Abstract
Objective:
This review aimed to update the progress of microRNA (miRNA) in early detection of ovarian cancer. We discussed the current clinical diagnosis methods and biomarkers of ovarian cancer, especially the methods of miRNA in early detection of ovarian cancer.
Data Sources:
We collected all relevant studies about miRNA and ovarian cancer in PubMed and CNKI from 1995 to 2015.
Study Selection:
We included all relevant studies concerning miRNA in early detection of ovarian cancer, and excluded the duplicated articles.
Results:
miRNAs play a key role in various biological processes of ovarian cancer, such as development, proliferation, differentiation, apoptosis and metastasis, and these phenomena appear in the early-stage. Therefore, miRNA can be used as a new biomarker for early diagnosis of ovarian cancer, intervention on miRNA expression of known target genes, and potential target genes can achieve the effect of early prevention. With the development of nanoscience and technology, analysis methods of miRNA are also quickly developed, which may provide better characterization of early detection of ovarian cancer.
Conclusions:
In the near future, miRNA therapy could be a powerful tool for ovarian cancer prevention and treatment, and combining with the new analysis technology and new nanomaterials, point-of-care tests for miRNA with high throughput, high sensitivity, and strong specificity are developed to achieve the application of diagnostic kits in screening of early ovarian cancer.
Keywords: Detection Method, MicroRNA, Ovarian Cancer
INTRODUCTION
Ovarian cancer is the most lethal gynecologic malignancy at present. Despite the recent new diagnostic possibilities and medical advances, the 5-year survival rate of patients with advanced ovarian cancer is only 25–35%.[1] Prognosis is usually very poor due to the late diagnosis, more than 70% of patients diagnosed with this cancer have reached late-stage (stage III-IV) when cancer has already spread beyond the ovaries.[2] One reason for this high mortality rate is the lack of an effective early detection method for ovarian cancer. Indeed, the 5-year survival rate at early-stage (stage I-II) can reach 90%.[3] Therefore, there is immediate need of reliable diagnostic biomarkers and novel therapeutic tools that can realize the early diagnosis of ovarian cancer. Among this, the recently discovered microRNAs (miRNAs) have been learned a lot. A total of 2578 human miRNAs have been found until now (Sanger miRBase database, version 20.0). Moreover, studies have found that miRNAs play a key role in various biological processes of ovarian cancer, such as development, proliferation, differentiation, apoptosis, and metastasis. These phenomena appear in early-stage, suggesting miRNA can be used as a new biomarker for early diagnosis of ovarian cancer.[4] This review summarizes the current clinical diagnosis method and diagnostic biomarkers of ovarian cancer, and the early detection methods of ovarian cancer miRNAs are explored.
CURRENT DIAGNOSIS METHODS FOR OVARIAN CANCER AND LIMITATIONS
Conventional diagnostic methods for ovarian cancer include the history of disease, physical examination, symptom index diagnosis and imaging diagnosis, etc. History of disease and physical examination can only understand the general condition of patients while symptom index diagnosis is an only preliminary clinical evaluation.[5] Imaging diagnostic methods mainly include ultrasonic, computed tomography (CT) scan, magnetic resonance imaging (MRI), and positron emission tomography-CT (PET-CT), etc.[6]
Ultrasound diagnosis is widely used due to the low price and low radiation hazard, which is an important diagnostic method for ovarian cancer, but it has limited specificity and sensitivity.[7] Therefore, ultrasound diagnosis should not be used alone as the screening method for ovarian cancer but should be combined with other test results to analyze comprehensively and diagnose early-stage ovarian cancer. CT imaging is better than ultrasound in display and preoperative staging of tumors. Its diagnostic sensitivity can reach 73.2%.[8] However, CT diagnosis has several defects such as low specificity and ionizing radiation. Compared with CT, MRI has the function of the multi-dimensional imaging. It provides better soft tissue contrast in the pelvic examination, and the sensitivity is obviously improved than CT (MRI for 90%, CT for 66%).[9] However, MRI is expensive, which is not suitable for ovarian cancer screening in the general population. Based on the tracking of radioactive tracer, PET-CT can provide information of cell anabolic metabolism rate, identify target cells in a specific organization and reflect the metabolic changes of tumor cells. Therefore, compared with CT or MRI, PET-CT has higher sensitivity in the diagnosis of ovarian cancer recurrence. However, its diagnostic accuracy is not ideal for early-stage, small, and low-grade malignant tumors. There may easily appear false positives and false negatives.[10]
Imaging diagnosis method is a normal method for clinical tumor diagnosis. But due to the various defects of the image device, it is usually to combine several kinds of technologies for diagnosis. Moreover because of the low sensitivity and specificity, the methods are often used in the diagnosis of high-risk groups, rather than early detection. Therefore, it is urgently needed to develop new methods for early diagnosis of ovarian cancer.
BIOMARKERS FOR OVARIAN CANCER DIAGNOSIS
In addition to the formation of ovarian cancer and the limitations of conventional diagnostic methods, the selection of biomarkers is also a big influencing factor on the difficulty of early diagnosis of ovarian cancer. A biomarker is a kind of chemical substance reflecting the existence of tumor. Occurrence and development of tumor can be understood through the existence and changes of biomarkers, thus assisting diagnosis, classification and prognosis judgment on tumor and guidance on tumor treatment.[11] The ideal biomarker needs good specificity, high sensitivity and should be closely related to tumor progression.
