.2) | |
| Age (years), median ± SD | 50.87 ± 11.38 | 52.68 ± 13.34 | 0.497 |
| Tumor location, n (%) | |||
| Head | 19 (48.7) | 31 (58.5) | 0.352 |
| Body/tail | 20 (51.3) | 22 (41.5) | |
| Symptoms, n (%) | |||
| No | 21 (53.8) | 23 (43.4) | 0.321 |
| Yes | 18 (46.2) | 30 (56.6) | |
| Surgery, n (%)* | |||
| Standard | 32 (82.1) | 49 (92.5) | 0.193 |
| Atypical | 7 (17.9) | 4 (7.5) | |
| Surgical approach, n (%) | |||
| Open | 19 (48.7) | 45 (84.9) | 0.000193 |
| Minimal invasive† | 20 (51.3) | 8 (15.1) | |
| Morbidity, n (%) | |||
| No | 16 (41.0) | 28 (52.8) | 0.296 |
| Yes | 23 (59.0) | 25 (47.1) | |
| Grade, n (%)‡ | |||
| Grade 1 | 31 (79.5) | 22 (41.5) | 0.001 |
| Grade 2 | 7 (19.9) | 17 (32.1) | |
| Grade 3 | 1 (2.6) | 14 (26.4) | |
| Primary tumor, n (%)§ | |||
| T1 | 29 (61.7) | 0 | 0.000000 |
| T2 | 17 (36.2) | 18 (34) | |
| T3 | 1 (2.1) | 30 (56.6) | |
| T4 | 0 | 5 (9.4) | |
| LN status, n (%) | |||
| Negative | 36 (92.3) | 36 (67.9) | 0.005 |
| Positive | 3 (7.7) | 17 (32.1) | |
| Angioinvasion, n (%) | |||
| No | 39 (100) | 38 (71.7) | 0.000282 |
| Yes | 0 | 15 (28.3) | |
| Perineural invasion, n (%) | |||
| No | 38 (97.4) | 47 (88.7) | 0.232 |
| Yes | 1 (2.6) | 6 (11.3) |
*Standard resections include pancreaticoduodenectomy and distal pancreatectomy. Atypical resections include middle pancreatectomy and enucleation; †Laparoscopic and robotic surgery; ‡WHO 2010 classification;[22] §ENETS recommended TNM staging system.[10] SD: Standard deviation; LN: Lymph node; WHO: World Health Organization; ENETS: European Neuroendocrine Tumor Society; TNM: Tumor-node-metastasis.
Table 5.
Predictive values of different radiological size cut-offs for LN metastasis in patients with NF-PNETs
| Cut-off (cm) | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | AUC |
|---|---|---|---|---|---|
| 1.5 | 90.0 | 22.2 | 88.9 | 24.3 | 0.561 |
| 2 | 85.0 | 33.3 | 88.9 | 26.2 | 0.592 |
| 2.5 | 85.0 | 50.0 | 92.3 | 32.1 | 0.675 |
| 3 | 70.0 | 55.6 | 87.0 | 30.4 | 0.628 |
| 3.5 | 65.0 | 62.5 | 86.5 | 32.5 | 0.638 |
| 4 | 65.0 | 69.4 | 87.7 | 37.1 | 0.672 |
NPV: Negative predictive value; PPV: Positive predictive value; AUC: Area under the curve; NF-PNETs: Nonfunctional pancreatic neuroendocrine tumors; LN: Lymph node.
DISCUSSION
NF-PNETs are relatively rare and heterogeneous pancreatic neoplasms with a remarkably increasing incidence.[2] Compared to functional neoplasms, NF neoplasms show a worse outcome in part due to the delay of diagnosis and higher malignant potential.[6,9] The optimal management for PNET is still controversial. Given its positive impact on survival,[23,24,25,26,27] surgical resection has become the treatment choice for most patients with NF-PNETs. In recent years, small NF-PNETs are increasingly being discovered incidentally in cross-sectional imaging for other purposes and routine regional lymphadenectomy when surgical resection is considered in such cases remains controversial. For these reasons, we conducted a mono-institutional retrospective study of resectable NF-PNETs with the purpose of (1) elucidating the clinical significance of LNM and (2) identifying reliable predictors of LNM to help surgeons to make informed treatment decisions.
