Abstract
Background:
The factors affecting the outcome of patients referred for lung transplantation (LTx) still have not been investigated extensively. The aim of this study was to characterize the patient outcomes and identify the prognostic factors for death while awaiting the LTx.
Methods:
From January 2003 to November 2013, the clinical data of 103 patients with end-stage lung disease that had been referred for LTx to Department of Thoracic Surgery, Shanghai Pulmonary Hospital were analyzed retrospectively. The relationship between predictors and survival was evaluated using the Kaplan–Meier method and the Cox proportional hazards model.
Results:
Twenty-five patients (24.3%) died while awaiting the LTx. Fifty patients (48.5%) underwent LTx, and 28 patients (27.2%) were still on the waitlist. Compared to the candidates with chronic obstructive pulmonary disease (COPD), patients with idiopathic pulmonary fibrosis (IPF) had a higher mortality while awaiting the LTx (40.0% vs. 12.3%, P = 0.003). Patients requiring mechanical ventilation (MV) had a higher mortality while waiting than others (50.0% vs. 20.2%, P = 0.038). Two variables, using MV and IPF but not COPD as primary disease, emerged as significant independent risk factors for death on the waitlist (hazard ratio [HR] = 56.048, 95% confidence interval [CI]: 3.935–798.263, P = 0.003 and HR = 14.859, 95% CI: 2.695–81.932, P = 0.002, respectively).
Conclusion:
The type of end-stage lung disease, pulmonary hypertension, and MV may be distinctive prognostic factors for death while awaiting the LTx.
Keywords: End-stage Lung Disease, Lung Transplantation, Prognosis, Waitlist
INTRODUCTION
In the past 30 years, lung transplantation (LTx) has emerged as a lifesaving therapy for patients with end-stage lung disease. In China, the first successful clinical procedure was performed in 1979.[1] An increasing number of lung transplants have been performed during the last decade. The growing number of LTx and the relative scarcity of suitable donor lungs have resulted in a longer wait-time for transplantation and an increasing mortality during the waiting period. Apart from the lung allocation score (LAS) in North America, no other reasonable score system is available in other countries, including China. LAS takes the type of native disease and medical urgency into account, but the specific factors affecting the survival in patients referred for LTx still have not been investigated extensively. There should be some differences in the spectrum of native disease, economic status, and health education of patients in different countries. The purpose of this retrospective study was to characterize the outcome of patients referred for LTx in the Chinese population and to identify the predictors of death while on the waitlist to be able to identify patients who should be prioritized for LTx.
METHODS
Patient population
This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine and performed in accordance with the principles of the Declaration of Helsinki. From January 2003 to November 2013, 249 patients with end-stage lung disease were registered for LTx in Shanghai Pulmonary Hospital. LTx was indicated for patients with severe pulmonary dysfunction, severe exercise intolerance, and medical treatment inefficacy.[2] Of these, 146 patients (58.6%) were excluded from the waitlist due to poor economic conditions and were unable to be followed up regularly after transplantation. Therefore, only 103 patients were enrolled in this study. The clinical data of patients were collected and reviewed, including demographic characteristics, lung function, and outcomes during the waiting period.
Pretransplantation assessment
These patients underwent a battery of examinations before transplantation, including routine blood tests, liver and renal function tests, pulmonary function tests, arterial blood gas analysis, 6-min walk test, chest imaging examination (plain chest X-ray and computed tomography), ventilation-perfusion lung scan, fiberoptic bronchoscopy, abdominal ultrasound scan, ultrasonic cardiogram, electrocardiogram, cardiac catheterization, and nutrition status evaluation.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation (SD) or median (range) and analyzed by the Student–Newman–Keuls or the Mann–Whitney U-test. Categorical variables were presented as numerical values and percentages and analyzed using Pearson's Chi-square or Fisher's exact test. Survival analysis was performed using the Kaplan–Meier method and compared with the log-rank test. All tests were two-sided. A P < 0.05 was considered statistically significant.