Ovarian cancer-related biomarkers at present are mainly carbohydrate antigen, lysophosphatide acid, human epididymis protein 4, nectin-4 and tyrosine kinase receptor, etc.[12] Until now, diagnostic index that is used in clinical for diagnosis, recurrence and prognosis of patients with ovarian cancer is still CA-125 only. However, CA-125 has many limitations in the diagnosis of ovarian cancer. First, its diagnostic sensitivity is low. The preoperative diagnostic sensitivity for patients with ovarian cancer of stage I and II is lower than 60%,[13] so it cannot realize the early detection on small and curable cancer. In addition, its diagnostic specificity is poor. CA-125 is increased to different degrees in lung cancer, liver cancer, pancreatic cancer, cervical cancer, colorectal cancer, and other malignant tumors,[14] so there are some false positives.[15,16]
For these reasons, European Group on Tumor Marker and National Academy of Clinical Biochemistry do not claim to use CA-125 as biomarkers in early diagnosis of ovarian cancer.[17,18] Therefore, a lot of researchers are devoted to seeking a new diagnostic biomarker with strong specificity.
MICRORNA - NEW BIOMARKER OF OVARIAN CANCER
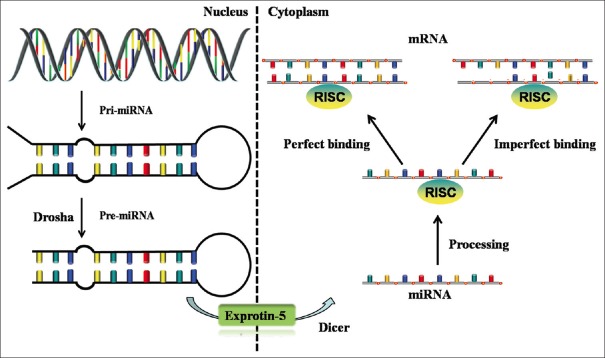
miRNA was discovered by Victor Ambros et al.[19] in 1993 in their study on lin-14 gene regulating development of nematodes. Mature miRNA is a kind of endogenous, noncoding, single-stranded small RNA, which is composed of about 20–22 nucleotides.[20] In this review, we summarized the biogenesis and function of miRNA [Figure 1].[21] MiRNA is involved in many physiological processes and is expressed abnormally in a variety of pathological conditions. These abnormal expressions of miRNA are closely related to the occurrence and development, and diagnosis and prognosis of the human disease, and form the specific characteristics of diseases spectrum. These characteristics contribute in the high specificity of miRNA to target gene. Compared to protein markers, miRNA changes before the protein and along with the progress of tumor.[22] In addition, miRNA is small and can effectively avoid degradation in an endogenous and exogenous fluid, so miRNA is more stable than DNA and messenger RNA (mRNA).
Figure 1.
Overview of microRNA biogenesis and function. At first, primary microRNA transcribed by genome is processed in the nuclei by Drosha enzyme to become precursor microRNA of stem-loop structure (70–100 nucleotides). Then precursor microRNA is transferred from nuclei to cytoplasm via RanGTP/exprotin-5 transport mechanism (RanGTP concentration is higher in nuclei, and exprotin-5 can promote precursor microRNA to release from Drosha complex and bring it to the outside by combining with it; while RanGTP is lower in cytoplasm and precursor microRNA is released by exprotin-5). At last, precursor microRNA is digested by another double-stranded RNA-specific ribonuclease (Dicer) to finally obtain the mature microRNA consisting of 20–22 nucleotides. MicroRNAs combined with RNA-induced silencing complex and bind to 3’-untranslated region of target messenger RNA. These mature microRNAs may set up complete or incomplete base pairing with messenger RNA to degrade target messenger RNA or lead to inhibitions on translation and further regulate the gene expressions after gene transcription.
Different kinds of tumors have different miRNA expression spectrum. A lot of studies over the comparison between normal ovary tissue and ovarian cancer tissues in miRNA expression showed that there were significant changes of miRNA in ovarian cancer tissue. Iorio et al.[23] compared 29 tissue miRNAs between epithelial ovarian cancer patients and healthy controls. The results showed that only 4 miRNAs (miR-141, miR-200a, miR-200b, and miR-200c) were up-regulated, and 25 miRNAs were down-regulated, where miR-125b-1, miR-140, miR-145, and miR-199a had the highest multiples of down-regulation. They suggested that the aberrant expression of miRNAs could exert on the pathogenesis and the development of different histotypes of ovarian carcinoma. Further study found that stage I epithelial ovarian cancer histotypes have their own characteristic miRNA expression and specific regulatory circuits.[24] The result showed that miR-30a-5p and miR-30a-3p were increased in clear cell histotype, indicated that miR-30a-5p and miR-30a-3p could be a stage-independent clear cell marker; whereas miR-192 and miR-194 were significantly highly expressed in mucinous histotype, suggested that miR-192 and miR-194 were key markers of mucinous subtypes. Recently, multiple studies prove that miRNA expression signature can be identified as potential prognostic indicators, which are summarized in Table 1.[25,26,27,28,29,30,31,32]
Table 1.