Our clinical data demonstrated that the 5-year DFS of resectable NF-PNETs was 64.1%. LNM is a significant prognostic predictor for most malignant tumors, although the impact of LNM on the survival of patients with NF-PNETs remains open to debate.[13,14,16,17,28,29,30] Many previous studies have yielded conflicting evidence regarding the prognostic value of LNM for PNET. The difference in patient selection and low lymph node sampling rates during resection for PNETs may account for these inconsistencies.[31] In accordance with our results, several previous studies have demonstrated LNM is significantly associated with a poor prognosis.[13,17,28,31,32,33,34] Both the ENETS-TNM staging system and the AJCC cancer staging system regard lymph node status as an important prognostic factor.[10,11] In our study, patients with LNM had a significantly higher risk (nearly 4-fold) of postoperative recurrence compared with those without LNM, which supports the necessity for regional lymphadenectomy in patients with high-risk of lymph node involvement.
Since LNM is apparently related to prognosis, the ability to distinguish patients with high-risk of LNM preoperatively is of great importance. NF-PNETs classified, according to the criteria of the 2010 WHO classifications, were divided into three groups using a grading scheme based on the Ki-67 index or mitotic count. Our study confirmed the significant correlation between the 2010 WHO grading system and postoperative recurrence, which is consistent with recent studies.[13] In addition, LNM occurred more frequently in patients with G2 or G3 NF-PNETs than in those with G1 tumors (G1, G2 vs. G3: 7.5%, 33.3%, 53.3%); therefore, it was presumed that these patients would benefit from node clearance and routine lymphadenectomy was recommended. However, classification of tumor grade usually depends on pathological examination and the possibility of a preoperative evaluation of Ki-67 is still questionable. Endoscopic ultrasonography (EUS) combined with fine-needle aspiration (FNA) or Tru-cut needle biopsy (TCB) can be used to evaluate tumor pathology preoperatively. Preoperative evaluation of Ki-67 index, combined with lesion size and imaging findings may help surgeons to decide on the best therapeutic approach and whether lymphadenectomy should be performed. However, there is a lack of data regarding the accuracy of FNA cytology in the assessment of Ki-67 value. All of the available reports describe studies with small sample sizes. In a recent study, the concordance rate for WHO classification between EUS FNA and resected specimens was 77.8%.[35] Use of EUS TCB needles can overcome many of the problems that reduce the accuracy of FNA, but they are not feasible for most patients as they are often performed only at high-volume centers, can be challenging for small masses, and are associated with a risk of pancreatitis and bleeding. Moreover, intratumoral Ki-67 heterogeneity limits the accurate preoperative evaluation of Ki-67 value by EUS FNA.[35]
Considering the limitations of preoperative evaluation of Ki-67 index, an alternative variable is required. Since tumor size can easily be obtained preoperatively by use of radiological techniques, it has been extensively studied to identify the patients with LNM. Increasing tumor size is strongly correlated with positive LN status and poor prognosis.[18,36,37] Partelli et al. reported that radiological tumor size ˃4 cm was an independent predictor of nodal metastasis in low and intermediate grade NF-PNETs.[13] In a retrospective study of 116 patients undergoing resection for NF-PNET, Toste et al. demonstrated that radiological tumor size ≥2 cm predicted nodal metastasis with a sensitivity of 93.8% and only two (7.4%) of 27 patients with tumor size <2 cm had LNM.[15] Hashim et al. reported that patients with tumor diameter >1.5 cm were 4.7 times more likely to have LNM compared to those with smaller tumor diameters. They also found two (12%) of 17 patients with tumor size ≤1 cm and five (13%) of 38 patients with tumor size ≤1.5 cm had LNM.[17] In contrast, some studies indicate that tumor size is not an accurate predictor of LNM. Parekh et al. reported that there was no difference in tumor size for patients with and without nodal metastasis (5.2 cm vs. 4.6 cm). Interestingly, 31% of patients with tumor size <3 cm had nodal metastasis, while only 1 patient in their series with a tumor <2 cm was LN positive.[38] Gratian et al. reported there was no association between decreasing tumor size and decreased percentage of cases presenting with regional nodal metastasis.[16] Our study suggests radiological tumor size is a sensitive predictor of lymph node status and demonstrates a strong correlation between increasing radiological tumor size and LNM. The incidence of LNM in patients with radiological size >2.5 cm was more than 4 times greater than that in patients with a tumor size ≤2.5cm. A cut-off of >2.5 cm was associated with a sensitivity of 85% for the presence of positive LNs. Three of 39 (7.7%) patients with a tumor size ≤2.5 cm had nodal metastasis and 2 patients (11.1%) with a tumor size ≤1.5 cm had nodal metastasis.