The relationship between predictors and survival was evaluated by Cox proportional hazards model. The patients that were transplanted were censored at the time of transplantation. In this model, the predictors included primary lung diseases (dummy variables for idiopathic pulmonary fibrosis [IPF] and “Other” with chronic obstructive pulmonary disease [COPD] as the reference), mechanical ventilation (MV), steroid use, oxygen use, mean pulmonary arterial pressure (mPAP), PaO2/FiO2, forced expiratory volume in 1 s of predicted value, blood type, body mass index, hypertension, and diabetes. Time zero was defined as the time from study entry to death before transplantation or to transplantation or last known contact alive.
RESULTS
Patient characteristics
In total, 103 patients with end-stage pulmonary diseases were included in the study, including 89 males (86.4%) and 14 females (13.6%) with a mean age of 54.6 ± 11.3 years (range: 11.0–76.0 years). The characteristics of patients are presented in Table 1. The median age of COPD patients was higher than that of others (58.0 years vs. 53.5 years). Most patients came from East China (73.8%, 76/103).
Table 1.
The demographic characteristics of patients referred for lung transplantation
| Characteristics | Total (N = 103) | The survival group (n = 78) | The death group (n = 25) | Statistical values | P |
|---|---|---|---|---|---|
| Age (years) | 54.6 ± 11.3 | 54.0 ± 11.0 | 56.2 ± 12.0 | 0.854* | 0.395 |
| Male, n (%) | 89 (86.4) | 69 (88.5) | 20 (80.0) | 0.546† | 0.460 |
| BMI (kg/m2) | 20.8 ± 3.9 | 20.7 ± 3.8 | 21.2 ± 3.9 | 0.567* | 0.572 |
| 6-MWD (m) | 269.7 ± 144.2 | 257.3 ± 163.5 | 303.8 ± 76.5 | 0.538* | 0.600 |
| mPAP (mmHg) | 43.6 ± 17.2 | 42.7 ± 14.7 | 54.3 ± 25.9 | 2.307* | 0.024 |
| Creatinine (μmol/L) | 60.9 ± 20.1 | 58.2 ± 13.9 | 66.8 ± 29.0 | 1.726* | 0.089 |
| Blood type, n (%) | |||||
| A | 27 (26.2) | 21 (26.9) | 6 (24.0) | 3.443† | 0.328 |
| B | 29 (28.2) | 25 (32.1) | 4 (16.0) | ||
| AB | 11 (10.7) | 7 (9.0) | 4 (16.0) | ||
| O | 36 (35.1) | 25 (32.1) | 11 (44.0) | ||
| Lung function test | |||||
| FEV1 (L) | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.9 ± 0.5 | 0.592* | 0.556 |
| FEV1 (%), predicted | 27.0 ± 17.9 | 25.4 ± 17.1 | 32.8 ± 20.2 | 1.337* | 0.186 |
| FVC (%), predicted | 45.9 ± 18.9 | 47.6 ± 20.1 | 40.0 ± 12.6 | −1.337* | 0.186 |
| Arterial blood gas analysis | |||||
| PaO2 (mmHg) | 62.5 ± 18.3 | 63.0 ± 17.8 | 61.2 ± 20.0 | −0.434* | 0.665 |
| PaCO2 (mmHg) | 46.4 ± 15.6 | 47.4 ± 15.2 | 43.5 ± 16.7 | −1.085* | 0.281 |
| PF ratio | 201.6 ± 59.7 | 203.8 ± 56.6 | 195.0 ± 69.3 | −0.637* | 0.525 |
| Complications, n (%) | |||||
| Hypertension | 19 (18.8) | 12 (15.8) | 7 (28.0) | 1.124† | 0.289 |
| DM | 9 (8.9) | 5 (6.6) | 4 (16.0) | 1.060† | 0.303 |
| Primary diseases, n (%) | |||||
| IPF | 30 (29.1) | 18 (23.1) | 12 (48.0) | 10.150† | 0.006 |
| COPD | 57 (55.3) | 50 (64.1) | 7 (28.0) | ||
| Others | 16 (15.5) | 10 (12.8) | 6 (24.0) | ||
| Use of steroid, n (%) | 20 (19.4) | 13 (17.3) | 7 (28.0) | 1.333† | 0.248 |
| Use of MV, n (%) | 14 (13.6) | 7 (9.0) | 7 (28.0) | 4.327† | 0.038 |
All data are shown as means ± SD unless otherwise indicated. *t values; †Chi-square values. BMI: Body mass index; 6-MWD: 6-min walk distance; mPAP: Mean pulmonary arterial pressure; FEV1: Forced expiratory volume at 1 s; FVC: Forced vital capacity; HBP: High blood pressure; DM: Diabetes mellitus; COPD: Chronic obstructive pulmonary disease; IPF: Idiopathic pulmonary fibrosis; PF: Pulmonary fibrosis; MV: Mechanical ventilation; SD: Standard deviation.