Potential prognostic miRNAs for ovarian cancer
| Tumor histology (n) | miRNA | Endpoint | Prognosis | Reference |
|---|---|---|---|---|
| EOC (48), PPC (7) | miR-200a, miR-200b, miR-429 | Overall survival | Good | [25], 2009 |
| SAC (185) | miR-410, miR-645 | Overall survival | Good | [26], 2011 |
| EOC (98) | miR-100 | Overall survival | Good | [27], 2012 |
| EOC (176) | miR-187 | Overall survival, recurrence-free survival | Good | [28], 2012 |
| EOC (100) | miR-150 | Overall survival, progression-free survival | Good | [29], 2014 |
| EOC (36) | miR-335 | Overall survival, recurrence-free survival | Good | [30], 2014 |
| EOC (146) | miR-196a | Overall survival, recurrence-free survival | Poor | [31], 2015 |
| EOC (211) | let-7a | Overall survival | Good | [32], 2015 |
SAC: Serous adenocarcinoma; EAC: Endometrioid adenocarcinoma; miRNAs: MicroRNAs; PPC: Primary peritoneal carcinomas.
Tissue miRNA cannot be used for early detection because tissue samples at or post diagnosis are required. Studies have shown that serum/plasma miRNA has obvious advantages as a tumor marker:[33] First, miRNA is involved in all stages of tumor including occurrence, development, and metastasis, with good specificity; next, serum/plasma miRNA, both stable and insensitive to ribonuclease, temperature, and pH, can be preserved for a long time while can be frozen and unfrozen repeatedly; finally, it is simple to detect serum/plasma miRNA, with low cost. These features make the serum/plasma miRNA become a promising potential noninvasive diagnostic marker. Resnick et al.[34] detected serum miRNA of 28 patients with serous ovarian cancer and 15 normal controls. The result showed that serum miR-92, miR-93, miR-21, miR-29b, and miR-126 in patients with ovarian cancer were significantly highly expressed, and miR-99b, miR-127, and miR-155 were lower expressed. It is worth mentioning that in these patients, miR-92, miR-93, and miR-21 have appeared before the increase of CA-125, suggesting these three kinds of miRNA can be used as the early diagnosis marker for serous ovarian cancer. Häusler et al.[35] using a miRNA microarray to analyze the whole blood-derived miRNA expression in 24 cases of serous ovarian cancer patients and 15 cases of healthy controls (HCs). The authors reported that miR-30c-1-3p was highly expressed and miR-181a-3p, miR-342-3p, and miR-450-5p were lowly expressed in serous ovarian cancer patients. In addition, these miRNAs had good specificity (88.1%) and sensitivity (86.7%) when compared with control group. Chung et al.[36] compared 2222 kinds of miRNAs between 18 ovarian cancer patients and 12 HCs. Four miRNAs (miR-132, miR-26a, let-7b, miR-145, and miR-143) were significantly under-expressed in the serum of serous ovarian cancer patients compared to controls. The results suggested that these miRNAs could be used as novel biomarkers of serous ovarian cancer. Zheng et al.[37] found that plasma miR-205 was up-regulated and plasma let-7f was down-regulated in ovarian cancer cases than in controls, and combined these miRNAs could be useful biomarkers for ovarian cancer detection, especially in patients with stage I disease (specificity was 77.8% and sensitivity was 90.0%), they also found that let-7f may be predictive of ovarian cancer prognosis. Hong et al.[38] showed that serum miR-221 may have a role as a noninvasive diagnostic marker in epithelial ovarian cancer. Furthermore, the over-expression of miR-221 was an independent unfavorable prognostic factor in epithelial ovarian cancer. Zuberi et al.[39] reported that the expression of miR-200a, miR-200b, and miR-200c were significantly up-regulated in the serum of epithelial ovarian cancer patients compared with normal controls. Interestingly, the results also showed that miR-200a over-expression was found be associated with tumor, histology, and stage; miR-200c over-expression was found be associated with lymph node metastasis. They suggested that serum miR-200a, miR-200b, and miR-200c were recognized as reliable markers to predict the prognosis and survival in epithelial ovarian cancer patients. Liang et al.[40] showed that serum miR-145 could discriminate patients with ovarian cancer from HCs, suggested that it could potentially serve as an outstanding biomarker for ovarian cancer. Gao et al.[41] reported that serum miR-200c and miR-141 were significantly increased in ovarian cancer patients compared with controls. In particular, the group with high miR-200c level achieved significantly a higher 2-year survival rate, while low miR-141 group showed a significantly higher survival rate. The results showed that miR-200c and miR-141 might be predictive biomarkers for ovarian cancer prognosis. All these studies prove the thought that the detection of ovarian cancer-associated miRNAs from the peripheral blood can be an important method for early diagnosis of this disease. These are summarized in Table 2.[34,35,36,37,38,39,40,41]
Table 2.