Given that smaller tumors are associated with low rates of LNM, better histology, and a better outcome than larger tumors, it is unclear whether lymphadenectomy should be avoided for small NF-PNETs. In a retrospective study of 1854 patients with NF-PNETs ≤2 cm, Gratian et al. demonstrated that there was no difference in 5-year overall survival in patients undergoing surgical resection between those who underwent lymphadenectomy and those who did not.[16] Interestingly, in this study, 29% of tumors ≤2 cm and 33% of tumors ≤0.5 cm presented with regional LNM with a median of eight lymph nodes sampled, which was unexpectedly higher than had been reported previously. However, patient selection bias might overestimate the malignant potential of these tumors and account for the high rate of LNM. NCCN guidelines suggest that lymphadenectomy should not be performed routinely for tumors <2 cm but should be considered in tumors that are 1–2 cm in size.[39] The benefits of lymphadenectomy in patients with small tumors is still unknown and more clinical data or well-designed clinical trials are needed to resolve this problem; however, such clinical trials are limited by the relative rarity and indolent behavior of small NF-PNETs. In our study, patients with small tumors (≤2.5 cm) has a very low-risk of LNM. In addition, recurrence occurred in only one of the patients with small tumors 84 months after surgery, and no deaths caused by these tumors were reported during the follow-up. These results suggest that lymphadenectomy should not perform routinely in these patients.
NCCN guidelines and current staging systems use the same cut-off of 2 cm as that used in pancreatic ductal adenocarcinoma,[10,11,12] although patients with NF-PNETs have a much better prognosis than those with PDA. A cut-off of 2 cm has a low specificity for estimating the risk of LNM; consequently, many patients undergo unnecessary lymphadenectomy under this criterion. Based on our results, a cut-off of 2.5 cm was shown to be appropriate and safe. Compared to 2 cm, a cut-off of 2.5 cm had a similar sensitivity, but higher specificity and negative predictive value, showing that a cut-off of 2.5 cm is more effective in distinguishing the patients who require LN resection. Given the previously demonstrated strict correlation between tumor diameter and poor survival after resection of NF-PNETs,[34,36] raising the threshold may result in some tumors with malignant potential being considered benign. Preoperative evaluation of Ki-67 index, combined with lesion size and imaging findings, may help surgeons to decide the best therapeutic approach. Since low-grade tumors are associated with a very low-risk of LNM and excellent survival, relaxing the indications for lymphadenectomy seems to be feasible.
Based on the results of this study, we identified radiological tumor size as a noninvasive and reliable factor to predict LNM in NF-PNETs. A radiological diameter ˃2.5 cm yielded a powerful correlation with the risk of LNM. The presence of symptoms at diagnosis was also shown to be a predictor of LNM. Although the symptoms of patients with NF-PNETs are always unspecific, they are thought to be related to local mass effect or metastatic disease; in other words, tumor burden.[7,18] Incidental detection of tumors is a strong prognostic factor for postoperative progression.[40] In this study, incidentally discovered tumors were slightly smaller in size (3.2 cm vs. 3.7 cm) and associated with a much lower risk of LNM (12.2% vs. 29.2%). Asymptomatic NF-PNETs smaller than 2 cm are unlikely to show aggressive behavior.[18,26,41] Bettini et al. reported only 6% of NF-PNETs ≤2 cm were malignant when discovered incidentally.[18] In our study, incidentally discovered small (≤2.5 cm) NF-PNETs had a very low-risk of LNM (4.2%) and no cases of postoperative recurrence or death due to the disease occurred. Considering the low-risk of LN involvement and the positive outcomes, routine lymphadenectomy is not recommended as an addition to pancreatic resection in cases of asymptomatic small NF-PNETs when surgery is considered.