Pulmonary function measurement
All patients needed oxygen therapy and 14 (13.6%) patients required MV. Forty-two patients (40.8%) were intolerant to the exercise of the pulmonary function test. The results of lung function test in the other 61 patients are shown in Table 1. Only 25 patients (24.3%) finished the 6-min walk test, the mean walking distance was 269.7 ± 144.2 m (range: 28.0–433.0 m).
Outcome and prognosis factors
The median follow-up time was 98 days (range: 1–2315 days). Twenty-five patients (24.3%) died while waiting. Fifty patients (48.5%) underwent LTx, and 28 patients (27.2%) are still on the waitlist. The mean wait-time was longer (386.2 days vs. 128.0 days, t = 2.133, P = 0.035), and the mPAP was lower (42.7 ± 14.7 mmHg vs. 54.3 ± 25.9 mmHg, P = 0.024) in the survival group than in the death group. In the death group, the mean survival time was shorter in patients with IPF than in other candidates (103.3 days vs. 154.8 days, t = 0.859, P = 0.400).
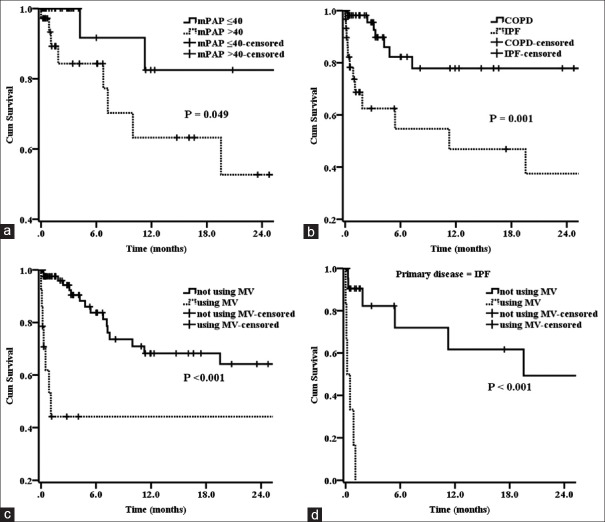
Compared to IPF patients, candidates with COPD had the higher chance of transplantation (54.4% vs. 43.3%, χ2 = 0.961, P = 0.328). However, patients with IPF had a higher mortality while awaiting LTx (40.0% vs. 12.3%, χ2 = 8.847, P = 0.003). There were significant differences in the survival rates between the primary diseases, the use of MV, and mPAP [all P < 0.05; Figure 1 and Table 1].
Figure 1.
(a-c) The differences in survival between patients with mean pulmonary arterial pressure ≤40 mmHg versus >40 mmHg, chronic obstructive pulmonary disease versus idiopathic pulmonary fibrosis, using mechanical ventilation versus not using mechanical ventilation were statistically significant (all P < 0.05). (d) The difference in survival between idiopathic pulmonary fibrosis patients using mechanical ventilation versus not using mechanical ventilation was also statistically significant (P < 0.001).