Potential diagnostic miRNAs for ovarian cancer
| Tumor histology | Sample | Elevated miRNA | Decreased miRNA | Control | References |
|---|---|---|---|---|---|
| SAC | Serum | miR-21, miR-92, miR-93, miR-126, miR-29b | miR-155, miR-127, miR-99b | HC | [34], 2009 |
| SAC | Whole blood | miR-30c-1-3p | miR-181a-3p, miR-342-3p, miR-450-5p | HC | [35], 2010 |
| SAC | Serum | miR-132, miR-26a, let-7b, miR-145 | HC | [36], 2013 | |
| SAC, MAC | Plasma | miR-205 | let-7f | HC | [37], 2013 |
| SAC, other | Serum | miR-221 | HC | [38], 2013 | |
| MAC | Serum | miR-200a, miR-200b, miR-200c | HC | [39], 2015 | |
| SAC, MAC, CAC, EAC, mixed | Serum | miR-145 | HC | [40], 2015 | |
| SAC, MAC, CAC, EAC, other | Serum | miR-200c, miR-141 | HC | [41], 2015 |
SAC: Serous adenocarcinoma; CAC: Clear cell adenocarcinoma; EAC: Endometrioid adenocarcinoma; MAC: Mucinous adenocarcinoma; HC: Healthy control; miRNAs: MicroRNAs.
miRNA plays a biological role via combining with 3′-untranslated region (3′UTR) of target mRNA, and then triggering either mRNA degradation or translational repression. Therefore, it can function as master coordinators, efficiently regulating and coordinating multiple cellular pathways and processes. Increasing evidence has led to the view that the expression of miRNAs is remarkably deregulated in ovarian cancer, suggesting that miRNAs may play important roles in multiple biological processes such as angiogenesis,[42] adhesion,[43] migration,[43,44] cell proliferation,[45] etc., and intervention on miRNA expression of known target genes and potential target genes can be novel treatment strategies for ovarian cancer therapy, as summarized in Table 3.[42,43,44,45,46,47,48,49]
Table 3.
Potential therapeutic miRNAs for ovarian cancer
| miRNA | Target gene | Cell line | Cellular function | References |
|---|---|---|---|---|
| miR-484 | VEGFB, VEGFR2 | MDAH-2774 (EOC), SKOV-3 (EOC) | Angiogenesis | [42], 2013 |
| miR-92a | ITGA5 | HeyA-8 (SAC), SKOV3ip1 (SAC), OVISE (CAC), RMG-1 (CAC), A2780 (SAC), CaOV3 (SAC) | Adhesion, migration | [43], 2013 |
| miR-200a, miR-200b | IL-8, CXCL1 | OVCAR8-ip1 (SAC), ES2 (SAC), HeyA-8 (SAC), OVCAR5 (SAC), A2780 (SAC), A2774 (SAC), IGROV1-ap1 (SAC), SKOV3ip1 (SAC) | Angiogenesis, migration | [44], 2013 |
| miR-506 | CDK4, CDK6 | SKOV3 (EOC), OVCA432 (EOC), OVCA433 (EOC), HeyA-8 (SAC) | Proliferation, senescence | [45], 2014 |
| miR-199b-5p | JAG1 | A2780 (EOC), SKOV3 (EOC), ES 2 (EOC) | Chemoresistance | [46], 2014 |
| miR-939 | APC2 | SKOV-3 (EOC), CAOV-3 (EOC), HO-8910 (EOC), ES-2 (EOC), A2780/DDP (EOC) | Proliferation | [47], 2015 |
| miR-661 | INPP5J | COV362 (EOC), SKOV3 (EOC), A2780 (EOC), OVCAR4 (EOC), EFO-21 (EOC), EFO-27 (EOC), TOV-21G (EOC), OV90 (EOC), OV56 (EOC) | Proliferation | [48], 2015 |
| miR-205 | VEGFR1, VEGFR2 | HO-8910PM (SAC), SKOV-3ip (SAC) | Invasion | [49], 2015 |
EOC: Epithelial ovarian carcinomas; SAC: Serous adenocarcinoma; CAC: Clear cell adenocarcinoma; miRNAs: MicroRNAs.
MICRORNA DETECTION METHODS FOR EARLY DIAGNOSIS OF OVARIAN CANCER
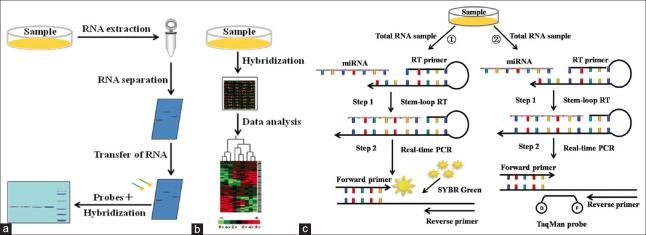
How to realize the early diagnosis of ovarian cancer and excellent detection method are the important factors to improve the detection sensitivity and specificity. The sequence of mature miRNA is generally very short, and there is interference of cross-hybridization of sequences between homologous miRNAs, so the new challenge is put forward in detection sensitivity and specificity, etc. A large number of miRNA detection methods are emerging in recent years. Common miRNA detection methods mainly include northern blotting, microarray, and reverse transcription-polymerase chain reaction (RT-PCR). Northern blotting can identify the expression of the corresponding gene by detecting the expression of RNA, and it can realize a semi-quantitative analysis of miRNA by combing with RNA marker. Lu et al.[50] applied northern blotting to explore the construction of eukaryotic expression vector miR-101 and its expression in human placental villi carcinoma, and the results showed miR-101 can significantly inhibit translation of endogenous EZH2, thus regulating tumor occurrence and infiltration. Northern blotting does not need to preamplify miRNA samples, but its sensitive is low, and the requirement of the sample is large. The microarray is a main technology realizing an analysis of miRNA expression spectrum and high-throughput detection of a variety of miRNAs at the same time. Its basic principle is based on the molecular hybridization technology. It can obtain expression spectrum of different miRNA specimens through detection on the intensity of hybridization signals, thus comparing the expression differences in miRNA specimens. Sorrentino et al.