In conclusion, LNM was identified as a significant predictor of postoperative recurrence in NF-PNETs. Tumor grade was associated with DFS and LNM. Preoperative prediction of LN involvement is feasible and radiological tumor size, which can be measured easily and accurately, was shown to be a useful and alternative variable correlated with LN involvement. Tumors ≤2.5 cm in preoperative imaging were unlikely to have positive LNs. The preoperative predictive model showed good predictive power for LN involvement. Our results suggest that parenchyma-sparing resection without regional lymphadenectomy is a reasonable option for selected patients with incidentally discovered NF-PNETs ≤2.5 cm when surgical resection is considered because of the low probability of LNM and a good outcome. In contrast, lymphadenectomy should be performed routinely in patients with NF-PNETs >2.5cm.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–81. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence B, Gustafsson BI, Chan A, Svesjda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. doi: 10.1016/j.ecl.2010.12.005. vii. [DOI] [PubMed] [Google Scholar]
- 3.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(1 Suppl):171–90. doi: 10.1002/1097-0142(19950101)75:1+<171::aid-cncr2820751306>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, Thayer SP, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: The Massachusetts General Hospital experience from 1977 to 2005. Arch Surg. 2007;142:347–54. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36:933–42. doi: 10.1007/BF01297144. [DOI] [PubMed] [Google Scholar]
- 7.Burns WR, Edil BH. Neuroendocrine pancreatic tumors: Guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34. doi: 10.1007/s11864-011-0172-2. [DOI] [PubMed] [Google Scholar]
- 8.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–33. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenna LR, Edil BH. Update on pancreatic neuroendocrine tumors. Gland Surg. 2014;3:258–75. doi: 10.3978/j.issn.2227-684X.2014.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compton CC, Byrd DR, Garcia-Aguilar J, Scott HK, Alexander O, Mary KW, et al. Springer Science & Business Media; 2012. AJCC cancer staging atlas: A companion to the seventh editions of the AJCC cancer staging manual and handbook. [Google Scholar]
- 12.Kulke MH, Shah MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, et al. Neuroendocrine Tumors, Version 1.2015. J Natl Compr Canc Netw. 2015;13:78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 13.Partelli S, Gaujoux S, Boninsegna L, Cherif R, Crippa S, Couvelard A, et al. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs) JAMA Surg. 2013;148:932–9. doi: 10.1001/jamasurg.2013.3376. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: Analysis of 3851 patients. Ann Surg. 2008;247:490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 15.Toste PA, Kadera BE, Tatishchev SF, Dawson DW, Clerkin BM, Muthusamy R, et al. Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg. 2013;17:2105–13. doi: 10.1007/s11605-013-2360-9. [DOI] [PubMed] [Google Scholar]
- 16.Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21:3515–21. doi: 10.1245/s10434-014-3769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashim YM, Trinkaus KM, Linehan DC, Strasberg SS, Fields RC, Cao D, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs) Ann Surg. 2014;259:197–203. doi: 10.1097/SLA.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75–82. doi: 10.1016/j.surg.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Cherif R, Gaujoux S, Couvelard A, Dokmak S, Vuillerme MP, Ruszniewski P, et al. Parenchyma-sparing resections for pancreatic neuroendocrine tumors. J Gastrointest Surg. 2012;16:2045–55. doi: 10.1007/s11605-012-2002-7. [DOI] [PubMed] [Google Scholar]
- 20.DiNorcia J, Lee MK, Reavey PL, Genkinger JM, Lee JA, Schrope BA, et al. One hundred thirty resections for pancreatic neuroendocrine tumor: Evaluating the impact of minimally invasive and parenchyma-sparing techniques. J Gastrointest Surg. 2010;14:1536–46. doi: 10.1007/s11605-010-1319-3. [DOI] [PubMed] [Google Scholar]
- 21.Falconi M, Zerbi A, Crippa S, Balzano G, Boninsegna L, Capitanio V, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621–7. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 22.Bosman FT, Carneiro F, Hruban RH, Theise ND. World Health Organization; 2010. WHO classification of tumours of the digestive system. [Google Scholar]