The influence of these parameters on survival while awaiting LTx was further illustrated by survival estimates using the Kaplan–Meier method [Figure 1]. Patients with mPAP ≤40 mmHg had better 1-year survival rate than those with mPAP >40 mmHg (82.5% vs. 63.2%, χ2 = 3.881, P = 0.049). Compared to those with COPD, candidates with IPF had a worse 1-year survival rate (46.9% vs. 77.9%, χ2 = 11.796, P = 0.001). There was a significant difference in 1-year survival rate between patients that required MV and those that did not (44.2% vs. 68.2%, χ2 = 15.473, P < 0.001). A stratified analysis showed that none of the patients with IPF that required MV survived to 1 year; however, the 1-year survival rate was 61.7% in those without using MV (P < 0.001).
Steroid use, mPAP level, the use of MV, and primary disease were further evaluated in the Cox proportional hazards mode, and the results are presented in Table 2. Two variables, using MV and IPF but not COPD as primary disease, emerged as significant independent risk factors for death on the waitlist (hazard ratio [HR] = 56.048, 95% confidence interval [CI]: 3.935–798.263, P = 0.003 and HR = 14.859, 95% CI: 2.695–81.932, P = 0.002, respectively).
Table 2.
Cox proportional hazards multivariable analyses results
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Use of steroid | 0.109 | 2.072 (0.851–5.043) | 0.280 | |
| mPAP >40 mmHg | 0.070 | 4.208 (0.890–19.892) | 0.261 | |
| Use of MV | <0.001 | 5.097 (2.069–12.553) | 0.003 | 56.048 (3.935–798.263) |
| Type of primary diseases | ||||
| IPF versus COPD | 0.002 | 4.448 (1.746–11.333) | 0.002 | 14.859 (2.695–81.932) |
| Others versus COPD | 0.387 | 1.721 (0.502–5.897) | 0.024 | 10.908 (1.367–87.014) |
| IPF versus others | 0.103 | 2.584 (0.827–8.077) | 0.726 | 1.362 (0.241–7.687) |
mPAP: Mean pulmonary arterial pressure; MV: Mechanical ventilation; COPD: Chronic obstructive pulmonary disease; IPF: Idiopathic pulmonary fibrosis; HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
There has been a long history of LTx in China, but so far the volume of LTx is very limited compared to that in the west countries. For a long time, Chinese legal progress in organ donation and allocation has lagged behind the advance of transplantation technique. However, in 2006 China developed a new national program for deceased-organ donation, which adheres to the World Health Organization guiding principles, is compliant with the Declaration of Istanbul, and respects the cultural and social values of the Chinese people.[3,4,5] In China, the first LTx, for which the donor lungs were obtained through brain death organ donation, was performed in 2007.[6] However, the shortage of legal and reasonable lung donors is still a fundamental bottleneck for LTx in China.
In addition to the limited reasonable lung donors, one of the most important problems hindering Chinese LTx is the patient's socioeconomic status. The Chinese government has made huge progress helping to provide citizens with medical insurance, and many diseases, including liver and renal transplantation, are covered by this insurance; however, LTx is often not covered. There is a significant demand for LTxs, but most patients with end-stage lung disease, especially in West and North China, cannot afford this expensive therapy. Most of the LTx procedures have been performed in prosperous East China, such as in Shanghai.
The timing of referral of a potential candidate to the LTx center has a great impact on patient survival during the wait period. According to data from China, the prevalence of spirometric-detected COPD in people aged ≥40 years has been reported to be 8% and COPD causes approximately 2.1 million deaths every year.[7,8] Our hospital is one of the most important medical centers for pulmonary diseases in China, but there have only been 249 referrals for LTx in our center during the last decade. The reasons vary. Besides the patients’ poor socioeconomic status, the majority of people including respiratory physicians do not know the prognosis of LTx. Many patients’ conditions were usually too severe to survive the long wait period when they were a referral for LTx. Eight patients (8/25, 32%) died in 30 days after registering for LTx in this study. Although heart-lung support technology develops rapidly along with the development of modern medicine, the mortality of severely sick patients is still very high when waiting for transplantation.[9] There were approximately 13.6% (14/103) patients that needed the MV pretransplantation in this study, and the mortality of these patients was significantly higher than that of others (50.0% vs. 20.2%, P = 0.038). Therefore, it is necessary to educate patients and their families as well as respiratory physicians that early evaluation for LTx is an important measure to decrease mortality during the wait period.