[51] used microarray to analyze the mechanisms of let-7e, miR-30c, miR-125b, miR-130a and miR-335 in drug-resistant cells A2780TAX, A2780TC1, A2780TC3 (paclitaxel), and A2780CIS (resistance to cisplatin), and found that down-regulation of miRNA can activate chemotherapy-resistance gene M-CSF. This result showed that resistance to drug of ovarian cancer was associated with the miRNA expression spectrum. Through microarray has the advantage of high flux, it is susceptible to the interference of cross hybridization of homologous miRNA sequences, and the equipment are expensive. RT-PCR can observe the real-time signal changes during the experiment by fluorescence signal changes. It has high sensitivity and practicability. Currently, main fluorescent quantitative PCR methods include fluorochrome method and hydrolysis probe method. The commonly used fluorochrome is SYBR Green. For example, Lou et al.[52] used SYBR Green I for fluorescence quantitative detection and found that low expression of miR-21 can obviously increase apoptosis of papillary ovarian cells OVCAR3 in vitro and reduce proliferation, invasion and migration ability of OVCAR3 cells. Suryawanshi et al.[53] used SYBR Green ROX for fluorescence quantitative detection and found low expressions of miR-15 and miR-16 in ovarian cancer. Moreover, it results in the growth of tumor cells by combining with 3′UTR in Brni-1 gene, suggesting tumor suppressor role of miR-15 and miR-16. The advantage of SYBR Green is easy to use. It does not need to design complex fluorescent, so it simplifies the detection methods and reduces the cost. However, it has a certain inhibition on PCR. Moreover, the fluorescence intensity is low and instable. TaqMan probe is the representative of hydrolysis probes. Cittelly et al.[54] confirmed ovarian cancer patients with low expression of targets (TUBB3) of antimicrotubular drugs had obviously longer survival period than patients with high expression of TUBB3 by TaqMan probe, suggesting miR-200c can increase the sensitivity of ovarian cancer tissues to chemotherapy drug taxol via target regulation on TUBB3. The emergence of TaqMan probe solves the non-specificity of fluorescent. At the reaction end, melting curve analysis is not needed, so the experimental time is shortened. But its price is high, and it is only suitable for a specific target and inconvenience to popularize and application. In addition, fluorescence group and quenching group on both sides of TaqMan probe are close to each other, so incomplete quenching results in high background. Since mature miRNA molecules only have 22 bases and the sequence is short, amplification reaction cannot be directly implemented by PCR. Figure 2 shows the schematic of these methods for expression profiling of miRNAs.[55]
Figure 2.
Schematic description of the methods for expression profiling of microRNAs. (a) Northern blotting method; (b) Microarray method; (c) Two reverse transcription-polymerase chain reaction methods: (1) The SYBR Green reverse transcription-polymerase chain reaction method; (2) The TaqMan probe reverse transcription-polymerase chain reaction method.
With the development of nano science and technology, researchers have developed more and more new methods used in detection on sensitivity and specificity of miRNAs. These methods can be divided into two categories, one detection method is based on the sample amplification reaction; another detection method is based on the probe hybridization (sample without amplification).
Sample amplification detection methods mainly include rolling circle amplification (RCA), hybridization chain reaction (HCR) and ligase chain reaction (LCR), etc. RCA is an isothermal amplification technique copying circular template based on displacement activity of DNA polymerase chain. Cheng et al.[56] induced RCA with miRNA as a primer. Moreover, the amplified product further induced branch rolling amplification as a primer, thus realizing detection on sensitivity and specificity of miRNA. Chapin and Doyle[57] established a new RCA method on this basis. They used a probe that was fixed in hydrogel particles to capture miRNA and can quantitatively detect a variety of miRNAs. This method is of high sensitivity and good specificity. It can distinguish the mismatching of a base and the linear range crosses 6 magnitudes (300 aM-40 pM). HCR triggers long chain of nucleic acids with a gap by trigger chain, and achieve amplification detection on the target by competitive hybridization between nucleic acids. Yang et al.[58] established a new method to detect miRNA based on the differences between grapheme oxide and single and double chains in acting forces and amplification of HCR signals. Ge et al.[59] designed a probe based on the tetrahedron nanostructures of DNA. The new probe and HCR amplification method can largely increase the sensitivity of detection on miRNA. And the detection limit can reach 0.1 fM. LCR uses DNA ligase to build covalent phosphate bond, which connects double-stranded DNA specifically. After heating denaturation, annealing, and connection, cycles is repeated for many times, thus target DNA is amplified to a large amount. Yuan et al.[60] established a new method detecting miRNA that was of high sensitivity by connecting LCR and fluorescence resonance energy transfer of cationic conjugated polymer assisted by lambda exonuclease. The new method can achieve high sensitivity detection on target miRNA (0.1 fM).