- 23.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: The impact of surgical resection on survival. Cancer. 2009;115:741–51. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 24.Martin RC, Kooby DA, Weber SM, Merchant NB, Parikh AA, Cho CS, et al. Analysis of 6,747 pancreatic neuroendocrine tumors for a proposed staging system. J Gastrointest Surg. 2011;15:175–83. doi: 10.1007/s11605-010-1380-y. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Rivera F, Stewart AE, Arnoletti JP, Vickers S, Bland KI, Heslin MJ. Surgical treatment of pancreatic endocrine neoplasms. Am J Surg. 2007;193:460–5. doi: 10.1016/j.amjsurg.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Zerbi A, Capitanio V, Boninsegna L, Pasquali C, Rindi G, Delle Fave G, et al. Surgical treatment of pancreatic endocrine tumours in Italy: Results of a prospective multicentre study of 262 cases. Langenbecks Arch Surg. 2011;396:313–21. doi: 10.1007/s00423-010-0712-4. [DOI] [PubMed] [Google Scholar]
- 27.Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: Incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–8. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 28.Bettini R, Boninsegna L, Mantovani W, Capelli P, Bassi C, Pederzoli P, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19:903–8. doi: 10.1093/annonc/mdm552. [DOI] [PubMed] [Google Scholar]
- 29.Wong J, Fulp WJ, Strosberg JR, Kvols LK, Centeno BA, Hodul PJ. Predictors of lymph node metastases and impact on survival in resected pancreatic neuroendocrine tumors: A single-center experience. Am J Surg. 2014;208:775–80. doi: 10.1016/j.amjsurg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Ito H, Abramson M, Ito K, Swanson E, Cho N, Ruan DT, et al. Surgery and staging of pancreatic neuroendocrine tumors: A 14-year experience. J Gastrointest Surg. 2010;14:891–8. doi: 10.1007/s11605-010-1173-3. [DOI] [PubMed] [Google Scholar]
- 31.Sarmiento JM, Farnell MB, Que FG, Nagorney DM. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: Long-term survival analysis. World J Surg. 2002;26:1267–71. doi: 10.1007/s00268-002-6714-9. [DOI] [PubMed] [Google Scholar]
- 32.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, et al. Prognostic factors in pancreatic endocrine neoplasms: An analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–42. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Tomassetti P, Campana D, Piscitelli L, Casadei R, Santini D, Nori F, et al. Endocrine pancreatic tumors: Factors correlated with survival. Ann Oncol. 2005;16:1806–10. doi: 10.1093/annonc/mdi358. [DOI] [PubMed] [Google Scholar]
- 34.Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, et al. Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–33. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa T, Yamao K, Hijioka S, Bhatia V, Mizuno N, Hara K, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32–8. doi: 10.1055/s-0033-1344958. [DOI] [PubMed] [Google Scholar]
- 36.Lombardi M, De Lio N, Funel N, Sardella C, Russo D, Urbani C, et al. Prognostic factors for pancreatic neuroendocrine neoplasms (pNET) and the risk of small non-functioning pNET. J Endocrinol Invest. 2015;38:605–13. doi: 10.1007/s40618-014-0219-x. [DOI] [PubMed] [Google Scholar]
- 37.Haynes AB, Deshpande V, Ingkakul T, Vagefi PA, Szymonifka J, Thayer SP, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: Short-term and long-term patient outcomes. Arch Surg. 2011;146:534–8. doi: 10.1001/archsurg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parekh JR, Wang SC, Bergsland EK, Venook AP, Warren RS, Kim GE, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: The UCSF experience with 149 patients. Pancreas. 2012;41:840–4. doi: 10.1097/MPA.0b013e31823cdaa0. [DOI] [PubMed] [Google Scholar]
- 39.Chu QD, Hill HC, Douglass HO, Jr, Driscoll D, Smith JL, Nava HR, et al. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol. 2002;9:855–62. doi: 10.1007/BF02557521. [DOI] [PubMed] [Google Scholar]
- 40.Cheema A, Weber J, Strosberg JR. Incidental detection of pancreatic neuroendocrine tumors: An analysis of incidence and outcomes. Ann Surg Oncol. 2012;19:2932–6. doi: 10.1245/s10434-012-2285-7. [DOI] [PubMed] [Google Scholar]
- 41.Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): Role for nonoperative management. Surgery. 2012;152:965–74. doi: 10.1016/j.surg.2012.08.038. [DOI] [PubMed] [Google Scholar]