The data released by the United Network for Organ Sharing (UNOS) in America showed that the type of end-stage lung disease is an important factor influencing the prognosis of patients during the wait period.[10] The pulmonary function of patients with some diseases such as IPF usually deteriorates too rapidly to undergo transplantation. Our study revealed that compared with patients with COPD, the mortality of patients with IPF during the wait period was much higher, and patients with COPD had a higher chance of transplantation. In contrast, the survival time of patients with IPF during the wait period was obviously shorter than for other patients. The UNOS study also reported that the mortality of patients with IPF was higher during the wait period and supported that those patients should have priority in LTx. The mortality of patients with different diseases varied very much, which implied that types of diseases should be strongly considered when listing patients for transplantation.
A lot of patients have end-stage lung disease complicated with pulmonary hypertension, which may progress and result in right heart failure. Arcasoy et al.[11] believed that pulmonary artery pressure gradually increased when awaiting LTx, and it was a strong predictor of death during the wait period. Our study also demonstrated that the mPAP was significantly higher in patients who died while awaiting transplantation (P = 0.024). Therefore, it is necessary to evaluate pulmonary hemodynamics and the right heart function precisely when the patient has pulmonary hypertension during the waiting period. In recent years, with the development of extracorporeal life support system, many severely ill patients have received effective treatment. However, it is controversial in the use of extracorporeal life support system prior to LTx can improve the mortality awaiting transplantation.[12,13,14,15,16,17] In addition, due to its high cost, it is not feasible to use this support system in developing countries, including China.
In our center, except for extremely severe patients, the majority of patients were listed for transplantation based on the length of their wait-time. Although early waitlisting is advantageous for those with an early stage of their disease, there is a major disadvantage for those with rapidly progressive illness or for those with late stage of their disease. Since the LAS was developed in 2005, the wait-time for critically ill patients has been decreased, and the waitlist mortality has been significantly reduced in the USA.[9] However, in China there is no related scoring system prioritizing patients based on the urgency of their diseases. Therefore, a reasonable LAS is urgently needed, in addition to a need to increase the pool of legal lung donation. Our study revealed that 1/4 of patients died during the wait period as a result of the limited donor lungs, which was similar to results from other studies.[18,19,20]
The primary limitation of this study was that it was a retrospective single-center study, and there were censored data in the survival analysis because almost half of the candidates underwent LTx successfully. Nevertheless, our study confirmed that the type of end-stage lung disease, pulmonary hypertension and the use of MV may be distinctive prognostic factors for death on the waitlist. When determining the lung allocation schemes, the type of disease and the medical urgency of transplantation should both be taken into account. It may offer some limited evidence for the development of a future LAS in China.
Financial support and sponsorship
This work was supported by the grants from the National Natural Science Foundation of China (No. 81100061 and No. 81170075).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Xin YL. A case of human lung transplantation (author's transl) (in Chinese) Chin J Surg. 1979;17:328–32. [PubMed] [Google Scholar]
- 2.International guidelines for the selection of lung transplant candidates. The American Society for Transplant Physicians (ASTP)/American Thoracic Society (ATS)/European Respiratory Society (ERS)/International Society for Heart and Lung Transplantation (ISHLT) Am J Respir Crit Care Med. 1998;158:335–9. doi: 10.1164/ajrccm.158.1.15812. [DOI] [PubMed] [Google Scholar]
- 3.Huang JF. Pragmatic solution for organ donation in response to challenges faced by the Chinese society: Summary for the National Donation after Circulatory Death Pilot Program. Chin Med J. 2013;126:569–73. doi: 10.3760/cma.j.issn.0366-6999.20123387. [PubMed] [Google Scholar]