Probe hybridization is a direct detection method, we do not need to be preamplified RNA samples. These methods mainly include nano-gold labeling technique and direct quantitative analysis of multiple miRNAs (DQAMmiR). The basic principle of nano-gold labeling technique is based on the molecular hybridization technology. Alkyl thiol modified oligonucleotides can be combined with nano-gold covalent, and then the probe may be formed with the target miRNA sequence hybridization. Degliangeli et al.[61] designed a probe based on the DNA-gold nanoparticle, which is immobilized on PEGylated gold nanoparticles (AuNPs). The DNA − AuNP probes can achieve high sensitivity detection on miR-203, and the detection limit can reach 0.2 fM. This new method can significantly advance the use of miRNAs as biomarkers in the clinical praxis. DQAMmiR is the new method to measure accurately amounts of multiple miRNAs in biological samples.[62] MiRNAs and excessive DNA probe hybridization, and separation by capillary electrophoresis. Due to different miRNAs corresponding modified amino acid DNA probe length is different, so the migration velocity also different. Using this method can simultaneously detect more than 10 kinds of miRNAs.
With the rapid development of the miRNAs detection technology, this is widely used in many aspects of cancer early detection. However, it is worth pointing out that PubMed search with “miRNAs and cancer” lists around 16,717 publications, whereas search with the term “miRNAs and ovarian cancer” lists around 616 publications. Obviously, miRNAs have great prospects in the early detection of ovarian cancer.
CONCLUSIONS AND PERSPECTIVES
Ovarian cancer has characteristics of high malignant degree, poor prognosis, and high mortality rate. In clinical, image detection is often used to find early the disease. Since miRNA is closely related to the occurrence and development of ovarian cancer, its expression is stable, and can be used as a marker for early diagnosis of ovarian cancer. Emerging evidence strongly supports the miRNAs have the potential to be useful diagnostic and prognostic biomarkers. Thus, in the near future, miRNA therapy could be a powerful tool for ovarian cancer prevention and treatment. It is worth noting that with the in-depth research of miRNAs, it is found the expression of miRNA is different in serum and plasma, and in patients’ body fluid including ascites, pleural effusion, urine and saliva, etc. It can be imagined that this simple, convenient and less painful test method has profound significance for diagnosis, treatment, and prognosis for patients with ovarian cancer.
On the other hand, as miRNA plays more and more important role in early diagnosis of ovarian cancer. Although miRNA detection technologies are quickly developed, these new miRNA detections have not yet been used widely in early diagnosis of ovarian cancer. Therefore, by combining the new analysis technology and new nano materials, point-of-care tests for miRNA with high throughput, high sensitivity, and strong specificity are developed to achieve the application of diagnostic kits in the screening of early ovarian cancer.
Financial support and sponsorship
This work was supported by a grant from Shanghai Health System Important Disease Joint Research Project (No. 2013ZYJB0201).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Scholz HS, Tasdemir H, Hunlich T, Turnwald W, Both A, Egger H. Multivisceral cytoreductive surgery in FIGO stages IIIC and IV epithelial ovarian cancer: Results and 5-year follow-up. Gynecol Oncol. 2007;106:591–5. doi: 10.1016/j.ygyno.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 3.Hensley ML. A step forward for two-step screening for ovarian cancer. J Clin Oncol. 2010;28:2128–30. doi: 10.1200/JCO.2009.26.6346. [DOI] [PubMed] [Google Scholar]
- 4.Kinose Y, Sawada K, Nakamura K, Kimura T. The role of microRNAs in ovarian cancer. Biomed Res Int 2014. 2014 doi: 10.1155/2014/249393. 249393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurie M. Development of an ovarian cancer symptom index: Possibilities for earlier detection. Cancer. 2007;110:226–7. doi: 10.1002/cncr.22749. [DOI] [PubMed] [Google Scholar]
- 6.Walczewska M, Mocarska A, Burdan F, Janczarek M, Zelazowska-Cieslinska I, Staroslawska E. Diagnosis of benign ovarian lesions using imaging techniques. Pol Merkur Lekarski. 2015;38:55–60. [PubMed] [Google Scholar]
- 7.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng JH. Comparison of diagnostic value of CT and MRI for ovarian cancer. Pract J Cancer. 2015;1:034. [Google Scholar]
- 9.Lambregts DM, Cappendijk VC, Maas M, Beets GL, Beets-Tan RG. Value of MRI and diffusion-weighted MRI for the diagnosis of locally recurrent rectal cancer. Eur Radiol. 2011;21:1250–8. doi: 10.1007/s00330-010-2052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: Comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med. 2015;40:371–7. doi: 10.1097/RLU.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 11.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LL. Advances on tumor markers for diagnosis of ovarian cancer. Int J Gynecol Obestet. 2012;39:348–51. [Google Scholar]
- 13.Yurkovetsky ZR, Linkov FY, E Malehorn D, Lokshin AE. Multiple biomarker panels for early detection of ovarian cancer. Future Oncol. 2006;2:733–41. doi: 10.2217/14796694.2.6.733. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XL, Zhou JP, Wang CM, Meng DP. Analysis of the clinical value of carbohydrate antigen 125 with multiple tumor markers protein biochip detective system. J Mod Oncol. 2007;15:415–6. [Google Scholar]
- 15.Block MS, Maurer MJ, Goergen K, Kalli KR, Erskine CL, Behrens MD, et al. Plasma immune analytes in patients with epithelial ovarian cancer. Cytokine. 2015;73:108–13. doi: 10.1016/j.cyto.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadducci A, Menichetti A, Guiggi I, Notarnicola M, Cosio S. Correlation between CA125 levels after sixth cycle of chemotherapy and clinical outcome in advanced ovarian carcinoma. Anticancer Res. 2015;35:1099–104. [PubMed] [Google Scholar]
- 17.Duffy MJ, Bonfrer JM, Kulpa J, Rustin GJ, Soletormos G, Torre GC, et al. CA125 in ovarian cancer: European group on tumor markers guidelines for clinical use. Int J Gynecol Cancer. 2005;15:679–91. doi: 10.1111/j.1525-1438.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- 18.Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brünner N, Chan DW, et al. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54:e11–79. doi: 10.1373/clinchem.2008.105601. [DOI] [PubMed] [Google Scholar]
- 19.Lee RC, Feinbaum RL, Ambros V. The C.elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 21.Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142–65. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 22.Wittmann J, Jäck HM. Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta. 2010;1806:200–7. doi: 10.1016/j.bbcan.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 24.Calura E, Fruscio R, Paracchini L, Bignotti E, Ravaggi A, Martini P, et al. miRNA landscape in stage I epithelial ovarian cancer defines the histotype specificities. Clin Cancer Res. 2013;19:4114–23. doi: 10.1158/1078-0432.CCR-13-0360. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–64. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Shih KK, Qin LX, Tanner EJ, Zhou Q, Bisogna M, Dao F, et al. A microRNA survival signature (MiSS) for advanced ovarian cancer. Gynecol Oncol. 2011;121:444–50. doi: 10.1016/j.ygyno.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Peng DX, Luo M, Qiu LW, He YL, Wang XF. Prognostic implications of microRNA-100 and its functional roles in human epithelial ovarian cancer. Oncol Rep. 2012;27:1238–44. doi: 10.3892/or.2012.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao A, Lin CY, Lee YS, Tsai CL, Wei PC, Hsueh S, et al. Regulation of ovarian cancer progression by microRNA-187 through targeting Disabled homolog-2. Oncogene. 2012;31:764–75. doi: 10.1038/onc.2011.269. [DOI] [PubMed] [Google Scholar]
- 29.Jin M, Yang Z, Ye W, Xu H, Hua X. MicroRNA-150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PLoS One. 2014;9:e103965. doi: 10.1371/journal.pone.0103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao J, Cai J, Huang D, Han Q, Chen Y, Yang Q, et al. miR-335 represents an independent prognostic marker in epithelial ovarian cancer. Am J Clin Pathol. 2014;141:437–42. doi: 10.1309/AJCPLYTZGB54ISZC. [DOI] [PubMed] [Google Scholar]
- 31.Fan Y, Fan J, Huang L, Ye M, Huang Z, Wang Y, et al. Increased expression of microRNA-196a predicts poor prognosis in human ovarian carcinoma. Int J Clin Exp Pathol. 2015;8:4132–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, Katsaros D, Risch HA, Canuto EM, Biglia N, Yu H, et al. MicroRNA let-7a Modifies the effect of self-renewal gene HIWI on patient survival of epithelial ovarian cancer. Mol Carcinog. doi: 10.1002/mc.22285. Epub 2015 Jan 28. [DOI] [PubMed] [Google Scholar]
- 33.Cortez MA, Welsh JW, Calin GA. Circulating microRNAs as noninvasive biomarkers in breast cancer. Recent Results Cancer Res. 2012;195:151–61. doi: 10.1007/978-3-642-28160-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112:55–9. doi: 10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Häusler SF, Keller A, Chandran PA, Ziegler K, Zipp K, Heuer S, et al. Whole blood-derived miRNA profiles as potential new tools for ovarian cancer screening. Br J Cancer. 2010;103:693–700. doi: 10.1038/sj.bjc.6605833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung YW, Bae HS, Song JY, Lee JK, Lee NW, Kim T, et al. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patients. Int J Gynecol Cancer. 2013;23:673–9. doi: 10.1097/IGC.0b013e31828c166d. [DOI] [PubMed] [Google Scholar]
- 37.Zheng H, Zhang L, Zhao Y, Yang D, Song F, Wen Y, et al. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS One. 2013;8:e77853. doi: 10.1371/journal.pone.0077853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong F, Li Y, Xu Y, Zhu L. Prognostic significance of serum microRNA-221 expression in human epithelial ovarian cancer. J Int Med Res. 2013;41:64–71. doi: 10.1177/0300060513475759. [DOI] [PubMed] [Google Scholar]
- 39.Zuberi M, Mir R, Das J, Ahmad I, Javid J, Yadav P, et al. Expression of serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological features. Clin Transl Oncol. 2015;17:779–87. doi: 10.1007/s12094-015-1303-1. [DOI] [PubMed] [Google Scholar]
- 40.Liang H, Jiang Z, Xie G, Lu Y. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumour Biol. 2015;36:5305–13. doi: 10.1007/s13277-015-3191-y. [DOI] [PubMed] [Google Scholar]