- 4.Huang J, Wang H, Fan ST, Zhao B, Zhang Z, Hao L, et al. The national program for deceased organ donation in China. Transplantation. 2013;96:5–9. doi: 10.1097/TP.0b013e3182985491. doi: 10.1097/TP.0b013e3182985491. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Millis JM, Mao Y, Millis MA, Sang X, Zhong S. A pilot programme of organ donation after cardiac death in China. Lancet. 2012;379:862–5. doi: 10.1016/S0140-6736(11)61086-6. doi: 10.1016/S0140-6736(11)61086-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu F, Chen JY, Ye SG, Zhang J, Chen ZH. Procurement of double lungs from brain death donor and lung transplantation: Case report. Chin J Transplant. 2009;3:30–3. doi: 10.3969/j.issn.1674-3903.2009.01.008. [Google Scholar]
- 7.Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, et al. Prevalence of chronic obstructive pulmonary disease in China: A large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–60. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 8.Reilly KH, Gu D, Duan X, Wu X, Chen CS, Huang J, et al. Risk factors for chronic obstructive pulmonary disease mortality in Chinese adults. Am J Epidemiol. 2008;167:998–1004. doi: 10.1093/aje/kwm393. doi: 10.1093/aje/kwm393. [DOI] [PubMed] [Google Scholar]
- 9.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–27. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 10.Barr ML, Bourge RC, Orens JB, McCurry KR, Ring WS, Hulbert-Shearon TE, et al. Thoracic organ transplantation in the United States, 1994-2003. Am J Transplant. 2005;5(4 Pt 2):934–49. doi: 10.1111/j.1600-6135.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 11.Arcasoy SM, Christie JD, Pochettino A, Rosengard BR, Blumenthal NP, Bavaria JE, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest. 2001;120:873–80. doi: 10.1378/chest.120.3.873. [DOI] [PubMed] [Google Scholar]
- 12.Mangi AA, Mason DP, Yun JJ, Murthy SC, Pettersson GB. Bridge to lung transplantation using short-term ambulatory extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2010;140:713–5. doi: 10.1016/j.jtcvs.2010.02.029. doi: 10.1016/j.jtcvs.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: A new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139:e137–9. doi: 10.1016/j.jtcvs.2009.12.021. doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Fadel E, Mercier O, Mussot S, Leroy-Ladurie F, Cerrina J, Chapelier A, et al. Long-term outcome of double-lung and heart-lung transplantation for pulmonary hypertension: A comparative retrospective study of 219 patients. Eur J Cardiothorac Surg. 2010;38:277–84. doi: 10.1016/j.ejcts.2010.02.039. doi: 10.1016/j.ejcts.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Strueber M, Hoeper MM, Fischer S, Cypel M, Warnecke G, Gottlieb J, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant. 2009;9:853–7. doi: 10.1111/j.1600-6143.2009.02549.x. doi: 10.1111/j.1600-6143.2009.02549.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmid C, Philipp A, Hilker M, Arlt M, Trabold B, Pfeiffer M, et al. Bridge to lung transplantation through a pulmonary artery to left atrial oxygenator circuit. Ann Thorac Surg. 2008;85:1202–5. doi: 10.1016/j.athoracsur.2007.12.032. doi: 10.1016/j.athoracsur.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Orsini B, Sage E, Olland A, Cochet E, Tabutin M, Thumerel M, et al. High-emergency waiting list for lung transplantation: Early results of a nation-based study. Eur J Cardiothorac Surg. 2014;46:e41–7. doi: 10.1093/ejcts/ezu259. doi: 10.1093/ejcts/ezu259. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Huang D, Mou X, Chen Y, Gong Y, He J. Investigation of quality of life and relevant influence factors in patients awaiting lung transplantation. J Thorac Dis. 2011;3:244–8. doi: 10.3978/j.issn.2072-1439.2010.08.03. doi: 10.3978/j.issn.2072-1439.2010.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shitrit D, Gershman Y, Peled N, Medalion B, Saute M, Amital A, et al. Risk factors for death while awaiting lung transplantation in Israeli patients: 1997-2006. Eur J Cardiothorac Surg. 2008;34:444–8. doi: 10.1016/j.ejcts.2008.03.068. doi: 10.1016/j.ejcts.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 20.Belkin RA, Henig NR, Singer LG, Chaparro C, Rubenstein RC, Xie SX, et al. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2006;173:659–66. doi: 10.1164/rccm.200410-1369OC. doi: 10.1164/rccm.200410-1369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]