- 41.Gao YC, Wu J. MicroRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer. Tumour Biol. 2015;36:4843–50. doi: 10.1007/s13277-015-3138-3. [DOI] [PubMed] [Google Scholar]
- 42.Vecchione A, Belletti B, Lovat F, Volinia S, Chiappetta G, Giglio S, et al. A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc Natl Acad Sci U S A. 2013;110:9845–50. doi: 10.1073/pnas.1305472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohyagi-Hara C, Sawada K, Kamiura S, Tomita Y, Isobe A, Hashimoto K, et al. miR-92a inhibits peritoneal dissemination of ovarian cancer cells by inhibiting integrin a5 expression. Am J Pathol. 2013;182:1876–89. doi: 10.1016/j.ajpath.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 44.Pecot CV, Rupaimoole R, Yang D, Akbani R, Ivan C, Lu C, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:275–89. doi: 10.1038/ncomms3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang D, et al. MiR-506 suppresses proliferation and induces senescence by directly targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol. 2014;233:308–18. doi: 10.1002/path.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu MX, Siu MK, Liu SS, Yam JW, Ngan HY, Chan DW. Epigenetic silencing of microRNA-199b-5p is associated with acquired chemoresistance via activation of JAG1-Notch1 signaling in ovarian cancer. Oncotarget. 2014;5:944–58. doi: 10.18632/oncotarget.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ying X, Li-ya Q, Feng Z, Yin W, Ji-hong L. MiR-939 promotes the proliferation of human ovarian cancer cells by repressing APC2 expression. Biomed Pharmacother. 2015;71:64–9. doi: 10.1016/j.biopha.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Zhu T, Yuan J, Wang Y, Gong C, Xie Y, Li H. MiR-661 contributed to cell proliferation of human ovarian cancer cells by repressing INPP5J expression. Biomed Pharmacother. 2015;75:123–8. doi: 10.1016/j.biopha.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Li L, Li Z, Gong G, Chen P, Liu H, et al. The role of miR-205 in the VEGF-mediated promotion of human ovarian cancer cell invasion. Gynecol Oncol. 2015;137:125–33. doi: 10.1016/j.ygyno.2015.01.531. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Liu ZX, Liu T, Cheng WW, Gao YT, Wang H. The built eukaryotic expression vector of microRNA-101 and the expression in the human placenta carcinoma cells (in Chinese) Chin J Clin Med. 2010;17:627–30. [Google Scholar]
- 51.Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol. 2008;111:478–86. doi: 10.1016/j.ygyno.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Lou Y, Cui Z, Wang F, Yang X, Qian J. miR-21 down-regulation promotes apoptosis and inhibits invasion and migration abilities of OVCAR3 cells. Clin Invest Med. 2011;34:281–9. doi: 10.25011/cim.v34i5.15671. [DOI] [PubMed] [Google Scholar]
- 53.Suryawanshi S, Vlad AM, Lin HM, Mantia-Smaldone G, Laskey R, Lee M, et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res. 2013;19:1213–24. doi: 10.1158/1078-0432.CCR-12-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cittelly DM, Dimitrova I, Howe EN, Cochrane DR, Jean A, Spoelstra NS, et al. Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol Cancer Ther. 2012;11:2556–65. doi: 10.1158/1535-7163.MCT-12-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Planell-Saguer M, Rodicio MC. Analytical aspects of microRNA in diagnostics: A review. Anal Chim Acta. 2011;699:134–52. doi: 10.1016/j.aca.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 56.Cheng Y, Zhang X, Li Z, Jiao X, Wang Y, Zhang Y. Highly sensitive determination of microRNA using target-primed and branched rolling-circle amplification. Angew Chem Int Ed Engl. 2009;121:3318–22. doi: 10.1002/anie.200805665. [DOI] [PubMed] [Google Scholar]
- 57.Chapin SC, Doyle PS. Ultrasensitive multiplexed microRNA quantification on encoded gel microparticles using rolling circle amplification. Anal Chem. 2011;83:7179–85. doi: 10.1021/ac201618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang L, Liu C, Ren W, Li Z. Graphene surface-anchored fluorescence sensor for sensitive detection of microRNA coupled with enzyme-free signal amplification of hybridization chain reaction. ACS Appl Mater Interfaces. 2012;4:6450–3. doi: 10.1021/am302268t. [DOI] [PubMed] [Google Scholar]
- 59.Ge Z, Lin M, Wang P, Pei H, Yan J, Shi J, et al. Hybridization chain reaction amplification of microRNA detection with a tetrahedral DNA nanostructure-based electrochemical biosensor. Anal Chem. 2014;86:2124–30. doi: 10.1021/ac4037262. [DOI] [PubMed] [Google Scholar]
- 60.Yuan Z, Zhou Y, Gao S, Cheng Y, Li Z. Homogeneous and sensitive detection of microRNA with ligase chain reaction and lambda exonuclease-assisted cationic conjugated polymer biosensing. ACS Appl Mater Interfaces. 2014;6:6181–5. doi: 10.1021/am500883q. [DOI] [PubMed] [Google Scholar]
- 61.Degliangeli F, Kshirsagar P, Brunetti V, Pompa PP, Fiammengo R. Absolute and direct microRNA quantification using DNA-gold nanoparticle probes. J Am Chem Soc. 2014;136:2264–7. doi: 10.1021/ja412152x. [DOI] [PubMed] [Google Scholar]
- 62.Cherney LT, Krylov SN. Theoretical estimation of drag tag lengths for direct quantitative analysis of multiple miRNAs (DQAMmiR) Analyst. 2013;138:553–8. doi: 10.1039/c2an36296a. [DOI] [PubMed] [Google Scholar]